Abstract
Introduction:
The ongoing marginalization of lesbian, gay, and bisexual (LGB) people has been hypothesized to produce poorer late-in-life cognitive outcomes, according to mechanisms posited by minority stress and allostatic load theories. Yet the existence of those outcomes remains understudied, and results of existing studies have been contradictory. Using a population-based longitudinal aging study, this paper will compare age at diagnosis of Alzheimer’s disease (AD) or a related dementia, and rates of cognitive decline between participants in same-sex relationships (SSRs) and different-sex relationships (DSRs).
Methods:
The study used longitudinal cognitive-health data from the Health and Retirement Study (HRS; 1998–2018; N = 26,344) to analyze the onset of cognitive impairment and AD/dementia and the rates of cognitive change between participants in SSRs and those in DSRs. We hypothesized that SSR participants would have worse overall cognitive functioning in old age and would experience earlier onset of cognitive impairment. Using multiple regression, we compared the ages at which participants in SSRs and DSRs first reported AD or dementia diagnoses, and the ages at which they first scored below cut-offs for cognitive impairment, not dementia (CIND) and possible dementia as determined using the cognitive assessment. The study then compared rates of cognitive decline over time across the SSR and DSR groups, including stratified analyses by education, race/ethnicity, wealth, and sex/gender.
Results:
Participants in SSRs reported dementia diagnoses (β = −12.346; p = .001), crossed the threshold into CIND (β = −8.815; p < .001) and possible dementia (β = −13.388; p < .001) at a younger age than participants in DSRs. When adjusted for covariates, participants in SSRs also had lower cognition at baseline (β = 0.745; p = .003), though having slower rates of cognitive decline when SSR was interacted with time (β = 0.066; p = .003). In separate analyses, cognitive differences for SSR participants were only found in participants without undergraduate degrees, with below-median household incomes, and women.
Conclusion:
Our findings support theories suggesting that marginalization and stigma cause premature cognitive impairment. Findings also suggest that higher education might mitigate the adverse effects of sexuality-minority status on cognitive aging. Results do not support these theories’ claims of more rapid cognitive decline; the lower slopes of cognitive decline with time are compatible with the possibility of slower rates of decline for aging individuals in SSRs.
Keywords: Alzheimer’s disease, dementia, same-sex relationships, cognition, lesbian, gay, bisexual (LGB), sexual orientation
Introduction
Alzheimer’s disease (AD), a progressive, age-related neurodegenerative disease that causes cognitive declines and behavioral and mood changes, is costly for patients, their caregivers and loved ones.[1] Our understanding of the aging experiences of lesbian, gay, and bisexual (LGB) people, including their burdens from aging-related diseases like AD and other dementias, is underdeveloped compared to other marginalized groups.[2] Only recently have researchers begun to describe the incidence, age of onset, and prevalence of AD and dementia for LGB people.[3–5]
Minority stress and allostatic load theories provide mechanisms linking LGB people’s experiences of marginalization, stigma, and discrimination to their cognitive health.[6] According to these theories, sexual-minority status affects cognitive outcomes via the same behavioral and physiological risk factors for AD and dementia that are in the general population, albeit at elevated levels: depression, stress, smoking, drug use, and drinking; and higher chronic-inflammation biomarkers and decreased hippocampal volume.[7] In response to discrimination, stigma, and marginalization, LGB people experience higher rates of these risk factors than do straight people. [6–9] Gay and bisexual men’s higher risk of human immunodeficiency virus (HIV) also increases their risk for dementia directly, through HIV-associated dementia; and indirectly, through associated illnesses, stigma, and social isolation.[10]
The educational and employment discrimination LGB people face also deprives them of “cognitive reserve” to protect against neuropathology.[11–13] These outcomes appear unavoidable by remaining “closeted”, as concealing stigmatized identities exacts its own health costs.[14] Along with discrimination and stigma, concealment places LGB people at greater risk of social isolation in older adulthood, also a risk factor for cognitive decline, AD, and dementia.[5]
These are all persistent problems that also intersect with other forms of marginalization, including race/ethnicity, sex/gender, and class.[6, 9] Yet some LGB people are able to cultivate coping mechanisms and may experience greater resilience that mitigates their risk of cognitive decline[8]. Whether the capacity for resilience and coping corresponds to these intersectional identities or other factors, like material resources, requires further study.
Taken together, these potential mechanisms present several aspects of LGB people’s experiences of cognitive aging that existing research has yet to address, namely, questions about the timing of AD and dementia onset and the timing and trajectories of cognitive decline. Minority stress and allostatic load literatures predict not only higher prevalence of AD and dementia among marginalized groups, which an earlier study demonstrates[3], but also experiencing accelerated cognitive decline, which results in their having AD and dementia at younger ages.[7] One study has found evidence of increased incidence and prevalence of dementia among LGB people compared to straight people[3], but questions remain about whether the former also experience more rapid cognitive decline and earlier onset of cognitive impairment, including AD and dementia.
Minority stress may harm cognition through material deprivation and social stigma; cognitive reserve theory suggests that the cognitive benefits of education and other forms of cognitive stimulation could improve outcomes in this population.[11, 15] What remains unclear and understudied is the ability of education to protect from—and possibly even compensate for—the deleterious effects of minority stress. In other words, do cognitive-reserve–enhancing factors like education offset the cognitive harms of minority status? If so, does this mitigation occur through direct cognitive benefits of education, or through the material advantages associated with education?
Extant research into LGB cognitive aging has been hampered by insufficient data and has shown conflicting results.[2] Like previous studies that rely on population-based data, we measure sexuality indirectly, by relying on the genders of study participants and their spouses/partners, treating participants in same-sex relationships (SSR) as proxies for LGB people, and those in different-sex relationships (DSR) as proxies for straight people. [3, 4] Using longitudinal data from the Health and Retirement Study (HRS), this study analyzed cognitive health differences between participants in SSRs and those in DSRs to test the following hypotheses:
SSR participants will experience more rapid cognitive decline and earlier onset of cognitive impairment no dementia (CIND) and AD and other dementias than DSR participants.
SSR participants will have worse overall cognitive performance than DSR participants, reflected in lower scores at baseline and for subsequent waves.
Disparities in cognitive performance and decline between DSR and SSR participants will be mitigated by education.
Disparities in cognitive performance and decline between DSR and SSR participants will be greater for people with intersecting marginalized identities, including people of color (POC), women, and less wealthy people.
Our study builds on and complements existing work on sexuality and cognitive health and addresses several gaps and contradictions in the literature. Several studies looked at prevalence within LGB populations but lack non-LGB samples for comparison.[5, 16] Results of existing population-based studies of dementia risk among LGB people have been contradictory: one study[3] reported increased incidence among people in SSRs versus those in DSR, but another[4] reported no differences. These studies also often fail to attend to important situational differences between DSRs and SSRs. For example, given that in the HRS, SSR participants are more likely than DSR participants to report being partnered instead of married, marital status has been hypothesized as an important factor in the cognitive differences between DSR and SSR HRS participants.[3] However, same-sex marriage was not legal anywhere in the US until 2004 and not nationwide until 2015 (HRS cognitive data is only available up to 2016). As a result, for most SSR respondents, there is no legal difference between “married” and “partnered” marital-status responses, and it seems likely that marital status for SSRs before 2015 is a proxy for other, unspecified differences. Moreover, the legalization of same-sex marriage altered the social and material consequences of marriage for LGB couples and affected their health.[17, 18]
Our emphasis on the rate of change and the age at which AD and dementia occur is reflected in our choice of outcome variable and study design: in addition to thresholds for CIND used in the earlier study[3], we have also performed our analysis on three other outcomes: possible dementia, based on cognitive-assessment cores; self-reported AD and dementia diagnoses; and raw cognitive-assessment score. We also utilize longitudinal methods to be able to investigate change across time within individuals. With AD and dementia cases, this enables us to better compare the ages at which AD and dementia occur; using raw cognitive-assessment scores enables comparison of both baseline scores (i.e., intercepts) and slopes of cognitive change across time.
Materials and Methods
Data
This study relies on data from the HRS, the largest population-based longitudinal study of the aging experiences of Americans aged 51 and older.[19] The HRS began in 1992; since 1998, the study has moved to a steady-state design, following participants biennially. Every six years, the study adds new participants from more recent cohorts as the youngest members of those cohorts reach age 51. For participants unable or unwilling to complete the questionnaire themselves, a proxy informant familiar with the sampled individual answers instead.
Data for this paper were drawn from two HRS data products: non-cognitive cross-wave data were taken from HRS data processed by RAND, while—to have the most up-to-date data possible—cross-wave cognitive data were taken from the HRS’s cognitive data. The study began administering full cognitive assessments in 1998 and asked for AD and dementia diagnoses beginning in 2010. While the most recently completed wave was in 2020, cognitive data is only available until 2016.
This project involves secondary analysis of anonymized, publicly available data. It is exempt from ethics review and has been certified as such by our institution’s Institutional Review Board. HRS obtained informed consent for all participants in its study before each wave of participation.[19]
Inclusion and Exclusion Criteria
We used data from 1998 to 2018; before applying our inclusion and exclusion criteria, there are 422,360 biannual observations from 42,236 participants. We included all HRS participants who gave their marital status as “married” or “partnered” for at least one wave of the HRS, and who had cognitive-assessment scores and diagnosis information for at least one wave of the study. This left our sample population with 26,344 participants producing 186,607 biannual observations.
Measures
Sexuality:
This study indirectly identifies LGB people through relationship data, relying on the sex/gender (HRS uses the terms interchangeably) of participants and their partners. The HRS relationship data also includes relationship status, with options for “partnered” and “married”; married and partnered participants are treated identically in all other relationship questions.
Unlike existing studies of SSRs in the HRS[3], we did not limit participants who were in relationships only for the duration of those relationships. To maximize observations, and better use relationship status as an indirect measure of sexuality, we included participants who were married or partnered for at least one wave of the study, even during the waves in which they were not in a relationship. For sexuality, we categorized participants based on their own and their partners’ sexes/genders: participants with a same-sex/gender partner for at least one wave of the HRS (referred to as “SSR participants”), and those who only had different sex/gender partners for all waves in which they were in a relationship (“DSR participants”). Thus, participants who were in both DSRs and SSRs (n = 10) during the study were categorized as SSR participants.
Cognitive Health and Functioning:
The HRS records cognitive functioning and health in several ways. First is self-reported AD/dementia diagnosis: from wave four through nine (1998–2008), this question asked only whether a doctor had told the participant they had a “memory-related disease”. In wave 10 (2010), HRS began specifying AD and dementia diagnoses in separate questions, including whether that diagnosis was new since the last wave; this data is available until 2018. For consistency, we only use responses to AD and dementia questions from 2010 onward; we combined the two responses into single variables for AD/dementia. HRS does not ask for the age at diagnosis, so we used the age at the time of the interview in which a new diagnosis was indicated. Proxy informants answer questions about AD/dementia diagnoses.
The second cognition measure is a cognitive assessment administered to HRS participants, available 1998–2016. The HRS collects immediate and delayed word-recall (10 words each) data; it also assesses mental status using a version of the Telephone Interview for Cognitive Status (TICS), which includes vocabulary, naming, and numeracy questions. Recall and mental-status scores are combined into a cognitive-function score. Beginning in 2000, the HRS imputed results for cases in which portions of the test are missing.[20] Interviews of proxy informants do not have cognitive-assessment scores and those scores are not imputed.
Following earlier studies[3], we used a modified score that consists of a subset of the HRS cognitive-assessment questions: immediate and delayed word-recall (10 points each), Serial 7s (subtracting 7 from a number, then again from the result, 5 times; 5 points), and counting backwards (2 points). There are thus 27 points possible; scores lower than 12 indicate likely cognitive impairment without dementia (CIND); those below 6 indicate possible dementia. This system, including the cutpoints for CIND and possible dementia, has been found to improve the correct classification of cognitive state compared to the score from the complete HRS cognitive battery.[21]
Time:
For our cross-sectional analysis for hypothesis 1, we used participants’ age in years. For longitudinal analyses (hypotheses 2–4), we used participants’ time-in-study measured in years; because of the age difference between DSR and SSR participants, and because of the strong relationship between age and cognitive health, we also adjusted for age above 51 years at baseline.
Covariates
We included several covariates, based on their relevance to both cognitive aging in the overall population and LGB people’s aging experiences, while also balancing the need for comprehensiveness against the limitations of small sample size with its attendant risk of overfitting the model. Because of limitations in HRS questions, experiences of historical discrimination, e.g., in education, are not recorded, and instead indirect measures, such as educational attainment, must be used.
Learning:
Participants’ cognitive-performance scores improve after their first assessment due to test familiarity.[15] To adjust for this learning effect, we included a dichotomous variable that indicates whether it is a participant’s first assessment.
Sex/gender:
The HRS asks participants for their sex/gender in their initial wave only. There is one sex/gender question with only two possible responses, “Male” and “Female”, so data about gender identity, including whether participants’ or their spouses/partners are transgender, gender nonbinary, or otherwise gender minorities, is not available.[22] Partners’ and spouses’ genders were asked directly of those persons, with the same limitations.
Education:
Because of the small sample size for participants in SSRs, education was coded using a binary variable for those who completed an undergraduate degree or greater and those who did not.
Race/ethnicity:
The HRS asked participants’ race and Hispanic ethnicity in separate questions, only in the initial interview. From these data, we created a binary variable: participants whose race is Black or Other, and those who indicated Hispanic ethnicity, were coded as “Person of Color” (POC); those who responded white and non-Hispanic were coded accordingly.
Wealth:
There are documented material disparities between LGB and straight people, in part because of discrimination.[23–27] Because this study uses data from a retirement survey, we opted to use household wealth (instead of wages or income) to account for the economic disadvantage some LGB people face. We rely on the HRS’s total household wealth variable, natural-logarithmically transformed.
Self-rated health:
LGB people face higher rates of many behavioral, health, and physiological risk factors for cognitive impairment, including workplace discrimination, smoking, alcohol use, high blood pressure, and systemic inflammation biomarkers. These, in turn, are all associated with poorer self-rated health, which is also associated with sexuality, as well as higher incident dementia.[28] The HRS asks respondents for their self-rated health on a five-point scale, with 5 being “Excellent” and 1, “Poor”.
Statistical Analyses
Summary statistics were compared between SSR and DSR participants using t-tests with unequal variance for the means of continuous variables, and Pearson χ2 tests for categorical variables.
To test hypothesis 1, we used multiple regression to compare the mean age of diagnosis for AD/dementia and the mean age at which the thresholds for CIND and possible dementia on the cognitive test were first crossed across the two subpopulations. We also adjusted for the above-named covariates.
To test hypotheses 2–4, we used mix-effects, multi-level model (MLM) regression to test the relationship between cognitive trajectories and sexuality, with the above-described 27-point cognitive-assessment score as the dependent variable. The higher-level, random-effects term is the cross-wave participant identifier: by including a random-effects term for individuals across each wave of the study, MLM regression adjusts for the lack of independence between observations from any single participant. Random slopes for time-in-study were calculated in addition to intercepts; we included the former to address the possibility of cognitive trajectory-heteroskedasticity with time across the sample population. To test hypothesis 2, we created two MLM models: in Model 1, independent fixed-effect variables were baseline age, time in study, learning effect, sexuality, and an interaction effect between sexuality and time; Model 2 included the above-named covariates.
MLM models were also used to test hypotheses 3 and 4. Stratified analyses are useful for accommodating unbalanced sample sizes such as ours, in which the population average for any given effect size may “drown out” the effect sizes across the primary subpopulations of interest. We performed stratified analyses by the above demographic factors: education, for hypothesis 3; and sex/gender, race/ethnicity, and baseline wealth for hypothesis 4. For education, we separated participants without undergraduate degrees from those with undergraduate degrees or more and ran separate MLM regression analyses on each subgroup. For household wealth, the two stratification groups were participants with above-median household wealth and those with below-median wealth at baseline.
Statistical significance is reported using p-values at a 95% confidence level (α = 0.05). All analyses were performed with STATA/IC 16.1 [StataCorp].
Results
Table 1 provides summary statistics of the SSR and DSR groups. SSR participants in their first wave of participation in the study tend to be younger (mean age: 55.2 years; standard deviation (SD) = 9.2) than DSR participants at baseline (mean age = 60.4 years; SD = 4.8; t = 15.276; p < .001); this age difference occurs both across the study and within each wave (maximum age of SSR participants is 96.3 years; for DSR it is 109.0 years). SSR respondents also tend to have joined the HRS later than those in DSRs and have participated in fewer waves, on average (t = 7.989; p < .001).
Table 1.
Summary Statistics of Health and Retirement Study (1998–2018) participants who are in a relationship for at least one wave of the study
| Participants in Same-sex Relationships | Participants in Different-sex Relationships | |||
|---|---|---|---|---|
|
| ||||
| N | Participants | 214 | 26,130 | |
| Observations | 1,043 | 185,564 | ||
|
| ||||
| Mean cognitive scorea | 16.8 b | 16.0 c | ||
| Mean agea | 55.2 | 60.4 | ||
| Report AD/dementia diagnosis | n % | 7 | 469 | |
| 3.3 | 1.8 | |||
| Crossed CIND threshold | 48 | 10,228 | ||
| 20.0 | 39.1 | |||
| Crossed possible-dementia threshold | 10 | 2,744 | ||
| 4.7 | 10.5 | |||
| Mean Self-rated healtha | 3.4 | 3.2 | ||
| Mean Ln(Wealth)a | 12.4 | 11.8 | ||
| Sex/Gender | ||||
| Women | 110 | 13,388 | ||
| 51.4 | 51.2 | |||
| Men | 104 | 12,742 | ||
| 48.6 | 48.8 | |||
| Race/Ethnicity | ||||
| People of Color | 50 | 8,080 | ||
| 23.4 | 31.0 | |||
| White, Non-Hispanic | 164 | 17,946 | ||
| 76.6 | 69.0 | |||
| Education | ||||
| No College Degree | 109 | 20,449 | ||
| 50.9 | 78.3 | |||
| College Degree + | 105 | 5,671 | ||
| 49.1 | 21.7 | |||
| Year joined (median) | 2010 | 1998 | ||
| Mean # of waves of participationd | 4.0 | 5.7 | ||
Note: Bolded numbers indicate a statistically significant difference between participants in same-sex relationships and those in different-sex relationships; AD = Alzheimer’s disease; CIND = cognitive impairment, no dementia
At baseline
Minimum: 0; Maximum: 27; Standard deviation [SD]: 4.4
Minimum: 3 Maximum: 26; SD: 3.9
Prior to death, being dropped from the sample, or up until wave 14 (2018)
Unadjusted for other variables, SSR participants also have higher cognitive scores at baseline (t = −2.851; p = .005), as well as higher mean self-reported health (t = −3.071; p = .002) and mean wealth (t = −4.500; p < .0001). While the sex/gender makeup does not differ (χ2 = 0.002; p = .961), HRS participants who have been in SSRs are more likely to be white than those in DSRs (χ2 = 5.857; p = .020); the former are also more likely to have college degrees or more (χ2 = 92.756; p < .001).
Table 2 reports the results of three regressions testing hypothesis 1, namely, the factors affecting the age at which self-reported AD/dementia diagnoses, CIND, and possible dementia are first recorded. For diagnoses, the coefficients for SSR participants are statistically significant and negative (β = −12.346; p = .001). Similarly, those SSR participants who cross the thresholds in cognitive-assessment scores for CIND (β = −8.815; p < .001) or possible dementia (β = −13.388; p < .001) do so at a younger age than DSR participants.
Table 2.
Regression results for age at which a diagnosis of Alzheimer’s disease or dementia is First self-reported or at which participants were initially characterized as cognitively impaired based on neuropsychological testing, Health and Retirement Study, 1998–2018
| Self-reported Alzheimer’s | Cognitive Assessment Threshold (1998–2016) |
|||
|---|---|---|---|---|
| Disease/Dementia Diagnosis (2010–2018) | Cognitive Impairment, Not Dementia | Possible Dementia | ||
|
| ||||
| Same-sex | β | −12.346 | −8.815 | −13.388 |
| Relationship | Standard Error | 3.855 | 1.645 | 3.699 |
| p | .001 | <.001 | <.001 | |
| College degree + | 0.772 | 2.023 | 1.223 | |
| 0.867 | 0.343 | 0.782 | ||
| .373 | <.001 | .118 | ||
| Woman | 0.566 | −0.194 | 0.637 | |
| 0.645 | 0.210 | 0.405 | ||
| .381 | .356 | .116 | ||
| Person of Color | −4.314 | −7.868 | −7.749 | |
| 0.767 | 0.231 | 0.428 | ||
| <.001 | <.001 | <.001 | ||
| Wealth | 0.086 | 0.620 | 0.474 | |
| 0.162 | 0.060 | 0.103 | ||
| .595 | <.001 | <.001 | ||
| Self-rated Health | 0.635 | −0.415 | 0.106 | |
| 0.291 | 0.097 | 0.180 | ||
| .030 | <.001 | .556 | ||
| Constant | 79.169 | 67.401 | 72.762 | |
| 2.034 | 0.741 | 1.285 | ||
| <.001 | <.001 | <.001 | ||
|
| ||||
| R2 | 0.062 | 0.167 | 0.164 | |
| Observations | 869 | 8,852 | 2,301 | |
Note: Wealth was transformed using the natural logarithm.
Table 3 presents MLM regression results for cognitive performance for hypothesis 2, tested using two models: time and sexuality alone (Model 1), and time and sexuality adjusted for covariates (Model 2). As expected, age and time-in-study had negative effects that were statistically significant in both Models 1 and 2. In Model 1, SSR did not have a statistically significant effect on the cognitive intercept (i.e., assessment score at age 51) (β = 0.308; p = .267); the interaction between time and sexuality was also not significant (β = 0.043; p = .149). In Model 2, SSR was statistically significant in the negative direction for the cognitive intercept (β = −0.745; p = .003); the SSR–time interaction was also statistically significant but positive (β = 0.066; p = .026). When adjusted for covariates, having been in a SSR has a negative effect on cognitive-score intercept, but a positive effect on the slope. In other words, while SSR participants start with lower cognitive scores (after adjusting for differences in the sample subpopulations), they also decline more slowly than participants in DSRs.
Table 3.
Results from mixed-effects multilevel regressions examining baseline level and longitudinal change in total cognitive scores, Health and Retirement Study, 1998–2016
| Model 1: Adjusting for Age, Learning, and Sexuality Only | Model 2: Adjusting for all covariates | ||
|---|---|---|---|
|
| |||
| Fixed Effects | |||
|
| |||
| Baseline Age | β | −0.124 | −0.146 |
| Standard Error | 0.003 | 0.003 | |
| p | <.001 | <.001 | |
| Time in Study | −0.196 | −0.191 | |
| 0.002 | 0.002 | ||
| <.001 | <.001 | ||
| Learning | −0.770 | −0.643 | |
| 0.035 | 0.036 | ||
| <.001 | <.001 | ||
| Same-sex Relationship | 0.308 | −0.745 | |
| 0.28 | 0.248 | ||
| .267 | .003 | ||
| Same-sex Relationship*Time | 0.043 | 0.066 | |
| 0.030 | 0.030 | ||
| .149 | .026 | ||
| College degree+ | 1.858 | ||
| 0.041 | |||
| <.001 | |||
| Woman | 0.09 | ||
| 0.041 | |||
| <.001 | |||
| Person of Color | −2.16 | ||
| 0.048 | |||
| <.001 | |||
| Wealth | 0.271 | ||
| 0.009 | |||
| <.001 | |||
| Self-rated Health | 0.267 | ||
| 0.011 | |||
| <.001 | |||
| Constant | 17.685 | 15.271 | |
| 0.038 | 0.119 | ||
| <.001 | <.001 | ||
|
| |||
| Random Effects | |||
|
| |||
| Slope | 0.027 | 0.025 | |
| 0.001 | 0.001 | ||
| Intercept | 10.705 | 6.920 | |
| 0.140 | 0.107 | ||
| Slope-Intercept Covariance | −0.086 | −0.096 | |
| 0.009 | 0.008 | ||
|
| |||
| Wald χ2 | 9568.63 | 18734.57 | |
| Observations | 124,472 | 116,507 | |
Note: Age and time in study were expressed in years. Wealth was transformed using the natural logarithm.
Figure 1 displays the predicted cognitive-assessment scores for the DSR and SSR populations for time-in-study, t, = 0–18 based on Model 2. The intercept (i.e., predicted score at t = 0, or ŷ0, adjusted for sample average age at baseline) for SSR participants is less than for DSR participants (ŷ0 SSR = 15.866; 95% Confidence Interval [CI]: 15.386–16.352; ŷ0 DSR = 16.611; CI: 16.562–16.660). However, because of the positive slope for SSR participants versus DSR participants, by t = 4 years, there is no statistically significant difference between the two groups’ predicted scores (ŷ4 SSR = 15.510; CI: 15.061–15.959; ŷ4 DSR = 15.990; CI: 15.949–16.031).
Fig. 1.
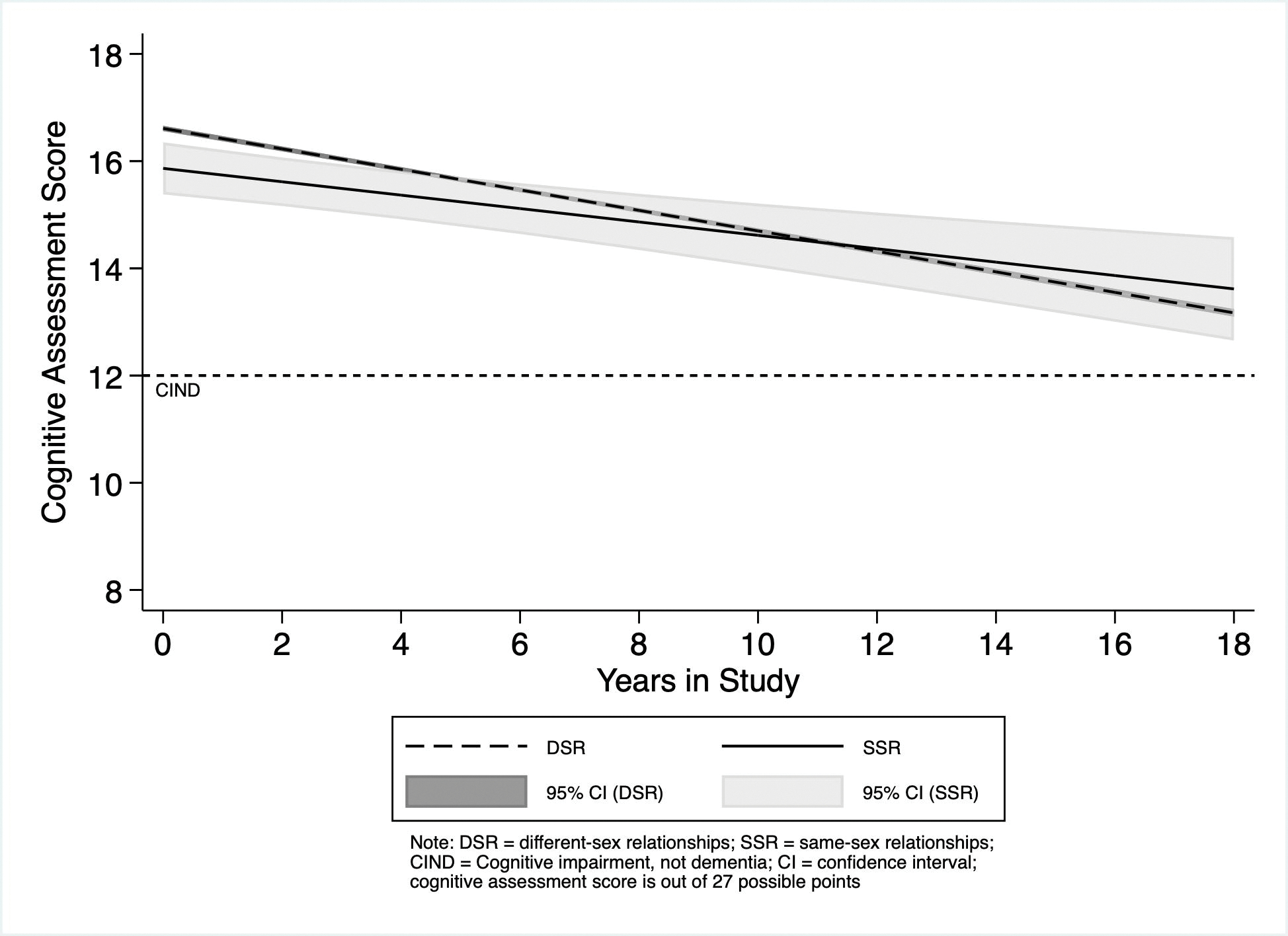
Predicted cognitive-assessment scores for Health and Retirement Study Participants in Same-sex and Different-sex Relationships
Stratified Analyses
Table 4 shows the results of the stratified analyses, testing hypotheses 3 and 4. Stratifying by education produces the same results for those without an undergraduate degree as the unstratified Model 2. For these less-educated participants, SSR (β = −1.025; p = .006) has a statistically significant, negative coefficient and SSR–time interaction has a statistically significant, positive one (β = 0.108; p = .009). For those with a college degree or higher education, however, the association of SSR status with cognition is not statistically significant (β = −0.317; p = .299), nor is its interaction with time (β =−0.005; p = .902). The differences in cognition between SSR and DSR participants are only present for those without college degrees.
Table 4.
Results of separate mixed-effects regressions examining total cognitive score, stratified by education level, sex/gender, and race/ethnicity, Health and Retirement Study, 1998–2016
| No College Degree | College Degree + | Women | Men | People of Color | Non-Hispanic White | Below-median Wealth | Above-median Wealth | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fixed Effects | |||||||||
|
| |||||||||
| Baseline Age | β | −0.148 | −0.140 | −0.140 | −0.152 | −0.149 | −0.144 | −0.153 | −0.146 |
| Std. Error | 0.003 | 0.005 | 0.004 | 0.004 | 0.007 | 0.003 | 0.004 | 0.003 | |
| p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Time in Study | −0.197 | −0.169 | −0.195 | −0.187 | −0.181 | −0.194 | −0.198 | −0.181 | |
| 0.003 | 0.005 | 0.003 | 0.003 | 0.005 | 0.003 | 0.004 | 0.003 | ||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Learning | −0.616 | −0.703 | −0.722 | −0.574 | −0.714 | −0.606 | −0.646 | −0.722 | |
| 0.044 | 0.065 | 0.054 | 0.049 | 0.068 | 0.043 | 0.055 | 0.050 | ||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Same-sex | −1.025 | −0.317 | −0.829 | −0.642 | −1.012 | −0.659 | −0.807 | −0.623 | |
| Relationship | 0.371 | 0.305 | 0.348 | 0.355 | 0.628 | 0.265 | 0.414 | 0.288 | |
| 0.006 | 0.299 | 0.017 | 0.071 | 0.107 | 0.013 | 0.051 | 0.031 | ||
| Same-sex | 0.108 | 0.005 | 0.124 | 0.009 | 0.154 | 0.058 | 0.060 | 0.059 | |
| Relationship*Time | 0.042 | 0.041 | 0.044 | 0.039 | 0.079 | 0.032 | 0.048 | 0.038 | |
| 0.009 | 0.902 | 0.005 | 0.818 | 0.050 | 0.069 | 0.208 | 0.118 | ||
| College Degree+ | - | - | 1.757 | 1.987 | 2.530 | 1.677 | 2.149 | 1.729 | |
| 0.073 | 0.068 | 0.118 | 0.054 | 0.087 | 0.053 | ||||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||
| Woman | 0.849 | 0.656 | - | - | 0.849 | 0.656 | 0.849 | 0.656 | |
| 0.049 | 0.073 | 0.049 | 0.073 | 0.049 | 0.073 | ||||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||
| Person of Color | −2.306 | −1.526 | −2.361 | −1.920 | - | - | −2.304 | −1.962 | |
| 0.055 | 0.095 | 0.067 | 0.068 | 0.061 | 0.064 | ||||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||
| Wealth | 0.271 | 0.235 | 0.264 | 0.280 | 0.217 | 0.297 | - | - | |
| 0.01 | 0.019 | 0.012 | 0.013 | 0.016 | 0.01 | ||||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||
| Self-rated Health | 0.267 | 0.258 | 0.280 | 0.251 | 0.248 | 0.271 | 0.308 | 0.286 | |
| 0.013 | 0.025 | 0.016 | 0.016 | 0.025 | 0.013 | 0.018 | 0.015 | ||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Constant | 13.731 | 15.843 | 14.569 | 13.541 | 12.216 | 13.318 | 16.435 | 17.293 | |
| 0.131 | 0.272 | 0.156 | 0.167 | 0.197 | 0.137 | 0.089 | 0.082 | ||
| <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||
|
| |||||||||
| Random Effects | |||||||||
|
| |||||||||
| Slope | Variance | 0.025 | 0.024 | 0.028 | 0.021 | 0.017 | 0.027 | 0.024 | 0.025 |
| Std. Error | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | |
| Intercept | 7.604 | 4.684 | 7.117 | 6.669 | 8.554 | 6.169 | 8.256 | 5.931 | |
| 0.132 | 0.165 | 0.151 | 0.151 | 0.244 | 0.114 | 0.175 | 0.130 | ||
| Slope-Intercept | −0.102 | −0.076 | −0.100 | −0.087 | −0.068 | −0.096 | −0.104 | −0.089 | |
| Covariance | 0.009 | 0.014 | 0.011 | 0.011 | 0.017 | 0.008 | 0.013 | 0.001 | |
|
| |||||||||
| Wald χ2 | 12000.000 | 3022.919 | 9526.229 | 8876.633 | 3231.987 | 13000.000 | 7971.677 | 10000.000 | |
| Observations | 88,711 | 27,796 | 64,084 | 52,423 | 26,884 | 89,623 | 48,759 | 67,748 | |
Note: Age and time in study were expressed in years. Wealth was transformed using the natural logarithm
Stratification by sex/gender produces similar results as for education: for women: SSR (β = −0.829; p = .017) and its interaction with time (β = 0.124; p = .005) are both significant with opposite signs. For men, neither SSR (β = 0.−0.642; p = .071) nor SSR*Time (β = 0.009; p = .818) is statistically significant. While there are statistically significant differences between SSR and DSR women, namely, lower cognitive intercepts but shallower slopes of decline, we did not find such differences between SSR and DSR men.
Stratification by race/ethnicity produces different results from the previous analyses: for POC, the intercept for SSR is not statistically significant (β = −1.012; p = .107), while it is for non-Hispanic whites (β = 0.659; p = .013). For both groups, the SSR*Time interaction is statistically significant and positive; the value is greater for POC (β = 0.154; p = .050) than for non-Hispanic whites (β = 0.058; p = .032). Thus, SSR non-Hispanic whites had lower average scores at baseline and declined more slowly than did DSR non-Hispanic whites. For SSR POCs, only the rate of cognitive decline differed significantly between SSR and DSR groups.
Stratification by wealth reverses the patterns from previous stratifications: among participants with below-median wealth, neither the intercept for SSR (β = −0.807; p = .051) nor the SSR*Time interaction (β = 0.060; p = .204) was significant. For participants with above-median wealth, SSR is statistically significant (β = −0.623; p = .031), but SSR*Time is not (β = −0.059; p = .118). Only wealthier SSR participants had lower cognitive intercepts relative to their wealthier DSR counterparts; however, the coefficient for the intercept for the less-wealthy participants was borderline significant. Neither group had significant DSR–SSR differences in the rate of cognitive decline.
Figure 2 displays the predicted cognitive-assessment scores between t = 0 and t = 18 for the DSR and SSR populations for the eight stratified regression analyses.
Fig. 2.
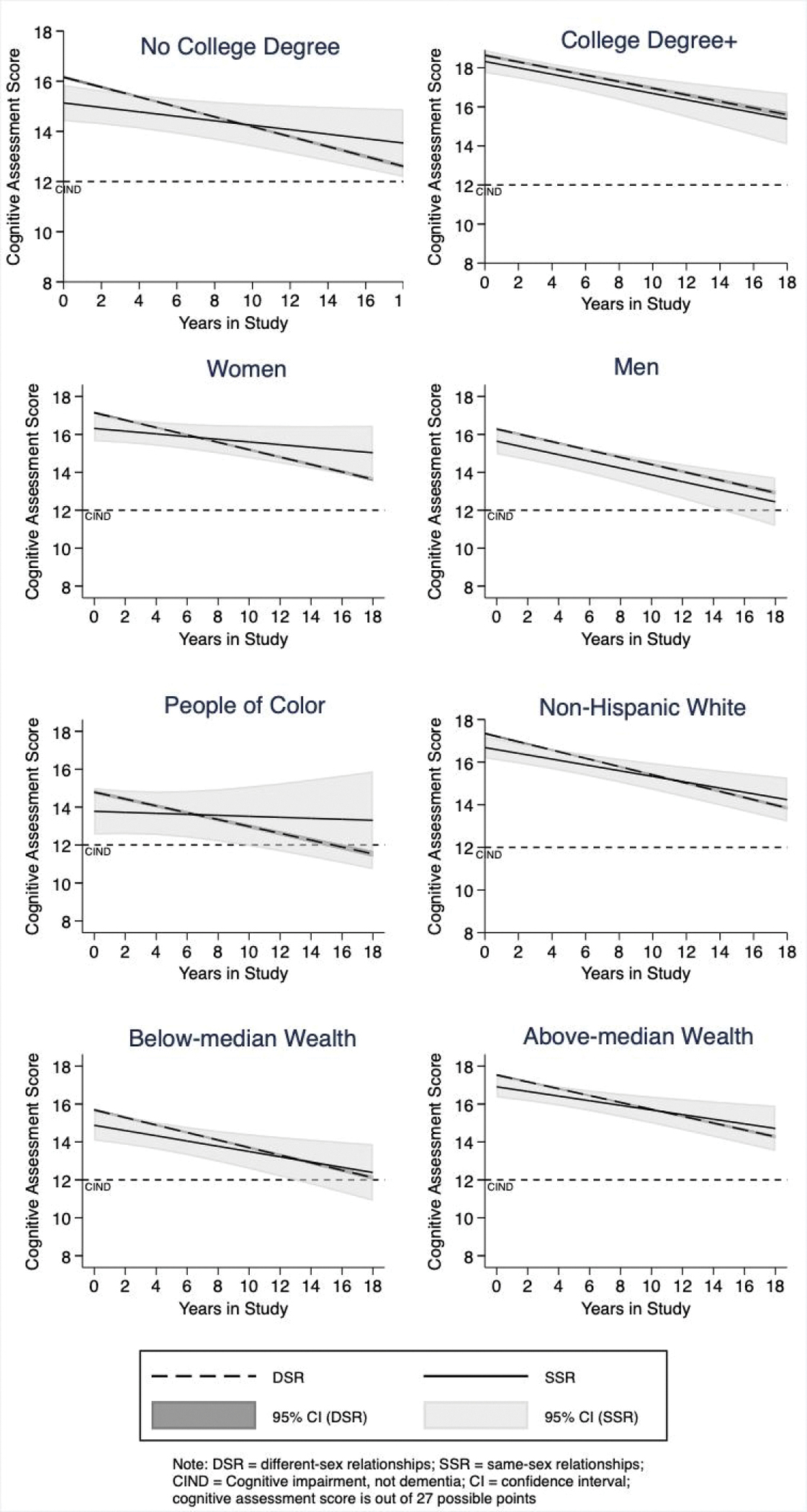
Predicted cognitive-assessment scores for Health and Retirement Study Participants in Same-sex and Different-sex Relationships, based on Separate Regression Analyses for Education level, Sex/Gender, Race/Ethnicity, and Wealth
Discussion
In this study, we found that HRS participants in SSRs reported AD/dementia diagnoses and crossed the cognitive-impairment threshold at younger ages than participants in DSRs; this is concordant with hypothesis 1. We also found that, after adjusting for covariates in Model 2, SSR participants have lower cognitive-assessment scores at younger ages, but that they decline more slowly than DSR participants as age increases. This supports part of hypothesis 2, regarding lower starting cognition, but poorer cognitive performance with age was not observed for SSR participants. The differences between these cognitive trajectories are observed between DSR and SSR participants without college degrees, women, non-Hispanic whites, and participants with above-median household wealth; but not for those with college degrees or men. These results help resolve conflicting results reported by previous studies[3, 4] by showing that sexuality-based differences are found in specific sub-populations, including women and those without university degrees; moreover, the aspects of cognitive trajectories may vary for differently marginalized groups.
This brings us to hypotheses 3 and 4, testing cognitive reserve and intersecting identities. Our results support hypothesis 3: education mitigates the relationship between sexuality and cognitive performance. This, in turn, supports the possibility that cognitive reserve, for which education is often a proxy[11], is protective against the cognitive effects of sexual-minority status. For hypothesis 4, our results provide mixed support, as cognitive differences are present along sex/gender hierarchies, but not for race/ethnicity or wealth. Intersecting marginal identities do not appear to compound cognitive harms for those groups.
Education may be directly cognitively protective, as cognitive reserve theory has demonstrated. In addition to cognitive reserve, educational differences may be due to several factors; SSR participants without college degrees may have faced anti-LGB discrimination that may have prevented them from that educational attainment. Greater education is also associated with decreased anti-LGB stigma at the individual level[29], in workplaces[30], and communities.[18] More educated people in SSRs may thus face less stigma and more social support from peers, coworkers, and neighbors than their less-educated counterparts, which may, in turn, decrease their overall stress and increase their cognitive health. They may also disclose their identities more, which has beneficial effects on psychological and overall well-being.[14]
Beyond the direct cognitive benefits of education, SSR participants with more college degrees may have had access to the resources to move to the locations[31] and workplaces[13, 32] of their choosing, for example. In addition to the burdens of sexuality-based stigma and discrimination, less-educated and poorer LGB people face class-based discrimination and stigma within LGB communities.[33] However, the lack of major differences between below-median and above-median wealth groups may indicate the importance of direct educational benefits through cognitive reserve over the associated material benefits often derived from education. Finally, education may be the result of cohort effects in the sample population: that SSR participants are more likely to have college degrees than DSR participants may be the result of their overall younger age and their later birth years; this may also explain the difference between DSR and SSR participants’ self-reported health at baseline.
Our results complicate the claims made in the minority stress and allostatic load literatures, namely, that the material disadvantage and social marginalization associated with sexual minority status accelerate the cognitive aging process before later adulthood.[7] As already discussed, with SSR participants overall, we do see earlier disease onset and lower initial cognition scores. Our findings for wealth stratification (Table 4) are concordant with the claims that anti-LGB social stigmatization is harmful, and disparities do not arise solely from antimaterial deprivation due to discrimination. POC participants in SSRs have the lowest average ages for AD/dementia diagnoses and crossing the CIND and possible dementia thresholds (Table 2). POC in SSRs also have the lowest mean intercept for cognition (Table 3). This suggests that minority stress theory’s claims about earlier cognitive decline and disease-onset are supported by the present study under certain circumstances. However, based on our stratified analyses, it does not appear that intersecting identities exacerbate the effects of minority stress. Moreover, groups with lower intercepts among SSR participants also tended to have lower rates of decline with age than did the corresponding DSR participants. This may demonstrate the possibility of resilience or other protective factors at work; the absence of differences in cognitive slope between DSR and SSR men and college-educated participants requires additional exploration as to how resilience may arise.
Survival bias is related to resilience and may also affect our results. Survival bias argues that the group-level differences in cognitive health are due to higher rates of early mortality amongst group members, so those at lower risk of poor cognitive health are more likely to live long enough to be included in the sample population. This is one potential explanation for the greater prevalence of AD/dementia among women than men: men die younger, and the factors that contribute to the longevity of those men who survive also increase their cognitive health during aging.[34] In the present study, survival bias may explain the lack of statistically significant differences in intercepts between SSR and DSR POC, men, and below-median wealth participants; survival bias may also contribute to inadequate sample sizes to detect an association in these cases.
The results from the stratification by sex/gender show that sexuality for people in relationships affects women’s cognition, but not men’s. These results are surprising because, in many respects, women in SSRs fare better than those in DSRs, while gay and bisexual men in relationships tend to fare worse than straight men. For example, women in SSRs do not pay the same penalties in terms of overall welfare and happiness that women in DSRs do, while men in SSRs benefit less than men in DSRs.[35] Furthermore, gay and bisexual men also face greater economic insecurity than straight men.[23, 26] In contrast, lesbians have historically had higher individual[27] and household incomes[24] than straight women, a pattern that persists for older women (though it is reversed among younger women). However, bisexual women experience an income penalty[24] and lesbian households are also more likely to experience poverty.[25]
Among older LGB people, differing lifecourses may offset these advantages. Lesbians and bisexual women from older cohorts are more likely to have been in DSRs and to have had children—both of which are potentially deleterious to cognitive health—than gay and bisexual men.[36, 37] SSRs among women also tend to dissolve at higher rates than DSRs or SSRs among men.[38] Discerning which of these factors contributed to our results here will require further study.
Our race/ethnicity and wealth findings may also evidence a “floor effect” of cognitive testing. Because POC status has the greatest negative effect on cognitive intercept, the putative effects of LGB identity may not be detectable. This is especially likely in cognitive assessments that rely heavily on verbal skills, such as the one used in this project, on which POC and poorer people tend to score lower. The clustering of these participants’ scores at the lower end of the range artificially compresses their variance, which, consequently, makes additional variables appear to have no effect.[39] Conversely, a “ceiling effect” may help explain the lack of difference between college-educated DSR and SSR participants, as more educated persons tend to perform well on verbal-heavy assessments.[40]
Limitations
There remains a shortage of data that allows for a more complete understanding of the experiences of LGB people as they age, including the burdens of cognitive decline, AD, and dementia, and the risks they face due to societal homophobia and heterosexism. Yet, efforts to study comparative rates of cognitive decline, AD, and dementia, among other aging-related illnesses, are more difficult to perform using population-based samples because of the historical exclusion of sexuality questions from studies like the HRS. As has been previously noted, studies such as this one would be much easier and more generalizable if questions about gender identity and sexuality were asked routinely in large population-based studies of aging.[22]
While we have at times referred here to “LGB people”, our indirect measurement gives only a partial picture of overall LGB cognitive aging. Having to rely only on participants in relationships is likely not representative of factors that disproportionately affect single LGB people. We cannot compare our results to individuals who are not currently in coresident relationships, or whose coresident partner does not reflect their sexual identity. These limited data also make it difficult—if not impossible in most cases—to know participants’ actual sexual identities, and to track how these identities may change over time. For example, the ten participants who were in both DSRs and SSRs during the study may be bisexual, may have come out of the closet belatedly, or may have been out but in a DSR relationship for other reasons. This missing information is an especially acute problem for studying bisexual people, who continue to face erasure and discrimination from society generally and within the LGB community, and who often experience worse outcomes in health and material security than straight or gay and lesbian people.[24, 26]
Moreover, because we only have the genders of participants’ partners during their time in the study, we lack information about LGB people who were in SSRs but have since divorced, separated, or been widowed. This is especially troubling in the context of gay men, who disproportionately suffered high mortality rates at the height of the HIV/AIDS epidemic in the 1980s and 1990s.[10, 41] The AIDS epidemic is likely to lead to the undercounting of many gay and bisexual men who lost partners to AIDS. The epidemic was a unique and sizeable source of trauma and loss at both the intimate and community level that is likely to have long-term health effects, potentially including on cognition. Similarly, without information on pre-study partners’ genders, the marital histories and identity formation of LGB men and women do not allow for lifecourse analyses.
The growth in the number and proportion of SSR participants in subsequent cohorts makes it likely that LGB respondents had varying levels of comfort disclosing their partners’ sex/gender and the nature of their relationships. While the HRS has begun gathering sexuality information, it is only doing so for participants who have joined the study in 2016 or after, so information on these older cohorts will remain incomplete. Additionally, since the HRS does not track changes in gender[22], we cannot examine the impact of changes to gender identity on reported results.
In addition to the above limitations, the small sample size of the SSRs makes it difficult to test the range of possible intersecting identities, risk factors, relationship changes, and outcomes for participants in SSRs. The relatively small number of POC in SSRs, especially once stratified by sex/gender, makes further study of racially and ethnically differentiated experiences among LGB people difficult to analyze statistically. Similarly, the lack of statistically significant findings in our analysis of participants with below-median wealth may have been due to small sample size and greater household wealth among SSR participants: while half the participants overall had below-median wealth at baseline, only 34.76% of SSR participants (n = 65) did.
Sample-size restrictions also build on existing limitations not specific to LGB people. For example, the lack of direct cognitive-assessment scores from proxy interviews likely biases cognitive assessment data, given the strong association between cognitive performance and the ability to complete a self-interview.[21] Given small sample sizes and the likely systematically different availability and knowledge of proxy respondents, comparisons of proxy informants’ information about sampled persons’ cognition for DSR and SSR participants are not possible.
Opportunities for Further Study
Pursuant to the above discussion, there are many avenues for future research to pursue that are likely to produce fruitful insights into the factors and experiences of cognitive aging among LGB people. To begin with, as the HRS gathers more waves of data that include sexuality questions, it will be possible to perform studies like this one on younger generations of LGB people with a more complete sampling of the LGB population beyond coupled persons. It will also be possible to test the effects of same-sex marriage legalization on LGB health, including cognition.[17]
Additional waves of data will also enable more complex analyses that can study changes in the rate of cognitive decline with age, including changepoint timing and the pre- and post-acceleration rates of decline. A previous study found significant differences for education using this method, but the analysis requires at least five waves of data (SSR participants average four waves) and large sample size to be adequately powered.[15] Incorporation of survival analyses, as that study does, can also begin to analyze resilience and survival bias in producing the outcomes we saw in this study.
Using HRS data, it may also be possible to look at the intersections of place, employment, and education on cognitive outcomes to better understand the nature of the education dynamics reported here. The limited marital history and reproductive data from the HRS may be similarly used to test hypotheses about sex/gender differences. If suitable data can be found, it may also be possible to test for various lifecourse differences, including employment history and career trajectory, lifetime relationship history, and experiences of violence and discrimination. HRS data may also be useful for analysis of potential mediating risk factors between sexuality and cognitive outcomes, such as depression, smoking, drinking, and social isolation. While there are surveys of LGB people that cover some of these topics, there remains a paucity of suitable data sources that would enable comparison between LGB and straight people.
Conclusion
Our results here have larger implications for the study of LGB people’s experiences of aging and scholarly, clinical, and policy responses to them. The lower intercepts for cognitive scores of people in SSRs, and their progression to cognitive impairment and possible dementia at younger ages, make it necessary to start younger in studying, diagnosing, intervening, and treating LGB people for cognitive impairment, including AD and dementia. For example, attempts to increase cognitive reserve through late-life education and enrichment activities will likely need to start earlier if they are to be effective for LGB people—especially LGB people of color. The model of elder-care provision in the US is private, often familial; and yet, many LGB people are childless, thus lacking a key caregiving resource. Given their earlier onset of AD or dementia, they may be potentially left with decreased working years and, as a result, diminished savings to pay for their own professional care. Further research into this area is necessary, and LGB-focused policy interventions and community support are likely essential.
Funding Sources:
This study was funded by National Institutes of Health/National Institute of Aging grant number RF1 AG058595.
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan.
Footnotes
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
Statement of Ethics:
Study approval: This study was exempted from full ethics review by Stony Brook University’s Institutional Review Board (IRB# 498619)
Informed Consent: Written informed consent from participants was obtained by the Health and Retirement Study itself; more information is available at the HRS website: hrs.isr.umich.edu/.
Data Availability:
HRS data is publicly available for free at the HRS’s website: hrs.isr.umich.edu/. Further enquiries can be directed to the corresponding author.
References
- 1.Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. [DOI] [PubMed] [Google Scholar]
- 2.Fredriksen-Goldsen KI, Jen S, Muraco A. Iridescent Life Course: LGBTQ Aging Research and Blueprint for the Future – A Systematic Review. Gerontology. 2019;65(3):253–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Hsieh N, Zhang Z, Zhang Y, Langa KM. Same-Sex Couples and Cognitive Impairment: Evidence From the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2020:gbaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perales-Puchalt J, Gauthreaux K, Flatt J, Teylan MA, Resendez J, Kukull WA, et al. Risk of dementia and mild cognitive impairment among older adults in same-sex relationships. Int J Geriatr Psychiatry. 2019;34(6):828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredriksen-Goldsen KI, Jen S, Bryan AEB, Goldsen J. Cognitive Impairment, Alzheimer’s Disease, and Other Dementias in the Lives of Lesbian, Gay, Bisexual and Transgender (LGBT) Older Adults and Their Caregivers: Needs and Competencies. J Appl Gerontol. 2018;37(5):545–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forrester SN, Gallo JJ, Whitfield KE, Thorpe RJ Jr. A Framework of Minority Stress: From Physiological Manifestations to Cognitive Outcomes. Gerontologist. 2018;59(6):1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correro AN, Nielson KA. A review of minority stress as a risk factor for cognitive decline in lesbian, gay, bisexual, and transgender (LGBT) elders. J Gay Lesbian Ment Health. 2020;24(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson CL, Andel R. Does Sexual Orientation Relate to Health and Well-Being? Analysis of Adults 50+ Years of Age. Gerontologist. 2020;60(7):1282–90. [DOI] [PubMed] [Google Scholar]
- 9.Walubita T, Forrester SN, Jesdale BM. Allostatic Load Among Black Sexual Minority Women. J Womens Health. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenfeld D, Bartlam B, Smith RD. Out of the Closet and Into the Trenches: Gay Male Baby Boomers, Aging, and HIV/AIDS. Gerontologist. 2012;52(2):255–64. [DOI] [PubMed] [Google Scholar]
- 11.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis DI, Harlow RE. Gay Youth and the Right to Education. Yale Law Policy Rev. 1986;4(2):446–78. [Google Scholar]
- 13.Gates TG, Viggiani PA. Understanding lesbian, gay, and bisexual worker stigmatization: a review of the literature. Int J Sociol Soc Policy. 2014;34(5/6):359–74. [Google Scholar]
- 14.Pachankis JE. The psychological implications of concealing a stigma: A cognitive-affective-behavioral model. Psychol Bull. 2007;133(2):328–45. [DOI] [PubMed] [Google Scholar]
- 15.Clouston SAP, Smith DM, Mukherjee S, Zhang Y, Hou W, Link BG, et al. Education and Cognitive Decline: An Integrative Analysis of Global Longitudinal Studies of Cognitive Aging. J Gerontol B Psychol Sci Soc Sci. 2019;75(7):e151–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flatt JD, Johnson JK, Karpiak SE, Seidel L, Larson B, Brennan-Ing M. Correlates of Subjective Cognitive Decline in Lesbian, Gay, Bisexual, and Transgender Older Adults. J Alzheimers Dis. 2018;64(1):91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kail BL, Acosta KL, Wright ER. State-level marriage equality and the health of same-sex couples. Am J Public Health. 2015;105(6):1101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ofosu EK, Chambers MK, Chen JM, Hehman E. Same-sex marriage legalization associated with reduced implicit and explicit antigay bias. PNAS. 2019;116(18):8846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for Social Research. Health and Retirement Study Ann Arbor, Michigan: University of Michigan; 2020. [Available from: hrs.isr.umich.edu/. [Google Scholar]
- 20.Fisher GG, Hassan H, Faul JD, Rodgers WL, Weir DR. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992–2014. Ann Arbor, Mich.: Survey Research Center; 2017. January 13. [Google Scholar]
- 21.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66 Suppl 1(Suppl 1):i162–i71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanes DW, Clouston SAP. Ask Again: Including Gender Identity in Longitudinal Studies of Aging. Gerontologist. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badgett MVL. The Wage Effects of Sexual Orientation Discrimination. Ind Labor Relat Rev. 1995;48(4):726–39. [Google Scholar]
- 24.Gorman BK, Oyarvide Z. Sexual Orientation, Socioeconomic Status, and Healthy Aging. Generations: Journal of the American Society on Aging. 2018;42(2):56–60. [Google Scholar]
- 25.Schneebaum A, Badgett MVL. Poverty in US Lesbian and Gay Couple Households. Feminist Economics. 2019;25(1):1–30. [Google Scholar]
- 26.Chai L, Maroto M. Economic Insecurity among Gay and Bisexual Men: Evidence from the 1991–2016 U.S. General Social Survey. Sociol Perspect. 2020;63(1):50–68. [Google Scholar]
- 27.Martell ME. Age and the new lesbian earnings penalty. International Journal of Manpower. 2020;41(6):649–70. [Google Scholar]
- 28.Stephan Y, Sutin AR, Luchetti M, Aschwanden D, Terracciano A. Self-rated health and incident dementia over two decades: Replication across two cohorts. Journal of Psychiatric Research. 2021;143:462–6. [DOI] [PubMed] [Google Scholar]
- 29.Treas J How Cohorts, Education, and Ideology Shaped a New Sexual Revolution on American Attitudes toward Nonmarital Sex, 1972–1998. Sociol Perspect. 2002;45(3):267–83. [Google Scholar]
- 30.Ragins BR, Singh R, Cornwell JM. Making the invisible visible: Fear and disclosure of sexual orientation at work. J Appl Psychol. 2007;92(4):1103–18. [DOI] [PubMed] [Google Scholar]
- 31.Spring AL. Declining Segregation of Same-Sex Partners: Evidence from Census 2000 and 2010. Popul Res Policy Rev. 2013;32(5):687–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escoffier J. Stigmas, Work Environment, and Economic Discrimination Against Homosexuals. Homosex Couns J. 1975;2(1):8–17. [Google Scholar]
- 33.Taylor Y. Complexities and Complications: Intersections of Class and Sexuality. J Lesbian Stud. 2009;13(2):189–203. [DOI] [PubMed] [Google Scholar]
- 34.Shaw C, Hayes-Larson E, Glymour MM, Dufouil C, Hohman TJ, Whitmer RA, et al. Evaluation of Selective Survival and Sex/Gender Differences in Dementia Incidence Using a Simulation Model. JAMA Network Open. 2021;4(3):e211001–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia MA, Umberson D. Marital Strain and Psychological Distress in Same-Sex and Different-Sex Couples. J Marriage Fam. 2019;81(5):1253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Monteflores C, Schultz SJ. Coming Out: Similarities and Differences for Lesbians and Gay Men. J Soc Issues. 1978;34(3):59–72. [Google Scholar]
- 37.Karim R, Dang H, Henderson VW, Hodis HN, St. John J, Brinton RD, et al. Effect of Reproductive History and Exogenous Hormone Use on Cognitive Function in Mid- and Late Life. J Am Geriatr Soc. 2016;64(12):2448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg AE, Garcia R. Predictors of relationship dissolution in lesbian, gay, and heterosexual adoptive parents. J Fam Psychol. 2015;29(3):394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L, Gonzalez J. Modeling Floor Effects in Standardized Vocabulary Test Scores in a Sample of Low SES Hispanic Preschool Children under the Multilevel Structural Equation Modeling Framework. Front Psychol. 2017;8:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco-Marina F, García-González JJ, Wagner-Echeagaray F, Gallo J, Ugalde O, Sánchez-García S, et al. The Mini-mental State Examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. International Psychogeriatrics. 2010;22(1):72–81. [DOI] [PubMed] [Google Scholar]
- 41.Jacquez JA, Koopman JS, Simon CP, Longini IM Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr (1988). 1994;7(11):1169–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HRS data is publicly available for free at the HRS’s website: hrs.isr.umich.edu/. Further enquiries can be directed to the corresponding author.


