Abstract
Background:
Nocardiosis occurs in up to 1.7% of the hematopoietic stem cell transplant (HSCT) population. Risk factors for its development and subsequent outcomes have been incompletely studied.
Objectives:
To evaluate risk factors for nocardiosis in HSCT recipients and an association with 12-month mortality following Nocardia infection.
Study Design:
We performed a nested case-control study of HSCT recipients at three transplant centers from 2011 to 2021. Allogeneic HSCT recipients were matched 1:4 to controls based on age, sex, date of transplant, and transplant site. Due to theorized differences of risk for nocardiosis between allogeneic and autologous HSCTs and a low number of infected autologous recipients, only allogeneic HSCT recipients were matched to controls. Associations with nocardiosis in the allogeneic group were assessed by multivariable conditional logistic regression. Outcomes of all HSCT recipients with nocardiosis included 12-month mortality and post-treatment recurrence.
Results:
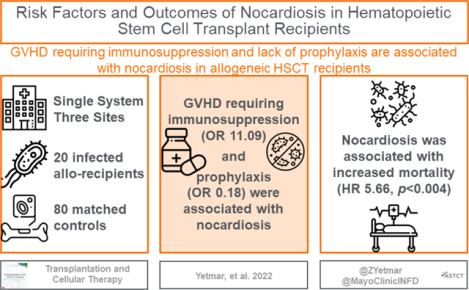
27 HSCT recipients were diagnosed with nocardiosis, including 20 allogeneic and 7 autologous HSCT patients. Twenty (74.1%) had localized pulmonary infection, 4 (14.8%) were disseminated, and 3 (11.1%) had localized skin infection. The allogeneic recipients were diagnosed a median 12.2 months after transplantation, compared to 41 months for the autologous recipients. All autologous HSCT recipients had alternative reasons for ongoing immunosuppression at diagnosis, mostly being therapy for relapsed hematologic disease. No infected patients were receiving trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis. In multivariable analysis of 20 allogeneic patients and 80 matched controls, graft-versus-host disease (GVHD) requiring current immunosuppression and lack of prophylaxis were associated with nocardiosis. Nocardiosis was significantly associated with subsequent mortality, with a 12-month mortality rate of 29.6%. However, no patients who completed treatment experienced Nocardia recurrence.
Conclusions:
Intensified immunosuppression following allogeneic HSCT, such as treatment for GVHD, is associated with development of nocardiosis. Nocardiosis occurs more distantly from transplantation in autologous recipients, possibly driven by therapy for relapsed hematologic disease. No patients receiving TMP-SMX prophylaxis developed nocardiosis. Nocardia infection is associated with high mortality and further strategies for prevention and treatment are needed.
Keywords: Nocardia, Opportunistic infection, Stem cell transplantation, Graft-versus-host disease, Trimethoprim-sulfamethoxazole
Graphical Abstract
Background
Nocardia is a ubiquitous gram-positive organism with a proclivity for causing infection in immunocompromised individuals. Nocardiosis has been estimated to occur in up to 1.7% of allogeneic hematopoietic stem cell transplant (HSCT) recipients.1,2 Incidence of nocardiosis may be higher in allogeneic HSCT recipients as compared to autologous recipients, with multiple case series describing their clinical and microbiologic characteristics.1–10 Outcomes from this infection are poor, with up to 40% of HSCT recipients dying within 1 year of nocardiosis diagnosis.1,6 Despite these data, little is known regarding associations with development of nocardiosis in this population.
Described risk factors for nocardiosis in other populations have largely included heightened pharmacologic immunosuppression, lymphopenia, and cytomegalovirus (CMV) infection.11,12 Additionally, limited data suggests that trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis may be effective in preventing nocardiosis in the HSCT population.4,5 However, there are currently no controlled studies examining risk factors for nocardiosis in HSCT recipients and studies examining TMP-SMX prophylaxis were largely compared to a historical cohort after a programmatic change in prophylaxis.
We aimed to perform a case-control study examining associations with development of nocardiosis in HSCT recipients, including theorized risk factors and impact of prophylaxis. Additionally, we sought to describe outcomes of nocardiosis in this population, including mortality and post-treatment Nocardia recurrence.
Methods
We performed a multicenter, matched nested case-control study of HSCT recipients with or without nocardiosis at three sites in Arizona, Florida, and Minnesota between the years 2011 and 2021. These sites share common transplantation protocols. Prophylaxis for Pneumocystis is standard. TMP-SMX dosed at 80–400 mg daily is the preferred prophylactic agent and dosing, which is started after engraftment and continued until day 100 in autologous recipients or three months after graft-versus-host disease (GVHD) immunoprophylaxis or treatment is discontinued in allogeneic recipients. Alternative agents may be used in cases of allergy or intolerance to TMP-SMX. Penicillin VK is also utilized as antibacterial prophylaxis during the first-year post-transplant for autologous recipients and is continued longer-term for allogeneic recipients except in those without GVHD, whose immunosuppression has been discontinued, and have been vaccinated for Streptococcus pneumoniae. In patients with penicillin allergy, doxycycline is substituted. In one commonly used GVHD prophylaxis regimen for matched related or unrelated donors, patients receive GVHD prophylaxis with oral tacrolimus starting 3 days prior to transplantation, followed by full or mini-dose methotrexate in the early post-transplant period. In those who do not develop acute GVHD, prophylaxis is typically tapered starting day 80–100 post-transplant, based on risk of relapse. Our institutional review board reviewed our study protocol and granted it an exempt status (#22-004936).
Inclusion and exclusion criteria
HSCT recipients were identified through our internal transplant center registry. These patients were cross-referenced in our microbiology database of patients with a culture growing a Nocardia species. These patients were then manually screened through pre-determined inclusion and exclusion criteria. Inclusion criteria were age ≥18 years on the date of nocardiosis diagnosis, hematopoietic stem cell transplantation prior to diagnosis, and culture growth of a Nocardia species with accompanying signs, symptoms, and/or radiographic findings consistent with nocardiosis. Exclusion criteria included Nocardia diagnosis prior to transplantation, lack of culture-confirmation of nocardiosis, or lack of research authorization per Minnesota statute. Case patients who had undergone allogeneic transplantation were the matched 1:4 to uninfected control patients. Matched characteristics included age, sex, date of transplant, and site of transplant. Control patients were required to have at least as much follow-up as the time from transplantation to diagnosis of their matched case patient. Due to a paucity of autologous recipients with nocardiosis and theorized differences in risk compared to allogeneic recipients, only allogeneic transplant recipients with nocardiosis were matched to an uninfected allogeneic recipient control group.
Once eligible patients were identified, data were manually extracted from the electronic medical record. Abstracted data included demographics, transplant-specific characteristics, transplant complications such as GVHD and immunosuppressive therapy, comorbid conditions, treatment details, and outcomes. These data were abstracted as of the index date, which was the date of diagnosis for case patients or the corresponding time from transplantation for matched control patients.
Definitions
Nocardiosis was defined as culture growth of a Nocardia species with clinical signs, symptoms, and/or radiographic features consistent with infection. Disseminated infection was defined as involvement of at least two non-contiguous organs, isolation of Nocardia in a blood culture, or isolated central nervous system involvement without recent neurologic surgery or penetrating skull injury. CMV infection and invasive fungal infection were defined according to published guidelines.13,14 Acute GVHD was graded per the Glucksberg criteria and chronic GVHD per the National Institutes of Health Consensus criteria.15,16 Lymphopenia was defined as an absolute lymphocyte count ≤0.5 ×109/L. Chronic kidney disease was defined as a baseline glomerular filtration rate of less than 60 mL/min/1.73 m2 using the 2021 CKD-EPI equation.17 Antimicrobial therapy included the initial treatment regimen for patients with nocardiosis.
Identification and susceptibility testing
The clinical microbiology laboratory at Mayo Clinic in Rochester, Minnesota, received specimens for culture, identification, and susceptibility testing from Mayo Clinic sites. Clinical specimens were cultured in BD Bactec mycobacterial growth indicator tube 960 broth in mycobacterial growth indicator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and on Middlebrook 7H11/7H11S agar biplates incubated at 35°C to 37°C for up to 6 weeks. Decontamination was performed for sputum cultures. Blood samples were collected in Myco F/Lytic bottles and incubated for up to 6 weeks. Positive mycobacterial growth indicator tube broth was subcultured to a Middlebrook 7H11 agar plate and isolated colony growth was identified using Sanger sequencing of a 500-bp region of the 16S rRNA gene. From August 2014, matrix-assisted laser desorption ionization time-of-flight mass spectrophotometry was added to supplement species identification using Sanger sequencing.18,19 Antimicrobial susceptibility testing was performed via broth microdilution using the Trek Sensititre Rapmyco plate and interpreted according to the Clinical and Laboratory Standards Institute guidelines during the respective period.20,21
Statistical analysis
The primary outcome was development of nocardiosis in allogeneic HSCT recipients. Presenting characteristics of the autologous HSCT recipients were described. Secondary outcomes included 12-month mortality and post-treatment nocardiosis recurrence. Continuous variables are presented as mean with standard deviation or median with interquartile range (IQR). Categorical variables are presented as number with percentage. Multivariable conditional logistic regression was used to analyze the primary outcome, incorporating theorized risk factors defined a priori based on available power while avoiding model overfitting. Several univariable exploratory analyses were conducted of other theorized associations. Survival between infected and uninfected allogeneic transplant recipients was compared by the Kaplan-Meier method with log-rank test and a site-stratified univariable Cox proportional hazards model. The proportionality assumption was assessed by examining Schoenfeld residuals. All analyses were performed using BlueSky Statistics version 7.40 software (BlueSky Statistics LLC, Chicago, Illinois).
Results
Nocardia characteristics
Twenty-seven HSCT recipients with nocardiosis were identified, including 20 allogeneic and 7 autologous transplant recipients. The most common Nocardia species was N. cyriacigeorgica (10, 37%), followed by N. farcinica (5, 18.5%; Figure 1). The most common presenting symptoms were cough (55.6%), fever (33.3%), and dyspnea (29.6%) at a median 9 days (IQR 6.0–20.5) prior to diagnosis. Eighteen (66.7%) underwent magnetic resonance imaging of the brain, 7 (25.9%) underwent computed tomography of the head, and 2 (7.4%) had no brain imaging. Four (14.8%) had disseminated infection, including one with a brain abscess, one with primary bacteremia, and two with pulmonary and bloodstream involvement. The patients with non-disseminated infection included 3 with localized skin infection and 20 with localized pulmonary infection. Four of the patients with pulmonary infection also had pleural involvement. Of these 20 patients with localized pulmonary infection, diagnosis was via bronchoalveolar lavage in 8 (40.0%), sputum culture in 5 (25.0%), and transbronchial biopsy in 3 (15.0%). The remaining four (20.0%) were those with pleural involvement, who were diagnosed by pleural fluid culture with radiographic changes in the pulmonary parenchyma. Most patients required hospitalization (81.5%), with 4 (14.8%) also requiring admission to the intensive care unit. Antibiotic susceptibility testing was performed in 26 isolates (Table 1). Initial treatment regimens included TMP-SMX in 19 (70.4%), linezolid in 6 (22.2%), ceftriaxone in 5 (18.5%), minocycline in 5 (18.5%), cefepime in 3 (11.1%), moxifloxacin in 3 (11.1%), and 1 each of amikacin, amoxicillin-clavulanate, clarithromycin, doxycycline, meropenem, azithromycin, and sulfadiazine. Patients initially received a median of 2.0 total treatment agents (range 1–3) and 1.5 active treatment agents (range 0–3). Twenty-one patients completed therapy with a median duration of 216 days (IQR 194–350).
Figure 1:
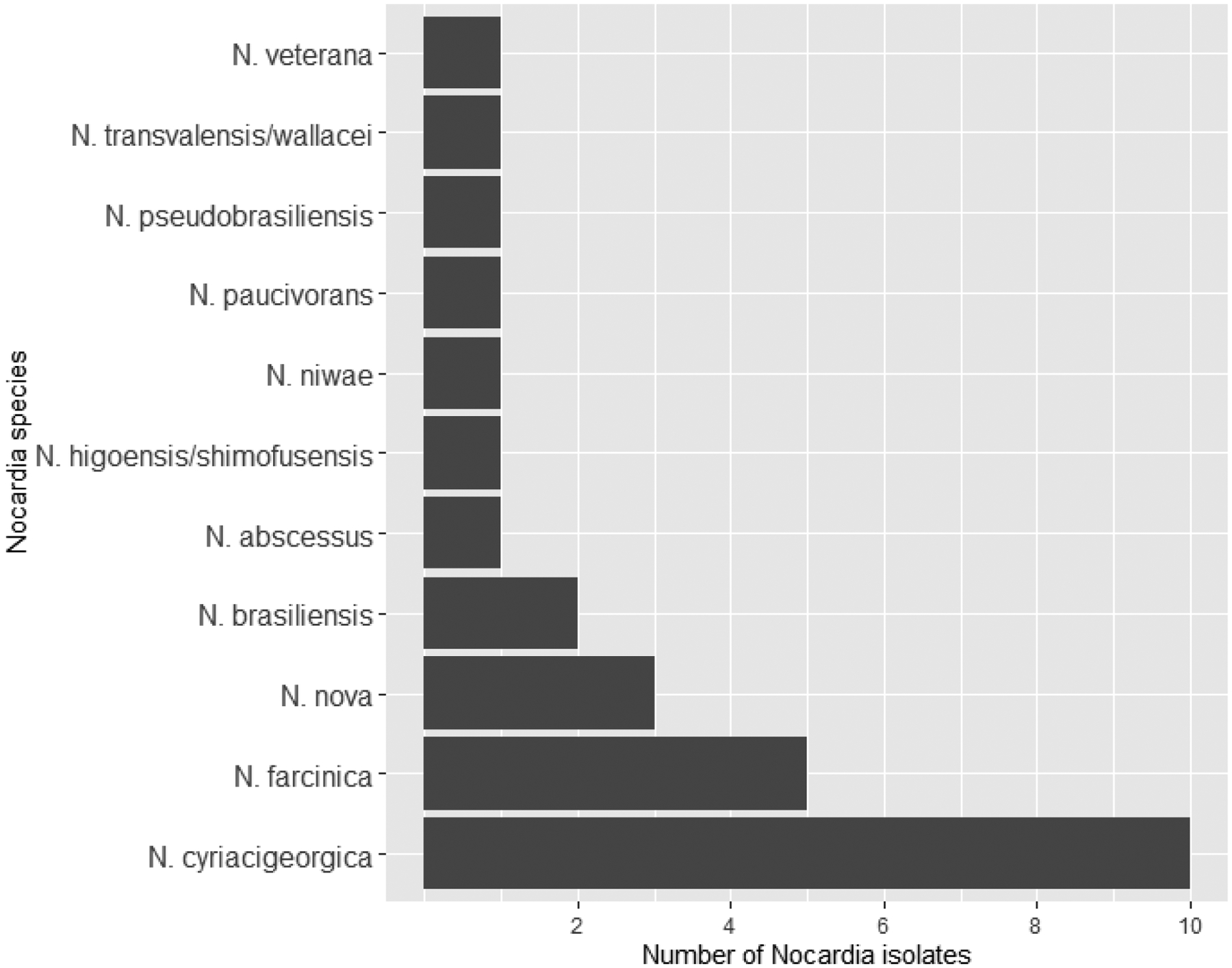
Bar chart of Nocardia species in 27 hematopoietic stem cell transplant recipients
Table 1:
Antimicrobial susceptibility testing results of 26 Nocardia isolates
| Drugs tested | Susceptible (N=26) |
|---|---|
| Amikacin | 24 (92.3) |
| Augmentin | 9 (34.6) |
| Ceftriaxone | 8 (30.8) |
| Ciprofloxacin | 6 (23.1) |
| Clarithromycin | 8 (30.8) |
| Doxycycline | 3 (11.5) |
| Imipenem | 22 (84.6) |
| Linezolid | 26 (100.0) |
| Minocycline | 4 (15.4) |
| Moxifloxacin | 10 (38.5) |
| Tobramycin | 15 (57.7) |
| Trimethoprim-sulfamethoxazole | 25 (96.2) |
Data are N (%)
Allogeneic transplant recipients
The 20 allogeneic recipients were matched to 80 uninfected controls with adequate post-transplant follow-up (Table 2). A majority underwent matched unrelated transplantation, with the most common indications including acute myeloid leukemia, myelodysplastic syndrome or myelofibrosis, chronic myeloid leukemia, and lymphoma. Nearly all patients developed GVHD, and most were still receiving immunosuppression at their index date. Fifty-six patients had chronic GVHD, whose severity was mild in 6 (10.7%), moderate in 36 (64.3%), and severe in 14 (25%). Acute GVHD was present in 25 patients, with a median global grade of 2 (IQR 1–2). This included a grade of 1 in 7 (28.0%), 2 in 13 (52.0%), 3 in 4 (16.0%), and 4 in 1 (4.0%) patient. No patients had both acute and chronic GVHD at their index date. The most common sites of GVHD were skin (47; 58.0%), gastrointestinal (36; 44.4%), and liver (23; 28.4%). No case patients were receiving TMP-SMX at diagnosis, compared to 37.5% of controls. Of the case patients, 19 (95%) were receiving an alternative Pneumocystis prophylaxis agent. This was most commonly inhaled pentamidine (13; 65%), followed by atovaquone (3; 15%) and dapsone (3; 15%). Alternative Pneumocystis prophylaxis was used due to an adverse reaction to TMP-SMX (17; 89.5%) or sulfa allergy (2; 10.5%). A small proportion of case and control patients were receiving doxycycline prophylaxis (15% and 6.2%, respectively). Nocardiosis was diagnosed a median of 12.2 months after transplantation (IQR 6.6–60.5 months).
Table 2:
Characteristics of 20 allogeneic stem cell transplant recipients with nocardiosis and 80 matched uninfected controls
| Case (N=20) | Control (N=80) | Total (N=100) | |
|---|---|---|---|
| Age, years, mean (SD) | 55.6 (14.8) | 53.7 (14.1) | 53.6 (14.2) |
| Sex | |||
| - Female | 5 (25.0) | 20 (25.0) | 25 (25.0) |
| - Male | 15 (75.0) | 60 (75.0) | 75 (75.0) |
| Race (N=98) | |||
| - American Indian or Alaska Native | 1 (5.0) | 0 (0.0) | 1 (1.0) |
| - Asian | 0 (0.0) | 1 (1.3) | 1 (1.0) |
| - Black or African American | 0 (0.0) | 2 (2.6) | 2 (2.0) |
| - White | 19 (95.0) | 73 (93.6) | 92 (93.9) |
| - Other | 0 (0.0) | 2 (2.6) | 2 (2.0) |
| Ethnicity (N=95) | |||
| - Hispanic or Latino | 0 (0.0) | 8 (10.5) | 8 (8.4) |
| - Not Hispanic or Latino | 19 (100.0) | 68 (89.5) | 87 (91.6) |
| Type of allogeneic transplant | |||
| - Cord blood | 0 (0.0) | 1 (1.2) | 1 (1.0) |
| - Haploidentical | 0 (0.0) | 5 (6.2) | 5 (5.0) |
| - Matched related | 9 (45.0) | 29 (36.2) | 38 (38.0) |
| - Matched unrelated | 9 (45.0) | 43 (53.8) | 52 (52.0) |
| - Mismatched | 2 (10.0) | 2 (2.5) | 4 (4.0) |
| Retransplant | 3 (15.0) | 9 (11.2) | 12 (12.0) |
| Transplant indication | |||
| - Acute lymphoblastic leukemia | 1 (5.0) | 11 (13.8) | 12 (12.0) |
| - Acute myeloid leukemia | 8 (40.0) | 23 (28.8) | 31 (31.0) |
| - Aplastic anemia | 0 (0.0) | 2 (2.5) | 2 (2.0) |
| - Chronic lymphocytic leukemia | 2 (10.0) | 2 (2.5) | 4 (4.0) |
| - Chronic myeloid leukemia | 1 (5.0) | 13 (16.2) | 14 (14.0) |
| - Lymphoma | 3 (15.0) | 11 (13.8) | 14 (14.0) |
| - Myelodysplastic syndrome/myelofibrosis | 4 (20.0) | 15 (18.8) | 19 (19.0) |
| - Plasma cell disorder | 1 (5.0) | 3 (3.8) | 4 (4.0) |
| Conditioning | |||
| - Myeloablative | 10 (50.0) | 48 (60.0) | 58 (58.0) |
| - Reduced intensity conditioning | 10 (50.0) | 32 (40.0) | 42 (42.0) |
| GVHD | 19 (95.0) | 62 (77.5) | 81 (81.0) |
| - Acute | 1 (5.3) | 24 (38.7) | 25 (30.9) |
| - Chronic | 18 (94.7) | 38 (61.3) | 56 (69.1) |
| Current immunosuppression | 19 (95.0) | 53 (66.2) | 72 (72.0) |
| - Tacrolimus | 10 (50.0) | 28 (35.0) | 38 (38.0) |
| - Cyclosporine | 3 (15.0) | 9 (11.2) | 12 (12.0) |
| - Mycophenolate | 3 (15.0) | 5 (6.2) | 8 (8.0) |
| - Corticosteroids | 15 (75.0) | 26 (32.5) | 41 (41.0) |
| - Sirolimus | 0 (0.0) | 5 (6.2) | 5 (5.0) |
| - Ruxolitinib | 4 (20.0) | 2 (2.5) | 6 (6.0) |
| - Chemotherapy | 1 (5.0) | 4 (5.0) | 5 (5.0) |
| - Infliximab | 1 (5.0) | 0 (0.0) | 1 (1.0) |
| Daily prednisone dose, mg, median (IQR) (N=41) | 20.0 (10.0, 55.0) | 16.2 (5.0, 30.0) | 17.5 (7.5, 40.0) |
| GVHD requiring current immunosuppression | 18 (90.0) | 44 (55.0) | 62 (62.0) |
| Current morphological remission | 16 (80.0) | 73 (91.2) | 89 (89.0) |
| HCT-CI, median (IQR) | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.2) |
| CMV infection within 6 months | 4 (20.0) | 4 (5.0) | 8 (8.0) |
| Invasive fungal infection within 6 months before diagnosis | 3 (15.0) | 1 (1.2) | 4 (4.0) |
| TMP-SMX prophylaxis | 0 (0.0) | 30 (37.5) | 30 (30.0) |
| - 160–800 mg twice-weekly | 0 (0.0) | 7 (23.3) | 7 (23.3) |
| - 160–800 mg thrice-weekly | 0 (0.0) | 13 (43.3) | 13 (43.3) |
| - 80–400 mg daily | 0 (0.0) | 9 (30.0) | 9 (30.0) |
| - 80–400 mg thrice-weekly | 0 (0.0) | 1 (3.3) | 1 (3.3) |
| Doxycycline prophylaxis | 3 (15.0) | 5 (6.2) | 8 (8.0) |
| TMP-SMX or doxycycline prophylaxis | 3 (15.0) | 34 (42.5) | 37 (37.0) |
| Leukocyte count, x109/L, median (IQR) | 7.4 (6.0, 9.8) | 6.4 (4.8, 8.6) | 6.7 (4.9, 8.9) |
| Neutrophil count, x109/L, median (IQR) | 5.2 (3.5, 8.2) | 3.7 (2.5, 5.3) | 3.9 (2.5, 6.2) |
| Lymphocyte count, x109/L, median (IQR) | 1.0 (0.3, 1.3) | 1.3 (1.0, 2.4) | 1.2 (0.8, 2.1) |
| Lymphocyte count ≤ 0.5 x109/L | 7 (35.0) | 5 (6.2) | 12 (12.0) |
| Chronic kidney disease | 6 (30.0) | 19 (23.8) | 25 (25.0) |
All data are N (%) unless otherwise specified.
Abbreviations: CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; GVHD, graft-versus-host disease; HSC-CI, hematopoietic cell transplantation comorbidity index; IQR, interquartile range; SD, standard deviation; TMP-SMX, trimethoprim-sulfamethoxazole.
In multivariable analysis, current receipt of immunosuppression for GVHD and lack of either TMP-SMX or doxycycline prophylaxis were both significantly associated with development of nocardiosis (Table 3). TMP-SMX prophylaxis alone was unable to be formally evaluated as no case patients were receiving TMP-SMX at diagnosis. Exploratory univariable analyses found a prednisone dose ≥20 mg/day, lymphopenia, CMV infection within the last 6 months, and invasive fungal infection within the last 6 months to be associated with nocardiosis.
Table 3:
Conditional logistic regression analyses of associations with nocardiosis in allogeneic recipients
| Variable | Univariable analyses | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Prophylaxisa | 0.23 | 0.06–0.87 | .030 | 0.18 | 0.05–0.70 | .013 |
| GVHD requiring current immunosuppression | 7.96 | 1.70–37.20 | .008 | 11.09 | 2.09–58.75 | .005 |
| Prednisone dose ≥20 mg/day | 3.68 | 1.23–11.01 | .020 | |||
| Lymphopeniab | 10.7 | 2.17–52.75 | .004 | |||
| Retransplant | 1.42 | 0.33–6.07 | .637 | |||
| Reduced intensity conditioning | 1.97 | 0.54–7.21 | .308 | |||
| Recent CMV infectionc | 4.00 | 1.00–15.99 | .049 | |||
| Recent invasive fungal infectionc | 12.00 | 1.25–115.36 | .031 | |||
| Morphologic remission | 0.39 | 0.10–1.49 | .168 | |||
Bold values indicate p <0.05
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; GVHD, graft-versus-host disease.
Includes current use of trimethoprim-sulfamethoxazole and/or doxycycline.
Lymphocyte count <0.5 x109/L.
Within 6 months before the index date.
Autologous transplant recipients
Seven autologous transplant recipients were diagnosed with nocardiosis at a median 41 months after transplantation (range 19.6–70.1 months). All patients underwent transplantation for a plasma cell disorder, all were of white race, one (14.3%) was of Hispanic or Latino ethnicity, and 3 (42.9%) were female with a mean age of 63.6 years (standard deviation 8.0 years). Six (85.7%) patients experienced disease recurrence after transplantation but prior to their index date. One patient without disease recurrence was also a kidney transplant recipient who was receiving tacrolimus, mycophenolate, and prednisone at diagnosis. All other patients were receiving hematologic disease-directed therapy at diagnosis, with agents including bendamustine and thalidomide, cyclophosphamide and carfilzomib, daratumumab, doxorubicin and cyclophosphamide, ixazomib and lenalidomide, and pomalidomide. Five patients were also receiving corticosteroids. No patients were receiving TMP-SMX prophylaxis, while one was receiving doxycycline.
Post-Nocardia outcomes
Six (30%) allogeneic transplant recipients died within 12 months of diagnosis, compared with 5 (6.2%) of the control patients. A Kaplan-Meier curve illustrating post-index date survival in case and control allogeneic transplant recipients is shown in Figure 2. Diagnosis of nocardiosis was associated with increased risk of 12-month mortality after the index date, with a hazard ratio of 5.66 (95% confidence interval 1.72–18.58; p=0.004). In addition, two (28.6%) autologous transplant recipients died within 12 months of diagnosis. Of the 8 patients with nocardiosis who died within 1 year of diagnosis, 4 were directly attributable to nocardiosis (1 autologous and 3 allogeneic recipients). The four whose deaths were not directly attributable to nocardiosis died of either refractory GVHD or relapsed hematologic malignancy (2 each).
Figure 2:
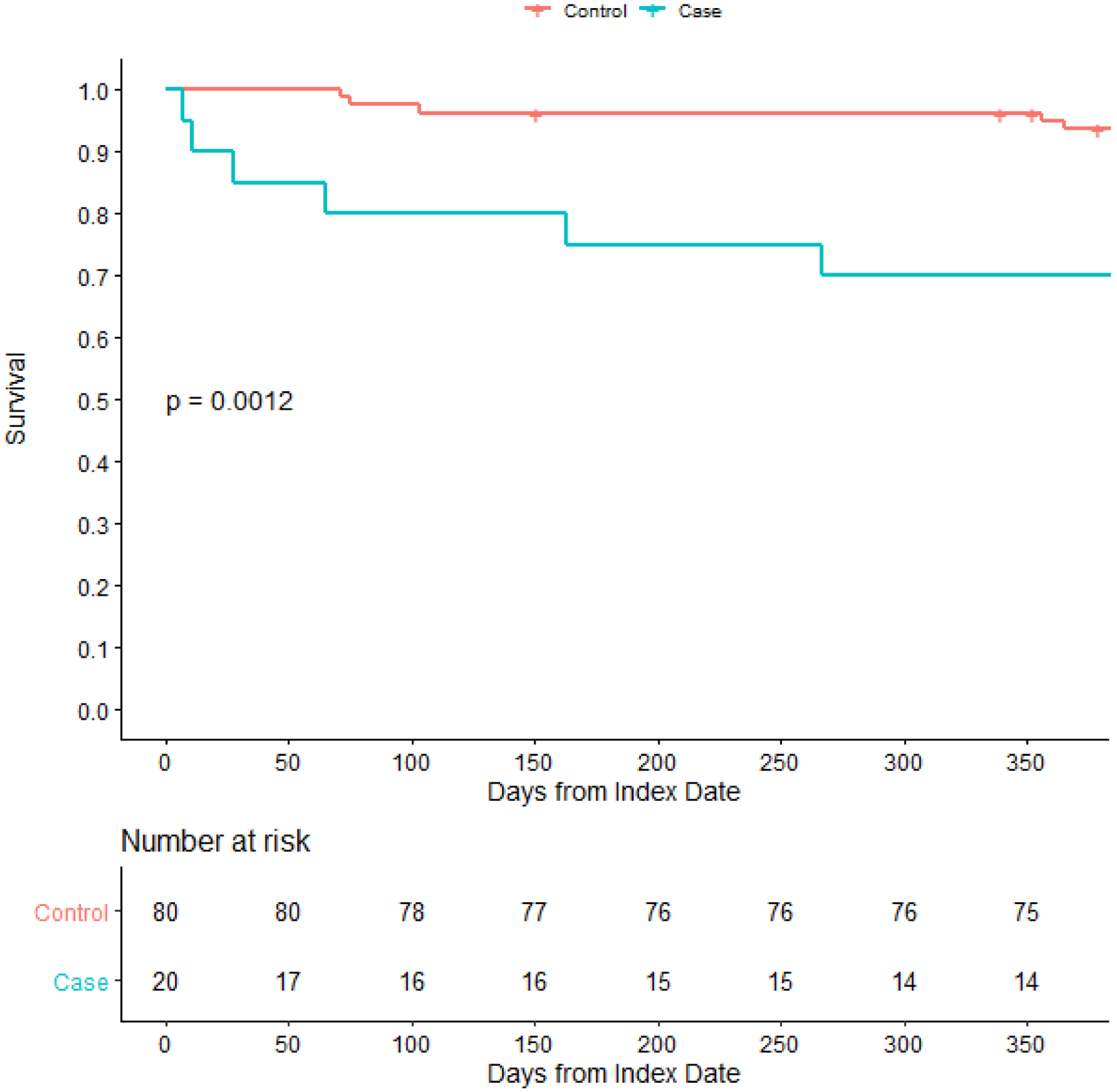
Kaplan-Meier curve comparing survival following diagnosis or matched date of follow-up between allogeneic hematopoietic stem cell transplant recipients with or without nocardiosis. The dashed lines on the Kaplan-Meier curve indicate a censored patient. The p-value is calculated via the log-rank test.
Among the 21 patients who completed therapy, 9 (42.9%) received secondary prophylaxis. Secondary prophylactic agents included TMP-SMX in 7, doxycycline in 1, and both TMP-SMX and azithromycin in 1. TMP-SMX was primarily dosed as 160–800 mg daily (6), with one each receiving 160800 mg twice-daily and 80–400 mg thrice-weekly. No patients experienced a recurrence of nocardiosis in a median post-treatment follow-up time of 637 days (IQR 314–1395).
Discussion
In this study, ongoing immunosuppression for GVHD and lack of Nocardia-active prophylaxis were associated with development of nocardiosis in allogeneic HSCT recipients. Additionally, diagnosis of nocardiosis in autologous HSCT recipients was relatively remote from transplantation and all these patients had alternative, non-transplant reasons for ongoing immunosuppression. Despite the common practice of administering secondary prophylaxis, no patients developed recurrent nocardiosis after completing primary therapy regardless of secondary prophylaxis use.
Current use of immunosuppressive therapy for GVHD was identified as a significant association with nocardiosis in limited multivariable analysis of the allogeneic transplant recipients. Additionally, exploratory unadjusted analyses showed high-dose prednisone use, lymphopenia, recent CMV infection, and recent invasive fungal infection were associated with nocardiosis. These risk factors have been identified in other, non-HSCT populations, most notably in studies of solid organ transplant recipients with nocardiosis.11,12 In these prior studies, multiple measures of immunosuppression have been associated with Nocardia infection. Similarly, development of CMV infection and invasive fungal infection are reflections of significant immunosuppression, and may signal risk for other co-infections, such as Nocardia.
The autologous transplant recipients were excluded from the risk factor analysis due to significant differences in their expected risk for specific complications such as GVHD, as well as practice differences such as immunosuppressant medication use. This is reflected in the difference in median time from transplantation to diagnosis, being 12.2 months for allogeneic recipients but 41 months for autologous patients. Furthermore, the autologous recipients had alternative reasons for ongoing immunosuppressant, with 6 having relapsed hematologic disease and 1 was receiving maintenance immunosuppressant therapy due to a prior solid organ transplantation. This implies that the risk for nocardiosis in autologous transplant recipients may be primarily driven by alternative factors than HSCT.
Use of prophylaxis with either TMP-SMX or doxycycline was associated with a reduced odds of nocardiosis after adjusting for GVHD immunosuppression. While doxycycline was included in this composite factor to allow quantitative analysis, it is remarkable that no patients who were receiving TMP-SMX prophylaxis developed nocardiosis. This is consistent with prior reports that have suggested lower rates of nocardiosis with TMP-SMX prophylaxis.4,5 However, this finding has not been universal. A recent multicenter cohort in Europe of HSCT recipients with nocardiosis found 42% were receiving TMP-SMX prophylaxis at diagnosis.6 While these data did not include uninfected controls, the high rate of TMP-SMX use suggested prophylaxis may not be effective. It should be noted that this report did not include TMP-SMX dosing, which may affect the effectiveness of prophylaxis, and there was also a higher rate of disseminated infection (57% versus 15.8% in the present study). There are theorized geographical differences in Nocardia species, which may have led to differences in virulence of Nocardia isolates between studies, which in turn may account for the different outcomes.22 Finally, given the lack of a control group, possible confounders could not be accounted for. While these studies were limited in their ability to account for other factors, prophylaxis remained significant after adjusting for GVHD-related immunosuppression, which is the primary driver of TMP-SMX prophylaxis use in our transplant program.
Nocardia infection has a high rate of 12-month mortality, with 30% of our patients with nocardiosis dying within this period. This is consistent with prior reports that also illustrated poor outcomes from nocardiosis.6,23,24 In a recent multicenter cohort of HSCT recipients with nocardiosis, 40% of patients died within 1 year.6 Factors associated with mortality in this prior study included lack of complete remission of the underlying hematologic disease and bacterial infection within 3 months of nocardiosis diagnosis. Factors associated with mortality after nocardiosis in other studies have included a higher number of comorbidities and recent invasive fungal infection, while disseminated infection has shown mixed results.23–26 While our study’s low number of patients limited our ability to analyze for factors associated with mortality, we did find that Nocardia is associated with a nearly 6-fold higher risk of mortality.
These data are notable as no patients experienced recurrence of nocardiosis following completion of therapy. This is despite less than half receiving secondary prophylaxis after primary therapy. Few studies have examined Nocardia recurrence, with a small study of HSCT recipients similarly showing no instances of recurrence, though only four patients had completed therapy.7 A larger study of solid organ transplant recipients showed this population to have a Nocardia recurrence rate of about 5%, which did not seem significantly impacted by use of secondary prophylaxis.27 However, it is possible that patients at highest risk for nocardiosis may have preferentially received secondary prophylaxis, such as those requiring ongoing immunosuppression, which may have affected these results. As the small numbers precluded controlling for potential confounding factors, further study is required to better define if specific populations of HSCTs would benefit from secondary prophylaxis.
This study has several limitations of note. It was conducted retrospectively and is therefore susceptible to sources of bias from this study design. Additionally, there are likely systematic differences between patients receiving different treatment regimens. There was an overall low number of patients with nocardiosis, which limited the number of included variables in the multivariable analysis. Specifically, there was a very low number of autologous transplant recipients with nocardiosis, and we could not formally examine associations with nocardiosis in this subpopulation. Patients also received different dosages of TMP-SMX prophylaxis, and we were unable to examine an optimal prophylaxis dose. We did not have information available regarding exposure variables such as occupation or recreational activities, which may have influenced individual patients’ risk of nocardiosis in the community.
Conclusion
Measures of heightened immunosuppression, such as the use of immunosuppressing medications for GVHD, high-dose corticosteroids, and lymphopenia, are associated with nocardiosis in allogeneic HSCT recipients while Nocardia-active prophylaxis was associated with lower odds of Nocardia infection. Specifically, no patients receiving TMP-SMX prophylaxis experienced nocardiosis, and its use over alternative prophylactic agents should be prioritized. Nocardiosis in autologous transplant recipients occurred remote from transplantation and closer in relationship to other causes of immunosuppression, such as relapsed hematologic disease, suggesting these factors may influence risk for Nocardia more than autologous transplantation. This population experienced high 12-month mortality following nocardiosis, though no patients in this study experienced recurrence following completion of therapy. Further study regarding strategies to prevent nocardiosis in HSCT recipients is needed as risk factors for infection are elucidated.
Highlights.
Nocardia is an opportunistic pathogen that primarily infects immunocompromised hosts.
HSCT recipients with nocardiosis have poor outcomes, but risk factors are ill defined.
Identified risk factors for nocardiosis in allogeneic recipients are GVHD requiring immunosuppression and lack of TMP-SMX prophylaxis.
Autologous recipients had alternative reasons for immune compromise, such as relapsed malignancy or solid organ transplantation.
Nocardiosis was associated with higher mortality, highlighting the need for preventative strategies.
Funding
This project was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CMV
Cytomegalovirus
- GVHD
Graft-versus-host disease
- HSCT
Hematopoietic stem cell transplant
- IQR
Interquartile range
- TMP-SMX
Trimethoprim-sulfamethoxazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
All authors have no conflicts of interest to report.
References
- 1.Daly AS, McGeer A, Lipton JH. Systemic nocardiosis following allogeneic bone marrow transplantation. Transpl Infect Dis. 2003;5(1):16–20. doi: 10.1034/j.1399-3062.2003.00007.x [DOI] [PubMed] [Google Scholar]
- 2.Kurosawa S, Sekiya N, Doki N, et al. The emergence of rare nocardiosis following allogeneic hematopoietic stem cell transplantation in the era of molecular taxonomy. Int J Infect Dis. 2019;89:154–162. doi: 10.1016/j.ijid.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 3.Mansi L, Daguindau E, Saas P, et al. Diagnosis and management of nocardiosis after bone marrow stem cell transplantation in adults: Lack of lymphocyte recovery as a major contributing factor. Pathol Biol. 2014;62(3):156–161. doi: 10.1016/j.patbio.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Molina A, Winston DJ, Pan D, Schiller GJ. Increased Incidence of Nocardial Infections in an Era of Atovaquone Prophylaxis in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2018;24(8):1715–1720. doi: 10.1016/j.bbmt.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 5.Gkirkas K, Stamouli M, Thomopoulos T, et al. Low-Dose Cotrimoxazole Administered in Hematopoietic Stem Cell Transplant Recipients as Prophylaxis for Pneumocystis jirovecii Pneumonia Is Effective in Prevention of Infection due to Nocardia. Biol Blood Marrow Transplant. 2019;25(9):e298–e299. doi: 10.1016/j.bbmt.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 6.Averbuch D, De Greef J, Duréault A, et al. Nocardia Infections in Hematopoietic Cell Transplant Recipients: A Multicenter International Retrospective Study of the Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation. Clin Infect Dis. 2022;75(1):88–97. doi: 10.1093/cid/ciab866 [DOI] [PubMed] [Google Scholar]
- 7.Hemmersbach-Miller M, Stout JE, Woodworth MH, Cox GM, Saullo JL. Nocardia infections in the transplanted host. Transpl Infect Dis. 2018;20(4):e12902. doi: 10.1111/tid.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo C, Antoniazzi F, Caira M, et al. Nocardia spp infections among hematological patients: Results of a retrospective multicenter study. Int J Infect Dis. 2013;17(8):e610–e614. doi: 10.1016/j.ijid.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 9.van Burik J, Hackman RC, Nadeem SQ, et al. Nocardiosis After Bone Marrow Transplantation: A Retrospective Study. Clin Infect Dis. 1997;24(6):1154–1160. doi: 10.1086/513654 [DOI] [PubMed] [Google Scholar]
- 10.Shannon K, Pasikhova Y, Ibekweh Q, Ludlow S, Baluch A. Nocardiosis following hematopoietic stem cell transplantation. Transpl Infect Dis. 2016;18(2):169–175. doi: 10.1111/tid.12499 [DOI] [PubMed] [Google Scholar]
- 11.Coussement J, Lebeaux D, Van Delden C, et al. Nocardia Infection in Solid Organ Transplant Recipients: A Multicenter European Case-control Study. Clin Infect Dis. 2016;63(3):338–345. doi: 10.1093/cid/ciw241 [DOI] [PubMed] [Google Scholar]
- 12.Peleg AV, Husain S, Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: A matched case-control study. Clin Infect Dis. 2007;44(10):1307–1314. doi: 10.1086/514340 [DOI] [PubMed] [Google Scholar]
- 13.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87–91. doi: 10.1093/cid/ciw668 [DOI] [PubMed] [Google Scholar]
- 14.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001 [DOI] [PubMed] [Google Scholar]
- 16.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel R MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61(1). doi: 10.1373/clinchem.2014.221770 [DOI] [PubMed] [Google Scholar]
- 19.Buckwalter SP, Olson SL, Connelly BJ, et al. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Mycobacterium species, Nocardia species, and Other Aerobic Actinomycetes. J Clin Microbiol. 2016;54(2). doi: 10.1128/JCM.02128-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CLSI. Susceptibility Testing of Mycobacteria, Nocardia Spp., and Other Aerobic Actinomycetes. 3rd Edition. CLSI Guideline M24. 3rd ed.; 2018.
- 21.CLSI. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia Spp., and Other Aerobic Actinomycetes. 1st Edition. CLSI Guideline M62. 1st ed.; 2018.
- 22.Traxler RM, Bell ME, Lasker B, Headd B, Shieh W-J, McQuiston JR. Updated Review on Nocardia Species: 2006–2021. Clin Microbiol Rev. Published online October 31, 2022. doi: 10.1128/cmr.00027-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takamatsu A, Yaguchi T, Tagashira Y, Watanabe A, Honda H. Nocardiosis in Japan: a Multicentric Retrospective Cohort Study. Antimicrob Agents Chemother. 2022;66(2). doi: 10.1128/aac.01890-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yetmar ZA, Challener DW, Seville MT, Bosch W, Beam E. Outcomes of Nocardiosis and Treatment of Disseminated Infection in Solid Organ Transplant Recipients. Transplantation. Published online October 28, 2022. doi: 10.1097/TP.0000000000004343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soueges S, Bouiller K, Botelho-Nevers E, et al. Prognosis and factors associated with disseminated nocardiosis: a ten-year multicenter study. J Infect. 2022;85(2):130–136. doi: 10.1016/j.jinf.2022.05.029 [DOI] [PubMed] [Google Scholar]
- 26.Lebeaux D, Freund R, Van Delden C, et al. Outcome and Treatment of Nocardiosis after Solid Organ Transplantation: New Insights from a European Study. Clin Infect Dis. 2017;64(10):1396–1405. doi: 10.1093/cid/cix124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yetmar ZA, Wilson JW, Beam E. Recurrent nocardiosis in solid organ transplant recipients: An evaluation of secondary prophylaxis. Transpl Infect Dis. 2021;23(6). doi: 10.1111/tid.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]


