Abstract
Targeted immunotherapy has improved patient survival in head and neck squamous cell carcinoma (HNSCC), but less than 20% of patients produce a durable response to these treatments. Thus, new immunotherapies that consider all key players of the complex HNSCC tumour microenvironment (TME) are necessary to further enhance tumour-specific T cell responses in patients. HNSCC is an ideal tumour type in which to evaluate immune and non-immune cell differences because of two distinct TME aetiologies (human papillomavirus (HPV)-positive and HPV-negative disease), multiple anatomic sites for tumour growth, and clear distinctions between patients with locally advanced disease and those with recurrent and/or metastatic disease. Recent technological and scientific advancements have provided a more complete picture of all cellular constituents within this complex TME and have evaluated the interplay of both immune and non-immune cells within HNSCC. Here, we include a comprehensive analysis of the complete ecosystem of the HNSCC TME, performed utilizing data-rich resources such as The Cancer Genome Atlas, and cutting-edge techniques, such as single-cell RNA sequencing, high-dimensional flow cytometry and spatial multispectral imaging, to generate improved treatment strategies for this diverse disease.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide (890,000 new cases and 450,000 deaths annually1,2) and occurs in the mucosal surfaces of four major anatomical sites: oral cavity, sinonasal cavity, pharynx and larynx3. A modest improvement in survival of patients with HNSCC has been demonstrated over the past decade, partially attributed to the decreased use of tobacco in the elderly population globally4. However, the incidence of HNSCC continues to rise, with a 30% anticipated increase by 2030 (refs.1,3,5), mostly attributed to the increase in human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) in younger individuals who is caused primarily by HPV16 and, to a lesser extent, HPV18. Indeed, the fraction of HPV-positive OPSCC in the United States increased from 16.3% in the 1980s to more than 72.7% in the 2000s3,6. As the effectiveness of prophylactic HPV vaccination is less well defined for OPSCC than for indices such as cervical cancer, a decreased incidence in HPV-related disease might not be evident before 2060. The prognosis of patients with OPSCC that is HPV-positive is more favourable than that of patients with HNSCC that is HPV-negative as they have improved responses to chemotherapy and radiotherapy owing to fewer co-existing conditions than patients with HPV-negative HNSCC, who are often physiologically compromised by chronic tobacco and alcohol use7. Further, HPV-positive OPSCCs display an increased immune infiltrate, and the overall microenvironment and cellular ecosystems of these tumours can be quite different to those from patients with HPV-negative disease3. In addition to HPV status, consideration of locally advanced versus recurrent and/or metastatic (R/M) disease is important owing to the potential impact of prior therapies and tumour progression in the tumour microenvironment (TME)8. Indeed, although the treatment of both locally advanced and R/M HNSCC has benefited a cohort of patients, there is room for improvement. HNSCC of the oral cavity is generally treated with surgical resection, followed by adjuvant chemotherapy plus radiotherapy3. Molecularly targeted agents, such as the epidermal growth factor receptor (EGFR) inhibitor cetuximab, have shown modest success in locally advanced disease9. Finally, two immune-checkpoint inhibitors (ICIs) targeting PD1 (nivolumab and pembrolizumab) are approved for the treatment of R/M HNSCC. Unfortunately, only 15–20% of patients with HNSCC achieve a durable response to these agents despite a twofold to threefold higher expression of PD1 and PDL1 within the tumour10. In addition to the clear genomic differences between HPV-positive and HPV-negative HNSCC (Table 1), which should be considered for next-generation immunotherapies, we can begin to assess how various microenvironmental drivers affect the immune and stromal landscape of these disease aetiologies. With the incidence of HNSCC increasing, a clear need exists for improved therapeutics for both locally advanced and R/M disease. Furthermore, locally advanced disease provides a window of opportunity to study the dynamic infrastructure of the HNSCC TME, knowledge of which is critical for improving therapies for patients that progress to R/M disease. This comprehensive Review of the cellular ecosystems of the two unique HNSCC TMEs (HPV positive and HPV negative) will discuss current treatment modalities and provide new combinatorial therapeutic avenues aimed at improving patient survival.
Table 1 |.
HNSCC: one disease, two distinct tumour microenvironments
| HNSCC type | Carcinogen | Anatomical site | Incidence | Tumour microenvironment |
|---|---|---|---|---|
| HPV-negative HNSCC | Tobacco, alcohol | Oral cavity, pharynx, larynx | Decreased since 2000s | Decreased immune infiltration |
| HPV-positive HNSCC | HPV | Oropharyngeal | Increased since 2000s | Increased immune infiltration |
HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus.
Interrogating cellular ecosystems of HNSCC
Important microenvironment differences between patients with HNSCC have been uncovered utilizing The Cancer Genome Atlas (TCGA). Specifically, the HNSCC cohort in TCGA (97 patients with HPV-positive disease and 423 patients with HPV-negative disease) contains bulk RNA sequencing data collected from tumour specimens resected from the different sites of HNSCC11. This rich resource has improved our understanding of the immunogenomic landscape in these two TMEs and how these landscapes are shaped by clinical features, including HPV status, tumour stage, mutational burden, and tobacco and alcohol use. Several reports using gene set enrichment analysis and unsupervised clustering to deconvolute and identify cell types stratified patients with HNSCC by immune signatures: cold (no immune infiltrate), lymphocyte (enrichment for CD4+ T cells, CD8+ T cells, B cells and plasma cells) and myeloid dendritic cell (DC) (enrichment of neutrophils, macrophages, monocytes, DCs, T regulatory (Treg) cells and eosinophils)12–15. Classifying patient tumours based on immune infiltrate alone is becoming more limited as assessing cellular neighbourhoods (that is, tertiary lymphoid structures; TLS) within these patients will offer key insights into the dynamic interplay of multiple cell types in patients with HNSCC (regardless of HPV status). Adding this further metric to immune high and immune low HNSCC tumours could be important in predicting response to immunotherapy as shown in adult sarcomas16. Further, TLS are important considerations in HNSCC as the presence of a mature TLS (that is, one with a germinal centre; GC) is associated with increased survival regardless of HPV status although survival is enhanced further in patients with HPV-positive tumours17. In terms of total abundance of each individual cell type, patients with HPV-positive HNSCC have a significant increase in B cells, plasma cells, T helper type 1 (TH1) CD4+ T cells, TH2 CD4+ T cells, natural killer (NK) cells and CD8+ T cells compared with patients with HPV-negative HNSCC. Monocytes, macrophages, NK T cells and neutrophils are higher in patients with HPV-negative HNSCC12,13,15. Reports regarding Treg cell abundance in HNSCC have been conflicting. One study reported via bulk RNA sequencing that the abundance of Treg cells was higher in patients with HPV-positive HNSCC than in patients with HPV-negative HNSCC while another reported no significant difference12,13. In general, lymphocyte gene signatures correlated with a longer overall survival than the cold and myeloid DC signatures, especially in patients with HPV-positive disease. Immune cold and myeloid DC gene signatures correlated with later-stage disease and shorter overall survival12,13,15. The molecular smoking signature, defined by the proportion of mutational processes in each tumour attributable to tobacco smoking, correlated inversely with immune infiltration in both HPV-positive and HPV-negative tumours and correlated positively with high total non-synonymous mutational burden (the total number of non-silent missense mutations per tumour)13.
Comparing individual differentially expressed immune-related genes (IRGs) in the TCGA-HNSCC cohort revealed a total of 65 IRGs that were associated with prognosis, 56 of which were significantly associated with overall survival rates in patients with HPV-negative HNSCC11,14. In this IRG prognosis model, SEMA3G, GNRH1, TNFRSF4 and ZAP70 were positively correlated with overall survival11 and PLAU, SH2D1A, CCL26, DKK1, GAST, PDGFA and STC1 were negatively correlated with overall survival. Further, the 5-year survival rate for patients whose tumours expressed more of the negatively correlated genes was 31.2% and that for patients whose tumours expressed more of the positively correlated genes was 66.8%11. This study focused on HPV-negative HNSCC tumours, which have low infiltration. Indeed, TCGA analyses that have focused on both TMEs have uncovered significant differences in several other immune pathways. For example, CD8+ T cells show significant upregulation of PD1, T cell immunoglobulin and mucin domain 3 (TIM3), lymphocyte-activation protein 3 (LAG3), and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) in HPV-positive tumours compared with HPV-negative tumours18–22. In addition, CD8+ T cells in HPV-positive tumours also showed increased expression of the T cell activation marker CD137 and ectonucleotidase CD39 compared with HPV-negative tumours23. KIR inhibitory receptor genes, which inhibit NK cell function, are increased in HPV-positive tumours and B cell-associated genes, such as CD200, VCAM1, BCL2 and ICOSLG, are also increased HPV-positive tumours13,18.
Bulk RNA sequencing datasets require deconvolution to identify cell types within samples and thus might under-represent the composition and distribution of cell types within a given sample24–27. Thus, such analyses have limited ability to detect intratumoural heterogeneity and cell-to-cell variability in gene expression. Further, differences in transcript quantification, read depth and sequencing platforms can compromise the reproducibility of TCGA analyses25,27. The development of single-cell RNA (scRNA) sequencing has revolutionized transcriptomic analysis of all cellular populations in the HNSCC TME (Fig. 1). Specifically, scRNA sequencing can uncover rare cell populations and novel protein–protein interactions, and track differentiation of cell types within a specific cell lineage24,27. Indeed, scRNA sequencing analyses paired with high-dimensional flow cytometry and spatial multispectral imaging (Fig. 1) have revealed immense differences among innate and adaptive immune cells within HPV-positive and HPV-negative HNSCC tumours that were not captured by initial bulk TCGA analyses28–30. Furthermore, non-immune cells, such as fibroblasts and mesenchymal stem cells, are also diverse in these TMEs and have now been included in scRNA sequencing landscape analyses28,31. In this Review, we discuss new technologies and analyses that more completely and discretely identify the differences in immune and non-immune compartments in patients with HPV-positive and HPV-negative HNSCC.
Fig. 1 |. Novel technologies have begun to elucidate the clear complexity of the HNSCC TME.
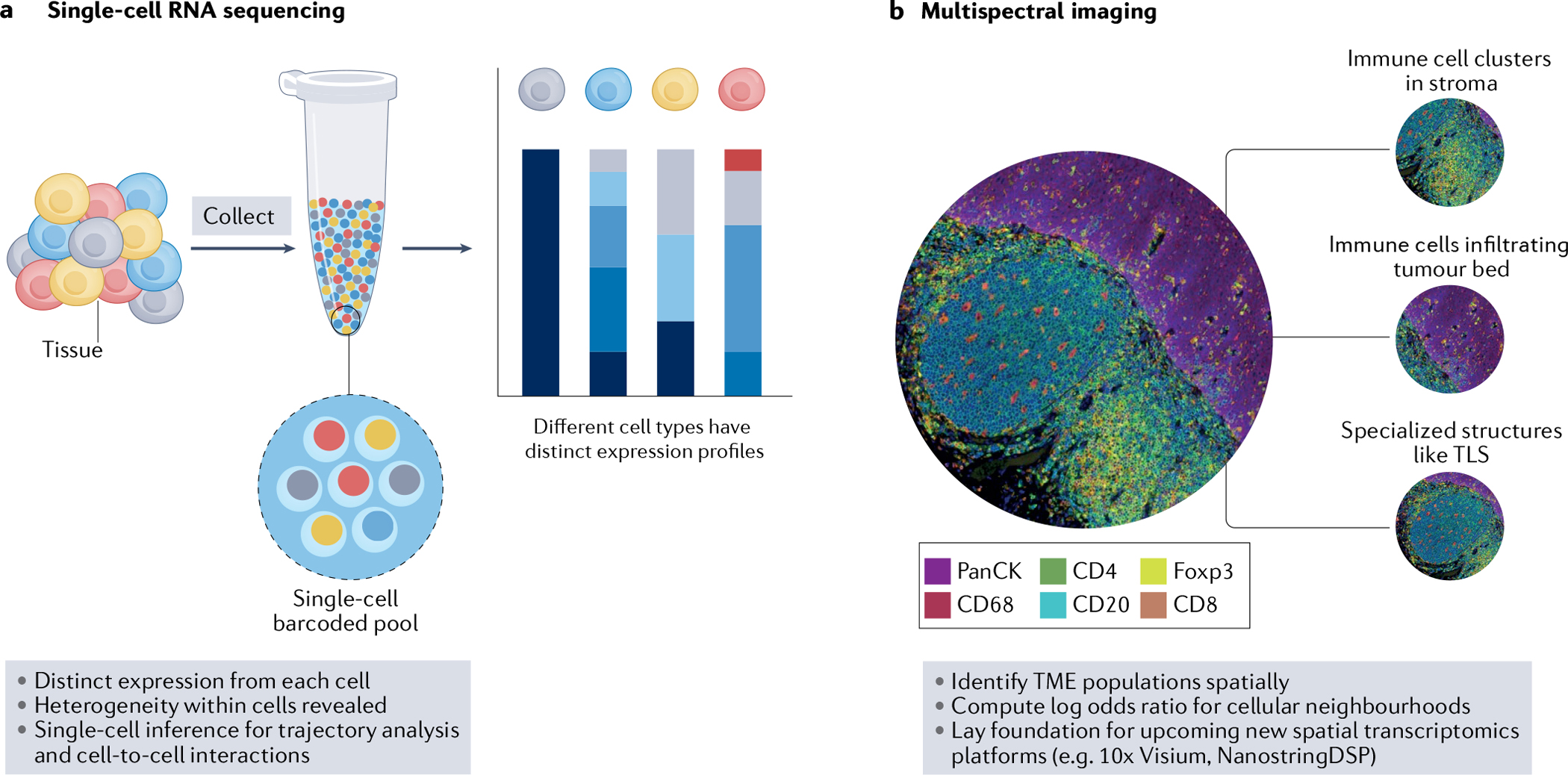
a, Single-cell RNA sequencing enables distinct expression from each cell and reveals heterogeneity within immune and non-immune cell subsets. Furthermore, bioinformatic analyses can provide single-cell inference for trajectory analysis and cell-to-cell interactions. b, Multispectral imaging is paramount to identifying tumour microenvironment (TME) populations spatially and analysing cellular interactions within and between tumour and stroma. Bioinformatic analyses can also determine cellular neighbourhoods within the head and neck squamous cell carcinoma (HNSCC) TME, and these imaging technologies have laid the foundation for new and upcoming spatial transcriptomic platforms that will increase our spatial knowledge of the HNSCC TME. TLS, tertiary lymphoid structure.
Maximizing cellular and humoral immunity
Functional CD8+ T cells are key to a robust immune response in HPV-positive and HPV-negative disease
CD8+ T cells can recognize antigens presented by tumour cells and directly kill them by releasing pro-inflammatory cytokines and cytolytic granules (Fig. 2). Although the overall abundance of CD8+ T cells decreases considerably in patients with HPV-negative HNSCC, those with comparable proportions of CD8+ T cells to proportions in those with HPV-positive tumours tend to have similarly favourable outcomes32–34. For example, patients with HPV-negative OPSCC who had comparable numbers of tissue-resident CD8+ T cells (CD103+) to patients with HPV-positive OPSCC demonstrated a similar outcome, suggesting a critical role for tissue-resident CD8+ T cells in HNSCC antitumour immunity irrespective of HPV status23,35.
Fig. 2 |. New T cell targets in the HNSCC TME.
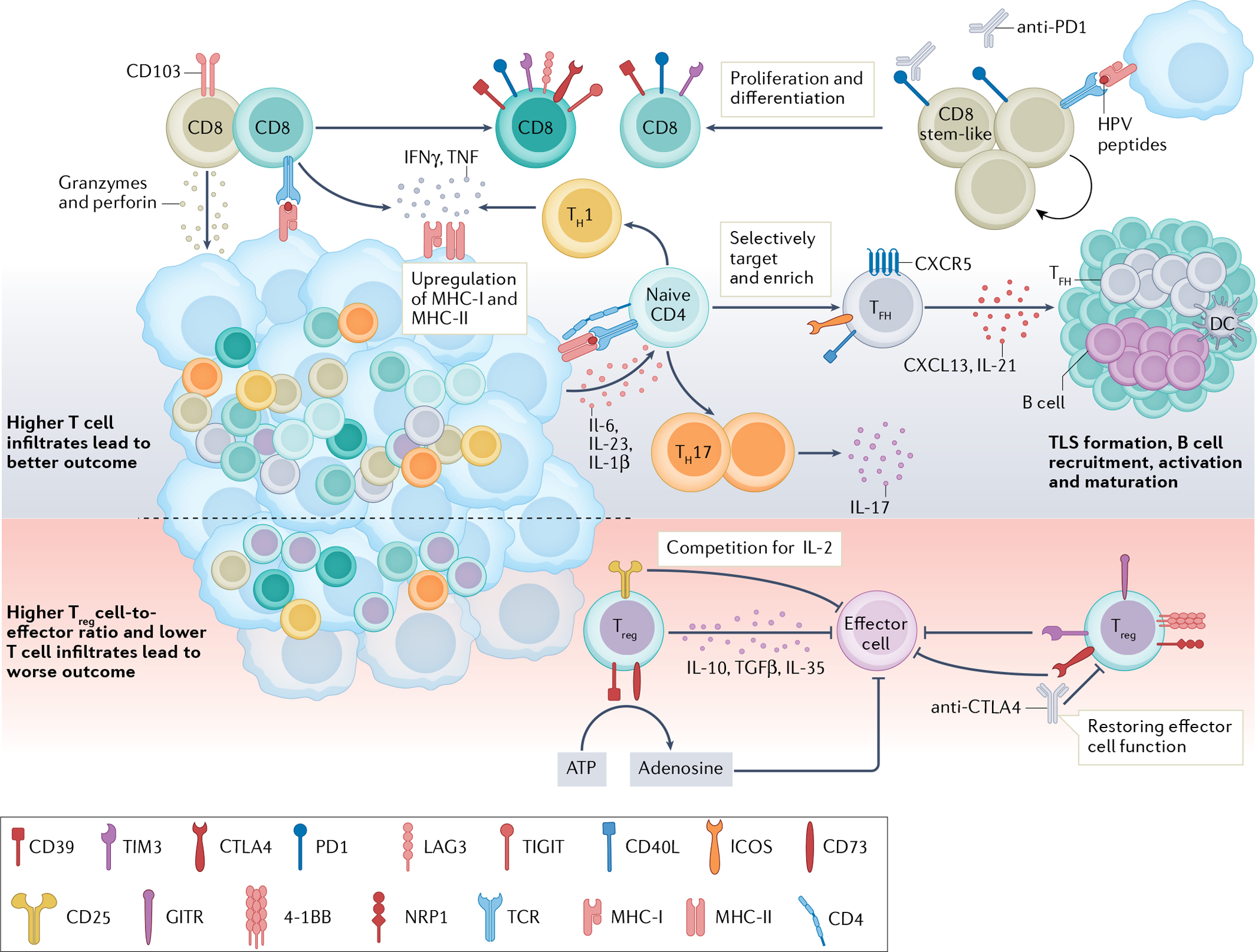
T cells are important in antitumour immunity, and human papillomavirus (HPV)-positive tumours generally show higher T cell infiltration and are associated with better outcomes than HPV-negative tumours32–34. CD8+ T cells can directly lyse target cells by releasing granzymes and perforin in an antigen-directed manner. Chronic engagement with tumour antigens leads to a dysfunctional state characterized by high expression of immune-checkpoint receptors (PD1, T cell immunoglobulin and mucin domain 3 (TIM3), lymphocyte-activation protein 3 (LAG3) and CTLA4)36–39. An HPV16-specific CD8+PD1+ T cell population in HPV-positive tumours contains a stem-like CD8+ T cell subset. These cells are capable of proliferating and differentiating into effector cells upon HPV peptide stimulation and represent a population of future therapeutic targets28. CD4+ T lymphocytes recognize major histocompatibility complex (MHC) class II antigens and differentiate into different subtypes upon antigen stimulation. A higher number of T helper 1 (TH1) and TH17 cells are found in HPV-positive tumours than in HPV-negative tumours48,49. TH1 cells release IFNγ and TNF and promote MHC class I and II upregulation on cancer cells, facilitating tumour elimination43,44. IL-17-releasing TH17 cells are induced by IL-6, IL-23 and IL-1β produced by primary tumour cells51,52. CXCR5+PD1+ICOS+CD40L+ T follicular helper (TFH) cells produce CXCL13 and IL-21, recruiting B cells into the tumour microenvironment (TME)56,57. TFH cells are essential for B cell activation, maturation and tertiary lymphoid structure (TLS) formation in tumours, and their presence is associated with improved outcomes29. T regulatory (Treg) cells have an immunosuppressive role in the TME. Treg cells can suppress effector cells through the release of IL-10, TGFβ and IL-35. High levels of CD25 on Treg cells compete with effector cells for local IL-2, so that effector cells might not have enough IL-2 to survive and function58–60. Treg cells also express CD39 and CD73, which convert extracellular ATP to adenosine and impair effector T cell function58,67. Co-expression of 4–1BB, GITR and neuropilin 1 (NRP1) on intratumoural Treg cells demonstrated an enhanced suppression function29,61,63. Immune-checkpoint receptors, such as TIM3 and CTLA4, are reportedly expressed by Treg cells in head and neck squamous cell carcinoma (HNSCC). Both are highly expressed by intratumoural Treg cells and able to suppress effector cell functions21,22,63,64. Therapeutically targeting Treg cells might help rejuvenate effector cell function. DC, dendritic cell; ICOS, inducible T cell co-stimulator; TCR, T cell receptor; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domains.
One of the hallmarks of the CD8+ T cell response in cancer and chronic viral infections is T cell dysfunction, sometimes termed exhaustion, a cell state characterized by hierarchal loss of effector function (production of IL-2, TNF and IFNγ) and co-expression of the immune inhibitory receptors PD1, TIM3, LAG3, CTLA4 and TIGIT36–39. Although flow cytometric studies revealed increased frequencies of PD1+CD8+ tumour-infiltrating lymphocytes (TILs) in HPV-positive tumours, the frequencies of CD8+PD1high TILs (which exhibit impaired IFNγ production) are higher in HPV-negative tumours40. Furthermore, PD1high expression on CD8+ TILs predicts response to anti-PD1/PDL1 therapies by patients with HPV-positive disease but has limited prognostic value in those with HPV-negative disease40. These data underscore the importance of further interrogating the state of CD8+ TILs in HPV-positive and HPV-negative HNSCC. scRNA sequencing that compared global changes in transcriptomic profiles of CD8+ TILs has revealed five broad cell states in HPV-positive and HPV-negative disease, including cycling, cytotoxic, pre-dysfunctional, early activated, and terminally exhausted T cells28,29. Diffusion pseudotime analysis revealed that CD8+ T cells follow a shared differentiation trajectory in HPV-positive and HPV-negative HNSCC, moving from early activated CD8+ T cells towards terminally differentiated CD8+ T cells marked by co-expression of inhibitory receptors29. Shared transcriptional profiles of exhausted CD8+ T cells have also been reported in paired lymph node metastases of patients who are treatment naive with locally advanced HPV-negative HNSCC30.
High-dimensional immune profiling studies using scRNA sequencing have revealed that CD8+ TILs are a phenotypically and functionally diverse population within the antigen-specific pool. scRNA sequencing analysis of HPV-specific T cells sorted from HPV-positive tumours and paired metastatic lymph nodes revealed three distinct subsets: stem-like (PD1+TIM3−CD39−TCF7+), transitory (PD1+IFNγhigh) and terminally differentiated (PD1+TIM3+CD39+)41. T cell receptor (TCR) clonotypes directed at HPV proteins E2, E5 and E6 were shared between the three subsets, which suggests that terminally differentiated CD8+ T cells derive from stem-like CD8+ T cells41. HPV-specific stem-like CD8+ T cells effectively proliferate upon in vitro HPV peptide stimulation and immune-checkpoint inhibition41. This finding might imply that strategic reinvigoration of HPV-specific stem-like CD8+ T cells by ICIs will benefit patients with HPV-positive HNSCC. Stem-like CD8+ T cells are also present in HPV-negative HNSCC tumours and produce abundant levels of TNF, although their antigen specificity was not determined42. More work is needed to determine how and if non-viral neoantigen-specific CD8+ T cells differentially contribute to response to therapy in both TMEs.
Leveraging conventional CD4+ T cells for improved help within the HNSCC TME
CD4+ T cells are also essential for eliciting antitumour immune responses through differentiation into distinct effector subtypes upon antigen stimulation and secretion of different cytokines43,44. TH1 CD4+ T cells produce pro-inflammatory cytokines, particularly IFNγ and TNF, which induce major histocompatibility complex (MHC) class I and II expression in the TME, enhancing tumour antigen recognition by other immune cells, leading to increased killing of tumour cells43,44. TH17 cells produce IL-17, IL-2, IL-8, and TNF and have an important role in pathogen protection45. Both pro-tumour and antitumour roles have been described for TH17 in both mouse and human cancer but studies are limited in human tumours46. Higher numbers of TH1 CD4+ T cells are observed in HPV-positive than in HPV-negative HNSCC. RNA sequencing analyses demonstrated that CD4+ TILs have increased RNA expression of the TH1 marker TBX21 (which encodes transcription factor T-bet and regulates IFNγ production) as well as increased expression of the TH17 markers RORA and RORC in HPV-positive HNSCC compared with HPV-negative HNSCC47. Further, HPV16-specific CD4+ T cells predominantly express IFNγ and IL-17, suggesting that TH1 and TH17 cells are enriched in the HPV-positive TME48,49. In addition, HPV16-specific CD4+ T cells release IFNγ and TNF and synergize with cisplatin-based therapy to control tumour cell growth, highlighting their importance inpatient response to cancer therapies50. TH17 cells also accumulate in the peripheral blood and tumour-draining lymph nodes of patients with HNSCC, and primary HNSCC tumours express the TH17-inducing cytokines IL-6, IL-23 and IL-1β51,52. Functionally, TH17 cells were able to suppress HNSCC tumour growth and decrease production of angiogenesis proteins by HNSCC tumour cells in vitro51,52. Future studies should further evaluate the antigen specificity and function of TH17 cells in both HPV-positive and HPV-negative HNSCC TMEs to determine their impact on patient outcomes. CD4+ T follicular helper (TFH) cells, which express CXCR5 and PD1, produce the chemokine CXCL13, which is important for recruiting B cells and CD8+ T cells into the tumour53–55. TFH cells are important for the activation and maturation of B cells through IL-21 production and expression of co-stimulatory ligands such as inducible T cell co-stimulator (ICOS) and the CD40 ligand56,57. Recent scRNA sequencing analysis demonstrated that a TFH gene signature is enriched in HPV-positive HNSCC and correlates with improved progression-free survival17,29. In addition, multispectral imaging revealed co-localization of TFH cells with B cells within TLS in HPV-positive HNSCC tumours17,29. More work is needed to evaluate the role of CD4+ T cell subsets in HNSCC and their potential for immunotherapeutic targeting. However, promoting the appropriate local intratumoural CD4+ T cell differentiation should be considered in the next generation of immunotherapies to augment CD8+ T cell function.
Refining Treg targeting to decrease immunosuppression in the TME
Treg cells are a subset of CD4+ T cells defined by the expression of transcription factor Foxp3 (forkhead box protein 3) and CD25, and they suppress immune effector cells and promote tumour progression58. Treg cells exert immunosuppressive functions in tumours through the secretion of inhibitory cytokines such as IL-10, IL-35 and TGFβ, upregulation of inhibitory receptors, disruption of metabolism via CD39 and CD73, and depriving the local TME of IL-2 via high CD25 expression58–60. Treg cells are present in the peripheral blood and tumours of patients with HNSCC60–62. Patients with HPV-positive HNSCC have higher frequencies of intratumoural Treg cells than those with HPV-negative HNSCC but recent scRNA sequencing analysis demonstrated that Treg cells share transcriptomic profiles in HPV-positive and HPV-negative HNSCC29. Interestingly, Treg cells exhibit unique transcriptional cell states distinguished by enrichment of interferon-response genes or TNF receptor (TNFR) family genes29. Interferon-response genes correlated with early activation of Treg cells while TNFR genes, such as GITR (also known as TNFRSF18), 4–1BB (also known as TNFRSF9) and OX40 (also known as TNFRSF4), were expressed later in differentiation, suggesting reciprocal control of Treg cell effector function29. Key inhibitory receptors, such as CTLA4, have also been described on Treg cells in HNSCC63. CTLA4 expression is high on intratumoural Treg cells in patients with HNSCC and is further upregulated after cetuximab treatment63. Further, CTLA4+ Treg cells inhibit NK cell function during cetuximab treatment, possibly owing to TGFβ production, and treatment with ipilimumab restores NK cell function by depleting Treg cells63,64. Other inhibitory receptors have now been more thoroughly examined in patients with HNSCC65,66. Expression of neuropilin 1 (NRP1) on intratumoural Treg cells was shown to correlate with GITR and 4–1BB expression on Treg cells61. NRP1+ Treg cells have enhanced suppressive function and correlate with overall poor prognosis in patients with HNSCC61. NRP1 expression on Treg cells is driven by T cell activation signals and can be reduced by inhibiting molecules within the TCR signalling pathway61. Intratumoural TIM3+ Treg cells also limit naive CD8+ T cell proliferation, thus inhibiting effector cell function in HNSCC21,22. Lastly, intratumoural Treg cells have high expression of CD39 and CD73 in HNSCC, which allows them to convert ATP to adenosine for T cell suppression via adenosine receptor 2A60,67. Taken together, these data suggest that Treg cells have diverse mechanisms to construct an immunosuppressive TME in HNSCC and that specifically inhibiting Treg cells in the HNSCC TME might enhance immuno-therapeutic response.
B cells within tertiary lymphoid structures increase antitumour humoral immunity
Several distinct subsets of B cells exist, and these subsets have diverse functional capacities that extend beyond antibody production and include antigen presentation, initiation of TLS formation, cytokine production and direct cell lysis68–74. Furthermore, studies have revealed the prognostic impact of B cells and TLS as predictors of patient survival and response to ICI therapy16,75–77. Thus, their role as potential immunotherapeutic targets warrants further investigation.
Infiltration by intratumoural B cells was significantly increased in samples from patients with HPV-positive HNSCC compared with samples from patients with HPV-negative disease, while infiltration of the invasive tumour margin was not significantly different between the two groups78–80. Overall, high densities of CD20+ B cells were associated with favourable outcomes in HNSCC79,81. B cells are predominantly found within TLS rather than as immune aggregates that have not formed a TLS in both HPV-positive and HPV-negative disease17. HPV-positive HNSCC tumours that occurred in the tonsil or tongue had more TLS than HPV-negative tumours that occurred in the same sites17. Multispectral imaging revealed that B cells co-localize with both CD8+ T and TFH cells in TLS in patients with HNSCC17,76,81,82. Strikingly, scRNA sequencing analysis revealed that, compared with other immune cells, B cells showed larger transcriptional differences between HPV-positive and HPV-negative disease17,29. Specifically, B cells within HPV-positive tumours were enriched for GC and naive B cell gene signatures whereas B cells in HPV-negative HNSCC were enriched for plasma cells and class-switched memory B cells (MBCs)29. Several other studies have quantified subsets of B cells within HNSCC tumours. B regulatory cell subsets, such as CD19+CD24hiCD38hi and CD19+CD25hi cells, were increased in HNSCC tumours compared with non-cancerous mucosa and healthy peripheral blood mononuclear cells78,83. Oropharyngeal sites of HNSCC (tonsil, base of tongue and oropharynx) have increased total levels of MBCs (CD38−IgD−), GC B cells (CD38+IgD−) and plasma cells (CD38hiIgD−) when frequencies of tumour-infiltrating B cells are high in the TME84. MHC class I, MHC class II, CD70, CD86 and CD40 expression were also significantly increased on B cells in these patients78,84. Intratumoural GC B cells that express canonical GC markers, such as BCL6 and semaphorin 4A (SEMA4A), are increased in HPV-positive HNSCC17. Intratumoural GC B cell subsets, including dark zone, light zone and a novel transitional GC population, were present in HPV-positive tumours with unique patterns of surface marker expression, suggesting that GC formation in HNSCC is not impaired17,76,82. An increased number of GC B cells correlated with superior overall survival in both HPV-positive and HPV-negative HNSCC17 (Fig. 3).
Fig. 3 |. Increasing cellular interactions with tertiary lymphoid structures in the HNSCC TME for maximal humoral and cellular immunity.
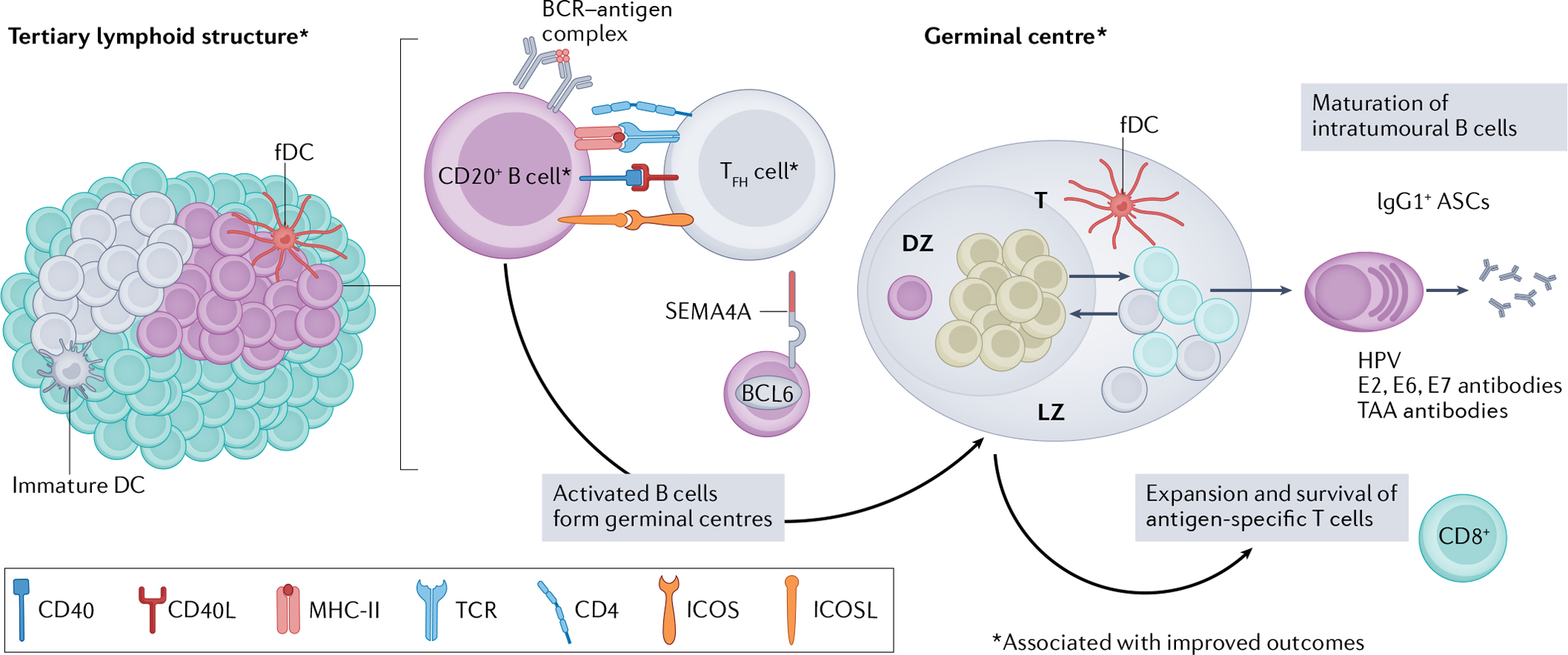
Tertiary lymphoid structures (TLS) form within the tumour or surrounding the tumour stroma in patients with head and neck squamous cell carcinoma (HNSCC)17,78,84. TLS vary in size and cell composition in patients, but an increased presence of TLS correlates with improved survival and reduced risk of recurrence in both human papillomavirus (HPV)-positive and HPV-negative HNSCC17. Within TLS, B cells co-localize with T follicular helper (TFH) cells and receive activation signals via CD40, inducible T cell co-stimulator ligand (ICOSL) and major histocompatibility complex class II (MHC-II) engagement17,29,84. Activation of B cells via TFH cells leads to germinal centre formation, which is regulated by transcription factor B cell lymphoma 6 (BCL6) and marked by surface expression of semaphorin 4A (SEMA4A) on germinal centre B cells17,165. Intratumoural germinal centre B cells in HPV-positive HNSCC have three distinct cell states: dark zone (DZ), light zone (LZ) and transitional (T), marked by unique patterns of gene expression that are important for the selection and maturation of B cells17. Formation of germinal centres within TLS is an independent predictor of superior progression-free survival in HNSCC17. As a result of successful germinal centre formation in HPV-positive HNSCC, germinal centres produce terminally differentiated somatic hypermutated antibody-secreting cells (ASCs) that have undergone immunoglobulin class-switching to an IgG1 isotype82. IgG1+ ASCs in patients with HPV-positive disease recognize HPV viral proteins E6, E7 and E2 (ref.82). Antibodies directed at tumour-associated antigens (TAAs) have also been detected in the serum of patients with HPV-positive and HPV-negative HNSCC78. Activated B cells might also provide co-stimulatory signals to CD8+ T cells, resulting in their expansion and survival in HNSCC tumours81,84. Co-localization of B cells with CD8+ T cells is associated with favourable outcomes in HNSCC81,84. Together, these features provide a new targetable axis for future immunotherapies, which should be directed at enhancing TLS formation, maturation of B cells and increasing B–T cell interactions within the TME of HNSCC. BCR, B cell receptor; fDC, follicular dendritic cell; ICOS, inducible T cell co-stimulator; TCR, T cell receptor.
The presence of active GCs in HNSCC tumours suggests that B cells undergo selection and could potentially have high-affinity antigen receptors directed at tumour-associated antigens. Indeed, IgG+ antibody-secreting cells (ASCs) specific for HPV E6 and E7 antigens were observed in both metastatic lymph node and primary tumours of patients with HPV-positive HNSCC82 (Fig. 3). Interestingly, IgG+ ASCs were also specific for HPV E2 and more ASCs produced antibodies specific for E2 than for E6 or E7 (ref.82). Intratumoural HPV-specific IgG+ ASC responses correlated positively with titres of HPV-specific IgG in patients. However, very few HPV-specific ASCs were found in the peripheral blood, suggesting that the generation of HPV-specific ASCs occurs locally in the TME82. HPV-specific IgG+ MBCs were also present in peripheral blood of patients. Circulating HPV-specific antibodies in patients have been shown to be predominantly of the IgG1 isotype, suggesting that HPV-specific antibodies can participate in activation of antibody-mediated killing of tumours via antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis85,86. HPV-specific human monoclonal antibodies (mAbs) were generated from intratumoural B cells and ASCs were shown to have a high degree of somatic hypermutation, which suggest that B cells in these tumours experience chronic antigen exposure82. Patients with HPV-negative disease have higher levels of circulating antibodies directed towards tumour-associated antigens, such as p53, melanoma antigen gene (MAGE) proteins and cyclin-dependent kinase inhibitor 2A (CDKN2A), compared with patients with HPV-positive disease78. The quality of tumour-associated antigen responses in HNSCC is yet to be evaluated. Taken together, these data indicate that distinct B cell-focused targeting strategies might be needed in HPV-positive and HPV-negative HNSCC but should focus on inducing TLS and driving antigen-specific intratumoural maturation of B cells.
Innate immune cells in the HNSCC TME
NK cells could improve tumour cell killing in patients with HNSCC
NK cells are highly malleable effector cells of the innate immune system that are regulated by a sophisticated interplay of activating and inhibitory surface receptors that scrutinize MHC class I expression and stress-induced ligands on potential target cells87.
NK cells are generally segregated into two major subsets: CD56brightCD16− and CD56dimCD16+. CD56brightCD16− cells are primarily known for their cytokine-producing capacity, while CD56dimCD16+ cells are best known for their cytotoxic capacity13,88. Increasing evidence indicates that CD56dimCD16− NK cells might be the third major subset89,90. CD56dimCD16− NK cells are primarily known for efficient IFNγ production in response to tumour challenge and display the most diverse chemokine receptor profile of the three subsets (CCR2+CCR3+CCR4+CCR5+CCR7+CXCR1+CXCR3+), making them highly capable of chemo-attraction89,91,92. Available studies suggest that the CD56dimCD16− subset might be the most frequent tumour-infiltrating NK cell subset in HNSCC, but little is known about how disease aetiology affects NK cell subset distribution93. This observation is highly important as cetuximab, a humanized IgG1 mAb used to treat EGFR-expressing tumours, is believed to mediate tumour elimination in part by promoting CD16/FcγRIII+ NK cell-mediated ADCC against EGFR+ tumours94–96. A decrease in CD16+ NK cell frequencies within the TME would suggest that ADCC might be a mechanism preventing R/M HNSCC but not of debulking the primary tumour. Increased understanding of NK cell subset functions within HNSCC is needed to optimize future immunotherapies.
Comparative transcriptomic analyses indicate that HNSCC have the highest median CD56dim NK cell infiltration amongst the most common tumour types13. When comparing HPV-positive and HPV-negative HNSCC aetiologies, NK cell infiltration is more prominent in HPV-positive tumour beds and surrounding stroma than in HPV-negative tumours and surrounding stroma89,92. An elevated NK cell infiltrate is associated with increased progression-free and overall survival in patients with HPV-negative and HPV-positive HNSCC (Fig. 4). The importance of NK cells in containing HPV-driven development of dysplastic lesions was highlighted in a case report of a patient with a dysfunctional NK cell compartment who displayed multiple recurrent HPV-associated skin and mucosal dysplasias93. The patient achieved long-term remission of all lesions following allogeneic haematopoietic cell transplantation that led to restoration of NK cell cytotoxicity93. Given the likely potency of NK cells in the TME, efforts should be made to increase their influx into the HNSCC TME.
Fig. 4 |. Innate cell interactions generate inflammatory signals that drive outcomes in patients with HNSCC.
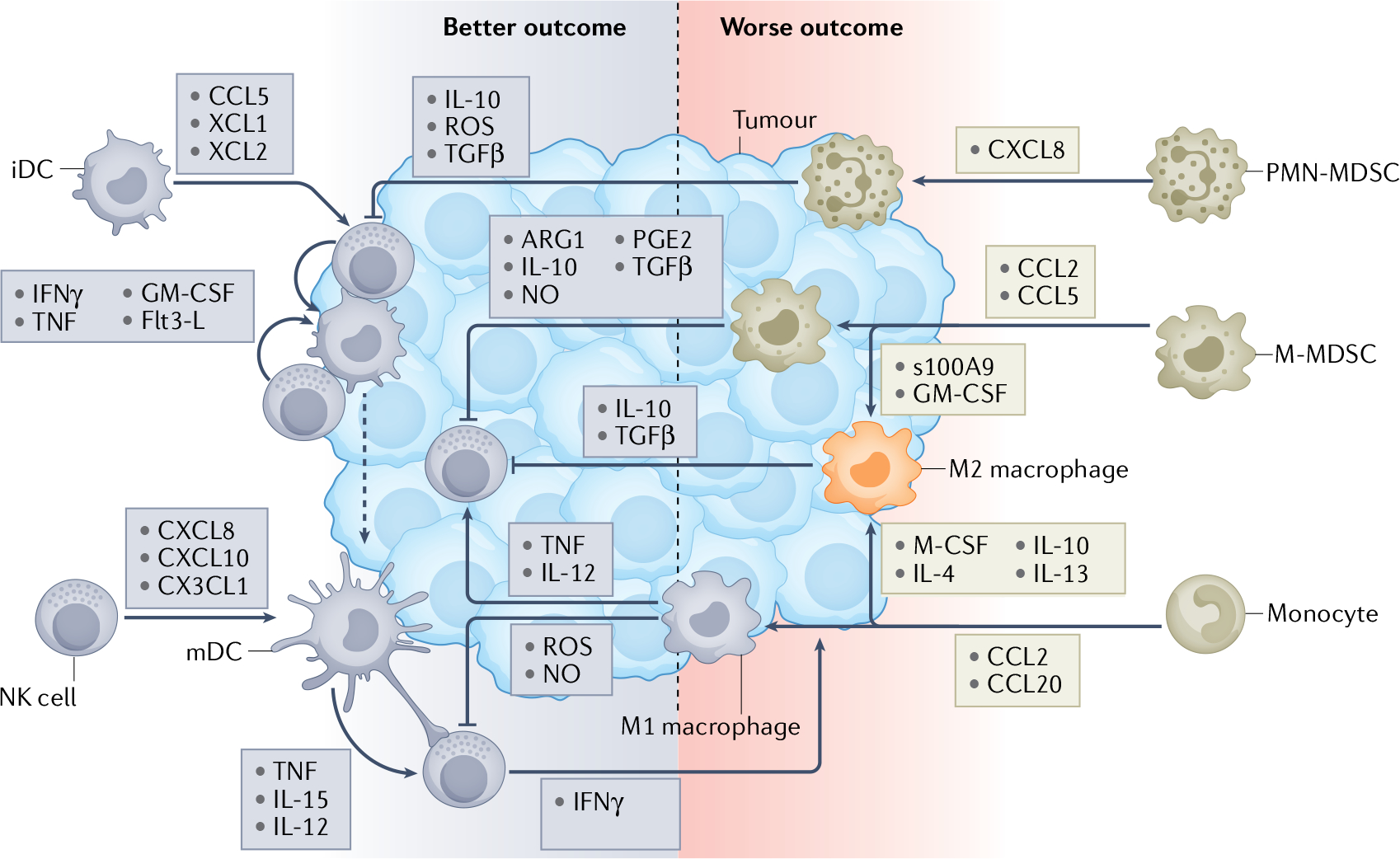
Innate immunity, an evolutionary defence against pathogens or tissue damage, plays a central role in antitumour defence as it dictates the robustness and function of tumour immune infiltrates. Human papillomavirus (HPV)-positive lesions generally have a greater level of innate immune infiltrates than HPV-negative lesions, which generally correlates with a better clinical outcome. Natural killer (NK) cell–dendritic cell (DC) crosstalk plays a pivotal role in antitumour immunity89,92. Enhanced NK cell and DC infiltrates correlate with better patient survival99,100. Tumour-infiltrating NK cells can recruit immature DC (iDCs) into the tumour microenvironment (TME) by releasing CCL5, XCL1 and XCL2 (ref.166). Once inside the tumour, iDCs can take up cell debris from killed tumour cells, which is the first step required for effective antigen presentation105. As antigen-loaded DCs start to mature, they can engage and crosstalk with NK cells with their long dendrites. NK cells enhance DC maturation and polarization through release of IFNγ, TNF, granulocyte–macrophage colony-stimulating factor (GM-CSF) and Flt3-L89,98. Maturing DCs (mDCs) in turn enhance NK cell activation through IL-12 secretion as well as cell-to-cell contact mediated by transmembrane TNF and trans-presented IL-15 (ref.104). Furthermore, they release a number of chemokines, including CXCL8, CXCL10 and CX3CL1, that enhance the number of NK cells infiltrating the tumour89,167. This cascade of events initiates and potentiates tumour-specific adaptive T cell responses that are required for effective tumour elimination105. The myeloid compartment is highly diverse and consists of various cell subsets that exhibit a broad range of immune functions. Myeloid cells commonly infiltrate the tumours and associated stroma and their presence influences outcomes in patients with head and neck squamous cell carcinoma (HNSCC). Patients with worse clinical outcome generally display higher numbers of tumour-infiltrating macrophages and myeloid-derived suppressor cells (MDSCs)107,118,119,123,125. Monocytes, monocytic MDSCs (M-MDSCs) and polymorphonuclear MDSCs (PMN-MDSCs) are recruited into a tumour by specific chemokines109,121. Once in the tumour, monocytes can differentiate into antitumour M1 macrophages and immunosuppressive M2 macrophages depending on the inflammatory signals they receive within the TME110. M-MDSCs can maintain their immature myeloid profile within the TME or can further differentiate into M2 macrophages under the influence of endogenous S100A9 and exogenous GM-CSF108,111–113. M2 macrophages, M-MDSCs and PMN-MDSCs all have a variety of mechanisms by which they can suppress NK cell and DC activation and skew their polarization towards an immunoregulatory phenotype. M1 macrophages, which can activate NK cells by IL-12 and TNF production, can also inhibit NK cell function by reactive oxygen species (ROS) and nitric oxide (NO) release116,117.
Conventional DCs bridge innate and adaptive immunity in the HNSCC TME
Known as professional antigen-presenting cells, conventional DCs are critical for the initiation and polarization of tumour antigen-specific immunity. By presenting tumour antigen-derived peptides in the context of MHC class I and II molecules, they can effectively prime and polarize naive CD4+ and CD8+ T cells, respectively97. Upon maturation, DCs can also effectively recruit NK cells (via CXCL8 (also known as IL-8), CXCL10 (also known as IP-10) and CX3CL1 (also known as fractalkine) release) and activate them (via IL-12p70 secretion and cell–cell contact via transmembrane TNF and trans-presented IL-15), which leads to reciprocal crosstalk and activation between these two cell types89,98 (Fig. 4).
In HNSCC, DC infiltration is directly associated with increased T cell and NK cell infiltration, decreased rates of tumour dissemination, and improved patient survival99,100. The impact of HPV status on DC infiltration and function remains ambiguous. Available studies suggest that HPV-positive tumours might have higher frequencies of tumour-infiltrating DCs than HPV-negative tumours; however, increased DC infiltrates are positively correlated with improved outcome only in patients with HPV-negative disease101. DC infiltration directly correlated with MIP3α/CCL20 expression102. The potential role of MIP3α/CCL20 in DC recruitment has also been implicated in HPV-driven cutaneous malignancies, where the HPV16 oncogenes E6 and E7 were shown to inhibit MIP3α transcription in human keratinocytes103. Two potential ways to improve the prognostic potential of DC-based immune scoring methods is to account for spatial relations of DC with other cell types found within the TME as well as their activation state and/or functional polarization. In the context of cetuximab therapy, ADCC-activated CD56dimCD16+ NK cells secrete IFNγ, which leads to increased DC maturation, type 1 skewing (characterized by increased production of IL-12p70, transmembrane TNF and trans-presented IL-15 as well as of NK cell-recruiting chemokines CXCL8, CXCL10 CX3CL1) and elevated expression of the NKG2D ligand MICA, which in turn further enhances NK cell activation98,104,105.
TAMs in patients with HNSCC have plasticity associated with HPV status
Tumour-associated macrophages (TAMs) are one of the major immune populations that infiltrate cancers106–108. In HNSCC, macrophage infiltration was elevated in tumours in comparison to normal mucosa and correlated with R/M disease and poor patient outcomes109. Increased TAM infiltration coincided with elevated MCP1/CCL2 and MIP3α/CCL20 expression in HNSCC109. Using scRNA sequencing, flow cytometry and multispectral imaging, TAMs were identified as the primary contributors of PDL1 within the HNSCC TME, regardless of disease aetiology28. Not only do TAMs express the highest levels of PDL1 on their cell membranes but PDL1+ macrophages spatially associate with CD8+ T cells within the HNSCC TME, which is imperative for cell–cell interactions28.
Macrophages are generally categorized into two functionally distinct/divergent subtypes of the activation–polarization continuum: M1 and M2 macrophages110. Tumour-ablating M1 macrophages are generated from monocytes polarized by IFNγ and granulocyte–macrophage colony-stimulating factor (GM-CSF)110. Pro-tumoural M2 macrophages can differentiate from monocytes or monocytic myeloid-derived suppressor cells (M-MDSCs)111. Monocyte-derived M2 macrophages are differentiated in the presence of macrophage colony-stimulating factor (M-CSF), IL-4, IL-10 and IL-13, while M-MDSC-derived M2 macrophages are generated in response to GM-CSF and endogenous S100A9 expression108,111–113. While M1 TAMs produce IL-12 for TH1-skewing and attack and eliminate tumour cells through reactive oxygen species (ROS) and nitric oxide (NO) release or by ADCC, M2 TAMs exhibit a variety of tumorigenic functions, including immune suppression via arginase 1, IL-10, PGE2 and TGFβ production, tumour vascularization, and promotion of tissue remodelling that facilitates tumour dissemination and radiotherapy resistance114–117. Thus, a high number of CD163+ M2 TAMs is associated with poor prognosis in HNSCC107,118,119 (Fig. 4). High infiltration of M1 TAMs and low infiltration of M2 TAMs were shown to correlate with superior prognosis in both HNSCC aetiologies, with HPV-positive tumours displaying a higher M1 to M2 TAM ratio15,47,115. Owing to the plasticity of TAMs, reprogramming tolerogenic M2 TAMs into antitumour M1 TAMs is a very promising tumour treatment strategy112.
Reducing immunosuppression in the HNSCC TME by targeting MDSCs
MDSCs are a heterogeneous group of highly immunosuppressive innate immune cells that expand owing to skewed haematopoiesis under pathological conditions such as cancer120,121. In nasopharyngeal carcinoma, a recent scRNA sequencing analysis demonstrated that MDSCs are developmentally similar to TAMs but are transcriptionally distinct cells122. They interact with T cells, DCs, macrophages, and NK cells and suppress their functions through a variety of mechanisms, including chronic production of ROS, NO, anti-inflammatory cytokines, arginase 1 and prostaglandin E2 (PGE2)120. Two key MDSC subsets have been identified: M-MDSCs and polymorphonuclear MDSCs (PMN-MDSCs)121. M-MDSCs and PMN-MDSCs are defined as CD14+CD11b+HLA-DRlo/–CD15− and CD15+CD11b+CD14−, respectively. M-MDSCs mediate immunosuppression primarily through arginase 1, NO, PGE2, TGFβ and IL-10, while PMN-MDSCs inhibit immune responses via ROS, TGFβ and IL-10 (ref.121). Tumour infiltration of these MDSC subsets is mediated by specific chemokines: MCP1/CCL2 and RANTES/CCL5 for M-MDSCs, and CXCL8 for PMN-MDSCs121.
Although there is no evidence to suggest that MDSC infiltration in HNSCC is driven by disease aetiology, elevated MDSC infiltration is observed in HNSCC in general, and correlates with high clinical stage and pathological grade123. In patients with melanoma, high numbers of circulating and tumour-infiltrating MDSCs are associated with poor patient prognosis and lack of responsiveness to immune-checkpoint blockade124,125. Immune-checkpoint blockade resistance can also be acquired as the number of intratumoural MDSCs increases following immune-checkpoint blockade124. To this end, a 2021 study reported that CD155+PDL1+ MDSCs are enriched in patients with HNSCC and that blockade of the TIGIT–CD155 axis effectively enhanced the response rate of patients with HNSCC to PDL1 blockade125.
Multiple strategies have been developed that target MDSC accumulation and differentiation (for example, all-trans-retinoic acid and sunitinib), tumour infiltration and function (for example, CXCR2-blocking and CSFR1-blocking antibodies, PDE5 inhibitors, triterpenoids, non-steroid anti-inflammatory drugs), or viability (for example, gemcitabine, 5-fluorouracil and DR5 antibodies)126–129. In vivo studies have shown that resistance to immune-checkpoint blockade can be partially alleviated by targeting MDSC accumulation (using, for example, anti-IL-18 antibodies, phenformin or selective inhibitors of soluble TNF), function (using PI3Kδ/γ and CSF1R inhibitors) and trafficking (using CXCR2)126,130,131. Clinical translation of these drugs has been hampered by their off-target toxicities, selective impact on specific MDSC subsets and limited antagonism of suppressive functions.
Stromal tissue regulates the HNSCC TME
When designing new therapeutics, consideration of the contribution of non-immune stromal cells is important as these cells can influence the phenotype and function of immune cells and control the nutritional and structural support for the TME (Fig. 5). Here, we focus on tumour stromal cells, including fibroblasts and mesenchymal stromal cells, and their interactions with malignant cells and immune cells in the HNSCC TME.
Fig. 5 |. The stromal microenvironment is functionally important for the HNSCC TME.
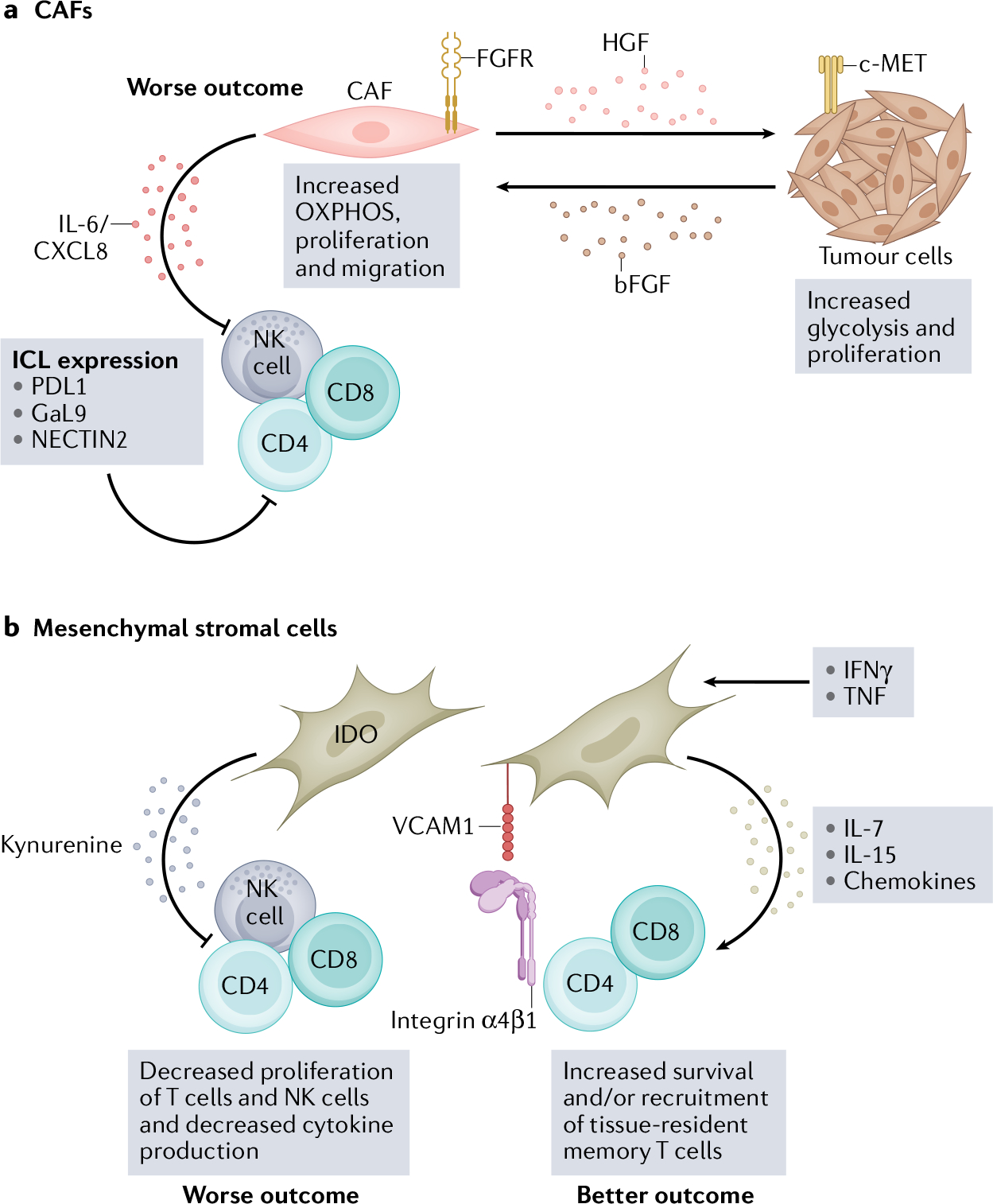
The head and neck squamous cell carcinoma (HNSCC) tumour microenvironment (TME) consists of a diverse stromal cell compartment — which includes cancer-associated fibroblasts (CAFs) and mesenchymal stromal cells (MSCs) — that interacts with neighbouring tumour cells and infiltrating immune cells106,143. These interactions primarily promote tumour growth, progression and metastases but, under some conditions, can provide a supportive environment for immune cells and recruit immune cells to the HNSCC TME132,141. Combining immune-checkpoint therapy with therapies that target the stromal compartment might improve patient outcomes in HNSCC. a, CAFs and tumour cells have a metabolic relationship mediated by hepatocyte growth factor (HGF) and basic fibroblast growth factor basic (bFGF), which increases oxidative phosphorylation (OXPHOS) in CAFs and glycolysis in tumour cells137. Increased oxidative phosphorylation in CAFs leads to IL-6 and CXCL8 production, which suppress immune cell function134,137. CAFs also express several immune-checkpoint ligands (ICL), including PDL1, galectin 9 (GaL9) and nectin cell adhesion molecule 2 (NECTIN2), which interact with corresponding inhibitory receptors on natural killer (NK) cells and T cells28. b, MSCs can also suppress immune cell function in vitro via indoleamine-pyrrole 2,3-dioxygenase (IDO), which metabolizes tryptophan into kynurenine and is cytotoxic to T cells and NK cells140,142,143. However, some in vitro studies suggest that MSCs can support tissue-resident memory T cells via IL-7 and IL-15 production in the presence of IFNγ and TNF144. VCAM1, vascular cell adhesion protein 1.
CAFs have vast functional differences in the HNSCC TME
scRNA sequencing performed on patients with treatment-naive HNSCC with primary tumours and lymph node metastasis revealed heterogeneity among tumour cells in patients with HPV-negative HNSCC tumours30. Seven expression patterns related to cell cycle, hypoxia, and epithelial genes and partial epithelial to mesenchymal transition (pEMT) were observed. Notably, pEMT-high cells were spatially localized to the leading edge of primary tumours near cancer-associated fibroblasts (CAFs) and expressed ligands that correspond to receptors expressed by malignant cells, particularly of interactions important for pEMT transition30,132. In late-stage HNSCC tumours, fibroblasts constitute up to 80% of the cellular composition and have multiple functions, including secreting collagen and fibrous macromolecules that build the extracellular matrix (ECM), maintenance of tissue homeostasis, recruitment of immune cells via secretion of cytokines, chemokines and growth factors, regulation of cell mobility within tissue through degradation of the ECM via matrix metalloproteinases (MMPs), and metabolic reprogramming. CAFs have been reported to be quite variable in phenotype in human solid tumours and thus to differ in function106,133–136.
The composition of CAFs in HNSCC was recently characterized in two independent scRNA sequencing studies28,31. In the first study, eight CAF subpopulations were uncovered using gene set enrichment analysis31. Seven of these subpopulations were significantly enriched in HNSCC tumour tissue compared to normal tissues. Subpopulations of myofibroblasts, EMT-positive CAFs, and MHC class II plus CAFs were associated with poor overall survival. Pathway enrichment analysis revealed that these three subpopulations had distinct biological functions related to the cyclic guanosine monophosphate-dependent protein kinase (cGMP–PKG) signalling pathway and the oxytocin signalling pathway, to oxidative phosphorylation and ECM receptor interactions, and antigen processing and presentation, respectively31. Distinctions between HPV-positive and HPV-negative HNSCC were not made in these analyses. In another scRNA sequencing study, nine subpopulations of fibroblasts were identified that could be grouped into three major subgroups: classical CAFs, normal activated fibroblasts and elastic fibroblasts28. Classical CAFs were enriched for genes encoding proteins such as fibroblast activation protein (FAP), platelet-derived growth factor receptor (PDGFRA), lysyl oxidase (LOX) and MMPs. Normal activated fibroblasts showed low expression of CAF markers. Elastic fibroblasts were enriched for tropoelastin (ELN), fibrillin 1 (FBLN1) and microfibril-associated protein 4 (MFAP4). The CAF gene signature correlated with poor overall survival in HPV-positive but not in HPV-negative HNSCC. Patients with HPV-positive HNSCC with both a low frequency of elastic fibroblasts and a classical CAF signature had the best overall survival28.
Functional analysis of CAFs derived from patients with HNSCC revealed that CAFs secrete hepatocyte growth factor (HGF), which promotes glycolysis in HNSCC tumour cells137,138. HNSCC tumour cells in turn secrete basic fibroblast growth factor (bFGF), which induces oxidative phosphorylation, proliferation and migration of CAFs by binding to the fibroblast growth factor receptor (FGFR). HGF regulates glycolysis in HNSCC tumour cells via the c-Met tyrosine kinase receptor expressed on HNSCC tumour cells. Inhibiting both c-Met and FGFR with small-molecule inhibitors attenuated CAF-associated HNSCC tumour growth. Interestingly, in 2D and 3D cultures, HPV-negative HNSCC cell line-conditioned media could activate normal oral fibroblasts to secrete HGF, IL-6, and CXCL8 and induce cell migration by fibroblasts while HPV-positive HNSCC cell lines could not134,139. Future studies should explore the secretory profile, proliferation and migration potential of CAFs in ex vivo HPV-positive and HPV-negative HNSCC tumours. The metabolic relationship between CAFs and tumour cells in HNSCC could be a potential targetable axis and enhance current ICI therapies. However, the metabolic relationship between CAFs and immune cells has not been fully elucidated. Notably, the heterogeneity in CAF phenotype and function can be patient specific and might provide a barrier to the therapeutic targeting of CAFs28,132,135.
MSCs can regulate immune responses in the HNSCC TME
Mesenchymal stromal cells (MSCs) are also referred to as mesenchymal stem cells because of their potential to differentiate into osteoblasts, chondrocytes, adipocytes or myocytes depending on environmental conditions136,140. This capability makes MSCs extremely important for tissue repair and regeneration. MSCs can also ameliorate tissue-damaging inflammation and immune responses through the inhibition of both innate and adaptive immune cells and can induce immune tolerance140. This function is mediated by the secretion of immunosuppressive soluble factors, such as PGE2, TGFβ, indoleamine 2,3-dioxygenase (IDO) and sHLA-G, and through the expression of inhibitory and apoptotic ligands such as PDL1, galectin 1 and 9, and FasL140,141. In malignant stroma, MSCs promote tumour progression and metastasis142.
MSCs are increased in HNSCC tumours compared to normal tissue, and when MSCs were isolated from patients with HNSCC, they inhibited in vitro cell proliferation and cytokine production by polyclonally stimulated CD4+ and CD8+ T cells143. HNSCC tumour-derived MSCs were also shown to inhibit T cell proliferation via IDO production and have a similar phenotype to normal bone marrow-derived MSCs143. Direct comparisons of MSCs in HPV-positive and HPV-negative tumours were not performed in this study most likely owing to the small number of patients included. However, of the 13 tumours analysed, 11 occurred in traditional HPV-negative sites. HNSCC-derived MSCs have also been reported to support survival of tissue-resident memory T cells through secretion of IL-7 and IL-15 (ref.144). Ex vivo, tissue residence markers (CD69 and CD103) were increased on CD4+ and CD8+ T cells in HNSCC and normal tissues compared to peripheral blood144. Co-culture of HNSCC-derived MSCs with healthy donor T cells increased CD69 and CD103 expression and migration of T cells. Vascular cell adhesion protein 1 (VCAM1) was shown to mediate interactions with HNSCC-derived MSCs and T cells, and blocking VCAM1 inhibited the upregulation of resident memory T cell markers144. Again, distinctions between HPV-positive and HPV-negative MSCs were not made; however, these studies suggest that HNSCC MSCs might have both pro-tumour and antitumour roles and their function in the TME warrants further investigation.
Current successes in targeting the TME
As highlighted in the Introduction, HNSCC can be divided into different diseases both biologically and clinically. The most common primary sites (oral cavity, larynx, hypopharynx, oropharynx) for HNSCC are divided by HPV status. Specifically, OPSCC is routinely divided into HPV-positive and HPV-negative with all other sites being predominantly HPV-negative (larynx, oral cavity, hypopharynx). In the setting of curative intent, locally advanced HPV-positive OPSCC can further be subdivided based on clinical staging and smoking status into low-risk disease with the best prognosis and intermediate-risk disease. For example, based on smoking status (≤10 pack-years or >10 pack-years) and nodal stage, 3-year overall survival of locally advanced low-risk HPV-positive, intermediate-risk HPV-positive and HPV-negative OPSCC was 90%, 70% and 30%, respectively145,146. For low-risk HPV-positive OPSCC, the field is evaluating de-escalation strategies through clinical trials, with the goal of maintaining the excellent outcomes in these patients but lowering the toxicity from treatment, especially long-term toxicity, which can negatively impact quality of life. Clinical trials continue to evaluate de-escalation strategies, such as reducing radiation dose and/or field, and/or replacing or omitting cisplatin chemotherapy with immunotherapy, either after minimally invasive surgery or in lieu of surgery. In the HPV-negative group, outcomes have not improved for decades in the locally advanced setting; ongoing trials therefore look to escalate therapy beyond our current therapeutics, including the addition of immunotherapy or novel radiosensitizers to standardize chemoradiation therapy (CRT). Patients with intermediate-risk HPV-positive OPSCC are often included in escalation strategies.
Paramount to the integration of immunotherapy in the locally advanced setting is an improved understanding of its interaction with standard CRT, with both standard modalities having stimulatory and suppressive immune effects. The first reported phase III escalation trial (JAVELIN 100) evaluating chemoradiation with or without the anti-PDL1 mAb avelumab given during and after CRT did not meet its primary objective of prolonging progression-free survival147. The lack of benefit with the addition of immunotherapy in this trial points to a need for understanding the best sequence of anti-PD1/PDL1 blockade and standard CRT. To this end, an ongoing randomized phase II trial specifically evaluating the timing of anti-PD1 (concurrent versus after CRT) has completed accrual with results currently pending (NCT02777385). Interestingly, in exploratory analysis of JAVELIN, the only subgroup in whom benefit of avelumab added to CRT was observed was that of patients who had high PDL1 (>25%) expression at baseline147. This finding suggests that perhaps only an immune-enriched subset would benefit from the addition of anti-PD1/PDL1 in the locally advanced setting. Additional phase III trials evaluating pembrolizumab concurrent with and after CRT (KEYNOTE412; NCT03040999) and atezolizumab starting after CRT (IMvoke 010; NCT03452137) are ongoing as is a phase III trial using anti-PD1 mAb therapy in the adjuvant setting post resection (RTOG 1216; NCT01810913).
Only 10% of patients with HNSCC present with distant metastatic disease but a large proportion, especially of those who are HPV negative, will either recur after curative intent therapy within the head and neck and/or develop metastasis to distant organs. These patients face tremendous morbidity and mortality and there is a great need for improvement in outcomes with systemic therapy. While patients with HPV-positive OPSCC have better outcomes than those with HPV-negative disease in the R/M setting, the difference is less than that seen in the locally advanced setting and systemic therapy alone is palliative148. However, great gains have been seen in the setting of R/M OPSCC over the past 5 years, first with the approval of nivolumab and pembrolizumab in 2016 in the platinum failure setting, and more recently with pembrolizumab with or without chemotherapy in the frontline setting in 2019 (refs.149–151). With this progress have come new questions, including how to improve outcomes in the anti-PD1-naive setting with combination therapy, an increased need for predictive biomarkers, and where immunotherapy fits after anti-PD1/PDL1 failure.
Efforts to improve outcomes in the frontline setting include immunotherapy combinations and immunotherapy in combination with targeted therapy. Unfortunately, two phase III trials evaluating anti-PD1/PDL1 plus anti-CTLA4 mAbs, Checkmate 651 and Kestrel, evaluating nivolumab plus ipilimumab and durvalumab plus tremelimumab, respectively, both failed to improve overall survival compared to chemotherapy for the primary trial populations evaluated, although the nivolumab plus ipilimumab regimen did appear to be superior to the EXTREME chemotherapy triplet152,153. A phase II/III trial with anti-PD1 plus an ICOS agonist did not achieve the efficacy needed to transition to the phase III portion154. Other potentially promising combinations for the frontline anti-PD1-naive setting include anti-PD1 plus anti-B7H3 mAb or cetuximab. Cetuximab in combination with pembrolizumab showed a response rate of 45% in patients who are anti-PD1 naive with R/M HNSCC, most of whom had failed platinum chemotherapy150. This result is nearly three times the response rate expected with pembrolizumab alone. Immunotherapeutics that can be given via intratumoural injection are also promising. Based on activity in melanoma, a frontline trial with the TLR agonist CMP-001 plus pembrolizumab is under way (NCT046332789). Although only a subset of patients with R/M HNSCC will have a lesion amenable to intratumoural injection, a reliable response at least in the accessible lesion could have the potential to reduce morbidity. Many novel combination immunotherapy strategies are under way in the anti-PD1/PDL1 failure setting. The majority are phase I studies with expansion cohorts that include HNSCC but two randomized phase III trials are ongoing. One trial is evaluating the multi-targeted tyrosine kinase inhibitor lenvatinib plus pembrolizumab (NCT04428151). The other is evaluating the natural killer group 2A (NKG2A) inhibitor monalizumab in combination with cetuximab compared with cetuximab alone in patients who have failed both platinum chemotherapy and anti-PD1 mAb therapy based on a response rate of 20% with the combination, with stable disease in an additional 38% of patients, from a single-arm trial of patients who had failed multiple lines of therapy, including immunotherapy155.
Most trials include all patients and stratify by HPV status but some trials are focusing solely on R/M HPV-positive OPSCC, primarily those investigating vaccines and adoptive T cell therapy. Treatment with ISA 101, a synthetic long-peptide HPV16 vaccine, in combination with nivolumab had an impressive response rate of 33% in a single-arm trial and a randomized phase II trial is ongoing, including frontline patients and those with platinum failure156. Data are limited on adoptive T cell therapy targeting HPV. Two small trials with autologous genetically engineered T cells expressing a TCR against either HPV16 E6 or E7 have been reported. A trial including 12 patients targeting E6 had 1 patient with OPSCC who achieved only stable disease, and another study with T cells targeting E7 included 4 patients with HNSCC, 2 of whom had a partial response and 2 of whom achieved stable disease. In both studies, the benefit was acute, only lasting 3–4 months157,158.
Although blockade of the anti-PD1/PDL1 pathway has revolutionized the field of oncology (including in R/M HNSCC), only a minority of patients benefit from these agents. The successes have been limited because efforts to target the complete HNSCC TME have not been made. Indeed, most immunotherapeutic regimens have been T cell centric (ICIs such as anti-CTLA4 and anti-PD1, HPV-specific vaccines, and adoptive T cell therapies). We have seen some new examples of targeting the diversity of the HNSCC TME (for example, cetuximab for Treg cell and NK cell targeting and intratumoural injection for increased myeloid cell function), but unique therapeutics are still limited. While efforts focusing on HPV are still ongoing, it is likely that we will have to take an even more personalized approach based on the characteristics of each individual patient’s TME for the field to take the next big leap with immunotherapy. Important to this goal are neoadjuvant window trials to better define the mechanism of action of current and novel agents and prospective biomarker-driven selection of combinations that also considers the known differences in HPV-positive and HPV-negative disease.
Lessons from anti-PD1 and novel treatments
With more therapeutic choices, including those in clinical trials, predictive biomarkers of efficacy are even more important. As biomarkers are being evaluated, we can learn a lot about the future of immunotherapeutics from anti-PD1 in patients with HNSCC. In anti-PD1-treated R/M HNSCC, increased PDL1, particular IFNγ-related immune gene expression profiles (GEP) and an increased tumour mutational burden (TMB) have all been associated with greater efficacy with anti-PD1 mAb monotherapy149,151,159. Multiple studies have shown that PDL1 shows increased predictive value when its expression on tumours and immune cells is combined versus its expression on tumour cells alone160,161. The predictive value of these biomarkers in combination has been evaluated in R/M HNSCC. PDL1, TMB and GEP were independently associated with response to anti-PD1 mAb therapy, with the highest efficacy in patients with high TMB and high GEP or high TMB and high PDL1, with correlation only found between PDL1 and GEP159. Remarkably, the response rate was still only 34% in patients with a favourable combination of these biomarkers159. Intratumoural hypoxia has also been shown to be predictive in R/M HNSCC and, importantly, is a biomarker that has the potential to be therapeutically targeted162. Data are conflicting as to whether HPV positivity is associated with a higher chance of response, with a more consistent increased response observed in HPV-positive than in HPV-negative disease when both groups expressed PDL1 (refs.159,162). What is clear and important is that both patients with HPV-positive and patients with HPV-negative HNSCC benefit from anti-PD1 mAb therapy over standard-of-care chemotherapy and that the magnitude of benefit is similar in both groups163. While data are consistent in that the TME is more immunogenic in patients with HPV-positive disease, the great majority of these data are from patients at the time of their initial presentation with earlier-stage disease. An analysis of paired primary and recurrent HNSCC tumours demonstrated a significant decrease in CD8+ T cells, NK cells and B cells in recurrent tumours, with chemoradiation as part of their initial therapy at diagnosis being associated with a more immune suppressed TME at recurrence164. Although more data are needed, these findings suggest that the immune infiltration and activation status of HPV-positive and HPV-negative tumours might be more similar at recurrence than it is at initial presentation, potentially accounting for comparable efficacy of anti-PD1 mAb therapy in HPV-positive and HPV-negative HSNCC tumours.
Conclusions
With these lessons in mind, the complete HNSCC TME needs to be considered when designing next-generation immunotherapies (Fig. 6). Specific to CD8+ T cells, additional work on how non-viral neoantigen-specific CD8+ T cells could differentially contribute to therapeutic responses in patients with both HPV-positive and HPV-negative tumours will be important. Furthermore, how to augment CD8+ T cells with local intratumoural CD4+ T cell differentiation should be considered in the next generation of immunotherapies while specific therapeutics to dampen tumour Treg cell activity should be employed to release the brake on CD8+ T cell function. Cell types other than T cells might also greatly enhance antitumour activity in patients with HNSCC. Distinct B cell targeting strategies might be needed in patients with HPV-positive and HPV-negative HNSCC tumours but, overall, these strategies should focus on inducing TLS formation and driving antigen-specific intratumoural B cell maturation. Furthermore, an increased understanding of NK cell subset functions in patients with HNSCC is necessary for improved immunity and, specifically, to increase the influx of NK cells into the TME and improve the spatial interplay with myeloid cells such as DCs and macrophages. In addition, reducing M2 polarization and decreasing MDSC-related immunosuppression could alleviate pro-tumour immunity in both HPV-positive and HPV-negative TMEs. Finally, future studies should explore the effect of CAFs and MSCs in the TME and in the regulation of immunity before and after immunotherapy. New technologies, such as scRNA sequencing, multiplexed spatial imaging and spatial transcriptomics, will also reveal new targets of the TME to consider as next-generation therapies combined with current immunotherapies such as anti-PD1; however, other technologies, such as immunoPET, and preclinical models will be necessary to validate and implement these new targets. ImmunoPET is a new technology that combines the specificity of mAbs with highly sensitive PET to enable a 3D probing of the TME, which will be particularly useful in imaging the complex cellular neighbourhoods in the TME, in particular, TLS formation and maturation. Furthermore, while current preclinical markers for HNSCC enable a basic evaluation of the TME, it will be important to develop, in the longer term, more physiological murine models that reflect the differences outlined in this Review for target testing and validation. In this Review, we have summarized several new targets to further interrogate, which go far beyond the T cell. It is not just one immune player that must be targeted but, rather, an entire orchestra of immune and non-immune players that must synchronize for robust improvements in therapeutic strategies for patients with HPV-positive and HPV-negative HNSCC tumours. These new technologies and robust analyses of the TME can help with biomarker identification and, more importantly, could lead to personalized therapeutics in patients with HNSCC.
Fig. 6 |. Immune and non-immune therapeutic targets in the HNSCC TME.
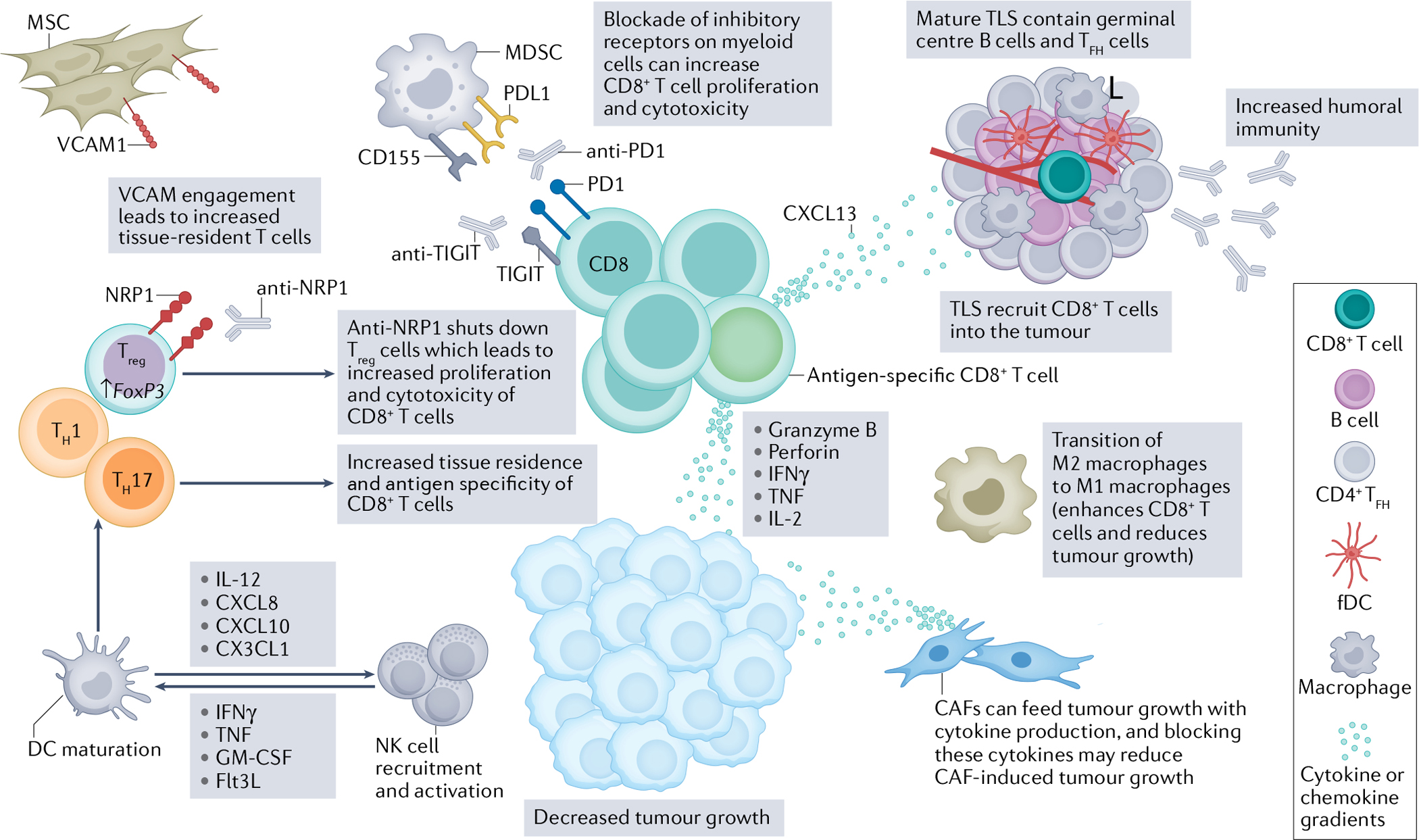
The head and neck squamous cell carcinoma (HNSCC) tumour microenvironment (TME) hosts a complex interplay of immune and non-immune cells that can amplify tumour killing. Augmenting aspects of antitumour immunity while suppressing pro-tumour immunity will be key in next-generation therapeutics. Although the CD8+ T cell has been the centre of immunotherapeutic strategies for several years, several other axes can be targeted for improved antitumour CD8+ T cell responses. First, blockade of the PD1–PDL1 pathway on myeloid cells, in particular myeloid-derived suppressor cells (MDSCs), has been an important consideration for improved intratumoural CD8+ T cell function. Additionally, other inhibitory receptors, like T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), can be blocked on myeloid cells to improve responses to anti-PD1 immunotherapy125. Further, reinvigoration of these antigen-specific CD8+ T cells can result in increased production of key chemokines, such as CXCL13, that can trigger tertiary lymphoid structure (TLS) development in the TME168. Amplifying mature, germinal centre-containing TLS will lead to increased recruitment of CD8+ T cells and increased antitumour humoral immunity. Further, directly enhancing germinal centre B cells and T follicular helper (TFH) cells could be paramount for increased antitumour immunity as they correlate with increased prognosis17,29. TFH cells also secrete CXCL13, which helps with further recruitment of B cells and CD8+ T cells into the TME and TLS53–55. Evidence exists to indicate that the transition of M2 macrophages to M1 macrophages will further enhance antitumour immunity112. Furthermore, dendritic cell (DC) maturation can be fuelled by natural killer (NK) cells via secretion of IFNγ, TNF, granulocyte–macrophage colony-stimulating factor (GM-CSF) and Flt3L89,98, which in turn will also help to activate and recruit more NK cells via IL-12, CXCL8, CXCL10 and CX3CL1 (refs.89,104,167). DC maturation is also instrumental in educating productive T helper 1 (TH1) and TH17 responses, which ultimately lead to increased tissue residence and antigen specificity of CD8+ T cells. While T regulatory (Treg) cells have dampened CD8+ T cells in the TME, new therapeutics, such as neuropilin 1 (NRP1) blockade, can specifically reduce immunosuppression without any effects on homeostatic balance in patients61,65. Finally, non-immune cells, such as cancer associated fibroblasts (CAFs) and mesenchymal stromal cells (MSCs), have a dichotomy of function in the TME. CAFs can feed tumour growth via cytokine production, and the metabolic crosstalk between CAFs and tumour cells could be a targetable axis to suppress tumour growth. MSCs can be advantageous in recruiting tissue-resident T cells to the TME in a vascular cell adhesion protein 1 (VCAM1)-dependent manner144. Ultimately, next-generation immunotherapies for HNSCC will be multi-faceted to improve patient response and treatment durability. fDC, follicular dendritic cell.
Footnotes
Competing interests
L.V. is a co-inventor of a methodology licensed to INmune Bio, Inc., where soluble TNF sequestration using DN-TNF can be used to prevent or treat malignancies. D.P.Z. declares competing interests with Blueprint Medicines (advisory board), Macrogenics (consulting), Prelude Therapeutics (advisory board), and Merck (advisory board) and research support (institutional) from Merck, BMS, AstraZeneca, GlaxoSmithKline, Aduro, Astellas, Macrogenics, Lilly, Bicara, Checkmate Pharma, and Novasenta. R.L.F. declares competing interests with Aduro Biotech, Inc. (consulting), AstraZeneca/MedImmune (clinical trial, research funding), Bristol-Myers Squibb (advisory board, clinical trial, research funding), EMD Serono (advisory board), MacroGenics Inc. (advisory board), Merck (advisory board, clinical trial), Novasenta (consulting, stock, research funding), Numab Therapeutics AG (advisory board), Pfizer (advisory board), Sanofi (consultant), Tesaro (research funding) and Zymeworks Inc. (consultant). T.C.B. declares competing interests with Walking Fish Therapeutics (Scientific Advisory Board). The other authors declare no competing interests.
Peer review information Nature Reviews Cancer thanks Zhijun Sun, Rieneke van de Ven and Moshe Elkabets for their contribution to the peer review of this work.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Ferlay J et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Sung H et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Johnson DE et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 6, 92 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HHS.gov. Smoking Cessation: A Report of the Surgeon General — Key Findings https://www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/2020-cessation-sgr-factsheet-key-findings/index.html#:~:Text=2020%20Surgeon%20General’s%20Report%20Findings,a%20decade%20to%20life%20expectancy (2020).
- 5.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29, 4294–4301 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell SF, Vu L, Spanos WC & Pyeon D The key differences between human papillomavirus-positive and -negative head and neck cancers: biological and clinical implications. Cancers 13, 5206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RL et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by prior cetuximab use. Clin. Cancer Res. 25, 5221–5230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner JA et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Cramer JD, Burtness B & Ferris RL Immunotherapy for head and neck cancer: recent advances and future directions. Oral. Oncol. 99, 104460 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L et al. Comprehensive immunogenomic landscape analysis of prognosis-related genes in head and neck cancer. Sci. Rep. 10, 6395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mito I et al. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci. Rep. 11, 16134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal R et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 1, e89829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang A-M et al. Tumor mutation burden, immune cell infiltration, and construction of immune-related genes prognostic model in head and neck cancer. Int. J. Med. Sci. 18, 226–238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X et al. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol. Immunol. 96, 28–36 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Petitprez F et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Ruffin AT et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 12, 3349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood O et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 7, 56781–56797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuerdemann N et al. LAG-3, TIM-3 and VISTA expression on tumor-infiltrating lymphocytes in oropharyngeal squamous cell carcinoma-potential biomarkers for targeted therapy concepts. Int. J. Mol. Sci. 22, 379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner A et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 8, 44418–44433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z et al. Novel effector phenotype of Tim-3+ regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin. Cancer Res. 24, 4529–4538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J-F et al. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 37, 44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duhen T et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang B, Lee JH & Bang D Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med. 50, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuksin M et al. Applications of single-cell and bulk RNA sequencing in onco-immunology. Eur. J. Cancer 149, 193–210 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry F et al. Single-cell RNA sequencing of the cardiovascular system: new looks for old diseases. Front. Cardiovasc. Med. 6, 173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X & Wang C-Y From bulk, single-cell to spatial RNA sequencing. Int. J. Oral. Sci. 13, 36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kürten CHL et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat. Commun. 12, 7338 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cillo AR et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity 52, 183–199.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puram SV et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q et al. Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveals distinct cancer-associated fibroblasts in head and neck squamous cell carcinoma. Ann. Transl. Med. 9, 1017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordfors C et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 49, 2522–2530 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Wuerdemann N et al. PD-L1 expression and a high tumor infiltrate of CD8+ lymphocytes predict outcome in patients with oropharyngeal squamous cells carcinoma. Int. J. Mol. Sci. 21, 5228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z et al. PD-L1 in combination with CD8+ TIL and HIF-1α are promising prognosis predictors of head and neck squamous cell carcinoma. Cancer Manag. Res. 12, 13233–13239 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Y et al. CD103+ T and dendritic cells indicate a favorable prognosis in oral cancer. J. Dent. Res. 98, 1480–1487 (2019). [DOI] [PubMed] [Google Scholar]
- 36.McKinney EF & Smith KG T cell exhaustion and immune-mediated disease-the potential for therapeutic exhaustion. Curr. Opin. Immunol. 43, 74–80 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Wherry EJ & Kurachi M Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wherry EJ T cell exhaustion. Nat. Immunol. 12, 492–499 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Beltra J-C et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kansy BA et al. PD-1 status in CD8+ T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 77, 6353–6364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberhardt CS et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 597, 279–284 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D et al. A comprehensive profile of TCF1+ progenitor and TCF1- terminally exhausted PD-1+CD8+ T cells in head and neck squamous cell carcinoma: implications for prognosis and immunotherapy. Int. J. Oral. Sci. 14, 8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borst J, Ahrends T, Bąbała N, Melief CJM & Kastenmüller W CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Tay RE, Richardson EK & Toh HC Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 28, 5–17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tesmer LA, Lundy SK, Sarkar S & Fox DA Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye J, Livergood RS & Peng G The role and regulation of human Th17 cells in tumor immunity. Am. J. Pathol. 182, 10–20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gameiro SF et al. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 7, e1498439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatt KH et al. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J. Exp. Med. 217, e20200389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albers A et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 65, 11146–11155 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Welters MJP et al. Intratumoral HPV16-specific T cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin. Cancer Res. 24, 634–647 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Kesselring R, Thiel A, Pries R, Trenkle T & Wollenberg B Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br. J. Cancer 103, 1245–1254 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Zhao Y, Zhang W & Zhang W Increased prevalence of T(H)17 cells in the peripheral blood of patients with head and neck squamous cell carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 112, 81–89 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Yang M et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J. Immunother. Cancer 9, e001136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niogret J et al. Follicular helper-T cells restore CD8+-dependent antitumor immunity and anti-PD-L1/PD-1 efficacy. J. Immunother. Cancer 9, e002157 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim ST et al. Human extrafollicular CD4+ Th cells help memory B cells produce Igs. J. Immunol. 201, 1359–1372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tahiliani V, Hutchinson TE, Abboud G, Croft M & Salek-Ardakani S OX40 Cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J. Immunol. 198, 218–228 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zotos D et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dadey RE, Workman CJ & Vignali DAA Regulatory T cells in the tumor microenvironment. Adv. Exp. Med. Biol. 1273, 105–134 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Sawant DV et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 20, 724–735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jie HB et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer 109, 2629–2635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chuckran CA et al. Prevalence of intratumoral regulatory T cells expressing neuropilin-1 is associated with poorer outcomes in patients with cancer. Sci. Transl. Med. 13, eabf8495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strauss L, Bergmann C, Gooding W, Johnson JT & Whiteside TL The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 13, 6301–6311 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Jie H-B et al. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 75, 2200–2210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oida T, Xu L, Weiner HL, Kitani A & Strober W TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J. Immunol. 177, 2331–2339 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Chuckran CA, Liu C, Bruno TC, Workman CJ & Vignali DA Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J. Immunother. Cancer 8, e000967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Economopoulou P, Kotsantis I & Psyrri A The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open 1, e000122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deaglio S et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janjic BM, Kulkarni A, Ferris RL, Vujanovic L & Vujanovic NL Human B cells mediate innate anti-cancer cytotoxicity through concurrent engagement of multiple TNF superfamily ligands. Front. Immunol. 13, 837842 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson BH CD20+ B cells: the other tumor-infiltrating lymphocytes. J. Immunol. 185, 4977–4982 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV & Chudakov DM B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 20, 294–307 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Yuen GJ, Demissie E & Pillai S B lymphocytes and cancer: a love-hate relationship. Trends Cancer 2, 747–757 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen P & Fillatreau S Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15, 441–451 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Germain C, Gnjatic S & Dieu-Nosjean M-C Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front. Immunol. 6, 67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruno TC et al. Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non-small cell lung cancer patients. Cancer Immunol. Res. 5, 898–907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sautès-Fridman C et al. Tertiary lymphoid structures and B cells: clinical impact and therapeutic modulation in cancer. Semin. Immunol. 48, 101406 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Kim SS et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer Res. 26, 3345–3359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helmink BA et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lechner A et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 8, 1535293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russell S et al. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head. Neck Oncol. 5, 24 (2013). [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int. J. Oral. Sci. 12, 24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pretscher D et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 9, 292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wieland A et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeske SS et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol. Immunother. 69, 1205–1216 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hladíková K et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. Immunother. Cancer 7, 261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Erp EA, Luytjes W, Ferwerda G & van Kasteren PB Fc-Mediated antibody effector functions during respiratory syncytial virus infection and disease. Front. Immunol. 10, 548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gül N & van Egmond M Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res. 75, 5008–5013 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Bald T, Krummel MF, Smyth MJ & Barry KC The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 21, 835–847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Concha-Benavente F et al. PD-L1 mediates dysfunction in activated PD-1+ NK cells in head and neck cancer patients. Cancer Immunol. Res. 6, 1548–1560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vujanovic L, Ballard W, Thorne SH, Vujanovic NL & Butterfield LH Adenovirus-engineered human dendritic cells induce natural killer cell chemotaxis via CXCL8/IL-8 and CXCL10/IP-10. Oncoimmunology 1, 448–457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vujanovic L et al. CD56dim CD16- natural killer cell profiling in melanoma patients receiving a cancer vaccine and interferon-α. Front. Immunol. 10, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stabile H et al. Reconstitution of multifunctional CD56lowCD16low natural killer cell subset in children with acute leukemia given α/β T cell-depleted HLA-haploidentical haematopoietic stem cell transplantation. Oncoimmunology 6, e1342024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wagner S et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer 138, 2263–2273 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Lisco A et al. Treatment of relapsing HPV diseases by restored function of natural killer cells. N. Engl. J. Med. 385, 921–929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srivastava RM et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 19, 1858–1872 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kurai J et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin. Cancer Res. 13, 1552–1561 (2007). [DOI] [PubMed] [Google Scholar]
- 96.López-Albaitero A et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol. Immunother. 58, 1853–1864 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma Y, Shurin GV, Peiyuan Z & Shurin MR Dendritic cells in the cancer microenvironment. J. Cancer 4, 36–44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerosa F et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195, 327–333 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jardim JF, Gondak R, Galvis MM, Pinto CAL & Kowalski LP A decreased peritumoral CD1a+ cell number predicts a worse prognosis in oral squamous cell carcinoma. Histopathology 72, 905–913 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Reichert TE, Scheuer C, Day R, Wagner W & Whiteside TL The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer 91, 2136–2147 (2001). [PubMed] [Google Scholar]
- 101.Partlová S et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 4, e965570 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kindt N et al. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral. Oncol. 62, 1–10 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Guess JC & McCance DJ Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J. Virol. 79, 14852–14862 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vujanovic L et al. Virally infected and matured human dendritic cells activate natural killer cells via cooperative activity of plasma membrane-bound TNF and IL-15. Blood 116, 575–583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wculek SK et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 106.Utispan K & Koontongkaew S Fibroblasts and macrophages: key players in the head and neck cancer microenvironment. J. Oral. Biosci. 59, 23–30 (2017). [Google Scholar]
- 107.Hu Y et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 35, 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mantovani A, Marchesi F, Malesci A, Laghi L & Allavena P Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marcus B et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer 101, 2779–2787 (2004). [DOI] [PubMed] [Google Scholar]
- 110.Mosser DM & Edwards JP Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kwak T et al. Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Rep. 33, 108571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cai H, Zhang Y, Wang J & Gu J Defects in macrophage reprogramming in cancer therapy: the negative impact of PD-L1/PD-1. Front. Immunol. 12, 690869 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sinha P, Clements VK, Bunt SK, Albelda SM & Ostrand-Rosenberg S Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Mantovani A et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004). [DOI] [PubMed] [Google Scholar]
- 115.Fu E et al. M2 macrophages reduce the radiosensitivity of head and neck cancer by releasing HB-EGF. Oncol. Rep. 44, 698–710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krneta T et al. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J. Leukoc. Biol. 101, 285–295 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Pan Y, Yu Y, Wang X & Zhang T Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ugel S, De Sanctis F, Mandruzzato S & Bronte V Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Invest. 125, 3365–3376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balermpas P et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer 111, 1509–1518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gabrilovich DI, Ostrand-Rosenberg S & Bronte V Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Veglia F, Sanseviero E & Gabrilovich DI Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gong L et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat. Commun. 12, 1540 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma X et al. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget 8, 42061–42075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meyer C et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63, 247–257 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mao L et al. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral. Oncol. 121, 105472 (2021). [DOI] [PubMed] [Google Scholar]
- 126.Highfill SL et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 6, 237ra67 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar V et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32, 654–668.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalinski P Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marvel D & Gabrilovich DI Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 125, 3356–3364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davis RJ et al. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kδ/γ. Cancer Res. 77, 2607–2619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sobo-Vujanovic A et al. Inhibition of soluble tumor necrosis factor prevents chemically induced carcinogenesis in mice. Cancer Immunol. Res. 4, 441–451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knops AM et al. Cancer-associated fibroblast density, prognostic characteristics, and recurrence in head and neck squamous cell carcinoma: a meta-analysis. Front. Oncol. 10, 565306 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou B et al. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. J. Oral. Pathol. Med. 43, 585–592 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Costea DE et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 73, 3888–3901 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Bienkowska KJ, Hanley CJ & Thomas GJ Cancer-associated fibroblasts in oral cancer: a current perspective on function and potential for therapeutic targeting. Front. Oral. Health 2, 686337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Valkenburg KC, de Groot AE & Pienta KJ Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar D et al. Cancer-associated fibroblasts drive glycolysis in a targetable signaling loop implicated in head and neck squamous cell carcinoma progression. Cancer Res. 78, 3769–3782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kang SH et al. Cancer-associated fibroblast subgroups showing differential promoting effect on HNSCC progression. Cancers 13, 654 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wheeler SE et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head. Neck 36, 385–392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu X et al. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 11, 345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kansy BA et al. The bidirectional tumor–mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res. Ther. 5, 95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu C et al. Bone marrow mesenchymal stem cells interact with head and neck squamous cell carcinoma cells to promote cancer progression and drug resistance. Neoplasia 23, 118–128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liotta F et al. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer 112, 745–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mazzoni A et al. Human T cells interacting with HNSCC-derived mesenchymal stromal cells acquire tissue-resident memory like properties. Eur. J. Immunol. 50, 1571–1579 (2020). [DOI] [PubMed] [Google Scholar]
- 145.Ang KK et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O’Sullivan B et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 17, 440–451 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Lee NY et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 22, 450–462 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Fakhry C et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 32, 3365–3373 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferris RL et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sacco AG et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 22, 883–892 (2021). [DOI] [PubMed] [Google Scholar]
- 151.Burtness B et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019). [DOI] [PubMed] [Google Scholar]
- 152.Argiris A et al. LBA36 Nivolumab (N) + ipilimumab (I) vs EXTREME as first-line (1L) treatment (tx) for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): final results of CheckMate 651. Ann. Oncol. 10.1016/j.annonc.2021.08.2113 (2021). [DOI] [Google Scholar]
- 153.Astrazeneca. Update on KESTREL Phase III trial of Imfinzi with or without Tremelimumab in the 1st-line Treatment of Recurrent or Metastatic Head and Neck Cancer https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/update-on-kestrel-phase-iii-trial-for-imfinzi.html (2021).
- 154.GSK. GSK Provides Update on Feladilimab, An Investigational Inducible T Cell Co-stimulatory (ICOS) Agonist https://www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-feladilimab-an-investigational-inducible-t-cell-co-stimulatory-icos-agonist/ (2021).
- 155.Cohen RB et al. Combination of monalizumab and cetuximab in recurrent or metastatic head and neck cancer patients previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors. J. Clin. Oncol. 38, 6516–6516 (2020). [Google Scholar]
- 156.Massarelli E et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 5, 67–73 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doran SL et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II Study. J. Clin. Oncol. 37, 2759–2768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nagarsheth NB et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 27, 419–425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seiwert TY et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 (2016). [DOI] [PubMed] [Google Scholar]
- 160.Ferris RL et al. Abstract CT021: Tumor-associated immune cell PD-L1 expression and peripheral immune profiling: Analyses from CheckMate 141. In Clinical Trials CT021–CT021 (American Association for Cancer Research, 2017). [Google Scholar]
- 161.Chow LQM et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J. Clin. Oncol. 34, 3838–3845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zandberg DP et al. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J. Immunother. Cancer 9, e002088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferris RL et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral. Oncol. 81, 45–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Watermann C et al. Recurrent HNSCC harbor an immunosuppressive tumor immune microenvironment suggesting successful tumor immune evasion. Clin. Cancer Res. 27, 632–644 (2021). [DOI] [PubMed] [Google Scholar]
- 165.Lu N et al. Human Semaphorin-4A drives Th2 responses by binding to receptor ILT-4. Nat. Commun. 9, 742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Böttcher JP et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park MH, Lee JS & Yoon JH High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J. Surg. Oncol. 106, 386–392 (2012). [DOI] [PubMed] [Google Scholar]
- 168.Schumacher TN & Thommen DS Tertiary lymphoid structures in cancer. Science 10.1126/science.abf9419 (2022). [DOI] [PubMed] [Google Scholar]


