Abstract
Chronic hepatitis C can lead to cirrhosis and hepatocellular carcinoma. We studied the safety and immunogenicity of a novel therapeutic hepatitis C virus (HCV) genotype 1a/1b consensus DNA vaccine, INO-8000, encoding HCV NS3, NS4A, NS4B and NS5A proteins alone or co-administered with DNA encoding interleukin-12 (IL-12) (INO-9012), a human cytokine that stimulates cellular immune function, in individuals with chronic hepatitis C. This was a phase I, multi-site dose-escalation trial with an expansion cohort evaluating doses of 0, 0.3, 1.0, and 3.0 mg of INO-9012 (IL-12 DNA) as an addition to 6.0 mg of (INO-8000) (HCV DNA vaccine). Vaccines were administered by intramuscular injection followed by electroporation at study entry and at weeks 4, 12, and 24. HCV-specific CD4+ and CD8+ T cell immune responses were measured by IFNγ ELISpot and flow cytometry-based assays. Transient, mild-to-moderate injection site reactions unrelated to interleukin-12 DNA dose were common. Increases in HCV-specific IFN-γ production occurred in 15/20 (75%) participants. Increases in the frequency of HCV-specific CD4+ and CD8+ T cells occurred at all dose-levels, with the greatest increases seen at 1.0 mg of INO-9012. HCV-specific CD8+ and CD4+ T cell activities increased in 16/18 (89%) and 14/17 (82%) participants with available data, respectively. The vaccine regimen was safe and induced HCV-specific CD4+ and CD8+ cellular immune responses of modest magnitude in most HCV-infected participants. The addition of 1.0 mg of IL-12 DNA provided the best enhancement of immune responses. The vaccine regimen had little effect on controlling HCV viremia.
Keywords: hepatitis C, interlekin-12, DNA vaccine, immunotherapy, hepatocellular carcinoma
Introduction
HCV infection is a global pandemic, with approximately 58 million individuals currently infected worldwide and 1.5 million new cases reported each year. There are 2.4 million people living with hepatitis C in the United States. Despite the availability of curative antiviral treatments, the incidence of acute HCV infection in the United States has been rising steadily since 2006 because of the injection drug use epidemic.
Of acutely infected individuals, 55–85% will fail to clear the virus and develop chronic infection, and up to a quarter of chronically infected patients will go on to develop cirrhosis (1–4). In addition to the morbidity and mortality associated with cirrhosis, these patients also face a 10–20 fold increased risk of developing hepatocellular cancer (HCC). This risk is lowered after effective antiviral therapy, but some risk remains. The risk of developing HCC once cirrhosis has developed in patients with chronic HCV infection ranges between 1 to 3 percent per year (5) and carries a poor prognosis (6). Worldwide, HCC is the seventh most common malignancy and the second leading cause of cancer-related deaths.
Current antiviral drugs are >95% effective in achieving a cure of chronic HCV infection. Although these treatments have favorable response rates and low occurrences of adverse events, there still are issues with drug interactions and treatment adherence. Furthermore, they do not protect against recurrence of HCV infection, a growing threat with the upsurge in injection drug use. These treatment issues combined with new concepts in immune therapy serve to underscore a need for the development of improved immunotherapies such as therapeutic vaccines for the treatment and prevention of chronic HCV infection. Currently, there is no vaccine for HCV. While the correlates of immune protection against HCV have yet to be fully elucidated, numerous studies have suggested that a strong, multi-specific Th1 T cell response is required for clearance of acute infection (7). Given the importance of T cells in control and clearance of HCV infection, it is becoming more likely that an effective vaccine strategy would be able to elicit a potent HCV-specific T cell response.
We studied a therapeutic application of a novel HCV genotype 1a/1b consensus DNA vaccine encoding HCV NS3, NS4A, NS4B and NS5A proteins. We previously showed that following immunization, rhesus macaques were able to mount HCV-specific responses strikingly like those reported in resolving patients, including strong NS3-specific IFN-γ responses, robust CD4+ and CD8+ T cell proliferation and induction of polyfunctional T cells (8). Additionally, results of fine epitope mapping revealed one animal that was able to mount a T cell response against a known HCV NS3 HLA-A2 epitope in humans (8). Systemic immunization in the C57BL/6 mouse model induced potent HCV-specific T cell responses able to traffic to and function within the tolerant environment of the liver. These T cell responses remained fully functional within the liver and had the ability to become highly activated upon liver expression of their cognate antigen as shown by up-regulation of IFN-γ and clearance of HCV antigen expressing hepatocytes (9,10). Taken together, these findings supported study of this HCV T-cell based immunization strategy for the treatment of HCV infection in humans.
A Phase I, multi-site trial (MAY2013-02-01), was conducted to determine the safety profile of the HCV DNA vaccine, consisting of INO-8000 (HCV antigen DNA) alone or co-administered with INO-9012 (IL-12 DNA) and to identify a dose of IL-12 DNA for co-administration with INO-8000 based on induction of HCV-specific interferon (IFN)-γ production by peripheral blood mononuclear cells (PBMC) at 26 weeks compared with baseline in HCV-infected participants. The dose of IL-12 DNA found to be safe and inducing the strongest HCV-specific immune responses, as determined by IFN-γ production, will be considered the “selected” dose and chosen for study of antiviral effectiveness in a subsequent Phase II trial.
Materials and Methods
The Phase I study was conducted through the NCI-funded, multi-center Cancer Prevention Network (CPN)(NCT02772003). All aspects of the study protocol were reviewed and approved by the appropriate Institutional Review Board for human research at each participating site. Mayo Clinic in Rochester, MN served as the coordinating research base. The study was conducted in accordance with the CONSORT study guidelines and the ethical guidelines of the Declaration of Helsinki, CIOMS, Belmont Report, and U.S. Common Rule. It was monitored twice annually by the Data and Safety Monitoring Board of the Mayo Clinic Cancer Center. All authors had access to the study data and reviewed and approved the final manuscript. Enrolling institutions included University of Puerto Rico, San Juan, PR; Temple University, Philadelphia, PA; Mayo Clinic in Florida, Jacksonville FL; and Mayo Clinic in Rochester, MN.
Study Participants
Prior to screening, all participants underwent informed consent and signed the approved informed consent document. The study population included males and females ≥18 years of age, with a documented history of chronic genotype 1 HCV infection with plasma HCV RNA >10,000 IU/mL. Other eligibility criteria included not receiving or in acute clinical need for HCV treatment; no evidence of cirrhosis or extensive bridging fibrosis; no active malignancy (not including non-melanoma skin cancer); no immune compromising illness; adequate organ and marrow function (or deemed not clinically significant by the medical monitor), 12-lead ECG showing normal heart rhythm; no diagnosis of HIV or HBV, no history of major organ transplant, no history of cardiac arrythmia or pre-excitation syndromes, no uncontrolled illness; no history of allergic reactions attributed to compounds similar to INO-8000 and IL-12 DNA; and not receiving any other investigational agents within 6 months of registration.
Study Intervention
INO-8000 was a combination of equal concentrations of pGX8005 (NS3/4A), pGX8006 (NS4B) and pGX8007 (NS5A) plasmids, formulated at a total DNA concentration of 10 mg/mL and filled in a 2 mL glass vial with a minimum recoverable volume of 0.8 mL. IL-12 DNA contained pGX6001 (hIL-12) plasmid, formulated at a total DNA concentration of 10 mg/mL and filled in a 2 mL glass vial with a minimum recoverable volume of 0.2 mL. Sterile water for injection (sWFI) was used as the diluent for study agent.
Trial Design
The Phase I trial comprised two parts. In Part 1, a conventional cohorts-of-three dose escalation design was employed. The first dose-level cohort (Dose Level 0) received INO-8000 (6 mg) alone with the remaining three dose-level cohorts evaluating IN0–8000 (6 mg) in combination with escalating doses of IL-12 DNA (Dose Level 1: 0.3 mg; Dose Level 2: 1.0 mg; Dose Level 3: 3.0 mg). In Part 2, additional participants were randomized across each of the dose levels deemed safe in Part 1 to achieve a maximum total of 24 study participants; randomization was based on the Pocock-Simon dynamic allocation procedure. All vaccines were administered by IM+EP at study entry (Day 0), and at study week 4, 12, and 24. Participants were contacted by telephone at Weeks 8, 14, 26, 36, 48, and 76 to assess adverse events (AEs). AEs were assessed by use of a participant-completed vaccine report card, which was collected at each subsequent visit. The primary safety endpoint was dose-limiting toxicity (DLT) defined as an adverse event (AE) occurring within 14 days after the initial vaccine administration (Day 0) or within 14 days after the second vaccine administration (Week 4) that was deemed to be anything other than not related to the intervention and met one of the criteria shown in Table 1. HCV-specific IFN-γ was measured by ELISpot in PBMC at baseline (Day 0) and at study week 26. The primary efficacy endpoint was defined as the within-participant change of HCV-specific IFN-γ at study week 26.
Table 1.
Dose limiting toxicity
| Toxicity | Dose-limiting Toxicity CTCAE v4.0 |
|---|---|
| Administration site reaction (erythema, swelling, pain, tenderness) | ≥ Grade 3 (recorded ≥ 2 hours after study treatment) |
| Fever | ≥ 39.0 degrees C (102.2 degrees F) |
| Decreased hemoglobin, WBC, platelets, lymphocytes, neutropenia | ≥ Grade 3 |
| Renal toxicity | Serum creatinine ≥ 2 times baseline |
| Allergic reaction | ≥ Grade 3 Note: Any allergic reaction that requires treatment with epinephrine or steroids, but may not require prolonged treatment or hospitalization, will qualify as a Grade 3 allergic reaction and DLT |
| Hepatic toxicity |
|
Immunology Assays
Peripheral blood mononuclear cells (PBMCs) were collected from whole blood after isolation by density gradient centrifugation on Ficoll-Paque (Amersham Biosciences). PBMC were cryopreserved and transferred to liquid nitrogen for batch testing. For in vitro stimulation, cryopreserved cells were thawed and rested overnight at 37°C in complete RPMI medium.
Peptides
Peptides 15 amino acids in length, overlapping by 8, that span the non-structural proteins (NS)3/4A, NS4B and NS5A of HCV genotype 1a were obtained from Genscript and reconstituted with DMSO at a concentration of 20 mg/mL. The 199 peptides were pooled into 6 separate pools: 3 pools for NS3/4A, 1 pool for NS4B, and 2 pools for NS5A.
ELISpot assays
IFN-γ ELISpot assays were performed using the Human Interferon-γ ELISPOTPRO kit (Mabtec) using pre-coated plates. PBMCs were plated at 2 × 105 cells/well in triplicate and cultured for 20 hours with HCV overlapping pools of peptides for NS3/4A, NS4B, and NS5A at a concentration of 2 μg/mL or controls (either media/ dimethyl-sulfoxide (DMSO) or PMA and Ionomycin). The plates were then processed according to the manufacturer’s protocol and read by a blinded independent investigator using an automated image analysis software (CTL). The antigen specific response is plotted and was calculated by subtracting any background in DMSO stimulated wells from HCV peptide stimulated wells.
Flow Cytometry
To identify antigen-specific T lymphocytes expressing markers of activation and lytic activity, PBMC (1 × 106/well) were cultured for 5 days at 37°C, 5% CO2 in the presence of HCV overlapping pool of peptides for NS3/4A, NS4B, and NS5A at a concentration of 2 μg/mL or controls in 96-wells U-bottom plates. Negative control wells included stimulation with an equimolar amount of DMSO or OVA peptides and were used for background subtraction. Concanavalin A was used as a positive control. After stimulation, cells were first stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) for 10 minutes at RT. Cells were then washed and stained with fluorescently labeled antibodies against surface markers CD14 (MjP9), CD16 (3G8), CD19 (SJ25C1), CD8a (RPA-T8), CD38 (HIT2) from BD Biosciences; CD4 (SK3), CD137 (4B4–1), CD69 (FN50), PD-1 (EH12.2H7) from Biolegend; TIM-3 (344823) from Novus and LAG-3 (30S223H) from Thermo Fisher and resuspended in FACS buffer (PBS plus BSA 5%, EDTA 2 mM, HEPES 20 mM) and incubated at 4°C in the dark for 30 minutes. Cells were then washed with FACS buffer and resuspended with FoxP3 Fix/Perm Kit (Thermo Fisher) and incubated for 45 minutes at 4°C in the dark. Cells were then washed twice with permeabilization buffer and stained with intracellular antibodies against Perforin (B-D48), Ki-67 (Ki-67) from Biolegend; CD3 (SK7), Granulysin (RB1), Granzyme B (GB11) from BD Biosciences and Granzyme A (CB9) from Thermo Fisher for 45 minutes at 4°C in the dark. After staining, cells were washed once with permeabilization buffer and resuspended in FACS buffer. All samples were acquired on a BD LSRFortessa (BD Biosciences, San Diego, CA) and analyzed with FlowJo v10 software (TreeStar, Ashland, OR). An acceptance criterion was applied to each sample for both CD4+ and CD8+ responses to ensure sample viability.
Sample Size Calculation
A total of 24 evaluable participants was targeted across Parts 1 and 2. Given the preliminary data on the safety profile of this regimen, we anticipated that 3 evaluable participants would be enrolled per dose level in Part 1 for a total of 12 evaluable participants. In Part 2, we anticipated that 12 additional evaluable participants (3 per dose level) would be randomized to one of the four dose levels in Part 1. With six evaluable participants per dose level across Parts 1 and 2, the study would have at least 80% power to detect an effect size of 1.8 in the mean change in production of HCV-specific IFN-γ at study week 26 based on a two-sided, two-sample t-test comparing each of the IL-12 DNA dose-level cohorts (0.3 mg; 1.0 mg; 3.0 mg) with the initial dose level cohort INO-8000 (6 mg). For each of these three pairwise comparisons, the effect size represents the expected difference in the means divided by the within group standard deviation. In this early phase study, no adjustment was made for performing multiple comparisons.
Statistical Methods
Statistical analyses were conducted by the Mayo Clinic Cancer Center Statistics and Data Management Center on a database frozen on 06/17/2021. The safety analysis population comprised all participants who received at least one dose of INO-8000 (with or without IL-12 DNA), while the efficacy analysis population comprised all participants who had HCV-specific IFN-γ production measured at baseline and at study week 26. Safety and preliminary efficacy results are presented according to dose-level cohort combining participants across Part 1 and Part 2.
The constellation of AEs as scored using the National Cancer Institute Common Terminology Criteria for Adverse Events, v4.0 (CTCAE v4.0) were summarized by reporting the number and percentage of participants based on the maximum grade for each type of AE experienced by a participant. Further, the maximum grade for any AE was calculated for each participant and the number and percentage of grade 1+ AEs were tabulated and summarized according to dose and overall. Grade 3+ AEs were described in detail and summarized separately as well as by attribution.
For the primary efficacy endpoint, the absolute and percentage change of production of HCV-specific IFN-γ by peripheral blood mononuclear cells from baseline to week 26 was summarized descriptively for each of the four dose levels. Per the protocol-defined primary analysis, a one-way analysis of variance (ANOVA) was conducted. The dependent variable was the within-participant absolute change from baseline at week 26 and the single factor was the dose-level cohort. Based on the ANOVA model, if the overall F-test achieved statistical significance at the 5% significant level, the following three prespecified pairwise comparisons were made: Dose Level 3 vs 0; Dose Level 2 vs 0; and Dose Level 1 vs 0. No adjustment was made for performing multiple comparisons with statistical significance assessed at the nominal 5% level. For each dose level, we reported the mean absolute (and percentage) change and 95% confidence interval (CI) as well as the mean [95% CI] of the between dose-group differences comparing dose levels 3, 2, and 1 versus 0. To facilitate the identification of the dose level to move forward to a Phase II study, we considered that dose level that achieved the greatest average increase provided that that dose level was also deemed to be safe.
There were three translational endpoints: (1) the binary outcome of whether the participant achieved a >1 log reduction in HCV RNA levels at study week 26; (2) the within-participant log reduction in HCV RNA levels at study week 26 calculated as log10 (baseline) – log10 (Week 26); and (3) the within-participant change from baseline at study week 26 in CD8 and CD4 T lymphocytes as measured by flow cytometry. We descriptively summarized these endpoints in a tabular and/or graphical fashion by dose level. To quantify the degree of uncertainty in the average changes from baseline, we also reported the corresponding 95% CI.
All statistical tests were two-sided. P values are reported as continuous quantities. Statistical analyses were performed using SAS, version 9.4.
Data Availability
Upon publication, data and materials available in the Cancer Data Access System must be requested using the standardized process.
Results
From 2016 to 2019, 23 participants were enrolled at four sites: 12 to Part 1 and 11 to Part 2. One participant in Part 2, who was randomized to Dose Level 0, withdrew consent prior to receiving the study intervention. Therefore, the safety analysis population comprised 22 participants across 4 dose levels: 6 Dose Level 0, 6 Dose Level 1, 5 Dose Level 2, and 5 Dose Level 3. Of these 22 participants, 20 had HCV-specific IFN-γ production measured at baseline and at study week 26: 5 Dose Level 0, 5 Dose Level 1, 5 Dose Level 2, and 5 Dose Level 3 (Figure 1).
Figure 1. CONSORT diagram of the progress of study participants in the clinical trial.
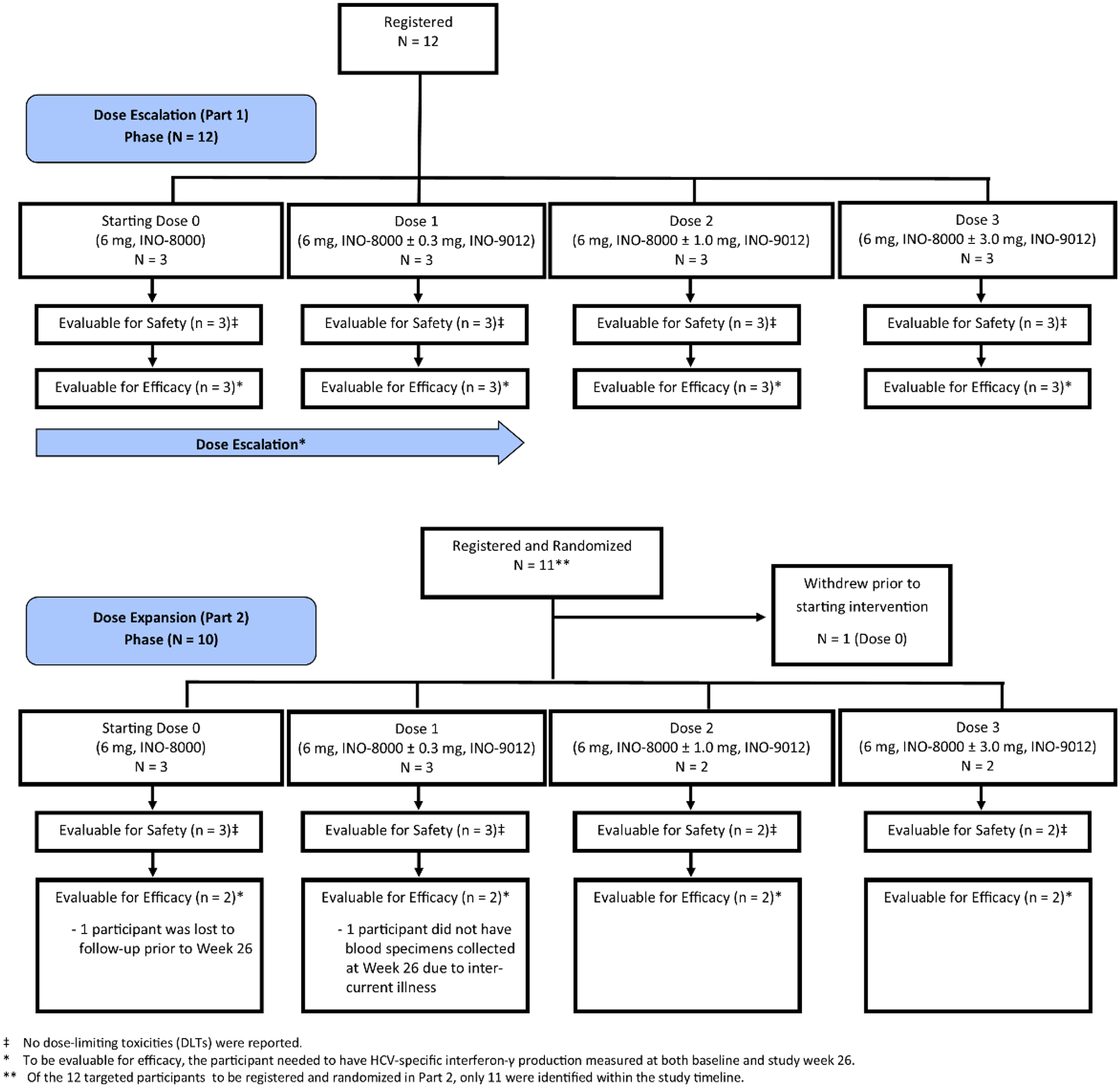
The participants enrolled, treated, and ultimately eligible for the primary safety and efficacy analyses are shown.
Table 2 shows the baseline characteristics for the 22 participants who received the study intervention. The median [range] age (in years) was 48.5 [34, 61]. 9 (40.9%) were female. Fourteen (63.6%) were White, 5 (22.7%) were Black or African American, and for the remaining 3 participants race was unknown. More than two-thirds of the study participants were Hispanic or Latino (72.7%).
Table 2.
Baseline Characteristics
| Dose Level 0 (N=6) |
Dose Level 1 (N=6) |
Dose Level 2 (N=5) |
Dose Level 3 (N=5) |
Total (N=22) |
|
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 48.0 (8.58) | 47.8 (8.80) | 53.6 (5.13) | 44.6 (9.24) | 48.5 (8.20) |
| Median | 45.5 | 49.0 | 55.0 | 45.0 | 48.5 |
| Range | 40, 61 | 35, 61 | 46, 58 | 34, 59 | 34, 61 |
| Sex, n (%) | |||||
| Female | 3 (50.0%) | 3 (50.0%) | 1 (20.0%) | 2 (40.0%) | 9 (40.9%) |
| Male | 3 (50.0%) | 3 (50.0%) | 4 (80.0%) | 3 (60.0%) | 13 (59.1%) |
| Race, n (%) | |||||
| Black or African American | 1 (16.7%) | 1 (16.7%) | 2 (40.0%) | 1 (20.0%) | 5 (22.7%) |
| Unknown | 2 (33.3%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 3 (13.6%) |
| White | 3 (50.0%) | 4 (66.7%) | 3 (60.0%) | 4 (80.0%) | 14 (63.6%) |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 3 (50.0%) | 3 (50.0%) | 5 (100.0%) | 5 (100.0%) | 16 (72.7%) |
| Not Hispanic or Latino | 3 (50.0%) | 3 (50.0%) | 0 (0.0%) | 0 (0.0%) | 6 (27.3%) |
| Dose Cohort, n (%) | |||||
| Part 1 (Dose Escalation) | 3 (50.0%) | 3 (50.0%) | 3 (60.0%) | 3 (60.0%) | 12 (54.5%) |
| Part 2 (Dose Expansion1) | 3 (50.0%) | 3 (50.0%) | 2 (40.0%) | 2 (40.0%) | 10 (45.5%) |
| HCV RNA Level < 800,000 IU/mL | 1 (33.3%) | 1 (33.3%) | 1 (50.0%) | 0 (0.0%) | 3 (30.0%) |
| HCV RNA Level ≥ 800,000 IU/mL | 2 (66.7%) | 2 (66.7%) | 1 (50.0%) | 2 (100.0%) | 7 (70.0%) |
All 22 participants received at least one dose of study intervention.
Abbreviations: SD, standard deviation; BMI, body mass index; HCV, hepatitis C virus; RNA, ribonucleic acid.
HCV RNA level reported for Part 2 only.
Primary Safety Endpoint: Adverse Events
No DLTs were observed across the four dose levels. Table 3 shows the AE profile according to dose level. There were no grade 4 or grade 5 AEs reported. In total, 3 / 22 (13.6%) particpants experienced a grade 3 AE, all of which were deemed unrelated to study intervention. One participant on dose level 0 experienced a grade 3 abdominal pain and a torn meniscus. Two participants on dose level 1 experienced a single grade 3 AE (non-cardiac chest pain; micro-invasive cervical cancer).
Table 3.
Summary of Grade 1+ Adverse Events based on maximum grade per participant per adverse event
| Patients with a maximum: | Dose Level | n / N (%) |
|---|---|---|
| Grade 1 Adverse Event | Dose Level 0 | 0 / 6 (0.0%) |
| Dose Level 1 | 1 / 6 (16.7%) | |
| Dose Level 2 | 1 / 5 (20.0%) | |
| Dose Level 3 | 0 / 5 (0.0%) | |
| Grade 2 Adverse Event | Dose Level 0 | 5 / 6 (83.3%) |
| Dose Level 1 | 2 / 6 (33.3%) | |
| Dose Level 2 | 4 / 5 (80.0%) | |
| Dose Level 3 | 5 / 5 (100.0%) | |
| Grade 3 Adverse Event | Dose Level 0 | 1 / 6 (16.7%) |
| Dose Level 1 | 2 / 6 (33.3%) | |
| Dose Level 2 | 0 / 5 (0.0%) | |
| Dose Level 3 | 0 / 5 (0.0%) | |
| Grade 4 Adverse Event | Dose Level 0 | 0 / 6 (0.0%) |
| Dose Level 1 | 0 / 6 (0.0%) | |
| Dose Level 2 | 0 / 5 (0.0%) | |
| Dose Level 3 | 0 / 5 (0.0%) | |
| Grade 5 Adverse Event | Dose Level 0 | 0 / 6 (0.0%) |
| Dose Level 1 | 0 / 6 (0.0%) | |
| Dose Level 2 | 0 / 5 (0.0%) | |
| Dose Level 3 | 0 / 5 (0.0%) |
Self-limited mild-to-moderate injection site pain and other local injection site reactions were common (20 / 22 or 86.4%); systemic reactions, such as nausea, myalgia, arthralgia, fatigue, were uncommon. Injection site reactions generally resolved within 3–4 days. There were no clear differences in these AEs across the dose levels.
Primary Efficacy Endpoint: Change in HCV-specific IFN-γ Production
All 20 participants who had HCV-specific IFN-γ production assayed at baseline and at study week 26 had received all 4 planned injections administered on Day 0 and at study weeks 4, 12, and 24. The mean absolute change (SFU/106 PBMCs) and mean percent change (%) in HCV-specific IFN-γ production from baseline to study week 26 for dose level 0 were −1.89 (95% CI, −6.78 to 3.00) and 27.9 (95% CI, −109.1 to 164.9), respectively; for dose level 1, 11.00 (95% CI, −20.47 to 42.47) and 2029.7 (95% CI, −3423.7 to 7483.1), respectively; for dose level 2, 29.89 (95% CI, −5.76 to 65.54) and 538.6 (95% CI, 220.5 to 856.8), respectively; for dose level 3, 18.67 (95% CI, −18.58 to 55.92) and 230.5 (95% CI, −101.6 to 562.6), respectively. Based on the one-way ANOVA model of the absolute change in HCV-specific IFN-γ expression at week 26, there were no statistically signficant differences between the dose groups (Overall F-test: P = 0.253). Dose level 2 achieved the largest numerical mean absolute change in HCV-specific IFN-γ expression at study week 26. (Figure 2)
Figure 2. Therapeutic vaccination with INO-8000 and INO-9012 induces HCV specific IFN-γ production.
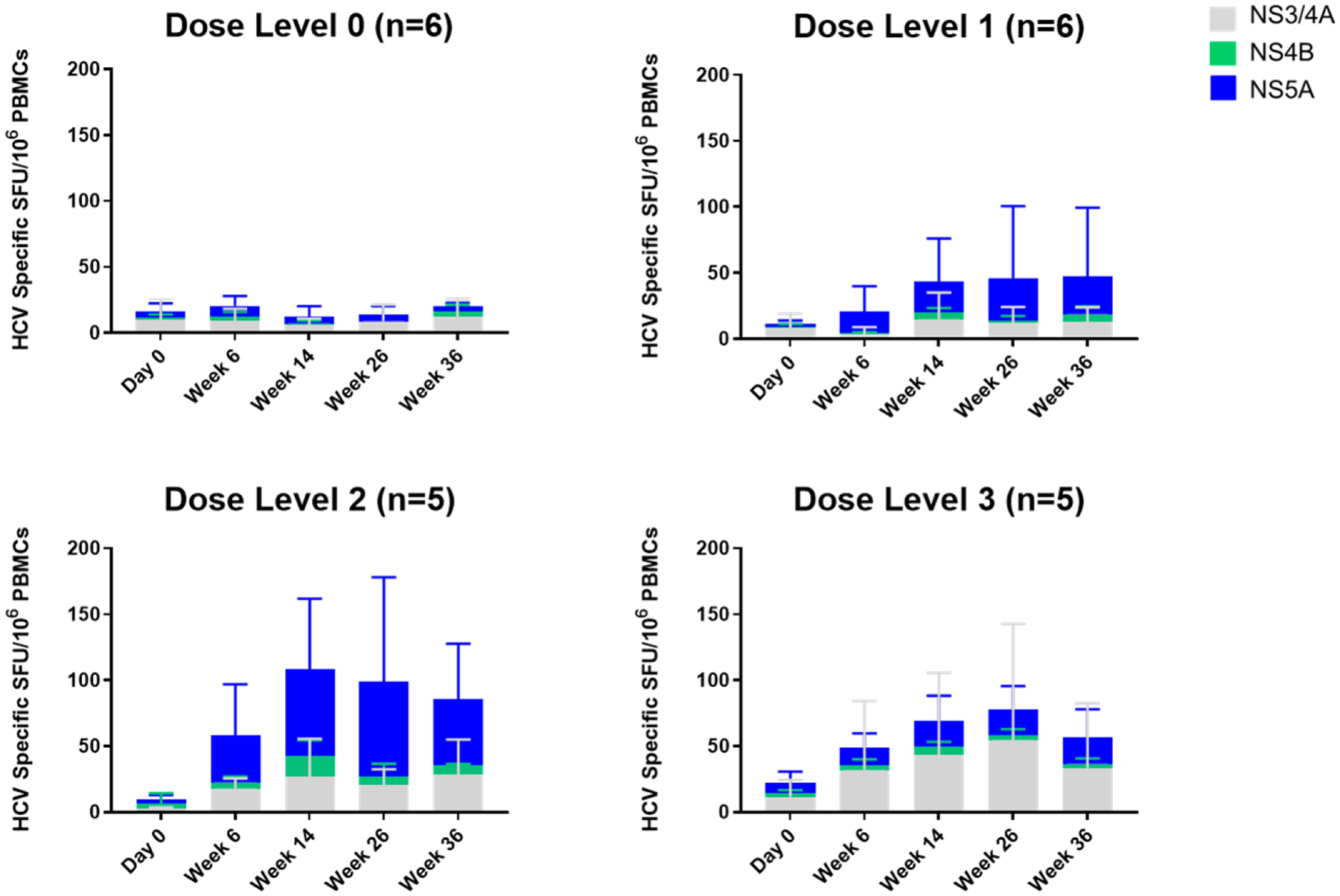
The average magnitudes of IFN-γ detected in each dose group following stimulation with HCV antigens encoded in INO-8000 (NS3/4A in gray, NS4B in green and NS5A in blue) at each study timepoint. Whiskers represent the standard deviation.
When looking at the longitudinal cellular immune responses by ELISpot assay at Day 0 and at Weeks 6, 14, 26, and 36 within each dose level, general trends can be observed (Figure 2). Cellular responses were observed to the multiple HCV antigens encoded by INO-8000, with the majority of responses to NS5A, followed by NS3/4A and the lowest reactivity to NS4B. The addition of IL-12 DNA trends with both numerically higher magnitudes of IFN-γ production as well as more participants with a positive response to HCV antigens at Week 26 compared to baseline. Overall, 15/20 (75%) achieved an increase in HCV-specific IFN-γ production at Week 26 compared with baseline. Within dose levels 0, 1, 2, and 3, the percentages of participants who achieved an increase at Week 26 were 60% (3/5), 60% (3/5), 100% (5/5), and 80% (4/5), respectively. Dose level 2 achieved the highest proportion of participants experiencing an increase over baseline by study week 26 (5/5 or 100%).
Flow Cytometry
To further characterize the cellular response to INO-8000, a flow cytometry-based assay was utilized. Activated and proliferative CD4+ T and CD8+ T lymphocytes were observed following treatment with INO-8000 (Figure 3), the average %CD38+CD69+Ki67+ expression on CD4 T cells for dose level 0 was 0.17 at baseline and 0.88 at week 26, with an average within-participant change of 0.61 (−0.275, 1.486; 95% CI); for dose level 1, 0.15 at baseline and 0.93 at week 26 with an average within-participant change of 0.76 (−1.296, 2.810); for dose level 2, 0.09 at baseline and 0.95 at week 26 with an average within-participant change of 1.36 (–1.016, 3.74); and for dose level 3, 0.05 at baseline and 0.45 at week 26 with an average within-participant change of 0.40 (−0.305, 1.101) (Figure 3A). The greatest increase in the frequency of this activated and proliferative CD4+ T cell population was observed at dose level 2.
Figure 3. Therapeutic vaccination with INO-8000 and INO-9012 induces activated HCV specific CD4+ and CD8+ T cells.
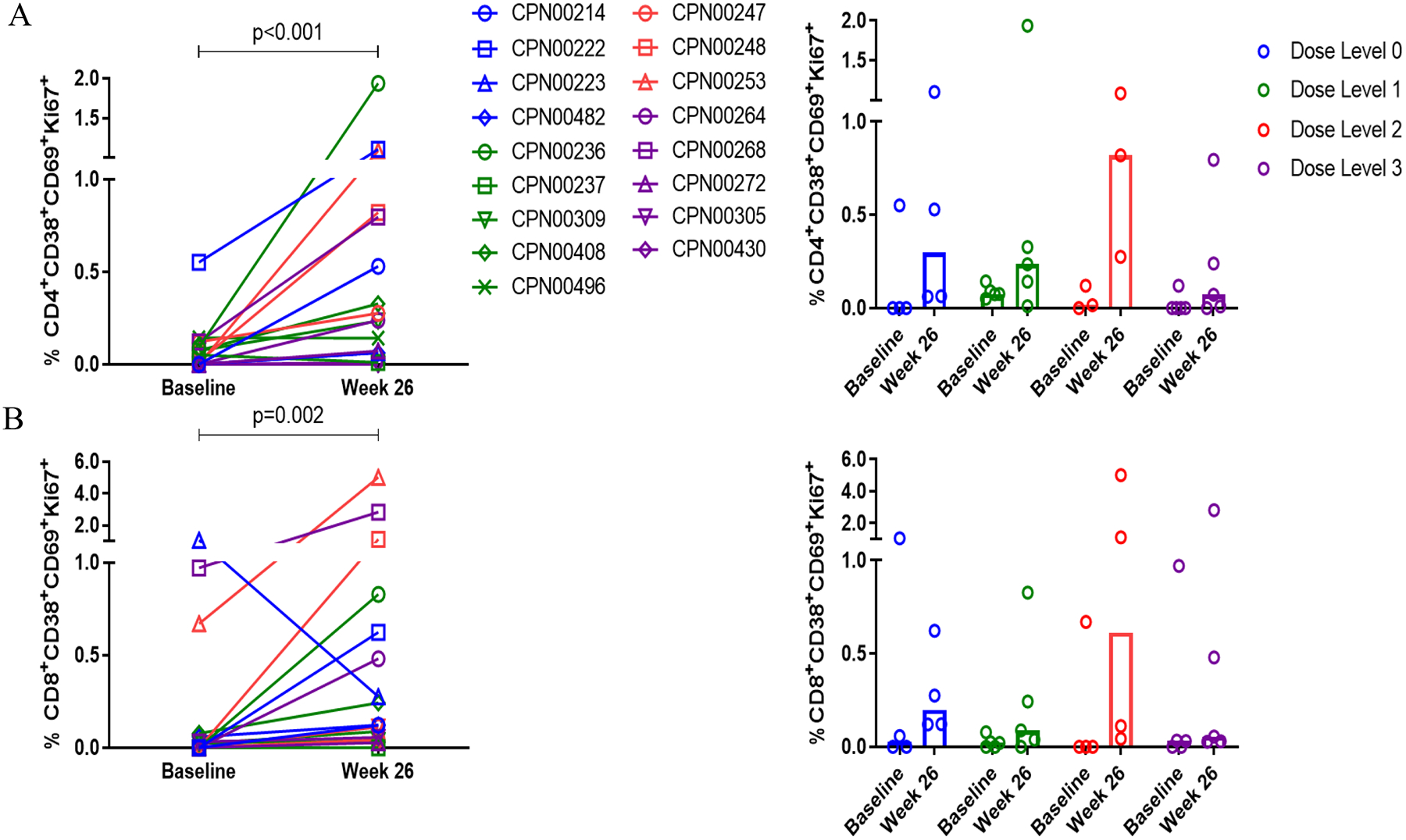
The frequency of HCV-specific CD4+ (A) and CD8+(B) T cells expressing the activation markers CD38 and CD69 and proliferation marker Ki67 at baseline and at week 26. Individual patients are represented with open symbols and are colored by dose group (Dose Level 0 in blue, Dose Level 1 in green, Dose Level 2 in red, and Dose Level 3 in purple). The Wilcoxon signed-rank test was performed. Bars represent the median frequency.
An increase in the frequency of activated and proliferative CD8+ T cells was also observed in the majority of participants following treatment with INO-8000 (Figure 3B). The average %CD38+CD69+Ki67+ expression on CD8 T cells for dose level 0 was 0.37 at baseline and 0.57 at week 26, with an average within-participant change of 0.02 (−1.817, 1.856); for dose level 1, 0.04 at baseline and 0.48 at week 26, with an average within-participant change of 0.43 (−0.439, 1.294); for dose level 2, 0.34 at baseline and 2.49 at week 26, with an average within-participant change of 2.75 (−3.757, 9.257); and for dose level 3, 0.41 at baseline and 1.35 at week 26, with an average within-participant change of 0.94 (−1.048, 2.921). As with the CD4 T cell responses, the largest increase in activated and proliferative CD8+ T cells was seen at dose level 2.
The generation of CD8+ T cells capable of targeting and killing cells infected with HCV following immunotherapy was also assessed. Activated HCV-specific CD8+ T cells with cytolytic potential (%CD38+KI67+GrB+Prf+) (Figure 4A) and %CD69+KI67+GrB+Prf+ (Figure 4B) were observed at frequencies greater than pre-dose levels in all dose-level groups. Again, the greatest increases were seen at dose level 2.
Figure 4. Therapeutic vaccination with INO-8000 and INO-9012 induces activated HCV specific CD8+ T cells with lytic potential.
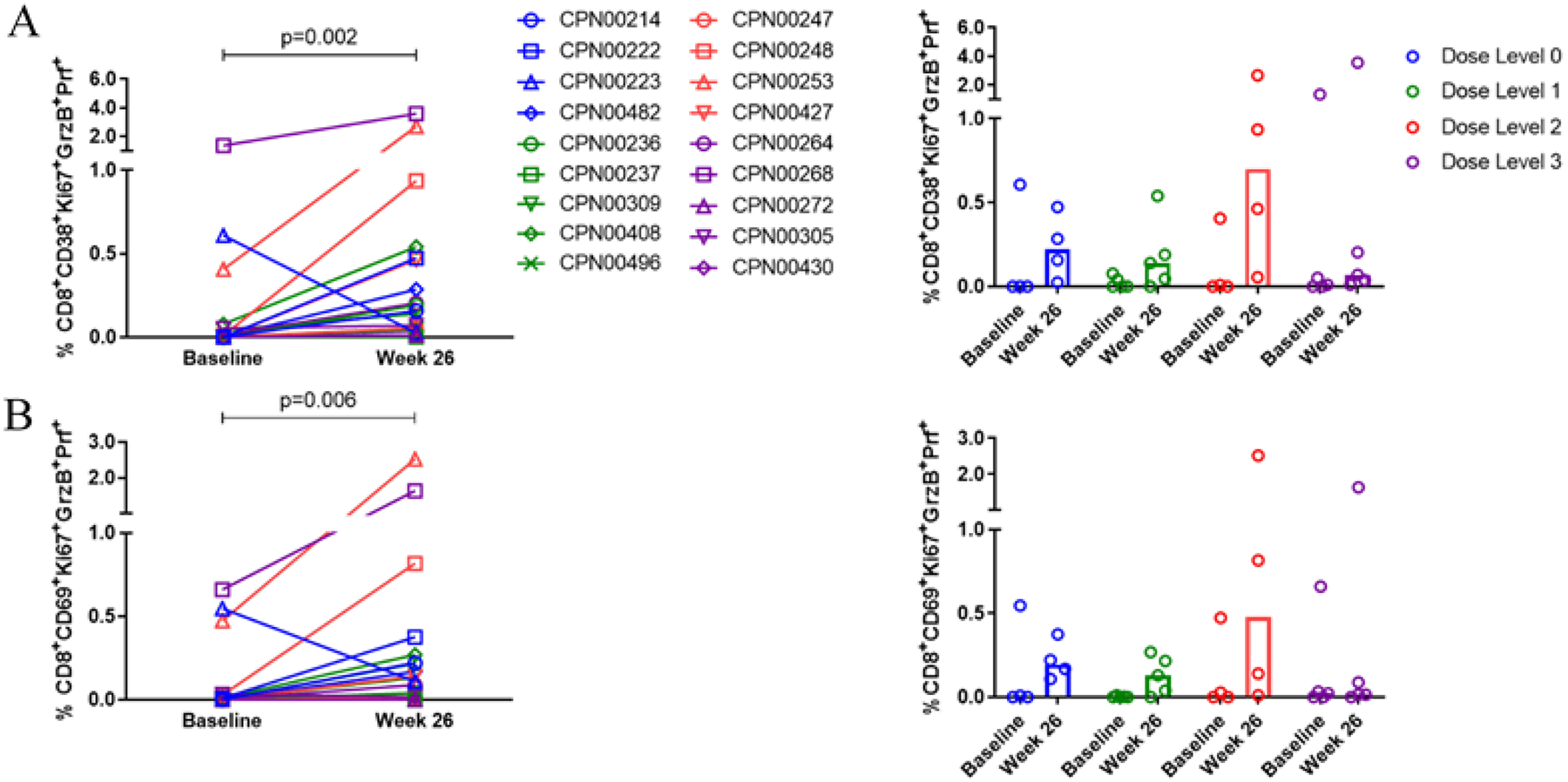
The frequency of activated HCV-specific CD38+ (A) or CD69+ (B) Ki67+ CD8+T cells expressing lytic markers granzyme B and perforin at baseline and at week 26. Individual patients are represented with open symbols and are colored by dose group (Dose Level 0 in blue, Dose Level 1 in green, Dose Level 2 in red, and Dose Level 3 in purple). The Wilcoxon signed-rank test was performed. Bars represent the median frequency.
When looking at the immunologic responses by flow cytometry across the 18 participants with available paired pre- and post-treatment samples at week 26, treatment with INO-8000 resulted in 16 participants having increases in CD8+T cell parameters of activation and lytic proteins, with 4 participants having responses greater than 0.5%. Of the 17 participants (one participant had a sample that failed the CD4 acceptance criterion) with available paired pre- and post-treatment samples at week 26 for CD4+ T cell parameters, 14 had increases in activated CD4+ T cells, reaching nearly 2% in one participant. T cell responses were targeted to more than one component of INO-8000, with the majority of responses to NS5A, followed by NS3/4A, and the lowest reactivity to NS4B.
Hepatic enzymes
No study participant developed higher AST or ALT elevations, and those who had increased values at baseline did not experience a change in the levels of these values.
Virological responses
One participant achieved a >1 log decrease (or undetectable) in HCV RNA level at Week 26; this participant was in dose level 2. At baseline, the participant had a log10 HCV RNA level of 6.825 while at Week 26, the log10 HCV RNA level was 4.726 corresponding to a log reduction of 2.099; furthermore, the baseline and Week 26 HCV-specific IFN-γ production was 2.776 and 22.220, corresponding to an increase of 19.443. Unfortunately, this participant did not have sufficient viable PBMCs available to run a flow cytometric analysis. No change in liver enzymes occurred in this participant. In the study as a whole, the average change at Week 26 in log10 HCV RNA levels was 0.113 (−0.323, 0.548) in dose level 0, −0.305 (−1.331, 0.721) in dose level 1, 0.459 (−1.361, 2.278) in dose level 2, and −0.099 (−0.542, 0.344) in dose level 3.
Discussion
INO-9012 is a synthetic DNA plasmid that encodes for interleukin-12 (IL-12), a cytokine shown to stimulate Th1-associated cellular immune responses. The inclusion of INO-9012 has been previously shown to elicit higher magnitudes of immune responses when co-administered with viral or self antigens in humans (11,12). Specifically, the addition of INO-9012 adjuvant has been shown to elicit higher magnitudes of cellular responses including IFN-γ production, activated CD4+ and CD8+T cells, and activated CD8+ CTLs (12). Therefore, we conducted the current study in HCV-infected participants to determine the safety profile of the HCV DNA vaccine (INO-8000) and to identify a dose of IL-12 DNA for co-administration with INO-8000 based on the induction of HCV-specific cellular immune responses.
In this Phase I trial, we determined that the INO-8000/INO-9012 HCV DNA vaccine, consisting of a plasmid encoding HCV antigens and a plasmid encoding different doses of IL-12, administered intramuscularly with electroporation, was safe and well tolerated. Injection site pain and other local reactions were common, but they were mild-to-moderate and transient, and unrelated to IL-12 DNA dose. The primary efficacy analysis, which assessed IFN-γ ELISpot cellular immune responses to HCV antigens induced by the vaccine regimen, did not find significant differences across the IL-12 dose level cohorts. However, the numbers of participants in each study cohort were small. When the cellular immune responses was assessed in individual study participants by either IFN-γ ELISpot or flow cytometry, immunogenicity was demonstrated, especially when higher doses of IL-12 DNA (dose level 2 and 3) were co-administered with INO-8000 (HCV antigen-encoding DNA vaccine).
Cellular responses were induced to all the HCV antigens encoded by INO-8000 (NS5A, NS3/4A, NS4B), with most of the responses to NS5A and the least to NS4B. Interestingly, a shift in antigen dominance in the ELISpot assay was seen when comparing dose level 2 to dose level 3, with NS5A contributing to the former and NS3/4A to the latter. While the mechanism of this shift is unclear, the only difference between these dose groups is the amount of IL-12 adjuvant in the formulation and this may have had an influence on antigen presentation or on the resulting immune responses. Higher magnitudes as well as more participants with HCV-specific CD4+ T and CD8+ T lymphocyte immune responses were observed. Fifteen of 20 (75%) study participants overall, 5 of 5 (100%) participants in the dose level 2 cohort, experienced an increase in HCV-specific IFN-γ production by ELISpot. By a flow cytometry-based assay, treatment with INO-8000 led to increases in the frequency of activated and proliferative CD4+ and CD8+ T cells, as well as activated CD8+ T cells potentially capable of targeting and killing cells infected with HCV, in all dose-level groups. The greatest increases were observed at dose level 2. Sixteen of 18 (89%) study participants with available data had increases in HCV-specific CD8+T cell activity and 14 of 17 (82%) participants had increases in HCV-specific CD4+ T cell activity. Nevertheless, only one study participant (dose level 2 – 1.0mg) achieved a >1 log reduction in HCV viral load. This participant had a high magnitude HCV-specific cellular immune response relative to other participants, as measured by ELISpot.
There have been other clinical studies of therapeutic vaccination for chronic HCV infection. A similar HCV DNA vaccine that encode only for HCV NS3/4A delivered by IM/EP also elicited IFN-γ ELISpot responses to NS3 in a phase 1 study (13). In an open-label, dose-escalating study of an MVA vaccine expressing HCV NS3, NS4, and NS5B, HCV-specific cellular immune responses were induced in 5 of the 15 participants, and plasma HCV RNA declined transiently in 8 patients (14). In a follow-up phase II open-label, randomized, controlled study, this vaccine was studied in combination with pegylated interferon α−2a and ribavirin (PEG-IFNα/RBV) for chronic HCV. When the vaccine regimen was administered before PEG-IFNα/RBV, twice as many study participants achieved transient early virologic responses compared with those receiving PEG-IFNα/RBV alone (15).
Autologous monocyte-derived dendritic cells transduced with a recombinant adenovirus encoding HCV NS3 protein were administered to five individuals with chronic HCV infection. CD4 and CD8 T cell response of low magnitude were induced. No changes in HCV viremia were observed (16). In another dose-escalation study, adenoviral vectors encoding HCV NS proteins administered alone or in combination with PEG-IFNα/RBV were found to elicit HCV-specific CD8+ T cell responses in 15 of 24 HCV-infected study participants, although of lower magnitude than in HCV-negative controls. Sequence analyses determined that vaccination mostly induced immune responses only when the vaccine immunogen differed from the recipient’s autologous virus. No effect on plasma HCV RNA levels was observed (17).
In a randomized, placebo-controlled trial of an HCV E1 protein vaccine, most vaccine recipients developed higher anti-E1 antibody levels and T cell proliferative responses. Although serum HCV RNA levels did not change, serum ALT levels declined in the group who received vaccine. Liver fibrosis improved in 38% of vaccine recipients, and this improvement was associated with the increases in anti-E1 antibody levels (18). A peptide-based vaccine incorporating CD4 and CD8 epitopes with poly L-arginine adjuvant induced higher CD4 and CD8 T cell responses in a majority of vaccine recipients (19,20). Transient declines of >1 log HCV RNA were observed in 3 of 36 vaccine recipients in one study (20). Another peptide vaccine induced peptide-specific cytotoxic t lymphocyte (CTL) activity in most vaccinees, but reduced viral load in only 2 of 25 study participants (21).
Finally, it is noteworthy that although a chimpanzee adenovirus 3 (ChAd3)-HCV NS prime/ modified vaccinia Ankara(MVA)-HCV NS boost vaccine regimen did not prevent chronic HCV infection in a double-blind, randomized, placebo-controlled trial, the peak plasma HCV RNA after infection was reduced in the vaccine group compared to control.22 This is consistent with the observations in the therapeutic vaccine studies that declines in HCV viral load occurred more often in individuals in whom higher magnitude anti-HCV cellular immune responses were induced (13–15,20,21). Thus, immunotherapy with an HCV vaccine is able to induce new immune responses against HCV antigens and this has translated to modest antiviral activity in some studies.
In summary, INO-8000, when administered IM followed by electroporation together with an IL-12 adjuvant, elicited CD4+ and CD8+ cellular immune reponses to the multiple HCV antigens encoded by the DNA vaccine in most study participants. The addition of IL-12 DNA enhanced these immune responses, with the 1.0 mg dose level providing the best adjuvant effect. Going forward, this should be the dose of IL-12 DNA administered with the 6.0 mg dose of INO-8000. No significant safety signal was observed. The results demonstrate the ability of IL-12 to enhance the immune responses of vaccines targeting viruses, including those with oncogenic potential. Unfortunately, the immune responses induced by this particular vaccine regimen were not sufficient to translate into a notable antiviral effect in persons already infected with HCV. Undoubtedly, immune-based therapeutics will not be able to match the remarkable potency and curative activities of directly acting antivirals in treating chronic hepatitis C infection.
Prevention Relevance Statement.
The administration of interleukin 12 DNA along with a hepatitis C viral antigen DNA vaccine enhanced the HCV-specific immune responses induced by the vaccine in individuals with chronic hepatitis C, an important cause of hepatocellular carcinoma. IL-12 could be an effective adjuvant in vaccines targeting HCV and other oncogenic viruses.
Acknowledgements
The authors gratefully acknowledge the clinical study coordinators at the participating organizations: Lori Bergstrom, Jessica Hernandez-Marrero, Lori Chase, Dana Kontras, Katelyn Register, Jenna Murray, Aleshia Thomas, and James Robinson. The authors acknowledge the Mayo Clinic Cancer Prevention Network (CPN) for their assistance with study design, administration, and manuscript preparation.
Financial Support
This work was sponsored by the National Cancer Institute, Division of Cancer Prevention (contract no. HHSN261201200042I; P.J. Limburg).
Conflict of Interest Disclosures:
Dr. Limburg serves as Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with Mayo Clinic. Dr. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement; Exact Sciences. K. Kraynyak, M. Morrow, J. Boyer, I. Marrero, A. Sylvester, J. Pawlicki, E. Gillespie and E. Barranco are employees of Inovio Pharmaceuticals and receive compensation in the form of salary and stock options.
Abbreviations:
- CPN
Cancer Prevention Network/Mayo Clinic Consortium
- EP
Electroporation
- HCV
Hepatitis C Virus
- IL-12
Interleukin-12
- PBMC
Peripheral Blood Mononuclear Cells
Footnotes
Opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute
References
- 1.AASLD/IDSA/IAS-USA Recommendations for testing, managing, and treating hepatitis C. [cited 2015]. Available from: http://www.hcvguidelines.org
- 2.Baumert TF, Wellnitz S, Aono S, Satoi J, Herion D, Tilman G, et al. Antibodies against hepatitis C virus-like particles and viral clearance in acute and chronic hepatitis C. Hepatology 2000;32: 610–17. [DOI] [PubMed] [Google Scholar]
- 3.DHHS Center for Disease Control and Prevention The ABCs of Hepatitis Fact Sheet. [cited 2004]. Available from: https://www.cdc.gov/hepatitis/hcv/profresourcesc.htm
- 4.Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002;36(5 Suppl 1):S35–46. [DOI] [PubMed] [Google Scholar]
- 5.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology 1999;29:1311–1316. [DOI] [PubMed] [Google Scholar]
- 6.Westbrook RH, & Dusheiko G (2014). Natural history of hepatitis C. Journal of hepatology, 61(1 Suppl), S58–68. doi: 10.1016/j.jhep.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 7.Flynn JK, Dore GJ, Hellard M, Yeung B, Rawlinson WD, White PA, Kaldor JM, Lloyd AR, French RA, ATAHC Study Group. Maintenance of Th1 hepatitis C virus (HCV)-specific responses in individuals with acute HCV who achieve sustained virological clearance after treatment. J Gastroenterol Hepatol. 2013. Nov;28(11)1770–81. doi: 10.111/jgh.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang Kuhs KA, Ginsberg AA, Yan J, Wiseman RW, Khan AS, Sardesai NY, et al. Hepatitis C virus NS3/NS4A DNA vaccine induces multiepitope T cell responses in rhesus macaques mimicking human immune responses. Mol Ther 2012;20: 669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang Kuhs KA, Toporovski R, Ginsberg AA, Olsen AL, Shedlock DJ, Morrow MP, et al. Peripheral immunization induces functional intrahepatic hepatitis C specific immunity following selective retention of vaccine-specific CD8 T cells by the liver. Hum Vaccin 2011;7:1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang Kuhs KA, Toporovski R, Yan J, Ginsberg AA, Shedlock DJ, Weiner DB. Induction of intrahepatic HCV NS4B, NS5A and NS5B-specific cellular immune responses following peripheral immunization. PLoS One 2012;7:e52165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebas P, Kraynyak KA, Patel A, Maslow JN, Morrow MP, Sylvester AJ, et al. Intradermal SynCon® Ebola GP DNA Vaccine is temperature stable and safely demonstrates cellular and humoral Immunogenicity advantages in healthy volunteers. J Infect Dis 2019;220:400–10. [DOI] [PubMed] [Google Scholar]
- 12.Vonderheide RH, Kraynyak KA, Shields AF, McRee AJ, Johnson JM, Weijing W, et al. Phase 1 study of safety, tolerability and immunogenicity of the human telomerase (hTERT)-encoded DNA plasmids INO-1400 and INO-1401 with or without IL-12 DNA plasmid INO-9012 in adult patients with solid tumors. J Immunother Cancer 2021;9:e003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiland O, Ahlén G, Diepolder H, Jung MC, Levander S, Fons M, et al. Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C. Mol Ther 2013;21:1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habersetzer F, Honnet G, Bain C, Maynard-Muet M, Leroy V, Zarski J-P, et al. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology 2011;141:890–9.e1-4. [DOI] [PubMed] [Google Scholar]
- 15.Di Bisceglie AM, Janczweska-Kazek E, Habersetzer F, Mazur W, Stanciu C, Carreno V, et al. Efficacy of immunotherapy with TG4040, peg-interferon, and ribavirin in a Phase 2 study of patients with chronic HCV infection. Gastroenterology 2014;147:119–131 e113 [DOI] [PubMed] [Google Scholar]
- 16.Zabaleta A, D’Avola D, Echeverria I, Llopiz D, Silva L, Villanueva L, et al. Clinical testing of a dendritic cell targeted therapeutic vaccine in patients with chronic hepatitis C virus infection. Mol Ther Methods Clin Dev 2015;2:15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly C, Swadling L, Capone S, Brown A, Richardson R, Halliday J, et al. Chronic hepatitis C viral infection subverts vaccine-induced T-cell immunity in humans. Hepatology 2016;63:1455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevens F, Roskams T, Van Vlierberghe H, Horsmans Y, Sprengers D, Elewaut A, et al. A pilot study of therapeutic vaccination with envelope protein E1 in 35 patients with chronic hepatitis C. Hepatology 2003;38:1289–96. [DOI] [PubMed] [Google Scholar]
- 19.Firbas C, Jilma B, Tauber E, Buerger V, Jelovcan S, Lingnau K, et al. Immunogenicity and safety of a novel therapeutic hepatitis C virus (HCV) peptide vaccine: a randomized, placebo controlled trial for dose optimization in 128 healthy subjects. Vaccine 2006:24:4343–53. [DOI] [PubMed] [Google Scholar]
- 20.Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, et al. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology 2008;134:1385–1395. [DOI] [PubMed] [Google Scholar]
- 21.Yutani S, Komatsu N, Shichijo S, Yoshida K, Takedatsu H, Itou M, et al. Phase I clinical study of a peptide vaccination for hepatitis C virus-infected patients with different human leukocyte antigen-class I-A alleles. Cancer Sci 2009;100:1935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N Engl J Med 2021;384:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon publication, data and materials available in the Cancer Data Access System must be requested using the standardized process.


