Abstract
Background
COVID-19 rebound is usually reported among patients experiencing concurrent symptomatic and viral rebound. But longitudinal viral RT-PCR results from early stage to rebound of COVID-19 was less characterized. Further, identifying the factors associated with viral rebound after nirmatrelvir-ritonavir (NMV/r) and molnupiravir may expand understanding of COVID-19 rebound.
Methods
We retrospectively analyzed clinical data and sequential viral RT-PCR results from COVID-19 patients receiving oral antivirals between April and May, 2022. Viral rebound was defined by the degree of viral load increase (ΔCt ≥ 5 units).
Results
A total of 58 and 27 COVID-19 patients taking NMV/r and molnupiravir, respectively, were enrolled. Patients receiving NMV/r were younger, had fewer risk factors for disease progression and faster viral clearance rate compared to those receiving molnupiravr (All P < 0.05). The overall proportion of viral rebound (n = 11) was 12.9%, which was more common among patients receiving NMV/r (10 [17.2%] vs. 1 [3.7%], P = 0.16). Of them, 5 patients experienced symptomatic rebound, suggesting the proportion of COVID-19 rebound was 5.9%. The median interval to viral rebound was 5.0 (interquartile range, 2.0–8.0) days after completion of antivirals. Initial lymphopenia (<0.8 × 109/L) was associated with viral rebound among overall population (adjusted odds ratio [aOR], 5.34; 95% confidence interval [CI], 1.33–21.71), and remained significant (aOR, 4.50; 95% CI, 1.05–19.25) even when patients receiving NMV/r were considered.
Conclusion
Our data suggest viral rebound after oral antivirals may be more commonly observed among lymphopenic individuals in the context of SARS-CoV-2 Omicron BA.2 variant.
Keywords: COVID-19 rebound, SARS-CoV-2, Omicron, Viral kinetics, Whole genome sequencing
Introduction
Oral nirmatrelvir-ritonavir (NMV/r) and molnupiravir disrupt the SARS-CoV-2 lifecycle and have been shown to be efficacious in preventing COVID-19 progression among at-risk individuals in clinical trials.1 , 2 Since December 2021, these oral antivirals have been used under emergency use authorization (EUA) globally.3, 4, 5 The occurrence of viral rebound, a given increase after initial decline of viral load, after patients took NMV/r was low (2.3%) in a phase 2–3, double-blind, randomized, controlled trial (EPIC-HR).6 As real-world data of oral antivirals accumulate, COVID-19 rebound (also named as rebound of SARS-CoV-2 infection or COVID-19 recrudescence) after treatment with NMV/r has been reported widely.7, 8, 9, 10, 11, 12
COVID-19 rebound is a unique phenomenon described as a relapse of symptoms after transient resolutions (symptomatic rebound) with concurrence of viral rebound, commonly reported as a negative-to-positive conversion by rapid antigen testing. But little is known viral kinetics determined by reverse-transcriptase-polymerase chain reaction (RT-PCR) testing regardless of whether symptomatic rebound was present.7, 8, 9, 10, 11 Also, COVID-19 rebound among patients taking molnupiravir were less characterized.13 , 14
To date, most investigations of COVID-19 rebound are triggered by symptomatic rebound, followed by confirmation of viral rebound. Given the observations that rebound symptoms were usually milder than initial symptoms, misclassification bias in symptom-driven design7, 8, 9, 10, 11, 12 or databank-analyses13 , 15 may underestimate the true prevalence of COVID-19 rebound. That is, if the investigation of COVID-19 rebound is initially triggered by the presence of viral rebound, followed by confirmation of symptomatic rebound, the prevalence may differ. Additionally, using symptomatic rebound to equivocate COVID-19 rebound after oral antivirals may misconstrue a clinical biphasic pattern16 , 17 or worsening of symptoms due to the inflammatory phase of COVID-19 or long COVID, if not correlated with viral kinetics.
Herein, we aimed to utilize sequential reverse-transcriptase-polymerase chain reaction (RT-PCR) testing to investigate the frequency of, and factors associated with viral rebound among mild-moderate COVID-19 patients receiving oral antivirals. We also sought to define whether viral rebound is associated with replication-competent virus or mutated virus and whether viral rebound is a reliable predictor of COVID-19 rebound.
Methods
Study setting
The government of Taiwan initially mandated all COVID-19 patients to be hospitalized and released from isolation later only after meeting a defined clinical and RT-PCR criteria during the second wave of SARS-CoV-2 epidemic caused by the Omicron BA.2 variant in mid-April, 2022.18 Specific treatment recommendations were adjusted to include the appropriate use of oral antiviral agents for patients with mild-moderate COVID-19 but risk factors for progression. Serial RT-PCR testing was performed to decide whether an isolated patient could be de-isolated.
This retrospective study was conducted at National Taiwan University Hospital (NTUH) between April 1st and May 25th, 2022. The principles for admission and release of COVID-19 patients were according to the regulation, which was periodically updated, by the Central Epidemic Command Center, Taiwan (Table S1).19 Under EUA in Taiwan, NMV/r was indicated for mild COVID-19 patients aged ≥12 years, and molnupiravir for those aged ≥18 years without available alternative treatments for COVID-19.3
In this study, we included only patients who completed oral antiviral treatments for their first episodes of COVID-19, with at least three nasopharyngeal specimens sequentially collected every 2–3 days for RT-PCR assays, for the purpose of tracking and documenting viral kinetics. The study was approved by the Research Ethics Committee of the NTUH (202206079RIND).
Clinical definitions
For each enrolled patient, index RT-PCR testing was defined as the 1st positive RT-PCR test. We obtained patient information from the electronic medical records. Daily symptoms were independently reviewed by two physicians (P-YC and J-TW). A symptom severity score was used to evaluate the symptom severity. It consisted of fever, headache/myalgia, malaise, cough/sputum, rhinorrhea/nasal congestion, sore throat/hoarseness, dyspnea, diarrhea, and dysosmia. Each symptom was rated on a scale of 0–2, indicating no symptoms, mild/improving, and persistent/deteriorating, respectively. All initial laboratory data within 1 day of index RT-PCR testing were recorded. Lymphopenia was defined as an absolute lymphocyte count (ALC) of ≤0.8 × 109/L.20 Symptomatic rebound was defined by an abrupt increase of symptom severity score after initial decrease.
Cycle threshold (Ct) values were determined by dual targeting regions, either RdRp/N-genes (Abbot ALINITY m SARS-COV-2 ASSAY) or the ORF 1a/b/E-genes (Roche the cobas® SARS-CoV-2 Test). Viral rebound was defined by an increase of viral load after an initial decrease of viral load with a ΔCt ≥ 5 units (ΔCt = Ct1-Ct2; Ct1, the maximum Ct value before the decline, except that of index RT-PCR; Ct2, the first Ct value lower than Ct1 ≥ 5 units during the decline). Viral clearance rate was defined as the difference between the value of Cthighest and that of Ctlowest divided by the interval between the detection date of Cthighest and that of Ctlowest (Cthighest, the highest Ct value before rebound among patients with viral rebound and that before end of follow-up [EOF] among those without viral rebound; Ctlowest, the lowest Ct value within 5 days of index RT-PCR).
EOF was defined as discharged from the hospital or up to28 days after index RT-PCR, whichever occurred first. Clinical outcomes were assessed daily by an 8-point pulmonary ordinal scale till EOF. Clinical improvement was defined as substantial decline of a pulmonary ordinal score ≥2 at EOF.21
SARS-CoV-2 viral cultures
Nasal swab specimens maintained in the viral-transport media were collected from the patients with COVID-19 and prospectively preserved at the Department of Laboratory Medicine, National Taiwan University Hospital. The clinical specimens at last date prior to viral rebound and at the viral rebound date for the first four patients with viral rebound after receiving oral antiviral agents were selected for viral cultures as previously prescribed.22 Briefly, the specimens were propagated in VeroE6 cells in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 g/L of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin (Sigma–Aldrich). Culture supernatants were harvested when more than 70% of cells showing cytopathic effects.
SARS-CoV-2 whole genome sequencing
Total nucleic acid was extracted from the supernatants of preserved media with the QIAamp viral RNA mini kit (QIAGEN, USA) according to the manufacturer's instructions. Samples were treated with Illumina COVIDSeq RUO Kits (cDNA was amplified using the ARTIC v4 Primer Pools) according to the manufacturer's instructions and sequenced with Illumina NovaSeq. A total of 1 Gb of de-multiplexed reads were generated from each sample. All de-multiplexed reads were trimmed off 15bp at their 5′ and 3′ terminal. Then, trimmed reads were mapped to the reference SARS-CoV-2 genome (MN908947.3) and consensus sequences were called by using a custom pipeline. This pipeline utilized BWA-mem23 to map reads onto the reference genome, and variants were called by bcftools mpileup (previously known as Samtools mpileup).24 Any SNP with a quality score >20 and IMF >0.51 was kept by a custom python script. Consensus sequences were finally called with bcftools consensus.
Statistical analysis
Continuous and categorical variables were compared by using Mann–Whitney U test and Fisher's exact test, respectively. ALC at baseline and at rebound/EOF in each patient were compared by Wilcoxon signed-rank test. Logistic regression analyses were performed to analyze the risk factors for viral rebound among patients receiving oral antiviral agents. All parameters were initially tested by univariable analysis and those with a P value < 0.15 were used for multivariate analysis. Parameters with collinearity were not simultaneously considered in the final model. Stepwise model comparison and Akaike's information criterion were used to determine the best model of multiple variables analysis. The sensitivity analyses were performed in NMV/r group and in viral rebound defined as a greater degree of viral load increase (ΔCt ≥ 12.4)10, respectively. For sensitivity analyses; only univariable logistic regression analysis were performed due to relatively small numbers of viral rebound and total patients. The analyses were performed using Stata software (version 14; StataCorp). Two-sided P values < 0.05 were considered significant.
Results
Clinical and virological comparisons between patients receiving NMV/r and molnupiravir
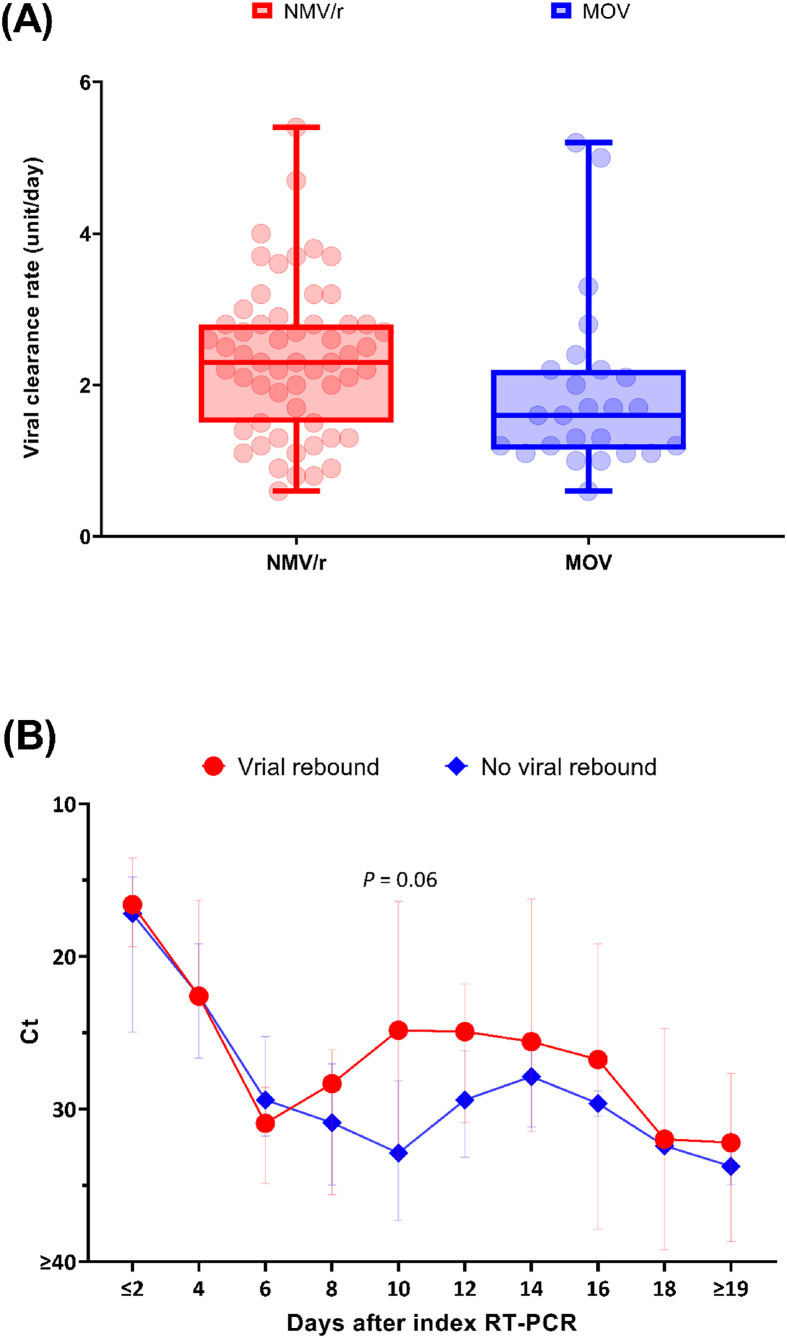
Among 261 patients receiving oral antivirals during the study period, 85 patients with sequential collection of nasopharyngeal swabs for RT-PCR assays and no antiviral switch were included (Fig. S1). Fifty-eight (68.2%) of them received NMV/r. Patients receiving NMV/r were younger, had better renal function, fewer risk factors for disease progression, and lower levels of inflammatory markers compared to those receiving molnupiravir (Table 1 ). The initial pulmonary ordinal scores, the median Ctlowest values within 5 days after index RT-PCR testing and time-to-initiation of antivirals from index date were not significantly different between two groups. The median viral clearance rate (interquartile, IQR) in NMV/r group was significantly faster than that in molnupiravir group (2.3 [1.6–2.8] vs. 1.6 [1.2–2.2] units per day) (Fig. 1 A).
Table 1.
Comparisons of clinical and virological characteristics, and outcomes among COVID-19 patients receiving nirmatrelvir/ritonavir and molnupiravir.
| Total (n = 85) | Nirmatrelvir/ritonavir (n = 58) | Molnupiravir (n = 27) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 58.5 (38.0–78.0) | 49.0 (34.0–72.0) | 73.3 (55.0–84.0) | 0.002 |
| Gender, male | 47 (55.3) | 32 (55.2) | 15 (55.6) | >0.99 |
| Vaccine status | 0.24 | |||
| No vaccinations | 37 (43.5) | 25 (43.1) | 12 (44.4) | |
| 1 dose | 14 (16.5) | 7 (12.1) | 7 (25.9) | |
| 2 doses | 8 (9.4) | 5 (8.6) | 3 (11.1) | |
| 3 doses | 26 (30.6) | 21 (36.2) | 5 (18.2) | |
| Comorbidities | ||||
| Diabetes mellitus | 19 (22.4) | 7 (12.1) | 12 (44.4) | 0.002 |
| Cardiovascular disorder | 18 (21.2) | 6 (10.3) | 12 (44.4) | 0.001 |
| Chronic lung disease | 3 (3.5) | 2 (3.5) | 1 (3.7) | >0.99 |
| Chronic kidney disease | 13 (15.3) | 1 (1.7) | 12 (44.4) | <0.001 |
| Chronic liver diseases | 6 (7.1) | 3 (5.2) | 3 (11.1) | 0.38 |
| Neurodevelopmental disorder | 0 (0) | 0 (0) | 0 (0) | >0.99 |
| Psychiatric disorder | 1 (1.2) | 0 (0) | 1 (3.7) | 0.32 |
| Dementia | 6 (7.1) | 1 (1.7) | 5 (18.5) | 0.01 |
| Active tuberculosis | 1 (1.2) | 0 (0) | 1 (3.7) | 0.32 |
| Obesity, BMI≥25 | 37 (43.5) | 23 (39.7) | 14 (51.9) | 0.35 |
| Cigarette smoker | 10 (11.8) | 8 (13.8) | 2 (7.4) | 0.49 |
| Immunosuppression | 17 (20.0) | 12 (20.7) | 5 (18.5) | >0.99 |
| Active cancer | 25 (29.4) | 17 (29.3) | 8 (29.6) | >0.99 |
| Numbers of risk factors | 2 (1-3) | 1.5 (1-3) | 4 (1-5) | <0.001 |
| Pulmonary ordinal scale at baseline | 3 (3-3) | 3 (3-3) | 3 (3-3) | >0.99 |
| Initial laboratory data | ||||
| White blood cell count ( × 109/L) | 6.8 (5.2–9.1) | 6.8 (4.9–9.0) | 6.7 (5.4–9.6) | 0.62 |
| Lymphocyte count ( × 109/L) | 1.1 (0.7–1.5) | 1.1 (0.7–1.6) | 1.1 (0.7–1.3) | 0.82 |
| Lymphopenia (<0.8 × 109/L) | 24 (31.2) | 16 (32.0) | 8 (29.6) | >0.99 |
| Neutrophil count ( × 109/L) | 4.8 (3.3–7.4) | 4.9 (3.2–6.9) | 4.7 (3.4–8.1) | 0.58 |
| Hemoglobin (g/dL) | 12.5 (10.4–13.7) | 12.8 (11.5–14.3) | 12.1 (10.1–13.3) | 0.09 |
| Platelet count ( × 109/L) | 218 (152–276) | 239 (165.5–280) | 200 (136–266) | 0.15 |
| C-reactive protein (mg/dL) | 1.6 (0.7–4.2) | 1.1 (0.5–3.4) | 2.1 (1.1–5.5) | 0.06 |
| Ferritin (ng/mL) | 299.9 (161.8–529.1) | 256.5 (121.4–421.9) | 391.1 (211.8–988.0) | 0.02 |
| Procalcitonin (ng/mL) | 0.10 (0.06–0.21) | 0.08 (0.05–0.15) | 0.15 (0.09–0.58) | 0.02 |
| D-dimer (mg/L) | 0.7 (0.3–1.8) | 0.4 (0.2–1.1) | 1.8 (0.7–5.2) | <0.001 |
| Albumin (g/dL) | 4.1 (3.6–4.6) | 4.3 (3.9–4.7) | 3.7 (3.6–4.0) | 0.02 |
| Total bilirubin (mg/dL) | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.6 (0.4–0.7) | 0.87 |
| AST (U/L) | 25.5 (18.5–35.5) | 22.5 (17.0–35.0) | 28.0 (21.0–37.0) | 0.33 |
| ALT (U/L) | 19.0 (12.0–29.0) | 20.0 (14.0–37.0) | 14.0 (12.0–23.0) | 0.11 |
| Lactate dehydrogenase (U/L) | 203.0 (170.0–247.5) | 203.0 (168.5–245.5) | 206.0 (176.5–247.5) | 0.46 |
| Creatine kinase (U/L) | 89 (36–164) | 86.5 (38.5–162.5) | 96 (35–187) | 0.61 |
| BUN (mg/dL) | 15.9 (11.1–23.8) | 13.6 (10.8–18.1) | 28.8 (16.6–41.4) | <0.001 |
| Creatinine (mg/dL) | 0.9 (0.7–1.2) | 0.9 (0.7–1.0) | 1.6 (0.7–3.1) | 0.02 |
| Virological parameter | ||||
| Ct value of index RT-PCR test | 16.6 (14.0–23.6) | 17.0 (14.1–23.9) | 15.6 (13.6–23.6) | 0.36 |
| Ctlowest value within 5 days of index RT-PCR testa | 16.2 (14.0–19.1) | 16.4 (14.0–19.7) | 15.6 (13.6–18.3) | 0.32 |
| Numbers of RT-PCR test | 3 (3-4) | 3 (3-4) | 3 (3-4) | >0.99 |
| Intervals between index and last RT-PCR test, days | 9 (8-12) | 8 (7-12) | 9 (8-12) | 0.44 |
| Time-to-initiation antiviral, days | ||||
| From index date | 1 (0–1) | 1 (0–2) | 1 (0–1) | 0.24 |
| From symptom onset | 2 (1-3) | 2 (1-3) | 1 (1-2) | 0.03 |
| Outcomes | ||||
| Viral rebound | 11 (12.9) | 10 (17.2) | 1 (3.7) | 0.16 |
| Time to viral rebound after index RT-PCR test, days | 10.0 (8.0–12.0) | 9.5 (8.0–11.0) | 16.0 | 0.15 |
| Time to viral rebound after completion of antivirals, days | 5.0 (2.0–8.0) | 4.5 (2.0–6.0) | 11.0 | 0.11 |
| Clinical improvementb | 73 (85.6) | 51 (87.9) | 22 (81.5) | 0.51 |
| Follow-up duration, days | 11 (9-16) | 10 (9-15) | 12 (10-22) | 0.04 |
Continuous variables, median (interquartile); categoric variables, n (%).
Abbreviation: BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; PCR, polymerase chain reaction.
Ctlowest, the lowest Ct value within 5 days of index PCR among patients with and without viral rebound.
Clinical improvement was defined as substantial decline of an ordinal score ≥2 at end of follow-up.
Figure 1.
Comparisons of viral kinetics between patients receiving nirmatrelvir/ritonavir and molnupiravir (A) and between patients with and without viral rebound (B). Panel A shows viral clearance rate, defined as the ratio of the difference between the value of Cthighest and that of Ctlowest to the interval between the detection date of Cthighest and that of Ctlowest. Of them, Cthighest is the highest Ct value before rebound among patients with viral rebound and that during follow-up among those without viral rebound. Ctlowest is the lowest Ct value within 5 days of index PCR test among patients with and without viral rebound. A boxplot displays median and interquartile range, and whiskers display minimum and maximum Ct values. Ct values above the upper limit of detection, 40, were assigned a value of ≥40. Patients with reverse viral decay kinetics were not included (1 in NMV/r group and 2 in MOV group). Panel B shows sequential median Ct values in patients with and without viral rebound by circles and diamonds, respectively. The difference of median Ct value between patients with and without viral rebound was approaching significantly on Day 10 after index RT-PCR. The I bars represent interquartile ranges. NMV/r, nirmatrelvir/ritonavir; MOV, molnupiravir.
Clinical and virological characteristics of viral rebound
As shown in Table 1, the proportions of clinical improvement were 87.9% and 81.5% of patients receiving NMV/r and molnupiravir, respectively. There were only one respiratory failure (patient P85) and no death. The overall proportion of viral rebound was 12.9%, with a numerically greater one among patients receiving NMV/r than those receiving molnupiravir (10 [17.2%] vs. 1 [3.7%], P = 0.16). The median time to viral rebound was 10.0 (8.0–12.0) days after the index RT-PCR testing and 5.0 (2.0–8.0) days after completion of antivirals (Fig. 1B); modest increases in viral load were common even among patients not meeting the criteria for viral rebound. Individual viral kinetics among patients with viral rebound were shown in Fig. S2.
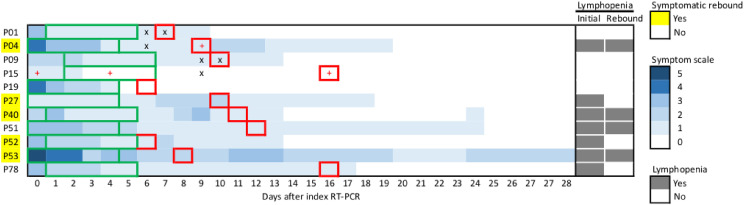
Among patients with viral rebound, the initial total symptom score was ≤5 on a scale of 0–18. During viral rebound, five of eleven patients (45.5%) experienced symptomatic rebound (Fig. 2 ). The major rebound symptom was cough/sputum (n = 3). Among patients without viral rebound, none developed symptomatic rebound, but one had disease progression to respiratory failure (P85) (Fig. S3). These findings suggested that the proportion of COVID-19 rebound was 5.9% (5/85). Among patients with viral rebound, patients with symptomatic rebound were more likely to have initial lymphopenia and earlier viral rebound, but not associated with the magnitude of viral rebound, viral clearance rates or existence of replication-competent virus at rebound (Fig. 2 & Table S2). The Ct values of swabs with detected replication-competent virus at rebound was lower than those without detection of replication competent virus (Ct values: 14.3 [P04] and 16.2 [P15] vs. 26.4 [P01] and 23.0 [P09]). WGS data derived from four sequential clinical specimens in a single patient with viral rebound (P15) demonstrated no additional mutations at 3C-like proteinase coding gene conferring NMV/r resistance (Table S3).
Figure 2.
The symptom heatmaps of 11 COVID-19 patients experiencing viral rebound. The y axis represents each patient numbers, and the x axis represents days after index PCR. A symptom score, consisting of fever, headache/myalgia, malaise, cough/sputum, rhinorrhea/nasal congestion, sore throat/hoarseness, dyspnea, diarrhea, and dysosmia, was used for daily symptom evaluation among them. A score of each symptom is assigned as a value of 0–2, indicating asymptomatic, improving or mild, and progressive or persistent, respectively. A highest daily symptom score is 18. Each green bar illustrates the treatment course of each patient receiving oral antivirals. Each red square illustrates the date of viral rebound. Paired viral cultures at the date of viral rebound and at the most closely date prior to viral rebound were performed in 4 patients (P01, P04, P09, and P15). Additional viral cultures at different dates were performed in patient P15. A symbol of plus indicates replication-competent virus, and a symbol of cross indicates unculturable virus.
Factors associated with viral rebound
Patients with viral rebound were more likely to have active cancer (63.6% vs. 24.3%) and initial lymphopenia (63.6% vs. 25.8%) compared to those without viral rebound (Table 2 ). The proportion of remaining lymphopenia among those at viral rebound was greater than that of those without viral rebound at EOF (40.0% vs. 12.7%). However, the ALCs of most patients at viral rebound had increased significantly compared to initial counts (Fig. S4). In multivariable logistic regression analysis, initial lymphopenia was the only factor associated with viral rebound (adjusted odds ratio, 5.34; 95% CI, 1.33–21.71), but not active cancer (P for interaction, 0.16).
Table 2.
Factors associated with viral rebound among COVID-19 patients receiving nirmatrelvir/ritonavir and molnupiravir.
| With viral rebound (n = 11) | Without viral rebound (n = 74) | Univariable |
Multivariabled |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |||
| Demographics | ||||||
| Age, years | 59.8 (38.3–77.0) | 57.4 (37.9–78.6) | 1.00 (0.97–1.03) | 0.99 | ||
| Gender, male | 9 (81.8) | 38 (51.4) | 4.26 (0.86–21.08) | 0.08 | ||
| Vaccine history | ||||||
| No vaccinations | 7 (63.6) | 30 (40.5) | 2.57 (0.69–9.54) | 0.16 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 2 (18.2) | 17 (23.0) | 0.75 (0.15–3.78) | 0.72 | ||
| Cardiovascular disorder | 1 (9.1) | 17 (23.0) | 0.34 (0.04–2.81) | 0.31 | ||
| Chronic lung disease | 0 (0) | 3 (4.1) | NA | NA | ||
| Chronic kidney disease | 2 (18.2) | 11 (14.9) | 1.27 (0.24–6.70) | 0.78 | ||
| Chronic liver diseases | 0 (0) | 6 (8.1) | NA | NA | ||
| Neurodevelopmental disorder | 0 (0) | 0 (0) | NA | NA | ||
| Psychiatric disorder | 0 (0) | 1 (1.4) | NA | >0.99 | ||
| Dementia | 1 (9.1) | 5 (6.8) | 1.38 (0.15–13.06) | NA | ||
| Active tuberculosis | 0 (0) | 1 (1.4) | NA | NA | ||
| Obesity, BMI≥25 | 5 (45.5) | 32 (43.2) | 1.09 (0.31–3.91) | 0.89 | ||
| Cigarette smoker | 3 (27.3) | 7 (9.5) | 3.59 (0.77–16.72) | 0.10 | ||
| Immunosuppression | 4 (36.4) | 13 (17.6) | 2.68 (0.68–10.52) | 0.16 | ||
| Active cancer | 7 (63.6) | 18 (24.3) | 5.44 (1.43–20.76) | 0.01 | ||
| Numbers of risk factors | 2 (2-3) | 2 (1-3) | 1.14 (0.80–1.63) | 0.45 | ||
| Initial laboratory data | ||||||
| White blood cell count ( × 109/L) | 6.7 (3.3–7.4) | 6.8 (5.2–9.3) | 1.00 (1.00–1.00) | 0.09 | ||
| Lymphopenia (<0.8 × 109/L)a | 7/11 (63.6) | 17/66 (25.8) | 5.04 (1.31–19.39) | 0.02 | 5.34 (1.33–21.71) | 0.02 |
| Remaining lymphopeniab | 4 (40.0) | 9 (12.7) | 4.59 (1.08–19.49) | 0.04 | ||
| Hemoglobin (g/dL) | 13.3 (8.5–14.7) | 12.5 (10.4–13.6) | 0.91 (0.70–1.18) | 0.46 | ||
| Thrombocytopenia (<100 × 109/L)a | 3/11 (27.3) | 3/72 (4.2) | 8.63 (1.48–50.12) | 0.02 | ||
| C-reactive protein >1.0 mg/dLa | 4/8 (50.0) | 40/63 (63.5) | 0.57 (0.13–2.52) | 0.46 | ||
| Ferritin >300 ng/mLa | 5/9 (55.6) | 32/66 (48.5) | 1.33 (0.33–5.39) | 0.69 | ||
| Procalcitonin (ng/mL) | 0.11 (0.06–0.16) | 0.10 (0.06–0.28) | 0.19 (0.00–18.98) | 0.48 | ||
| D-dimer >0.5 mg/La | 3/7 (42.9) | 30/52 (57.7) | 0.55 (0.11–2.71) | 0.46 | ||
| Albumin (g/dL) | 4.5 (3.7–4.5) | 4.0 (3.6–4.6) | 1.33 (0.48–3.67) | 0.58 | ||
| Total bilirubin (mg/dL) | 0.58 (0.38–0.82) | 0.57 (0.45–0.74) | 1.05 (0.88–1.25) | 0.58 | ||
| AST (U/L) | 30 (21–66) | 24 (18–35) | 1.00 (0.99–1.01) | 0.50 | ||
| ALT (U/L) | 37 (19–50) | 17 (12-27) | 1.00 (1.00–1.01) | 0.91 | ||
| Lactate dehydrogenase >245 U/La | 2/7 (28.6) | 15/57 (26.3) | 1.12 (0.20–6.40) | 0.90 | ||
| Creatine kinase >185 U/La | 1/7 (14.3) | 13/58(22.4) | 0.58 (0.06–5.23) | 0.63 | ||
| BUN (mg/dL) | 16.3 (10.5–19.8) | 15.9 (11.4–25.8) | 0.97 (0.91–1.03) | 0.33 | ||
| Creatinine (mg/dL) | 1.0 (0.8–1.2) | 0.9 (0.7–1.1) | 0.76 (0.36–1.58) | 0.46 | ||
| Virological parameter | ||||||
| Ctlowest value within 5 days of index RT-PCR testc | 16.3 (13.1–17.0) | 16.2 (14.0–19.7) | 0.93 (0.81–1.09) | 0.39 | ||
| Viral clearance ratec | 2.6 (1.9–3.7) | 2.2 (1.3–2.8) | 1.35 (0.79–2.31) | 0.27 | ||
| Therapeutic parameter | ||||||
| Time-to-initiation of antiviral (days), from index date | 1 (0–1) | 1 (0–1) | 0.77 (0.39–1.51) | 0.44 | ||
| Time-to-initiation of antiviral (days), from symptom onset | 2 (1-2) | 2 (1-3) | 0.91 (0.57–1.43) | 0.67 | ||
| Nirmatrelvir/ritonavir | 10 (90.9) | 48 (64.9) | 5.42 (0.66–44.69) | 0.12 | 7.05 (0.81–61.18) | 0.08 |
Continuous variables, median (interquartile); categoric variables, n (%).
Abbreviation: BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; PCR, polymerase chain reaction; OR, odd ratios; CI, confidence interval; aOR, adjusted odds ratio; NA, not applicable.
Denominators represented the numbers of patients receiving a specific laboratory test.
Remaining lymphopenia: lymphopenia remained at viral rebound among patients with viral rebound and at end of follow-up among those without viral rebound.
Ctlowest is the lowest Ct value within 5 days of index PCR among patients with and without viral rebound. Cthighest is the highest Ct value before rebound among patients with viral rebound and that during follow-up among those without viral rebound. Viral clearance rate is defined as the ratio of the difference between the value of Cthighest and that of Ctlowest to the interval between the detection date of Cthighest and that of Ctlowest.
Goodness-of-fit: Pearson test, P = 0.46 > 0.05; Hosmer–Lemeshow test, P = 0.76 > 0.05.
By sensitivity analyses, association between initial lymphopenia and viral rebound remained significant among patients receiving NMV/r (4.50 [1.05–19.25]; P = 0.04). If the viral rebound was defined as higher degrees of ΔCt (≥12.4), 10 the proportion of patients experiencing viral rebound was only 4.7% (n = 4), and all of them received NMV/r. Under such definition, initial lymphopenia was associated with viral rebound (ΔCt ≥ 12.4) with a borderline significance (7.43 [0.73–75.53]; 4.50 [1.05–19.25]; P = 0.09).
Discussion
The prevalence of COVID-19 rebound previously ranged from 0.4% to 5.9% among outpatient patients receiving NMV/r or molnupiravir.6 , 13, 14, 15 , 25 Previous studies utilized symptomatic rebound as a trigger event to investigate the presence of COVID-19 rebound. But rebound symptoms are usually milder than initial symptoms and multifactorial.7, 8, 9, 10, 11, 12 Therefore, previous observations suggest viral rebound may be a reasonable alternative trigger to investigate COVID-19 rebound.7, 8, 9, 10, 11, 12 Hence, we delineate viral kinetics by utilizing sequential RT-PCR methods among patients receiving oral antivirals, alongside careful daily documentation of evolving clinical symptoms and laboratory parameters to underpin COVID-19 rebound. In our cohort, the proportion of viral rebound and COVID-19 rebound was 12.9% and 5.9%, respectively.
By the cut-off values (ΔCt ≥ 5) for viral rebound triggering investigation of COVID-19 rebound, several findings in the present study were consistent with previous results. 6, 7, 8, 9, 10, 11, 12 First, the interval to viral rebound was 5.0 (2.0–8.0) days after completion of antivirals; this corresponded to the typical COVID-19 rebound trajectory reported. Second, modest increases, if any, in symptom severity scores, among our patients experiencing viral rebounds attest to the milder rebound symptoms as previously reported. Third, at time of viral rebound, presence of replication-competent viruses was noted in some patients. Fourth, we confirm the achievement of viral clearance without additional treatment courses of antivirals in at least 63.6% of patients (7/11) with viral rebound (Fig. S2). Collectively, our data support viral rebound might be a reasonable trigger event for investigating COVID-19 rebound.
However, the optimal cut-offs (ΔCt) for viral rebound have yet to be defined. By defining viral rebound as negative-to-positive conversion of RT-PCR testing results, recent two studies found extremely low rates of viral rebound (≤1%) after oral antivirals.14 , 26 But both studies had their own caveats. One of these two studies found the interval to viral rebound from symptom onset was about 30 days, much longer than those in previous reports.6, 7, 8, 9, 10, 11, 12 And no symptomatic rebound was noted during viral rebound in that report.26 The other one from Hong Kong enrolled unusually high proportions of patients (around 50%) concomitantly receiving oral antivirals and corticosteroids for COVID-19 in their early disease course.14 Current treatment guidelines all recommend against corticosteroids use in the early phase of COVID-19.3, 4, 5, 14 Hence, the results from HK were incomparable to the current and other previous studies.6, 7, 8, 9, 10, 11, 12 Further, some studies has found concurrent symptomatic and viral rebound after NMV/r may develop even before a RT-PCR Ct value or a rapid antigen test turned to be negative.6 , 10 Therefore, the aforementioned findings suggest that negative-to-positive conversion of RT-PCR testing results may underestimate the prevalence of viral rebound and COVID-19 rebound after oral antivirals.
As shown in Fig. 1B, the decrease of Ct value among patients between Day 10 and 14 after the date of index PCR who did not meet the criteria of viral rebound by the present study might be due to mild fluctuation of viral load during the disease course (indeed, the difference between median Ct value on Day 10 and 14 [31.8 and 27.9, respectively] was less than 5).27 , 28 Or it might just reflect that patients with higher Ct values on Day 10 were discharged accordingly, and those with lower Ct values remained hospitalized throughout Day 14 and thus had opportunity to receive repeatedly RT-PCR testing, which therefore resulted in a relative lower pooled median Ct value on Day 14.
Furthermore, we found viral rebound was associated with initial lymphopenia. Lymphopenia at diagnosis may reflect preexisting immunocompromised status or an unfavorable clinical consequence of infected patients with low antibody titers.29 Our findings highlight early initiation of oral antivirals prevented lymphopenic patients from developing respiratory failures and deaths, but did not deter viral rebound.20 In light of previous report that rising antibody levels against SARS-CoV-2 are associated with remission of COVID-19 rebound7 and that a cytotoxic CD8+ T cell response is correlated with effective viral clearance,30 lymphopenic patients may take longer time to clear a vaccine-mismatched virus completely after rapid viral suppression by oral antivirals. Subsequently, these patients are more likely to experience viral rebound.
Direct comparison of viral rebound rates between NMV/r and molnupiravir are complex owing to different treatment indications and viral clearance rates. We found the net effect of slow viral eradication causing prolonged viral exposure among aging and comorbid patients receiving molnupiravir might translate towards less viral rebound. But based on the small number of events in our molnupiravir cohort we hesitate to draw any firm conclusions.
The strengths of the current study were as follows. First, only hospitalized patients were enrolled, so the confounding effect of different health care-seeking behavior and adherence to antivirals was minimized. Moreover, reinfection was excluded in the context of hospital-wide active surveillance of inpatient-healthcare personnel transmission in our hospital.31 Our WGS data further consolidated that NMV/r resistance was not correlated with COVID-19 rebound. 8-10
Our retrospective study had inevitable flaws. By using viral kinetics, we avoided record bias of symptom evolution, but by excluding patients who had received less than three consecutive RT-PCR tests (for example: patients with milder disease and those who didn't require hospitalization), we may have generated selection bias. Our population is relatively unique, and our results may not be generalizable. The fact that our patients had more underlying comorbidities and lower vaccination rates compared to previous reports15 , 25 suggests that rates of viral rebound depend on the adequacy and speed of the immune response generated. Although no comparisons of immunological profiles between patients with and without viral rebound in the current study, our finding that initial lymphopenia, even at rebound, was a predictor for viral rebound provides a clue for further in-depth characterization of both B cell and T cell response in patients receiving oral antivirals, especially focusing on SARS-CoV-2-targeted antibody Fc-receptor functions to facilitate viral clearance.32
In conclusion, this proof-of-concept study characterizes the phenomenon of viral rebound after antivirals which complements our current understanding of COVID-19 rebound. We further found initial lymphopenia account, in part, for viral rebound after a standard course of oral antivirals. Further investigations to ascertain the underlying mechanisms of COVID-19 rebound are needed to resolve unmet needs.
Funding
This study has been funded by the Ministry of Science and Technology, Taiwan (111-2321-B-002-017; 111-2740-B-002-006), the Ministry of Health and Welfare (MOHW111-TDU-B-211-134002), and the National Taiwan University Hospital (NTUH.MM022-1).
Data availability
The sequencing data from four sequential clinical specimens of P15 used in the current study have been deposited in GISAID (https://www.gisaid.org/CoV2020/). The accession codes and the mutations in these viral isolates are summarized in Supplementary Table S3.
Author contributions
P-YC, conceptualization, data collection and analysis, visualization and writing original draft; J-TW, conceptualization, funding acquisition, data collection and analysis, writing original draft; S-YC, conceptualization, funding acquisition, virological experiments, writing original draft; S-CC, conceptualization, resources, and critically reviewing; editing and proofreading. All authors reviewed, revised and approved the final version of the manuscript.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
The authors would like to thank Dr. Shiou-Hwei Yeh for assistance with sequencing, and the services provided by the Biosafety Level-3 Laboratory of the First Core Laboratory from National Taiwan University College of Medicine; the Biosafety Level- 3 Laboratory from National Taiwan University Hospital.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfma.2023.02.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines for clinical management of SARS-CoV-2 infection, Taiwan centers for disease control [Tranditinal Chinese version]. Available at https://www.cdc.gov.tw/Category/Page/xCSwc5oznwcqunujPc-qmQ [Accessed January 31, 2023].
- 4.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. [Accessed December 8, 2022]. [PubMed]
- 5.Update to living WHO guideline on drugs for covid-19. BMJ. 2022;377:o1005. doi: 10.1136/bmj.o1005. [DOI] [PubMed] [Google Scholar]
- 6.Anderson A.S., Caubel P., Rusnak J.M., Investigators E.-H.T. Nirmatrelvir-ritonavir and viral load rebound in covid-19. N Engl J Med. 2022;387(11):1047–1049. doi: 10.1056/NEJMc2205944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelli G., Focosi D., Turriziani O., Tuccori M., Brandi R., Fillo S., et al. Virological and clinical rebounds of COVID-19 soon after nirmatrelvir/ritonavir discontinuation. Clin Microbiol Infect. 2022;28(12):1657–1658. doi: 10.1016/j.cmi.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucau J., Uddin R., Marino C., Regan J., Flynn J.P., Choudhary M.C., et al. Characterization of virologic rebound following nirmatrelvir-ritonavir treatment for COVID-19. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlin A.F., Clark A.E., Chaillon A., Garretson A.F., Bray W., Porrachia M., et al. Virologic and immunologic characterization of COVID-19 recrudescence after nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charness M.E., Gupta K., Stack G., Strymish J., Adams E., Lindy D.C., et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med. 2022;387(11):1045–1047. doi: 10.1056/NEJMc2206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulson J.M., Adams A., Gray L.A., Evans A. COVID-19 "Rebound" associated with nirmatrelvir/ritonavir pre-hospital therapy. J Infect. 2022;85(4):436–480. doi: 10.1016/j.jinf.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epling B.P., Rocco J.M., Boswell K.L., Laidlaw E., Galindo F., Kellogg A., et al. Clinical, virologic, and immunologic evaluation of symptomatic coronavirus disease 2019 rebound following nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Berger N.A., Davis P.B., Kaelber D.C., Volkow N.D., Xu R. COVID-19 rebound after paxlovid and molnupiravir during January-June 2022. medRxiv. 2022 doi: 10.1101/2022.06.21.22276724. [DOI] [Google Scholar]
- 14.Wong G.L., Yip T.C., Lai M.S., Wong V.W., Hui D.S., Lui G.C. Incidence of viral rebound after treatment with nirmatrelvir-ritonavir and molnupiravir. JAMA Netw Open. 2022;5(12) doi: 10.1001/jamanetworkopen.2022.45086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malden D.E., Hong V., Lewin B.J., Ackerson B.K., Lipsitch M., Lewnard J.A., et al. Hospitalization and emergency department encounters for COVID-19 after paxlovid treatment - California. MMWR Morb Mortal Wkly Rep. 2022;71(25):830–833. doi: 10.15585/mmwr.mm7125e2. December 2021-May 2022. [DOI] [PubMed] [Google Scholar]
- 16.CDC Health Advisory: COVID-19 rebound after Paxlovid treatment. Atlanta, GA: US Department of Health and Human Services, CDC. Available at https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf. [Accessed July 20, 2022].
- 17.Smith D.M., Li J.Z., Moser C., Yeh E., Currier J.S., Chew K.W., et al. Recurrence of symptoms following a 2-day symptom free period in patients with COVID-19. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.38867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nextstrain. Available at https://nextstrain.org/ncov/gisaid/global/6m. [Accessed December 8, 2022].
- 19.Crucial policies for combating COVID-19. Available at https://covid19.mohw.gov.tw/en/mp-206.html. [Accessed July 20, 2022].
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y.W., Chao T.L., Li C.L., Wang S.H., Kao H.C., Tsai Y.M., et al. D614G substitution of SARS-CoV-2 spike protein increases syncytium formation and virus titer via enhanced furin-mediated spike cleavage. mBio. 2021;12(4) doi: 10.1128/mBio.00587-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranganath N., O'Horo J.C., Challener D.W., Tulledge-Scheitel S.M., Pike M.L., Michael O'Brien R., et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Gao M., You H., Zhang P., Pan Y., Li N., et al. Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deo R., Choudhary M.C., Moser C., Ritz J., Daar E.S., Wohl D.A., et al. Viral and symptom rebound in untreated COVID-19 infection. medRxiv. 2022 doi: 10.1101/2022.08.01.22278278. [DOI] [Google Scholar]
- 28.Pandit J.A., Radin J.M., Chiang D., Spencer E.G., Pawelek J.B., Diwan M., Roumani L., Mina M.J. The COVID-19 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated with Nirmatrelvir Plus Ritonavir Versus Untreated Controls. Clin Infect Dis. 2023 doi: 10.1093/cid/ciad102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M.H., Nam Y., Son N.H., Heo N., Kim B., Kang E., et al. Antibody level predicts the clinical course of breakthrough infection of COVID-19 caused by delta and Omicron variants: a prospective cross-sectional study. Open Forum Infect Dis. 2022;9(7):ofac262. doi: 10.1093/ofid/ofac262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 31.Pan S.C., Hsu M.C., Chang H.H., Wang J.T., Lai Y.L., Chen P.C., et al. Prospective health surveillance for COVID-19 among health care workers at a university medical center in Taiwan, January to June 2020. J Formos Med Assoc. 2022;121(3):613–622. doi: 10.1016/j.jfma.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C., Li Y., Kaplonek P., Gentili M., Fischinger S., Bowman K.A., et al. The kinetics of SARS-CoV-2 antibody development is associated with clearance of RNAemia. mBio. 2022;13(4) doi: 10.1128/mbio.01577-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data from four sequential clinical specimens of P15 used in the current study have been deposited in GISAID (https://www.gisaid.org/CoV2020/). The accession codes and the mutations in these viral isolates are summarized in Supplementary Table S3.