Abstract
Purpose:
The Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs) polycythemia vera, essential thrombocythemia, and primary myelofibrosis are characterized by JAK/STAT pathway activation. JAK inhibitors are approved for MPN treatment, but persistence has been observed, due to JAK/STAT reactivation.
Experimental Design:
Using MPN patient samples, JAK2-mutated cell lines, and MPN mouse models, we examined both the efficacy and mechanism by which crizotinib, the ALK/MET/RON/ROS1 inhibitor approved for the treatment of non-small cell lung cancer, alters MPN cell proliferation and JAK/STAT activation.
Results:
We found that crizotinib suppresses proliferation and activation of JAK2/STAT signaling, and decreases the disease burden in the JAK2V617F mouse model of MPN. Furthermore, we found that crizotinib could overcome JAK inhibitor persistence to ruxolitinib. Interestingly, phosphorylation of the crizotinib target RON kinase was enhanced in ruxolitinib-persistent cells. We show that phospho-JAK2 and phospho-RON can physically interact to sustain JAK/STAT signaling, and that the combination of crizotinib and ruxolitinib disrupts this interaction. Furthermore, RON knockdown suppresses proliferation and activation of JAK/STAT signaling in JAK2-mutated cells, and RON deletion in a JAK2V617F mouse MPN model decreases the disease burden. We also observed RON hyperactivation in MPN patient cells, suggesting that RON may be an important target of crizotinib in MPN.
Conclusions:
In summary, we demonstrate that crizotinib has preclinical efficacy in MPN patient cells, JAK2-mutated cell lines, and a JAK2-mutated mouse model, and that the combination of crizotinib with JAK inhibitors suppresses JAK inhibitor persistence. Our work suggests that crizotinib should be investigated for the treatment of MPN patients.
Keywords: crizotinib, JAK/STAT, MPN, persistence, myeloproliferative
Statement of Translational Relevance:
The ALK/MET/RON/ROS1 inhibitor crizotinib is approved for the treatment of non-small cell lung cancer and has limited hematologic toxicities. Philadelphia-negative myeloproliferative neoplasms (MPNs), which are characterized by JAK/STAT pathway activation, have developed persistence mechanisms to JAK inhibitor treatment, leading to loss of response. We found that crizotinib suppresses proliferation and JAK/STAT signaling in MPN patient cells, and decreases the disease burden in vivo in a mouse MPN model. Importantly, treatment of persistent MPN cells with crizotinib overcomes resistance to the JAK inhibitor ruxolitinib, and this may be due to disruption of the JAK2 interaction with RON kinase in MPN cells. Our data suggests that crizotinib should be investigated for the treatment of MPN patients, particularly in the setting of JAK inhibitor resistance.
INTRODUCTION
The Philadelphia chromosome (BCR-ABL)-negative myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are clonal HSC disorders characterized by the dysregulated proliferation of one or more myeloid lineage compartments. In most cases of MPN, activation of the JAK/STAT pathway is observed, primarily due to the acquisition of driver mutations in Janus Kinase 2 (JAK2), the thrombopoietin receptor (MPL), or calreticulin (CALR).1 The only curative treatment for MPNs is stem cell transplantation, but many patients are poor candidates for transplantation due to advanced age or comorbidities.2 While first generation JAK inhibitors, such as ruxolitinib and fedratinib, are approved for myelofibrosis, and are effective in reducing the disease burden, there are several limitations. JAK inhibitors are not effective in eliminating the malignant MPN-initiating cell or in reversing bone marrow fibrosis, and they cause dose-limiting cytopenias.2 Furthermore, it has been observed both in MPN mouse models and in MPN patients that JAK2 mutant clones can persist despite JAK inhibitor treatment.3,4 Thus, there is an urgent need for new treatment options that can lead to a long-term reduction in disease burden in MPN patients with minimal toxicity to normal hematopoietic cells.
We observed that crizotinib, a potent inhibitor of ALK, ROS1, MET, and RON kinases5, could suppress the proliferation of a JAK2-V617F mutant clone in the blood in a patient with EML4-ALK rearranged lung cancer. Importantly, we show that crizotinib inhibits the proliferation of MPN patient CD34+ cells, and that this is independent of the JAK inhibitor treatment history. We also show that crizotinib inhibits activation of the JAK/STAT signaling pathway in MPN patient cells, JAK2-mutated cell lines, and the JAK2V617F;Vav1-Cre knock in mouse model of MPN. Furthermore, we found that the combination of crizotinib with ruxolitinib can re-sensitize JAK inhibitor persistent cells to ruxolitinib, and that these effects can be due to the inhibition of RON kinase. Overall, our findings suggest that crizotinib should be investigated for the treatment on MPN.
MATERIALS AND METHODS
Mice:
Mice were maintained under pathogen-free conditions in a barrier facility in microisolator cages, and experiments were conducted based on protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Albert Einstein College of Medicine (AECOM) (#00001181 and #00001165). JAK2V617FloxP/+ mice were purchased from The Jackson Laboratory (Stock No. 031658; RRID:IMSR_JAX:031658).6 Stk−/− mice were provided as a gift from Dr. Pamela Hankey-Giblin.7 Experimental mice always included both males and females. Peripheral blood was obtained under isoflurane anesthesia by facial vein bleeding. Peripheral blood counts were analyzed on Genesis (Oxford Science) blood analyzer.
Mononuclear Cell and CD34 Isolation from Patient Samples:
As per the Albert Einstein IRB protocol # 2017-7529, MPN patient blood samples were obtained through the Montefiore Hospital hematology biobank (IRB protocol # 2013-2918) with informed written consent, in accordance with the Declaration of Helsinki. Samples were diluted 1:4 with pre-warmed sterile filtered MACS buffer (1L 1X PBS, 0.5% BSA, 2mM EDTA, pH 7.2), then layered 1:1 on top of Ficoll-Paque PLUS (GE Healthcare, Cat #17-1440-02). Samples were then centrifuged at 1800rpm for 1 hour at 23 degrees Celsius. The mononuclear layer was collected, washed, and subjected to CD34 isolation using the human CD34 Microbead kit (Miltenyi Biotec, Cat #130-046-702).
Methylcellulose Colony Forming Unit Assays:
Primary patient or healthy donor CD34+ mononuclear cells or HEL cells (RRID:CVCL_0001) were resuspended in IMDM with L-glutamine and 25mM HEPES media (Life Technologies, Cat #12440-053) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Pre-warmed 2.5mL human StemMACS complete media containing EPO (Miltenyi Biotec, Cat #130-091-280) aliquots were treated with the indicated inhibitor or 30% DMSO in 1X PBS as control. For primary patient CD34+ mononuclear cells, a final concentration of 4000 cells was added to the media and drug suspension. For HEL cells, a final concentration of 10,000 cells was added to the media and drug suspension. Plates were incubated at 37 degrees Celsius and colonies formed were counted 10-12 days post plating. For mouse samples, whole bone marrow or spleen cells were subjected to RBC lysis (RBC lysis solution, QiAGEN) and resuspended in IMDM with L-glutamine media containing 10% FBS and 1% penicillin-streptomycin. For myeloid colony assays, cells were seeded at 10,000 cells per dish in methylcellulose semisolid medium (M3434, STEMCELL Technologies). The colonies were scored and phenotyped at 7-10 days after plating. For erythroid colony assays, 50,000 cells were seeded in M3334 (STEM CELL Technologies). BFU-E colonies were counted at 3 days after plating, while CFU-E colonies were counted at 7 days.
Phospho-Flow Cytometry on Patient and Donor Blood Samples:
This protocol was adapted from previous phospho-flow cytometry protocols on peripheral blood.8-11 In brief, patient peripheral blood samples were treated with human EPO at 3 units/mL, human SCF at 50 ng/mL, and human HGF at 50 ng/mL for 5 minutes at 37C. Samples were then fixed with 4% paraformaldehyde at room temperature for 10 minutes, followed by RBC lysis with 0.1% Triton X-100 at 37C for 20 minutes. Samples were washed using FACS buffer, and cells were either immediately permeabilized by adding 900 uL; of 50% methanol and incubating for 10 minutes, or frozen in −20C using PBS supplemented with 10% glycerol and 20% FBS. Samples were then centrifuged and then stained simultaneously with cell surface and intracellular phospho-antibodies (Table S1) for 30 minutes at 4°C, and were analyzed on the BD FACS LSRII flow analyzer.
In-vivo crizotinib treatment:
JAK2V617FloxP/+;Vav1-Cre mice were bled at 6 weeks to confirm elevated blood counts and excision of the STOP cassette.6 Crizotinib (Pfizer) was diluted at 14 mg/kg in HCl acidified water, as advised by Pfizer. Control mice were treated with HCl acidified water. Mice were then treated with crizotinib at 100mg/kg by oral gavage for 5 days a week for 3 weeks. Animals were humanely euthanized after 3 weeks of treatment.
Histology on Bone Marrow:
Harvested murine tissues were fixed in 10% buffered formalin (Thermo Fisher Scientific, SF1004). At the Einstein Histopathology Core Facility samples were embedded in paraffin, cut into thin slices, and mounted on a slide. Mounted tissues were stained with hematoxylin and eosin stain (H&E). Histology images were acquired using the Olympus BX43 microscope with a digital camera, using 10X magnification. Image acquisition was performed using Infinity software.
Retroviral Bone Marrow Transplantation:
JAK2-V617F retroviral bone marrow transplantation was performed as previously described.12 To generate the MSCV-JAK2V617F-GFP retroviral supernatant, 5,000,000 293T cells were plated in 10mL DMEM media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin for overnight incubation at 37 degrees Celsius. 5μg of the MSCV-JAK2V617F-GFP plasmid (gift from the Gilliland lab) and 5μg of the EcoPak Psi packaging plasmid were diluted in 500μL DMEM. 15μL of Trans293 (Origene, Cat #TT500002P5) was added, and the mixture was incubated at room temperature for 15 minutes. 6mL of DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin was added, and this mixture was added dropwise to 293T cells. Cells were incubated at 37 degrees Celsius overnight, after which the media was changed. The cell culture supernatant was collected after an additional 24 and 48 hours, then centrifuged at 3000 RPM for 5 minutes and filtered through a 0.45 micron syringe filter. Donor bone marrow cells from 10-12 week old C57BL/6 mice (RRID:IMSR_JAX:000664) were harvested, RBCs were lysed, and cKIT enrichment was performed using CD117 microbeads (MACS Miltenyi Biotec Cat #130-091-224). Cells were then plated in 6 well plates containing 3mL of RPMI media supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 10μg/mL of recombinant mouse IL-3 (Peprotech Cat #213-13), SCF (Peprotech Cat #250-03), and IL-6 (Peprotech Cat #216-16). Donor cells were subjected to two spin infections, in which 500μL of concentrated viral supernatant (System Biosciences Cat #LV810A-1), 30μL HEPES Buffer, and 2 μL of polybrene were added to the cells. Plates were centrifuged at 2500 RPM for 90 minutes at 32C, and then were incubated at 37C for 1 hour. Recipient mice were given a single dose of irradiation (950 Gy) at least 3 hours prior to transplantation, and 1x106 cells were injected retro-orbitally per animal. Recipient mice were given 0.9 ml of 100mg/ml Baytril 100 (Bayer) in 250ml drinking water for 3 weeks after transplantation. Animals were humanely euthanized when they had palpable splenomegaly or were moribund.
Cell Lines:
HEL(RRID:CVCL_0001), and SET2 (RRID:CVCL_2187) cells were a gift from Dr. Ulrich Steidl. After authentication by STR profiling (performed by the Einstein Genomics Core on 4/2019), HEL and SET2 cells were cultured in RPMI 1640 with L-glutamine (Corning, Cat #10-040-CV) supplemented with 10% fetal bovine serum (Gemini, Cat #900-108) and 1% penicillin/streptomycin (Corning, Cat #30-002-CI). SET2- and HEL-ruxolitinib-persistent cells were generated and cultured as previously described 3. HEK 293T cells (RRID:CVCL_0063) were cultured in DMEM with 4.5 g/L glucose and without L-glutamine and sodium pyruvate (Corning, Cat #15-017-CV) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cell lines were routinely tested for mycoplasma via PCR using GPO (5’-actcctacgggaggcagcagt-3’) and MGSO (5’-tgcaccatctgtcactctgttaacctc-3’) primers.
Antibodies and Reagents:
The antibodies against phospho-JAK2 (Cat #3771; RRID:AB_330403), JAK2 (Cat #3230; RRID:AB_2128522), phospho-STAT3 (Cat #9145; RRID:AB_2491009), STAT3 (Cat#12640; RRID:AB_2629499), phospho-STAT5 (Cat #9314; RRID:AB_2302702), STAT5 (Cat #94205; RRID:AB_2737403) RON (Cat #2654; RRID:AB_2298153), phospho-MET (Cat #3077; RRID:AB_2143884), and MET (Cat #3127; RRID:AB_331361) were purchased from Cell Signaling Technology. The antibody against phospho-RON (Cat #AF1947; RRID:AB_2846934) was purchased from R&D Systems. The antibodies against vinculin (Cat #V9131; RRID:AB_10872742) and beta-actin (Cat #A2228; RRID:AB_476697) were purchased from Sigma-Aldrich. The secondary antibodies goat anti-mouse (Cat #926-68070; RRID:AB_10956588) and goat anti-rabbit (Cat #926-32211; RRID:AB_621843) were purchased from LI-COR. The human cytokines EPO (Cell Sciences, Cat #CRE600B), SCF (Peprotech, Cat #300-07), and HGF (Peprotech, Cat #10039H) were used for stimulating cell lines and patient peripheral blood samples at concentrations of 3 units/mL and 100ng/mL, respectively. For in vitro studies, the inhibitors crizotinib (Cat #S1068), ruxolitinib (Cat #S1378), ceritinib (Cat #s7083), and alectinib (Cat #s2762) were purchased from Selleckchem. For in vivo studies, crizotinib was obtained through a material transfer agreement from Pfizer. Human donor bone marrow CD34+ cells were purchased from All Cells.
Western Analysis:
To prepare lysates for Western analysis, cells were serum starved in RPMI media with L-glutamine supplemented with 2% fetal bovine serum and 1% penicillin/streptomycin. Lysates were prepared by harvesting 1 million cells and treating with the indicated inhibitor or 30% DMSO in 1X PBS as a control for 2-4 hours at 37 degrees Celsius. Cells were then stimulated with 0.1% BSA in 1X PBS control or with indicated cytokines for 5 minutes at 37 degrees Celsius, centrifuged at 2500rpm for 5 minutes at 4 degrees Celsius, washed with 1X PBS containing sodium orthovanadate (Na3VO4), and centrifuged at 11,000rpm for 5 minutes at 4 degrees Celsius. Cell pellets were resuspended in lysis buffer containing NP-40 (Alfa Aesar, Cat #J60902), Na3VO4, PMSF, and Pierce protease and phosphatase inhibitor tablet (Thermo Scientific, Cat #A32961) and kept on ice for 30 minutes. Cells were centrifuged at full speed for 5 minutes at 4 degrees Celsius and the supernatant (protein lysate) was transferred to a new sterile 1.5mL microcentrifuge tube. To prepare lysates for co-immunoprecipitation, lysates we treated with 3μg of antibody overnight in 4C, then incubated with Protein A beads (Thermo Fisher Scientific Cat #22810) for 1 hour in 4C. Samples were washed 3 times then loaded for Western analysis. Lysates were run on NuPAGE 7% Tris-Acetate protein gels (Thermo Fisher, Cat #EA0358BOX) at 150V with NuPAGE 1X Tris-Acetate SDS Running Buffer (Thermo Fisher, Cat #LA0041) and transferred onto nitrocellulose membrane at 30V with NuPAGE 1X Transfer Buffer (Thermo Fisher, Cat #NP0006). Membranes were blocked with 5% milk TBS-T for one hour at room temperature. Primary antibody incubation was done overnight at 4 degrees Celsius in 5% BSA TBS-Tween 20. Loading control antibodies against vinculin and beta-actin were done for one hour at room temperature in 5% BSA TBS-Tween 20, followed by secondary antibodies for one hour at room temperature in 5% BSA TBS-Tween 20. Membranes were imaged using LI-COR imaging software.
Proliferation Assays:
Cell viability after drug treatment was measured using the Cell Titer-Glo® Luminescent Cell Viability Assay (Promega, Cat #G9241). 50 μL of drug dilutions were added to 50 μL of 2x105 cells/mL cells in 96 well plates. Drug dilutions were prepared in cell growth media such that final concentrations would be 50 nM, 100 nM, 250 nM, 500 nM, 1 μM and 2.5 μM. 100μL of growth medium without drugs or cells was used to obtain background luminescence. Plates were equilibrated for 30 minutes at room temperature, and then 100 μL of Cell Titer-Glo® reagent was added to each well to make 200 μL final volume. Plates were incubated for 10 minutes at room temperature with gentle nutation. Luminescence was measured with bidirectional reading and emission at 520 nm on the Victor X plate reader. For synergy analysis in SET2-persistent cells, all wells were normalized to the DMSO-only control such that viability of those cells was presumed to be 100%. ZIP synergy scoring was performed using SynergyFinderPlus13,14.
Lentiviral Production:
Human shRNA plasmid kits for RON (Cat # TL320425) and MET (Cat #TL320418) were purchased from Origene. Each kit contained 4 unique 29mer shRNA constructs in a lentiviral GFP vector. As a control, a non-effective 29-mer scrambled shRNA cassette in pGFP-C-shLenti vector was used (Origene, Cat #TR30021). Each shRNA plasmid was transformed into DH5alpha competent bacterial cells (Life Technologies, Cat #18265017) and selected for using chloramphenicol supplemented agar plates. Colonies were picked and grown in antibiotic-supplemented LB media for DNA purification using the Qiagen HiSpeed Plasmid Maxi Kit (Cat #12663). For virus generation, 5,000,000 293T cells were plated in 10mL DMEM media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin for overnight incubation at 37 degrees Celsius. 5μg of the desired lentiviral construct was diluted in 500μL Opti-MEM, in addition to 6.7μg of pCMVR8.74 packaging plasmid (RRID:Addgene_22036) and 4.46μg of pCMV-VSVg empty backbone plasmid (RRID:Addgene_8453) (gifts from David Shechter’s Lab). 45μL of 1μg/μL PEI was added, and the mixture was incubated at room temperature for 15 minutes. 6mL of DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin was added, and this mixture was added dropwise to 293T cells. Cells were incubated at 37 degrees Celsius for 6 hours, at which time the media was changed and cells were incubated at 37 degrees Celsius for another 72 hours. The cell culture supernatant was then centrifuged at 3000 RPM for 5 minutes and filtered through a 0.45 micron syringe filter.
Lentiviral Transduction:
For transduction into HEL or SET2 cells, cells were combined with lentiviral supernatant and polybrene transfection reagent (Millipore Sigma, Cat #TR-1003-G) was added for a final concentration of 8μg/mL. Cells were incubated for 24 hours at 37 degrees Celsius, the media was changed to fresh RPMI 1640 with L-glutamine supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and cells were incubated for another 48 hours at 37 degrees Celsius. Cells were then either collected and stained with DAPI (MP Biomedicals, Cat #157574) for flow cytometry at the Einstein Flow Cytometry Core Facility, or subjected to antibiotic selection using puromycin at 2 μg/mL. shRNA efficiency in transduced cells was detected by qPCR using the sense primer 5’-TATCCTGCAGGTGGAGCTG-3’ and the antisense primer 5’-ATGAAATGCCATGCCCTTAG-3’ for RON and the sense primer 5’-GCATTTTTACGGACCCAATC-3’ and antisense primer 5’-TGTTCGATATTCATCACGGC-3’ for MET.
Competitive Bone Marrow Transplantation:
500,000 WT or Stk−/− CD45.2+ whole bone marrow donor CD45.2+ cells and 500,000 competitor cells from age matched CD45.1+ B6.SJL WT mice were transplanted into 6-8 week old lethally irradiated B6.SJL mice. Recipient mice were given a single dose of irradiation (950 Gy) at least 3 hours prior to transplantation. Donor bone marrow was retro-orbitally injected and recipient mice were given 0.9 ml of 100mg/ml Baytril100 (Bayer) in 250ml drinking water for 3 weeks after transplantation. Peripheral blood was collected from recipient mice every 4 weeks, and animals were sacrificed at 16 weeks post-transplantation.
Flow Cytometry Analysis:
For HSPC flow cytometry analysis, bone marrow from crushed or flushed bones was subjected to RBC lysis, and cells were stained with a lineage cocktail of anti-mouse biotin-labeled lineage antibodies for 30 minutes at 4°C, and anti-mouse fluorochrome-conjugated surface antibodies for 30 minutes at 4°C (Table S2), as previously described.15,16 For flow cytometry analysis of erythroid maturation, bone marrow and spleen cells were stained without RBC lysis with CD71 (BD Biosciences, Cat #BD-561937) and Ter119 (Thermo Fisher, Cat #17-5921-82; RRID:AB_469473) antibodies.17 Flow cytometry analysis was performed on the BD FACS LSR II or CYTEK AURORA. For HSPC analysis, the absolute number of each population per femur was calculated based on the number of whole bone marrow cells flushed from one femur. Analysis of all flow cytometry data was performed using FlowJo software (V9, V10).
Statistics:
GraphPad Prism 8 was used for all statistical analyses. For the comparison of 2 experimental groups, the unpaired 2-tailed Student’s t test was used. For comparisons of more than 2 groups, ANOVA was used with the Tukey’s multiple-comparisons test, unless otherwise indicated. The specific statistical test used is also indicated in each figure legend. In all graphs, error bars indicate mean ± SEM. A P value less than 0.05 was considered significant.
Data sharing statement:
The data generated in this study are available upon request from the corresponding author.
RESULTS
Crizotinib suppresses proliferation and JAK/STAT signaling in erythroid progenitors from a polycythemia vera patient
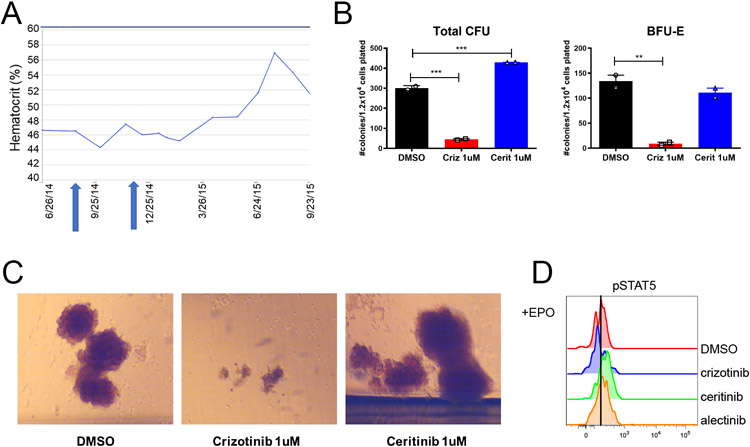
In a patient with stage IV EML4-ALK positive non-small cell lung cancer (NSCLC), we unexpectedly observed an increase in the hematocrit after the treatment was switched from crizotinib to another ALK inhibitor ceritinib (Fig. 1A, Table S3). Crizotinib is an FDA approved ALK/ROS1/MET/RON inhibitor indicated for the treatment of ALK or ROS1-positive metastatic NSCLC5, while ceritinib inhibits ALK, ROS1, and IGF1R.18 Molecular testing of the patient’s peripheral blood identified the JAK2-V617F mutation with a variant allele frequency of 23%, leading to the diagnosis of PV. This case prompted the question of whether crizotinib inhibited the expansion of a pre-existing JAK2-V617F clone. To determine whether crizotinib can inhibit the proliferation of JAK2-V617F hematopoietic progenitors, we isolated CD34+ cells from the patient’s peripheral blood and plated them in methylcellulose media supplemented with myeloid growth factors in the presence of crizotinib, ceritinib, or DMSO control. Crizotinib reduced the numbers and size of both total colony-forming units (CFU) and burst-forming units (BFU-E; erythroid precursors), while ceritinib increased the number of total CFUs (Fig. 1B-C). We also observed by intracellular phospho-flow cytometry that ex vivo treatment of this patient’s peripheral blood cells with crizotinib, but not with ceritinib or alectinib (another ALK inhibitor), could inhibit STAT5 phosphorylation in CD71+ mononuclear cells (Fig. 1D). These data suggest that crizotinib may have unique therapeutic efficacy in JAK2-mutated PV cells, which is different from the effects of other ALK inhibitors.
Figure 1: Crizotinib suppresses proliferation of JAK2V617F PV cells in vivo and in vitro.
(A) Trend in hematocrit of a patient with EML4-ALK lung cancer while on ALK inhibitors (B) Number of total colony forming units (CFU) and burst forming units-erythroid (BFU-E) colonies from CD34+ cells from this patient’s peripheral blood treated ex vivo with ALK inhibitors. ANOVA with Tukey’s multiple-comparison tests was used. (C) Representative BFU-E colonies (D) Phospho-flow cytometry analysis of STAT5 phosphorylation in this patient’s erythroblasts with crizotinib, ceritinib, or alectinib inhibitor treatment stimulated with EPO ex vivo. Cells are gated on the CD71hi CD34neg doublet negative cells population *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data represent mean ± SEM.
Crizotinib inhibits proliferation and JAK/STAT signaling in MPN
To determine whether the inhibitory effects of crizotinib are conserved in additional MPN subtypes, we plated peripheral blood CD34+ cells from Ph- MPN patients with a variety of diagnoses, molecular findings, and treatment histories (Table S3) in methylcellulose media supplemented with myeloid growth factors in the presence of crizotinib. We discovered that crizotinib inhibits both total CFU and BFU-E colony formation by MPN patient cells in a dose-dependent manner across a spectrum of MPN subtypes, including PV, ET, and PMF, and different driver mutations, including JAK2, MPL, and CALR (Fig. 2A, Fig. S1A-D, Table S3). Importantly, we also observed an inhibitory effect of crizotinib on colony formation by MPN cells from patients who were on JAK inhibitor treatment at the time of sample acquisition (Fig. 2B). To determine the effects of crizotinib at these doses on normal hematopoiesis, we performed colony assays with crizotinib on bone marrow CD34+ cells collected from 3 healthy donors. While we found that crizotinib significantly inhibits colony formation at the 1μM dose, likely due to the roles of STAT3 and STAT5 in normal hematopoietic progenitors, we observed minimal toxicity at the 500 nM dose or at lower doses (Fig. S1E-F). As MPN cell proliferation is driven by the JAK/STAT signaling pathway, we tested whether crizotinib can inhibit JAK/STAT signaling in PMF patient cells by intracellular phospho-flow cytometry. We observed that both crizotinib and ruxolitinib could inhibit STAT5 and STAT3 phosphorylation in CD71+ mononuclear cells (Fig. 2C, Fig. S2A).
Figure 2: Crizotinib inhibits proliferation and JAK/STAT signaling in MPN cells in a dose-dependent manner.
(A) Colony formation assays of MPN peripheral blood CD34+ cells with ex vivo crizotinib treatment (0.5μM: N=7, 1μM: N=19). ANOVA with Tukey’s multiple-comparison test was used (B) Colony formation assay of MPN CD34+ cells with ex vivo crizotinib treatment, categorized by the patient’s current JAK inhibitor (JAKi) status (JAK2i treated: N=7, untreated: N=5) (A-B) Colony numbers were normalized to the DMSO control for each patient. (C) Flow cytometry analysis of STAT3 and STAT5 phosphorylation in PMF patient erythroblasts with crizotinib (criz) or ruxolitinib (rux) inhibitor treatment ex vivo stimulated with EPO, SCF, and hepatocyte growth factor (HGF) (D) Schematic for treatment of JAK2V617F-loxP/+;Vav1-Cre mice and JAK2V617F-loxP/+ controls with crizotinib or vehicle (E) Spleen weights of JAK2V617F-loxP/+;Vav1-Cre mice and controls after 3 week treatment with crizotinib (N=7) or vehicle (N=12) (F) Representative H&E images of bone marrow from JAK2V617F-loxP/+;Vav1-Cre mice treated with crizotinib or vehicle. Images were taken at 10X magnification. (G) Dose-response curves from cell viability assays in the JAK2-V617F mutant cell lines HEL and SET2 and the EML4-ALK rearranged non-small cell lung cancer cell line H3122 after 3 days of crizotinib treatment. Experiments were performed at least three times, with similar results (H) Western blot analysis of JAK/STAT signaling in HEL cells treated with crizotinib, ceritinib, or ruxolitinib and stimulated with erythropoietin (EPO) and stem cell factor (SCF). Representative data from one of at least three independent experiments is shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data represent mean ± SEM.
To determine whether crizotinib has efficacy in MPN in vivo, we used the JAK2-V617F knock-in model of MPN, which is characterized by a lethal PV-like MPN with elevated hematocrit and splenomegaly.6 We treated JAK2V617Flox/+;Vav1-Cre mice with crizotinib 100mg/kg or vehicle 5 days a week by oral gavage beginning at 6 weeks of age, after the hematocrit and white blood cell (WBC) counts became elevated (Fig. 2D). After 3 weeks of treatment, we sacrificed the mice and examined the spleen weights and blood counts. We found that crizotinib reduced the spleen size of JAK2V617FloxP/+;Vav1-Cre mice, accompanied by a trend in decrease in hemoglobin and WBC count (Fig. 2E, Fig. S2B-C). The reduction in spleen size is likely due to decreased myeloid infiltration of the spleens of crizotinib-treated mice, as we observed improved spleen architecture in the crizotinib-treated group (Fig. 2F). Importantly, we did not observe any effects of crizotinib on the blood counts or spleen weights of littermate controls with the genotypes JAK2V617FloxP/+ or JAK2+/+;Vav1-Cre (Fig. 2E, Fig. S2B-C). These results suggest that crizotinib has therapeutic efficacy in MPN in vivo, with minimal hematologic toxicity.
To examine the mechanism by which crizotinib inhibits proliferation and JAK/STAT signaling in MPN cells, we tested crizotinib in two JAK2-mutated hematopoietic cell lines, SET2 and HEL, using the Cell Titer Glo (Promega) proliferation assay. We found that crizotinib inhibits cell proliferation in a dose dependent manner in HEL cells (IC50=0.1036 μM) and SET2 cells (IC50=0.0572 μM), though to a lesser extent than of the H3122 EML4-ALK rearranged lung cancer cell line (IC50= 0.003548 μM) (Fig. 2G). In contrast, the ALK inhibitor ceritinib only inhibited the proliferation of H3122 cells (IC50= 0.003548 μM), but not of HEL cells (Fig. S2D). We also found that crizotinib, but not ceritinib, inhibited the phosphorylation of JAK2 and STAT3 (Fig. 2H, Fig. S2E).
Crizotinib promotes a sustained response to JAK inhibitor treatment
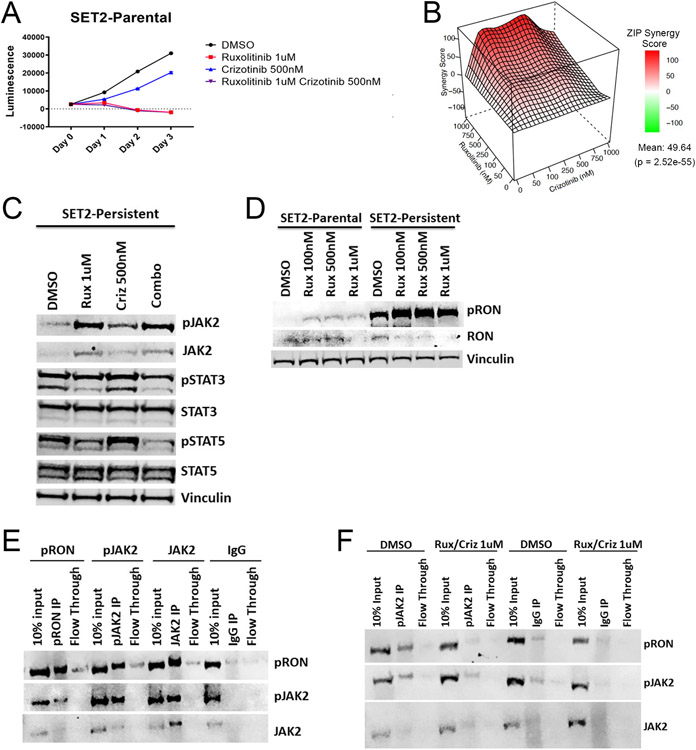
The JAK inhibitor ruxolitinib is approved by the FDA for the treatment of myelofibrosis and polycythemia vera, and it inhibits JAK/STAT signaling by stabilizing the phosphorylated version of JAK2.19 One limitation of current MPN treatment is JAK inhibitor persistence, described as sustained JAK/STAT signaling in MPN cells undergoing chronic JAK inhibitor treatment.3 It has been demonstrated that persistence can develop due to enhanced heterodimerization between JAK2 and other JAK family members JAK1 and TYK2 to potentiate STAT phosphorylation, and via an increase in total JAK2 expression through an epigenetic mechanism.3 To determine whether the addition of crizotinib could ameliorate JAK inhibitor persistence, we generated SET2 ruxolitinib persistent cells3, and confirmed their sustained signaling and proliferation when treated with ruxolitinib. Interestingly, we found that, unlike JAK inhibitor naïve cells (SET2-parental), SET2 ruxolitinib-persistent cells no longer respond to crizotinib treatment (Fig. 3A-B). However, they do respond to the combination of ruxolitinib and crizotinib, and these drugs have a synergistic relationship in SET2 persistent cells based on ZIP synergy analysis (ZIP synergy score 49.64, p = 2.52e-55) (Fig. 3B). Furthermore, we observed that the addition of crizotinib to ruxolitinib led to a decrease in STAT5 and STAT3 phosphorylation (Fig. 3C). Importantly, we also found that the effects on pSTAT5 and pSTAT3 with this drug combination is conserved in HEL ruxolitinib-persistent cells (Fig. S3A). Therefore, treatment of ruxolitinib-persistent cells with crizotinib can resensitize them to ruxolitinib.
Figure 3: Treatment of JAK inhibitor persistent cells with crizotinib is important for overcoming persistence.
(A) Cell Titer Glo proliferation assays of SET2-parental cells treated with crizotinib and/or ruxolitinib. For the final timepoint, ANOVA with Tukey’s multiple-comparison test was used (B) ZIP synergy scores for dose response matrix of SET2-persistent cells treated with crizotinib and/or ruxolitinib for 3 days (red represents synergy; green represents antagonism). (C) Western analysis of JAK/STAT signaling in SET2-persistent cells treated with ruxolitinib and/or crizotinib and stimulated with EPO and SCF (D) RON activation in SET2-parental or SET2-persistent cells (E-F) Co-immunoprecipitation and Western analysis of phospho-RON, phospho-JAK2, and JAK2 in SET2-ruxolitinib persistent cells in (E) untreated conditions or (F) with crizotinib and ruxolitinib combination treatment (Rux/Criz). Representative data from one of at least three independent experiments is shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data represent mean ± SEM.
RON kinase potentiates JAK/STAT signaling in ruxolitinib-persistent cells
Due to the differential responses of JAK2-mutated cells to two different ALK inhibitors, and the low ALK expression levels reported in hematopoietic stem and progenitor cells20, we reasoned that ALK is unlikely to be the main target of crizotinib in JAK2-mutated hematopoietic cells. Examination of the non-overlapping targets of crizotinib, ceritinib, and alectinib, as reported in the literature5,18,21,22 revealed that crizotinib, but not the other ALK inhibitors, is also a potent inhibitor of MET and RON kinases. Therefore, we hypothesized that MET and/or RON are the most likely therapeutic targets of crizotinib in MPN cells.
Interestingly, the crizotinib target RON kinase has known roles in promoting erythroid cell proliferation following EPO-R-JAK2 activation23, thereby highlighting a potential role for RON signaling in MPN cell pathogenesis. Of note, RON kinase was originally identified as an essential gene for cellular transformation by Friend erythroleukemia virus via activating STAT324,25, and has also been demonstrated to play a role in the inflammatory response and in tumorigenesis.7,26,27 However, the role of RON in MPN pathogenesis in unknown. Interestingly, we found that phosphorylation of RON is significantly increased in both SET2 and HEL persistent cells compared to parental cells (Fig. 3D, Fig. S3B), and crizotinib is unable to inhibit RON activation and subsequent STAT3/5 phosphorylation in persistent cells, likely due to the high levels of RON phosphorylation in these cells (Fig. 3C-D, Fig. S3A-B).
It has been reported that RON can be activated by erythropoietin receptor (EPO-R) downstream of JAK2 to promote erythroid progenitor expansion.23 When overexpressed in COS cells, RON forms a complex with EPO-R and can serve as a substrate for JAK2.23 However, it is not known whether JAK2 and RON interact in JAK2-mutated cells at endogenous expression levels. Given the requirement for combination treatment with crizotinib and ruxolitinib to achieve an inhibitory response in persistent cells, we hypothesized that phosphorylated RON and JAK2 form a complex. Therefore, we performed co-immunoprecipitation assays between phospho-RON (pRON) and phospho-JAK2 (pJAK2), or pRON and JAK2 in persistent cells. Consistent with our hypothesis, we found that pJAK2 and JAK2 can be immunoprecipitated using an anti-pRON antibody, and reciprocally, pRON can be immunoprecipitated with an anti-pJAK2 antibody, suggesting that pRON and pJAK2 physically interact in persistent cells at endogenous expression levels (Fig. 3E). Importantly, we show that dual inhibition of RON and JAK with crizotinib and ruxolitinib disrupts this interaction in both SET2 and HEL persistent cells (Fig. 3F, Fig. S3C-F). These data suggest that phospho-RON physically interacts with phospho-JAK2 to sustain JAK-inhibitor persistence, and that crizotinib impairs this interaction and subsequently decreases JAK/STAT signaling and cell proliferation.
RON kinase is an important target of crizotinib in MPN
To confirm that RON is a target of crizotinib in JAK2-mutated cells, we treated SET2 and HEL cells with crizotinib and examined RON phosphorylation. We confirmed that crizotinib inhibits RON phosphorylation in a dose dependent manner (Fig. 4A, Fig. S4A). We then performed shRNA knockdown of RON (encoded by MST1R) in HEL cells and examined the effects on proliferation and JAK/STAT signaling. Four different MST1R-targeted shRNA constructs were lentivirally transduced into HEL cells, and quantitative RT-PCR (qPCR) confirmed a relative decrease in RON mRNA expression (Fig. 4B). We determined that shRNA knockdown of RON inhibits colony formation by HEL cells (Fig. 4C). Western analysis revealed that shRNA knockdown of RON phenocopies the inhibitory effects of crizotinib in JAK2-mutated cells, causing decreased phosphorylation of JAK2, STAT3, and STAT5, suggesting that RON is a mediator of JAK-STAT signaling (Fig. 4D-E). Importantly, we observed a similar inhibitory effect on JAK2-mutated cell proliferation upon shRNA knockdown of RON in SET2 cells (Fig. S4B-C).
Figure 4: RON kinase is an important target of crizotinib in MPN.
(A) Western analysis of RON in SET2 cells treated with crizotinib and stimulated with erythropoietin (EPO), stem cell factor (SCF), and hepatocyte growth factor (HGF). Representative data from one of at least three independent experiments is shown (B) qPCR of MST1R (RON) expression in HEL cells transduced with 4 different MST1R shRNA constructs (C) Colony formation assay of transduced HEL cells in methylcellulose with myeloid growth factors. Experiments were performed at least three times, with similar results. ANOVA with Tukey’s multiple-comparison test was used. (D) Western analysis of RON, STAT3 and STAT5 phosphorylation in shRNA-transduced HEL cells stimulated with EPO, SCF, and HGF. Representative data from one of three independent experiments are shown. (E) Western analysis of JAK2 in shRNA-transduced HEL cells stimulated with EPO, SCF, and HGF (F) Flow cytometry analysis of RON phosphorylation in MPN patient or control donor peripheral blood CD45lo population with ex vivo EPO and SCF stimulation (G) Schematic for JAK2-V617F-GFP retroviral bone marrow transplantation using bone marrow from Stk−/− (RON−/−) mice (H) Peripheral blood analysis of WT (N=8) or Stk−/− (N=9) JAK2-V617F mice over time. WBC= white blood cells; Hb=hemoglobin; PLT=platelets (I) Percentage of GFP in the peripheral blood over time in WT (N=8) or Stk−/− (N=9) JAK2-V617F mice, quantified by flow cytometry. Student’s t-test was used *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data represents mean ± SEM.
As crizotinib is also a potent inhibitor of MET kinase, we also performed shRNA knockdown of MET in HEL cells to determine if MET signaling plays an important role in MPN pathogenesis. We confirmed a relative decrease in MET mRNA expression by qPCR (Fig. S4D). However, Western analysis revealed that MET knockdown does not decrease STAT3 and STAT5 phosphorylation (Fig. S4E). Furthermore, treatment of HEL cells with capmatinib, a MET-selective inhibitor that has no activity against RON kinase, did not decrease STAT3 or STAT5 phosphorylation, unlike crizotinib (Fig. S4F). Overall, these experiments suggest that RON can potentiate JAK-STAT signaling in JAK2-mutated cells, and is likely to be the more important target of crizotinib in MPN.
To determine whether RON activation is also enhanced in MPN patients, we performed intracellular phospho-flow cytometry on peripheral blood samples from JAK2-mutated MPN patients. Importantly, we observed a modest but significant increase in RON activation in MPN patient mononuclear cells (MNCs) compared to control donor MNCs (Fig. 4F). This further supports an oncogenic role for RON in MPN pathogenesis.
To determine whether genetic deletion of RON impairs MPN disease in vivo, we used the JAK2-V617F mouse bone marrow transplantation model.12 Stk (the mouse ortholog for RON) knockout mice were previously generated by homologous recombination to delete an 850 base pair region of exon 1.7 These mice develop normally, but exhibit increased inflammation in response to IFNγ.7 We transduced cKit+ bone marrow cells from Stk−/− mice and C56 Bl/6 WT controls with the JAK2V617F-GFP retrovirus, confirmed similar %GFP in the bone marrow in both groups (Fig. S5A-B), and transplanted the cells into lethally irradiated recipients (Fig. 4G). Although JAK2-V617F retroviral transplantation in the C57BL/6 strain does not cause lethal disease, and has variable effects on spleen size in this strain (Fig. S5C)12, we observed a significant decrease in WBC, hemoglobin, and the % of GFP+ cells in the peripheral blood in the Stk−/− group over time (Fig. 4H-I), despite similar engraftment at 4 weeks post-transplantation (Fig. 4I). These results demonstrate that RON plays an important role in the proliferation of JAK2-mutated cells in vivo.
Genetic deletion of RON in mice impairs terminal erythropoiesis, but has no impact on normal hematopoietic stem and progenitor cells
For inhibition of RON kinase by crizotinib to be a safe therapeutic target in MPN patients, the role of RON must be more important in MPN cells than in healthy hematopoietic cells. We confirmed that Stk−/− mice have normal white blood cell counts, hemoglobin levels, and platelet counts, as was previously reported7 (Fig. S6A). Stk−/− mice also have normal spleen and liver weights (Fig. S6B). As RON has already been implicated in erythroblast proliferation23, we used flow cytometry for Ter119 and CD71 to examine erythroid differentiation in the bone marrow and spleen of Stk−/− and age-matched WT C57 Bl/6 control mice17 (Fig. 5A-C, Fig S6C). While the percentage of Ter119+ erythroid cells was unchanged in Stk−/− bone marrow, we observed an increase in the frequency of immature erythroid cells (population II, basophilic erythroblasts) and a subsequent decrease in the frequency of mature erythroid cells (population IV, orthrochromatophilic erythroblasts), suggesting impaired erythroid differentiation (Fig. 5B-C). The overall percentage of Ter119+ cells was decreased in Stk−/− spleens (Fig. 5B), with a decrease specifically in populations III and IV (Fig. S6C), suggesting an overall decrease in terminal erythropoiesis in the spleen. Additionally, plating of Stk−/− bone marrow or spleen cells in methylcellulose media supplemented with erythropoietin (M3334, Stem Cell Technologies) revealed that Stk−/− bone marrow cells produce fewer colony-forming units-erythroid (CFU-E) colonies than control cells, while there was no difference in BFU-E colony numbers (Fig. 5D-E). We did not observe any differences in BFU-E or CFU-E formation by Stk−/− spleen cells (Fig. S6D). These data suggest that deletion of Stk results in a subtle defect in terminal erythropoiesis, but this does not result in anemia.
Figure 5: Stk (RON) genetic deletion perturbs erythropoiesis in the bone marrow.
(A) Flow cytometry gating strategy of Ter119+ cells in WT (N=5) or Stk−/− (N=5) bone marrow or spleen (B) Quantification of flow cytometry analysis of Ter119+ cells in WT (N=5) or Stk−/− (N=5) bone marrow or spleen. Representative data from one of three independent experiments is shown. (C) Flow cytometry analysis of erythroid maturation with CD71 and Ter119 in WT (N=6) or Stk−/− (N=6) bone marrow cells, showing the gating strategy on erythroid progenitor populations in the bone marrow. Representative data from one of three independent experiments is shown. Quantification of population frequencies of total Ter119+ cells is shown on the right. (D-E) Colony formation assays by WT or Stk−/− bone marrow in methylcellulose media supplemented with erythropoietin. Data is pooled from three independent experiments (N=9 per genotype). For all experiments, an unpaired 2-tailed Student’s t test was performed. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data represent mean ± SEM.
To determine whether Stk deletion impairs hematopoietic stem and progenitor cells, we first analyzed stem cell and progenitor populations using an established flow cytometry panel16. We observed that Stk−/− mice have similar numbers of hematopoietic stem cell and progenitor cells compared to WT controls, except for a subtle increase in the megakaryocyte-erythroid primed multi-potential progenitor (MPPMk/E) population in Stk−/− bone marrow (Fig. S7A-B). Furthermore, plating and serial re-plating of Stk−/− bone marrow cells in methylcellulose media with myeloid growth factors revealed no significant defects in colony formation, differentiation, or self-renewal (Fig. S7C-D). To determine whether Stk deletion impairs HSC repopulation, we performed competitive bone marrow transplantation, in which CD45.2+ Stk−/− and CD45.1+ WT competitor bone marrow cells were transplanted in 1:1 ratio into lethally irradiated recipients (Fig. S8A). Donor chimerism in all of the blood lineages in Stk−/− recipients was not significantly different from that of WT recipients, suggesting that Stk−/− HSPCs have normal functional capacity for long-term multi-lineage repopulation (Fig. S8B). Furthermore, we did not observe any differences in donor chimerism within any HSPC populations at 16 weeks post-transplantation (Fig. S8C). Overall, these data confirm a role for RON signaling in terminal erythroid differentiation, but suggest that RON plays a minimal role in hematopoietic stem and progenitor cells. Therefore, we expect that pharmacological inhibition of RON will have minimal toxicity to the healthy hematopoietic system.
DISCUSSION
In this study, we identify a novel therapeutic indication for the ALK/MET/RON/ROS1 inhibitor crizotinib in myeloproliferative neoplasms, as treatment of MPN patient cells and JAK2-mutated cell lines with crizotinib impairs proliferation and decreases JAK/STAT pathway activation. Furthermore, we show that crizotinib decreases the disease burden in the JAK2V617F knock in model of MPN. Importantly, we uncover a role for crizotinib in modulating JAK inhibitor persistence, which is a known limitation of JAK inhibitor treatment of MPNs. We show that JAK inhibitor persistent cells are resistant to crizotinib as a single agent, but when combined with ruxolitinib, crizotinib confers a sustained response to JAK inhibition. Furthermore, our analysis of Stk−/− (Ron knockout) mice reveals minimal effects on healthy hematopoiesis, except for a subtle defect in terminal erythropoiesis. This is consistent with the favorable safety profile of crizotinib in lung cancer patients, with few reported hematologic toxicities.28 Therefore, we expect that crizotinib could have efficacy in MPN patients, and that a therapeutic window can likely be achieved with crizotinib in this patient population.
This work was initiated by the index patient’s diagnosis of JAK2-V617F+ PV after the ALK inhibitor for EML4-ALK rearranged lung cancer was changed from crizotinib to ceritinib. It is possible that this patient had undiagnosed PV prior to ALK inhibitor treatment, which was suppressed with crizotinib treatment. However, it is well known that JAK2-V617F mutations are common in clonal hematopoiesis.29 Alternatively, he could have had clonal hematopoiesis that progressed to PV when he began ceritinib treatment. Our colony assays on this patient’s peripheral blood CD34+ cells are consistent with crizotinib inhibiting proliferation of the JAK2-mutated clone. Interestingly, we also noted an increase in CFUs by this patient’s CD34+ cells with ceritinib treatment (Fig. 1B-C). While this result is consistent with his progression to PV while on ceritinib, understanding whether this is a biologically and clinically significant finding requires further investigation.
We found that the crizotinib target RON kinase is hyperactivated in JAK inhibitor persistent cells, and that phospho-RON and phospho-JAK2 interact in these cells to sustain JAK/STAT pathway activation. We show that treatment with crizotinib can disrupt this interaction, resulting in suppression of JAK/STAT pathway activation. Given that the JAK inhibitor persistence phenotype is mediated by increased heterodimerization between JAK2 and its family members JAK1 and TYK2,3 and that the JAK family members bind to cytokine receptor tyrosine kinases, it is possible that RON also binds to other members of the JAK family to promote JAK/STAT signaling, and/or that JAK2 can bind to RON to drive the persistence phenotype. This may also be possible through other docking molecules like GRB2, GAB1, and GAB2, as both RON and JAK2 have been shown to recruit and phosphorylate these proteins for activation of downstream signaling pathways.25,30,31 Interestingly, JAK2 and RON tyrosine activation is similar, in that Tyr 966 in both proteins is required for maximal kinase activity.32 This may be important for delineating the mechanism by which RON modulates JAK/STAT signaling in JAK2 inhibitor persistent cells, especially since inhibition of both kinases is required to disrupt complex formation in persistent cells.
In addition to the important role for RON in JAK inhibitor persistence, we have begun to delineate a mechanism for RON in JAK/STAT signaling and MPN pathogenesis in JAK inhibitor naïve MPN cells. We show that shRNA knockdown of RON in JAK2-mutated cells phenocopies the inhibitory effects of crizotinib, and that deletion of RON in JAK2V617F cells decreases the MPN disease burden in vivo, suggesting that RON may be a novel mediator of JAK/STAT signaling in MPN.
The reported interaction between EPO-R and RON could be one potential mechanism for how RON interacts with the JAK/STAT pathway in MPN, as JAK2 also associates with EPO-R. The binding of a truncated version of RON to EPO-R is required for erythroleukemia development through the RON-mediated activation of STAT3.24,25,30,33 Furthermore, co-expression of RON and EPO-R in COS cells was shown to result in EPO-R phosphorylation23, further supporting that RON is part of the EPO-R complex. Given this, along with our data revealing that RON inactivation inhibits JAK/STAT phosphorylation and that phospho-RON and phospho-JAK2 interact in persistent cells, it is likely that RON can potentiate EPO-R/JAK2 signaling (Fig. 6). This role for RON downstream of EPO-R is consistent with the defect in terminal erythropoiesis that we observed in Stk knockout mice. It is also consistent with our findings of crizotinib efficacy, even in MPN cells from patients who were on JAK inhibitor treatment (Fig. 2B), as it suggests that RON cooperates with JAK2 in MPN pathogenesis, but could also play a JAK2-independent role. It is also possible that EPO-R signaling via JAK2 may induce RON activation, which could lead to a further increase in JAK/STAT signaling, resulting in a positive feedback loop.
Figure 6: Model for the proposed mechanism of crizotinib in MPN.
RON physically interacts with JAK2 to potentiate JAK/STAT signaling. This interaction is disrupted with the RON inhibitor crizotinib, leading to inactivation of JAK/STAT signaling. Created with Biorender.com.
Our study identifies RON kinase as one possible target of crizotinib in MPN. While our data suggests that RON plays an important role in MPN cell proliferation and JAK/STAT signaling, it is possible that other targets of crizotinib are also important in MPN pathogenesis. Our findings of the low efficacy of the other ALK inhibitors ceritinib and alectinib in JAK2-mutated hematopoietic cells suggest that ALK is not the target of crizotinib in MPN. The only major kinase targets of crizotinib that are not shared by other ALK inhibitors are RON and MET5,18,21, which share some homology and can heterodimerize and phosphorylate each other to potentiate downstream signaling.34 While our data suggests that MET kinase plays a minimal role in JAK/STAT signaling, a role for its ligand hepatocyte growth factor (HGF) has previously been reported in erythroblast signaling and in MPN.35-37 Therefore, it is possible that RON and MET cooperate in MPN pathogenesis, and that they are both important targets of crizotinib in MPN. Furthermore, recent evidence suggests that crizotinib may also have some activity in inhibiting JAK2, though its potency at JAK2 is at least 10-fold lower than at MET or RON22. Additionally, we found that crizotinib can inhibit the proliferation of CD34+ cells from MPN patients who were on JAK inhibitor treatment, and that crizotinib can re-sensitize JAK inhibitor persistent cells to ruxolitinib, suggesting that JAK2 is not the most important target of crizotinib in MPN.
Overall, this study demonstrates the efficacy of crizotinib in primary MPN patient cells and in a mouse MPN model. Furthermore, we show that combination treatment with ruxolitinib and crizotinib overcomes JAK inhibitor persistence, suggesting that crizotinib can lead to a more sustained JAK inhibitor response. Therefore, this should prompt the testing of crizotinib in MPN, either as an individual agent after first line treatment with a JAK inhibitor, or in combination with a JAK inhibitor to re-sensitize patients who have stopped responding to JAK inhibitors. In addition, it would be interesting to address whether upfront treatment with crizotinib and ruxolitinib can prevent the development of persistence. In addition, crizotinib could be tested as an alternative to JAK inhibitors for patients who cannot tolerate JAK inhibitors due to cytopenias. In a disease with few treatment options, crizotinib could be a valuable therapeutic agent for MPN treatment, either as a single agent, or in combination with JAK inhibitors.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all past and current members of the Gritsman laboratory for their helpful suggestions and discussions. We thank Daqian Sun and Swathi-Rao Narayangari of the AECOM Stem Cell Isolation and Xenotransplantation Facility for assistance with in vivo crizotinib treatment and flow cytometry and sorting (funded through New York Stem Cell Science grant C029154), Dr. Jinghang Zhang for assistance with flow cytometry, and Amanda Beck of the AECOM Histology and Comparative Pathology Facility. We thank Dr. Anne Bresnick for her assistance with co-immunoprecipitation experiments. We thank Peter Schultes of the AECOM Department of Cell Biology for technical assistance. This work was supported by a Research Scholar Grant RSG-19-130-01-DDC from the American Cancer Society to Kira Gritsman, MD, PhD, the National Cancer Institute of National Institutes of Health (NIH) Grant R01CA196973 (K.Gritsman), startup funds from the Albert Einstein College of Medicine (K.Gritsman), National Cancer Institute/NIH fellowship F31CA247172 (L.Gurska), the Training Program in Cellular and Molecular Biology and Genetics at Albert Einstein College of Medicine #5T32GM007491-44 (L.Gurska), the NHLBI/NIH Ruth L. Kirschstein National Research Service Award F32HL146119 (K. Ames), the IRACDA/BETTR training Institutional Research and Academic Career Development Award 2K12GH102779-07A1 (K.Ames), the American Society of Hematology HONORS Award (D.Choi), the NIH Medical Scientist Training Program Training Grant #T32GM007288 (A.Schurer), Eclipse Training Grant #TL1 TR002557 (S. Glushakow-Smith), National Cancer Institute of NIH P01 CA108671 11 (R.L. Levine), National Cancer Institute of NIH grant 1K99CA252005-01A1 (L.A. Miles), Albert Einstein Cancer Center core support grant (P30CA013330). The Cytek Aurora Multiparameter Flow Cytometer was purchased with funding from the National Institutes of Health SIG grant #1S10OD026833-01. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations
- MPN
Myeloproliferative neoplasm
- PV
Polycythemia vera
- ET
Essential thrombocytosis
- PMF
Primary myelofibrosis
- JAK2
Janus kinase 2
- STAT3
Signal Transducer and Activator of Transcription 3
- STAT5
Signal Transducer and Activator of Transcription 5
- MPL
Thrombopoietin receptor
- CALR
Calreticulin
- ALK
Anaplastic lymphoma kinase
- RON
Recepteur d'origine nantais
- MET
Mesenchymal Epithelial Transition
- ROS1
ROS Proto-Oncogene 1
- IGF1R
Insulin-like growth factor 1 receptor
- EPO-R
Erythropoietin receptor
- EPO
Erythropoietin
- HGF
Hepatocyte growth factor
- SCF
Stem cell factor
- NSCLC
Non-small cell lung cancer
- MNCs
Mononuclear cells
- WBC
White blood cell
- RBC
Red blood cell
- JAK1
Janus kinase 1
- TYK2
Tyrosine kinase 2
- GRB2
Growth factor receptor bound protein 2
- GAB1
GRB2 Associated Binding Protein 1
- GAB2
GRB2 Associated Binding Protein 2
- CFU
Colony forming units
- CFU-E
Colony forming units-erythroid
- BFU-E
Burst forming units-erythroid
- HSC
Hematopoietic stem cell
- ST-HSC
Short-term hematopoietic stem cell
- MPPMk/E
Megakaryocyte/erythrocyte multi-potential progenitor
- MPPG/M
Granulocyte/macrophage multi-potential progenitor
- CMP
Common myeloid progenitor
- GMP
Granulocyte-macrophage progenitor
- MEP
Megakaryocyte-erythroid progenitor
Footnotes
Conflicts of interest
K.G. has received research funding from ADC Therapeutics and iOnctura, SA. B.H. receives grants or contracts from any entity from Boehringer Ingelheim, Astra Zeneca, Merck, BMS, Advaxis, Amgen, AbbVie, Daiichi, Pfizer, GSK, Beigene, and Janssen. B.H. has consulting fees with Astra Zeneca, Boehringer Ingelheim, Veracyte, Janssen, Takeda, Merck, BMS, Genentech, Pfizer, and Eli-Lilly. R.L.L. is on the supervisory board of Qiagen and is a scientific advisor to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, Syndax, C4 Therapeutics and Isoplexis which each include equity interest. R.L.L. receives research support from Calico, Constellation, Ajax, and Prelude and has consulted for Jubilant, Incyte, Celgene, Janssen, Astellas, Morphosys and Novartis. R.L.L. has received honoraria from Astra Zeneca, Roche, Lilly and Amgen for invited lectures and from Gilead for grant reviews. L.A.M. is on the speakers bureau for and has received honoraria for invited lectures from Mission Bio, Inc. All other authors declare no competing financial interests.
REFERENCES
- 1.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017;129:667–79. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A Myeloproliferative neoplasms: A decade of discoveries and treatment advances. American Journal of Hematology 2016;91:50–8. [DOI] [PubMed] [Google Scholar]
- 3.Koppikar P, Bhagwat N, Kilpivaara O, et al. Heterodimeric JAK–STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature 2012;489:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gisslinger H, Schalling M, Gisslinger B, Skrabs C, Müllauer L, Kralovics R. Restoration of response to ruxolitinib upon brief withdrawal in two patients with myelofibrosis. American Journal of Hematology 2014;89:344–6. [DOI] [PubMed] [Google Scholar]
- 5.Giroux-Leprieur E, Fallet V, Cadranel J, Wislez M. Spotlight on crizotinib in the first-line treatment of ALK-positive advanced non-small-cell lung cancer: patients selection and perspectives. Lung Cancer (Auckl) 2016;7:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010;17:584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correll PH, Iwama A, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Funct 1997;1:69–83. [DOI] [PubMed] [Google Scholar]
- 8.Chow S, Hedley D, Grom P, Magari R, Jacobberger JW, Shankey TV. Whole blood fixation and permeabilization protocol with red blood cell lysis for flow cytometry of intracellular phosphorylated epitopes in leukocyte subpopulations. Cytometry A 2005;67:4–17. [DOI] [PubMed] [Google Scholar]
- 9.Perl AE, Kasner MT, Tsai DE, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res 2009;15:6732–9. [DOI] [PubMed] [Google Scholar]
- 10.Marvin J, Swaminathan S, Kraker G, Chadburn A, Jacobberger J, Goolsby C. Normal bone marrow signal-transduction profiles: a requisite for enhanced detection of signaling dysregulations in AML. Blood 2011;117:e120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woost PG, Solchaga LA, Meyerson HJ, Shankey TV, Goolsby CL, Jacobberger JW. High-resolution kinetics of cytokine signaling in human CD34/CD117-positive cells in unfractionated bone marrow. Blood 2011;117:e131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera–like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 2006;107:4274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav B, Wennerberg K, Aittokallio T, Tang J. Searching for Drug Synergy in Complex Dose–Response Landscapes Using an Interaction Potency Model. Computational and Structural Biotechnology Journal 2015;13:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng S, Wang W, Aldahdooh J, et al. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genomics, Proteomics & Bioinformatics 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmati S, Sinclair T, Tong M, Bartholdy B, Okabe R, Ames K et al. , JCI Insight 2019;4(13):e125832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challen GA, Pietras EM, Wallscheid NC, Signer RAJ. Simplified murine multipotent progenitor isolation scheme: Establishing a consensus approach for multipotent progenitor identification. Exp Hematol 2021;104:55–63. [DOI] [PubMed] [Google Scholar]
- 17.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood 2001;98:3261–73. [DOI] [PubMed] [Google Scholar]
- 18.Au TH, Cavalieri CC, Stenehjem DD. Ceritinib: A primer for pharmacists. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners 2017;23:602–14. [DOI] [PubMed] [Google Scholar]
- 19.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366:787–98. [DOI] [PubMed] [Google Scholar]
- 20.Cabezas-Wallscheid N, Klimmeck D, Hansson J, et al. Identification of Regulatory Networks in HSCs and Their Immediate Progeny via Integrated Proteome, Transcriptome, and DNA Methylome Analysis. Cell Stem Cell 2014;15:507–22. [DOI] [PubMed] [Google Scholar]
- 21.Ly AC, Olin JL, Smith MB. Alectinib for advanced ALK-positive non-small-cell lung cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 2018;75:515–22. [DOI] [PubMed] [Google Scholar]
- 22.Davis MI, Hunt JP, Herrgard S, et al. Comprehensive analysis of kinase inhibitor selectivity. Nature Biotechnology 2011;29:1046–51. [DOI] [PubMed] [Google Scholar]
- 23.van den Akker E, van Dijk T, Parren-van Amelsvoort M, et al. Tyrosine kinase receptor RON functions downstream of the erythropoietin receptor to induce expansion of erythroid progenitors. Blood 2004;103:4457–65. [DOI] [PubMed] [Google Scholar]
- 24.Persons DA, Paulson RF, Loyd MR, et al. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet 1999;23:159–65. [DOI] [PubMed] [Google Scholar]
- 25.Ni S, Zhao C, Feng GS, Paulson RF, Correll PH. A novel Stat3 binding motif in Gab2 mediates transformation of primary hematopoietic cells by the Stk/Ron receptor tyrosine kinase in response to Friend virus infection. Mol Cell Biol 2007;27:3708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson CB, Ray M, Lutz M, Sharda D, Xu J, Hankey PA. The RON receptor tyrosine kinase regulates IFN-gamma production and responses in innate immunity. J Immunol 2008;181:2303–10. [DOI] [PubMed] [Google Scholar]
- 27.Yao H-P, Zhou Y-Q, Zhang R, Wang M-H. MSP–RON signalling in cancer: pathogenesis and therapeutic potential. Nature Reviews Cancer 2013;13:466. [DOI] [PubMed] [Google Scholar]
- 28.Roberts PJ. Clinical use of crizotinib for the treatment of non-small cell lung cancer. Biologics 2013;7:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKerrell T, Park N, Chi J, et al. JAK2 V617F hematopoietic clones are present several years prior to MPN diagnosis and follow different expansion kinetics. Blood Adv 2017;1:968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teal HE, Ni S, Xu J, et al. GRB2-mediated recruitment of GAB2, but not GAB1, to SF-STK supports the expansion of Friend virus-infected erythroid progenitor cells. Oncogene 2006;25:2433–43. [DOI] [PubMed] [Google Scholar]
- 31.Wolf A, Eulenfeld R, Gabler K, et al. JAK2-V617F-induced MAPK activity is regulated by PI3K and acts synergistically with PI3K on the proliferation of JAK2-V617F-positive cells. JAKSTAT 2013;2:e24574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argetsinger LS, Stuckey JA, Robertson SA, et al. Tyrosines 868, 966, and 972 in the kinase domain of JAK2 are autophosphorylated and required for maximal JAK2 kinase activity. Mol Endocrinol 2010;24:1062–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelstein LD, Ney PA, Liu QP, Paulson RF, Correll PH. Sf-Stk kinase activity and the Grb2 binding site are required for Epo-independent growth of primary erythroblasts infected with Friend virus. Oncogene 2002;21:3562–70. [DOI] [PubMed] [Google Scholar]
- 34.Chang K, Karnad A, Zhao S, Freeman JW. Roles of c-Met and RON kinases in tumor progression and their potential as therapeutic targets. Oncotarget 2015;6:3507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galimi F, Bagnara GP, Bonsi L, et al. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol 1994;127:1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iguchi T, Sogo S, Hisha H, et al. HGF activates signal transduction from EPO receptor on human cord blood CD34+/CD45+ cells. Stem Cells 1999;17:82–91. [DOI] [PubMed] [Google Scholar]
- 37.Boissinot M, Vilaine M, Hermouet S. The Hepatocyte Growth Factor (HGF)/Met Axis: A Neglected Target in the Treatment of Chronic Myeloproliferative Neoplasms? Cancers 2014;6:1631–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.