Abstract
Importance
Patient satisfaction is a crucial indicator for assessing quality of care in healthcare settings. However, patient satisfaction benchmark for cancer hospitals in China is not established.
Objective
To examine patient satisfaction levels in tertiary cancer hospitals in China, and inter-hospital variations after case-mix adjustment.
Design
A nationwide cross-sectional hospital performance survey conducted from January to March 2021.
Settings
At 30 tertiary cancer hospitals in China.
Participants
A total of 4,847 adult inpatients consecutively recruited at 30 tertiary cancer hospitals were included.
Exposures
Patient characteristics included demographic characteristics (sex, age, education, and annual family income), clinical characteristics (cancer type, cancer stage, self-reported health status, and length of stay), and actual respondents of questionnaire.
Main outcomes and measures
Patient satisfaction was measured using 23 items covering five aspects, administrative process, hospital environment, medical care, symptom management, and overall satisfaction. Responses to each item were recorded using a 5-point Likert scale. Patient satisfaction level for each aspect was described at individual and hospital levels. Using multilevel logistic regression, patient characteristics associated with patient satisfaction were examined as case-mix adjusters and inter-hospital variation were determined.
Results
The satisfaction rates for symptom management, administrative process, hospital environment, overall satisfaction, and medical care aspects were 74.56, 81.70, 84.18, 84.26, and 90.86% with a cut-off value of 4, respectively. Significant predictors of patient satisfaction included sex, age, cancer type, cancer stage, self-reported health status, and actual respondent (representative or patient) (all P < 0.05). The ranking of the hospitals' performance in satisfaction was altered after the case-mix adjustment was made. But even after the adjustment, significant variation in satisfaction among hospitals remained.
Conclusions and relevance
This study pointed to symptom management as a special area, to which a keen attention should be paid by policymakers and hospital administrators. Significant variation in satisfaction among hospitals remained, implying that future studies should examine major factors affecting the variation. In review, target interventions are needed in low-performing hospitals.
Keywords: patient experience, cancer, tertiary hospital, hospital performance, China
Key points
Question: What is the patient satisfaction level with different healthcare aspects in China's cancer hospitals? Does the patient satisfaction vary across hospitals?
Findings: Drawing from a national hospital performance survey that included 4,847 inpatients in China's 30 cancer hospitals, patient satisfaction level with clinical aspects of healthcare is found to be high, while there was a perceived need for improvement in symptom management and the cumbersome hospital admission process. The ranking of the hospitals' performance in satisfaction was altered after the case-mix adjustment was made, while significant variation in satisfaction among hospitals remained.
Meanings: This study pointed to symptom management as a special area, to which a keen attention should be paid by policymakers and hospital administrators and discrepancy of hospital performance in patient satisfaction remains to be narrowed.
Introduction
Cancer is the second leading cause of death and a major contributor to the disease burden worldwide and in China (1). Cancer patients take efforts in seeking hospitals offering safe, effective and comfortable care. In China, cancer patients could receive treatment and care in various hospitals, among which cancer hospitals provide the most specialized and skilled oncology treatment and deliver a large proportion of cancer care. Besides, cancer hospitals in China were graded as three levels based on their technology and resources, including primary, secondary and tertiary cancer hospitals (2). The tertiary cancer hospitals represent China's national and regional medical centers for cancer care and regularly offer treatments for numerous patients with more severe and complicated conditions. In China with the increase of cancer patients, annual admissions of patients by cancer hospitals have increased from 1.10 million to 3.24 million from 2010 to 2020, which posed a great challenge for these hospitals (3).
Patients' perception of their care is a vital source of healthcare quality assessment alone or in combination with clinical outcomes (4–6). Patient experience or satisfaction as the most commonly used indicator of patients' perception has become a key component of performance assessment and certification in healthcare settings (7, 8), helping guide quality improvement actions, regulations, and incentive payments (9, 10). National initiatives involving patient experience surveys have been developed for decades, such as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey in the USA, the National Health Service (NHS) National Inpatient Survey in England, and other surveys in other countries or regions (11–13). Several initiatives in cancer care settings also have been gradually developed, including the CAHPS (Consumer Assessment of Healthcare Providers and Systems) Cancer Care Survey, NCPES (National Cancer Patient Experience Survey), and ECCQI (European Cancer Consumer Quality Index) (14–19). In China some exploratory studies were conducted on this topic but usually with small sample and either in one single cancer center or on single cancer type (20–23). The nationwide, multi-center surveys covering patients with various cancer types in China are lacking.
Previous studies on cancer patient experience mainly focused on impact factors and subgroup differences in patient satisfaction (24, 25). Age, sex, education, income, race or ethnicity, disease characteristics, treatment files, and survey methods are associated with satisfaction in some advanced countries and regions (24–28). These studies mainly focused on the level of patient satisfaction and associated factors, but did not explored the inter-hospital variation, which is important for targeted improvement of cancer care for specific hospital. Case-mix adjustment has been widely used in hospital comparison studies and it helps precisely measure inter-hospital variation after removing the effect of patient constitution (29–34).
Based on a multi-center cross-sectional patient survey in China, this study measured cancer patient satisfaction in tertiary cancer hospitals across China. We employed a case-mix model for each aspect of patient satisfaction, demonstrating the effects of the model on hospital comparisons. Inter-hospital variation in patient satisfaction after case-mix adjustment was determined.
Methods
Based on the national hospital performance survey, a multi-center cross-sectional study of patient satisfaction in tertiary cancer hospitals was conducted between January and March 2021 (13, 35, 36). This survey was commissioned by the China National Health Commission to investigate patients' perceptions and the performance of tertiary public hospitals. This study followed the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Sampling
A total of 30 tertiary cancer hospitals across 28 provinces in China were included, covering all national and most provincial-level cancer hospitals, accounting for approximately half of tertiary cancer hospitals in China. The sample size was at least 1,746 to estimate a satisfaction rate of 80% with a precision of plus or minus 2% at the 95% confidence level; and set an invalid proportion of collected data of 10%. We set a minimum sample size of 150 inpatients in each of the 30 hospitals, so the planned sample size in 30 tertiary cancer hospitals was 4,500 in total.
Data collection
At least 150 inpatients per hospital were consecutively recruited from January to March 2021. Patients on the day of or the day prior to discharge, aged 18 and over and with clear consciousness were invited to participate in this survey. They were asked to fill in the questionnaire using their electronic devices under the guide of trained investigators. If patients could not respond to the questionnaire independently due to inconvenience or inability, the family members present at the hospital provided responses based on the patients' perspectives. All patients provided informed consent for their participation in the study. Finally, a total of 4,847 eligible adult patients took part in this survey and were included in our analyses.
Survey instrument and variables
The patient satisfaction questionnaire regarding cancer care was designed based on international surveys [including the CAHPS (37, 38), NCPES (16, 28), ECCQI (19), and other area-related surveys (11, 12, 39, 40)] and domestic policies related to improving care quality (13, 18). Cancer patient satisfaction in this questionnaire were measured with 23 items over five aspects including administrative process, hospital environment, medical care, symptom management and overall satisfaction. Responses to each item were recorded using a 5-point Likert scale (5-strongly satisfied to 1-strongly dissatisfied) or “not applicable.” Table 1 showed the items and average score of each aspect. Every aspect got its score ranged from 1 to 5 based on the average score of corresponding satisfaction items. Higher scores represent higher patient satisfaction.
Table 1.
Patient satisfaction, internal consistency, and average aspect scores.
| Aspect | Items | Questions | Cronbach's α | Aspect score mean, SD |
|---|---|---|---|---|
| Administrative process | 4 | -I am satisfied with the waiting time for admission | 0.794 | 4.67 (0.52) |
| -I am satisfied with the admission procedure | ||||
| -I am satisfied with the accessibility of medical complains | ||||
| -I am satisfied with the punctuality of surgery | ||||
| Hospital environment | 5 | -I am satisfied with the cleanness and absence of smells in ward and toilet | 0.880 | 4.71 (0.46) |
| -I am satisfied with the quietness of the ward | ||||
| -I am satisfied with the fall prevention equipment in the hospital | ||||
| -I am satisfied with the signage in the hospital | ||||
| -I am satisfied with the accessibility of hand-washing solution in the ward | ||||
| Medical care | 9 | -I am satisfied that the doctor took my treatment preferences into account | 0.946 | 4.82 (0.36) |
| -I am satisfied with the protection of patient's privacy of medical staffs | ||||
| -I am satisfied with medication instructions of medical staffs | ||||
| -I am satisfied that the doctor carefully explain about treatment schemes (pros and cons) | ||||
| -I am satisfied with the doctors' care | ||||
| -I am satisfied with the doctors' careful inquiry about my illness conditions | ||||
| -I am satisfied with the nurses' timely help when I need | ||||
| -I am satisfied with the courtesy and respect of medical staffs | ||||
| -I am satisfied with the nurses' manners | ||||
| Symptom managementa | 3 | -I am satisfied with psychological counseling regarding stress, anxiety and depression | 0.942 | 4.67 (0.61) |
| -I am satisfied with diet instructions regarding nausea and vomiting | ||||
| -I am satisfied with pain management regarding severe hurt | ||||
| Overall satisfaction | 2 | -Overall, I am satisfied with this hospitalization | 0.753 | 4.77 (0.43) |
| -I would like to recommend the hospital to my relatives and friends |
Sample size of symptom management was 2,276 because not all patients had undergone clinical symptoms surveyed.
This questionnaire was validated through three rounds of expert consultation and a small-scale pilot test. The internal consistency of five aspects was tested using Cronbach's α coefficient. Cronbach's α values were 0.753–0.942 at an acceptable level, which indicating a sound reliability of the survey questionnaire.
This questionnaire also collected patient information including demographic characteristics (sex, age, education, and annual family income), clinical characteristics (cancer type, cancer stage, self-reported health status, and length of stay), and actual respondents (whether the patient or a representative completed the questionnaire). Actual respondents were categorized as patients or representatives. Representatives completed the questionnaire when patients were unavailable or unable to respond independently.
Statistical analysis
We transferred the patient satisfaction score for each aspect into a binary variable at a cutoff point of 4 (patient satisfaction = 1 if average score >4). The patient satisfaction rates for each aspect at individual level were calculated and those at hospital level were depicted at box graphs. The Pearson's chi-square tests were used to compare the satisfaction rate for each aspect and preliminarily identified the relationship between patient characteristics and satisfaction level.
Because our data have a hierarchical structure and patients were nested in multiple hospitals, multilevel regressions were used to explore case-mix factors related to patient satisfaction. We performed multilevel logistic regression for each aspect. All covariates identified in this paper were included in multilevel logistic regression analyses for all these factors were statistically significant or clinical significance. Intraclass correlation coefficient (ICC) was inspected, which indicated the extent to which the overall variation in patient satisfaction could be attributed to hospital effect (41). The ICCs ranged from 6.9 to 15.5% across five aspects evaluated in this study, reconfirming the necessity for multilevel modeling (Table 3).
Table 3.
Multilevel logistic regression of patient satisfaction (OR with 95% confidence interval).
| Specification | Administrative process | Hospital environment | Medical care | Symptom managementa | Overall satisfaction | |
|---|---|---|---|---|---|---|
| Sex | Female | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Male |
0.73 (0.61, 0.87)*** |
0.90 (0.75, 1.09) |
0.76 (0.60, 0.96)* |
0.93 (0.74, 1.16) |
0.88 (0.73, 1.06) |
|
| Age | 18–45 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 45–64 | 1.19 (0.98, 1.45) |
1.40 (1.14, 1.73)*** |
1.19 (0.91, 1.55) |
1.13 (0.87, 1.45) |
1.13 (0.92, 1.39) |
|
| 65–85 | 1.09 (0.85, 1.39) |
1.11 (0.86, 1.45) |
0.91 (0.66, 1.27) |
1.10 (0.79, 1.52) |
1.01 (0.78, 1.32) |
|
| Education | College or above | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Junior or high school | 1.02 (0.84, 1.25) |
1.04 (0.84, 1.29) |
0.96 (0.74, 1.27) |
1.12 (0.87, 1.44) |
0.92 (0.74, 1.14) |
|
| Primary School or less | 0.91 (0.70, 1.19) |
0.89 (0.67, 1.18) |
0.72 (0.51, 1.02) |
1.06 (0.76, 1.49) |
0.80 (0.60, 1.06) |
|
| Monthly family income | <30,000 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 30,000–60,000 | 0.92 (0.76, 1.11) |
0.92 (0.75, 1.13) |
1.06 (0.82, 1.36) |
0.94 (0.73, 1.20) |
1.03 (0.84, 1.26) |
|
| ≥60,000 | 1.15 (0.93, 1.43) |
1.08 (0.86, 1.36) |
1.12 (0.84, 1.49) |
1.04 (0.79, 1.37) |
1.11 (0.88, 1.39) |
|
| Cancer type | Lung cancer |
0.63 (0.50, 0.80)*** |
0.82 (0.63, 1.06) |
0.75 (0.54, 1.06) |
0.73 (0.54, 0.98)* |
0.75 (0.58, 0.98)* |
| Digestive tract cancer (esophagus, gastric, colorectal) | 0.89 (0.71, 1.11) |
0.96 (0.76, 1.22) |
0.84 (0.61, 1.15) |
0.77 (0.58, 1.02) |
0.91 (0.72, 1.16) |
|
| Breast or cervical cancer |
0.56 (0.44, 0.72)*** |
0.78 (0.60, 1.02) |
0.49 (0.35, 0.68)*** |
0.54 (0.40, 0.74)*** |
0.64 (0.49, 0.83)*** |
|
| Other types | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Non-cancer | 1.07 (0.80, 1.43) |
1.21 (0.89, 1.64) |
1.01 (0.69, 1.47) |
1.17 (0.76, 1.79) |
1.22 (0.90, 1.65) |
|
| Cancer stage | 0–2 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 3–4 | 0.92 (0.75, 1, 14) |
1.10 (0.88, 1.38) |
1.28 (0.95, 1.71) |
0.93 (0.72, 1.19) |
1.13 (0.90, 1.42) |
|
| unknown |
0.81 (0.65, 0.99)* |
0.79 (0.63, 0.99)* |
0.80 (0.61, 1.06) |
0.66 (0.51, 0.87)** |
0.70 (0.56, 0.87)*** |
|
| Self-reported health status | Worse | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Moderate | 1.03 (0.86, 1.24) |
1.15 (0.95, 1.39) |
0.99 (0.78, 1.26) |
1.09 (0.87, 1.36) |
1.06 (0.88, 1.28) |
|
| Better | 1.14 (0.94, 1.39) |
1.55 (1.25, 1.92)*** |
1.25 (0.95, 1.64) |
1.52 (1.16, 1.99)** |
1.60 (1.29, 1.99)*** |
|
| Length of stay | 1–14 days | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| >14 days | 0.86 (0.72, 1.03) |
0.91 (0.75, 1.11) |
0.85 (0.67, 1.08) |
0.83 (0.66, 1.04) |
0.99 (0.81, 1.21) |
|
| Respondent | Patient | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Representative |
0.82 (0.69, 0.98)* |
0.80 (0.66, 0.97)* |
1.07 (0.84, 1.36) |
0.76 (0.61, 0.95)* |
0.88 (0.73, 1.07) |
|
| ICC(%)b | 0.108 (0.063, 0.181) |
0.155 (0.093, 0.247) |
0.161 (0.092, 0.265) |
0.069 (0.034, 0.136) |
0.144 (0.084, 0.235) |
|
Sample size of symptom management was 2,276 because not all patients had undergone clinical symptoms surveyed.
P < 0.05,
P < 0.01,
P < 0.001.
Represent ICC and its 95%confidence interval after case-mix adjustment. Bold OR with 95% confidence interval is beyond or below 1.
ICC, intraclass correlation coefficient.
The 30 tertiary hospitals included 3 national-level hospitals, 27 provincial-level hospitals. The 3 national-level hospitals were labeled “NA,” “NB,” and “NC,” and the other cancer hospitals were labeled “A,” “B,” “C,” etc. A caterpillar plot for each aspect was generated to depict inter-hospital variation in patient satisfaction (41). The y-axis shows the estimate of the hospital residuals with 95% confidence intervals for patient satisfaction after modeling multilevel multivariate regressions; the x-axis shows the hospital rank based on the hospital's residual, ranging from low to high. The residuals represent hospital departures from the overall mean, so a hospital whose confidence interval does not overlap the line at zero (zero line representing the mean satisfaction rate across sampled hospitals) is said to differ significantly from the average at 5% level. At the left-hand side of the plot, there is a cluster of hospitals whose mean satisfaction rate is lower than average (categorized as worse); at the other extreme, there is a cluster with above-average satisfaction rate (categorized as better); when overlapping the line at zero, there is a cluster with average satisfaction rate (categorized as average) (42).
Statistical analyses were conducted using Stata/SE 15.0 software (Stata Corp. LP, College Station, TX, USA). All tests were two-sided, and P < 0.05 indicated statistical significance.
Results
Patient characteristics
Of the 4,847 patients sampled in 30 hospitals, 2,291 (47.27%) were male, 2,637 (54.40%) were 45–64 years of age, and 2,631 (54.28%) had a junior or high school education. Lung, digestive tract, or breast/cervical cancer was present in 14.98, 19.89, and 17.80% of the patients, respectively; 30.62% had stage 3–4 cancer, and the hospital stay exceeded 14 days in 20.51% (Table 2). Some patients did not experience clinical symptoms during their hospital stay; thus, the symptom management aspect did not apply to them. The symptom management aspect analysis included 2,276 patients.
Table 2.
Patient characteristics and the rate of patient satisfaction.
| Specification | Number of inpatients, N (%) | Administrative process | Hospital environment | Medical care | Symptom managementa | Overall satisfaction |
|---|---|---|---|---|---|---|
| Total | 4,847 (100) | 3,960 (81.70) | 4,080 (84.18) | 4,404 (90.86) | 1,697 (74.56) | 4,084 (84.26) |
| Sex | ||||||
| Male | 2,291 (47.27) | 1,841 (80.36) | 1,925 (84.02) | 2,072 (90.44) | 808 (75.30) | 1,923 (83.94) |
| Female | 2,556 (52.73) | 2,119 (82.90) | 2,155 (84.31) | 2,332 (91.24) | 889 (73.90) | 2,161 (84.55) |
| P | 0.02 | 0.79 | 0.34 | 0.44 | 0.56 | |
| Age | ||||||
| 18–44 | 1,190 (24.55) | 968 (81.34) | 983 (82.61) | 1,077 (90.50) | 394 (73.78) | 1,001 (84.12) |
| 45–64 | 2,637 (54.40) | 2,173 (82.40) | 2,263 (85.82) | 2,417 (91.66) | 946 (75.20) | 2,239 (84.91) |
| 65–85 | 1,020 (21.04) | 819 (80.29) | 834 (81.76) | 910 (89.22) | 357 (73.76) | 844 (82.75) |
| P | 0.31 | 0.002 | 0.06 | 0.63 | 0.27 | |
| Education | ||||||
| College or above | 1,280 (26.41) | 1,064 (83.13) | 1,090 (85.16) | 1,175 (91.80) | 430 (74.14) | 1,102 (86.09) |
| Junior or high school | 2,631 (54.28) | 2,153 (81.83) | 2,229 (84.72) | 2,399 (91.18) | 939 (75.30) | 2,217 (84.26) |
| Primary School or less | 936 (19.31) | 743 (79.38) | 761 (81.30) | 830 (88.68) | 328 (73.05) | 765 (81.73) |
| P | 0.08 | 0.03 | 0.03 | 0.62 | 0.02 | |
| Annual family income | ||||||
| <30,000 | 1,359 (28.04) | 1,084 (79.76) | 1,118 (82.27) | 1,214 (89.33) | 508 (73.41) | 1,108 (81.53) |
| 30,000–60,000 | 1,626 (33.55) | 1,298 (79.83) | 1,348 (82.90) | 1,472 (90.53) | 563 (73.69) | 1,364 (83.89) |
| ≥60,000 | 1,862 (38.42) | 1,578 (84.74) | 1,614 (86.68) | 1,718 (92.27) | 626 (76.34) | 1,612 (86.57) |
| P | <0.001 | 0.001 | <0.001 | 0.34 | <0.001 | |
| Cancer type | ||||||
| Lung cancer | 726 (14.98) | 565 (77.82) | 602 (82.92) | 658 (90.63) | 292 (73.55) | 605 (83.33) |
| Digestive tract cancer (esophagus, gastric, colorectal) | 964 (19.89) | 795 (82.47) | 815 (84.54) | 878 (91.08) | 394 (73.64) | 819 (84.96) |
| Breast or cervical cancer | 863 (17.80) | 683 (79.14) | 717 (83.08) | 768 (88.99) | 288 (68.90) | 712 (82.50) |
| Other types cancer | 1,528 (31.52) | 1,291 (84.49) | 1,302 (85.21) | 1,420 (92.93) | 585 (78.84) | 1,323 (86.58) |
| Non-cancer | 766 (15.80) | 626 (81.72) | 644 (84.07) | 680 (88.77) | 138 (75.00) | 625 (81.59) |
| P | 0.001 | 0.56 | 0.004 | 0.005 | 0.01 | |
| Cancer stage | ||||||
| 0–2 | 1,403 (28.95) | 1,167 (83.18) | 1,198 (85.39) | 1,283 (91.45) | 518 (76.97) | 1,206 (85.96) |
| 3–4 | 1,484 (30.62) | 1,227 (82.68) | 1,277 (86.05) | 1,384 (93.26) | 653 (76.11) | 1,296 (87.33) |
| Unknown | 1,960 (40.44) | 1,566 (79.90) | 1,605 (81.89) | 1,737 (88.62) | 526 (70.60) | 1,582 (80.71) |
| P | 0.03 | 0.001 | <0.001 | 0.01 | <0.001 | |
| Self-reported health status | ||||||
| Worse | 17,84 (36.81) | 1,439 (80.66) | 1,462 (81.95) | 1,611 (90.30) | 695 (72.32) | 1,473 (82.57) |
| Moderate | 1,684 (34.74) | 1,372 (81.47) | 1,410 (83.73) | 1,517 (90.08) | 576 (73.94) | 1,399 (83.08) |
| Better | 1,379 (28.45) | 1,149 (83.32) | 1,208 (87.60) | 1,276 (92.53) | 426 (79.48) | 1,212 (87.89) |
| P | 0.15 | <0.001 | 0.04 | 0.009 | <0.001 | |
| Length of stay | ||||||
| 1–14 days | 3,853 (79.49) | 3,187 (82.71) | 3,279 (85.10) | 3,531 (91.64) | 1,322 (75.93) | 3,270 (84.87) |
| >14 days | 994 (20.51) | 773 (77.77) | 801 (80.58) | 873 (87.83) | 375 (70.09) | 814 (81.89) |
| P | <0.001 | 0.001 | <0.001 | 0.007 | 0.02 | |
| Respondent | ||||||
| Representative | 1,470 (30.33) | 1,161 (78.98) | 1,195 (81.29) | 1,330 (90.49) | 543 (70.70) | 1,212 (82.45) |
| Patient | 3,377 (69.67) | 2,799 (82.88) | 2,885 (85.43) | 3,074 (91.03) | 1,154 (76.53) | 2,872 (85.05) |
| P | 0.001 | <0.001 | 0.54 | 0.003 | 0.02 | |
Bold P-values < 0.05.
Sample size of symptom management was 2,276 because not all patients had undergone clinical symptoms surveyed.
Patient satisfaction level
The satisfaction scores associated with the administrative process, hospital environment, medical care, symptom management, and overall satisfaction were 4.67, 4.71, 4.82, 4.67, and 4.77, respectively (Table 1). The respective satisfaction rates were 81.70, 84.18, 90.86, 74.56, and 84.26% (Table 2). The satisfaction level was highest in the medical care aspect and comparatively lower in the symptom management and administrative process aspects for respondents. Besides, patients in different sociodemographic groups showed different satisfaction levels. For example, female patients expressed higher satisfaction in administrative process aspect, and patients in the middle age expressed higher satisfaction in hospital environment aspect.
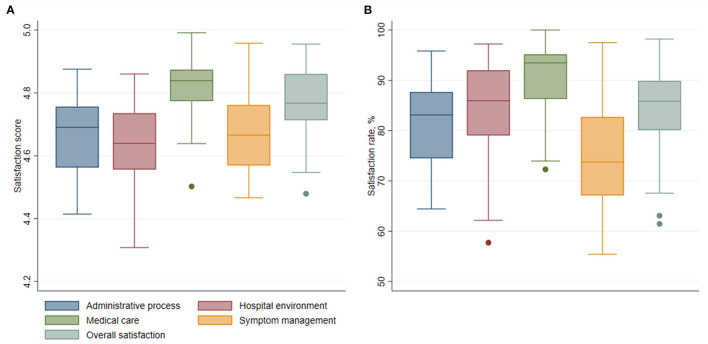
The aggregated satisfaction scores and rates for each hospital are shown in Figure 1. At the hospital level, the symptom management and process aspects also showed the lower level of satisfaction, and the medical care aspect showed the highest. The aggregated satisfaction scores ranged from 4.31 to 4.99 in all aspects across hospitals; and the satisfaction rates ranged from 55.41 to 100.0%, indicating substantial differences in hospital performance (Supplementary Table 1).
Figure 1.
Patient satisfaction aggregated at hospital level over five aspects. (A) Box plot of patient satisfaction scores over five aspects. (B) Box plot of patient satisfaction rates over five aspects.
Predictors on patient satisfaction
In the multivariate multi-level regression models, statistically significant predictors for at least one aspect were sex, age, cancer type, cancer stage, self-reported health status, and actual respondent (Table 3). Patients aged 45–64 years were 1.40 times more likely to be satisfied with the hospital environment than those aged <45 years (OR: 1.40, 95% CI: 1.14–1.73). Compared with those with worse self-reported health status, patients with better self-reported health status were 1.55 (95% CI: 1.25–1.92) and 1.52 (95% CI: 1.16–1.99) times more likely to be satisfied with the hospital environment and symptom management, respectively, and their overall satisfaction was 1.60 (95% CI: 1.29–1.99) times higher.
Male patients had 27% (OR: 0.73, 95% CI: 0.61–0.87) and 24% (OR: 0.76, 95% CI: 0.60–0.96) lower odds of being satisfied with the administrative process and medical care than their counterparts. Compared with those with other cancer types, patients with lung cancer had 37% (OR: 0.63, 95% CI: 0.50–0.80) and 27% (OR: 0.73, 95% CI: 0.54–0.98) lower odds of being satisfied with the administrative process and symptom management, respectively, and their overall satisfaction was 25% (OR: 0.75, 95% CI: 0.58–0.98) lower; patients with breast or cervical cancer had 44% (OR: 0.56, 95% CI: 0.44–0.72), 51% (OR: 0.49, 95% CI: 0.35–0.68), 46% (OR: 0.54, 95% CI: 0.40–0.74), and 36% (OR: 0.64, 95% CI: 0.49–0.83) lower odds of being satisfied with the administrative process, medical care, symptom management and overall satisfaction aspects. Compared with those with cancer stages 0–2, patients who did not know their cancer stage had relatively lower odds of being satisfied with the four aspects, except for the medical care aspect. When representatives completed the questionnaire, satisfaction rates with the administrative process, hospital environment, and symptom management were ~18–24% lower than when patients completed the survey (Table 3).
Effect of case-mix adjustment
Hospital performance altered after case-mix adjustment. Hospital ranking based on patient satisfaction with various aspects (within a range of 5) changed (Supplementary Figure 1). Hospital performance based on patient satisfaction (better, average, or worse than average) was slightly changed in the overall satisfaction aspect and symptom management aspect after risk adjustment (Supplementary Table 2).
Inter-hospital variation of patient satisfaction
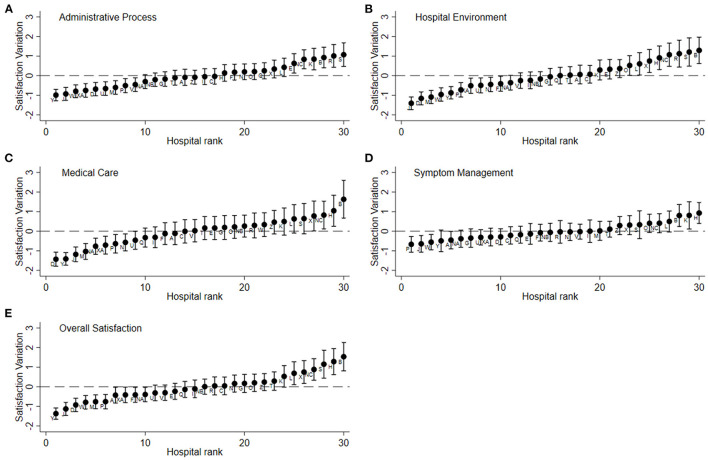
The caterpillar plots revealed wide patient satisfaction variation in the five aspects across hospitals (Figure 2). Even after the adjustment, significant variations in satisfaction among hospitals remained. For each aspect, high-performing hospitals with a confidence interval over 0 and low-performing hospitals with a confidence interval below 0 could be identified. The plots of the five aspects revealed that one hospitals (B) performed better in all aspects than the average, and two hospitals (J, P) performed worse in all aspects (Supplementary Table 3).
Figure 2.
Satisfaction variation among hospitals after case-mix adjustment. (A) Administrative process, (B) hospital environment, (C) medical care, (D) symptom management, (E) overall satisfaction. Y axis, satisfaction variation (random effect and standard error) by hospitals; X axis, hospital rank sorted by random effect. All models are adjusted with variables: sex, age, education, annual family income, cancer type, cancer stage, self-reported health status, length of stay and actual respondent.
Discussion
Patient satisfaction with various aspects in tertiary cancer hospitals in China was investigated based on a multi-center patient survey. The satisfaction level in the medical care aspect was highest, while that in the symptom management aspect was lowest. We examined patient-level characteristics associated with patient satisfaction among cancer hospitals, and using those factors as case-mix adjusters, we found there were changes in the relative level of hospital patient satisfaction after the adjustment. These findings indicate that it is necessary to standardize the patient composition in hospital evaluation researches. However, the discrepancy in hospital performance remained substantial among cancer hospitals in China after case-mix adjustment. The factors embedded in hospital structure that affect hospital performance need to be explored in the future. Overall, the methods and findings in our study were helpful for benchmarking patient satisfaction with cancer hospitals within countries and guiding hospital quality improvement efforts.
Measuring patient satisfaction with five aspects of cancer care in China provides a greater understanding of cancer care. This study presented patients' perceptions of their care including the symptom management aspect, which was rarely used in international patient experience or satisfaction surveys (14, 15, 24, 43). In this study, we found that the symptom management aspect was associated with the lowest satisfaction level (satisfaction rate of 74.56% and satisfaction score of 4.67) among Chinese cancer patients. Cancer patients worldwide may experience a high symptom burden during disease development and treatment, such as depression, pain, sleep disturbance, fatigue, and malnutrition (44, 45). As a part of palliative care, appropriate symptom management helps prolong life, enhance prognoses, and improve health-related quality of life (46, 47). More attention should be paid to symptom management aspect when conducting hospital assessment worldwide, which may promote medical education and training on this topic for medical staff and patients.
In addition, we found that patients were most satisfied with their medical care, and their satisfaction with the administrative process was comparatively lower. These findings are in accordance with previous studies for China's tertiary general hospitals, which also revealed better performance in medical care and poorer performance in process management (36). The influx of patients at tertiary hospitals in China has resulted in process management challenges (2). Within the process aspect, patients were least satisfied with waiting times, which should be addressed as a priority. Regarding the hospital environment, patients were least satisfied with the quietness and cleanliness items. The perceived need for process and environment improvement by patients alerts us to take efforts in the future.
Patient-level factors including sociodemographic characteristics (sex and age) and clinical characteristics (cancer type, cancer stage, and self-reported health status) were identified as influencing factors in the five aspects using multilevel regression models. Female and middle-aged patients were more likely to be satisfied with their service. Advanced cancer stage and worse self-reported health were risk factors associated with patient satisfaction, consistent with a previous study conducted among older cancer patients in the USA (43). The aforementioned patients are usually more depressed, worried about their disease, and concerned with care plans, leading to greater expectations and more negative perceptions. Some of patients in our study did not know their cancer stage, potentially due to undetermined staging or family members concealing the cancer stage from the patient (48). Uncertainty regarding the status of the disease may result in patient anxiety and weakens their communication flexibility with medical staff (49, 50). Actual respondent was a significant impact factor in the process, environment, and symptom management aspects. Patients who required a representative may have had more severe conditions, and the information provided by representatives reflected the collective perceptions of the patients and their representatives.
Because patient characteristics influence their perceptions of medical service, the direct comparison between hospitals with regard to patient satisfaction might be biased if there is a significant difference in patient constitution (51–53). It is suggested that when comparing satisfaction levels across hospitals, patient composition should be taken into account, and case-mix adjusted satisfaction level should be used as a benchmark (32, 54). For example, the composition of cancer types greatly differed across cancer hospitals in our study. The proportion of lung cancer patients among the sampled hospitals ranged from 6.1 to 28.7%, and that of breast cancer patients ranged from 3.8 to 39.1%. As the results of this study suggested, cancer type is a significant factor influencing patient satisfaction; thus, using it as a case-mix adjuster is essential for hospital comparison.
The patient satisfaction for each aspect was case-mix adjusted for age, sex, education, income, cancer type, cancer stage, self-reported health status, length of stay, and respondent using multilevel models. Case-mix adjustment had a modest effect on hospital comparison. First, case-mix adjustment had a modest effect on hospital rank (the rank changed within a range of 5). Second, hospital performance categories (categorized into better, average, or worse) of patient satisfaction were slightly changed in the overall satisfaction aspect and symptom management aspect after risk adjustment. Hospital performance categories were more robust compared with hospital rank before and after risk adjustment. The study on national cancer patient experience in England found 6–10 hospitals moved out of the extreme performance categories after case mix adjustment (54). The results of the national surgical quality survey in the USA showed the sufficiency of risk adjustment for accurate comparisons of hospital quality (55). While the effect of case-mix adjustment was modest for some practices in previous studies, they also found that case-mix adjustment corrected significant underestimation of scores or rates for a small proportion of practices serving vulnerable patients (56).
Wide variation in patient satisfaction among hospitals was shown after adjustment, indicating that the case-mix constitution was not decisive for difference of hospital performance in cancer care. These remaining inter-hospital variation may result from hospital features and the quality of health services provided. Therefore, for narrowing disparity of patient satisfaction among hospitals, efforts to improve the resource endowment and service quality of poor-performing hospitals should be take into consideration. The high-performing hospitals identified in analyses can act as valuable cases to offer experiences. Besides, the caterpillar plots readily revealed specific hospitals that performed worse in various aspects, which helps policymakers, researchers, and hospital managers easily identify discrepancies in hospital performance, and conduct targeted interventions for specific hospitals and corresponding aspects.
This study has several strengths. First, this was the first nationwide multi-center patient satisfaction study of cancer hospitals in China, filling a gap in the knowledge of cancer care in China. Second, this study broke down patient satisfaction into five aspects and explored substantial aspects of hospital improvement opportunities. Third, this study eliminated possible statistical inaccuracies due to the cluster effect of patients and examined the variation across hospitals using multilevel models. The inter-hospital variation was clearly visualized in the caterpillar plots. This study also has some limitations. First, this study sampled tertiary cancer hospitals in China, and the generalization of these findings was limited for other hospitals at a lower level. Second, patient information was self-reported, and recall bias may exist. However, recall bias is estimated to be low because the information collected was relatively explicit, and the recall period was short (interviews were conducted on the discharge day or the day prior). Third, our study did not include hospital characteristics and contextual factors as explanatory variables, which may reveal more sources of inter-hospital variation. Future studies should be conducted to explore the effect of health system contexts and hospital characteristics associated with patient satisfaction.
Conclusions
This is the first nationwide multi-center patient survey regarding patient satisfaction in tertiary cancer hospitals in China. The results revealed the need to improve quality, especially in process optimization and symptom management. Substantial inter-hospital variation remained after case-mix adjustment, revealing the substantial discrepancy in patient satisfaction across hospitals and the need of efforts in quality improvement in low-performing hospitals. This study is helpful for policymakers, researchers, and hospital managers to identify problems in service quality, conduct targeted interventions, and address deficient aspects of healthcare services in cancer hospitals. Future studies could further explore factors regarding institutional features associated with patient satisfaction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Institute of the Medical Biology of the Chinese Academy of Medical Sciences (IPB-2020-23). The patients/participants provided their written informed consent to participate in this study.
Author contributions
ML is the guarantor. All authors contributed to the planning, conduct, analysis, and writing of this study.
Funding Statement
This work was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences—A Strategic Study on Healthy China Development and Health System Reform (2021-I2M-1-046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1059878/full#supplementary-material
References
- 1.Global Burden of Disease 2019 Cancer Collaboration. Kocarnik JM, Compton, Dean, Fu W, Gaw, BL, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. (2022) 8:420–44. 10.1001/jamaoncol.2021.6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Gong W, Kong X, Mueller O, Lu G. Factors associated with outpatient satisfaction in tertiary hospitals in china: a systematic review. Int J Environ Res Public Health. (2020) 17:7070. 10.3390/ijerph17197070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.China National Health Commission . China Health Statistical Yearbook 2020. Beijing: Peking Union Medical College Press; (2021). [Google Scholar]
- 4.Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. (2010) 67:27–37. 10.1177/1077558709341065 [DOI] [PubMed] [Google Scholar]
- 5.Marsh C, Peacock R, Sheard L, Hughes L, Lawton R. Patient experience feedback in UK hospitals: what types are available and what are their potential roles in quality improvement (QI)? Health Expect. (2019) 22:317–26. 10.1111/hex.12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Pusic AL, Hall BL, Ko CY, Temple LK. Combining clinical outcomes and patient-reported experiences to evaluate hospital cancer surgery quality. Conference Abstract. Ann Surg Oncol. (2018) 25:S14-15. 10.1245/s10434-018-6349-1 [DOI] [Google Scholar]
- 7.Roland M. Linking physicians' pay to the quality of care–a major experiment in the United Kingdom. N Engl J Med. (2004) 351:1448–54. 10.1056/NEJMhpr041294 [DOI] [PubMed] [Google Scholar]
- 8.Hays R. The Consumer Assessment of Healthcare Providers and Systems (CAHPS) approach to assessing patient experiences with care in the United States. Qual Life Res. (2018) 27:S12. 10.1007/s11136-018-1946-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanLare JM, Conway PH. Value-based purchasing–national programs to move from volume to value. N Engl J Med. (2012) 367:292–5. 10.1056/NEJMp1204939 [DOI] [PubMed] [Google Scholar]
- 10.Revere L, Langland-Orban B, Large J, Yang Y. Evaluating the robustness of the CMS Hospital Value-Based Purchasing measurement system. Health Serv Res. (2021) 56:464–73. 10.1111/1475-6773.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Ichinose Y, Matsuki M, Wakita T, Toida T, Masuda M, et al. Experiences of patients with cancer at health care facilities in Japan: results from a nationwide survey. BMC Health Serv Res. (2021) 21:1180. 10.1186/s12913-021-07184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu TH, Chung KP, Tung YC, Tsai HY. Insight into patients' experiences of cancer care in Taiwan: an instrument translation and cross-cultural adaptation study. Int J Environ Res Public Health. (2018) 15:1772. 10.3390/ijerph15081772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Ding H, Liu S, Wang Z, Hu G, Liu Y. Influence of patient and hospital characteristics on inpatient satisfaction in China's tertiary hospitals: a cross-sectional study. Health Expect. (2020) 23:115–24. 10.1111/hex.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evensen CT, Yost KJ, Keller S, Arora NK, Frentzel E, Cowans T, et al. Development and testing of the CAHPS cancer care survey. J Oncol Pract. (2019) 15:e969–78. 10.1200/JOP.19.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla N, Urato M, Ambs A, Schussler N, Hays RD, Clauser SB, et al. Unveiling SEER-CAHPS®: a new data resource for quality of care research. J Gen Intern Med. (2015) 30:641–50. 10.1007/s11606-014-3162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nartey Y, Stewart I, Khakwani A, Beattie V, Wilcock A, Woolhouse I, et al. Is the English cancer patient experience survey representative? A comparative analysis with the National Lung Cancer Audit. Lung Cancer. (2020) 140:27–34. 10.1016/j.lungcan.2019.11.023 [DOI] [PubMed] [Google Scholar]
- 17.Esser P, Sautier L, Sarkar S, Schilling G, Bokemeyer C, Koch U, et al. Development and preliminary psychometric investigation of the German Satisfaction with Comprehensive Cancer Care (SCCC) Questionnaire. Health Qual Life Outcomes. (2021) 19:147. 10.1186/s12955-021-01784-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evensen C, Yost KJ, Keller S, Cowand T, Frentzel E, Jenkins S. CAHPS cancer care survey: development, testing, and final content of a survey of patient experience with cancer care. J Clin Oncol. (2017) 35(8) 10.1200/JCO.2017.35.8_suppl.227 [DOI] [Google Scholar]
- 19.Wind A, Roeling MP, Heerink J, Sixma H, Presti P, Lombardo C, et al. Piloting a generic cancer consumer quality index in six European countries. BMC Cancer. (2016) 16:711. 10.1186/s12885-016-2752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam WWT, Kwong A, Suen D, Tsang J, Soong I, Yau TK, et al. Factors predicting patient satisfaction in women with advanced breast cancer: a prospective study. BMC Cancer. (2018) 18:162. 10.1186/s12885-018-4085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Dai Z, Cheng S, Xie S, Woo SM, Luo Z, et al. Validation of EORTC IN-PATSAT32 for Chinese cancer patients. Support Care Cancer. (2015) 23:2721–30. 10.1007/s00520-015-2636-6 [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Dai Z, Cheng S, Xie S, Woo SM-L, Luo Z, et al. Analysis and evaluation of satisfaction of inpatients in a cancer hospital. China Cancer, (2017) 26:333–7. [Google Scholar]
- 23.Li H, Wang N, Wu A. Analysis of present situation and influencing factors of patient satisfaction in a tumor hospital. Chin Hosp Manag. (2019) 39:62–4. 23078100 [Google Scholar]
- 24.Mollica MA, Buckenmaier SS, Halpern MT, McNeel TS, Weaver SJ, Doose M, et al. Perceptions of care coordination among older adult cancer survivors: A SEER-CAHPS study. J Geriatr Oncol. (2021) 12:446–52. 10.1016/j.jgo.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 25.Mollica MA, Weaver KE, McNeel TS, Kent EE. Examining urban and rural differences in perceived timeliness of care among cancer patients: a SEER-CAHPS study. Cancer. (2018) 124:3257–65. 10.1002/cncr.31541 [DOI] [PubMed] [Google Scholar]
- 26.Pham TM, Gomez-Cano M, Salika T, Jardel D, Abel GA, Lyratzopoulos G. Diagnostic route is associated with care satisfaction independently of tumour stage: evidence from linked English Cancer Patient Experience Survey and cancer registration data. Cancer Epidemiol. (2019) 61:70–8. 10.1016/j.canep.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 27.Younger E, Husson O, Bennister L, Whelan J, Wilson R, Roast A, et al. Age-related sarcoma patient experience: results from a national survey in England. BMC Cancer. (2018) 18:991. 10.1186/s12885-018-4866-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham TM, Abel GA, Gomez-Cano M, Lyratzopoulos G. Predictors of postal or online response mode and associations with patient experience and satisfaction in the English cancer patient experience survey. J Med Internet Res. (2019) 21:e11855. 10.2196/11855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadilhac DA, Kilkenny MF, Levi CR, Lannin NA, Thrift AG, Kim J, et al. Risk-adjusted hospital mortality rates for stroke: evidence from the Australian Stroke Clinical Registry (AuSCR). Med J Austral. (2017) 206:345–50. 10.5694/mja16.00525 [DOI] [PubMed] [Google Scholar]
- 30.Glance LG, Maddox KJ, Johnson K, Nerenz D, Cella D, Borah B, et al. National quality forum guidelines for evaluating the scientific acceptability of risk-adjusted clinical outcome measures: a report from the National Quality Forum Scientific Methods Panel. Ann Surg. (2020) 271:1048–55. 10.1097/SLA.0000000000003592 [DOI] [PubMed] [Google Scholar]
- 31.Bailit JL, Grobman WA, Rice MM, Spong CY, Wapner RJ, Varner MW, et al. Risk-adjusted models for adverse obstetric outcomes and variation in risk-adjusted outcomes across hospitals. Am J Obstetr Gynecol. (2013) 209:446.e1–30. 10.1016/j.ajog.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vos EL, Lingsma HF, Jager A, Schreuder K, Spronk P, Peeters M-JTFDV, et al. Effect of case-mix and random variation on breast cancer care quality indicators and their rankability. Value Health. (2020) 23:1191–99. 10.1016/j.jval.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 33.Cai T, Zaslavsky AM. Bayesian hierarchical modeling of substate area estimates from the Medicare CAHPS survey. Stat Med. (2019) 38:1662–77. 10.1002/sim.8068 [DOI] [PubMed] [Google Scholar]
- 34.Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. (2010) 67:56–73. 10.1177/1077558709339066 [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Hu G, Ma J, Chen Y, Wu L, Liu Q, et al. Consumer satisfaction with tertiary healthcare in China: findings from the 2015 China National Patient Survey. Int J Qual Health Care. (2017) 29:213–21. 10.1093/intqhc/mzw160 [DOI] [PubMed] [Google Scholar]
- 36.Hu G, Chen Y, Liu Q, Wu S, Guo J, Liu S, et al. Patient experience of hospital care in China: major findings from the Chinese Patient Experience Questionnaire Survey (2016–2018). BMJ Open. (2019) 9:e031615. 10.1136/bmjopen-2019-031615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halpern MT, Mollica M, Lines LM, Cohen J, Kent EE. SEER-CAHPS: A national population-based data resource to evaluate patient-centered cancer care. Conference Abstract. Cancer Res. (2019) 79:3366. 10.1158/1538-7445.SABCS18-3366 [DOI] [Google Scholar]
- 38.Halpern MT, Urato MP, Kent EE. The health care experience of patients with cancer during the last year of life: analysis of the SEER-CAHPS data set. Cancer. (2017) 123:336–44. 10.1002/cncr.30319 [DOI] [PubMed] [Google Scholar]
- 39.Coulter A, Cleary PD. Patients' experiences with hospital care in five countries. Health Affairs. (2001) 20:244–52. 10.1377/hlthaff.20.3.244 [DOI] [PubMed] [Google Scholar]
- 40.Jean-Pierre P, Fiscella K, Freund KM, Clark J, Darnell J, Holden A, et al. Structural and reliability analysis of a patient satisfaction with cancer-related care measure: a multisite patient navigation research program study. Cancer. (2011) 117:854–61. 10.1002/cncr.25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bottle A, Faitna P, Aylin PP. Patient-level and hospital-level variation and related time trends in COVID-19 case fatality rates during the first pandemic wave in England: multilevel modelling analysis of routine data. BMJ Qual Saf. (2022) 31:211–20. 10.1136/bmjqs-2021-012990 [DOI] [PubMed] [Google Scholar]
- 42.Centre for Multilevel Modelling, University of Bristol . LEMMA (Learning Environment for Multilevel Methods and Applications). Available online at: https://www.cmm.bris.ac.uk/lemma/ (accessed July 19, 2022).
- 43.Halpern MT, Urato MP, Lines LM, Cohen JB, Arora NK, Kent EE. Healthcare experience among older cancer survivors: analysis of the SEER-CAHPS dataset. J Geriatr Oncol. (2018) 9:194–203. 10.1016/j.jgo.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bubis LD, Davis L, Mahar A, Barbera L, Li Q, Moody L, et al. Symptom burden in the first year after cancer diagnosis: an analysis of patient-reported outcomes. J Clin Oncol. (2018) 36:1103–11. 10.1200/JCO.2017.76.0876 [DOI] [PubMed] [Google Scholar]
- 45.Bubis LD, Davis LE, Canaj H, Gupta V, Jeong Y, Barbera L, et al. Patient-reported symptom severity among 22,650 cancer outpatients in the last six months of life. J Pain Sympt Manag. (2020) 59:58–66.e4. 10.1016/j.jpainsymman.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 46.Hoerger M, Greer JA, Jackson VA, Park ER, Pirl WF, El-Jawahri A, et al. Defining the elements of early palliative care that are associated with patient-reported outcomes and the delivery of end-of-life care. J Clin Oncol. (2018) 36:1096–102. 10.1200/JCO.2017.75.6676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama N, Matsumura C, Tahara Y, Sako M, Kurosawa H, Nomura T, et al. Symptom clusters and their influence on prognosis using EORTC QLQ-C15-PAL scores in terminally ill patients with cancer. Support Care Cancer. (2022) 30:135–43. 10.1007/s00520-021-06380-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gan Y, Zheng L, Yu NX, Zhou G, Miao M, Lu Q. Why do oncologists hide the truth? Disclosure of cancer diagnoses to patients in China: A multisource assessment using mixed methods. Psychooncology. (2018) 27:1457–63. 10.1002/pon.4545 [DOI] [PubMed] [Google Scholar]
- 49.Guan T, Santacroce SJ, Chen DG, Song L. Illness uncertainty, coping, and quality of life among patients with prostate cancer. Psychooncology. (2020) 29:1019–25. 10.1002/pon.5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lines LM, Cohen J, Kirschner J, Barch DH, Halpern MT, Kent EE, et al. Associations between illness burden and care experiences among Medicare beneficiaries before or after a cancer diagnosis. Journal of geriatric oncology (2022) 13:731–37. 10.1016/j.jgo.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment of the CAHPS Hospital Survey. Health Serv Res. (2005) 40(6 Pt 2):2162–81. 10.1111/j.1475-6773.2005.00470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta RH, Liang L, Karve AM, Hernandez AF, Rumsfeld JS, Fonarow GC, et al. Association of patient case-mix adjustment, hospital process performance rankings, and eligibility for financial incentives. JAMA. (2008) 300:1897–903. 10.1001/jama.300.16.1897 [DOI] [PubMed] [Google Scholar]
- 53.National Cancer Institute . Case-Mix Adjustment Guidance for SEER-CAHPS Analyses. Available online at: https://healthcaredelivery.cancer.gov/seer-cahps/researchers/adjustment_guidance.html. Published 2020 (accessed January 20, 2020).
- 54.Abel GA, Saunders CL, Lyratzopoulos G. Cancer patient experience, hospital performance and case mix: evidence from England. Fut Oncol. (2013) 10:1589–98. 10.2217/fon.13.266 [DOI] [PubMed] [Google Scholar]
- 55.Merkow RP, Bentrem DJ, Winchester DP, Stewart AK, Ko CY, Bilimoria KY. Effect of including cancer-specific variables on risk-adjusted hospital surgical quality comparisons. Ann Surg Oncol. (2013) 20:1766–73. 10.1245/s10434-013-2867-z [DOI] [PubMed] [Google Scholar]
- 56.Paddison C, Elliott M, Parker R, Staetsky L, Lyratzopoulos G, Campbell JL, et al. Should measures of patient experience in primary care be adjusted for case mix? Evidence from the English General Practice Patient Survey. BMJ Qual Saf. (2012) 21:634–40. 10.1136/bmjqs-2011-000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.