Abstract
Cannabis allergy is a burgeoning field; consequently, research is still in its infancy and allergists’ knowledge surrounding this topic is limited. As cannabis legalization expands across the world, it is anticipated that there will be an increase in cannabis use. Thus, we hypothesize that a concomitant rise in the incidence of allergy to this plant can be expected. Initiatives aimed at properly educating health care professionals are therefore necessary. This review presents the most up-to-date information on a broad range of topics related to cannabis allergy. Although the clinical features of cannabis allergy are becoming more well described and recognized, the tools available to make a correct diagnosis are meager and often poorly accessible. In addition, research on cannabis allergy is still taking its first steps, and new and potentially groundbreaking findings in this field are expected to occur in the next few years. Finally, although therapeutic approaches are being developed, patient and physician education regarding cannabis allergy is certainly needed.
Introduction
Today, more than 150 million people use cannabis on a regular basis: one of the most popular drugs worldwide. The first evidence of cannabis use can be dated back to 2500 years BC1; however, the evidence on the existence of cannabis allergy (CA) is relatively new, as it was not described until 1971.2 Although recent research has started to reveal a clinical picture and diagnosis of immunoglobulin (Ig)E-mediated CA, a lot is still to be explored. Today, CA could be a potentially life-threatening allergy with a risk of extensive cross-reactivities and, so, merits the attention of clinical allergists around the globe.
This review provides the current knowledge about the epidemiology of cannabis, the clinical picture of CA, and the described cross-reactive patterns. Furthermore, this review elaborates on different diagnostic and therapeutic options including recommendations for clinical practice on how to approach this new allergy entity.
Cannabis as an Allergenic Source
Cannabis is a genus of dioecious annual herbaceous plants in the family Cannabaceae. Among them, Cannabis sativa, Cannabis indica, and Cannabis ruderalis have been cultivated by humans for thousands of years for their psychoactive and medical properties, as food, and for textile production.3 Through hybridization work among these 3 species, numerous cultivars with differing characteristics have been selected.4
The psychoactive and medical effects of cannabis are related to its cannabinoid content, including tetrahydrocannabinol (THC) and cannabidiol (CBD).3 The ways in which cannabis is consumed are numerous. Recreationally, it is usually smoked in the form of marijuana, dried inflorescences of the female plant, or hashish, compressed and dried resin, or it can be eaten in the form of cakes or sweets, injected, or inhaled through vaping. In addition, products such as nabiximols5 are used in the medical field and hemp seeds are used as a food ingredient.6,7 Hemp oil can be obtained by cold extraction from hemp seeds and used as a cosmetic or as a food because of its polyunsaturated fatty acid and antioxidant content.8 Oils extracted from other parts of the plant such as inflorescences, stems, and leaves (ie, CBD and cannabis oils) can also be used for vaping, and some cases of eosinophilic pneumonia induced by their use have been described.9
In the past decades, numerous C sativa allergens have been isolated and studied.10-13 To date, 4 are recognized by the World Health Organization and International Union of Immunological Societies Allergen Nomenclature Sub-Committee, which are as follows: Can s 2, a profilin; Can s 3, a nonspecific lipid transfer protein (nsLTP); Can s 4, an oxygen-evolving enhancer protein 2 (OEEP2); and Can s 5, a pathogenesis-related protein 10 (PR10). Although part of the research has focused exclusively on C sativa allergens, selective sensitization to cannabis cultivars has been observed, probably related to the role of C indica in hybrid selection.4
Epidemiology of Cannabis
Incidence of Cannabis Use
Cannabis is the third most used psychoactive substance worldwide, after alcohol and tobacco.14 The legal status of cannabis for medical and recreational purposes varies internationally.14 North America includes some of the first jurisdictions to legalize medical cannabis use and second to legalize nonmedical use.15,16
Most information comes from survey studies. The data suggest that cannabis use is common and that consumption will continue to increase as attitudes change. According to 2019 United Nations reports, it is estimated that 4.0% of the global population (200.4 million) aged 15 to 64 years have used cannabis with use most prevalent in North America (14.5%), Australia and New Zealand (12.1%), followed by west and central Africa (9.4%).14
The National Survey on Drug Use and Health in the United States estimates that in 2019 to 2020, 46% (127.1 million) of the population aged 12 years or older have used cannabis at least once and approximately 17.9% (49.6 million) reported using cannabis in the last year.17 Young adults between the ages of 18 and 25 years have the highest past-year prevalence (35.4%) of cannabis use, followed by those 26 to 49 years old (21.7%).17 According to The Canadian Tobacco, Alcohol and Drugs Survey in 2020 approximately 27.0% of people reported having used cannabis within the past year.18 People between the ages of 16 to 24 years reported the highest percentage of cannabis use.18 In Europe, it is estimated that in 2019 approximately 90 million individuals aged 15 to 64 years used cannabis at least once, and from 2015 to 2020, approximately 15.4% of European Union inhabitants aged 15 to 35 years used cannabis in the previous year.19,20
A survey study conducted between 2016 and 2019 of US adults in 21 states revealed that higher frequency of cannabis use is more common among the young, males, Black and native American individuals, and respondents with low socioeconomic status.21 Being married and identifying as Asian or Hispanic were associated with lower frequency of cannabis use.21 Higher frequency of cannabis use was also common among persons who use tobacco, including electronic cigarettes, and alcohol with smoking being the most common form of cannabis use.21
Predictions for the Future (International Regulatory Framework and Predictions of Trends in Cannabis Use)
The increase in cannabis use may be partly attributable to the recent implementation of legalization policies in several countries and states. The prevalence of cannabis use was higher in US states with recreational legal cannabis than other states with nonlegal use.21 Legalization of recreational cannabis use has led to reduced prices, increased potency, and made cannabis more available to adult users.22 Furthermore, with the increasing availability of portable electronic “vaporized” systems, vaping cannabis is becoming more common among youth.23 We hypothesize that cannabis use will continue to increase owing to increased legalization policies, increased social acceptability of cannabis use, and a reduction of the perceived risk of cannabis use.24 Increased cannabis use may translate to higher incidences of cannabis sensitization either because of direct use of cannabis or because of an increase of secondhand smoke; however, no study has evaluated changes in sensitization rates.
Incidence of Cannabis Allergy
It is likely that CA is increasing partly because of its evolving legal status.25,26 Presently, the diagnosis of cannabis-related allergies requires a medical history of hypersensitivity reaction to cannabis products and positive skin testing result using crude extracts from buds and leaves.26 Even though there are several reports of allergies to different members of the Cannabaceae family, such as industrial hemp, descriptions of genuine IgE-dependent allergic reactions in cannabis users are less common, partly because of the illegal status of cannabis in many parts of the world.25 This makes it difficult to determine the prevalence of CA25; thus, the true prevalence of IgE-mediated C sativa allergy remains unknown. A Spanish allergy group investigated the prevalence of CA and found that 0.4% of patients presenting to their allergy clinic with respiratory and/or cutaneous symptoms had clinically relevant cannabis sensitization.27 However, this may not represent true prevalence as patients were consulting their allergy clinic and not involving the general population.
Knowledge, Attitudes, and Practice: Update of Patient and Physician Knowledge, Attitudes, and Practice and the International Cannabis Allergy Knowledge, Attitudes, and Practice Collaboration Study
Patients with allergy and asthma could potentially be at high risk from consuming cannabis by inhaled routes (smoking and/or vaping) which could potentially increase asthma exacerbation.28,29 To investigate the impact of cannabis use in the patients with allergy and asthma, a survey study was completed with participants recruited through the Allergy & Asthma Network. The survey inquired about cannabis knowledge, attitudes, and practice (KAP).30 Of 489 respondents, 18% (n = 88) used cannabis in the past 2 weeks. Among the cannabis users, 58% had current asthma (39.2% of whom were uncontrolled), 50% smoked cannabis, and 25% of those who smoked cannabis had cough from it. In the total sample, 40.9% indicated that their allergist asked about cannabis use. In noncannabis users, 2.5% reported that they had a CA. For those with asthma who smoke cannabis, adequate counseling about routes of administration is imperative.
To determine KAP among allergists, an international survey of 445 allergists from the United States, Canada, and the European Union was undertaken.31 Physicians with more cannabis knowledge had more progressive attitudes toward cannabis which affected real-world practice (ie, communication with patients regarding cannabis). In terms of comfort to speaking to patients about cannabis, 67.9% of the allergists indicated that they were; however, only 35.5% asked about cannabis on intake forms or verbally. Approximately 43% of the surveyed allergists indicated that they consulted with patients with suspected CA and 54.7% had done skin prick testing and/or in vitro cannabis testing. These dual studies of the allergy and asthma population represent the only demographics in which both patients and physicians have been surveyed in terms of their KAP.
Allergists indicated that educational programs are integral to improving their knowledge.31 It has been found that lack of education about cannabis and the endocannabinoid system is a common barrier to interacting with patients regarding cannabis among physicians from multiple specialties.32,33 Patients with allergy/asthma would also benefit from counseling regarding which routes of administration are safest and how to protect their lungs. To fill this unmet need among allergists, a series of educational modules is currently being created.
Clinical Features of Cannabis Allergy
Clinical Manifestations of Cannabis Allergy
CA is usually reported as an immediate-type hypersensitivity reaction that occurs after exposure to cannabis products through various modes of intake (including passive cannabis smoke inhalation34), whether for recreational, medical, or occupational reasons.35 Although smoking is the most frequently associated route of intake, cannabis consumption of edibles such as space cakes, oils, hemp seeds, and marijuana tea can also give rise to allergic reactions.36,37
Symptoms usually occur rapidly, within 30 minutes, and usually involve the respiratory system.36 Both the upper and lower airways can be affected, with features of rhinoconjunctivitis and asthma.36 Another organ most often involved is the skin. Generalized itching, localized38 and generalized urticaria, and angioedema have been described as part of the clinical manifestations of CA.36 Cardiovascular and gastrointestinal symptoms are rare.36,39
Nevertheless, anaphylaxis seems to be a common manifestation of CA, as up to 20% of patients with CA have experienced such a clinical event.36 This can potentially be fatal, although the only reported case of death owing to cannabis anaphylaxis occurred after parenteral use, an unusual mode of intake.40 Anaphylaxis can also occur after smoking cannabis products40 and after ingesting hemp seed41 or marijuana tea.37
In any case, symptoms of CA should always be distinguished from common adverse effects related to cannabis use that may in part have a similar presentation. Table 1 illustrates the common adverse effects related to cannabis consumption and the distinctive features compared with CA.42-53 Also worth mentioning is the possible role of cannabis contaminants, for example, molds,54 which can potentially cause symptoms in sensitized individuals which are difficult to distinguish from genuine CA.
Table 1.
Differential Diagnosis Between Cannabis-Related Adverse Effects and Cannabis Allergy Symptoms
| System involved | Cannabis adverse effects | Cannabis allergy symptoms |
|---|---|---|
| Eye | Clear looking, red, dilated conjunctival vessels40,41 | Conjunctivae are muddy, red |
| No itching | Itching | |
| Nose | Nasal congestion | Nasal congestion |
| No itching or sneezing | Itching and sneezing | |
| Mouth/throat | No itching | Itchy palate and throat |
| Phenytoin-like diffuse gingival hyperplasia, coral pink color40,42,43 | Palate and uvula may swell, glassy appearance | |
| Other: xerostomia, leukoderma, candidiasis,40,42 caries, hoarse voice | None | |
| Heart | Dose-dependent increase within few minutes of blood pressure and heart rate44,45 | Hypotension and bradycardia mainly in anaphylaxis |
| Toxic reaction resembling inherited Brugada syndrome45 | None | |
| Pulmonary | Wheezing/cough | Wheezing/cough |
| Case reports of pneumothorax and pneumomediastinum46-48 | No | |
| Case report of eosinophilic pneumonia49 | No | |
| GI tract | Cannabis hyperemesis syndrome50,51 | Abdominal cramping can occur in anaphylaxis |
Abbreviation: GI, gastrointestinal.
The effect of cofactors that may act as facilitators of allergic reactions such as alcohol, physical exercise, and nonsteroidal anti-inflammatory drugs55 is not known in CA; however, a role for them should never be overlooked.
In addition, populations residing near hemp crops may experience cannabis-related rhinoconjunctivitis caused by exposure to cannabis pollen.56
Anecdotally, some cases of delayed-type dermatitis related to cannabis harvesting or cannabis oil use have also been described.8,57,58
Cannabis-Related Food Allergy
Frequently Involved Foods
A case report published in 2007 listed a number of foods causing symptoms in an individual with an allergy to C sativa.13 Subsequently, tomatoes, hazelnut, walnut, peanut, peaches and other Rosaceae fruits, kiwifruit, banana, tomato, citrus fruits, and grapes have been reported to provoke reactions in individuals sensitized or allergic to C sativa.27,36,59,60 Such reactions are considered to be owing to homology between Can s 3, the nsLTP in C sativa, and nsLTP in these foods.61 Wine and beer could also trigger reactions in individuals sensitized to C sativa because of cross-reactivity between the nsLTP allergens in barley, grapes, and Can s 3.39 Furthermore, for reasons that are not yet fully known, a history of anaphylaxis to cannabis or sensitization to Can s 3 seems to be risk factors for cofactor-associated food allergic reactions in patients with CA with clinical reactivity to plant-derived foods.36
Existence of Regional Differences in Cannabis-Associated Food Allergy
The phenomenon of food allergic reactions occurring in individuals sensitized to C sativa has only been reported in Europe. Studies from the United States have not found Can s 3 to be a relevant allergen in individuals with CA, possibly because of a lack of reported sensitization and/or allergy involving nsLTP in these populations.12,37,62 Although other allergens in C sativa, Can s 2 (profilin), Can s 5 (PR10), and ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO), are also relevant allergens in plant foods, there is no evidence as yet that they are responsible for cross-reactions to foods.11,12,61
Hemp Seed Allergy
Hemp seeds, a variety of C sativa grown commercially, are high in protein, have a good ratio of omega 3-omega 6 polyunsaturated fatty acids, and contain a much lower level of THC and higher quantity of CBD.63,64 The high level of CBD, the nutrient profile and link to health benefits, is fueling the increase in the availability and consumption of foods containing hemp seed. A case report suggests a link between cannabis use and allergic reactions to hemp seed.65 However, although a case series found that most individuals reacting to hemp seed were cannabis users, 27% were not, suggesting hemp seeds can provoke allergy independently of sensitization to C sativa.66
Occupational Allergy to Cannabis
CA has been described among cannabis growers, factory workers, and laboratory personnel experiencing both respiratory and cutaneous symptoms on exposure.67 Cannabis varieties such as hemp have been an essential component in industries as an important source of fiber, paper, food, medicine, and in textile industries.35,68 The expansion of cannabis access has resulted in a growing workforce which brings into focus an increased concern for the health and well-being of workers who are involved with manufacturing, handling, and dispensing of the cannabis plant and its products,68 including increased concern for potential allergic reactions.68 Occupational sensitization to cannabis has mostly centered on the cannabis exposures of law enforcement officers and forensic investigators.35 Prolonged occupational exposure to cannabis typically results in respiratory irritation, airflow obstruction, and inflammation. However, because cannabis is often not the only allergen present during the manufacturing and growing processes, symptoms can be elicited by other allergens and irritants associated with the cannabis plant, including fungi, bacterial endotoxin, pesticides, and hemp dust, which is not explained by allergic sensitization to cannabis.35,68,69
However, reports suggest that the origins of these symptoms are likely owing to nonimmune reactions rather than true IgE-mediated allergy.67 The use of appropriate personal protective equipment may assist in reducing the number of symptoms reported on duty, independent of their origin.69
From Diagnosis to Treatment
Component Resolved Diagnosis
Developing diagnostic approaches to definitively establish clinically relevant sensitization to cannabis has been fraught with multiple issues that limit accessibility to cannabis and impede development of tools for research and diagnostics. Although sociopolitical and medical views to cannabis are evolving worldwide, accessibility to the plant in the United States and in many other countries is illegal, thus undermining scientific progress in the field. In the United States, the 2018 Hemp Farming Act improved access to the hemp plant (cannabis plant containing <0.3% THC) paving the way for research use. Although this has been a boost on the laboratory research front, significant restrictions continue in developing clinical allergy testing. Over the years, multiple groups in North America and in Europe have adopted the approach of testing sera from patients with presumed allergy to cannabis to identify specific immunoreactive allergens. These collaborative efforts have helped identify specific component allergens of cannabis.
The Primary Role for Cannabis Sativa Nonspecific Lipid Transfer Protein Can s 3
Can s 3 is one of the widely studied allergens of cannabis. Can s 3 rose to prominence in Europe, where multiple independent studies identified it as a major allergen.13,27,36 Moreover, Can s 3 shares its molecular features with homologous nsLTPs from other plants, driving the paradigm of cannabis-fruit/vegetable allergy syndrome.62 Preliminary unpublished investigations in the United States have revealed that Can s 3 is a relevant allergen among North American cohorts, although it does not explain all sensitizations.12 Can s 3 also does not seem to be relevant in occupational allergies, with 1 study in Belgium reporting low frequency of sensitization to this allergen among symptomatic law enforcement officers.67 Nevertheless, Can s 3 is a major allergen with immense clinical relevance, particularly among nonoccupational cohorts.35,70
Profilin, Oxygen-Evolving Enhancer Protein 2, and Pathogenesis-Related Protein 10
Recombinant version of proteins Can s 2 (cannabis profilin), 4 (cannabis oxygen-evolving enhancer protein 2), and 5 (cannabis PR-10) have also been used to evaluate cannabis sensitization.10,11 Although Can s 2 and Cans 4 sensitization is relatively less common, IgE binding to Can s 5 allergen seems to be more significant. Furthermore, integration of functional studies such as basophil activation test (BAT) and mast cell studies has also provided insights into clinical relevance of these allergens.10,11
Other Allergens (RuBisCo Conundrum)
Other allergens of cannabis have been reported but have not been validated using component resolved diagnostics approach.12 For instance, the abundant plant protein RuBisCo was reported as a potential allergen of cannabis; however, it is unclear whether this is a false-positive finding and an artifact of immunoproteomics approach. Finally, there are some reports of sensitization to cross-reactive carbohydrate determinants; however, this is yet to be explored in great detail.
Diagnostic Algorithm
Detecting Sensitization to Cannabis
Diagnosis of immediate-type IgE-mediated CA begins with a thorough history that considers the routes of exposure and filters adverse effects associated with the use of this substance.70-73
If the history seems to be suggestive for a possible CA, the physician needs to demonstrate sensitization to cannabis as such. Traditionally, the first step in this process is to perform a prick-by-prick test with buds, leaves, and other raw parts of the plant,35 possibly of different varieties, because selective sensitization to one rather than the other is possible.4 Another option is to perform skin tests using handmade cannabis extracts, possibly enriched with Can s 3.34,36,60,74 The use of cannabis-derived oils to perform skin tests, including their allergen content, has not yet been investigated.
Alternatively, an in vitro test for hemp-specific IgE can be performed. Unfortunately, only a singleplex test (FEIA ImmunoCAP, Special Allergen Service, Phadia Thermo Fisher Scientific, Waltham, Massachusetts) (available on request for research purposes) and a commercial assay that is part of a multiplex panel (Allergy Explorer-Alex 2, Macroarray Diagnostics, Vienna, Austria) are accessible to date. Calculating the ratio of hemp-specific IgE to total IgE allows the specificity of the test to be improved.75 In addition, cell activation tests such as BAT and mast cell activation tests using passively sensitized cells (pMAT) using a whole raw cannabis extract can be also used to find sensitization to cannabis.11,34,36,60,74
Detecting Sensitization to Cannabis Components
If all the test results for the detection of cannabis sensitization are negative, CA is ruled out with reasonable certainty. Otherwise, sensitization to the molecular components of cannabis should be studied through component resolved diagnosis (CRD) to confirm the diagnosis (Fig 1). In fact, CRD allows the identification of any sensitization to profilin, PR10, or cross-reactive carbohydrate determinants that can often be harbingers of false-positive results.35 At present, only 1 assay for the determination of Can s 3-specific IgE is commercially available (as part of the multiplex panel mentioned earlier). However, to detect sensitization against single molecular components of cannabis, it is alternatively possible to perform an in-house cytofluorimetric bead assay, an immunoblot, or a cell activation test.10,11,36,70,74,76 Table 2 illustrates a comparison of diagnostic performances of different diagnostic tests in patients with CA. Table 3 summarizes possible applicable tools for the detection of IgE-mediated sensitization to cannabis.
Figure 1.
Diagnostic flowchart for IgE-mediated cannabis allergy. CRD, component resolved diagnosis; IgE, immunoglobulin E.
Table 2.
Comparison of Diagnostic Performance of 5 Different Diagnostic Tests in Cannabis Allergy (Adapted From Decuyper et al34)
| Test performance | SPT with a Can s 3 enriched extract | sIgE hemp (ImmunoCAP) | sIgE Can s 3 | BAT with crude CS extract | BAT with Can s 3 |
|---|---|---|---|---|---|
| Sensitivity | 58% (49-67) | 82% (74-89) | 47% (38-56) | 49% (37-60) | 45% (35-55) |
| Specificity | 81% (71-88) | 32% (20-45) | 87% (78-93) | 67% (55-78) | 85% (76-92) |
| PPV | 80% (72-86) | 70% (66-74) | 82% (72-89) | 64% (54-73) | 78% (67-86) |
| NPV | 58% (53-64) | 47% (34-61) | 56% (51-60) | 52% (46-59) | 57% (52-62) |
Abbreviations: BAT, basophil activation test; CI, confidence interval; IgE, immunoglobulin E; NPV, negative predictive value; PPV, positive predictive value; sIgE, specific IgE; SPT, skin prick test.
NOTE. Comparison between patients with cannabis allergy and individuals with cannabis tolerance. Values are expressed as percentages and CI 95%.
Table 3.
Diagnostic Tools for the Diagnosis of IgE-Mediated Allergy to Cannabis
| Hemp (Cannabis sativa) | ImmunoCAPa |
| Allergy Explorer-Alex 2b | |
| CBA | |
| Immunoblot | |
| BAT | |
| pMAT | |
| Cannabis allergens | Allergy Explorer-Alex 2b,c |
| CBA | |
| Immunoblot | |
| BAT | |
| pMAT |
Abbreviations: BAT, basophil activation test; CBA, cytofluorimetric bead assay; IgE, immunoglobulin E; pMAT, passive mast cell activation test.
Available for research purposes on request.
Commercially available in Europe.
Only Can s 3 is available.
Cannabis Challenge Test and Miscellaneous
To date, challenge testing with cannabis, taken in the form of smoke or edibles, is not yet part of the diagnostic algorithm because of repercussions related to possible adverse effects and legal fallout.35
Finally, for those cases of contact dermatitis with cannabis, use of patch testing has also been described.57,58
Novel Insights and Unmet Needs in the Diagnosis of Cannabis Allergy
Accessibility to Diagnostics
Currently, there are still many unmet needs in the diagnosis of CA. The absence on the market of cannabis extracts for in vivo testing and the difficult accessibility of in vitro testing are the major obstacles to standardized and reliable diagnosis worldwide.35,70
In addition, as far as the United States is concerned, legal restrictions are a major limitation. In fact, cannabis is currently classified as a Schedule I substance. Access to samples of the plant is virtually impossible except for strictly regulated research purposes.77 Performing skin tests with the fresh parts or extracts is therefore impossible outside of these contexts. Similarly, there are no commercially available in vitro tests, and cell activation tests must be performed with plant extracts, which therefore fall under the same limitations as described previously. This completely limits the possibility of performing a CA diagnosis outside of centers where this is done for academic reasons.
Component Resolved Diagnosis
As for the CRD, identification of specific allergens relevant to cannabis sensitization may seem to be a piecemeal approach; however, it has been effective in identifying and characterizing allergens, which will eventually contribute toward understanding the gestalt of CAs. Availability of assays to specifically measure Can s 3 levels in the serum of sensitized patients and in environmental samples has developed from adoption of the CRD approach. There are also efforts to develop ImmunoCAP platforms with existing allergens. Development of sensitive and specific assays will help resolve the sensitization profile in many patients where current approaches fail to establish IgE immunoreactivity to specific allergens.
As a final note, much of the research on CRD has focused on detecting C sativa allergens. However, it is possible that a small percentage of patients actually have selective sensitization to C indica. This is reflected in variable susceptibility to products derived from hybrids of these 2 cannabis species.4 These patients could benefit from a species-specific diagnostic approach.
Cell Activation Tests
Cell activation tests such as BAT and pMAT certainly represent promising diagnostic tools, both in clinical and research settings.10,11,36,60,70,74 Unfortunately, they are hampered by some important limitations. Up to 15% of the patients do not respond in the BAT, and in these cases the results cannot be correctly interpreted.78 In addition, to provide reliable results, the test must be performed with fresh blood and in experienced centers equipped with state-of-the art infrastructures. The pMAT aims to overcome the limitations related to nonresponder status that plagues the BAT.78-80 The test can also be performed on frozen plasma samples giving way to a centralization of the analysis that allows for greater standardization of results.79 Unfortunately, pMAT is currently available in only a few centers and is still far from being widely accessible in daily practice, also because of the issues related to the need for fresh mast cell cultures or mast cell lines.
Cannabis Challenge Test
Finally, the risk of adverse effects and the legal difficulties of challenge testing for cannabis, potentially the reference standard, further complicate the physician’s path to a correct diagnosis. Future research to find safer ways to perform a cannabis challenge (eg, a hemp seed challenge test39) is definitely needed.
Treatment
To date, the only effective therapy for CA is complete avoidance of the culprit.35 When this is not possible, such as in an occupational setting, or when exposure occurs inadvertently (second hand), management is not unlike that of other allergens. This involves symptomatic treatment and prescription of 2 epinephrine autoinjectors to patients who have experienced anaphylaxis.35 Patients with cannabis-related food allergy should also avoid foods implicated in clinical cross-reactivity.39 In addition, the use of appropriate personal protective equipment and engineering and administrative precautions could mitigate the risk to cannabis industry and police workers.68
Regarding active therapeutic approaches, omalizumab was successfully used in a single case of a policewoman who had several episodes of anaphylaxis after exposure to cannabis and could not avoid possible future contact in the workplace.81 After 4 months of omalizumab 300 mg subcutaneously every 4 weeks, she was able to be exposed to large amounts of cannabis without experiencing anaphylaxis. Furthermore, the use of crude cannabis protein immunotherapy has only currently been used to treat a case of cannabis pollen-related rhinitis and asthma.82 However, the development of desensitization protocols for patients who have experienced allergy after the intake of cannabis products looks to be an interesting future therapeutic approach.
Physician and Patient Education
Given its increased use and widespread legalization, physicians should incorporate questions about cannabis as part of every medical history.83 This screening should be considered for all patients in their practices at least once for cannabis use. Groups with higher rates of use and groups at higher risk of harm should be screened more often, at least annually.84 Specific questions on route of use (ingestion, inhalation, topical, etc), including on the various cannabis product(s) used, are essential.85 Cannabis use should be scrutinized with the same diligence as we scrutinize tobacco use. The use of cannabis should be assessed and documented at each visit, both quantitatively and qualitatively, as precisely as possible, and patients should be provided with counseling using the same methods we use in treating tobacco addiction.86 The medical history should be performed in a nonjudgmental and respectful fashion.83,87 Physicians should also be aware of their own personal biases regarding cannabis.87 Any recommendations should be made based on the available scientific evidence.85 Patient-specific materials, including written and web-based information, are valuable complements to the office visit.35 Because of ongoing cannabis legalization and the introduction of new products to the market, physician education on cannabis, at local, state, and national levels, is paramount.35
Conclusion
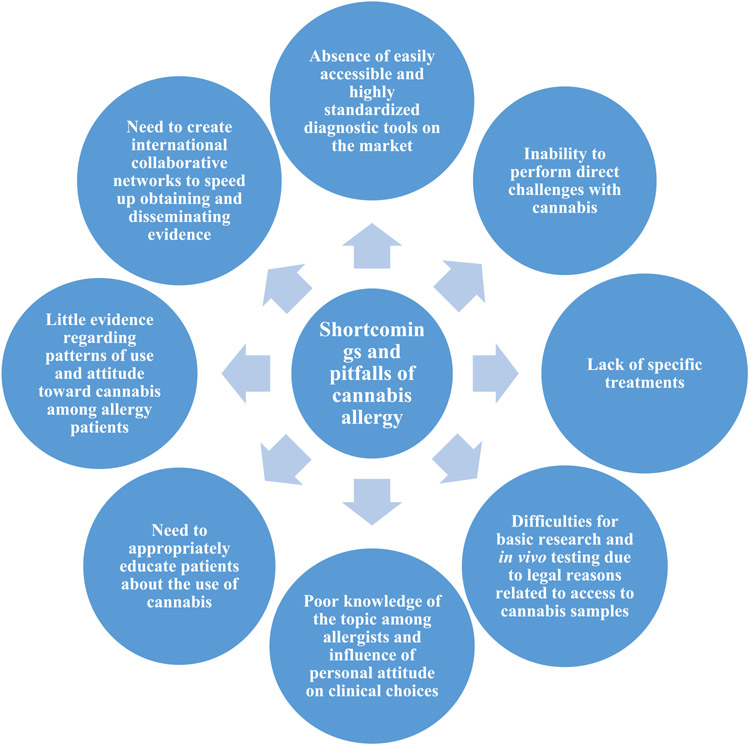
Prospects for large-scale legalization of cannabis indicate the need of acquiring even more knowledge about CA. At present, little is yet known about the epidemiology and inter-regional clinical differences that exist among patients. Furthermore, much remains to be discovered about cannabis allergens and their role in different clinical pictures, in cannabis-associated vegetable food allergy, and the influence of chemical and physical modifications that may occur in relation to common modes of cannabis intake (eg, combustion). The fate of diagnostic research will be inextricably linked to the evidence obtained in these areas. In this latter domain, a major breakthrough will only be possible when standardized and commercially available tests will become readily accessible to allergists, which at present is still far from being achieved. Finally, therapeutic treatments have been largely neglected by research so far, and the existing literature is limited to anecdotal cases. Patients who cannot avoid contact with cannabis for professional reasons would be the first to benefit. However, the constant search for more knowledge must be coupled with adequate and widespread dissemination of this information among physicians. Currently, in fact, awareness of this topic is still limited, and this is often associated with the personal attitudes of physicians. This has immediate repercussions on the quality of care provided to patients, increasing the risk of missing possible diagnoses and providing the most appropriate advice. In addition, at present, attitudes and pattern of use of cannabis among patients with allergy/asthma have only been investigated in 1 study.30 A choral effort by those involved in the management of CA is therefore necessary. Properly educating physicians and patients will have certainly immediate positive effects. As a final point, the importance of international collaboration cannot but be stressed. CA, in any case, remains a relatively rare disease, and only large case series originating from these initiatives will allow rapid advancement. Ventures such as the International Allergist Canna KAP consortium act as a point of contact between allergy societies and professionals from different continents and represents the right path to pursue to keep alive and grow this field of allergy that is probably still in its infancy. Figure 2 summarizes the main shortcomings and pitfalls of CA in its current state.
Figure 2.
Shortcomings and pitfalls of cannabis allergy.
Key Messages.
Cannabis is the most consumed illicit drug in the world, but allergy to this substance is probably overlooked because of little knowledge of this topic among health care professionals.
The most common form of allergy to cannabis is an immediate-type hypersensitivity characterized almost invariably by skin and respiratory symptoms.
Diagnosis of cannabis allergy is based on a stepwise approach, starting with skin tests.
No cannabis-specific treatment is currently available for cannabis allergy.
The allergy and asthma population are the only demographics in which both patients and physicians have been surveyed in terms of their knowledge, attitude, and practice.
Acknowledgments
The authors thank the support of the members of the International Cannabis Allergy Collaboration and extend their gratitude to Miriam Standish, from the American College of Allergy, Asthma and Immunology, who supported the work of the group by arranging meetings.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
References
- 1.Ren M, Tang Z, Wu X, Spengler R, Jiang H, Yang Y, et al. The origins of cannabis smoking: chemical residue evidence from the first millennium BCE in the Pamirs. Sci Adv. 2019;5(6):eaaw1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liskow B, Liss JL, Parker CW. Allergy to marihuana. Ann Intern Med. 1971;75(4):571–573. [DOI] [PubMed] [Google Scholar]
- 3.Pisanti S, Bifulco M. Medical cannabis: a plurimillennial history of an evergreen. J Cell Physiol. 2019;234(6):8342–8351. [DOI] [PubMed] [Google Scholar]
- 4.Stepaniuk P, Kanani A. Selective cannabis strain allergy in a patient presenting with a local allergic reaction. Allergy Asthma Clin Immunol. 2021;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueberall MA, Essner U, Silván CV, Mueller-Schwefe GHH. Comparison of the effectiveness and tolerability of nabiximols (THC:CBD) oromucosal spray versus oral dronabinol (THC) as add-on treatment for severe neuropathic pain in real-world clinical practice: retrospective analysis of the German pain e-registry. J Pain Res. 2022;15:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson R Hemp as an agricultural commodity. Congressional Research Service. 2018. Available at: https://sgp.fas.org/crs/misc/RL32725.pdf. Accessed March 15, 2022. [Google Scholar]
- 7.Odieka AE, Obuzor GU, Oyedeji OO, Gondwe M, Hosu YS, Oyedeji AO. The medicinal natural products of Cannabis sativa Linn.: a review. Molecules. 2022;27 (5):1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark E, Nilsson U, Samaran Q, Raison-Peyron N. Allergic contact dermatitis from Cannabis sativa (hemp) seed oil. Contact Dermatitis. 2022;87(3):292–293. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, Cool CD, Maloney JP. Histopathological correlation of acute on chronic eosinophilic pneumonitis caused by vaporized cannabis oil inhalation. Chest. 2021;159(3):e137–e139. [DOI] [PubMed] [Google Scholar]
- 10.Decuyper II, Rihs HP, Mertens CH, van Gasse AL, Elst J, de Puysseleyr L, et al. A new cannabis allergen in Northwestern Europe: the oxygen-evolving enhancer protein 2 (OEEP2). J Allergy Clin Immunol Pract. 2020;8(7):2421–2424. e2. [DOI] [PubMed] [Google Scholar]
- 11.Ebo DG, Decuyper II, Rihs HP, Mertens C, van Gasse AL, van der Poorten MLM, et al. Clinical communications IgE-binding and mast celleactivating capacity of the homologue of the major birch pollen allergen and profilin from Cannabis sativa. Available at: www.jaci-inpractice.org. Accessed August 30, 2022. [DOI] [PubMed] [Google Scholar]
- 12.Nayak AP, Green BJ, Sussman G, Berlin N, Lata H, Chandra S, et al. Characterization of Cannabis sativa allergens. Ann Allergy Asthma Immunol. 2013;111(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamboa P, Sanchez-Monge R, Sanz ML, Palacín A, Salcedo G, Diaz-Perales A. Sensitization to Cannabis sativa caused by a novel allergenic lipid transfer protein, Can s 3.J Allergy Clin Immunol. 2007;120(6):1459–1460. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Office on Drugs and Crime. World Drug Report 2021. Global overview: drug demand drug supply. Available at: www.unodc.org/unodc/en/data-and-analysis/wdr2021.html. Accessed August 30, 2022. [Google Scholar]
- 15.Rotermann M Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Health Rep. 2019;30(6):3–13. [DOI] [PubMed] [Google Scholar]
- 16.Leung J, Chan G, Stjepanović D, Chung JYC, Hall W, Hammond D. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacol (Berl). 2022;239(5):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Behavioral Health Statistics and Quality. Results From the 2019 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2020. [Google Scholar]
- 18.Health Canada. Canadian tobacco, alcohol and drugs survey. Canadian cannabis survey 2021: summary. 2021. Available at: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2021-summary.html. Accessed August 30, 2022. [Google Scholar]
- 19.Manthey J, Freeman TP, Kilian C, López-Pelayo H, Rehm J. Public health monitoring of cannabis use in Europe: prevalence of use, cannabis potency, and treatment rates. Lancet Reg Health Eur. 2021;10:100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabri AC, Galanti MR, Orsini N, Magnusson C. Changes in cannabis policy and prevalence of recreational cannabis use among adolescents and young adults in Europe–an interrupted time-series analysis. PLoS One. 2022;17(1): e0261885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffers AM, Glantz S, Byers A, Keyhani S. Sociodemographic characteristics associated with and prevalence and frequency of cannabis use among adults in the US. JAMA Netw Open. 2021;4(11): e2136571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall W, Lynskey M. Assessing the public health impacts of legalizing recreational cannabis use: the US experience. World Psychiatry. 2020;19(2):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond CJ, Chaney A, Hendrickson B, Sharma P. Cannabis use among U.S. adolescents in the era of marijuana legalization: a review of changing use patterns, comorbidity, and health correlates. Int Rev Psychiatry. 2020;32(3):221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han BH, Funk-White M, Ko R, Al-Rousan T, Palamar JJ. Decreasing perceived risk associated with regular cannabis use among older adults in the United States from 2015 to 2019. J Am Geriatr Soc. 2021;69(9):2591–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decuyper II, van Gasse AL, Cop N, Sabato V, Faber MA, Mertens C, et al. Cannabis sativa allergy: looking through the fog. Allergy. 2017;72(2):201–206. [DOI] [PubMed] [Google Scholar]
- 26.Decuyper I, Ryckebosch H, van Gasse AL, Sabato V, Faber M, Bridts CH, et al. Cannabis allergy: what do we know Anno 2015. Arch Immunol Ther Exp (Warsz). 2015;63 (5):327–332. [DOI] [PubMed] [Google Scholar]
- 27.Larramendi CH, López-Matas MÁ, Ferrer Á, Huertas ÁJ, Pagán JA, Navarro LÁ, et al. Prevalence of sensitization to Cannabis sativa. Lipid-transfer and thaumatin-like proteins are relevant allergens. Int Arch Allergy Immunol. 2013;162(2):115–122. [DOI] [PubMed] [Google Scholar]
- 28.Chatkin JM, Zani-Silva L, Ferreira I, Zamel N. Cannabis-associated asthma and allergies. Clin Rev Allergy Immunol. 2019;56(2):196–206. [DOI] [PubMed] [Google Scholar]
- 29.Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeiger JS, Silvers WS, Winders TA, Hart MK, Zeiger RS. Cannabis attitudes and patterns of use among followers of the Allergy & Asthma Network. Ann Allergy Asthma Immunol. 2021;126(4):401–410. e1. [DOI] [PubMed] [Google Scholar]
- 31.Zeiger JS, Silvers WS, Naimi DR, Skypala IJ, Ellis AK, Connors L, et al. Impact of cannabis knowledge and attitudes on real-world practice. Ann Allergy Asthma Immunol. 2022;129(4):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hordowicz M, Klimkiewicz A, Jarosz J, Wysocka M, Jastrzębska M. Knowledge, attitudes, and prescribing patterns of cannabis and cannabinoid-containing medicines among European healthcare workers: a systematic literature review. Drug Alcohol Depend. 2021;221: 108652. [DOI] [PubMed] [Google Scholar]
- 33.Ng JY, Gilotra K, Usman S, Chang Y, Busse JW. Attitudes toward medical cannabis among family physicians practising in Ontario, Canada: a qualitative research study. CMAJ Open. 2021;9(2):E342–E348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decuyper II, Faber MA, Sabato V, Bridts CH, Hagendorens MM, Rihs HP, et al. Where there’s smoke, there’s fire: cannabis allergy through passive exposure. J Allergy Clin Immunol Pract. 2017;5(3):864–865. [DOI] [PubMed] [Google Scholar]
- 35.Skypala IJ, Jeimy S, Brucker H, Nayak AP, Decuyper II, Bernstein JA, et al. Cannabis-related allergies: an international overview and consensus recommendations. Allergy. 2022;77(7):2038–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decuyper II, van Gasse AL, Faber MA, Elst J, Mertens C, Rihs HP, et al. Exploring the diagnosis and profile of cannabis allergy. J Allergy Clin Immunol Pract. 2019;7 (3):983–989. e5. [DOI] [PubMed] [Google Scholar]
- 37.Tessmer A, Berlin N, Sussman G, Leader N, Chung EC, Beezhold D. Hypersensitivity reactions to marijuana. Ann Allergy Asthma Immunol. 2012;108(4):282–284. [DOI] [PubMed] [Google Scholar]
- 38.Rojas Pérez-Ezquerra P, Sánchez-Morillas L, Davila-Ferandez G, Ruiz-Hornillos FJ, Carrasco García I, Herranz Mañas M, et al. Contact urticaria to Cannabis sativa due to a lipid transfer protein (LTP). Allergol Immunopathol (Madr). 2015;43(2):231–233. [DOI] [PubMed] [Google Scholar]
- 39.Decuyper II, Rihs HP, van Gasse AL, Elst J, de Puysseleyr L, Faber MA, et al. Cannabis allergy: what the clinician needs to know in 2019. Expert Rev Clin Immunol. 2019;15(6):599–606. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert JD, Grabowski M, Byard RW. Intravenous administration of cannabis and lethal anaphylaxis. Med Sci Law. 2017;57(2):91–94. [DOI] [PubMed] [Google Scholar]
- 41.Stadtmauer G, Beyer K, Bardina L, Sicherer SH. Anaphylaxis to ingestion of hempseed (Cannabis sativa). J Allergy Clin Immunol. 2003;112(1):216–217. [DOI] [PubMed] [Google Scholar]
- 42.Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des. 2014;20(25):4112–4118. [DOI] [PubMed] [Google Scholar]
- 43.Wang MTM, Danesh-Meyer HV. Cannabinoids and the eye. Surv Ophthalmol. 2021;66(2):327–345. [DOI] [PubMed] [Google Scholar]
- 44.Singh H, Katz J, Saleh W, Cha S. Impact of cannabis on the port of entry-oral tissues: an overview. Int J Oral Dent Health. 2019;5(3):1–5. [Google Scholar]
- 45.Baddour HM, Audemorte TB, Layman FD. The occurrence of diffuse gingival hyperplasia in a patient using marijuana. J Tenn Dent Assoc. 1984;64(2):39–43. [PubMed] [Google Scholar]
- 46.Subramaniam VN, Menezes AR, DeSchutter A, Lavie CJ. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116 (2):146–153. [PMC free article] [PubMed] [Google Scholar]
- 47.Pratap B, Korniyenko A. Toxic effects of marijuana on the cardiovascular system. Cardiovasc Toxicol. 2012;12(2):143–148. [DOI] [PubMed] [Google Scholar]
- 48.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10 (3):239–247. [DOI] [PubMed] [Google Scholar]
- 49.Lee MH, Hancox RJ. Effects of smoking cannabis on lung function. Expert Rev Respir Med. 2011;5(4):537–547. [DOI] [PubMed] [Google Scholar]
- 50.Martinasek MP, McGrogan JB, Maysonet A. A systematic review of the respiratory effects of inhalational marijuana. Respir Care. 2016;61(11):1543–1551. [DOI] [PubMed] [Google Scholar]
- 51.Liebling PD, Siu S. A novel cause of eosinophilic pneumonia. J Bronchol Interv Pulmonol. 2013;20(2):183–185. [DOI] [PubMed] [Google Scholar]
- 52.Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicolson SE, Denysenko L, Mulcare JL, Vito JP, Chabon B. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53 (3):212–219. [DOI] [PubMed] [Google Scholar]
- 54.Kagen S, Kurup V, Sohnle P, Fink J. Marijuana smoking and fungal sensitization. J Allergy Clin Immunol. 1983;71(4):389–393. [DOI] [PubMed] [Google Scholar]
- 55.Niggemann B, Beyer K. Factors augmenting allergic reactions. Allergy. 2014;69 (12):1582–1587. [DOI] [PubMed] [Google Scholar]
- 56.Mayoral M, Calderóan H, Cano R, Lombardero M. Allergic rhinoconjunctivitis caused by Cannabis sativa pollen. J Investig Allergol Clin Immunol. 2008;18(1):73–74. [PubMed] [Google Scholar]
- 57.Foster E, Nguyen C, Norris P. Contact Buzz: Allergic Contact Dermatitis to Cannabis. Dermatitis. 2018;29(4):223–224. 10.1097/DER.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 58.Azim SA, Yim K, Higgins S, Wurzer J, Adler BL. Contact allergy to cannabis and related essential oils [e-pub ahead of print]. Dermatitis. 10.1097/DER.0000000000000869, accessed August 30, 2022. [DOI] [PubMed] [Google Scholar]
- 59.de Larramendi CH, Carnés J, García-Abujeta JL, García-Endrino A, Muñoz-Palomino E, Huertas AJ, et al. Sensitization and allergy to Cannabis sativa leaves in a population of tomato (Lycopersicon esculentum)-sensitized patients. Int Arch Allergy Immunol. 2008;146(3):195–202. [DOI] [PubMed] [Google Scholar]
- 60.Ebo DG, Swerts S, Sabato V, Hagendorens MM, Bridts CH, Jorens PG, et al. New food allergies in a European non-Mediterranean region: is Cannabis sativa to blame? Int Arch Allergy Immunol. 2013;161(3):220–228. [DOI] [PubMed] [Google Scholar]
- 61.Rihs HP, Armentia A, Sander I, Brüning T, Raulf M, Varga R. IgE-binding properties of a recombinant lipid transfer protein from Cannabis sativa. Ann Allergy Asthma Immunol. 2014;113(2):233–234. [DOI] [PubMed] [Google Scholar]
- 62.Skypala IJ, Asero R, Barber D, Cecchi L, Diaz Perales A, Hoffmann-Sommergruber K, et al. Non-specific lipid-transfer proteins: allergen structure and function, cross-reactivity, sensitization, and epidemiology. Vol. 11, Clinical and Translational Allergy. 11. Chichester: John Wiley & Sons Inc; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson TE. Controlled substances chaos: the Department of Justice’s new policy position on marijuana and what it means for industrial hemp farming in North Dakota. North Dakota Law Rev. 2015;90:613. [Google Scholar]
- 64.Rupasinghe HPV, Davis A, Kumar SK, Murray B, Zheljazkov VD. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules. 2020;25(18):4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhatia P, Chen M, Christiansen S. Marijuana and stoned fruit. Ann Allergy Asthma Immunol. 2018;120(5):536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alkhammash S, Tsui H, Thomson DMP. Cannabis and hemp seed allergy. J Allergy Clin Immunol Pract. 2019;7(7):2429–2430. e1. [DOI] [PubMed] [Google Scholar]
- 67.Decuyper II, van Gasse A, Faber MA, Mertens C, Elst J, Rihs HP, et al. Occupational cannabis exposure and allergy risks. Occup Environ Med. 2019;76(2):78–82. [DOI] [PubMed] [Google Scholar]
- 68.Decuyper II, Green BJ, Sussman GL, Ebo DG, Silvers WS, Pacheco K, et al. Occupational allergies to cannabis. J Allergy Clin Immunol Pract. 2020;8(10):3331–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sussman GL, Beezhold DH, Cohn JR, Silvers WS, Zeiger JS, Nayak AP. Cannabis: an emerging occupational allergen? Ann Work Expo Health. 2020;64(7):679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toscano A, Elst J, van der Poorten ML, Beyens M, Heremans K, Decuyper II, et al. Establishing diagnostic strategies for cannabis allergy. Expert Rev Clin Immunol. 2022;18(10):1015–1022. [DOI] [PubMed] [Google Scholar]
- 71.Tashkin DP, Tan WC. Inhaled marijuana and the lung [e-pub ahead of print]. J Allergy Clin Immunol Pract. 10.1016/j.jaip.2022.05.009. accessed August 30, 2022. [DOI] [PubMed] [Google Scholar]
- 72.Yazulla S Endocannabinoids in the retina: from marijuana to neuroprotection. Prog Retin Eye Res. 2008;27(5):501–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ocampo TL, Rans TS. Cannabis sativa: the unconventional “weed” allergen. Ann Allergy Asthma Immunol. 2015;114(3):187–192. [DOI] [PubMed] [Google Scholar]
- 74.Decuyper II, Faber MA, Lapeere H, Mertens C, Rihs HP, van Gasse AL, et al. Cannabis allergy: a diagnostic challenge. Allergy. 2018;73(9):1911–1914. [DOI] [PubMed] [Google Scholar]
- 75.Decuyper II, Rihs HP, Mertens CH, van Gasse AL, Elst J, Faber MA, et al. In search of the golden ratio for cannabis allergy: utility of specific allergen-to-total IgE ratios. Allergy. 2021;76(11):3522–3525. [DOI] [PubMed] [Google Scholar]
- 76.Faber M, van Gasse A, Sabato V, Hagendorens MM, Bridts CH, de Clerck LS, et al. Marihuana allergy: beyond the joint. J Investig Allergol Clin Immunol. 2015;25(1):70–72. [PubMed] [Google Scholar]
- 77.The Health Effects of Cannabis and Cannabinoids. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 78.Ebo DG, Bridts CH, Mertens CH, Sabato V. Principles, potential, and limitations of ex vivo basophil activation by flow cytometry in allergology: a narrative review. J Allergy Clin Immunol. 2021;147(4):1143–1153. [DOI] [PubMed] [Google Scholar]
- 79.Ebo DG, Heremans K, Beyens M, van der Poorten MLM, van Gasse AL, Mertens C, et al. Flow-based allergen testing: can mast cells beat basophils? Clin Chim Acta. 2022;532:64–71. [DOI] [PubMed] [Google Scholar]
- 80.Elst J, van der Poorten MM, van Gasse AL, de Puysseleyr L, Hagendorens MM, Faber MA, et al. Mast cell activation tests by flow cytometry: a new diagnostic asset? Clin Exp Allergy. 2021;51(11):1482–1500. [DOI] [PubMed] [Google Scholar]
- 81.Engler DB, Malick AA, Saraf SK, Dargel LA. Severe marijuana allergy controlled with omalizumab. J Allergy Clin Immunol. 2013;131(2):AB215. [Google Scholar]
- 82.Kumar R, Gupta N. A case of bronchial asthma and allergic rhinitis exacerbated during cannabis pollination and subsequently controlled by subcutaneous immunotherapy. Indian J Allergy Asthma Immunol. 2013;27(2):143. [Google Scholar]
- 83.Silvers WS. A Colorado allergist’s experience with marijuana legalization. Ann Allergy Asthma Immunol. 2016;116(2):175–177. [DOI] [PubMed] [Google Scholar]
- 84.Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60(9):801–808. e423-e432. [PMC free article] [PubMed] [Google Scholar]
- 85.Slawek D, Meenrajan SR, Alois MR, Comstock Barker P, Estores IM, Cook R. Medical cannabis for the primary care physician. J Prim Care Community Health. 2019;10: 215013271988483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lutchmansingh D, Pawar L, Savici D. Legalizing cannabis: a physician’s primer on the pulmonary effects of marijuana. Curr Respir Care Rep. 2014;3(4):200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.George M, Avila M, Speranger T, Bailey HK, Silvers WS. Conducting an integrative health interview. J Allergy Clin Immunol Pract. 2018;6(2):436–439. e3. [DOI] [PubMed] [Google Scholar]