Abstract
Introduction
Brain tumors cause morbidity and mortality in part through peritumoral brain edema. The current main treatment for peritumoral brain edema are corticosteroids. Due to the increased recognition of their side-effect profile, there is growing interest in finding alternatives to steroids but there is little formal study of animal models of peritumoral brain edema. This study aims to summarize the available literature.
Methods
A systematic search was undertaken of 5 literature databases (Medline, Embase, CINAHL, PubMed and the Cochrane Library). The generic strategy was to search for various terms associated with “brain tumors”, “brain edema” and “animal models”.
Results
We identified 603 reports, of which 112 were identified as relevant for full text analysis that studied 114 peritumoral brain edema animal models. We found significant heterogeneity in the species and strain of tumor-bearing animals, tumor implantation method and edema assessment. Most models did not produce appreciable brain edema and did not test for observable manifestations thereof.
Conclusion
No animal model currently exists that enable the investigation of novel candidates for the treatment of peritumoral brain edema. With current interest in alternative treatments for peritumoral brain edema, there is an unmet need for clinically relevant animal models.
Keywords: Animal model, Brain tumor, Edema
Introduction
Peritumoral brain edema is a key contributor to morbidity and mortality in brain tumors resulting in mass effect and raised intracranial pressure [1, 2].
Brain edema is broadly divided into fluid accumulation within cells (cytotoxic edema) or in the interstitial space (vasogenic edema) [3], although the two usually coexist to a greater or lesser extent [4]. Intracellular or “cytotoxic” edema is thought to arise from disordered metabolism (e.g. as a consequence of ischaemia) inside cells allowing fluid to enter the cells [4]. By contrast, extracellular or “vasogenic” edema is thought to arise from dysfunction in the Starling forces that govern the passive exchange ingress and egress of fluid between the vasculature and interstitial space [4]. In intrinsic high-grade, metastatic brain tumors and some meningiomas extracellular vasogenic edema is usually the major contributor and is believed to result from leakiness of the blood–brain barrier (BBB), driven by factors such as abnormal neovascularization, and changes at the subcellular level with disrupted tight junctions, fenestrations of endothelia, increasing pinocytic vesicles and abnormalities of the basal membrane [5]. At the molecular level, vascular endothelial growth factor (VEGF) and inflammatory cytokines such as leukotriene C4, nitrous oxide (NO) and prostaglandin E2 have been implicated.
Dexamethasone, a synthetic corticosteroid, has been used to control peritumoral brain edema since the 1950s [6, 7] and up to a fifth of patients with a malignant brain tumor take steroids for the remainder of their lives (23.3 weeks) from the time of diagnosis [8]. However, corticosteroids have significant adverse effects that increase over time as the cumulative dose increases [8–11]. In practice, it can be very challenging to balance the therapeutic and adverse effects of corticosteroids; indeed, recent studies suggest that corticosteroids may actually increase mortality in patients with GBM [12, 13]. There is, therefore, increasing interest in finding alternative agents for the control of peritumoral brain edema. Accordingly, various candidates have been investigated in animal models as potential alternatives to dexamethasone [13–15].
Since their first use in the late 1940s, numerous categories of animal model have arisen, each with their own strengths and weaknesses [16]. These animal models can be broadly divided according to the host animals’ species (e.g. mouse, rat, cat etc.) and strain (e.g. Fischer vs BDIX vs Sprague–Dawley rats). They can also be categorized according to the method used to generate tumors in the host animals, which include chemical induction of tumors and implantation/injection of established tumor cell lines, and implantation/injection of xenogeneic implants of either established cell lines or patient-derived tissue in immunodeficient animals. In primary brain cancer models, cancer cells are injected orthotopically into the brain. This same method is frequently used to study macrometastases in the brain. However, hematogenous brain metastasis models, where cancer cells are administered intracardiac or into the internal carotid artery and colonize the brain from the blood stream, better recapitulate metastatic disease [17, 18]. More recently, genetically engineered mouse models which reproducibly develop intracranial tumors and humanized mouse models allowing transplantation of human tumors into partially immunocompetent animals have been developed [16, 19]. Understandably, in developing these models, investigators have sought greater levels of fidelity in replicating the histopathological and genetic makeup of human tumors in animals; replicating the effects of the resulting tumor on the surrounding normal brain tissues, including the development of intratumoral brain edema, may not have been a design priority.
When testing new agents for the treatment of peritumoral brain edema, pre-clinical models should be carefully assessed regarding their suitability for this task. We here present a systematic scoping review of different pre-clinical brain tumor models claiming to study peritumoral brain edema. As steroids are most commonly necessary for long periods of time in the treatment of primary and secondary tumours within the brain parenchyma, where radiotherapy is a frequent adjunct and surgery not always possible, we limit the review to studies modelling such tumours. This deliberately excludes meningiomata, where peritumoral edema does occur, but is a more variable phenomenon.
Methods
Literature search
A systematic search was undertaken of 5 literature databases (Medline, Embase, CINAHL, PubMed and the Cochrane Library). No limits were set for date of publication or language. The generic strategy was to search for various terms associated with “intracranial tumors”, “brain edema” and “animal models”. The detailed search strategy for each database can be found in Appendix A.
Report selection
Reports were screened in two passes. First, two reviewers (MWJS and SPS) screened report titles and clearly unrelated reports were eliminated. Subsequently, two reviewers (MWJS and M-T L) screened the abstract of the remaining reports and eliminated any remaining reports that did not meet the inclusion criteria and those in non-English languages. The inclusion criteria were any form of animal model and any form of intracranial tumor induction method (e.g. injection/implantation of tumor cells, genetically-induced tumors, chemically-induced tumors) was deemed sufficiently relevant to proceed to data extraction. Disagreements at both stages were resolved by discussion. The remaining reports underwent full text review and data were extracted by a single author (MWJS). Data were extracted for each animal model – some reports studied more than one – and included: host animal characteristics (species; strain; immunity status), tumor characteristics (induction method; cell line, where relevant; syn- or xenogeneity), and reported measures of edema (e.g. histopathology; neuroimaging; brain water content (BWC) etc.).
Imaging review
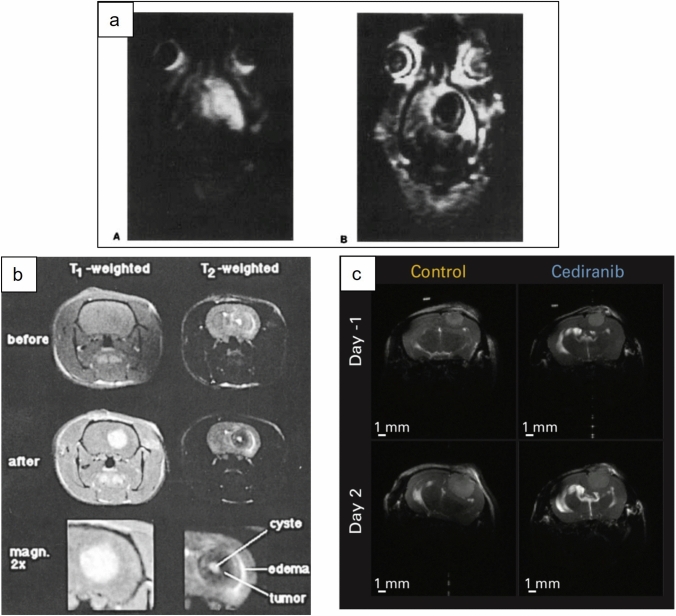
The subset of selected reports which showed MRI sequences of peritumoral brain edema were reviewed separately. Of twenty-eight reviewed articles, four were excluded as they were devoid of MR imaging. Of the remaining 24 research papers 16 were rat models, 4 were mouse, 3 were cat, and 1 was dog. MR images were reviewed by a consultant neuroradiologist (SC) and assigned the presence or absence of peritumoral edema. Cases with peritumoral edema were classified in to one of two patterns, either ‘halo’, i.e. a rim of edema around the tumor or ‘infiltrative’ i.e. edema extending along white matter tracts and also classified by extent depending on whether the volume of edema was volume less, or greater than the volume of the tumor volume, see Fig. 1.
Fig. 1.
Representative MR images of different edema patterns obtained in animal models. a an example of infiltrative tumor edema captured on T1 IR snapshot FLASH imaging from rats bearing F98 glioma, reproduced with permission[52]. b an example of a peritumoral ‘halo’ of edema, demonstrated in T1- and T2-weighted MR images in Fischer rats bearing F98 glioma, reproduced with permission[30]. c an example of tumor with no detectable edema on T2-weighted MR in nude mice bearing U87 tumors, reproduced with permission [25]
Results
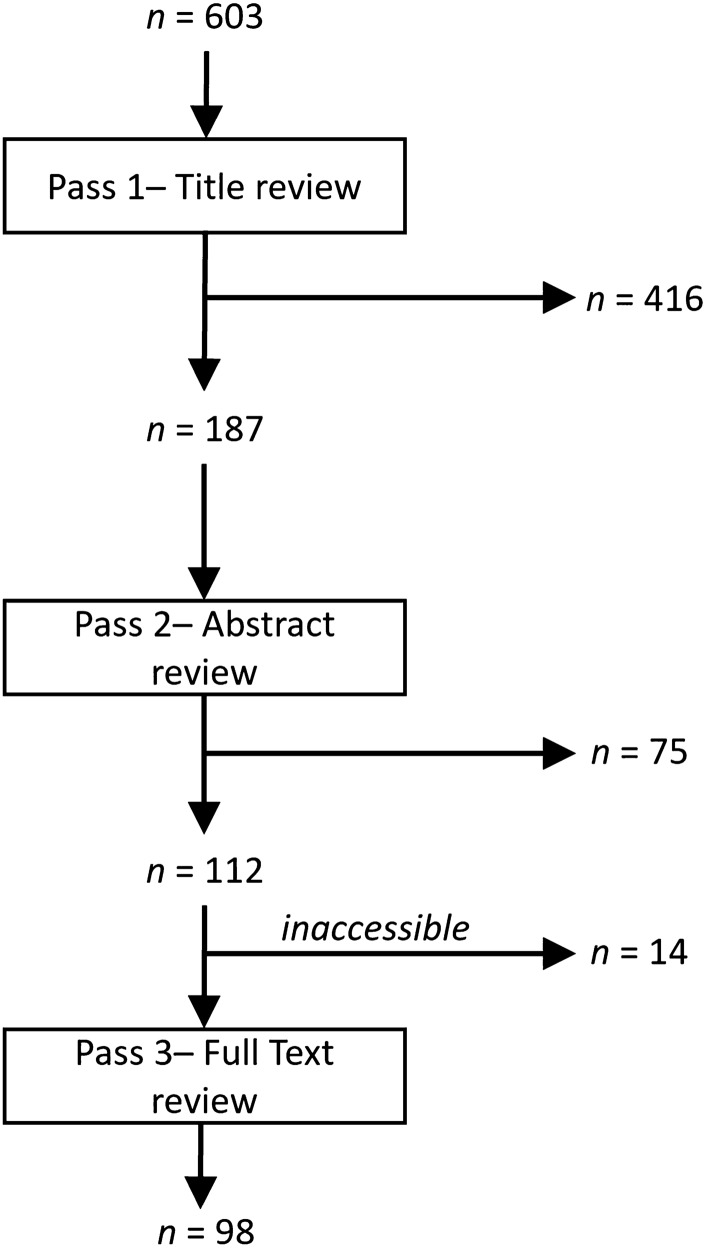
Our search identified 603 reports which were narrowed down to 112 for full text review; 14 reports could not be accessed (Fig. 2) due to our library lacking access. Some of the remaining 98 reports alluded to more than one animal model, so the final number of animal models under evaluation (114) was greater than the number of reports. 53 studies involved the assessment of a particular anti-edema agent and were therefore specifically designed to assess peritumoral edema.
Fig. 2.
Report selection process
We report our findings in two separate ways. First, we will take a high-level view of the type of model used (see Table 1). We then progressively break down the proportion of animal models by host species, the host strain and the method of tumor generation. The most common animal model types were syngeneic graft models (n = 77), followed by xenogeneic graft models (n = 31), chemically-induced (n = 4), virally induced (n = 1) and genetically engineered mouse (n = 1); no patient-derived xenograft models or carotid-/cardiac-injection metastasis models were identified. These can be further divided by species (Table 2). Again, we found an apparent near uniform consensus in that the most used species were rat and mouse, accounting for over 70% of all animal models (Table 3). While investigators seemed to favor immunocompetent rat strains, especially Fischer or Sprague–Dawley, there was marked variation in the utilization of murine strains. There was also considerable variation in the investigators’ choice of tumor implant; most investigators implanted cultured rat glioma cells (59 models) but there was considerable variation in the cell line used.
Table 1.
Summary of animal model categories
| Immunocompetent | Chemically-induced | Animals are exposed (often in utero) to carcinogens such as N-ethylnitrosurea or methylchloranthene to cause spontaneous generation of tumors in vivo. Tumors develop with delay and in an unpredictable fashion, with some animals developing several tumors while some may not develop any in the allocated time | [29, 49–55] | |
| Virally-induced | Animals are exposed to carcinogenic viruses such as Rous Sarcoma Virus to cause spontaneous generation of tumors | [56] | ||
| Implanted/Injected (syngeneic) | From culture | Tumors induced in animals of the same species as the prospective host organism are taken and stored in cell culture prior to transplantation, e.g. F98, 9L, C6 or RG2 cell lines into rat; B16F10 into mouse, VX2 into rabbit | [36, 53–61] | |
| From tumor biopsy | Tumors induced in animals of the same species as the prospective host organism are taken from a tumor-bearing animal and implanted into another of the same species. The tumors may be spontaneous or may have been induced by other means, e.g. chemical | [62] | ||
| Implanted/Injected (xenogeneic) | From culture | Tumors induced in animals of a different species as the prospective host organism are taken and stored in cell culture prior to transplantation, e.g. rat lines like RG2 and F98 in outbred cat | [50, 51, 63] | |
| Genetically-engineered animals | Animals are genetically engineered to overexpress oncogenes or lose critical tumor suppressor gene function meaning that tumors can be generated either spontaneously or by use of triggers | [13] | ||
| Immunocompromised | Implanted/injected (xenogeneic) | From culture | Tumor cells obtained from animals of a different species as the prospective host organism are propagated in cell culture prior to transplantation into an immunocompromised animals (i.e. mice and rats), e.g. human cell lines like U87, LX1 etc. or mice or rat cell lines such as C6 | [28, 64] |
| From tumor biopsy | Tumors obtained from animals of a different species as the prospective host organism are transplanted directly into an immunocompromised animal | Not represented in this review |
Table 2.
Number of animal models reported by species and species strain of tumor-induction method used
| Tumor model Species–cell line/origin |
No of reports | Tumor model Species–cell line/origin |
No of reports |
|---|---|---|---|
| Rat—C6 | 22 | Rat—RGl2.2 | 3 |
| Rat—RG2 | 11 | Rat—Induced | 3 |
| Rat—9L | 11 | Rat—Walker 256 | 2 |
| Rat—F98 | 10 | Rat—A15A5 | 2 |
| Rat—RN6 | 2 | Rat—Miscellaneous | 8 |
| Human—Miscellaneous | 9 | Mouse—B16F10 | 2 |
| Human—U87 | 5 | Mouse—Miscellaneous | 8 |
| Rabbit—Miscellaneous | 1 | Mouse—GEM | 1 |
| Rabbit—VX2 | 7 | Mouse—Induced | 1 |
| Dog—Miscellaneous | 1 | Other—Induced | 1 |
| Unknown etiology | 4 |
All injected/implanted cell lines were implanted intracranially, no models used intracarotid or -cardiac injection
Table 3.
Number of animal models reported by species and species strain of tumor-bearing animal used
| Host species | Total (species) | Immune status | No of reports | Strain | No of reports |
|---|---|---|---|---|---|
| Rat | 61 | Competent | 57 | Fischer | 30 |
| Sprague–Dawley | 10 | ||||
| Wistar | 9 | ||||
| BD-IX | 6 | ||||
| NOS | 2 | ||||
| Compromised | 4 | Nude | 4 | ||
| Mouse | 26 | Competent | 12 | C57 Bl/6 variants | 7 |
| Knockout mice | 2 | ||||
| C3H | 1 | ||||
| VM/Dk | 1 | ||||
| NOS | 1 | ||||
| Compromised | 14 | Other nude | 8 | ||
| BALB/c nude | 6 | ||||
| Rabbit | 8 | ||||
| Macaque | 1 | ||||
| Dog | 2 | ||||
| Guinea pig | 4 | ||||
| Cat | 12 | ||||
All injected/implanted cell lines were implanted intracranially, no models used intracarotid or -cardiac injection
The most common combination of tumor-bearing animal and method of tumor generation was a variant of a rat-derived tumor cell line implanted into immunocompetent rats, accounting for 53 of all animal models. For example, the single biggest subgroup, defined as using the same strain and same cell line were Fischer rats implanted with 9L rat glioma cells but this accounted for only 9 of the 61 individual studies using rats.
Finally, there was variation in the methodology used (Table 4) for assessing the extent of edema, and, where applicable, response to treatment. The most prevalent method was assessment by histopathology (70% of studies) with studies of BWC and extravasation reported in just over 40% of studies, symptomatology in 38%, imaging in 31%, electrolyte composition assays in 10% and permeability studies in 6% of animal models.
Table 4.
Summary of peritumoral edema measures
| Edema measure | Description |
|---|---|
| Histology | We included studies that used any type of histopathological technique. These included “simple” staining and light microscopy, immune-staining and light-microscopy as well as scanning- and transmission electromicrography techniques. Factors such as distance between cells can then be used as a measure of edema. Histopathological techniques require post-mortem tissue for analysis and therefore allow only “snapshot” sampling of edema. Histopathological techniques were frequently used concurrently with extravasation of immune-stainable substances not normally found in brain tissue, either endogenous (albumin) or exogenous (horseradish peroxidase) to provide some measure of “leakiness” of cerebral vasculature |
| Brain water content (BWC) | Brain water content studies predominantly use the difference between wet and dried weight of an aliquot of brain tissue to estimate the water content of the tissue. Tissue is harvested post-mortem, weighed and then dried according to protocol (usually in an oven) and weighed again afterwards. This method cannot distinguish between increased extracellular fluid, as seen in peritumoral vasogenic edema, and increased water content e.g. due to cytotoxic edema |
| Extravasation studies | Extravasation studies include any studies that endeavor to establish the concentration of a chemical that is not normally present in brain tissue but occurs either naturally in blood, or is injected. The most commonly used substances were dyes such as Evans blue, exogenous proteins like horseradish peroxidase and endogenous proteins like albumin. The presence or concentration of these can then be detected in tissue post-mortem by a variety of techniques such as immunostaining microscopy, Western blotting and spectroscopy. This technique is an indirect measure of blood–brain barrier “leakiness” rather than edema, but the former is often used by investigators as a proxy for the latter |
| Imaging | Imaging can be used to investigate animals in vivo for radiographic appearances suggestive of tumor and/or peritumoral edema. The most common imaging modality is MRI where one would expect areas of high T2/FLAIR and low T1 signal relative to surrounding grey/white matter around the tumor to correspond to edema. However, imaging interpretation can be complex, especially in tumors that form complex heterogeneous masses which makes interpretation of imaging more difficult. MRI scans are considerably more expensive than the other techniques outlined here |
| Symptomatology | Assessing symptomatology in animals can be done in a number of ways; the simplest is to use a measure of mortality e.g. the time from the tumor instigation until death. More in-depth techniques have been used in some studies, for instance, observing for unilateral weakness or pupillary mydriasis (signs of considerable localizing mass effect) or observing for behavioral change (failure to feed, failure to groom). This has the benefit of measuring what would likely constitute a primary outcome in any human trial (morbidity/mortality) and can be done cheaply and repeatedly over time. It should be noted, however, that these measures do not differentiate between tumor growth and isolated tumor edema |
| Electrolyte composition assay | Similar to BWC, an aliquot of brain tissue is homogenized and ashened before quantifying the electrolyte content for instance with liquid chromatography. This technique is occasionally seen reported together with BWC, with which it shares significant methodological overlap |
| Permeability studies | We included in this category studies that used infusion of substances such as radio-labelled small molecules like aminoisobutyric acid, to allow calculation of a permeability constant. This is a more formal quantification of the permeability of brain vasculature to that particular substance than can be obtained by simply injecting a bolus of a substance as with extravasation studies. These techniques require sacrificing the animal |
Of the 24 articles containing MR images, 11 had no clear peritumoral edema (all rodent models), 4 showed a pattern of peritumoral halo all with edema volume less than tumor volume (3 rat glioma clone F98 tumor models, 1 rat 9L gliosarcoma model), 6 revealed infiltrative edema with volume less than tumor volume (4 rats, 1 mouse, 1 dog) and the remaining 3 (all cat models) showed infiltrative edema with greater volume than the tumor (Table 5). Eleven of the 24 studies where involved the assessment of an anti-edema agent.
Table 5.
Table classifying extent of peritumoral edema as shown on the MR images provided in studies of animal brain tumor models
| No | 1st Author (year) | Animal | Tumor model | MRI magnet strength (Tesla, T) | Images provided | Edema pattern |
|---|---|---|---|---|---|---|
| 1 | Yang et al. [39]* | Mouse (4–6-week-old BALB/c (nu/nu) mice) | Rat C6 glioma cells | 0.5 T | T1 pre & post Gd, T2 | No clear edema |
| 2 | Yamamoto et al. [38] | Rat (Fischer) | Rat 9L glioma/gliosarcoma cells | 2.4 T | T1 post-ATN-10 | No clear edema |
| 3 | Whelan et al. [43] | Dog | Canine gliosarcoma cells | 0.5 T | T1 pre & post Gd, T2 | Infiltrative, less than tumor volume |
| 4 | Tjujajev et al. [15]* | Rat (Fischer) | Rat RG2 gliomas | 4.7 T | T1 post Gd, proton density | Infiltrative, less than tumor volume |
| 5 | Tjuvajev et al. [62] | Rat (Fischer) | Rat RG2 gliomas | 4.7 T | T1 post Gd | No clear edema |
| 6 | Thompson et al. [21] | Rat (nude) | Human Small cell lung carcinoma (SCLC) LX1 or A2058 melanoma cells | 11.75 T | T1 pre & post Gd DCE-MRI | No clear edema |
| 7 | Takahashi et al. [37] | Rat (Wistar) | Rat C6 glioma cells | Not specified | T1 pre & post Gd, T2 | Infiltrative, less than tumor volume |
| 8 | Shevtsov et al. [22] | Rat (Wistar) | Rat C6 glioma cells | Not specified | T1, T2 and DWI with ADC maps | No clear edema |
| 9 | Sehm et al. [34]* | Rat (Fischer) | Rat glioma clone F98 | 3 T | T1 post Gd & T2 | Peritumoral halo, less than tumor volume |
| 10 | Pitter et al. [13]* | Mouse (Ntv-a/ink4a-arf-/- and Gli-luc;Ntv-a;Ink4a-Arf-/- mice) | RCAS-PDGFB-HA-transfected DF-1 cell suspension | 9.4 T | T1 post Gd & T2 | Infiltrative, less than tumor volume |
| 11 | Mazurchuk et al. [36] | Rat (Fisher) | Rat 9L glioma/gliosarcoma | 1.5 T | T1 & T2 | Peritumoral halo, less than tumor volume |
| 12 | Li et al. [35]* | Rat (Wistar) | Rat C6 glioma cells | 3 T | T2 | No clear edema |
| 13 | Kamoun et al. [25]* | Mouse (Nude) | Human U87 or U118 or Rat CNS1 tumors | 9.4 T | T2 | No clear edema |
| 14 | Ito et al. [60] | Rat (Wistar) | Rat C6 glioma cells | 1.5 T | T1 post-Gd | No clear edema |
| 15 | Hossmann et al. [23] | Cat | Rat glioma clone F98 | Not specified | T1 pre & post Gd, T2 | Infiltrative, greater than tumor volume |
| 16 | Hoehn-Berlage et al. [41] | Rat (Fischer) | Rat glioma clone F98 | 4.7 T | IR snapshot FLASH images pre and post MnTPPS | Infiltrative, less than tumor volume |
| 17 | Hoehn-Berlage et al. [52] | Cat | Rat glioma clone F98 | 4.7 T | T2 | Infiltrative, greater than tumor volume |
| 18 | Hoehn-Berlage et al. [32] | Rat (Fischer) | Rat glioma (F98), schwannoma (RN6), or neuroblastoma (E367) | 4.7 T | T2 & T1 post Gd | Infiltrative, less than tumor volume |
| 19 | Engelhorn et al. [31] | Rat (Fischer) | Rat glioma clone F98 | 1.5 T | CISS, T2, T1 post Gd | Peritumoral halo, less than tumor volume |
| 20 | Eis et al. [59] | Rat (Fischer) | Rat F98 glioma, RN6 schwannoma and E367 neuroblastoma | 4.7 T | T1, T2, PD, ADC maps | No clear edema |
| 21 | Chae et al. [28]* | Mouse (Nude) | Human U87 glioma cells | 9.4 T | T2 | No clear edema |
| 22 | Bulnes et al. [29]* | Rat (Sprague–Dawley) | Ethylnitrosourea (ENU) administration | Not specified | T1 post Gd & T2 | No clear edema |
| 23 | Bockhorst et al. [30] | Rat (Fischer) | Rat glioma clone F98 | 4.7 T | T1 pre & post Gd, T2 | Peritumoral halo, less than tumor volume |
| 24 | Bayens-Simmonds et al.[42] | Cat | 9L glioma/gliosarcoma | 2.35 T | Varying echo pulse | Infiltrative, greater than tumor volume |
MnTPPS Tumor-enhancing Contrast agent manganese(III) tetraphenylporphine sulfonate. Gd Gadolinium, intravenous contrast agent
ATN-10 (Manganese-metalloporphyrin)
*Studies involved in the assessment of an anti-edema agent
Discussion
We here report the findings from a systematic search and review of the literature on animal brain tumor models in which brain edema was reported. Most of the studies reviewed used indirect measures of cerebral edema (BWC, pathology, blood–brain barrier permeability) and these may not equate to the edema seen in patients with high grade tumors. Even in those studies where radiology was used, few of the animal models showed peritumoral brain edema in keeping with that seen in patients—and in the models which did show impressive edema, most notably in an immunocompetent feline model with an implanted xenograft tumor, there would be concern that the mechanism of edema formation is different to that seen in glioma. Overall, we found no evidence for a definitive animal model to use when assessing changes in peritumoral brain edema following treatment. The reports that have been published show considerable heterogeneity in design; this likely reflects peritumoral edema having been of secondary concern in designing brain tumor animal models.
Our results demonstrate that there is considerable heterogeneity in the combinations of tumor-bearing animal and tumor-induction method combinations and the measures used to assess the extent of edema. Edema measures in the literature presented here can be characterized as measuring BWC, measuring blood vessel leakiness, measuring symptoms or detecting radiological signs of edema (Table 4). There was often a disconnect between the results of these measures. For instance, Kamoun et al. [25] used BWC, imaging and clinical outcomes taken together in an immunosuppressed mouse model implanted with cultured human-derived glioma-derived cell lines. In their study, there was a significant increase in BWC in the ipsilateral cerebral hemisphere of tumor-bearing animals compared with tumor-free controls and this reduced following treatment with cediranib. Cediranib, which inhibits vascular endothelial growth factor (VEGF) receptor tyrosine kinase, limiting the growth of new blood vessels, also significantly delayed mortality in this model. However, the MRI images presented appeared not to show convincing T2 signal attributable to edema. This suggests that in this model, BWC changes occur without radiological evidence of peritumoral brain edema. This disconnect is important because most animal studies use histopathology and BWC to estimate peritumoral brain edema with imaging only occasionally being used. By contrast, in clinical practice, the reverse is true: clinical signs and radiology are routinely used to inform treatment decisions.
The radiological appearances of edema in the animal models were also very different compared to that seen in humans. On review of the imaging shown in the reports selected in this review, none of the animal tumor models showed a clear resemblance to those of humans with high grade brain tumors. The majority showed no peritumoral edema although some did show a halo pattern of edema. The halo gives the impression of a capsule or boundary within which the edema is confined. In contrast, in patients with GBM and metastases there is often widespread edema which migrates along the white matter tracts. In human clinical trials there are no standardized criteria for measuring/recording this cerebral edema—although there are standardized methods for recording radiological response to treatment (e.g. RANO [26]). Carlson et al. [27] devised a grading system for peritumoral brain edema:—grade 0 being no edema; grade 1 showing edema up to 2 cm from the tumor margin and grade 3 having edema extending more than 2 cm from the tumor margin. These authors noted that in GBM patients, 23% were grade 0, 23% were grade 1 and 54% showed grade 2, edema i.e. over 75% of patients had significant peritumoral brain edema.
In the animal models reviewed here, the extent of peritumoral edema appeared to be model dependent, with immunocompromised animals, perhaps not surprisingly, showing essentially no T2 signal attributable to peritumoral brain edema [25, 28]. Genetically-engineered mouse models and ENU-induced tumors in rats also showed no MRI evidence of edema [13, 29]. In immunocompetent rats implanted with rat-derived tumors only relatively minor edema is seen surrounding the tumor, often with a peritumoral ‘halo’, with the ratio of tumor volume to edema volume reported as between 50 and 150% [30–34]. In other reports, no or almost no T2 signal attributable to peritumoral edema can be seen on images in the manuscript [35–39]. This is different to human high grade tumors where it is common to see edema propagating through white matter, reaching large volumes and sometimes even crossing the midline into the macroscopically tumor-free hemisphere [40]. Most of the models discussed above have demonstrated edema using measures such as histopathological assessment or BWC, or by studying blood vessel permeability. Given that the extent of peritumoral edema in humans is often vastly greater radiologically, it is questionable how well these other measures would predict treatment response in humans.
We have found only one group of animal model that appears to show peritumoral edema similar to that seen in humans, namely cats injected with one of several rat glioma cell lines, e.g. F98 [41], RG2, 9L or C6. Studies have shown that the edema occurs preferentially in white matter and has increased albumin content, confirming the vasogenic origin of the fluid in these models [42].
It is not clear what drives these differences in peritumoral brain edema between the different animal models and between pre-clinical and clinical appearances. We can propose several possible explanations. Firstly, small rodents have a comparatively limited amount of white matter in the brain compared to larger animals. As radiologically demonstrable edema in humans has a predilection for white matter tracts, this may affect the limited amount of edema visible in rodent models. This may be supported by reports of spontaneously occurring canine gliomas which show considerable white matter tract edema [43] and by the edema seen in feline models. Furthermore, there may be less apparent intrinsic differences in the preponderance of different species’ organs for developing tumors [44, 45]—given that spontaneous brain tumors in rodents are very rare but do occur in larger animals, e.g. dogs [43, 46], there may be hitherto undescribed differences in the brains of small rodents and other animals that lead to different behavior of brain tumors, including edema. It must also be noted that the most common implantation site in rodents is the dorsal striatum which is different from where we see tumors in patients and may have an effect of the initiation and expansion of edema.
Secondly, there are considerable differences in immune status between the different models. Given the importance of inflammatory mediators in generating vasogenic edema, this may account for at least some of the variability in peritumoral brain edema. In the model where a rat or mouse-derived xenograft is implanted into immunocompetent cats, considerable edema is seen. Interestingly, while there is good evidence that the rat brain is an immune privileged site [47–49] and does not tend to generate much of an immune response to injected tissue unless the tumor material contacts non-brain parenchyma (such as in infiltration into the cerebral ventricles, or concurrent subdermal and intracerebral injection). By comparison, cats injected with rat tumor lines show features of immunological rejection [50] with leuko- and lymphocytic infiltration, which strongly implies the cat immune system behaves differently to that of rats. One would expect a xenograft to generate a near-maximal immunological reaction; this immune response and/or rejection may contribute to loss of integrity of the blood brain barrier and an edematous reaction resembling that seen in humans with gliomata in effect but produced by a mechanism that is not proven to be similar to human peritumoral brain edema and may be more akin to the edema seen in patients with cerebral abscess. Conversely, immunocompetent rats implanted with rat glioma cell lines had much less edema, and immunocompromised rats generated no edema. The clinical course in these experiments mirrors the radiological findings, with the immunocompetent cats bearing xenografts having a relatively fulminant course with neurological decline in the initial 2 weeks followed by either death or recovery and eventual regression of the tumor [51], which is compatible with immune clearance of the implanted foreign material and does not reflect the experience of patients with high grade tumors.
We note that the studies included in this systematic search do not encompass all of the animal model literature: we present a disproportionate number of studies that model gliomata compared with models of metastases, and a notable paucity or absence of certain models. For instance, we present only one genetically-engineered mouse model, and our search did not return any porcine models, despite their existence and the presence of edema on scans [65]. Given our robust and inclusive search strategy, we believe this lends further credence to the hypothesis that the study of edema in animal model research has been limited.
In summary, differences in the edema generated appear to be partly driven by factors relating to immunogenicity of the tumor/tumor-bearing animal combination, but also the macrostructure of the brain of the tumor-bearing animal. Further, the implanted tumors behave somewhat unpredictably, with considerable between-subjects differences reported in the growth rate of the tumor and the degree of associated brain edema [42].
To conclude, we have shown that there are considerable limitations to the use of the brain tumor animal models presented herein to study tumor-induced brain edema and hence to study potential alternatives to steroids treatment.
We postulate that for an animal model of peritumoral edema to be considered valid, it should fulfill the following criteria:
-
(i)
The tumor must produce an immune response that credibly mimics that of human brain to human tumor, i.e. it must not be driven by a xenogeneic immune reaction.
-
(ii)
It must produce a relevant volume of radiologically detectable edema, with manifestations that temporarily improve or resolve with steroid treatment with corresponding changes in other measures of edema such as BWC, symptomatology, extravasation studies and so forth.
-
(iii)
The model should be affordable and compatible with the highest standards of animal welfare.
Regrettably, none of the existing models of peritumoral brain edema reviewed here satisfy these fundamental requirements. However, there is opportunity here: there are tumor animal models have not been formally assessed as models of brain tumor edema yet, but could prove to be useful. We have highlighted two key areas of interest: immunocompetent models, and large animal models. Meanwhile, unless a relevant peritumoral brain edema animal model can be characterised, it is likely that potential alternatives to steroids will need to be trialed directly on patients. We would also argue that the neuro-oncology community should agree on standard outcome measures for recording peritumoral brain edema in both animal models and in patients on clinical trials.
Appendix A-Search strategies
Medline
1 Medline ("brain edema").ti,ab 5880
2 Medline "BRAIN EDEMA"/ 13,665
3 Medline ("brain hematoma").ti,ab 35
4 Medline "HEMATOMA, EPIDURAL, CRANIAL"/ 3331
5 Medline ("brain edema").ti,ab 857
6 Medline ("brain swelling").ti,ab 1469
7 Medline ("cerebral edema").ti,ab 4674
8 Medline ("cerebral edema").ti,ab 1160
9 Medline ("cerebral swelling").ti,ab 258
10 Medline ("cytotoxic brain edema").ti,ab 113
11 Medline ("intracranial edema").ti,ab 14
12 Medline ("vasogenic brain edema").ti,ab 252
13 Medline ("vasogenic cerebral edema").ti,ab 53
14 Medline (1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13) 23,492
15 Medline (astrocytoma).ti,ab 10,355
16 Medline ASTROCYTOMA/ 13,983
17 Medline (astroglioma).ti,ab 195
18 Medline ("brain cancer").ti,ab 2419
19 Medline "BRAIN NEOPLASMS"/ 100,154
20 Medline (brain AND (tumor* OR tumor*)).ti,ab 71,923
21 Medline ((brain AND (tumor* OR tumor*)) AND metastasis).ti,ab 4989
22 Medline ("brain neoplasm*").ti,ab 764
23 Medline ("brain neoplasm*" AND benign).ti,ab 38
24 Medline ("brain stem" AND (tumor* OR tumor*)).ti,ab 1842
25 Medline "BRAIN STEM NEOPLASMS"/ 1453
26 Medline ((brain AND (tumor* OR tumor*)) AND malignan*).ti,ab 14,988
27 Medline ((brain AND (tumor* OR tumor*)) AND primary).ti,ab 14,143
28 Medline ((brain AND (tumor* OR tumor*)) AND recurrent).ti,ab 2936
29 Medline ("cerebellar neoplasm*").ti,ab 46
30 Medline "CEREBELLAR NEOPLASMS"/ 8589
31 Medline (cerebellar AND (tumor* OR tumor*)).ti,ab 4313
32 Medline (cerebellum AND (tumor* OR tumor*)).ti,ab 2449
33 Medline ("cerebral astrocytoma").ti,ab 114
34 Medline ("cerebral ventricle" AND (tumor* OR tumor*)).ti,ab 54
35 Medline "CEREBRAL VENTRICLE NEOPLASMS"/ 3390
36 Medline ("cerebral ventricle" AND neoplasm*).ti,ab 2
37 Medline (cerebroventricular AND neoplasm*).ti,ab 0
39 Medline ("cerebellopontine" AND (tumor* OR tumor*)).ti,ab 2045
40 Medline ("choroid plexus neoplasm*").ti,ab 37
41 Medline "CHOROID PLEXUS NEOPLASMS"/ 597
42 Medline ("choroid plexus" AND (tumor* OR tumor*)).ti,ab 1396.
43 Medline (ependymoma).ti,ab 3316
44 Medline EPENDYMOMA/ 4715
45 Medline (ependymoblastoma).ti,ab 246
46 Medline "NEOPLASMS, NEUROEPITHELIAL"/ 807
47 Medline ("fibrillary astrocytoma").ti,ab 167
48 Medline (ganglioglioma).ti,ab 947
49 Medline GANGLIOGLIOMA/ 828
50 Medline ("gemistocytic astrocytoma").ti,ab 68
51 Medline (glioma).ti,ab 36,642
52 Medline GLIOMA/ 34,564
53 Medline (glioma AND astrocytic).ti,ab 785
54 Medline (glioma AND mixed).ti,ab 602
55 Medline (glioma AND subependymal).ti,ab 58
56 Medline (glioblastoma).ti,ab 26,720
57 Medline GLIOBLASTOMA/ 20,942
58 Medline ("glial cell" AND (tumor* OR tumor*)).ti,ab 699
59 Medline ("hypothalamic cancer").ti,ab 0
60 Medline ("hypothalamic neoplasm*").ti,ab 6
61 Medline ("hypothalamic teratoma").ti,ab 0
62 Medline "HYPOTHALAMIC NEOPLASMS"/ 712
63 Medline (hypothalamus AND (tumor* OR tumor*)).ti,ab 1650
64 Medline (hypophysis AND (tumor* OR tumor*)).ti,ab 310
65 Medline ("infratentorial cancer").ti,ab 0
66 Medline "INFRATENTORIAL NEOPLASMS"/ 781
67 Medline ("infratentorial neoplasm*").ti,ab 4
68 Medline (infratentorial AND (tumor* OR tumor*)).ti,ab 1001
69 Medline ("intracranial astrocytoma").ti,ab 12
70 Medline ("intracranial neoplasm*").ti,ab 1053
71 Medline (glioma AND malignan*).ti,ab 9955
72 Medline ("medullary neoplasm*").ti,ab 19
73 Medline (medullary AND (tumor* OR tumor*)).ti,ab 6216
74 Medline "NEOPLASMS, DUCTAL, LOBULAR, AND MEDULLARY"/ 70
75 Medline (medulloepithelioma).ti,ab 316
76 Medline MEDULLOBLASTOMA/ 639
77 Medline ("mesencephalic neoplasm*").ti,ab 0
78 Medline (midbrain AND (tumor* OR tumor*)).ti,ab 538
79 Medline (midbrain AND neoplasm*).ti,ab 49
80 Medline ("myxopapillary ependymoma").ti,ab 309
81 Medline "GLIOMA, SUBEPENDYMAL"/ 166
82 Medline (neurocytoma).ti,ab 596
83 Medline NEUROCYTOMA/ 560
84 Medline (neuroectodermal AND (tumor* OR tumor*)).ti,ab 5157
85 Medline exp "NEUROECTODERMAL TUMORS"/ 265,949
86 Medline (neurohypophysial AND neoplasm*).ti,ab 4
87 Medline (oligoastrocytoma).ti,ab 478
88 Medline (oligoastrocytic AND (tumor* OR tumor*)).ti,ab 37
89 Medline (oligodendroglioma).ti,ab 2318
90 Medline OLIGODENDROGLIOMA/ 3409
91 Medline (oligodendrocytosis).ti,ab 5
92 Medline (parenchymal AND (tumor* OR tumor*)).ti,ab 3837
93 Medline PINEALOMA/ 1782
94 Medline ("pilocytic astrocytoma").ti,ab 1211
95 Medline ("pineal gland" AND (tumor* OR tumor*)).ti,ab 580
96 Medline (pineoblastoma).ti,ab 295
97 Medline (pinealoma).ti,ab 251
98 Medline (pineocytoma).ti,ab 187
99 Medline (PNET).ti,ab 2115
100 Medline ("primitive neuroectodermal" AND (tumor* OR tumor*)).ti,ab 3212
101 Medline ("pons angle" AND (tumor* OR tumor*)).ti,ab 2
102 Medline ("pontine neoplasm").ti,ab 2
103 Medline (pontine AND (tumor* OR tumor*)).ti,ab 970
104 Medline (pontine AND glioma).ti,ab 505
105 Medline ("posterior fossa" AND neoplasm).ti,ab 110
106 Medline ("posterior fossa" AND (tumor* OR tumor*)).ti,ab 2887
107 Medline (spongioblastoma).ti,ab 109
108 Medline "NEUROECTODERMAL TUMORS, PRIMITIVE"/ 1661
109 Medline (subependymoma).ti,ab 265
110 Medline ((subtentorial AND tumor*) AND tumor*).ti,ab 2
111 Medline ("supratentorial neoplasm*").ti,ab 28
112 Medline "SUPRATENTORIAL NEOPLASMS"/ 2001
113 Medline (tentorial AND meningioma).ti,ab 176
114 Medline (cerebri AND (tumor* OR tumor*)).ti,ab 484
115 Medline (ventrical AND (tumor* OR tumor*)).ti,ab 8
116 Medline (ventricular AND (tumor* OR tumor*)).ti,ab 5805
117 Medline (15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30) 128,452
118 Medline (31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 60 OR 62 OR 63 OR 64) 90,403
119 Medline (66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72 OR 73 OR 74 OR 75 OR 76 OR 78 OR 79 OR 80 OR 81 OR 82 OR 83 OR 84 OR 85 OR 86 OR 87 OR 88 OR 89 OR 90) 276,190
120 Medline (91 OR 92 OR 93 OR 94 OR 95 OR 96 OR 97 OR 98 OR 99 OR 100 OR 101 OR 102 OR 103 OR 104 OR 105 OR 106 OR 107 OR 108 OR 109 OR 110 OR 111 OR 112 OR 113 OR 114 OR 115 OR 116) 23,358
121 Medline (14 AND 117) 1867
122 Medline (14 AND 118) 864
123 Medline (14 AND 119) 944
124 Medline (14 AND 120) 195
125 Medline (animal* OR "animal health" OR "animal population" OR "animal research" OR "animal study" OR "animal studies" OR "laboratory animal" OR primate OR rabbit OR rodent OR rat).ti,ab 1,865,196
126 Medline ANIMALS/ OR "ANIMAL POPULATION GROUPS"/ 6,167,987
127 Medline "MODELS, ANIMAL"/ OR "ANIMAL EXPERIMENTATION"/ 41,734
128 Medline (125 OR 126 OR 127) 6,410,366
129 Medline (121 AND 128) 300
130 Medline (122 AND 128) 195
131 Medline (123 AND 128) 203
132 Medline (124 AND 128) 12
133 Medline 129 [Animals] 284
134 Medline 130 [Animals] 185
135 Medline 131 [Animals] 193
136 Medline 132 [Animals] 11
PubMed
1 PubMed ("brain edema").ti,ab 16,319
2 PubMed ("brain hematoma").ti,ab 55
3 PubMed ("brain edema").ti,ab 864
4 PubMed ("brain swelling").ti,ab 1484
5 PubMed ("cerebral edema").ti,ab 4773
6 PubMed ("cerebral edema").ti,ab 1172
7 PubMed ("cerebral swelling").ti,ab 260
8 PubMed ("cytotoxic brain edema").ti,ab 114
9 PubMed ("intracranial edema").ti,ab 15
10 PubMed ("vasogenic brain edema").ti,ab 253
11 PubMed ("vasogenic cerebral edema").ti,ab 54
12 PubMed (1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11) 20,554
13 PubMed (astrocytoma).ti,ab 35,937
14 PubMed (astroglioma).ti,ab 36,037
15 PubMed ("brain cancer").ti,ab 2644
16 PubMed (brain AND (tumor* OR tumor*)).ti,ab 7655
17 PubMed ((brain AND (tumor* OR tumor*)) AND metastasis).ti,ab 8827
18 PubMed ("brain neoplasm*").ti,ab 289
19 PubMed ("brain neoplasm*" AND benign).ti,ab 7
20 PubMed ("brain stem" AND (tumor* OR tumor*)).ti,ab 411
21 PubMed ((brain AND (tumor* OR tumor*)) AND malignan*).ti,ab 20,587
22 PubMed ((brain AND (tumor* OR tumor*)) AND primary).ti,ab 20,651
23 PubMed ((brain AND (tumor* OR tumor*)) AND recurrent).ti,ab 4955
24 PubMed ("cerebellar neoplasm*").ti,ab 23
25 PubMed (cerebellar AND (tumor* OR tumor*)).ti,ab 948
26 PubMed (cerebellum AND (tumor* OR tumor*)).ti,ab 464
27 PubMed ("cerebral astrocytoma").ti,ab 121
28 PubMed ("cerebral ventricle" AND (tumor* OR tumor*)).ti,ab 234
29 PubMed ("cerebral ventricle" AND neoplasm*).ti,ab 3420
30 PubMed (cerebroventricular AND neoplasm*).ti,ab 2
31 PubMed ("choroid plexus neoplasm*").ti,ab 5
32 PubMed ("choroid plexus" AND (tumor* OR tumor*)).ti,ab 119
33 PubMed (ependymoma).ti,ab 6103
34 PubMed (ependymoblastoma).ti,ab 37,355
35 PubMed ("fibrillary astrocytoma").ti,ab 166
36 PubMed (ganglioglioma).ti,ab 1296
37 PubMed ("gemistocytic astrocytoma").ti,ab 70
38 PubMed (glioma).ti,ab 84,055
39 PubMed (glioma AND astrocytic).ti,ab 2494
40 PubMed (glioma AND mixed).ti,ab 1474
41 PubMed (glioma AND subependymal).ti,ab 622
42 PubMed (glioblastoma).ti,ab 33,206
43 PubMed ("glial cell" AND (tumor* OR tumor*)).ti,ab 40
44 PubMed ("hypothalamic cancer").ti,ab 0
45 PubMed ("hypothalamic neoplasm*").ti,ab 2
46 PubMed ("hypothalamic teratoma").ti,ab 0
47 PubMed (hypothalamus AND (tumor* OR tumor*)).ti,ab 517
48 PubMed (hypophysis AND (tumor* OR tumor*)).ti,ab 598
49 PubMed ("infratentorial cancer").ti,ab 0
50 PubMed ("infratentorial neoplasm*").ti,ab 2
51 PubMed (infratentorial AND (tumor* OR tumor*)).ti,ab 101
52 PubMed ("intracranial astrocytoma").ti,ab 12
53 PubMed ("intracranial neoplasm*").ti,ab 295
54 PubMed (glioma AND malignan*).ti,ab 18,717
55 PubMed ("medullary neoplasm*").ti,ab 6
56 PubMed (medullary AND (tumor* OR tumor*)).ti,ab 835
57 PubMed ("mesencephalic neoplasm*").ti,ab 0
58 PubMed (midbrain AND (tumor* OR tumor*)).ti,ab 89
59 PubMed (midbrain AND neoplasm*).ti,ab 1325
60 PubMed ("myxopapillary ependymoma").ti,ab 314
61 PubMed (neurocytoma).ti,ab 745
62 PubMed (neuroectodermal AND (tumor* OR tumor*)).ti,ab 419
63 PubMed (neurohypophysial AND neoplasm*).ti,ab 31
64 PubMed (oligoastrocytoma).ti,ab 4711
65 PubMed (oligoastrocytic AND (tumor* OR tumor*)).ti,ab 2
66 PubMed (oligodendrocytosis).ti,ab 6
67 PubMed (parenchymal AND (tumor* OR tumor*)).ti,ab 264
68 PubMed ("pilocytic astrocytoma").ti,ab 1265
69 PubMed ("pineal gland" AND (tumor* OR tumor*)).ti,ab 132
70 PubMed (pineoblastoma).ti,ab 1943
71 PubMed (pinealoma).ti,ab 1833
72 PubMed (pineocytoma).ti,ab 1879
73 PubMed (PNET).ti,ab 38,072
74 PubMed ("primitive neuroectodermal" AND (tumor* OR tumor*)).ti,ab 152
75 PubMed ("pons angle" AND (tumor* OR tumor*)).ti,ab 14
76 PubMed ("pontine neoplasm").ti,ab 2
77 PubMed (pontine AND (tumor* OR tumor*)).ti,ab 223
78 PubMed (pontine AND glioma).ti,ab 1058.
79 PubMed ("posterior fossa" AND neoplasm).ti,ab 3846.
80 PubMed ("posterior fossa" AND (tumor* OR tumor*)).ti,ab 244
81 PubMed (spongioblastoma).ti,ab 37,261
82 PubMed (subependymoma).ti,ab 483
83 PubMed ((subtentorial AND tumor*) AND tumor*).ti,ab 1
84 PubMed ("supratentorial neoplasm*").ti,ab 7
85 PubMed (tentorial AND meningioma).ti,ab 260
86 PubMed (cerebri AND (tumor* OR tumor*)).ti,ab 99
87 PubMed (ventrical AND (tumor* OR tumor*)).ti,ab 3
88 PubMed (ventricular AND (tumor* OR tumor*)).ti,ab 762
89 PubMed (69 OR 70 OR 71 OR 72 OR 73 OR 74 OR 75 OR 76 OR 77 OR 78 OR 79 OR 80 OR 81 OR 82 OR 83 OR 84 OR 85 OR 86 OR 87 OR 88) 45,658
90 PubMed (49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68) 27,601
91 PubMed (29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48) 122,455
92 PubMed (13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28) 75,219
93 PubMed (animal* OR "animal health" OR "animal population" OR "animal research" OR "animal study" OR "animal studies" OR "laboratory animal" OR primate OR rabbit OR rodent OR rat).ti,ab 21,587,741
94 PubMed (12 AND 92 AND 93) 867
95 PubMed (12 AND 91 AND 93) 832
96 PubMed (12 AND 90 AND 93) 272
97 PubMed (12 AND 89 AND 93) 102
98 PubMed (human).ti,ab 17,558,986
99 PubMed 94 not 98 76
100 PubMed 95 not 98 121
101 PubMed 96 not 98 18
102 PubMed 97 not 98 8
CINAHL
1 CINAHL ("brain edema").ti,ab 332
2 CINAHL "CEREBRAL EDEMA"/ 920
3 CINAHL ("brain hematoma").ti,ab 1
4 CINAHL ("brain swelling").ti,ab 94
5 CINAHL ("cerebral edema").ti,ab 406
6 CINAHL ("cerebral edema").ti,ab 84
7 CINAHL ("cerebral swelling").ti,ab 14
8 CINAHL ("cytotoxic brain edema").ti,ab 4
9 CINAHL ("intracranial edema").ti,ab 1
10 CINAHL ("vasogenic brain edema").ti,ab 4
11 CINAHL ("vasogenic cerebral edema").ti,ab 4
12 CINAHL (1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11) 1367
13 CINAHL (astrocytoma).ti,ab 333
14 CINAHL (astroglioma).ti,ab 1
15 CINAHL ("brain cancer").ti,ab 263
CINAHL "BRAIN NEOPLASMS"/ 4757
17 CINAHL (brain AND (tumor* OR tumor*)).ti,ab 3251
18 CINAHL ((brain AND (tumor* OR tumor*)) AND metastasis).ti,ab 393
19 CINAHL ("brain neoplasm*").ti,ab 39
20 CINAHL ("brain neoplasm*" AND benign).ti,ab 1
21 CINAHL ("brain stem" AND (tumor* OR tumor*)).ti,ab 29
22 CINAHL ((brain AND (tumor* OR tumor*)) AND malignan*).ti,ab 534
23 CINAHL ((brain AND (tumor* OR tumor*)) AND primary).ti,ab 723
24 CINAHL ((brain AND (tumor* OR tumor*)) AND recurrent).ti,ab 139
25 CINAHL ("cerebellar neoplasm*").ti,ab 1
26 CINAHL (cerebellar AND (tumor* OR tumor*)).ti,ab 122
27 CINAHL (cerebellum AND (tumor* OR tumor*)).ti,ab 61
28 CINAHL ("cerebral astrocytoma").ti,ab 1
29 CINAHL ("cerebral ventricle" AND (tumor* OR tumor*)).ti,ab 1
30 CINAHL ("cerebral ventricle" AND neoplasm*).ti,ab 0
31 CINAHL (cerebroventricular AND neoplasm*).ti,ab 0
32 CINAHL (cerebellopontine AND (tumor* OR tumor*)).ti,ab 68
33 CINAHL ("choroid plexus neoplasm*").ti,ab 0
34 CINAHL ("choroid plexus" AND (tumor* OR tumor*)).ti,ab 18
35 CINAHL (ependymoma).ti,ab 182
36 CINAHL (ependymoblastoma).ti,ab 0
37 CINAHL ("fibrillary astrocytoma").ti,ab 2
38 CINAHL (ganglioglioma).ti,ab 21
39 CINAHL (glioma).ti,ab 1326
40 CINAHL GLIOMA/ 2607
41 CINAHL (glioma AND astrocytic).ti,ab 20
42 CINAHL (glioma AND mixed).ti,ab 28
43 CINAHL (glioma AND subependymal).ti,ab 1
44 CINAHL (glioblastoma).ti,ab 1097
45 CINAHL ("glial cell" AND (tumor* OR tumor*)).ti,ab 22
46 CINAHL ("hypothalamic cancer").ti,ab 0
47 CINAHL ("hypothalamic neoplasm*").ti,ab 0
48 CINAHL ("hypothalamic teratoma").ti,ab 0
49 CINAHL (hypophysis AND (tumor* OR tumor)).ti,ab 1
50 CINAHL ("infratentorial cancer").ti,ab 0
51 CINAHL "INFRATENTORIAL NEOPLASMS"/ 73
52 CINAHL "HYPOTHALAMIC NEOPLASMS"/ 9
53 CINAHL ("infratentorial neoplasm*").ti,ab 1
54 CINAHL (infratentorial AND (tumor* OR tumor*)).ti,ab 17
55 CINAHL ("intracranial astrocytoma").ti,ab 1
56 CINAHL ("intracranial neoplasm*").ti,ab 37
57 CINAHL (glioma AND malignan*).ti,ab 358
58 CINAHL ("medullary neoplasm*").ti,ab 0
59 CINAHL (medullary AND (tumor* OR tumor*)).ti,ab 134
60 CINAHL "NEOPLASMS, DUCTAL, LOBULAR, AND MEDULLARY"/ 324
61 CINAHL (medulloepithelioma).ti,ab 3
62 CINAHL "NEUROECTODERMAL TUMORS, PRIMITIVE"/ 80
63 CINAHL (medulloblastoma).ti,ab 284
64 CINAHL ("mesencephalic neoplasm*").ti,ab 0
65 CINAHL (midbrain AND (tumor* OR tumor*)).ti,ab 16
66 CINAHL (midbrain AND neoplasm*).ti,ab 2
67 CINAHL ("myxopapillary ependymoma").ti,ab 16
68 CINAHL (neurocytoma).ti,ab 29
69 CINAHL (neuroectodermal AND (tumor* OR tumor*)).ti,ab 171
70 CINAHL (neurohypophysial AND neoplasm*).ti,ab 0
71 CINAHL (oligoastrocytoma).ti,ab 45
72 CINAHL (oligoastrocytic AND (tumor* OR tumor*)).ti,ab 1
73 CINAHL (oligodendroglioma).ti,ab 112
74 CINAHL (oligodendrocytosis).ti,ab 0
75 CINAHL (parenchymal AND (tumor* OR tumor*)).ti,ab 102
76 CINAHL ("pilocytic astrocytoma").ti,ab 26
77 CINAHL ("pineal gland" AND (tumor* OR tumor*)).ti,ab 15
78 CINAHL (pineoblastoma).ti,ab 5
79 CINAHL PINEALOMA/ 29
80 CINAHL "INFRATENTORIAL NEOPLASMS"/ 73
81 CINAHL (pinealoma).ti,ab 2
82 CINAHL (pineocytoma).ti,ab 3
83 CINAHL (PNET).ti,ab 100
84 CINAHL ("primitive neuroectodermal" AND (tumor* OR tumor*)).ti,ab 135
85 CINAHL ("pons angle" AND (tumor* OR tumor*)).ti,ab 1
86 CINAHL ("pontine neoplasm").ti,ab 0
87 CINAHL (pontine AND (tumor* OR tumor)).ti,ab 34
88 CINAHL (pontine AND glioma).ti,ab 25
89 CINAHL ("posterior fossa" AND neoplasm*).ti,ab 10
90 CINAHL ("posterior fossa" AND (tumor* OR tumor*)).ti,ab 108
91 CINAHL (spongioblastoma).ti,ab 1
92 CINAHL "NEOPLASMS, NEUROEPITHELIAL"/ 81
93 CINAHL ((subtentorial AND tumor*) AND tumor*).ti,ab 0
94 CINAHL ("supratentorial neoplasm*").ti,ab 2
95 CINAHL (tentorial AND meningioma).ti,ab 1
96 CINAHL MENINGIOMA/ 493
97 CINAHL (cerebri AND (tumor* OR tumor*)).ti,ab 20
98 CINAHL (ventrical AND (tumor* OR tumor*)).ti,ab 0
99 CINAHL (ventricular AND (tumor* OR tumor*)).ti,ab 294
100 CINAHL (13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72 OR 73 OR 74 OR 75 OR 76 OR 77 OR 78 OR 79 OR 80 OR 81 OR 82 OR 83 OR 84 OR 85 OR 86 OR 87 OR 88 OR 89 OR 90 OR 91 OR 92 OR 93 OR 94 OR 95 OR 96 OR 97 OR 98 OR 99) 9685.
101 CINAHL (animal* OR "animal health" OR "animal population" OR "animal research" OR "animal study" OR "animal studies" OR "laboratory animal" OR primate OR rabbit OR rodent OR rat).ti,ab 44,880.
102 CINAHL "ANIMALS, LABORATORY"/ OR "ANIMAL POPULATION GROUPS"/ OR ANIMALS/35299
103 CINAHL "ANIMAL STUDIES"/ 43,675
104 CINAHL (101 OR 102 OR 103) 90,301
105 CINAHL (12 AND 100) 78
106 CINAHL (103 AND 105) 11
107 CINAHL (102 AND 105) 12
Appendix B-MeSH terms
Brain edema
Brain hematoma
Brain edema
Brain swelling
Cerebral edema
Cerebral edema
Cerebral swelling
Cytotoxic brain edema
Cytotoxic cerebral edema
Intracranial edema
Vasogenic brain edema
Vasogenic cerebral edema
Brain Disease, Edema (< 1992)
Astrocytoma
Astroglioma
Brain cancer
Brain tumor(s)
Brain tumor(s)
Brain Tumor Metastasis
Brain neoplasm(s)
Brain neoplasm(s), benign
Brain stem tumor(s)
Brain stem tumor(s)
Brain tumor, malignant
Brain tumor, malignant
Brain tumor, primary
Brain tumor, primary
Brain tumor, recurrent
Brain tumor, recurrent
Cerebellar Neoplasm(s)
Cerebellar Tumor(s)
Cerebellar Tumor(s)
Cerebellum tumor
Cerebral Astrocytoma
Cerebral Ventricle Tumor(s)
Cerebral Ventricle Tumor(s)
Cerebral Ventricle Neoplasm(s)
Cerebroventricular Neoplasm(s)
Cerebello Pontine Angle Tumor
Choroid Plexus Neoplasms
Choroid Plexus Tumors
Ependymoma
Ependymoblastoma
Fibrillary Astrocytoma
Ganglioglioma
Gemistocytic Astrocytoma
Glioma
Glioma, Astrocytic
Glioma, Mixed
Glioma, Subependymal
Glioblastoma, Giant Cell
Gliosarcoma
Glial Cell Tumor(s)
Glial Cell Tumor(s)
Hypothalamic Cancer
Hypothalamic Neoplasm(s)
Hypothalamic Teratomas
Hypothalamic Tumor(s)
Hypothalamic Tumor(s)
hypothalamus tumor
Hypophysis tumor
Infratentorial Cancer
Infratentorial Neoplasm(s)
Infratentorial Tumor(s)
Infratentorial Tumor(s)
Intracranial Astrocytoma
Intracranial Neoplasm(s)
Malignant Glioma
Medullary Neoplasm(s)
Medullary Tumor(s)
Medullary Tumor(s)
Medulloepithelioma
Mesencephalic Neoplasm(s)
Midbrain Tumor(s)
Midbrain Tumor(s)
Myxopapillary Ependymoma
Neoplasm Metastases
Neoplasm Micrometastases
Neurocytoma
Neuroectodermal Tumors, Primitive
Neurohypophysial Region Neoplasms
Oligoastrocytoma.
Oligoastrocytic tumors
Oligodendroglioma
Oligodendrocytosis
Parenchymal Tumor
Pilocytic Astrocytoma
Pineal Gland Tumor
Pineal Gland Tumor
Pineal Tumor(s)
Pineal Tumor(s)
Pineoblastoma
Pinealoma
Pineocytoma
Pineocytoma-Pineoblastoma
PNET
Pons angle tumor
Pontine Neoplasm(s)
Pontine Tumor(s)
Pontine Tumor(s)
Pontine Glioma
Posterior Fossa Neoplasm(s)
Posterior Fossa Tumor
Posterior Fossa Tumor
Primitive Neuroepithelial Neoplasms
Spongioblastoma
Subependymoma
Subtentorial Tumor
Supratentorial Neoplasm(s)
Tentorial Meningioma
Tentorium Meningioma
Tumor, Cerebri
Ventricle Tumor, Brain
Ventricular Tumors, Brain
Animal(s)
Animal health
Animal population
Animal research
Animal studies
Laboratory animals
Non-human data
Primates
Rabbits
Rats
Rodents
Animal v Human comparative study
Author contributions
All authors contributed to the study conception and design of search strategy. Review of the reports and analyzes was performed by MWJS, PC, MtL and SPS. The first draft of the manuscript was written by MWJS and LJL. All authors read and approved the final manuscript.
Funding
Brain Research UK,Candlelighters,Yorkshire's Brain Tumour Charity,Engineering and Physical Sciences Research Council (EPSRC),National Institute for Health and Care Research
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kellie G. Appearances observed in the dissection of two individuals; death from cold and congestion of the brain. Trans Med-Chir Soc Edinb. 1824;1:84. [PMC free article] [PubMed] [Google Scholar]
- 2.Monro A. Observations on the Structure and Functions of the Nervous System. 1783.
- 3.Klatzo I. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967;26(1):1–14. doi: 10.1097/00005072-196701000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol (Berl) 1987;72(3):236–239. doi: 10.1007/BF00691095. [DOI] [PubMed] [Google Scholar]
- 5.Stummer W. Mechanisms of tumor-related brain edema. Neurosurg Focus. 2007;22(5):1–7. doi: 10.3171/foc.2007.22.5.9. [DOI] [PubMed] [Google Scholar]
- 6.Jelsma R, Bucy PC. The Treatment of glioblastoma multiforme of the Brain. J Neurosurg. 1967;27(5):388–400. doi: 10.3171/jns.1967.27.5.0388. [DOI] [PubMed] [Google Scholar]
- 7.Kofman S, Garvin J, Nagamani D, Taylor S. Treatment of cerebral metastases from breast carcinoma with prednisolone. J Am Med Assoc. 1957;163(16):1473–1476. doi: 10.1001/jama.1957.02970510039008. [DOI] [PubMed] [Google Scholar]
- 8.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support Care Cancer. 2002;10(4):322–328. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 9.Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Care Res. 2006;55(3):420–426. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 10.Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 11.Sarin R, Murthy V. Medical decompressive therapy for primary and metastatic intracranial tumours. Lancet Neurol. 2003;2(6):357–365. doi: 10.1016/S1474-4422(03)00410-1. [DOI] [PubMed] [Google Scholar]
- 12.Hui CY, Rudra S, Ma S, Campian JL, Huang J. Impact of overall corticosteroid exposure during chemoradiotherapy on lymphopenia and survival of glioblastoma patients. J Neurooncol. 2019 doi: 10.1007/s11060-019-03146-7. [DOI] [PubMed] [Google Scholar]
- 13.Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(5):1458–1471. doi: 10.1093/brain/aww046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones-Bolin S, Zhao H, Hunter K, Klein-Szanto A, Ruggeri B. The effects of the oral, pan-VEGF-R kinase inhibitor CEP-7055 and chemotherapy in orthotopic models of glioblastoma and colon carcinoma in mice. Mol Cancer Ther. 2006;5(7):1744–1753. doi: 10.1158/1535-7163.MCT-05-0327. [DOI] [PubMed] [Google Scholar]
- 15.Tjuvajev J, Uehara H, Desai R, et al. Corticotropin-releasing factor decreases vasogenic brain edema. Cancer Res. 1996;56(6):1352–1360. [PubMed] [Google Scholar]
- 16.Huszthy PC, Daphu I, Niclou SP, et al. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro-Oncol. 2012;14(8):979–993. doi: 10.1093/neuonc/nos135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balathasan L, Beech JS, Muschel RJ. Ultrasonography-guided intracardiac injection: an improvement for quantitative brain colonization assays. Am J Pathol. 2013;183(1):26–34. doi: 10.1016/j.ajpath.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Zitvogel L, Pitt JM, Daillère R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16(12):759–773. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 20.Tjuvajev J, Gansbacher B, Desai R, Beattie B, Kaplitt M, Matei C, Koutcher J, Gilboa E, Blasberg R (1995) RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin 2 and interferon-γ. Cancer Res 55(9):1902–1910 [PubMed]
- 21.Thompson EM, Pishko GL, Muldoon LL, Neuwelt EA (2013) Inhibition of SUR1 decreases the vascular permeability of cerebral metastases. Neoplasia 15(5):535–543 [DOI] [PMC free article] [PubMed]
- 22.Shevtsov MA, Nikolaev BP, Yakovleva LY, Dobrodumov AV, Zhakhov AV, Mikhrina AL, Pitkin E, Parr MA, Rolich VI, Simbircev AS (2015) Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma. Neoplasia 17(1):32–42 [DOI] [PMC free article] [PubMed]
- 23.Hossmann KA, Szymas J, Seo K, Assheuer J, Krajewski S (1989) Experimental transplantation gliomas in the adult cat brain. Acta Neurochir 98(3):189–200. 10.1007/BF01407347 [DOI] [PubMed]
- 24.Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–520. doi: 10.1038/s41571-019-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen PY, Chang SM, den Van M, B, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. doi: 10.1200/JCO.2017.72.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson MRJ, Pope WB, Horvath S, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13(9):2592–2598. doi: 10.1158/1078-0432.CCR-06-2772. [DOI] [PubMed] [Google Scholar]
- 28.Chae S-S, Kamoun WS, Farrar CT, et al. Angiopoietin-2 interferes with anti-VEGFR2–induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16(14):3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulnes S, Argandona EG, Bengoetxea H, Leis O, Ortuzar N, Lafuente JV. The role of eNOS in vascular permeability in ENU-induced gliomas. In: Czernicki Z, Baethmann A, Ito U, Katayama Y, Kuroiwa T, Mendelow D, editors. Brain Edema XIV. Berlin: Springer; 2010. pp. 277–282. [DOI] [PubMed] [Google Scholar]
- 30.Bockhorst K, Els T, Kohno K, Hoehn-Berlage M. Localization of Experimental Brain Tumors in MRI by Gadolinium Porphyrin. In: Baethmann A, Hossmann K-A, editors. Ito U. Springer Vienna: Brain Edema IX; 1994. pp. 347–349. [DOI] [PubMed] [Google Scholar]
- 31.Engelhorn T, Savaskan NE, Schwarz MA, et al. Cellular characterization of the peritumoral edema zone in malignant brain tumors. Cancer Sci. 2009;100(10):1856–1862. doi: 10.1111/j.1349-7006.2009.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoehn-Berlage M, Bockhorst K. Quantitative magnetic resonance imaging of rat brain tumors: In vivo NMR relaxometry for the discrimination of normal and pathological tissues. Technol Health Care. 1994;2(4):247–254. doi: 10.3233/THC-1994-2404. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Liu X, Liu Z, Su Z. Interactions of connexin 43 and aquaporin-4 in the formation of glioma-induced brain edema. Mol Med Rep. 2015;11(2):1188–1194. doi: 10.3892/mmr.2014.2867. [DOI] [PubMed] [Google Scholar]
- 34.Sehm T, Fan Z, Ghoochani A, et al. Sulfasalazine impacts on ferroptotic cell death and alleviates the tumor microenvironment and glioma-induced brain edema. Oncotarget. 2016;7(24):36021. doi: 10.18632/oncotarget.8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Meng C, Zhang X, et al. Effect of photodynamic therapy combined with torasemide on the expression of matrix metalloproteinase 2 and sodium-potassium-chloride cotransporter 1 in rat peritumoral edema and glioma. Oncol Lett. 2016;11(3):2084–2090. doi: 10.3892/ol.2016.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazurchuk R, Zhou R, Straubinger RM, Chau RI, Grossman Z. Functional magnetic resonance (fMR) imaging of a rat brain tumor model: implications for evaluation of tumor microvasculature and therapeutic response. Magn Reson Imaging. 1999;17(4):537–548. doi: 10.1016/S0730-725X(98)00208-2. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi H, Hamada H, Teramoto A. Effect of Niravoline (RU51599), a κ-opioid receptor agonist, on tumour-origin brain oedema. Acta Neurochir (Wien) 1999;141(7):771–778. doi: 10.1007/s007010050374. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Matsumura A, Shibata Y, et al. Manganese-metalloporphyrin (ATN-10) as a tumor-localizing agent: magnetic resonance imaging and inductively coupled plasma atomic emission spectroscopy study with experimental brain tumors. Neurosurgery. 1998;42(6):1332–1337. doi: 10.1097/00006123-199806000-00083. [DOI] [PubMed] [Google Scholar]
- 39.Yang L-J, Lin ZX, Kang D-Z, et al. Effects of endostatin on C6 glioma-induced edema. Chin Med J (Engl) 2011;124(24):4211–4216. [PubMed] [Google Scholar]
- 40.Reulen H-J, Graber S, Huber P, Ito U. Factors affecting the extension of peritumoural brain oedema. A CT-study Acta Neurochir (Wien) 1988;95(1):19–24. doi: 10.1007/BF01793077. [DOI] [PubMed] [Google Scholar]
- 41.Hoehn-Berlage M, Tolxdorff T, Bockhorst K, Okada Y, Ernestus R-I. In vivo NMR T2 relaxation of experimental brain tumors in the cat: a multiparameter tissue characterization. Magn Reson Imaging. 1992;10(6):935–947. doi: 10.1016/0730-725X(92)90448-9. [DOI] [PubMed] [Google Scholar]
- 42.Bayens-Simmonds J, Boisvert DPJ, Castro ME, Johnson ES. A feline model for experimental studies of peritumor brain edema. J Neurooncol. 1988;6(4):371–378. doi: 10.1007/BF00177435. [DOI] [PubMed] [Google Scholar]
- 43.Whelan HT, Clanton JA, Moore PM, Tolner DJ, Kessler RM, Whetsell WO. Magnetic resonance brain tumor imaging in canine glioma. Neurology. 1987;37(7):1235–1235. doi: 10.1212/WNL.37.7.1235. [DOI] [PubMed] [Google Scholar]
- 44.Vittecoq M, Ducasse H, Arnal A, et al. Animal behaviour and cancer. Anim Behav. 2015;101:19–26. doi: 10.1016/j.anbehav.2014.12.001. [DOI] [Google Scholar]
- 45.Albuquerque TAF, Val LD, do, Doherty A, Magalhães JP de. From humans to hydra: patterns of cancer across the tree of life. Biol Rev. 2018;93(3):1715–1734. doi: 10.1111/brv.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song RB, Vite CH, Bradley CW, Cross JR. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, Age, and body weight. J Vet Intern Med. 2013;27(5):1143–1152. doi: 10.1111/jvim.12136. [DOI] [PubMed] [Google Scholar]
- 47.Medwar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 48.Shirai Y. On the transplantation of the rat sarcoma in adult heterogenous animals. Jap Med World. 1921;1:14–15. [Google Scholar]
- 49.Lorger M, Andreou T, Fife C, James F. Immune checkpoint blockade – how does it work in brain metastases? Front Mol Neurosci. 2019 doi: 10.3389/fnmol.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hossmann K-A, Wechsler W, Wilmes F. Experimental peritumorous edema. Acta Neuropathol (Berl) 1979;45(3):195–203. doi: 10.1007/BF00702671. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler W, Szymas J, Bilzer T, Hossmann K-A. Experimental transplantation gliomas in the adult cat brain. Acta Neurochir (Wien) 1989;98(1):77–89. doi: 10.1007/BF01407181. [DOI] [PubMed] [Google Scholar]
- 52.Hoehn-Berlage M, Norris D, Bockhorst K, et al. T1 snapshot flash measurement of rat brain glioma: kinetics of the tumor-enhancing contrast agent manganese (iii) tetraphenylporphine sulfonate. Magn Reson Med. 1992;27(2):201–213. doi: 10.1002/mrm.1910270202. [DOI] [PubMed] [Google Scholar]
- 53.Purves D, Dayan A. A preliminary investigation of promotion of brain tumours by hexachlorophane in Sprague-Dawley rats transplacentally exposed to N-ethylnitrosourea. Neuropathol Appl Neurobiol. 1992;18(3):259–264. doi: 10.1111/j.1365-2990.1992.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 54.Spencer AT, Smith WT. Behaviour of intracerebral autografts of mouse Tail skin pre-treated with a single application of 20-methylcholanthrene. Nature. 1965;207(4997):649. doi: 10.1038/207649a0. [DOI] [PubMed] [Google Scholar]
- 55.Yamada K, Hayakawa T, Ushio Y, Arita N, Kato A, Mogami H. Regional blood flow and capillary permeability in the ethylnitrosourea-induced rat glioma. J Neurosurg. 1981;55(6):922–928. doi: 10.3171/jns.1981.55.6.0922. [DOI] [PubMed] [Google Scholar]
- 56.Sipe JC, Vick NA, Bigner DD. Grey matter edema: The marginal zone of autochthonous virally-induced gliomas. J Neurol Sci. 1972;17(2):185–191. doi: 10.1016/0022-510X(72)90139-6. [DOI] [PubMed] [Google Scholar]
- 57.Deane BR, Greenwood J, Lantos PL, Pratt OE. The vasculature of experimental brain tumours. J Neurol Sci. 1984;65(1):59–68. doi: 10.1016/0022-510X(84)90067-4. [DOI] [PubMed] [Google Scholar]
- 58.Deane BR, Papp MI, Lantos PL. The vasculature of experimental brain tumours: Part 3. Permeability studies J Neurol Sci. 1984;65(1):47–58. doi: 10.1016/0022-510X(84)90066-2. [DOI] [PubMed] [Google Scholar]
- 59.Eis M, Els T, Hoehn-Berlage M. High resolution quantitative relaxation and diffusion MRI of three different experimental brain tumors in rat. Magn Reson Med. 1995;34(6):835–844. doi: 10.1002/mrm.1910340608. [DOI] [PubMed] [Google Scholar]
- 60.Ito S, Rachinger W, Stepp H, Reulen HJ, Stummer W. Oedema formation in experimental photo-irradiation therapy of brain tumours using 5-ALA. Acta Neurochir (Wien) 2005;147(1):57–65. doi: 10.1007/s00701-004-0422-1. [DOI] [PubMed] [Google Scholar]
- 61.Papadopoulos MC, Binder DK, Verkman AS. Enhanced macromolecular diffusion in brain extracellular space in mouse models of vasogenic edema measured by cortical surface photobleaching. FASEB J. 2004;19(3):425–427. doi: 10.1096/fj.04-2834fje. [DOI] [PubMed] [Google Scholar]
- 62.Aleu FP, Edelman FL, Katzman R, Scheinberg LC. Ultrastructural and biochemical analysis in cerebral edema associated with experimental mouse gliomas. J Neuropathol Exp Neurol. 1964;23(2):253–263. doi: 10.1097/00005072-196404000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Hossmann K-A, Bothe H-W, Bodsch W, Paschen W. Pathophysiological Aspects of Blood-Brain Barrier Disturbances in Experimental Brain Tumors and Brain Abscesses. In: Hossmann K-A, Klatzo I, editors. Cerebrovascular Transport Mechanisms. Berlin: Springer; 1983. pp. 89–102. [DOI] [PubMed] [Google Scholar]
- 64.Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm Res. 2009;58(9):619–624. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 65.Selek L, Seigneuret E, Nugue G, et al. Imaging and histological characterization of a human brain xenograft in pig: The first induced glioma model in a large animal. J Neurosci Methods. 2014;221:159–165. doi: 10.1016/j.jneumeth.2013.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.