Summary
Targeting coinhibitory receptors on dysfunctional T cells may improve response to anti-PD-(L)1 in the interferon-γ associated T cell-inflamed tumor microenvironment. The bispecific LAG-3 and PD-L1 blocking antibody FS118, potentially through LAG-3 shedding, represents a promising strategy to improve immune-checkpoint blockade. Soluble LAG-3 is an intriguing biomarker for LAG-3 drug activity.
In this issue of Clinical Cancer Research, Yap and colleagues investigated the safety and preliminary activity of bispecific antibody FS118 targeting lymphocyte activation gene-3 (LAG-3) and programmed death ligand 1 (PD-L1) in patients with anti-PD-(L)1 therapy-resistant advanced solid tumors (Figure 1).(1) LAG-3 is an inhibitory receptor that is absent on naïve T cells and is predominantly expressed on stimulated CD4+ and CD8+ T cells, leading to decreased T cell activation, function, and memory. LAG-3 expression is correlated with interferon (IFN)-γ production by CD4+/CD8+ T cells in a roughly similar paradigm as PD1.(2,3) Upregulation of LAG3 after PD1 blockade and the poor outcomes to immune checkpoint blockade therapy in patients with increased peripheral blood LAG3+CD8+ cells nominate LAG3 as a priority therapeutic target for combination immunotherapy. The canonical ligand for LAG3 is MHC class II, however roles for other ligands such as Galectin-3, Fibrinogen-like Protein 1, L-sectin as well as direct contact with TCR:CD3 complex have been reported. The relevance of these specific ligand:receptor interactions in clinical LAG3 immunotherapy awaits further investigation.(4)
Figure 1.
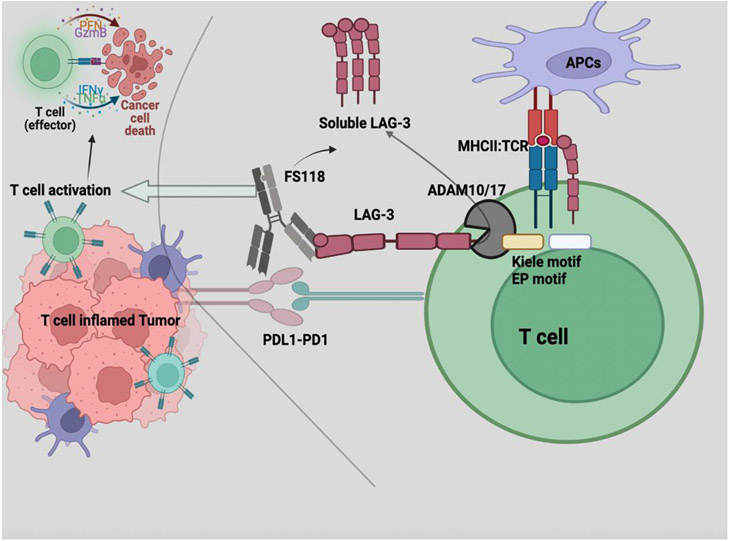
Lymphocyte activation gene-3 is expressed on stimulated T cells. ADAM10/17 metalloproteinases cleave LAG-3 from cell surface and result in increase in soluble LAG-3. Bispecific antibody FS118 targets LAG-3 and programmed death ligand 1 (PD-L1) in the T cell inflamed tumor microenvironment. The treatment results in increase in soluble LAG3, T cell activation and cancer cell death. Created with BioRender.com.
Co-expression of LAG-3 and PD-1 on tumor infiltrating lymphocytes and synergy between targeting these two pathways in murine models set the stage for LAG3 clinical development.(5) Relatlimab, an IgG4 monoclonal antibody targeting LAG-3, was the first drug in the LAG-3 pathway to be approved by FDA, when administered with nivolumab. This combination, compared against nivolumab alone, improved progression-free survival (PFS; HR=0.75) and was deemed well tolerated in patients with advanced melanoma. Median PFS was greater across all patients whose tumors had expression of LAG-≥1%, though benefit was also observed to a lesser extent in the LAG3 low population.(6) Nivolumab plus relatlimab has also demonstrated intriguing data in the neoadjuvant melanoma setting with a pathological complete response rate of 57% and associated increases in intra-tumoral and peripheral blood CD8+ and CD4+ memory T cells.(7) As of this writing, there are 26 actively recruiting clinical trials of relatlimab in ClinicalTrials.gov in combination with anti-PD1 therapy, with or without chemotherapy for various cancers. Across these studies, it is apparent of an intent to replace the indication of single-agent anti-PD1 therapy in both metastatic and peri-operative settings. Additionally, there are a large number of other LAG-3 targeting antagonistic antibodies, bispecific molecules, LAG3-Ig agonists which are being studied in early-phase clinical trials.
In the study by Yap et al., FS118 was well tolerated demonstrating 4.7% grade ≥3 treatment-related adverse events, though preliminary assessment of efficacy remains to be determined. Evaluation of clinical activity is difficult in this population of PD-(L)1 refractory patients though a disease control rate was described as 46.5% amongst all patients. Notably, less clinical benefit was observed in patients who demonstrated primary resistance to immune-checkpoint blockade therapy. Given the IFN-γ inducible nature of LAG3 expression, LAG3 targeted therapy would be presumed to have the highest likelihood of clinical benefit in tumors with T cell-inflamed tumor microenvironments. As such, patients with tumors where anti-PD1 has frontline activity and those that developed acquired resistance may represent the most appropriate cohorts for studying the role of LAG-3 immunotherapy. The development of relatlimab in melanoma emphasizes this paradigm where the phase II clinical trial of nivolumab plus relatlimab in anti-PD1 refractory disease showed only a modest suggestion of efficacy and yet the randomized phase III study in the treatment naïve setting met the primary endpoint.
In the study of FS118, approximately 60% of evaluable tumors were positive for both LAG-3 and PD-L1, and double expression was associated with a longer duration of therapy. Patients with stable disease demonstrated increase in CD4+ and CD8+ T cells from baseline in comparison to those with progressive disease. FS118 resulted in a dose-dependent increase in soluble LAG-3 (sLAG-3) with no significant differences being observed between 10mg/kg and 20mg/kg doses. The former was declared as the recommended phase 2 dose for further evaluation of the efficacy of this compound.
LAG-3 shedding has been described as a mediator of anti-PD1 efficacy with resistance to PD1 blockade observed in the absence of metalloprotease activity. Shedding of LAG3 is mediated by the ADAM10 and ADAM17 metalloproteinases and high LAG3:ADAM10 ratio in peripheral blood CD4+ T cells is associated with poor survival and resistance to anti-PD1 therapy in patients with head and neck squamous cell cancer (HNSCC) and melanoma.(8) In a preclinical system designed to express a non-cleavable LAG-3 mutant protein, a decrease in T cell proliferation and reduced production of interleukin-2 and IFN-γ was observed. Serum sLAG-3 had no impact on T-cell activation in the same study.(9) In contrast to this finding, sLAG-3 decreased the differentiation of monocytes to dendritic cells and may potentially impair T-cell response through reduced antigen presentation.(10) LAG-3 is also expressed on plasmacytoid dendritic cells and those served as a major source for sLAG-3 in vivo.(11) In patients with hepatocellular carcinoma sLAG-3 was significantly elevated in comparison to healthy controls. Elevated sLAG-3 is associated with poor prognosis in patients with HNSCC though in patients with gastric cancer it has been associated with better outcomes.
The emerging data surrounding LAG-3 in activation of antigen presentation and subsequent clinical development of sLAG-3 protein analysis is promising yet many questions remain outstanding. Particularly, the exact sources of sLAG3 as well as LAG3 expression on dendritic cells, and antitumor immune responses when combined with other immune-checkpoint blockade approaches awaits clarification.(12) Although treatment with FS118 resulted in sustained elevation of sLAG3, this study does not report correlations between response to therapy and levels of sLAG3. Mention is made that sLAG3 levels did not vary at baseline between PD1 primary- and acquired-resistant patients, emphasizing an unmet need to better understand sLAG-3 as a biomarker of treatment response.
After approximately a decade of development, LAG-3 immunotherapy has become the third clinically validated checkpoint molecule and the field is expanding quickly with new agents and novel approaches to leverage the pathway. For FS118, a clinical trial of FS118 combined with chemotherapy is on-going in patients with HNSCC with acquired resistance to anti-PD1 therapy. This study is notably attempting to enrich for LAG-3 positive tumors. Another area of potential development for LAG-3 therapies is in lymphoma where there is demonstrated expression on malignant B cells and treatment response before and after chimeric antigen receptor T cell therapy has been observed.(13,14) The regulatory role of LAG-3 cleavage on inhibitory functioning of this pathway makes sLAG-3 an interesting biomarker to study as a correlate for drug activity. More broadly however the immunologic association of LAG-3 with IFN-γ responses, T cell dysfunction and the T cell-inflamed tumor microenvironment remain the primary biology guiding further clinical and translational development surrounding this pathway.
Funding support acknowledgment:
JJL acknowledges NIH UM1CA186690-06, P50CA254865-01A1, P30CA047904-32, and R01DE031729-01A1.
Footnotes
Disclosures:
LK: None; JJL: DSMB: Abbvie, Immutep, Evaxion; Scientific Advisory Board: (no stock) 7 Hills, Affivant, Bright Peak, Exo, Fstar, Inzen, RefleXion, Xilio (stock) Actym, Alphamab Oncology, Arch Oncology, Duke Street Bio, Kanaph, Mavu, NeoTx, Onc.AI, OncoNano, physIQ, Pyxis, Saros, STipe, Tempest; Consultancy with compensation: Abbvie, Agenus, Alnylam, Atomwise, Bayer, Bristol-Myers Squibb, Castle, Checkmate, Codiak, Crown, Cugene, Curadev, Day One, Eisai, EMD Serono, Endeavor, Flame, G1 Therapeutics, Genentech, Gilead, Glenmark, HotSpot, Kadmon, KSQ, Janssen, Ikena, Inzen, Immatics, Immunocore, Incyte, Instil, IO Biotech, Macrogenics, Merck, Mersana, Nektar, Novartis, Partner, Pfizer, Pioneering Medicines, PsiOxus, Regeneron, Replimmune, Ribon, Roivant, Servier, STINGthera, Synlogic, Synthekine; Research Support: (all to institution for clinical trials unless noted) AbbVie, Astellas, Astrazeneca, Bristol-Myers Squibb, Corvus, Day One, EMD Serono, Fstar, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, Macrogenics, Merck, Moderna, Nektar, Next Cure, Numab, Palleon, Pfizer, Replimmune, Rubius, Servier, Scholar Rock, Synlogic, Takeda, Trishula, Tizona, Xencor; Patents: (both provisional) Serial #15/612,657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof)
References:
- 1.Yap TA, LoRusso PM, Wong DJ, Hu-Lieskowen S, Papdopoulous KP, Holz J-B, et al. A phase 1 first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1 in patients with advanced cancer and PD-L1 resistance. Clin Cancer Res 2022. [DOI] [PubMed] [Google Scholar]
- 2.Bruniquel D, Borie N, Hannier S, Triebel F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 1998;48(2):116–24 doi 10.1007/s002510050411. [DOI] [PubMed] [Google Scholar]
- 3.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 1990;171(5):1393–405 doi 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews LP, Cillo AR, Karapetyan L, Kirkwood JM, Workman CJ, Vignali DAA. Molecular Pathways and Mechanisms of LAG-3 in Cancer Therapy. Clin Cancer Res 2022. doi 10.1158/1078-0432.Ccr-21-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72(4):917–27 doi 10.1158/0008-5472.Can-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386(1):24–34 doi 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaria RN, Postow M, Burton EM, Tezlaff MT, Ross MI, Torres-Cabala C, et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022;611(7934):155–60 doi 10.1038/s41586-022-05368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews LP, Somasundaram A, Moskovitz JM, Szymczak-Workman AL, Liu C, Cillo AR, et al. Resistance to PD1 blockade in the absence of metalloprotease-mediated LAG3 shedding. Sci Immunol 2020;5(49) doi 10.1126/sciimmunol.abc2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. Embo j 2007;26(2):494–504 doi 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buisson S, Triebel F. LAG-3 (CD223) reduces macrophage and dendritic cell differentiation from monocyte precursors. Immunology 2005;114(3):369–74 doi 10.1111/j.1365-2567.2004.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol 2009;182(4):1885–91 doi 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson V, Khattak A, Haydon A, Eastgate M, Roy A, Prithviraj P, et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer 2020;8(2) doi 10.1136/jitc-2020-001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keane C, Law SC, Gould C, Birch S, Sabdia MB, Merida de Long L, et al. LAG3: a novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv 2020;4(7):1367–77 doi 10.1182/bloodadvances.2019001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke JJ, Sharma M, Sanborn RE, Cote GM, Bendell JC, Weiss GJ, et al. A phase I, first-in-human, open label, dose-escalation and cohort expansion study of MGD019, a bispecific DART protein binding PD-1 and CTLA-4 in patients with unresectable or metastatic neoplasms. Journal of Clinical Oncology 2019;37(15_suppl):TPS2661–TPS doi 10.1200/JCO.2019.37.15_suppl.TPS2661. [DOI] [Google Scholar]