Abstract
The most common cancer caused by human papillomavirus (HPV) infection in the United States is oropharyngeal cancer (OPC), and its incidence has been rising since the turn of the century. Due to substantial longterm morbidities with chemoradiation and the favorable prognosis of HPV-positive (HPV+) OPC, identifying the optimal deintensification strategy for this group has been a keystone of academic head-and-neck surgery, radiation oncology, and medical oncology for over the past decade. However, the first generation of randomized chemotherapy deintensification trials failed to change the standard of care, triggering concern over the feasibility of de-escalation. National database studies estimate that up to one-third of patients receive non-standard de-escalated treatments, which have subspecialty-specific nuances. A synthesis of the multidisciplinary deintensification data and current treatment standards is important for the oncology community to reinforce best practices and ensure optimal patient outcomes. In this review, the authors present a summary and comparison of prospective HPV+ OPC de-escalation trials. Chemotherapy attenuation compromises outcomes without reducing toxicity. Limited data comparing transoral robotic surgery (TORS) to radiation raise concern over toxicity and outcomes with TORS. There is promising data to support de-escalating adjuvant therapy after TORS, but consensus on treatment indications is needed. Encouraging radiation deintensification strategies have been reported (upfront dose-reduction and induction chemotherapy-based patient selection), but level one evidence is years away. Ultimately, stage and HPV status may be insufficient to guide de-escalation. The future of deintensification may lie in incorporating intra-treatment response assessments to harness the powers of personalized medicine and integrate real-time surveillance.
Introduction
The most common cancer caused by human papillomavirus (HPV) infection in the United States is oropharyngeal cancer (OPC), and its incidence has been rising since the turn of the century1,2 Even further, OPC incidence is projected to rise until the mid-2030s despite the availability of an HPV vaccine.3 In 2019, only 54% of adolescents and 21% of young adults were up-to-date with their HPV vaccines, and studies have reported drastic (up to 77%) declines in vaccine uptake in 2020 due to the Coronavirus Disease 2019 pandemic and vaccine hesitancy.3 Although increasing prevalence of oropharyngeal HPV infection has been implicated in the rising incidence of pathogenesis of the disease, increases in OPC survival have fortunately also been attributed to an increasing proportion of OPC cases resulting from HPV infection and a decline in OPC attributable to other causes such as tobacco.1
Regardless of HPV status, ample level one evidence established definitive concurrent cisplatin-based chemoradiotherapy (CRT) to 70Gy as one standard of care for locally advanced oropharyngeal cancer (OPC).4–10 Altered fractionation radiotherapy (AFRT), the addition of cetuximab, and induction chemotherapy failed to improve outcomes.4,5,6,11,12 Although 70Gy CRT cures the majority of patients treated, treatment comes at the cost of significant longterm toxicities. Unfortunately, up to 25% of ten-year survivors develop pharyngeal/laryngeal toxicity and 47% develop oral cavity toxicity.13 Pooled analysis of RTOG trials demonstrated that up to 43% of CRT patients experienced severe late toxicities including gastrostomy tube dependence, infection, fistula, or death.14
HPV-associated (HPV+) OPC has such a favorable prognosis that investigators have sought to deintensify treatment to reduce longterm morbidities in patients who are likely to live many years beyond their diagnoses. Recursive partitioning analysis (RPA) of RTOG 0129 defined “low-risk” as any HPV+ OPC with ≤10 pack-year smoking history (PYSH) or N0-N2a disease with >10 PYSH, and “intermediate-risk” as any HPV+ OPC with N2B-3 disease and >10 PYSH.4 Some deintensification trials included only low-risk while others also included intermediate-risk patients, highlighting discord even among experts on the most appropriate candidates for de-escalation.
However, enthusiasm for deintensification was tempered by apprehension of its viability when the first randomized controlled trials (RCTs) of chemotherapy attenuation and omission demonstrated the importance of standard of care (SOC) cisplatin to outcomes in HPV+ OPC.15–17 A recent meta-analysis of prospective and retrospective studies reported inferior overall survival (OS), progression-free survival (PFS), locoregional control (LRC), and distant metastasis-free survival (DMFS) with treatment deintensification in HPV+ OPC compared to SOC therapy.18 Ultimately, the American Society of Clinical Oncology (ASCO) issued a clinical provision that treatment de-escalation for HPV+ OPC “is a hypothesis that requires appropriate testing” and that “current treatment recommendations have not changed.19 Despite the failure of chemotherapy deintensification, the drive to decrease toxicity continues through other strategies: de-escalation through transoral robotic surgery (TORS) or deintensification of radiation therapy (RT).
A National Cancer Database (NCDB) registry analysis from 2010–2013 revealed that one-third of postoperative T1-T2 HPV+ OPC patients with intermediate-risk factors (2–4 involved nodes, lymphovascular invasion (LVI), or microscopic extracapsular extension (ECE)) received de-escalated adjuvant RT.20 Given that 85% of cancer patients are treated in the community but only 3% enroll on clinical trials, the vast majority of those de-escalated presumably were treated off clinical trial.21 The preponderance of deintensification trials and divergence in treatments administered despite no change in the SOC warrant an analysis of the data. Given subspecialty-specific nuances to de-escalation, interdisciplinary knowledge is ever more critical for these cancers which require multidisciplinary care. The aim of this review is to provide a synthesis of prospective deintensification trials (chemotherapy, surgery, and radiation), summarize current treatment standards, and explore consensuses and controversies in the management of HPV+ OPC to help reinforce best practices and ensure optimal patient outcomes.
Chemotherapy Deintensification Approaches
Cisplatin Attenuation
Considering its significant acute and chronic toxicities (nephrotoxicity, nausea/vomiting, ototoxicity, neuropathy), cisplatin attenuation was the first deintensification approach attempted. Table 1 illustrates eligibility criteria, treatment details, and outcomes from various chemotherapy attenuation and omission clinical trials. Given validated efficacy in head and neck cancer (HNC), the epidermal growth factor receptor (EGFR) inhibitor cetuximab became a promising alternative to cisplatin given seemingly less severe toxicities in the skin, gastrointestinal tract, and oropharyngeal mucosa.22 Another EGFR inhibitor (panitumumab) had failed to replace cisplatin in American Joint Committee on Cancer 7th Edition (AJCC7) Stage III/IV HNC.23,24 However, subset analysis from a randomized trial confirmed LRC, PFS, and OS benefits with cetuximab over RT alone in HPV+ OPC, warranting further comparison of cetuximab against cisplatin in this subset of patients.25 RTOG 1016 and De-ESCALaTE were the first chemotherapy deintensification RCTs, and both reported inferior outcomes with cetuximab with no improvements in acute or late severe toxicity (Table 1).15,16 In RTOG 1016, cisplatin conferred superior LRC, PFS, and OS.15 De-ESCALaTE showed significantly inferior LRC, DMFS, and OS, along with triple the recurrences (18% vs. 6%), with cetuximab.16 Ironically, attenuation of chemotherapy was not profoundly less toxic; although the mean number of grade 3–4 acute adverse events per patient was lower with cetuximab (2.35 cetuximab vs. 3.19 cisplatin, p<0.0001), the proportion of patients experiencing any grade 3–4 toxicity was similar in both groups (77.4% cetuximab vs. 81.7% cisplatin, p=0.16). While these trials did not change the SOC, they established high standards for modern 2-year PFS in HPV+ OPC (~85–94%) which appear favorable compared to historical RTOG studies (~80–87%).26
Table 1.
Chemotherapy Deintensification Trials
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| Chemotherapy Attenuation | |||
| De-ESCALaTE 16 | SOC def RT 70Gy (IMRT, 5 fxs/wk) with: | 2 yr PFS (p=0.0007): 94.0% vs. 83.9%, HR 3.4 [1.6–7.2] | Similar overall acute grade 3–5 toxicity: 87.6% vs. 87.9% |
| (Phase III) | HD cis 100 mg/m2 × 3c (n=166) | 2 yr LRF (p=0.0026): 3% vs. 12% | Similar overall late grade 3–5 toxicity: 29.5% vs. 22.4% |
| p16+ OPC. | VS. | 2 yr DM (p=0.0092): 3% vs. 9% | |
| AJCC7 T1N1-T4N3, T3-T4N0. | cetux wkly (n=168) | 2 yr OS (p=0.01): 97.5% vs. 89.4%, HR 5.0 [1.7–14.7] | |
| If >10 PYSH: N0-N2a. | Median FU = 25.9 months | 2 yr OS AJCC8 I/II (p=0.043): 98.4% vs. 93.2%, HR 4.3 [0.9–19.8] | |
| RTOG 1016 15 | SOC def AFRT 70Gy (IMRT, 6 fxs/wk) with: | 5 yr PFS (p=0.0002): 78.4% vs. 67.3%, HR 1.7 [1.3–2.3] | Similar overall acute grade 3–4 toxicity: 81.7% vs. 77.4% |
| (Phase III) | HD cis 100 mg/m2 × 2c (n=406) | 5 yr LRF (p=0.0005): 9.9% vs. 17.3%, HR 2.1 [1.4–3.1] | Similar overall late grade 3–4 toxicity: 20.4% vs. 16.5% |
| p16+ OPC. | VS. | 5 yr DM (p=0.09): 8.6% vs. 11.7%, HR 1.5 [0.9–2.4] | |
| AJCC7 T1N2a-T4N3, T3-T4N0-N3. | cetux wkly (n=399) | 5 yr OS (p=0.0163): 84.6% vs. 77.9%, HR 1.4 [upper CI 2.1] | |
| Any PYSH. | Median FU = 4.5 years | 5 yr OS AJCC8 I (p=NS): 92.4% vs. 85.9% | |
| TROG 12.01 29 | SOC def RT 70Gy (IMRT, 5 fxs/wk) with: | 3 yr PFS (p=0.015): 93% vs. 80%, HR 3.0 [1.2–7.7] | No difference in primary endpoint of symptom severity. |
| (Phase III) | LD cis 40 mg/m2 × 7c (n=92) | 3 yr LRF (p=0.11): 2% vs. 8%, HR 3.4 [0.7–16.6] | Similar acute Grade 3 dysphagia: 50% vs. 46% |
| p16+ OPC. | VS. | 3 yr DM (p=0.018): 3% vs. 12%, HR 4.1 [1.2–14.9] | Similar acute Grade 3 oral mucositis: 63% vs. 72% |
| AJCC7 T3N0-N2c, T1-T2 N2a-N2c; | wkly cetux (n=90) | 3 yr OS (p=0.32): 98% vs. 96%, HR 2.3 [0.4–12.7] | No difference in enteral feeding: 61% vs. 58% |
| excluded N2b-N2c if >10 PYSH. | Median FU = 4.1 years | AJCC8 I subset : 13 events, 121 pts, HR ~2.5 | |
| Immunotherapy | |||
| NCT03410615 /CCTG HN.934 | SOC def CRT (70Gy + HD cis 100 mg/m2 × 3c) | Ongoing, not reported | Ongoing, not reported |
| (Phase II, randomized) | VS. | ||
| p16+ OPC. | durva + RT + (adj durva or tremelimumab+durva) | ||
| AJCC7 T1–3 N2 (any PYSH); | |||
| if ≥ 10PYSH: T1–2N1 or T3N0–1. | |||
| NRG-HN005 | SOC def CRT (70Gy+HD cis × 2c) | Ongoing, not reported | Ongoing, not reported |
| (Phase II/III) | VS. | ||
| p16+ OPC. | Reduced dose def CRT (60Gy+HD cis × 2c) | ||
| AJCC7 T1–2 N1–2B, T3 N0–2B. | VS. | ||
| <10 PYSH. | Reduced dose def RT + IO (60Gy+nivolumab) | ||
| MDACC / NCT03799445 | Reduced def RT (50–66 Gy) with: | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | Anti-CTLA4 (ipilimumab) + | ||
| p16+OPC. | Anti-PD-1 (nivolumab) | ||
| AJCC7 T1N2A-2CM0, T2N1–2CM0 | |||
| Chemotherapy Omission | |||
| NRG-HN002 17 | Reduced dose def CRT | Null hypothesis 2 yr PFS ≤85%: | Higher acute toxicity with CRT. |
| (Phase II, randomized) | (60Gy IMRT + LD cis 40 mg/m2 × 6c, n=157): | - CRT 90.5% [84.5–94.7], p=0.0350, rejecting null hypothesis | Acute Grade 3–4 (p<0.001): 79.6% vs. 52.4% |
| p16+ OPC. | VS. | - AFRT 87.6% [81.1–92.5], p=NS, failing to reject null hypothesis | Late Grade 3–4 toxicity similar (p=NS): 21.3% vs. 18.1% |
| AJCC7 T1–2 N1–2b, T3 N0–2b. | AFRT alone (60Gy/5 wks IMRT, n=149): | 2 yr LRF (p=0.02): 3.3% vs. 9.5%, HR 2.56 [1.11–5.88] | |
| <10 PYSH. | 19.9% Unilateral RT. 80.1% Bilateral RT. | 2 yr DM (p=0.58): 4.0% vs. 2.1%, HR 0.70 [0.20–2.50] | |
| Median FU = 2.6 years | 2 yr OS (p=0.93): 96.7% vs. 97.3%, HR 1.05 [0.34–3.23] | ||
| Osaka Multicenter 38 | SOC def RT (IMRT, n=39). | 2/3 yr PFS: 94% [81–99] / 89% [74–96] | Acute grade 3 mucositis: 26% |
| (Phase II) | 70Gy/35 fxs or 70.4Gy/32fxs. | 2/3 yr OS: 100% / 97% [84–99] | Acute grade 3 dysphagia: 18% |
| p16+ and HPV+ OPC. | No chemo. | 2yr cumulative incidence of DM: 5% [1–15] | No late grade 3 toxicity. |
| AJCC7 Stage III/IV. | Median FU = 51 months | ||
| T1N1, T4, and N3 excluded. | |||
| Any PYSH. | |||
AFRT= altered fractionation radiation therapy, AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, c=cycles, cetux=cetuximab, cis=cisplatin, CRT=chemoradiation therapy, def=definitive, DM=distant metastasis, durva=durvalumab, FU=follow-up, fxs= fractions, Gy=gray, HD=high-dose, HR=hazard ratio, IMRT=intensity-modulated radiation therapy, LD=low-dose, LRF= locoregional failure, NS=not significant, OPC= oropharyngeal cancer, OS=overall survival, PFS=progression-free survival, PYSH=pack-year smoking history, RT=radiation therapy, SOC=standard of care, wk=week, wkly=weekly, yr=year.
Not only should cetuximab not replace SOC bolus high-dose (HD) cisplatin (100 mg/m2 q3weeks x3 cycles) in HPV+ OPC, it should not replace low-dose (LD) cisplatin (30–40 mg/m2 weekly) either. ARTSCAN III randomized HNC patients (~75% HPV+ OPC) to LD cisplatin versus cetuximab; three-year locoregional failure (LRF) was 23% with cetuximab versus 9% with LD cisplatin, and with similar toxicity.27 Subgroup analysis of HPV+ OPC patients in a phase two trial showed trends for inferior local control (LC) and OS with cetuximab versus LD cisplatin.28 Finally, TROG12.01 randomized patients with HPV+ OPC to cetuximab versus LD cisplatin (40 mg/m2), and 3-year PFS was inferior with cetuximab (93% vs. 80%, p=0.015) without less toxicity (Table 1).29
The potential synergism of immunotherapy in this immunosuppressive virus-associated cancer was hypothesis-generating and instigated much investigation. Survival advantages with immunotherapy in recurrent/metastatic disease piqued interest in its utilization in the definitive setting.30,31 REACH (GORTEC 2017–01) is a randomized trial comparing concurrent avelumab, cetuximab, and radiation followed by twelve months of adjuvant avelumab versus two SOC therapies (against HD cisplatin for cisplatin-eligible, and against weekly cetuximab for cisplatin-ineligible) in locally advanced HNC; results from the safety phase of the trial showed that the addition of avelumab was tolerable with no difference in grade ≥IV across groups (12% avelumab/cetuximab, 14% HD cisplatin, and 10% cetuximab).32 The PembroRAD study randomized cisplatin-ineligible HNC patients to cetuximab-RT versus the anti-PD1 pembrolizumab with RT; pembrolizumab-RT did not improve cancer outcomes but appeared to have less acute grade ≥3 toxicity (74% vs 92%, p=0.006).33 Table 1 illustrates three HPV+ OPC-specific immunotherapy trials which will assess durvalumab followed by adjuvant durvalumab or tremelimumab/durvalumab (with SOC 70Gy RT), nivolumab (HN005, which will also de-escalate RT to 60Gy), and ipilimumab and nivolumab (which will also de-escalate RT to 50–66Gy).34 While there is a need to discover appropriate upfront uses of immunotherapy, there is cause for caution as well. JAVELIN Head and Neck 100 uncovered a possible antagonistic effect of concurrent immune checkpoint inhibition with definitive CRT.35 Corroborating this, a randomized phase two study comparing concurrent versus sequential pembrolizumab with CRT in HNC showed numerically higher 2-year PFS with sequential administration (78% versus 89%) and recommended sequential pembrolizumab as the preferred regimen to compare with SOC CRT in HNC in a phase three trial.36 While not powered for subgroup analysis, HPV+ HNC patients on JAVELIN did not benefit from immunotherapy. Furthermore, a systematic review and meta-analysis of HNC immunotherapy trials found no significant difference in response when results were stratified by HPV status.37 At this time, there is no data to support that HPV status influences the decision to use immunotherapy, highlighting the need to identify biomarkers to predict response.
Cisplatin Omission
Even further than attenuation are attempts at chemotherapy omission. A multicenter phase two trial from Osaka treated HPV+ OPC with intensity-modulated RT (IMRT) alone, reporting 94% 2-year PFS (Table 1).38 HN002 (Table 1) was a phase two randomized trial which hypothesized that modestly reduced CRT (60Gy IMRT with LD cisplatin) and AFRT (60Gy/5 weeks IMRT alone) would both achieve 2-year PFS ≥85%.17 Two-year PFS without cisplatin did not meet the threshold to support omission. While there were more acute grade 3–4 toxicities with cisplatin, late grade 3–4 toxicities were comparable at ~20%. Given that HN002 randomized over 300 patients and included more favorable patients than the Osaka trial (n=39), the best available data clearly refute the omission of chemotherapy.
Given canonical studies, the supremacy of CRT in HPV+ OPC is not surprising. The Meta-Analysis of Chemotherapy on Head and Neck Cancer (MACH) reported absolute 5-year LC and OS benefits of 9% and 7%, respectively, with concomitant chemotherapy.39,40 Subsite analysis from the MACH showed the greatest survival benefit in early-stage OPC (p=0.02): Stage I/II (hazard ratio (HR) 0.75, n=362), Stage III (HR 1.01, n=1606), and Stage IV (HR 0.83, n=3679).41 However, the effect of stage did not retain significance on multivariate analysis. Furthermore, AFRT does not compensate for the absence of chemotherapy; the Meta-Analysis of Radiotherapy in Carcinomas of Head and Neck (MARCH) showed a 5.8% 5-year OS benefit with chemotherapy plus conventional RT over AFRT alone in non-metastatic HNC.42 Given that these landmark analyses included trials when HPV prevalence was lower and before HPV testing was routine, there was a conception that the benefits of chemotherapy may not extend to low-risk HPV+ OPC. However, the first randomized deintensification trials firmly concluded that chemotherapy improves outcomes—even in HPV+ OPC.
Optimal Cisplatin Administration
Not only does cisplatin remain SOC with RT, but HD bolus remains its standard administration. In the postoperative adjuvant setting, there are conflicting data on the supremacy of HD cisplatin. An RCT from India of mostly postoperative HNC patients showed inferior LRC with LD cisplatin (30 mg/m2) versus HD cisplatin, with trends towards inferior PFS and OS.43 An RCT from Japan of exclusively postoperative HNC patients showed noninferiority with LD cisplatin (40 mg/m2) versus HD cisplatin, although the HR noninferiority margin was set high at 1.32.44 As a current standard for postoperative HPV+ OPC with high-risk features, ECOG E3311 administered adjuvant radiation therapy with LD cisplatin (40 mg/m2 weekly) and reported a favorable 2-year PFS of 90.7% [90% confidence interval (CI) 86.2–95.4].45 Taken together, although HD cisplatin is SOC in the postoperative HNC setting, LD cisplatin at 40 mg/m2 weekly is defensible while adjuvant LD cisplatin at 30 mg/m2 weekly is not. However, in the definitive setting, HD cisplatin remains the SOC. A small retrospective study of CRT in specifically HPV+ OPC found more local and distant failures with LD (40 mg/m2 weekly) versus HD cisplatin (2-year PFS 75% vs. 96%, p=0.04), although OS was similar.46 ConCERT was a randomized noninferiority trial of HD versus LD (40 mg/m2 weekly) cisplatin for definitive CRT in OPC (87% HPV-negative), laryngeal cancer, hypopharyngeal cancer, and oral cavity cancer; 2-year LRC with LD cisplatin was within the 10% non-inferiority margin, but only about 20 patients in the trial had HPV+ OPC.47 Although not limited to HPV+ OPC, NRG-HN009 will randomize definitive CRT HNC patients to LD (40 mg/m2) versus SOC HD cisplatin. Until published, HD cisplatin remains the SOC for definitive CRT patients.
Summary
In summary, three RCTs (De-ESCALaTE, RTOG 1016, TROG 12.01) confirmed PFS benefits, two RCTs (De-ESCALaTE, RTOG 1016) confirmed OS benefits, two RCTs (De-ESCALaTE, TROG 12.01) confirmed DMFS benefits, and two RCTs (De-ESCALaTE, RTOG 1016) confirmed LRC benefits with cisplatin over cetuximab. Cisplatin omission also compromises outcomes (HN002), and bolus HD cisplatin remains its standard administration with definitive CRT in HPV+ OPC. The failure of chemotherapy deintensification prompted international cooperative groups to caution against deviation from the SOC and advocate that “harm minimization techniques should also be evaluated as an alternative to de-escalation.”48 It is important to highlight that modern deintensification trials report about half the late toxicities (~20%) with SOC CRT compared to historical RTOG trials (~40%).14,15,17 This may be in part due to better supportive care and the utilization of IMRT technology which has level one evidence to support toxicity improvements over conventional RT.49 The optimization of treatment delivery and harm minimization are important practices to sustain and enhance.
Surgical Deintensification Approaches Through Transoral Surgery (TORS)
TORS to Replace Definitive Radiation
Weighted average results from 51 series from North American academic institutions showed similar disease control and survival outcomes but higher severe or fatal complications with open transmandibular or transcervical surgery versus definitive RT for OPC.50 A meta-analysis of HPV+ OPC showed no difference with RT versus surgery for the combined endpoint of death, recurrence, or progression.51 However, the advent of TORS raised the question if the balance might now favor minimally invasive surgical intervention. In 2016, a comprehensive review of small-volume primary OPC showed no high-quality evidence comparing TORS to RT.52 But TORS utilization rapidly expanded and only five years later by 2021, a systematic review and meta-analysis of HPV+ OPC treatments concluded that TORS was associated with worse performance on certain measures of patient-reported swallow and overall function compared to CRT; additionally, there was a trend favoring CRT for gastrostomy tube dependence at 24–36 months (10.5% TORS vs. 3.3% CRT with cisplatin, p=0.06).53
Table 2 shows eligibility criteria, treatment details, and outcomes from various TORS trials. ORATOR compared quality-of-life (QOL) in patients randomized to SOC 70Gy RT (with chemotherapy for AJCC7 N1-N2 disease) versus TORS + neck dissection (ND) with pathology-directed adjuvant therapy (SOC 60Gy RT ± chemotherapy).54 There was no difference in outcomes (3-year PFS: 96.3% RT vs. 93.3% TORS, p=0.32; 3-year OS: 96.3% RT vs. 90.0% TORS, p=0.58) and longitudinal assessment demonstrated superior, but not clinically meaningful, dysphagia with RT over time.55 Post hoc subgroup analysis with longterm follow-up from the ORATOR trial revealed that the statistically significant and clinically meaningful superiority of dysphagia with RT was driven entirely by base of tongue tumors, with no difference in dysphagia between modalities for tonsil cancers.56 TORS patients had more pain, trismus, and bleeding, while RT patients had more mild ototoxicity, xerostomia, and mild neutropenia. ORATOR2 randomized only p16-positive (p16+) HPV+ OPC patients to de-escalated RT (60Gy ± LD cisplatin) versus TORS + ND ± de-escalated 50Gy postoperative RT (PORT); the trial explicitly attempted to avoid trimodality therapy.57 The trial closed early to accrual due to two deaths in the TORS arm: cervical spine osteomyelitis and oropharyngeal hemorrhage (despite trial-mandated external carotid artery ligation). At median follow-up of 17 months, all four PFS events had occurred in the TORS arm; 2-year PFS was inferior with TORS (83.5% TORS vs. 100% RT), but statistics could not be reported as survival data was immature due to early closer for unforeseen excess grade 5 toxicity events in the TORS arm.57 Of note, both definitive and adjuvant RT doses were de-escalated on ORATOR2. The Comparativeness Effectiveness Trial (Table 2) will attempt to provide further clarity regarding safety and efficacy with TORS compared to RT, using SOC definitive and adjuvant doses. And, other studies will address the role of TORS versus RT in resectable HNC, but are not limited to HPV+ OPC: the EORTC “Best Of” trial (NCT02984410) will randomize T1–2N0–1 OPC of any HPV status or supraglottic larynx cancer or T1N0 hypopharynx cancer to TORS versus SOC 66–70Gy RT and the QoLATI trial (NCT04124198) will randomize T1–2N0–1 OPC (any HPV status) to accelerated RT ± chemotherapy versus TORS with staging ND prior to planned TORS for clinically node-positive patients. Critical to these, and any, surgical de-escalation strategy is careful patient selection, complete staging workup, and implementation at a high-volume center with robotic surgery expertise.
Table 2.
Surgical Trials: TORS to Replace Definitive RT
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| ORATOR 54–56 | SOC def RT 70Gy ± chemo (n=34) | For p16+ subset (88% of pts in both arms): | RT patients had superior (but not clinically meaningful) |
| (Phase II, randomized) | - Chemo for N1–2 (n=23, 68%), HD cis preferred. | 3 yr PFS: 96.3% [76.5–99.5] vs. 93.3% [75.9–98.3], p=0.32 | swallowing-related QOL (p=0.049). |
| Resectable OPC. | VS. | 3 yr OS: 96.3% [76.5–99.5] vs. 90.0% [72.1–96.7], p=0.58 | RT pts had more dry mouth over time (p=0.0491). |
| AJCC7 T1-T2 (≤4 cm), | TORS+ND ± SOC 60Gy PORT ± chemo (n=34) | TORS patients used more nutritional supplements at 3 yrs (p=0.015). | |
| N0-N2, any PYSH. | - PORT for: <2 mm margin, ≥1 LN, +LVI, ≥T3 | ||
| Median FU = 45 months | |||
| ORATOR2 57 | Stratified by PYSH, then 1:1 randomization: | 2 yr PFS: 100% [100–100] vs. 83.5% [60.8–93.7] | 1 yr mean MDADI scores (P = 0.85): |
| (Phase II, randomized) | Reduced def RT 60Gy ± chemo (n=30) | 2 yr OS: 100% [100–100] vs. 89.1% [69.6–96.4] | RT: 85.7 ± 15.6 vs. TORS: 84.7 ± 14.5 |
| p16+ or HPV+ OPC. | - LD cis (40 mg/m2) for LN+ (n=21, 72%) | p-values not reported for OS and PFS comparisons. | |
| AJCC8 T1-T2 (≤4 cm), | VS. | Trial was reported with immature survival outcomes. | N=1 in each arm required a peg, none at 1 year. |
| N0-N2, any PYSH. | TORS + ND ± Reduced PORT 50–60Gy (n=31) | Trial closed to accrual in November 2020 due two treatment- | |
| - 60Gy: +margin or +ECE | related deaths from complications in the TORS arm. | Grade 2–5 toxicities: RT 67% vs. TORS+ND 71% | |
| - 50Gy: <3mm margin, >1 LN, LN > 3cm, LVI, ≥T3 | All 4 PFS events occurred in TORS arm (1 bleed, 1 cervical | -significantly more anorexia and dysgeusia in the RT arm | |
| Median FU = 17 months | osteomyelitis, 1 myocardial infarction, 1 local recurrence). | ||
| Comparativeness Effectiveness Trial/ | TORS + ND +/− 56–66Gy adj CRT | Ongoing, not reported | Ongoing, not reported |
| NCT03691441. (Phase IV) | vs. | ||
| p16+ OPC resectable by TORS. | SOC def CRT 70–72Gy | ||
| AJCC7 T1N2a-c, T2N1–2c, T3N0–2c. | HD cis preferred. | ||
| NECTORS 71–72 | Experimental (n=55): | 5 yr PFS (p=0.03): | Peg-dependence 12 months post-treatment (p < 0.0001): |
| (Phase II) | NAC (cis 75 mg/m2 + doce) → TORS + selective ND | - NAC + S: 96.1% [90.8–100] | 0% NAC+TORS vs. 24.5% CRT |
| p16+ OPC. | - CRT historical cohort: 67.6% [50.7–84.5] | Severe Grade 3+ events: | |
| AJCC7 T1–4N0–2c. | CRT historical cohort (n=145): HD cis + IMRT | Pathologic CR rates: 72% primary and 57% nodal. | 12.7% NAC+TORS vs. 24.6% CRT |
| Median FU 20.4 months. | Only 2 of 55 TORS pts received adj CRT. | ||
| OPTIMA II 73–74 | NAC carbo/nab-pacl/nivolumab x3c → RECIST response: | Among 9 TORS patients, 66.7% had pCR. | 1 patient died during NAC. |
| (Phase II) | De-escalated treatment administered in 84.9%. | ||
| p16+ and HPV+ OPC. | |||
| LR: | LR with ≥50% reduction: | ||
| T1-T2 non-bulky tonsil or well-lateralized BOT ≤3 cm, non- | - TORS+selective ND (n=9) | ||
| bulky N2A-2B with ≤2 non-lower neck LNs measuring ≤5 cm. | - Reduced dose def RT 50Gy(n=28): did not | ||
| HR: | qualify for/refused TORS | ||
| T4, N2C-3, >20 PYSH, or non-HPV16 subtype. |
adj=adjuvant, AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, chemo=chemotherapy, cis=cisplatin, CRT=chemoradiation therapy, def=definitive, ECE=extracapsular extension, FU=follow-up, Gy=gray, HD=high-dose, IMRT=intensity-modulated radiation therapy, LD=low-dose, LN=lymph node, LVI=lymophovascular invasion, NAC=neoadjuvant chemotherapy, ND=neck dissection, NS=not significant, OPC= oropharyngeal cancer, OS=overall survival, +=positive, peg=percutaneous endoscopic gastrostomy, PORT=postoperative radiation therapy, PFS=progression-free survival, PYSH=pack-year smoking history, RT=radiation therapy, SOC=standard of care, TORS=transoral robotic surgery, wkly=weekly, wks=weeks, yr=year.
TORS to Attenuate Adjuvant Therapies
Another approach de-escalates adjuvant therapy after TORS. Eastern Cooperative Oncology Group (ECOG) E3311 (Table 3) randomized resected HPV+ OPC with intermediate-risk factors (close margin <3 mm, perineural invasion (PNI), LVI, 2–4 involved lymph nodes (LN), or <=1 mm ECE) to reduced-dose (50Gy) versus standard-dose (60Gy) PORT.45 Patients with ECE >1 mm, positive margins, or pN2 disease (≥5 LNs) were not de-escalated and received SOC 60–66 Gy PORT with LD cisplatin (40 mg/m2). TORS with reduced-dose 50Gy PORT retained outstanding 94.9% 2-year PFS, raising the question if TORS + reduced-dose PORT may obviate chemotherapy and should be compared against SOC 70Gy CRT in a phase III trial. It is important to note that although 2-year PFS with 50Gy PORT was comparable to SOC 60Gy PORT (96.0%), there was no difference in patient-reported outcomes, raising the question if modest dose-reduction meaningfully improves toxicity. Figure 1a illustrates radiation dosimetry with SOC 60Gy (pink 6000 line) PORT to the neck. Figure 1b illustrates radiation dosimetry with reduced-dose 50Gy PORT to the neck (magenta 5000 line). With IMRT, medium/high 40–45Gy dose spillover (teal 4000 line, blue 4500 line) to central swallowing structures like the esophagus (pink) can be effectively minimized without significant differences between 50Gy and 60Gy dose prescriptions.Although E3311 was not a comparison of TORS versus RT as both ORATOR trials were, there was only one grade 5 hemorrhage among 495 TORS patients on E3311, providing proof of concept that TORS can be incorporated into de-escalation protocols when supported by a comprehensive credentialing process.
Table 3.
Surgical Deintensification Trials: TORS to Deintensify Adjuvant Therapy
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| ECOG-ACRIN E3311 45 | TORS+ND → risk-adapted adj therapy: | 2 yr PFS OBS: 96.9% [90%CI 91.9–100] VS. | Grade ≥3 toxicity: |
| (Phase II) | 50Gy: 94.9% [90%CI 91.3–98.6] VS. | OBS 17% | |
| Resected HPV+ OPC. | LR (T1–2N0–1, ≥ 3mm margin, no ECE/PNI/LVI) → OBS (n=38) | 60Gy: 96.0% [90%CI 92.8–99.3] VS. | 50Gy: 15% |
| AJCC7 T1–2N1–2b with RFs: | IR → SOC PORT 60Gy (n=108) vs. Reduced PORT 50Gy (n=100) | 60–66Gy CRT: 90.7% [90%CI 86.2–95.4] | 60Gy: 24% |
| IR: close margin <3 mm, PNI, LVI, LN>3 cm, | HR → SOC adj CRT 60–66Gy + LD cis 40 mg/m2 wkly (n=113) | 2 yr OS OBS: 100% VS. | 60–66Gy CRT: 61% |
| 2–4 LNs, or ≤1 mm ECE | 50Gy: 99.0% [90%CI 9.3–100] VS. | ||
| HR: +margin, ECE >1mm, ≥ 5 LNs | Median FU = 35 months | 60Gy: 98.1% [90%CI 95.9–100] VS. | PROs did not differ between 50Gy and 60Gy PORT. |
| 60–66Gy CRT: 96.3% [90%CI 93.3–99.3] | |||
| Mayo MC1273 58–59 | All had surgery + ND with margin neg resection. 95% TORS. | 2 yr PFS: 91.1% overall, 97.2% IR, 86.0% HR | 1 patient required a peg. |
| (Phase II) | DART + wkly doce for all. | 2 yr LRC: 96.2% overall, 100.0% IR, 93.0% HR | 0% grade 3 or worse toxicity 2 yrs after treatment. |
| p16+ OPC resected with neg margins AND ≥1 RF: | IR (n=37) → 30Gy (1.5Gy BID) | 2 yr OS: 98.7% overall, 100.0% IR, 97.7% HR | |
| IR: ≥T3, ≥N2, PNI, LVI | HR (n=43) → 36Gy (1.5Gy BID/1.8Gy BID SIB) | 2 yr DMFS: 94.9% | |
| HR: ECE | |||
| AJCC7 Stage III/IV, ≤10 PYSH. | Median FU = 35.7 months | 21% of HR ECE pts recurred (5 of 9 distant) | |
| DART-HPV/MC1675 61–62 | IR: | 2 yr PFS: 86.5% DART vs. 95.1% SOC | Grade ≥3 toxicity at 3 months (p=0.058): |
| (Phase III) | DART 30Gy + wkly doce (n=53) | 2 yr LRRFS: 95.5% DART vs. 97.9% SOC | 1.6% DART vs. 7.1% SOC |
| p16+ OPC resectable by TORS. | VS. | 2 yr OS: 96.1% DART vs. 97.0% SOC | |
| Neg margins (≤2 excisions to clear a margin edge) | SOC 60Gy + LD cis 40 mg/m2 wkly (n=26) | If pN2 and +ECE: | Pegs (p<0.0001): |
| AND at least one risk factor: | - 2 yr LRC 77.0% DART vs. 100% SOC | 1.6% DART vs. 27.4% SOC | |
| IR: ≥T3, ≥N2, PNI, LVI | HR: | - 2 yr DMFS 59.4% DART vs. 100% SOC | |
| HR: ECE | DART 36Gy + wkly doce (n=77) | - 2 yr PFS 42.9% DART vs. 100% SOC | |
| AJCC7 Stage III/IV, ≤10 PYSH. | VS. | If pN0–1 and +ECE: | |
| SOC 60Gy + LD cis 40 mg/m2 wkly (n=38) | - 2 yr LRC 95.8% DART vs. 100% SOC | ||
| - 2 yr DMFS 96.4% DART vs. 95.8% SOC | |||
| - 2 yr PFS 89.6% DART vs. 95.8% SOC | |||
| MC1273/MC1675 pooled analysis, 2 yr PFS: | |||
| ECE+: 85.2% [78.6%−92.5%] | |||
| ECE+/pN1: 92.5% [86.9%−98.5%] | |||
| ECE+/pN2: 54.5% [36.4%−81.7%] | |||
| No ECE: 97.7% [94.6%−100.0%] | |||
| No ECE/pN0–1: 97.5% [94.7%−100.0%] | |||
| No ECE/pN2: 100.0% | |||
| SIRS Sinai Robotic Surgery Trial/ NCT02072148 | TORS, and then based on pathology (n=200): | Ongoing, not reported | Ongoing, not reported |
| (Parallel Assignment) | - LR (>1 mm margin for tonsil, >3 mm margin for tongue, no LVI, | ||
| p16+ and HR HPV subtype (16/18/33/35,etc) OPC. | no PNI, <3 +LNs, no ECE, no matted/level IV/level V LNs): OBS | ||
| AJCC7 T1–2N0–2b. | - IR: 50Gy Reduced PORT | ||
| IR: <1 mm margin tonsil, <1 mm margin tongue, | - HR: 50Gy Reduced PORT + LD cis 40 mg/m2 wkly | ||
| LVI, PNI, <3 LNs, ≤1 mm ECE | - HR incomplete resection (+margins, >1 mm ECE, matted LNs): | ||
| HR: ≥3 +LNs, >1 mm ECE, contralateral or | 56Gy Reduced PORT + LD cis 40 mg/m2 wkly | ||
| supraclavicular LNs | |||
| Univ of Pittsburgh/NCT03715946 | Reduced PORT (45 or 50Gy) | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | + immunotherapy (concurrent+adj nivolumab) | ||
| p16+ OPC resected by TORS. | |||
| T0–3 with at least 1 IR factor. | |||
| <10PYSH: >N2b (>5 LNs), >1 mm ECE, +margin | |||
| >10PYSH: N2, > 1 mm ECE, +margin | |||
| Minimalist Trial(MINT)/ NCT03621696 | LR (neg margin, no T4, no N3): 42Gy Reduced PORT | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | IR (+margin or ECE): 42Gy Reduced PORT + HD cis x1c | ||
| p16+ or HPV+ OPC, resectable by TORS. | HR (T4 or N3): SOC 60Gy adj PORT + HD cis x3c | ||
| AJCC8 I-III. | |||
| PATHOS / NCT02215265 | TORS → risk-adapted adj therapy: | Ongoing, not reported | Ongoing, not reported |
| (Phase III) | |||
| p16+ and HPV+ OPC. | T3, N2a or N2b, +PNI, +LVI, 1–5 mm margin: | ||
| AJCC7 T1–3 N0–2B. | SOC 60Gy PORT vs. 50Gy Reduced PORT | ||
| +ECE or +margin: SOC 60Gy PORT ± cis (HD or LD) | |||
| No risk factors: OBS | |||
| AVOID 63 | Omission of PORT to resected primary site (n=60). | 2 yr PFS: 92.1% [80.2–97.0] | No pegs during RT. |
| (Phase II) | SOC 60–66Gy PORT to at-risk neck. | 2 yr LRC: 97.9% [86.1–99.7] | |
| HPV+ OPC resected by TORS, | Chemo for ECE. | 2 yr RRFS: 97.9% [86.3–99.7] | - 2 pegs (3.3%) due to surgery for recurrence |
| pT1–2N1–3, neg margin (≥2 mm), no PNI, no LVI. | - LD cis 40 mg/m2 wkly (n=9); HD cis (n=2); cetux (n=2) | 2 yr DMFS: 96.2% [85.5–99.0] | with soft tissue necrosis, both at 3 months after RT. |
| Median FU= 2.4 years | Only 1 of 60 pts recurred at the omitted primary site | Both pegs subsequently removed. | |
| ADEPT / NCT01687413 | Omission of adjuvant chemo in +ECE patients: | Trial terminated due to poor accrual | Trial terminated due to slow accrual |
| (Phase III) | SOC PORT (60Gy) VS. CRT (60Gy + LD cis 40 mg/m2 wkly) | ||
| p16+ OPC. | |||
| AJCC7 T1–4a N1–3. | |||
| TORS + ND with neg margin and must have +ECE. | |||
| ADAPT/ NCT03875716 | Omission of adjuvant chemo in +ECE or +margin patients: | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | LR (pT0–2N0–1, neg margin, no ECE, ≤2 LNs in Level II/III, ≥15 LNs | ||
| p16+ or HPV+ OPC, resectable by TORS. | dissected): OBS | ||
| AJCC8 cT0–2N0–1, ≤20PYSH. | IR: 46Gy Reduced PORT | ||
| IR: >2 LNs, <15 LNs dissected, +Level IB/IV/V LN, | HR: 60Gy SOC PORT, but no SOC chemo | ||
| ≤1 mm ECE, close margins, or +contralateral LN. | |||
| HR: >1 mm ECE or microscopic +margin. |
adj=adjuvant, AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, BID=bidaily, c=cycle, chemo=chemotherapy, CI=confidence interval, cis=cisplatin, CRT=chemoradiation therapy, DART=de-escalated adjuvant radiation therapy, def=definitive, DMFS=distant metastasis free survival. doce=docetaxel, ECE=extracapsular extension, FU=follow-up, Gy=gray, HD=high-dose, HPV=human papillomavirus, HR=high-risk, IMRT=intensity-modulated radiation therapy, IR=intermediate-risk, LD=low-dose, LN=lymph node, LR=low-risk, LRC=locoregional control, LRRFS=locoregional relapse free survival, LVI=lymophovascular invasion, NAC=neoadjuvant chemotherapy, ND=neck dissection, neg=negative, OBS=observation, OPC= oropharyngeal cancer, OS=overall survival, +=positive, peg=percutaneous endoscopic gastrostomy, PNI=perineural invasion, PORT=postoperative radiation therapy, PFS=progression-free survival, PORT=postoperative radiation therapy, PRO=patient-reported outcome, PYSH=pack-year smoking history, RF=risk factor, RRFS=regional relapse free survival, RT=radiation therapy, SIB=simultaneous integrated boost, SOC=standard of care, TORS=transoral robotic surgery, wkly=weekly, wks=weeks, yr=year.
Figure 1.
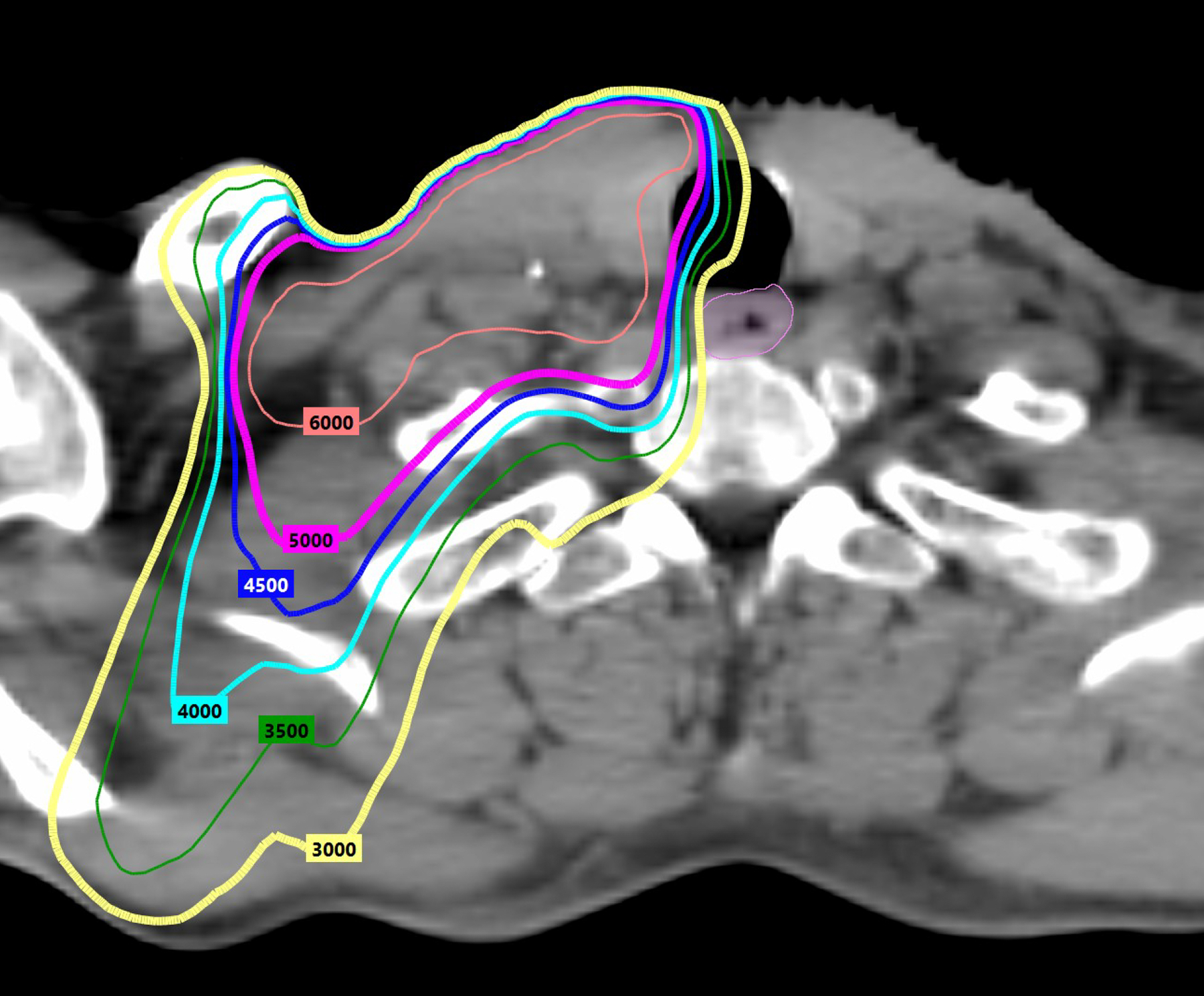
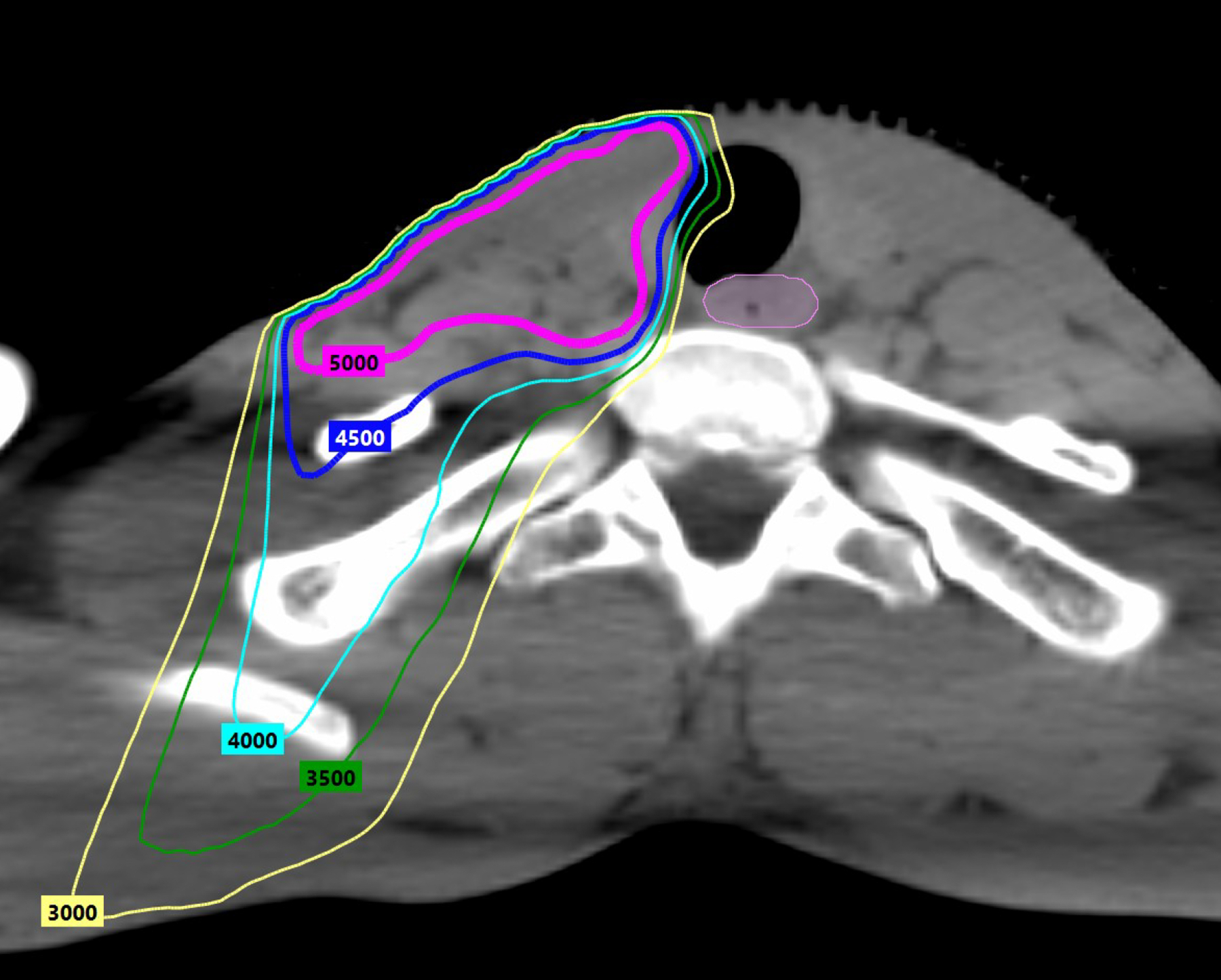
Radiation dosimetry with standard versus reduced PORT doses. Figure 1a illustrates radiation dosimetry with SOC 60Gy (pink 6000 line) PORT to the neck. Figure 1b illustrates radiation dosimetry with reduced-dose 50Gy PORT to the neck (magenta 5000 line). IMRT can limit medium/high dose scatter to central swallowing structures like the esophagus (pink). There is no notable difference in medium/high dose spillover of 45Gy (blue 4500 line) or 40Gy (teal 4000 line) between 60Gy and 50Gy dose prescriptions.
The Mayo Clinic MC1273 trial (Table 3) enrolled p16+ OPC patients with negative margins after surgery and <10 PYSH to even greater reductions in de-escalated adjuvant RT (DART).58,59 Intermediate-risk patients (≥T3, any LN>3 cm, ≥2 LNs, LVI, or PNI) received DART to 30Gy CRT bidaily (BID) with docetaxel, while high-risk ECE patients received 36Gy CRT BID with docetaxel. Two-year LRC and PFS were 96.2% and 91.1%, with no grade ≥3 toxicity within two years of treatment. Of note, 8 of 59 (14%) of N2 patients experienced progression, half of which were distant and raised questions about adequate radiosensitization and distant protection with docetaxel. Accelerated BID radiation is known to increase late toxicity, so these results warranted a toxicity comparison of lower total dose BID versus standard doses conventionally fractionated.60 Accordingly, 30–36Gy DART BID with docetaxel was compared against SOC 60Gy PORT with LD cisplatin (40 mg/m2) in the DART-HPV/MC1675 RCT (Table 3). Two-year PFS with DART 30–36Gy BID with docetaxel was 86.5% [95% CI 80.2–93.3] versus 95.1% [95% CI 88.8–100.0] with SOC. However, the DART arm experienced significantly smaller percentage that required feeding tubes (1.6% versus 27.4%, p>0.0001) and fewer grade ≥3 toxicities at three months (1.6% versus 7.1%, p=0.058).61 Again, progression was predominantly observed in patients with American Joint Committee on Cancer 8th edition (AJCC8) pN2 (>4 LNs) disease and ECE treated with DART, who experienced 42.9% 2-year PFS (versus 100% with SOC); conclusions in pN2 patients without ECE cannot be drawn as only two such patients were enrolled. A pre-planned pooled analysis of MC1273 and MC1675 reported 2-year PFS of 91.1% [95% (CI), 87.2%−95.3%]; this was both non-inferior to the target 92.3% PFS for HN005 (p = 0.29) and also higher than the HN005 acceptable PFS threshold of 86.9% (p=0.043).62 The ECE-positive cohort did not achieve the target 92.3% or acceptable 86.9% PFS thresholds (85.2% and 78.6%, respectively), which was driven by failures in N2/ECE-positive patients.62 A true noninferiority trial is estimated to require upwards of 4,000 patients to achieve a 1% noninferiority margin (more than all the TORS performed annually across all US academic centers), so these pooled results may be the best that can reasonably be achieved to address the question of DART after TORS. Ultimately, 30Gy DART with docetaxel appeared to meet target PFS thresholds with notable toxicity benefits in well-selected patients with intermediate-risk factors (those without ECE and with negative margins); 36Gy DART with docetaxel did not meet acceptable PFS thresholds in high-risk patients with ECE. Of note, postoperative patients with ≤1 mm ECE and negative margins were treated favorably with 50Gy without chemotherapy on E3311. So the question remains whether 50Gy PORT would meet the target and lower boundaries of acceptable 2-year PFS in a RCT, and whether modestly-reduced 50Gy PORT or DART with 30Gy BID with docetaxel is the optimal postoperative de-escalation regimen with intermediate-risk factors.
Based on E3311, MC1273, and MC1675, 2-year PFS estimates with DART doses ranging from 30Gy BID with docetaxel to 50Gy PORT alone range between ~86–95% for patients with postoperative intermediate-risk factors.45,59,61 It is important to note that adjuvant therapy indications varied widely between trials. On E3311, PORT was administered for LVI, PNI, <3mm margin, ECE ≤1mm, or 2–4 LNs; chemotherapy was added for ≥ 5 LNs, ECE >1 mm or positive margin. On MC1273/MC1675 both PORT and chemotherapy were administered for >1 LN, LN >3 cm, T3, any ECE, LVI or PNI. Table 3 shows four additional ongoing trials (SIRS, University of Pittsburgh, MiNT, PATHOS) exploring PORT doses ranging from 42–56Gy. A unique PORT deintensification strategy was volume reduction studied on AVOID (Table 3), which harnessed a negative ≥2 mm margin after TORS (no PNI or LVI) to omit primary site PORT; this strategy has profound implications as primary site mucosal axis RT causes the bulk of toxicity (mucositis, stomatitis, dysphagia, xerostomia). SOC 60–66Gy PORT was administered to the at-risk neck and 2-year local recurrence-free survival was 97.9%.63
Positive margins and ECE have been longstanding high-risk pathology indications for adjuvant chemotherapy with PORT.64 Despite only these two universal indications for adjuvant CRT, in practice a majority of surgical patients receive adjuvant CRT due to limitations in current preoperative staging methods and discrepancies in postoperative therapy indications. An NCDB study on surgery versus CRT for cT1–2N1–2B OPC showed no difference in OS but 59% of surgical patients received adjuvant CRT, illustrating that many seeking surgical deintensification end up escalated to trimodality therapy.65 One limitation of this study is that all surgical patients (including those undergoing non-definitive operations like simple palatine tonsillectomy for diagnostic workup of unknown primary cancers) were included, suggesting rampant adjuvant CRT rates with surgery. Another NCDB series focused on a cleaner subset of HPV+ OPC patients who had TORS (excluding open simple palatine tonsillectomy), reported 33% adjuvant CRT rates.66 As a standard, on ORATOR and E3311, adjuvant chemotherapy was administered to only 24% and 31% of TORS patients respectively. Of note, they had different chemotherapy indications (ORATOR for any ECE or positive margins and E3311 for >1 mm ECE, positive margins, or ≥5 LNs).45,54 The prognostic value of ECE in HPV+ OPC has been questioned, with some retrospective data suggesting no survival benefit for chemotherapy with PORT for HPV+ OPC.67,68 However, a multi-institutional retrospective study of HPV+ OPC patients who refused standard adjuvant therapy after TORS reported a 52% 3-year relapse rate in patients with high-risk pathologic features, highlighting the risk conferred by ECE.69 Future trials like PATHOS, ADEPT, and ADAPT (Table 3) will assess if ECE and positive margins remain indications for adjuvant chemotherapy in resected HPV+ OPC.
Finally, the utilization of TORS to improve primary site identification for HPV+ unknown primary cancers (UPC) is an emerging, powerful application for TORS to facilitate treatment deintensification. The FIND trial incorporated TORS to reduce radiation volumes in HPV+ UPCs; the pharynx was omitted from the radiation fields if primary tumors were excised with ≥ 3 mm margins or if no primary tumor was found. Half of patients qualified for omission of pharynx radiation, and 2-year LRC and DFS were 100% and 95%, respectively.70 The favorable disease control results highlight the promise of TORS as a diagnostic and potentially therapeutic tool that may facilitate substantial treatment de-escalation in HPV+ UPC.
Neoadjuvant therapy before TORS
Neoadjuvant immunotherapy is nascent, with current trends typically favoring its use in the very locally advanced setting. NECTORS (Table 2) administered induction cisplatin/docetaxel before TORS in AJCC7 T1–4 N0–2c HPV+ OPC and reported 94% 3-year cancer-specific survival (CSS).71,72 One phase IB/II trial will administer neoadjuvant stereotactic body RT and immunotherapy prior to TORS for AJCC7 T0–3 N0-N2B HPV+ OPC (NCT03618134). In AJCC7 T1–2 non-bulky N2A-2B HPV+ OPC, OPTIMA II (Table 2) administered induction carboplatin/nab‐paclitaxel/nivolumab, and reported 66.7% pathological complete response at TORS among nine low-risk patients with ≥50% tumor reduction after induction.73,74
Summary
In the definitive setting, one phase II randomized trial (ORATOR) reported no difference in PFS or OS and no clinically meaningful difference in toxicity with TORS + SOC PORT versus definitive RT, while another phase II randomized trial (ORATOR2) comparing TORS + de-escalated PORT versus de-escalated definitive RT closed early due to excessive grade 5 toxicities in the TORS arm. In the adjuvant setting for intermediate-risk patients, a phase II trial reported the feasibility of PORT dose to 50Gy without chemotherapy (E3311). A preplanned pooled analysis of phase II (MC1273) and III (MC1675) trials reported decreased toxicity with 30–36Gy BID DART plus docetaxel versus SOC PORT, but de-escalation in high-risk ECE patients did not meet acceptable PFS thresholds. There is considerable variability in adjuvant RT and chemotherapy criteria, and robotic surgery expertise is of paramount importance in strategies incorporating TORS as a part of the treatment paradigm.
Radiation Therapy Deintensification Approaches
Reduction of Elective Radiation Doses and Volumes
Radiation deintensification (volume and/or dose) is a promising approach with ample literature. Adaptive RT, modifying volumes to account for weight loss or tumor shrinkage, is already common clinical practice. Subclinical/elective radiation doses and volumes have also evolved and decreased. The Infield trial reduced elective nodal irradiation (ENI) volumes to involved and adjacent levels only and decreased subclinical radiation doses from a standard of ~44–63Gy to 40Gy in oropharyngeal and laryngeal cancers.75 RAVD (Table 4) was a trial of induction chemotherapy in locally advanced HNC; those with ≥50% shrinkage on CT or MRI received no ENI and >90% of locoregional failures (LRF) developed within high-dose RT volumes.76 Specific to HPV+ OPC, a phase II trial from Montreal (Table 4) achieved 100% LRC with 43.2Gy ENI and contralateral retropharyngeal and level IV LN omission in CRT patients.77 OPTIMA was an induction chemotherapy trial in HPV+ OPC which limited prophylactic lymph node RT to only the first echelon of uninvolved nodes in all patients, and reduced ENI dose to 30Gy for favorable responders in the de-escalated CRT arm.78,79 Two trials, SAVER and EVADER, are currently investigating ENI volume reductions (Table 4). At Memorial Sloan Kettering, both subclinical dose (to 30Gy) and ENI volume (omission of levels IB and V) are reduced off clinical trial in all HPV+ OPC receiving cisplatin.80,81 Figure 2 illustrates radiation dosimetry with ENI to 56Gy with omission of level IB LNs (right) versus inclusion (left); there is a dramatic difference in anterior oral cavity dose spillover with level IB omission. Thus, elective radiation dose and volume reduction are harm-mitigation strategies which have already been implemented.
Table 4.
Radiation Deintensification Trials: Elective Radiation Dose or Volume Reduction
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| RAVD 76 | IND cis/pacl/cetux x2c ± everolimus (n=89) → | HPV+ OPC (n=59): | Acute pegs (p=0.040): |
| (Phase I/II) | GR ≥50% shrinkage (n=37) → | 2yr PFS (p=0.10): 93.1% GR vs. 74.0% NR | 50.0% GR vs. 73.5% NR |
| AJCC7 IVA-IVB HNSCC. | No ENI. 75Gy to gross disease (BID every other wk). | 2yr OS: 92.1% GR vs. 95.2% NR | Peg dependence at 6 months (p = 0.005): |
| Oral cavity, OPC, Larynx, | NR <50% shrinkage (n=52) → | 5.7% GR vs. 32.6% NR | |
| Hypopharynx, Unknown Primary | 45Gy ENI (to next LN station) + 75Gy to gross disease. | >90% of LRFs developed in the highest risk/dose RT volume. | |
| cancers all included. | Mean FU = 24 months | ||
| Montreal 77 | IMRT + concurrent carbo/5FU (n=29). | 2 yr PFS : not reported | Acute grade 3 mucositis: 52% |
| (Phase II) | 2 yr LRC: 100% | ||
| p16+ OPC | SOC def CRT 70Gy to HR volume, 59.4Gy IR volume | 2 yr OS: 100% | All patient-reported QOL outcomes (except dry mouth) |
| AJCC7 III/IV | Reduced ENI dose: 43.2 Gy/24 fxs | returned to baseline within 10 months of treatment end. | |
| T1-4N0–2b and N3 with primary not extending beyond midline and ipsilateral nodes only; smokers permitted. | Reduced ENI volume: no contralateral RP or level IV LNs. | ||
| Median FU = 44 months | |||
| EVADER / NCT03822897 | SOC def RT 70Gy | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | SOC def CRT 70Gy + HD cis x3c | ||
| HPV+ OPC. | |||
| AJCC8 T1–3 N0–1. | ENI volumes reduced. | ||
| SAVER / NCT04609280 | ENI volumes reduced. Omission of contralateral LNs. | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | Treat only ipsilateral level II and III LN levels. | ||
| p16+ OPC. | If PORT: 54 Gy SOC | ||
| AJCC8 T1–4 and N0, N1, or N3. | If def RT: 69.96Gy SOC |
5FU=5-fluorouracil, AFRT= altered fractionation radiation therapy, AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, BID=bidaily, c=cycle, carbo=carboplatin, cetux=cetuximab, cis=cisplatin, CRT=chemoradiation therapy, def=definitive, ENI=elective nodal irradiation, FU=follow-up, fxs= fractions, GR=good response, Gy=gray, HD=high-dose, HNSCC=head and neck squamous cell carcinoma, IMRT=intensity-modulated radiation therapy, IND=induction, LN=lymph node, LRF= locoregional failure, NR=no response, OPC= oropharyngeal cancer, OS=overall survival, pacl=paclitaxel, PFS=progression-free survival, PORT=postoperative radiation therapy, QOL=quality of life, RP=retropharyngeal, RT=radiation therapy, SOC=standard of care, yr=year.
Figure 2.
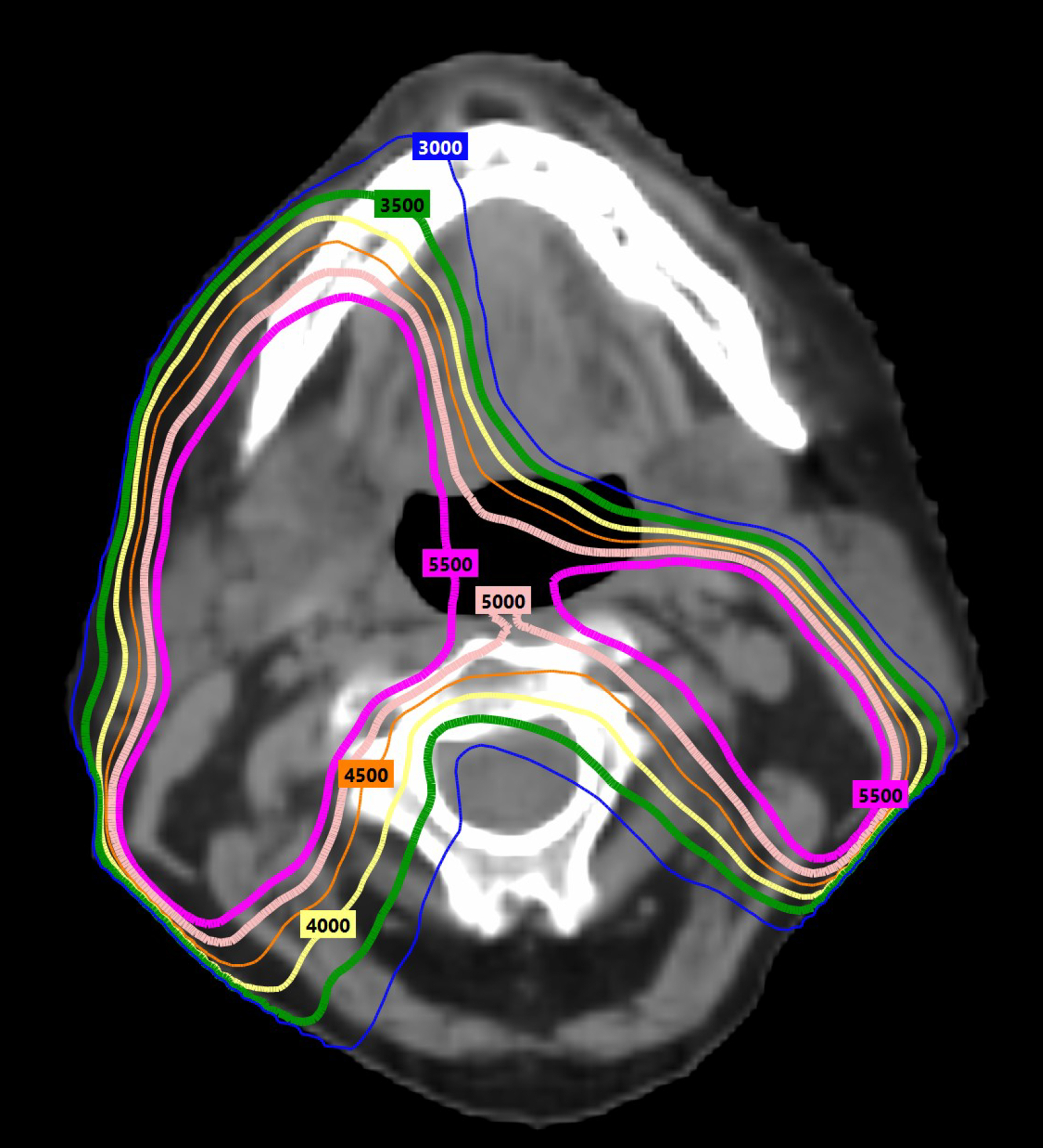
Radiation dosimetry with nodal irradiation to 56Gy (magenta 5600 line) with inclusion of level IB LNs (left) versus omission (right). There is a drastic difference in anterior oral cavity dose spillover with level IB omission.
Reduction of Definitive Radiation: Upfront Modest Dose Reduction
Radiation dose reduction to gross disease has been investigated in multiple phase II trials with two main strategies: upfront (empirically based on stage and PYSH) or selective (based on response to induction therapy). Four phase II trials (Table 5) have reported success with upfront modest dose reductions in p16+ OPC based on stage (AJCC7 T1-T3,N0-N2) and ≤10 PYSH. HN002 reported 90.5% 2-year PFS with LD cisplatin (40 mg/m2) and 60Gy IMRT.17 UNC/UF treated similar patients to 60Gy IMRT, reserving LD cisplatin (30 mg/m2) for T3 or N2 disease, and reported 86% 2-year PFS.82,83 LCCC1612 administered 60Gy IMRT or proton therapy with LD cisplatin and reported 92–93% 1-year PFS.84 PacCIS randomized AJCC7 Stage III/IV HNC patients to SOC 70Gy with cisplatin/5FU or reduced-dose 63Gy with cisplatin/paclitaxel and reported no difference in outcomes, but <15% of patients had p16+ OPC.85 Although all four trials showed that modest radiation dose reduction may be acceptable with chemotherapy, none used SOC HD cisplatin. Ultimately, results from HN005 (Table 5) will elucidate whether 60Gy is non-inferior to SOC 70Gy with SOC HD cisplatin in both arms. Based on HN002 and UNC/UF, 2-year PFS outcomes approach 90% with LD cisplatin and upfront modest RT dose reduction to 60Gy.
Table 5.
Radiation Deintensification Trials: Upfront Radiation Dose Reduction
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| NRG-HN002 17 | Reduced dose def CRT | Null hypothesis 2 yr PFS ≤85%: | Higher acute toxicity with CRT. |
| (Phase II, randomized) | (60Gy IMRT + LD cis 40 mg/m2 × 6c, n=157): | - CRT 90.5% [84.5–94.7], p=0.0350, rejecting null hypothesis | Acute Grade 3–4 (p<0.001): 79.6% vs. 52.4% |
| p16+ OPC. | VS. | - AFRT 87.6% [81.1–92.5], p=NS, failing to reject null hypothesis | Late Grade 3–4 toxicit (p=NS): 21.3% vs. 18.1% |
| AJCC7 T1–2 N1–2b, T3 N0–2b. | AFRT alone (60Gy/5 wks IMRT, n=149): | 2 yr LRF (p=0.02): 3.3% vs. 9.5%, HR 2.56 [1.11–5.88] | |
| <10 PYSH. | 19.9% Unilateral RT. 80.1% Bilateral RT. | 2 yr DM (p=0.58): 4.0% vs. 2.1%, HR 0.70 [0.20–2.50] | |
| Median FU = 2.6 years | 2 yr OS (p=0.93): 96.7% vs. 97.3%, HR 1.05 [0.34–3.23] | ||
| UNC/UF 82–83 | Reduced def CRT for all (n=114): | 2 yr PFS: 86% [77.5–91.3] | Acute ≥ Grade 3 toxicity: |
| (Phase II) | 60Gy RT + LD cis 30 mg/m2 wkly | 2 yr LRC: 95% [88.6–97.9] | - 2% xerostomia |
| p16+ OPC. | No chemo for T0-T2 N0–1. | 2 yr DMFS: 91% [83.9–95.4] | - 34% mucositis |
| AJCC7 T0–3 N0–2C and ≤10 PYSH | 2 yr OS: 95% [89.3–98.1] | - 21% dysphagia | |
| or > 5 yrs smoking abstinence. | Median FU = 31.8 months | - 4 local failures, half in T2N0–1 treated without chemo. | - 38/113 (33%) patients required peg |
| LCCC1612 84 | Reduced def CRT for all | 1 yr PFS was comparable: 92% IMPT, 93% IMRT | More short-term pegs with IMRT: 8% vs. 39% |
| (Phase II). | 60Gy RT + LD cis 30 mg/m2 wkly | ||
| p16+ OPC. | IMPT (n=25) vs. IMRT (n=46) | IMPT patients had better patient-report QOL and swallowing symptom scores than IMRT. | |
| AJCC7 T0–3 N0–2C and ≤10 PYSH | No chemo for T0–2 N0–1. | ||
| or > 5 yrs smoking abstinence. | Median FU = not reported | ||
| If ≥10 PYSH: only wild-type p53 eligible. | |||
| PacCIS 85 | SOC def CRT 70.6Gy + cis/5FU | p16+ OPC subset analysis: | Grade ≥3 acute dysphagia: |
| (Phase III) | (n= 105 total; 19 p16+ OPC) | 3yr PFS (p=0.653): 83.9% SOC VS. 84.6% 63Gy | 77.1% SOC, 81.1% 63Gy |
| AJCC7 Stg III/IV HNC. | VS. | ||
| Reduced def CRT 63Gy + cis/pacl | 3yr OS (p=0.76): 83.5% SOC VS. 92.3% 63Gy | Grade ≥3 acute functional mucositis: | |
| (n= 111 total; 13 p16+ OPC) | 64.2% SOC, 59.4% 63Gy | ||
| Median FU = 3.7 yrs | |||
| NRG-HN005 | SOC def CRT (70Gy+HD cis × 2c) | Ongoing, not reported | Ongoing, not reported |
| (Phase II/III) | VS. | ||
| p16+ OPC. | Reduced def CRT (60Gy+HD cis × 2c) | ||
| AJCC7 T1–2 N1–2B, T3 N0–2B. | VS. | ||
| <10 PYSH. | Reduced dose def RT + IO (60Gy+nivolumab) | ||
| PROTEcT / NCT04104945 | Reduced def CRT 60Gy + chemo | Ongoing, not reported | Ongoing, not reported |
| p16+ OPC. | Chemo options: HD cis, LD cis 40 mg/m2, cetux | ||
| AJCC8 T1–3 N1–2. | Omission of IB from ENI volume. |
5FU=5-fluorouracil, AFRT= altered fractionation radiation therapy, AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, c=cycle, cetux=cetuximab, chemo=chemotherapy, cis=cisplatin, CRT=chemoradiation therapy, def=definitive, DM=distant metastasis, DMFS=distant-metastasis free survival, FU=follow-up, fxs= fractions, Gy=gray, HD=high-dose, HNC=head and neck cancer, HR=hazard ratio, IMPT=intensity-modulated proton therapy, IMRT=intensity-modulated radiation therapy, IO=immunotherapy, LD=low-dose, LRF= locoregional failure, NS=not significant, OPC= oropharyngeal cancer, OS=overall survival, peg=percutaneous endoscopic gastrostomy, PFS=progression-free survival, PYSH=pack-year smoking history, QOL=quality of life, RT=radiation therapy, SOC=standard of care, wk=week, wkly=weekly, yr=year.
Reduction of Definitive Radiation: Induction Chemotherapy-Based Patient Selection
Five phase II trials (Table 6) administered induction chemotherapy to select the most favorable patients: early responders. None excluded T4, N3, or smokers, and only one used the SOC induction regimen of docetaxel/cisplatin/fluorouracil. Radiation was attenuated for those with complete response (CR) or partial response (PR) to induction. In ECOG E1308, responders to induction cisplatin/paclitaxel/cetuximab received 54Gy with cetuximab; cohort 2-year PFS of 80% fell short of the target (85%), but was 96% for the AJCC7 T1-T3 N1-N2B and <10 PYSH subset.86 The University of California multi-institutional trial administered induction carboplatin/paclitaxel followed by paclitaxel with 54Gy if CR/PR or 60Gy RT if <PR/no response); it reported 92% 2-year PFS (three of four failures were in 60Gy patients with minimal response to induction, one failure in a 54Gy patient with PR).87 On OPTIMA, low-risk and high-risk patients received induction carboplatin/nab-paclitaxel followed by both response- and risk-adjusted therapy ranging from 50Gy RT alone or 45Gy CRT in responders, to 75Gy CRT in non-responders; 2-year PFS was 94.5% for the entire cohort.78–79 OPTIMA II added immunotherapy to the induction regimen, and early results reported 96.3% 2-year PFS in low-risk patients with ≥50% tumor shrinkage; 2-year PFS fell to 85.8% in low-risk patients with ≥30 but <50% response or high-risk patients with ≥50% response, although median follow-up was less than two years.73–74 The Quarterback Trial administered induction docetaxel/cisplatin/fluorouracil and randomized responders by HPV-genotype to carboplatin with SOC 70Gy versus 56Gy; 2-year PFS with 56Gy was 84.4% [95% CI 66.5–93.2] with all recurrences occurring in high-risk patients with T4, N2c, ECE, or non-HPV16 disease.88–89 Overall, two-year PFS appears to approximate 90% with induction chemotherapy and reduced-dose RT in patients with good response to induction therapy without high-risk features.
Table 6.
Radiation Deintensification Trials: Induction Chemotherapy to Select Patients from Radiation Dose Reduction
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| ECOG 1308 86 | IND cis/pacl/cetux (n=80). | 2 yr PFS : 81% [69–89] 54Gy, 67% [38–85] SOC | Acute ≥ Grade 3 toxicity: |
| (Phase II) | 2 yr OS : 93% [83–97] 54Gy, 87% [56–96] SOC | - Mucositis : 30% 54Gy, 47% SOC | |
| p16+ and/or HPV+ OPC. | Response to IND (n=51) → Reduced dose def CRT 54Gy + cetux | - Dysphagia : 15% 54Gy, 29% SOC | |
| AJCC7 III, IVA, IVB. | 2 yr PFS, 54Gy in T1–3,N1-N2b,<10 PYSH: 96% [76–99] | At 12 months: | |
| T4 included, N3 allowed but none enrolled. Smokers permitted. | No response to IND (n=15) → SOC CRT 69.3Gy | Primary site clinical CR to IND (n=51) | - Difficulty swallowing solids: 40% 54Gy, 89% SOC |
| Median FU = 35.4 months | CR/PR/SD (n=62) | - Impaired nutrition: 10% 54Gy, 44% SOC | |
| University of CA CCRO-022 87 | IND carbo/pacl (n=45). | 2 yr PFS: 92% [77–97] | Acute ≥ Grade 3 toxicities in 39%: |
| (Phase II) | 2 yr LRC: 95% [80–99] | 9% mucositis, 9% dysphagia | |
| p16+ OPC. | CR/PR (n=24) → Reduced dose def CRT (54Gy + pacl) | 2 yr OS: 98% [85–100] | 3 pegs before and 3 pegs during RT |
| AJCC7 Stage III or IV. T4 and N3 enrolled. | <PR/no response (n=20) → Reduced dose def CRT (60Gy + pacl) | ||
| Smokers permitted. | Median FU = 2.5 years | 3 of 4 failures in 60Gy with minimal response to IND | |
| OPTIMA 78–79 | IND carbo/nab-pacl→ | 2 yr PFS: 95% overall, 95% LR, 94% HR | Acute ≥ Grade 3 mucositis: |
| (Phase II) | - LR ≥50% response (n=30): | 2 yr LRC: 98% overall, 100% LR, 97% HR | 30% 50Gy RT, 63% 45Gy CRT, 91% 75Gy CRT |
| p16+ OPC. AJCC7 | Reduced dose def RT 50Gy | 2 yr DMFS: 100% overall, 100% LR, 100% HR | |
| T1–4 N2–3 and T3–4 N0–3. Smokers permitted. | - LR 30–50% response, HR ≥50% response (n=45): | 2 yr OS: 98% overall, 100% LR, 97% HR | Peg tubes: 3% RT50, 33% CRT45, 80% CRT75 |
| LR (n=44): ≤T3, ≤N2B, and ≤10 PYSH | Reduced dose def CRT 45Gy BID + pacl/5FU/HU | ||
| HR (n=46): T4, ≥N2C, or >10 PYSH) | BID on days 1–5, 15–19, 29–33 (over 6 wks) | 3 recurrences within 2 yrs: 2 in 45Gy CRT, 1 in 50Gy RT | |
| - All others 75Gy BID + pacl/5FU/HU (n=15) | |||
| Note: prophylactic LN RT was limited to only | BID on days 1–5,15–19,29–33,43–47,57–61 (over 10 wks) | ||
| the first echelon of uninvolved LNs | Median FU = 4.2 years | ||
| OPTIMA II 73–74 | IND carbo/nab-pacl/nivolumab x3c → RECIST response: | 2yr PFS 90.4%[79.3–95.7]: | Peg tubes: |
| (Phase II) | De-escalated treatment administered in 84.9%. | 50Gy RT: 96.3% | 50Gy RT 7.1% |
| p16+ and HPV+ OPC. | LR with ≥50% reduction: | 45–50Gy CRT: 85.8% | 45–50Gy CRT 44.1% |
| LR: | - TORS+selective ND (n=9) | 70–75Gy CRT: 100% | 70–75Gy CRT 75.0% |
| T1-T2 non-bulky tonsil or well-lateralized BOT ≤3 cm, | - Reduced dose def RT 50Gy(n=28): did not qualify for/refused TORS | ||
| non-bulky N2A-2B with ≤2 non-lower neck LNs measuring ≤5 cm. | 2yr OS 93.3%[82.4–97.5]: | Grade 4 toxicity: | |
| HR: | LR <50% but ≥30% reduction, HR with ≥50% reduction: | 50Gy RT: 96.0% | 50Gy RT 7.1% |
| T4, N2C-3, >20 PYSH, or non-HPV16 subtype. | - Reduced dose def CRT (n=34): 50Gy+HD cis x2c or 45Gy+THFXx3c | 45–50Gy CRT: 91.9% | 45–50Gy CRT 8.8% |
| 70–75Gy CRT: 100% | 70–75Gy CRT 10.0% | ||
| LR <30% reduction, HR <50% reduction: | |||
| - SOC def CRT (n=10): 70Gy+HD cis x3c or 75Gy+TFHX x5c | −3 local failures, 0 distant failures | 1 patient died during IND. | |
| Adjuvant nivolumab x6 months in all. | - among 9 TORS patients, 66.7% had pCR. | ||
| Median FU = 23.1 months | |||
| Quarterback 88–89 | IND doce/cis/5FU x3c (n=20). | Initial results: | After enrolling first 4/12 patients in 56Gy arm, due to |
| (Phase III) | If response → randomized 1:2 to | 2 yr PFS (p=0·85): 87·5% SOC vs. 83·3% 56Gy | higher than expected mucositis and 2 pegs, protocol |
| p16+ and HPV+ OPC. HPV subtype assessed. | SOC def CRT 70Gy (n=8) or Reduced dosed def CRT 56Gy (n=12) | - 1 LRF in SOC, 2 LRF in 56Gy | was amended. Remaining 8/12 paties in 56Gy arm |
| AJCC7 Stage III/IV. | CRT=RT+carbo | - PFS hazard ratio: 1·46 [0·13–16·12] | received carbo only. |
| T4 and N3 enrolled. | Including phase 2b update (n= 32 patients 56Gy): | - 6 pegs in total: 4 (33%) 56Gy, 2 (25%) 70Gy | |
| T1N1 permitted, none enrolled. | Phase 2b update, IND doce/cis/5FU x3c and responders (n=20): | - 2yr PFS: 84.4% [66.5–93.2] | - no therapy-related mortality |
| Smokers permitted. | Reduced dose def CRT 56Gy + carbo | - 2yr LRC: 87.4% [69.8–95.1] | - Phase 2b trial toxicity to be reported |
| - 3yr LRC 85% was considered non-inferior to SOC CRT | - 2yr OS: 90.6% [73.7–96.9] | ||
| - 3yr PFS 80% was considered acceptable | 72% had poor risk factors (ECE, T4, N2c, Non-HPV16) | ||
| - All 5 recurrences occurred within 1 yr | |||
| Median FU = 50 months | - All 5 recurrences had ≥1 poor risk factors |
5FU=5-fluorouracil, AFRT= altered fractionation radiation therapy, AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, BID=bidaily, c=cycle, carbo=carboplatin, cetux=cetuximab, chemo=chemotherapy, cis=cisplatin, CR=complete response, CRT=chemoradiation therapy, def=definitive, DM=distant metastasis, DMFS=distant-metastasis free survival, doce=docetaxel, FU=follow-up, Gy=gray, HD=high-dose, HPV=human papillomavirus, HR=high-risk, HU=hydroxyurea, IMPT=intensity-modulated proton therapy, IMRT=intensity-modulated radiation therapy, IND=induction, LD=low-dose, LN=lymph node, LR= low-risk, LRC=locoregional control, LRF= locoregional failure, ND=neck dissection, OPC= oropharyngeal cancer, OS=overall survival, pacl=paclitaxel, pCR=pathological complete response, peg=percutaneous endoscopic gastrostomy, PFS=progression-free survival, PR=partial response, PYSH=pack-year smoking history, RT=radiation therapy, SD=stable disease, SOC=standard of care, THFX=paclitaxel,5FU,HU, TORS=transoral robotic surgery, wk=week, wkly=weekly, yr=year.
Summary
Induction-based dose-reduction seems to achieve favorable outcomes, but critics question whether the toxicity of neoadjuvant chemotherapy followed by reduced-dose CRT truly constitutes deintensification. Incidence of grade 3+ mucositis was 63% with definitive CRT on TROG 12.01 compared to 56% and 63% with induction plus 54Gy or 45Gy CRT on E1308 and OPTIMA, respectively.29,78,86 In contrast, grade 3 mucosal toxicities occurred in 34% with upfront dose reduction to 60Gy CRT on the UNC/UF trial.82 However, induction offers advantages of incorporating in vivo tumor behavior into the algorithm and extends eligibility to a broader spectrum of more advanced-stage HPV+ OPC patients typically excluded from other trials. Upfront dose-reduction could be considered with smaller volume disease, but induction with response-guided reduced-dose CRT could be considered for more advanced HPV+ OPC.
Despite abundant data from phase II studies showing 2-year PFS on the order of 85–95% and OS over 90% with modest RT dose reductions from 45–60Gy, it is important to note that there is yet no level one evidence.17,78,82–84,86–88 ASCO issued a statement that de-escalated RT should only be administered on protocol.15 Nevertheless, two NCDB analyses revealed that ~15% of HPV+ OPC patients received de-escalated RT doses <66Gy, with the vast majority presumably treated off of protocol and without the associated extensive eligibility workup, multidisciplinary discussion, and close surveillance typical of clinical trials.90–91
Controversies in Deintensification
Small-volume primary T1–2N1 HPV+ OPC
Four historical RCTs which included small-volume AJCC7 T1–2N1 OPC patients showed LRC, DMFS, PFS, and CSS benefits with concurrent cisplatin over RT alone.92–96 Because AJCC7 T1–2N1 OPC comprised only a minority of patients and the studies preceded the discovery of the prognostic relevance of HPV in OPC, the utility of these data was limited and chemotherapy use for AJCC7 T1–2N1 HPV+ OPC has been inconsistent--even amongst experts. Current National Comprehensive Cancer Network Guidelines favor TORS with risk-adapted adjuvant therapy or RT alone for these patients; CRT is assigned a category 2b recommendation. In contrast, United Kingdom National Multidisciplinary Guidelines recommend CRT for T1N1 OPC.97 While there is no level one evidence in HPV+ OPC, there is strong level one evidence that chemotherapy improves DMFS, PFS, and OS versus RT alone in EBV-associated T1–2N1 nasopharyngeal cancer.98 According to four different medical, surgical, and radiation professional societies, the SOC for AJCC7 T1-T2N1 HPV+ OPC includes the option of CRT after careful consideration (Table 7).99–102
Table 7.
Society guidelines for chemoradiation in the management of T1-T2 N1 oropharyngeal cancer.
| ASTRO Clinical Practice guideline 99 | “After a careful discussion of patient preferences and the limited evidence supporting its use, concurrent systemic therapy may be delivered to patients with T1-T2 N1 OPSCC receiving definitive RT who are considered at particularly significant risk for locoregional recurrence. (Conditional, LQE, 100%)” |
| “However, certain patients with T1–2 N1 OPSCC who are considered at particularly significant risk for locoregional recurrence may receive concurrent systemic therapy, because the absolute benefit of combined modality therapy may justify its toxicities in this population.” | |
| ACR Appropriateness Criteria 100 | “Patients with T1–2 N1–2aM0 disease can receive radiation, chemoradiation, or transoral surgery with neck dissection and appropriate adjuvant therapy.” |
| AHNS guidelines 101 | “Nevertheless, treatment strategies remain the same despite HPV status. Further research is necessary to determine the safety and efficacy of altering the management for patients with HPV‐positive HNSCC with respect to the new staging.” |
| ASCO guidelines 102 | “Concurrent systemic therapy may be delivered to patients with T1-T2 N1 OPSCC receiving definitive radiotherapy who are considered at particularly significant risk for locoregional recurrence, after a careful discussion of patient preferences and the limited evidence supporting its use (Recommendation strength: conditional, Quality of evidence: low).” |
AJCC7 Stage III OPC (T1–2N1, T3N0–1) encompassed a heterogeneous population with various treatment options and consequently wide practice patterns. Although guidelines reveal discrepancies amongst experts on chemotherapy in the small-volume AJCC7 T1–2N1 subset, in practice most received CRT. An NCDB analysis showed that 70% (2379 of 3399 patients) of T1–2N1 OPC received CRT, with no evidence that patients with HPV+ versus HPV-negative (HPV-) OPC benefitted differentially from chemotherapy.103 With AJCC8, N1 expanded to include any ipsilateral LNs <6 cm and ≤4 LNs (formerly AJCC7 N1–2B). Despite downgrading of T1–2N1 from Stage III in AJCC7 to Stage I in AJCC8, contemporary data still suggest a benefit with chemotherapy. An NCDB study of HPV+ OPC confirmed a survival detriment with RT alone compared to CRT in AJCC8 Stage I patients (HR 1.798, 95% CI 1.064–3.039, P=0.029).104 And among all AJCC7 T1–2N1 HNC in the NCDB, subset analysis actually showed the greatest survival benefit with CRT in OPC (HR 0.74, 95% CI 0.65–0.85, P <0.001).103
The majority of recent randomized deintensification trials administered CRT as SOC for AJCC7 T1–2N1 HPV+ OPC (Table 8), and all retained CRT as the SOC. Subset analysis from De-ESCALaTE revealed that even AJCC8 stage I/II HPV+ OPC (n=276) had a 5% 2-year OS advantage with cisplatin over cetuximab (98.4% versus 93.2%, p=0.043).16 On HN002, two of ten T1N1 patients treated with RT alone experienced LRF.17 The International Collaboration on Oropharyngeal Cancer Network for Staging found no difference in 5-year OS between AJCC7 N0, N1-N2A, and N2B HPV+ OPC patients: 80% (95% CI 73–87) versus 87% (95% CI 83–90) versus 83% (95% CI 80–86), respectively.105 This reflects shortcomings of nodal staging to stratify risk, with N1 HPV+ OPC patients having comparable prognosis to N2 patients (for whom chemotherapy is the consensus).
Table 8.
Eligibility for Chemoradiation Deintensification Trials
| T1-2N1 Included | T4 or N3 included | Smokers included | |
|---|---|---|---|
| RTOG 1016 15 | ☑ | ☑ | |
| De-ESCALaTE 16 | ☑ | ☑ | Only N0-N2a if >10PYSH |
| TROG 12.01 29 | Only N0-N2a if >10PYSH | ||
| HN002 17 | ☑ | Only <10PYSH | |
| ORATOR2 57 | ☑ | ☑ | |
| UNC/UF 82–83 | <10PYSH or >5 yrs abstinence from smoking | ||
| ECOG 1308 86 | ☑ | ☑ | ☑ |
| OPTIMA 78–79 | ☑ | ☑ | |
| Univ of CA 87 | ☑ | ☑ | ☑ |
| Quarterback 88–89 | ☑ | ☑ | ☑ |
| MSK 30 ROC 137 | ☑ | ☑ | |
| HN005 | ☑ | Only <10 PYSH |
PYSH= pack-year smoking history
Although limited, the available evidence suggests that omitting or de-escalating chemotherapy from RT in AJCC7 T1–2N1 HPV+ OPC results in inferior outcomes. The next generation of randomized deintensification trials (like HN005) administers HD cisplatin with RT as their SOC. There are differing opinions among experts, but the randomized trials which included these patients consistently showed superior outcomes when HD cisplatin was administered with RT. Ultimately, since distant metastasis is their predominant mode of failure, chemotherapy should be considered in eligible AJCC7 T1–2N1 patients.
Bulky, very locally advanced T4 or N3 HPV+ OPC
The inclusion of very advanced (T4 or N3) patients in deintensification studies was variable (Table 8). Subset analysis of T4 or N3 patients from De-ESCALaTE showed dismal 2-year OS with cetuximab versus cisplatin (67.1% vs. 93.3%, p=0.03).16 E1308 and Quarterback did not meet the target 92% 2-year PFS thresholds, largely due to poor outcomes in T4 patients.86,88 Given these patients’ propensity for DM and LRF, many trials escalate treatment in this cohort (e.g. KEYCHAIN, NCT03383094, is randomizing T4 or N3 HPV+ OPC patients to SOC 70Gy CRT with or without concurrent/adjuvant pembrolizumab). Considering their poor prognosis with cetuximab and their inclusion in treatment escalation trials, caution should be exercised before considering these patients for deintensification.
Smokers
Smoking was a defining factor in the original RTOG RPA and is believed to negate some of the prognostic benefits of HPV positivity.4,106 However, there is dissonance on the impact of smoking and it was ultimately not included into the AJCC8 staging system. Subset analysis of MARCH patients with known p16 and smoking status from four RCTs showed that p16+ former/current smokers had significantly worse PFS (HR 0.49, 95% CI 0.33–0.75) and an 18,7% 5-year OS detriment compared to never smokers.107 In contrast, a more contemporary multi-institutional RPA demonstrated that only current smokers experienced 2-year PFS below 91%, and any PYSH former smokers experienced 2-year PFS over 91%.108 Another RPA projected that AJCC7 T1–2N0–1 HPV+ OPC with ≤20 PYSH would fall into RPA-I with 89% 5-year OS, while the same patient with >20 PYSH would fall into RPA-II with 64% 5-year OS.109 In contrast, a nomogram based off RTOG 0129, RTOG 0522, and RTOG 9003 estimates that an AJCC7 T1–2N0–1 p16+ OPC nonsmoker or smoker should achieve 5-year OS of ~88% or 87% respectively.110 Ultimately, smoking is an eligibility factor for some trials and not others (Table 8). E3311 patients were stratified by PYSH, and >10 versus ≤10 PYSH smoking did not affect PFS on subsequent analysis.45 Even more recent analysis of outcomes on E3311 showed no significant PFS or OS differences when comparing current versus former smokers.111 Thus, the 10 PYSH rule may not apply to all early-stage HPV+ OPC, and former smokers can likely be included on deintensification trials but treatment should be stratified by smoking history when feasible.
Lessons Learned from Modern Deintensification Trials
HPV disrupts staging and inspires evolution in treatment paradigms, but is it enough to guide de-escalation?
It is widely accepted that HPV+ OPC constitutes a distinct clinical entity with more favorable biology and treatment responsiveness than their HPV-counterparts.112–113 Multiple meta-analyses have reported a 28–66% reduced risk of death in HPV+ OPC.107,114–116 Six RCTs with post-hoc stratification of HPV status (RTOG 0129, RTOG 0522, TROG 02.02, TAX 324, RTOG 9003, PET-NECK) showed improved OS (HR 0.49) and disease-free survival (DFS) (HR 0.41) in HPV+ OPC cohorts.117–118 However, it is important to note that not all HPV+ OPCs are equally favorable, outcomes are heterogeneous, and de-escalation may thus compromise outcomes for some patients. Genotypic heterogeneity has been identified, with The Cancer Genome Atlas and Quarterback Trial corroborating inferior outcomes with non-HPV16 sutypes.88,119 However, most trials do not mandate HPV subtyping and positivity on p16 immunohistochemistry is considered an adequate surrogate for HPV+ disease (although p16 immunohistochemistry does not discriminate between HPV16 and non-HPV16 subtypes). P53 mutations are enriched in recurrent/metastatic HPV+ OPC and PIK3CA mutation may be a biomarker of more aggressive disease.120–121 Additionally, there is a 2.6-fold greater risk of death in black versus non-Hispanic white patients after adjustment for HPV status.122 These findings underscore risk variations not captured by P16 status alone.
Given limitations of the staging system to discriminate risk, stage-based empirical deintensification efforts seem susceptible to failure. As cautionary tale, RTOG 0022 was a trial of accelerated IMRT (66 Gy over 6 weeks) without chemotherapy for the earliest stage AJCC7 T1–2N0–1 OPC patients (unknown HPV status): 2-year LRF was 9% and 2-year DFS was only 82.0%.123 While 2-year LRF rates of 9% without chemotherapy may seem reasonable at first glance, long-term follow-up of higher stage T1–2N1–2B or T3N0–2B p16+ OPC patients from RTOG 0129 reported 13.5% 8-year LRF with CRT—a metric that would not likely be achieved with 9% LRF sustained in the first two years after RT alone.5 Additionally, 82% DFS in the first two post-treatment years does not meet the currently accepted 92% threshold. Ultimately, there was no difference in 5-year PFS between AJCC8 Stage I and II patients treated with CRT on RTOG 0129 and RTOG 0522, suggesting that Stage I patients do not have a substantially superior prognosis warranting less therapy.26
Finally, the importance of long-term follow-up cannot be overemphasized. A meta-analysis of HPV+ tonsil cancer showed superior PFS to HPV-disease in the first three years that was not sustained at years four or five.124 The surgical literature estimates 88.7% 5-year CSS for T1–2N0–1 OPC after surgery ± adjuvant RT or CRT.125 However, when considering deintensification, given the morbidity of salvage, PFS is a critical consideration in addition to CSS. A retrospective study defined the ideal deintensification candidate as a T1–3N0–2C HPV+ OPC patient with 95% 3-year LRC and 93% 3-year DMFS.126 A viable deintensification approach must achieve excellent outcomes and maintain them on longer follow-up before it can change practice.
Re-examining the Radiation Dose-Response Relationship in HPV+ OPC
Seminal radiation dose-response curves for tumor control probability (TCP) were modeled at the turn of the century, and tonsil cancer curves were observed to be shallow due to underlying tumor heterogeneity. Doses of 55–75Gy achieved LRC rates ranging from ~60–85% for T2 tumors to ~35–70% for T3 tumors (Figure 3a); after accounting for treatment time and stage and assuming LC of 40–80%, each 1Gy increase of dose was estimated to improve TCP by 1.75%.127 This translated into an effort to escalate dose to maximize outcomes. Of note, these radiobiology models were built upon data from patients treated with RT alone, predating concurrent chemotherapy and the discovery of HPV as a prognostic factor in OPC.
Figure 3.
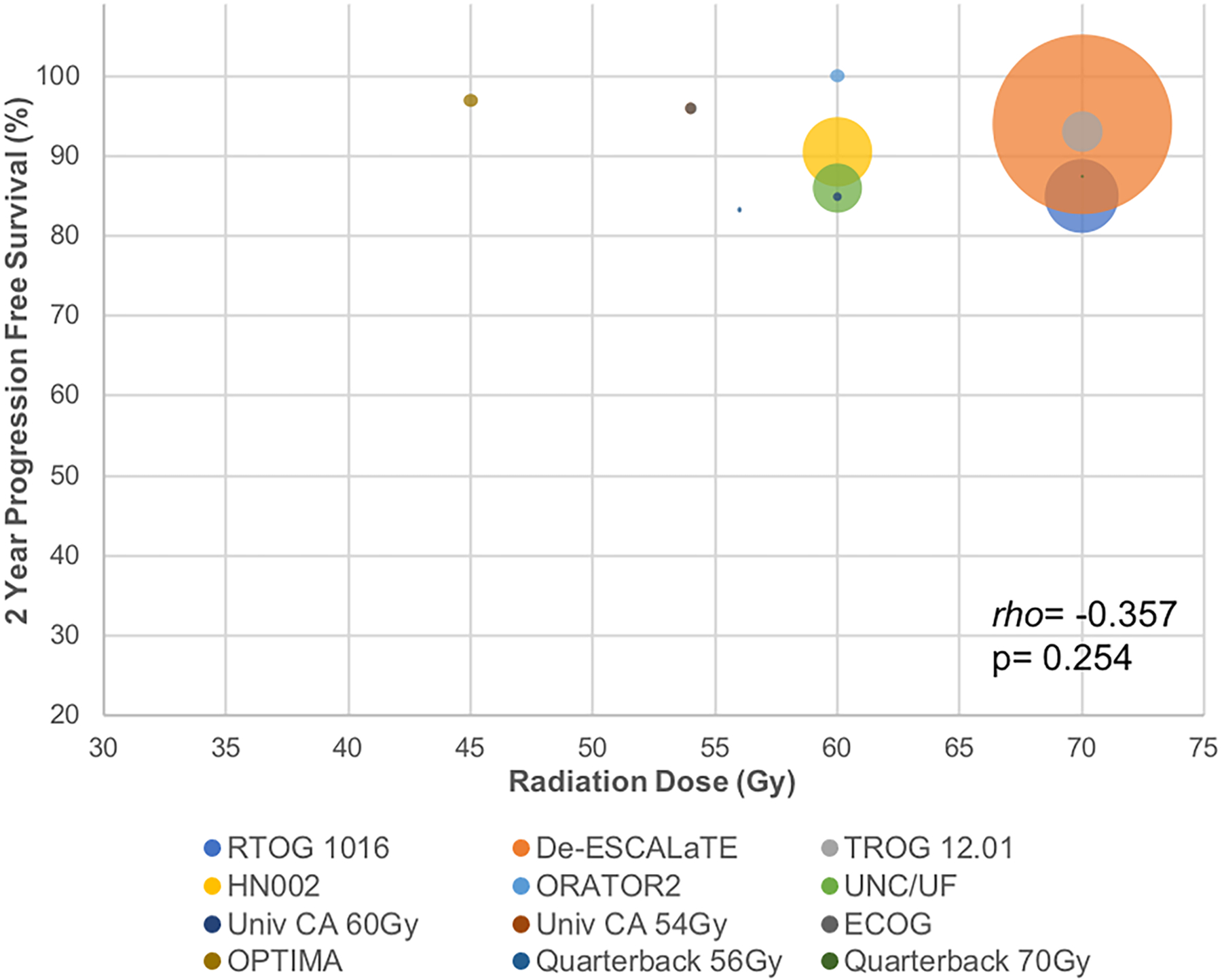
Tumor control probability (TCP) dose-response curves with definitive chemoradiation in HPV+ OPC.
(A) Bubble plot of LRC rates from modern chemoradiation trials (colored circles) shows no relationship between radiation dose and LRC (rho= 0.009, p= 0.978). Superimposed seminal T2 and T3 tonsil cancer TCP curves with radiation (black lines) modeled at the turn of the century117 (predating concurrent chemotherapy use) show shallow LRC improvements when increasing radiation doses from 55–75Gy.
(B) Bubble plot of PFS rates from modern chemoradiation trials show no relationship between radiation dose and PFS (rho= −0.357, p= 0.254).
We performed an exploratory analysis to reassess the dose-response relationship in AJCC7 Stage III-IV HPV+ OPC treated with modern definitive CRT. Dose-response relationships between radiation dose and LRC and PFS were analyzed with the Spearman rank correlation test (an ideally suited non-parametric test that does not assume a linear relationship and makes the least assumptions on the data). It yields a p-value (significant at <0.05) and correlation coefficient rho (ranging from −1 to +1). Robustness was performed by using Grubbs’s test to remove outliers at a level of p<0.05 (two-tailed); no outliers were identified. When plotting TCP for HPV+ OPC patients treated with CRT on modern trials (colored dots), we found no correlation between RT dose and LRC (Spearman’s rho=0.009, p=0.978, Figure 3a). We also found no correlation between RT dose and PFS (Spearman’s rho=−0.357, p=0.254, Figure 3b). Several notable trends were observed. First, the absolute TCP was much higher than historical rates, approximating 85–100% LRC with 45–70Gy CRT. Second, TCP in the 54–70Gy range appears flat and shallower than the original model (black lines, Figure 3a), suggesting even less of a response to dose-escalation.127 Finally, the TCP appears to be left-shifted, with high cure rates at lower doses and raising the question: how low can we reduce RT dose in the setting of CRT and still maintain excellent outcomes in HPV+ OPC?
THE FUTURE OF DE-ESCALATION: Intratreatment Response Assessments to Guide Treatment Deintensification
Although most HPV+ OPCs have favorable prognoses, distant metastasis is the predominant mode of failure and often occurs later and sometimes with a more disseminated pattern.126,128 While p53 mutational status is being used to guide RT de-escalation (NCT03077243, Table 9), no dominant predictive biomarker has been firmly established. However, technological advances in liquid biopsy and imaging allow us to monitor disease responses much earlier--even during treatment. Thus, until we have discovered a better biomarker, currently available tools can be utilized to assess response, personalize treatment, and perhaps even change course if needed.
Table 9.
Personalized Radiation Deintensification Trials
| Study | Treatment Arms | Outcomes | Toxicities |
|---|---|---|---|
| MSKCC 30 ROC136–137 / NCT03323463 | Baseline FMISO-PET scan prior to CRT start. | Pilot Study (n=15 de-escalated patients): | 0 grade 3 RT-related toxicities on the pilot trial. |
| (Phase II). | If first FMISO-PET +hypoxia, second FMISO-PET performed 2 wks after CRT start. | −2 yr LRC 100% | |
| p16+ OPC. | −2 yr PFS 92.9% | Acute grade 3 toxicity on the Phase II trial: | |
| AJCC7 T1–2 N1–2C. Any PYSH. | Hypoxia-neg: 30Gy CRT (with HD cis) | −2 yr OS 92.9% | - 2 diarrhea, 2 syncope, 1 vasovagal, 1 dysphagia |
| Pilot Study: | - n=19 Pilot trial (15 de-escalated) | −11 of 15 pathological CR. | - No grade 3 mucositis, 0 pegs. |
| - primary site resection before CRT | - n=158 Phase II trial (128 de-escalated) | Phase II (whole cohort): | |
| - planned ND at 4 months post-CRT. | −1 yr LRC 94% | ||
| Phase II FU: | Hypoxia+: 70Gy CRT (with HD cis) | −1 yr DMFS 100% | |
| - primary site resection before CRT | Median FU = 34 months (pilot study) | −1 yr PFS 94% | |
| - no planned ND at 4 months post-CRT. | Median FU = 12 months (Phase II). | −1 yr OS 100% | |
| - No 30Gy patients failed in the primary site | |||
| - 8 patients with LN recurrences, all | |||
| successfully salvaged with surgery. | |||
| UNC+UF / NCT03077243 | >10 PYSH AND p53 wild-type, Or ≤10 PYSH: | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | Deintensified def CRT (60Gy + LD cis 30–40 mg/m2 wkly) | ||
| p16+ and/or HPV+ OPC. | p53 mutant: SOC CRT 70Gy + LD cis 30–40 mg/m2 wkly | ||
| AJCC7 T0-3 N0-2C. | - ctHPVDNA will be prospectively assessed and correlated with outcomes. | ||
| The ReACT Study/ NCT04900623 | NavDX ctHPVDNA test (detects HPV16) at baseline, 4 wks and end of treatment. | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | LR: 5–6 wks reduced dose def RT ± chemo | ||
| p16+ and HPV+ OPC. | HR: 7–8 wks SOC def RT ± chemo | ||
| AJCC8 Stage I, II, or III. | Chemo: HD cis, LD cis, or carbo/pacl. | ||
| MSKCC / NCT05307939 | Must have preoperative NavDX ctHPVDNA >50 copies/mL. | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | Check NavDX ctHPVDNA levels (Frag/mL) postoperatively and every 3–6 months | ||
| HPV-16 OPC or unknown primary | to select patients for active surveillance (OBS) or SOC adjuvant therapy | ||
| cancer with no residual disease on | LR: <5 (OBS), 5–7 (repeat NavDX), >7 (SOC adjuvant therapy) | ||
| postoperative imaging. | IR: <5 (OBS), ≥5 (SOC adjuvant therapy) | ||
| T1N1-T4N3. No positive margin or ECE. | |||
| Univ of Michigan / NCT03416153 | Pre- and mid-treatment PET scans to select patients for de-escalation. | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | Deintensified def CRT (54Gy + carbo/pacl) | ||
| p16+ or HPV+ OPC. | |||
| AJCC8 Stage I or II. | |||
| NYU / NCT03215719 | Interval CT scan at 4 wks to select responders. | Ongoing, not reported | Ongoing, not reported |
| (Phase II) | >40% LN shrinkage → reduced dose def RT (60Gy + cis) | ||
| p16+ OPC. | |||
| AJCC7 T1–3 N1–2B. |
AJCC7=American Joint Committee on Cancer 7th Edition, AJCC8=American Joint Committee on Cancer 8th Edition, carbo=carboplatin, chemo=chemotherapy, cis=cisplatin, CRT=chemoradiation therapy, ctHPVDNA=circulating tumor HPV DNA, def=definitive, DM=distant metastasis, FU=follow-up, Gy=gray, HD=high-dose, HPV=human papillomavirus, HR=high-risk, IR= intermediate-risk, LD=low-dose, LN=lymph node, LR=low-risk, LRC= locoregional control, OBS=observation, OPC= oropharyngeal cancer, OS=overall survival, +=positive, pacl=paclitaxel, PET=positron emission tomography, PFS=progression-free survival, PYSH=pack-year smoking history, RT=radiation therapy, SOC=standard of care, wkly=weekly, wks=weeks, yr=year.
Circulating tumor HPV DNA
Circulating tumor HPV DNA (ctHPVDNA) holds promise to assess treatment response and early recurrence.129 A prospective clinical trial of CRT in HPV+ OPC found that post-treatment ctHPVDNA positivity had a 100% negative predictive value, and two consecutively positive values had a 94% positive predictive value with median 3.9 month lead-time from ctHPVDNA positivity to biopsy-proven recurrence.130 Of note, approximately 10–20% of patients had undetectable baseline ctHPVDNA and higher pretreatment levels were not associated with prognosis, so the use of liquid biopsy to assess intratreatment response or prescribe treatment is promising but still in need of further study. The ReACT Study (Table 9) will use ctHPVDNA levels to guide RT dose de-escalation in low-risk HPV+ OPC. Memorial Sloan Kettering (Table 9) will use ctHPVDNA to select patients for adjuvant therapy omission in postoperative low/intermediate-risk HPV+ OPC.
Radiomics and Functional Imaging
Another approach harnesses radiomics, using intratreatment imaging to identify early responders. A study from NYU showed that low-risk OPC with ≥43% nodal decrease on cone-beam computed tomography (CT) by treatment day 20 had superior 2-year LRC.131 Consequently, NCT03215719 (Table 9) will use interval scan at four weeks to identify responders and adaptively de-escalate to 60Gy in those with >40% nodal shrinkage. NCT03656133 will investigate if the individual patient proliferation saturation index (a mathematical model incorporating pre-treatment CT) can select RT fractionation to increase rapid response (≥ 32% volume reduction at 4 weeks) in p16+ AJCC8 T1–3N0–1 OPC. In addition to CT changes, decreased uptake on 18FDG-PET as early as one to two weeks into treatment (at ~10Gy or 20Gy) has also been found to predict PFS and OS in HPV+ OPC.132–133 NCT03416153 (Table 9) will use pre- and mid-treatment PET to selectively de-escalate patients to 54Gy CRT. Incorporating intratreatment volumetric or functional assessments to guide deintensification offers the advantage of not subjecting patients to extra therapy (i.e. induction chemotherapy), but modest 10–16Gy reductions in RT dose may be insufficient to translate into improved toxicity and QOL compared to SOC therapy. For example, radiation dose goals to swallowing structures were similar with SOC 70Gy on RTOG 1016 versus modest reductions to 54Gy and 60Gy on the CCRO022 and HN002 deintensification trials: oral cavity (mean <30Gy), esophagus (mean <30Gy), and uninvolved pharynx (<1/3 over 50Gy).15,17,87 More dramatic prescription dose reductions are likely necessary to achieve meaningful dosimetric and clinical toxicity advantages.
There is both preclinical and clinical data to support the consideration of more drastic dose de-escalation. A model using pre- and early-treatment (week four) CT tumor volume dynamics to estimate personalized RT doses was applied to a cohort of HNC patients from Moffitt and MD Anderson Cancer Centers; the in silico trial of dynamics-adapted dose personalization estimated that 77% of patients treated with SOC 66–70Gy were overdosed by an average of 39Gy.134 Even further beyond this mathematical model predicting that a majority of patients may only need ~30Gy was the most drastic dose de-escalation trial to date which delivered 30Gy to the majority of patients: the 30 ROC trial from Memorial Sloan Kettering. Hypoxic tumors are known to be treatment-resistant and have poor prognosis.135 The 30 ROC trial utilized functional PET imaging to select more favorable hypoxia-negative patients for drastic RT dose reduction to 30Gy (a 60% reduction from SOC 70Gy). In the pilot trial, nineteen p16+ OPC patients had upfront primary site resection and planned ND 4 months post-CRT; 15 patients had no hypoxia on baseline or early interval 18F-FMISO-PET and were de-escalated to 30Gy CRT.136 On planned post-30Gy CRT ND, 11 of 15 patients had pathological CR and 2-year LRC and PFS among de-escalated patients was 100% and 92.9%, respectively. Diffusion-weighted MR changes correlated with pathological CR, but tumor volume and ctHPVDNA changes did not. The follow-up Phase II trial enrolled 158 patients with primary site resection but no planned ND after 30Gy CRT; one-year LRC and PFS were 94%, with all 8 nodal recurrences successfully salvaged with surgery.137 Without induction chemotherapy and by incorporating functional imaging, about 80% of patients received drastically de-escalated therapy. The next cohort of patients on 30 ROC will proceed with hypoxia-guided definitive CRT de-escalation to 30Gy without any surgery at all. The incorporation of early interval, intra-treatment response assessments has the potential to revolutionize the de-escalation paradigm.
Genomics
Given lack of consensus on who and how to de-escalate patients, there is a need to identify novel therapeutic targets and pathways implicated in disease pathogenesis, response, and progression. If the ultimate goal of deintensification is toxicity mitigation, there is still much room to improve the therapeutic ratio by identifying biomarkers of radiosensitivity and perhaps reducing dose in these patients first.138 Moffitt Cancer Center combined a gene-expression-based radiosensitivity index with the linear quadratic model to generate an algorithm to predict radiation response.139 The genomic-adjusted radiation dose (GARD) score was significantly associated with time to first recurrence and OS, with a higher score predicting a greater therapeutic effect of RT.140 Interestingly, total radiation dose was not associated with recurrence or OS, suggesting that inherent tumor biology supersedes the radiation dose-response. Perhaps one of the most impactful uses of the GARD score would be to identify patients for whom radiation de-escalation (or escalation) should be considered. Accordingly, the Moffitt group is developing a trial of genomically-guided dose prescription for HPV+ OPC.141 In the 30 ROC trial, whole-genome sequencing identified a DNA repair defect predictive of response that was confirmed on an independent cohort from the MC1273 de-escalation study.136 Moving forward, the combination of biology-driven patient selection and confirmatory intra-treatment assessments seems ideal to personalize therapy and most safely guide de-escalation.
CONCLUSIONS
Although the favorable prognosis of HPV+ OPC changed the staging system, the first-generation of randomized de-escalation trials failed to justify a change in its management. HD cisplatin with RT remains the de facto SOC in HPV+ OPC (RTOG 1016, De-ESCALaTE). For primary management, TORS has more grade 5 toxicity compared to definitive RT (ORATOR2) and appears to have inferior PFS as well (ORATOR2). However, there is promising data that PORT may be de-escalated to 50Gy (E3311) in patients with intermediate-risk features. An RCT (MC1675) compared 30–36Gy BID DART plus docetaxel versus SOC 60Gy PORT (2-year PFS 86.5% versus 95.1%). However, a preplanned pooled analysis (MC1273, MC1675) reported outcomes above accepted PFS thresholds in patients with intermediate-risk features (negative margins, no ECE). Moving forward, an RCT will assess PORT de-escalation to 50Gy with intermediate-risk postoperative factors and establish consensus on chemotherapy guidelines with high-risk pathology (PATHOS). The data to date suggest that radiation dose may empirically be modestly reduced to 60Gy with concurrent cisplatin in non-bulky HPV+ OPC (HN002, UNC/UF), but level one evidence is years away (HN005). Induction chemotherapy to select early responders may permit RT dose reductions to 45–56Gy (OPTIMA, CCRO-022, E1308, Quarterback) in more advanced HPV+ OPC. However, the utilization of intratreatment assessments may allow de-escalation without induction chemotherapy. Even more drastic RT dose de-escalation with biology-driven tumor characterization via functional hypoxia imaging appears feasible (30 ROC). Although the SOC has not yet changed, there is justifiable optimism that with careful selection criteria, intratreatment response assessments, and sufficient longterm follow-up, one (or more) feasible de-escalation strategies will be established. Table 10 summarizes these findings.
Table 10.
Summary of Deintensification Trials
| De-Escalation Strategy | Relevant Trials | Results |
|---|---|---|
| Definitive CRT (SOC=HD cisplatin) | ||
| Attenuation with cetuximab | RTOG 1016 15 | Cetuximab is inferior to HD cisplatin |
| De-ESCALaTE 16 | Cetuximab is inferior to HD cisplatin | |
| TROG 12.01 29 | Cetuximab is inferior to LD cisplatin | |
| Omission of cisplatin | HN002 17 | Cisplatin cannot be omitted from Definitive RT |
| Attenuation with LD cisplatin | HN009 (not exclusive to HPV+ OPC) | Trial ongoing |
| Surgery (SOC=Open/Transmandibular/Transcervical Resection + ND) | ||
| TORS + ND ± adj RT VS. Def RT ± chemo | ORATOR 54–56 | TORS does not have less toxicity than def RT |
| ORATOR2 57 | TORS has inferior PFS and more grade 5 toxicity than def RT | |
| Adjuvant Radiation Therapy (SOC= 60Gy PORT) | ||
| TORS + ND + De-escalated Adj Therapy | ECOG 3311 45 | 50Gy PORT appears feasible with postoperative IR factors |
| MC1273 58–59 , MC1675 61–62 | 30Gy BID PORT + chemo is comparable to SOC with postoperative IR factors | |
| PATHOS | RCT ongoing (50Gy PORT vs. SOC for IR factors; SOC ± chemo for HR factors) | |
| Definitive Radiation Therapy (SOC=70Gy) | ||
| De-escalate Def RT Dose | ECOG E1308 86 , OPTIMA 78–79 , Univ of CA 87 , Quarterback 88–89 | NAC with deintensification to 45–60Gy in responders with bulky HPV+ OPC is feasible |
| HN002 17 , UNC/UF 82–83 | Upfront de-escalation to 60Gy CRT appears feasible in non-bulky HPV+ OPC | |
| 30ROC 137 | Selective de-escalation to 30Gy CRT appears feasible in hypoxia-negative patients | |
| HN005 | RCT ongoing (SOC 70Gy CRT vs. upfront de-escalation to 60Gy CRT) | |
adj=adjuvant, chemo=chemotherapy, CRT=chemoradiation therapy, def=definitive, HD=high-dose, HPV=human papillomavirus, HR=high-risk, IR=intermediate-risk, LD=low-dose, NAC=neoadjuvant chemotherapy, ND=neck dissection, OBS=observation, OPC=oropharyngeal cancer, +=positive, PFS=progression-free survival, PORT=postoperative radiation therapy, RCT=randomized control trial, RT=radiation therapy, SOC=standard of care, TORS=transoral robotic surgery
As we await the next generation of deintensification trials, it is important to evaluate lessons learned and clinical gaps identified. Small volume AJCC7 T1–2N1 HPV+ OPC is a cohort whose risk may have been underestimated and is thus at danger for undertreatment. De-ESCALaTE reported inferior outcomes with cetuximab and HN002 confirmed that chemotherapy omission is inappropriate. Although RT alone is recommended by some guidelines, it does not reflect the practice amongst many cooperative groups and experts conducting trials who administer cisplatin with RT as their SOC (HN005,ORATOR2). On the opposite end of the spectrum, some deintensification trials included locally advanced T4 and N3 HPV+ OPC patients. Historically, patients with bulky disease have been considered for treatment escalation (often induction chemotherapy), so it seems that practice trends against deintensification in this group. Finally, risk quantification based on smoking history/status is in need of further refinement; although those with >10PYSH (and especially current smokers) were considered high-risk, contemporary trials in HPV+ OPC fail to show an impact on PFS and smoking ultimately did not impact staging.
The optimal de-escalation approach remains unknown. While many clinical trials have reported promising results, it should be explicitly noted that the only strategy (chemotherapy attenuation) formally compared against the SOC in RCTs failed. There are ongoing deintensification trials evaluating the efficacy and toxicity of other strategies including radiation dose reduction or TORS to de-escalate adjuvant therapy. However, the adoption of multiple deintensification approaches simultaneously may confound our ability to understand which treatment(s) can be decreased, or potentially, omitted. There is already level two evidence to support various promising de-escalation strategies, but conducting multiple randomized phase three trials powered to confirm non-inferiority of each feasible deintensification method against the SOC is unfortunately not practicable. This raises the question: what level of evidence is required to change clinical practice? In principle, level one evidence would be necessary for de-escalation to become the standard of care. In practice, national registry data demonstrates that clinical management has already changed for some despite the absence of level one evidence; although no radiation deintensification strategy has been proven noninferior to the SOC, one-third of HPV+ OPC patients had received reduced postoperative and ~15% had received reduced definitive RT doses before 2015 (years prior to the publication of recent de-escalation studies).20,90,91 Given the limitations of national registry data, it is not possible to know whether these reduced doses were due to physician recommendation, patient request, toxicity, or non-compliance. However, it is important to point out that 69% of patients would not risk a potential 0–5% drop in survival to de-escalate treatment.142 Alternatively stated, 31% of patients might be willing to risk a potential 0–5% drop in survival to deintensify their therapy. Of note, on the RTOG 1016, De-ESCALaTE, TROG 12.01, and MC1675 phase III trials, PFS decrements ranged from 8.6–13% in the de-escalated compared to the SOC arms.15,16,29,61 But as practitioners who will be challenged with caring for patients who are ineligible or do not provide informed consent for SOC therapy, how can we navigate which de-escalation strategies are most suitable for clinical practice? Given that a number of potentially viable deintensification approaches have been reported, informed decision-making would rely on patient-specific factors (eligibility for TORS, systemic agents, or radiation), multidisciplinary consensus (all published deintensification trials were multidisciplinary efforts), resources (infrastructure, supplies, clinician expertise), patient preferences, and the data (how well the patient fits the trial eligibility criteria for the deintensification method being employed, multi-institutional studies may have less center-specific biases or be potentially more broadly applicable).
Ultimately, with the discovery of novel biomarkers and the development of new systemic therapies, future trials will eventually redefine and elevate the SOC. In the interim, as deintensification efforts continue, clinical trials and even clinical practice may benefit from utilizing currently available pre-, intra-, and early post-treatment parameters (ctHPVDNA, functional imaging) to better select, monitor, and personalize therapy for patients.
Funding:
Yhis study was supported by Cancer Center Support Grant P30 CA008748 from the NIH/National Cancer Institute (NCI).
Disclosures:
Nadeem Riaz is supported by research funding (ArcherDx and Repare Therapeutics Inc) and consulting fees (Illumina, Inc, and PaigeAI) outside the submitted work. C. Jillian Tsai reports receiving consultation fees from Varian Medical Systems outside the submitted work. Lara A. Dunn is supported by research funding (from Regeneron Pharmaceuticals, Inc, Eisai Co, Ltd, CUE-101, and Replimune Group Inc) and serves on the advisory board of Merck & Co, Inc, outside the submitted work. Alan Ho is supported by research funding (from Lilly, Genentech/Roche, AstraZeneca, Bayer, Kura Oncology, Kolltan Pharmaceuticals, Eisai, Bristol Myers Squibb, Astellas Pharma, Novartis, Merck, Pfizer, Ayala Pharmaceuticals, Allos Therapeutics, Daiichi Sankyo, Elevar Therapeutics), serves a consulting/advisory role (for Bristol Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Elevar Therapeutics, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia Biotech, InxMed), serves on the speakers’ bureau (Medscape, Omniprex America, Novartis), and receives travel/accommodations/expenses (from Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, KLUS Pharma) outside the submitted work. Bhuvanesh Singh reports serving on the scientific advisory board of ClinRx, outside the submitted work. Eric J. Sherman is supported by institutional research funding from Merck outside the submitted work. David G. Pfister reported receiving grants from the National Institutes of Health (NIH) and the Philanthropy–Serra Fund during the conduct of the study and research support from Hookipa Pharma Inc outside the submitted work. Nancy Y. Lee reports consultation fees (from Merck & Co, Inc, Merck EMD, Mirati Therapeutics, Roche, and Elsie Pharmaceuticals) outside the submitted work.
The remaining authors made no disclosures.
REFERENCES
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29(32): 4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damgacioglu H, Sonawane K, Zhu Y, et al. Oropharyngeal Cancer Incidence and Mortality Trends in All 50 States in the US, 2001–2017. JAMA Otolaryngol Head Neck Surg. 2022; 148(2): 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damgacioglu H, Sonawane K, Chhatwal J, et al. Long-term impact of HPV vaccination and COVID-19 pandemic on oropharyngeal cancer incidence and burden among men in the USA: A modeling study. Lancet Reg Health Am. Published online December 15, 2021. doi: 10.1016/j.lana.2021.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol 2014; 32: 3858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32: 2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–8. [DOI] [PubMed] [Google Scholar]
- 8.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999; 91: 2081–6. [DOI] [PubMed] [Google Scholar]
- 9.Denis F, Garaud P, Bardet E, et al. Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004; 22: 69–76. [DOI] [PubMed] [Google Scholar]
- 10.Olmi P, Crispino S, Fallai C, et al. Locoregionally advanced carcinoma of the oropharynx: conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy--a multicenter randomized trial. Int J Radiat Oncol Biol Phys 2003; 55: 78–92. [DOI] [PubMed] [Google Scholar]
- 11.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 2013; 14: 257–64. [DOI] [PubMed] [Google Scholar]
- 12.Hitt R, Grau JJ, Lopez-Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 2014; 25: 216–25. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Ridge JA, Li T, et al. Long-term toxicities in 10-year survivors of radiation treatment for head and neck cancer. Oral Oncol 2017; 71:122–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008; 26:3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019; 393(10166):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019; 393(10166):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J Clin Oncol 2021;39(9):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrelli F, Luciani A, Ghidini A, et al. Treatment de-escalation for HPV+ oropharyngeal cancer: A systematic review and meta-analysis. Head Neck. 2022;44(5):1255–1266. [DOI] [PubMed] [Google Scholar]
- 19.Adelstein DJ, Ismaila N, Ku JA, et al. Role of Treatment Deintensification in the Management of p16+ Oropharyngeal Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol 2019; 37(18): 1578–1589. [DOI] [PubMed] [Google Scholar]
- 20.Cramer JD, Ferris RL, Kim S, Duvvuri U. Primary surgery for human papillomavirus-associated oropharyngeal cancer: Survival outcomes with or without adjuvant treatment. Oral Oncology. 2018;87:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Copur MS, Ramaekers R, Gönen M, et al. Impact of the National Cancer Institute Community Cancer Centers Program on Clinical Trial and Related Activities at a Community Cancer Center in Rural Nebraska. J Oncol Pract. 2016;12(1):67–8, e44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2006; 354:567–578. [DOI] [PubMed] [Google Scholar]
- 23.Siu LL, Waldron HN, Chen BE, et al. Effect of Standard Radiotherapy With Cisplatin vs Accelerated Radiotherapy With Panitumumab in Locoregionally Advanced Squamous Cell Head and Neck Carcinoma: A Randomized Clinical Trial. JAMA Oncol 2017; 3(2):220–226. [DOI] [PubMed] [Google Scholar]
- 24.Giralt J, Trigo J, Nuyts S, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):221–232. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal DI, Harari PM, Giralt J, et al. Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol. 2016;34(12):1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebre-Medhin M, Brun E, Engström P, et al. ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy With Cisplatin Versus Cetuximab in Patients With Locoregionally Advanced Head and Neck Squamous Cell Cancer. J Clin Oncol. 2021;39(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buglione M, Maddalo M, Corvò R, et al. Subgroup Analysis According to Human Papillomavirus Status and Tumor Site of a Randomized Phase II Trial Comparing Cetuximab and Cisplatin Combined With Radiation Therapy for Locally Advanced Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2017; 97(3):462–472. [DOI] [PubMed] [Google Scholar]
- 29.Rischin D, King M, Kenny L, et al. Randomised trial of radiotherapy with weekly cisplatin or cetuximab in low risk HPV associated oropharyngeal cancer (TROG 12.01) - a Trans-Tasman Radiation Oncology Group study. Int J Radiat Oncol Biol Phys. 2021;111(4):876–886. [DOI] [PubMed] [Google Scholar]
- 30.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. [DOI] [PubMed] [Google Scholar]
- 31.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao Y, Aupérin A, Sun X, et al. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017–01 (REACH). Eur J Cancer. 2020; 141:21–29. [DOI] [PubMed] [Google Scholar]
- 33.Bourhis J, Sire C, Tao Y, et al. Pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced head and neck squamous cell carcinoma (LA-HNSCC): Results of the GORTEC 2015–01 “PembroRad” randomized trial. Annals of Oncol. 2020;31(S4):S1168. [DOI] [PubMed] [Google Scholar]
- 34.Spreafico A, Sultanem K, Chen B, et al. A randomized phase II study of cisplatin plus radiotherapy versus durvalumab plus radiotherapy followed by adjuvant durvalumab versus durvalumab plus radiotherapy followed by adjuvant tremelimumab and durvalumab in intermediate risk, HPV-positive, locoregionally advanced oropharyngeal squamous cell cancer (LA-OSCC) (Canadian Cancer Trials Group HN.9). Ann Oncol. 2018;29(suppl_8):viii372–viii399. [Google Scholar]
- 35.Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 2021;22(4):450–462. [DOI] [PubMed] [Google Scholar]
- 36.Clump DA, Zandberg DP, Skinner HD, et al. A randomized phase II study evaluating concurrent or sequential fixed-dose immune therapy in combination with cisplatin and intensity-modulated radiotherapy in intermediate- or high-risk, previously untreated, locally advanced head and neck cancer (LA SCCHN). J Clin Oncol. 2022; 40(16_suppl):6007–6007. [Google Scholar]
- 37.Patel JJ, Levy DA, Nguyen SA, Knochelmann HM, Day TA. Impact of PD-L1 expression and human papilloma-virus status in anti-PD1/PDL1 immuno-therapy for head and neck squamous cell carcinoma—systematic review and meta-analysis. Head Neck. 2020;42:774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemoto N, Seo Y, Nakahara S, et al. Radiation Therapy Alone for Human Papillomavirus-Related Squamous Cell Carcinoma of the Oropharynx: A Single-Arm, Phase 2 Study. Int J Radiat Oncol Biol Phys. 2021;110(2):403–411. [DOI] [PubMed] [Google Scholar]
- 39.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000; 355: 949–55. [PubMed] [Google Scholar]
- 40.Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4–14. [DOI] [PubMed] [Google Scholar]
- 41.Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol 2011; 100: 33–40. [DOI] [PubMed] [Google Scholar]
- 42.Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol 2017; 18: 1221–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noronha V, Joshi A, Patil VM, et al. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J Clin Oncol 2018; 36(11): 1064–1072. [DOI] [PubMed] [Google Scholar]
- 44.Kiyota N, Tahara M, Mizusawa J, et al. ; Head and Neck Cancer Study Group of the Japan Clinical Oncology Group (JCOG-HNCSG). Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J Clin Oncol. 2022. Mar 1:JCO2101293. doi: 10.1200/JCO.21.01293. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferris RL, Flamand Y, Weinstein GS, et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J Clin Oncol. 2022; 40(2):138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez CA, Wu X, Amsbaugh MJ, et al. High-dose versus weekly cisplatin definitive chemoradiotherapy for HPV-related oropharyngeal squamous cell carcinoma of the head and neck. Oral Oncol. 2017;67:24–28. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A, Kumar M, Bhasker S, et al. An open-label, noninferiority phase III RCT of weekly versus three weekly cisplatin and radical radiotherapy in locally advanced head and neck squamous cell carcinoma (ConCERT trial). J Clin Oncol. 2022; 40(16_suppl):6004–6004. [Google Scholar]
- 48.Mehanna H, Rischin D, Wong SJ, et al. De-Escalation After DE-ESCALATE and RTOG 1016: A Head and Neck Cancer InterGroup Framework for Future De-Escalation Studies. J Clin Oncol. 2020;38(22):2552–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nutting CM, Morden JP, Harrington KJ, et al. , on behalf of the PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011; 12:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer 2002; 94: 2967–80. [DOI] [PubMed] [Google Scholar]
- 51.Wang MB, Liu IY, Gornbein JA, et al. HPV-positive oropharyngeal carcinoma: a systematic review of treatment and prognosis. Otolaryngol Head Neck Surg 2015; 153: 758–69. [DOI] [PubMed] [Google Scholar]
- 52.Howard J, Masterson L, Dwivedi RC, et al. Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma. Cochrane Database Syst Rev 2016; 12: CD010963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quan DL, Sukari A, Nagasaka M, Kim H, Cramer JD. Gastrostomy tube dependence and patient-reported quality of life outcomes based on type of treatment for human papillomavirus-associated oropharyngeal cancer: Systematic review and meta-analysis. Head Neck. 2021; 43(11):3681–3696. [DOI] [PubMed] [Google Scholar]
- 54.Nichols AC, Theurer J, Prisman E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol 2019; 20(10):1349–1359. [DOI] [PubMed] [Google Scholar]
- 55.Nichols AC, Theurer J, Prisman E, et al. Randomized Trial of Radiotherapy Versus Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma: Long-Term Results of the ORATOR Trial. J Clin Oncol. 2022;40(8):866–875. [DOI] [PubMed] [Google Scholar]
- 56.Nichols AC, Theurer J, Prisman E, et al. Randomized Trial of Radiotherapy Versus Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma: Long-Term Results of the ORATOR Trial. J Clin Oncol. 2022; 40(8):866–875. [DOI] [PubMed] [Google Scholar]
- 57.Palma DA, Prisman E, Berthelet E, et al. Assessment of Toxic Effects and Survival in Treatment Deescalation With Radiotherapy vs Transoral Surgery for HPV-Associated Oropharyngeal Squamous Cell Carcinoma: The ORATOR2 Phase 2 Randomized Clinical Trial. JAMA Oncol. Published online April 28, 2022. doi: 10.1001/jamaoncol.2022.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma DJ, Price KA, Moore EJ, et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. JCO 2019;37(22): 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma DJ, Price K, Eric MJ, et al. Long-Term Results for MC1273, A Phase II Evaluation of De-Escalated Adjuvant Radiation Therapy for Human Papillomavirus Associated Oropharyngeal Squamous Cell Carcinoma (HPV+ OPSCC). Int J Radiat Oncol Biol Phys. 2021;111(3S):S61. [Google Scholar]
- 60.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014; 89: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma DM, Price K, Moore EJ, et al. MC1675, a Phase III Evaluation of De-Escalated Adjuvant Radiation Therapy (DART) vs. Standard Adjuvant Treatment for Human Papillomavirus Associated Oropharyngeal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2021; 111(3): S61. [Google Scholar]
- 62.Ma DM, Price K, Moore EJ, et al. Non-Inferiority Margin and Nodal Analysis of De-Escalated Adjuvant Radiation Therapy (DART) for HPV-Related Oropharyngeal Squamous Cell Carcinoma (OPSCC): A Preplanned Pooled Analysis of MC1273 & MC1675. International Journal of Radiation Oncology, Biology, Physics 2022; 112(5):e3–e4. [Google Scholar]
- 63.Swisher-McClure S, Lukens JN, Aggarwal C, et al. A Phase 2 Trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): Omission of the Resected Primary Tumor Bed After Transoral Robotic Surgery for Human Papilloma Virus-Related Squamous Cell Carcinoma of the Oropharynx. Int J Radiat Oncol Biol Phys. 2020; 106(4):725–732. [DOI] [PubMed] [Google Scholar]
- 64.Bernier J, Cooper, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancer: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck 2005; 27(10):843–50. [DOI] [PubMed] [Google Scholar]
- 65.Kelly JR, Park HS, An Y, et al. Comparison of survival outcomes among human papillomavirus-negative cT1–2 N1–2b patients with oropharyngeal squamous cell cancer treated with upfront surgery vs definitive chemoradiation therapy: an observational study. JAMA Oncol 2017; 3: 1107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baliga S, Kabarriti R, Jiang J, et al. Utilization of Transoral Robotic Surgery (TORS) in patients with Oropharyngeal Squamous Cell Carcinoma and its impact on survival and use of chemotherapy. Oral Oncol. 2018; 86:75–80. [DOI] [PubMed] [Google Scholar]
- 67.Sinha P, Lewis JS Jr., Piccirillo JF, et al. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118:3519–3530. [DOI] [PubMed] [Google Scholar]
- 68.Kharytaniuk N, Molony P, Boyle S, et al. Association of extracapsular spread with survival according to human papillomavirus status in oropharynx squamous cell carcinoma and carcinoma of unknown primary site. JAMA Otolaryngol Head Neck Surg. 2016;142:683–690. [DOI] [PubMed] [Google Scholar]
- 69.Routman DM, Funk RK, Tangsriwong K, et al. Relapse rates with surgery alone in human papillomavirus-related intermediate- and high-risk-group oropharynx squamous cell cancer: a multi-institutional review. Int J Radiat Oncol Biol Phys. 2017;99:938–946. [DOI] [PubMed] [Google Scholar]
- 70.de Almeida JR, Goldstein DP, Martino R, et al. Transoral robotic surgery (TORS)-guided radiotherapy (RT) volume de-intensification in p16-positive unknown primary squamous cell carcinoma (SCC) of the neck: A phase 2 trial (FIND). J Clin Oncol. 2022; 40(16_suppl):6067–6067. [Google Scholar]
- 71.Sadeghi N, Khalife S, Mascarella MA, et al. Pathologic response to neoadjuvant chemotherapy in HPV-associated oropharynx cancer. Head Neck. 2020;42:417–425. [DOI] [PubMed] [Google Scholar]
- 72.Sadeghi N, Mascarella MA, Khalife S, et al. Neoadjuvant chemotherapy followed by surgery for HPV-associated locoregionally advanced oropharynx cancer. Head Neck. 2020;42(8):2145–2154. [DOI] [PubMed] [Google Scholar]
- 73.Rosenberg AJ, Agrawal N, Pearson A et al. Low risk HPV-associated oropharyngeal squamous cell carcinoma treated with induction chemoimmunotherapy followed by TORS or radiotherapy. Presented at: the Multidisciplinary Head and Neck Cancers Symposium; February 27–29, 2020; Scottsdale, AZ. [Google Scholar]
- 74.Rosenberg AJ, Agrawal N, Pearson A, et al. Nivolumab, nabpaclitaxel, and carboplatin followed by risk/response adaptive de-escalated locoregional therapy for HPV-associated oropharyngeal cancer: OPTIMA II trial. Journal of Clinical Oncology. 2021. 39:15_suppl, 6011–6011 [Google Scholar]
- 75.Sher DH, Sen N, Shah JL, et al. Recurrence and Quality-of-Life Following Elective Nodal Volume and Dose De-Escalation for Oropharyngeal and Laryngeal Cancer: Initial Results from the Infield Trial. Int J Radiat Oncol Biol Phys 2019; 105(1): S53–S54. [Google Scholar]
- 76.Villaflor VM, Melotek JM, Karrison TG et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27:908–913. [DOI] [PubMed] [Google Scholar]
- 77.Bahig H, Lambert L, Filion E, et al. Phase II study of de-intensified intensity-modulated radiotherapy and concurrent carboplatin/5-fluorouracil in lateralized p16-associated oropharyngeal carcinoma. Head Neck. 2020;42(12):3479–3489. [DOI] [PubMed] [Google Scholar]
- 78.Siewert TMJ, Foster CC, Blair EA, et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol 2019; 30(2): 297–203. [DOI] [PubMed] [Google Scholar]
- 79.Rosenberg AJ, Agrawal N, Pearson A, et al. Risk and response adapted de-intensified treatment for HPV-associated oropharyngeal cancer: Optima paradigm expanded experience. Oral Oncol. 2021;122:105566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai CJ, McBride SM, Riaz N, et al. Reducing the Radiation Therapy Dose Prescription for Elective Treatment Areas in Human Papillomavirus-Associated Oropharyngeal Carcinoma Being Treated With Primary Chemoradiotherapy at Memorial Sloan Kettering Cancer Center. Pract Radiat Oncol. 2019; 9(2):98–101. [DOI] [PubMed] [Google Scholar]
- 81.Tsai CJ, McBride SM, Riaz N, et al. Evaluation of Substantial Reduction in Elective Radiotherapy Dose and Field in Patients With Human Papillomavirus–Associated Oropharyngeal Carcinoma Treated With Definitive Chemoradiotherapy. JAMA Oncol. Published online January 20, 2022. doi: 10.1001/jamaoncol.2021.6416. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chera BS, Amdur RJ, Green R, et al. Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus–Associated Oropharyngeal Squamous Cell Carcinoma. J Clin Oncol 2019; 37(29): 2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chera BS, Amdur RJ, Tepper JE, et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2018;124(11):2347–2354. [DOI] [PubMed] [Google Scholar]
- 84.Dagan R, Holtzman AL, Bryant CM, et al. De-Intensified (DI) IMPT vs. IMRT(Chemo) for HPV-Associated Oropharynx Cancer (HPV-OPC): Initial Quality of Life (QOL) Results From a Prospective, Multi-Institutional Trial. Int J Radiat Oncol Biol Phys. 2021;111(3S):e406–e407. doi: 10.1016/j.ijrobp.2021.07.1172. [DOI] [Google Scholar]
- 85.Fietkau R, Hecht M, Hofner B, et al. ; PacCis-Study Group. Randomized phase-III-trial of concurrent chemoradiation for locally advanced head and neck cancer comparing dose reduced radiotherapy with paclitaxel/cisplatin to standard radiotherapy with fluorouracil/cisplatin: The PacCis-trial. Radiother Oncol. 2020;144:209–217. [DOI] [PubMed] [Google Scholar]
- 86.Marur S, Li S, Cmelak AJ, et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol 2017; 35: 490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen AM, Felix C, Wang PC, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol 2017; 18: 803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Misiukiewicz K, Gupta V, Miles BA, et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: The Quarterback trial. Oral Oncol 2019; 95:170–177. [DOI] [PubMed] [Google Scholar]
- 89.Posner MR, Misiukiewicz K, Miles BA, et al. Survival (OS) and progression-free survival (PFS) results after induction chemotherapy (IC) followed by de-escalated chemoradiotherapy (RDCRT) for locally advanced (LA) HPV positive oropharynx cancer (HPVOPC). Journal of Clinical Oncology 2021; 39(15_suppl):6058–6058. [Google Scholar]
- 90.White R, Abel S, Hasan S, et al. Practice patterns and outcomes following radiation dose de-escalation for oropharyngeal cancer. Laryngoscope 2020;130(4):E171–E176. [DOI] [PubMed] [Google Scholar]
- 91.Gabani P, Lin AJ, Barnes J, et al. Radiation therapy dose de-escalation compared to standard dose radiation therapy in definitive treatment of HPV-positive oropharyngeal squamous cell carcinoma. Radiother Oncol 2019;134:81–88. [DOI] [PubMed] [Google Scholar]
- 92.Ghosh-Laskar S, Kalyani N, Gupta T, et al. Conventional radiotherapy versus concurrent chemoradiotherapy versus accelerated radiotherapy in locoregionally advanced carcinoma of head and neck: Results of a prospective randomized trial. Head Neck 2016; 38: 202–7. [DOI] [PubMed] [Google Scholar]
- 93.Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 2004; 22: 4665–73. [DOI] [PubMed] [Google Scholar]
- 94.Ghadjar P, Simcock M, Studer G, et al. Concomitant cisplatin and hyperfractionated radiotherapy in locally advanced head and neck cancer: 10-year follow-up of a randomized phase III trial (SAKK 10/94). Int J Radiat Oncol Biol Phys 2012; 82: 524–31. [DOI] [PubMed] [Google Scholar]
- 95.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 1998; 338: 1798–804. [DOI] [PubMed] [Google Scholar]
- 96.Haddad E, Mazeron JJ, Martin M, et al. [Comparison of concomitant radiotherapy and chemotherapy with radiotherapy alone in advanced cancers of the head and neck: results of a randomized trial]. Bull Cancer Radiother 1996; 83: 97–103. [PubMed] [Google Scholar]
- 97.Mehanna H, Evans M, Beasley M, et al. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S90–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011;103(23):1761–70. [DOI] [PubMed] [Google Scholar]
- 99.Sher DJ, Adelstein DJ, Bajaj GK, et al. Radiation therapy for oropharyngeal squamous cell carcinoma: Executive summary of an ASTRO Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol 2017; 7: 246–53. [DOI] [PubMed] [Google Scholar]
- 100.Beitler JJ, Quon H, Jones CU, et al. ACR Appropriateness Criteria((R)) Locoregional therapy for resectable oropharyngeal squamous cell carcinomas. Head Neck 2016; 38: 1299–309. [DOI] [PubMed] [Google Scholar]
- 101.Fulcher CD, Haigentz M Jr., Ow TJ, et al. AHNS Series: Do you know your guidelines? Principles of treatment for locally advanced or unresectable head and neck squamous cell carcinoma. Head Neck 2018; 40: 676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quon H, Vapiwala N, Forastiere A, et al. Radiation therapy for oropharyngeal squamous cell carcinoma: American Society of Clinical Oncology endorsement of the American Society for Radiation Oncology evidence-based clinical practice guideline. J Clin Oncol 2017; 35: 4078–90. [DOI] [PubMed] [Google Scholar]
- 103.Zumsteg ZS, Kim S, David JM, et al. Impact of concomitant chemoradiation on survival for patients with T1–2N1 head and neck cancer. Cancer 2017; 123: 1555–65. [DOI] [PubMed] [Google Scholar]
- 104.Cheraghlou S, Yu PK, Otremba MD, et al. Treatment deintensification in human papillomavirus-positive oropharynx cancer: Outcomes from the National Cancer Data Base. Cancer 2018; 124: 717–26. [DOI] [PubMed] [Google Scholar]
- 105.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol 2016; 17: 440–51. [DOI] [PubMed] [Google Scholar]
- 106.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol 2012; 30: 2102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lassen P, Lacas B, Pignon JP, et al. Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: The MARCH-HPV project. Radiother Oncol 2018; 126: 107–15. [DOI] [PubMed] [Google Scholar]
- 108.Broughman JR, Xiong DD, Moeller BJ, et al. Rethinking the 10-pack-year rule for favorable human papillomavirus-associated oropharynx carcinoma: A multi-institution analysis. Cancer 2020; 126(12):2784–2790. [DOI] [PubMed] [Google Scholar]
- 109.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol 2015; 33: 836–45. [DOI] [PubMed] [Google Scholar]
- 110.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol 2017; 35: 4057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehra R, Flamand Y, Quon H, et al. Outcomes by tobacco history in E3311, a phase II trial of transoral surgery (TOS) followed by pathology-based adjuvant treatment in HPV-associated (HPV+) oropharynx cancer (OPC): A trial of the ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2022; 40(16_suppl):6077–6077. [Google Scholar]
- 112.Galloway TJ, Zhang QE, Nguyen-Tan PF, et al. Prognostic value of p16 status on the development of a complete response in involved oropharynx cancer neck nodes after cisplatin-based chemoradiation: a secondary analysis of NRG Oncology RTOG 0129. Int J Radiat Oncol Biol Phys 2016; 96: 362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28(27):4142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 2007; 121: 1813–20. [DOI] [PubMed] [Google Scholar]
- 115.D’Souza G, Anantharaman D, Gheit T, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: a comparison of 1362 cases across three continents. Oral Oncol 2016; 62: 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sedghizadeh PP, Billington WD, Paxton D, et al. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? A systematic review and meta-analysis. Oral Oncol 2016; 54: 15–27. [DOI] [PubMed] [Google Scholar]
- 117.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer 2014; 50: 2636–48. [DOI] [PubMed] [Google Scholar]
- 118.Mehanna H, Wong WL, McConkey CC, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016; 374: 1444–54. [DOI] [PubMed] [Google Scholar]
- 119.Bratman SV, Bruce JP, O’Sullivan B, et al. Human papillomavirus genotype association with survival in head and neck squamous cell carcinoma. JAMA Oncol 2016; 2: 823–6. [DOI] [PubMed] [Google Scholar]
- 120.Morris LGT, Chandramohan R, West L, et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol 2017;3:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beaty BT, Moon DH, Shen CJ et al. PIK3CA mutation in HPV-associated OPSCC patients receiving deintensified chemoradiation. J Natl Cancer Inst 2019;112:855–858. [DOI] [PubMed] [Google Scholar]
- 122.Goodman MT, Saraiya M, Thompson TD, et al. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer 2015; 51: 2759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00–22). Int J Radiat Oncol Biol Phys 2010; 76: 1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmadi N, Chan M, Huo YR, et al. Survival outcome of tonsillar squamous cell carcinoma (TSCC) in the context of human papillomavirus (HPV): A systematic review and meta-analysis. Surgeon 2019; 17(1):6–14. [DOI] [PubMed] [Google Scholar]
- 125.Psychogios G, Mantsopoulos K, Agaimy A, et al. Prognostic factors in limited (T1–2, N0–1) oropharyngeal carcinoma treated with surgery +/− adjuvant therapy. Head Neck 2013; 35: 1752–58. [DOI] [PubMed] [Google Scholar]
- 126.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol 2013; 31: 543–50. [DOI] [PubMed] [Google Scholar]
- 127.Withers HR, Peters LJ, Taylor JM, et al. Local control of carcinoma of the tonsil by radiation therapy: an analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys 1995;33(3):549–62. [DOI] [PubMed] [Google Scholar]
- 128.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol 2013;49(1):79–85. [DOI] [PubMed] [Google Scholar]
- 129.Routman DM, Chera BS, Jethwa KR et al. Detectable HPV ctDNA in post-operative oropharyngeal squamous cell carcinoma patients is associated with progression. Int J Radiat Biol Phys. 2019;105:682–683. [Google Scholar]
- 130.Chera BS, Kumar S, Shen C et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol. 2020:38:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Byun DJ, Tam MM, Jacobson AS, et al. Prognostic potential of mid-treatment nodal response in oropharyngeal squamous cell carcinoma. Head Neck. 2020. Sep 21. doi: 10.1002/hed.26467. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mowery YM, Vergalasova I, Rushing CN, et al. Early 18F-FDG-PET Response During Radiation Therapy for HPV-Related Oropharyngeal Cancer May Predict Disease Recurrence. Int J Radiat Oncol Biol Phys 2020;108(4):969–976. [DOI] [PubMed] [Google Scholar]
- 133.Hentschel M, Appold S, Schreiber A, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 2011;38(7):1203–11. [DOI] [PubMed] [Google Scholar]
- 134.Zahid MU, Mohamed ASR, Caudell JJ, et al. Dynamics-Adapted Radiotherapy Dose (DARD) for Head and Neck Cancer Radiotherapy Dose Personalization. Journal of Personalized Medicine. 2021; 11(11):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008;8(3):180–92. [DOI] [PubMed] [Google Scholar]
- 136.Riaz N, Sherman E, Pei X, at al. Precision Radiotherapy: Reduction in Radiation for Oropharyngeal Cancer in the 30 ROC Trial. J Natl Cancer Inst. 2021;113(6):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee NY, Sherman E, Pei X, et al. The 30 ROC trial: Precision intra-treatment imaging guiding major radiation reduction in human papillomavirus related oropharyngeal cancer. Journal of Clinical Oncology. 2021;39(15_suppl):6019. [Google Scholar]
- 138.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer 2009;9(2):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol 2017;18(2):202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scott JG, Sedor G, Ellsworth P, et al. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): a cohort-based pooled analysis. Lancet Oncol. 2021;22(9):1221–1229. [DOI] [PubMed] [Google Scholar]
- 141.Caudell JJ, Torres-Roca JF, Gillies RJ, et al. The future of personalised radiotherapy for head and neck cancer. Lancet Oncol. 2017;18(5):e266–e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brotherston DC, Poon I, Le T, et al. Patient preferences for oropharyngeal cancer treatment de-escalation. Head Neck. 2013; 35: 151–9. [DOI] [PubMed] [Google Scholar]


