Abstract
The use of Post-Transplant Cyclophosphamide (PTCy) as Graft Versus Host Disease (GVHD) prophylaxis has resulted in reductions in GVHD and improved outcomes in allogeneic hematopoietic cell transplant (HCT) using HLA-mismatched related donors. We report the 3-year outcomes of the first multi-center prospective clinical trial using PTCy in the setting of mismatched unrelated donor (MMUD) bone marrow HCT. The study enrolled 80 patients (Either myeloablative (MAC) (N=40) or reduced intensity conditioning (RIC) (N=40)) with the primary endpoint of 1-year overall survival (OS). The median follow-up for this report is 34 months (range 12–46) in RIC and 36 months (range 18–49) in MAC. Three-year OS and non-relapse mortality (NRM) were 70% and 15%, and 62% and 10% in the RIC and MAC strata, respectively. No GVHD was reported after 1 year. Relapse incidence was 29% and 51% in RIC and MAC strata. OS did not differ based on HLA match grade (63% in the 7/8 strata and 71% in the 4–6/8 strata). These encouraging outcomes, sustained 3 years post-HCT, support the continued exploration of MMUD HCT using a PTCy platform. Important future areas to address include relapse reduction and furthering our understanding of optimal donor selection based on HLA and non-HLA factors.
INTRODUCTION
Post-transplant cyclophosphamide (PTCy) as graft versus host disease (GVHD) prophylaxis to facilitate hematopoietic cell transplantation (HCT) across HLA barriers has become widely accepted1. Pioneered in the haploidentical related donor (haplo) setting, this approach now has established efficacy. While the use of haplo donors expands access to HCT for those without a matched donor available, there remain patients for whom a haplo donor is not available2 or where this may not be the best donor choice (e.g., presence of donor specific antibodies, familial disease syndromes or age of the donor)3,4. To meet this need, we investigated the use of PTCy in mismatched unrelated donor (MMUD) HCT in a phase II multicenter prospective trial. The trial met its primary endpoint of overall survival >65% at 1 year (1 year OS was 76%; 90% CI: 67.3–83.3)5. Here we report 3-year follow-up data.
METHODS
Patient eligibility was previously described5. The study enrolled 80 patients with hematological malignancies, who had no suitable matched donor available, at 11 United States (US) centers from 12/2016 to 3/2019. There were two non-randomized conditioning intensity strata, either reduced intensity (RIC) or myeloablative conditioning (MAC). Following conditioning, patients received a fresh bone marrow (BM) graft on day 0, PTCy on days +3, +4, and sirolimus/mycophenolate mofetil starting on day +5. The study was approved by the National Marrow Donor Program (NMDP) central institutional review board (IRB) (n=2) or the transplant center (TC) IRB (n=9) (NCT02793544). All patients provided written informed consent.
Three-year follow-up data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR) database. Data within one-year post-HCT were reconciled with data collected in the clinical trial. Relapse, non-relapse mortality (NRM), and chronic GVHD (cGVHD) were estimated by cumulative incidence function with death without relapse, relapse, and death without cGVHD as competing risks, respectively. Patients without the event were censored at date of second transplant or last contact. OS, progression-free survival, and GVHD-free, relapse-free survival (GRFS) were estimated using the Kaplan-Meier method6. Survival probabilities were calculated from the date of HCT to the date of death or last contact.
RESULTS AND DISCUSSION
Patient, donor and HCT characteristics are shown in Table 1. Considering HLA-A, B, C and DRB1 at high resolution, the HLA match grade was 7/8 in 61% and 4–6/8 in 39% of transplants (43% in RIC, 36% in MAC). 48% of patients were from a racial/ethnic minority group.
Table 1:
Baseline characteristics for clinical trial patients by conditioning intensity
| Characteristic | MAC | RIC | Total |
|---|---|---|---|
|
| |||
| No. of patients | 40 | 40 | 80 |
| No. of centers | 9 | 8 | 11 |
| HIV infection pre-HCT - no. (%) | |||
| No | 40 | 36 (90) | 76 (95) |
| Yes | 0 | 4 (10) | 4 (5) |
| Age at HCT, years - no. (%) | |||
| Median (min-max) | 48.5 (18–66) | 59.5 (23–70) | 51.5 (18–70) |
| 15–29 | 8 (20) | 3 (7.5) | 11 (13.8) |
| 30–49 | 13 (32.5) | 11 (27.5) | 24 (30) |
| 50–70 | 19 (47.5) | 26 (65) | 45 (56.3) |
| Sex - no. (%) | |||
| Male | 23 (58) | 19 (48) | 42 (53) |
| Female | 17 (43) | 21 (53) | 38 (48) |
| Race - no. (%) | |||
| American Indian or Alaska Native | 1 (3) | 0 | 1 (1) |
| Asian | 1 (3) | 1 (3) | 2 (3) |
| Black or African American | 9 (23) | 6 (15) | 15 (19) |
| White | 29 (73) | 31 (78) | 60 (75) |
| Not reported/unknown | 0 | 2 (5) | 2 (3) |
| Ethnicity - no. (%) | |||
| Hispanic or Latino | 12 (30) | 7 (18) | 19 (24) |
| Not Hispanic or Latino | 28 (70) | 33 (83) | 61 (76) |
| Race/ethnicity - no. (%) | |||
| White/non-Hispanic | 17 (43) | 25 (63) | 42 (53) |
| Others | 23 (58) | 15 (38) | 38 (48) |
| Karnofsky score - no. (%) | |||
| 70 | 2 (5) | 1 (3) | 3 (4) |
| 80 | 12 (30) | 12 (30) | 24 (30) |
| 90 | 21 (53) | 17 (43) | 38 (48) |
| 100 | 5 (13) | 10 (25) | 15 (19) |
| HCT-CI - no. (%) | |||
| 0 | 4 (10) | 9 (23) | 13 (16) |
| 1 | 2 (5) | 8 (20) | 10 (13) |
| 2 | 10 (25) | 4 (10) | 14 (18) |
| 3+ | 24 (60) | 19 (48) | 43 (54) |
| Disease status at HCT - no. (%) | |||
| AML | 23 (57.5) | 14 (35) | 37 (46.3) |
| CR1 | 22 | 10 | 32 |
| CR2+ | 1 | 2 | 3 |
| PIF | 0 | 2 | 2 |
| ALL | 10 (25) | 7 (17.5) | 17 (21.3) |
| CR1 | 7 | 6 | 13 |
| CR2+ | 3 | 1 | 4 |
| CLL | 0 | 3 (7.5) | 3 (3.8) |
| CR | 0 | 3 | 3 |
| MDS | 2 (5) | 0 | 2 (2.5) |
| CR | 1 | 0 | 1 |
| HI | 1 | 0 | 1 |
| Other acute leukemia | 4 (10) | 0 | 4 (5) |
| CR1 | 3 | 0 | 3 |
| CR2+ | 1 | 0 | 1 |
| NHL | 1 (2.5) | 11 (27.5) | 12 (15) |
| CR1 | 0 | 5 | 5 |
| CR2+ | 1 | 3 | 4 |
| Relapse | 0 | 2 | 2 |
| PIF | 0 | 1 | 1 |
| HL | 0 | 5 (12.5) | 5 (6.3) |
| CR1 | 0 | 2 | 2 |
| Relapse | 0 | 1 | 1 |
| PIF | 0 | 2 | 2 |
| Refined disease risk index - no. (%) | |||
| Low | 3 (8) | 6 (15) | 9 (11) |
| Intermediate | 29 (73) | 21 (53) | 50 (63) |
| High | 3 (8) | 7 (18) | 10 (13) |
| Very high | 0 | 3 (8) | 3 (4) |
| N/A | 5 (13) | 3 (8) | 8 (10) |
| CMV serostatus - no. (%) | |||
| Negative | 16 (40) | 18 (45) | 34 (43) |
| Positive | 24 (60) | 22 (55) | 46 (58) |
| Time between diagnosis to HCT - no. (%) | |||
| < 6 months | 14 (35) | 10 (25) | 24 (30) |
| >= 6 months | 26 (65) | 30 (75) | 56 (70) |
| Number of prior auto HCTs - no. (%) | |||
| 0 | 38 (95) | 37 (93) | 75 (94) |
| 1 | 2 (5) | 3 (8) | 5 (6) |
| Infused total nucleated cells, ×108/kg - median (min-max) | 2.81 (0.6–520.8) | 2.8 (0.76–5.8) | 2.8 (0.76–520.8) |
| Infused CD34+ cells, ×106/kg - median (min-max) | 2.72 (0.89–5.24) | 2.2 (0.39–6.23) | 2.66 (0.39–6.23) |
| Conditioning regimen - no. (%) | |||
| TBI/Cy/Flu | 0 | 40 | 40 (50) |
| Bu/Cy | 3 (8) | 0 | 3 (4) |
| Bu/Flu | 31 (78) | 0 | 31 (39) |
| TBI/Cy | 6 (15) | 0 | 6 (8) |
| HLA match - no. (%) | |||
| 7/8 | 26 (65) | 23 (58) | 49 (61) |
| 6/8 | 8 (20) | 11 (28) | 19 (24) |
| 5/8 | 5 (13) | 2 (5) | 7 (9) |
| 4/8 | 1 (3) | 4 (10) | 5 (6) |
| Donor age, years - no. (%) | |||
| Median (min-max) | 27 (18–56) | 29 (21–44) | 29 (18–56) |
| 18–29 | 24 (60) | 23 (58) | 47 (59) |
| 30–39 | 9 (23) | 11 (28) | 20 (25) |
| 40–49 | 4 (10) | 6 (15) | 10 (13) |
| 50–59 | 3 (8) | 0 | 3 (4) |
| Donor weight, kg - median (min-max) | 77 (55–103) | 77 (52–104) | 77 (52–104) |
| Donor sex - no. (%) | |||
| Male | 20 (50) | 24 (60) | 44 (55) |
| Female | 20 (50) | 16 (40) | 36 (45) |
| Donor/recipient sex - no. (%) | |||
| M-M | 12 (30) | 12 (30) | 24 (30) |
| M-F | 8 (20) | 12 (30) | 20 (25) |
| F-M | 11 (28) | 7 (18) | 18 (23) |
| F-F | 9 (23) | 9 (23) | 18 (23) |
| Donor/recipient CMV serostatus - no. (%) | |||
| +/+ | 16 (40) | 13 (33) | 29 (36) |
| +/− | 8 (20) | 7 (18) | 15 (19) |
| −/+ | 8 (20) | 9 (23) | 17 (21) |
| −/− | 8 (20) | 11 (28) | 19 (24) |
| Donor/recipient ABO match - no. (%) | |||
| Matched | 20 (50) | 24 (60) | 44 (55) |
| Minor mis-match | 12 (30) | 5 (13) | 17 (21) |
| Major mis-match | 8 (20) | 8 (20) | 16 (20) |
| Bi-directional |
0 | 3 (8) | 3 (4) |
Survival and toxicity
The median follow-up (data lock: September 2021) was 34 months (range 12–46) in RIC and 36 months (range 18–49) in MAC, with OS at 3 years of 70% in the RIC strata and 62% in the MAC strata (Figure 1a). Table 2 shows all outcomes at 3 years. The incidence of GVHD was low at one year (particularly in the RIC strata5), and notably no additional cases of GVHD of any type or grade were reported after 1 year, likely contributing to the NRM of less than 15% seen across all patients at 3 years (Figure 1b).
Figure 1:
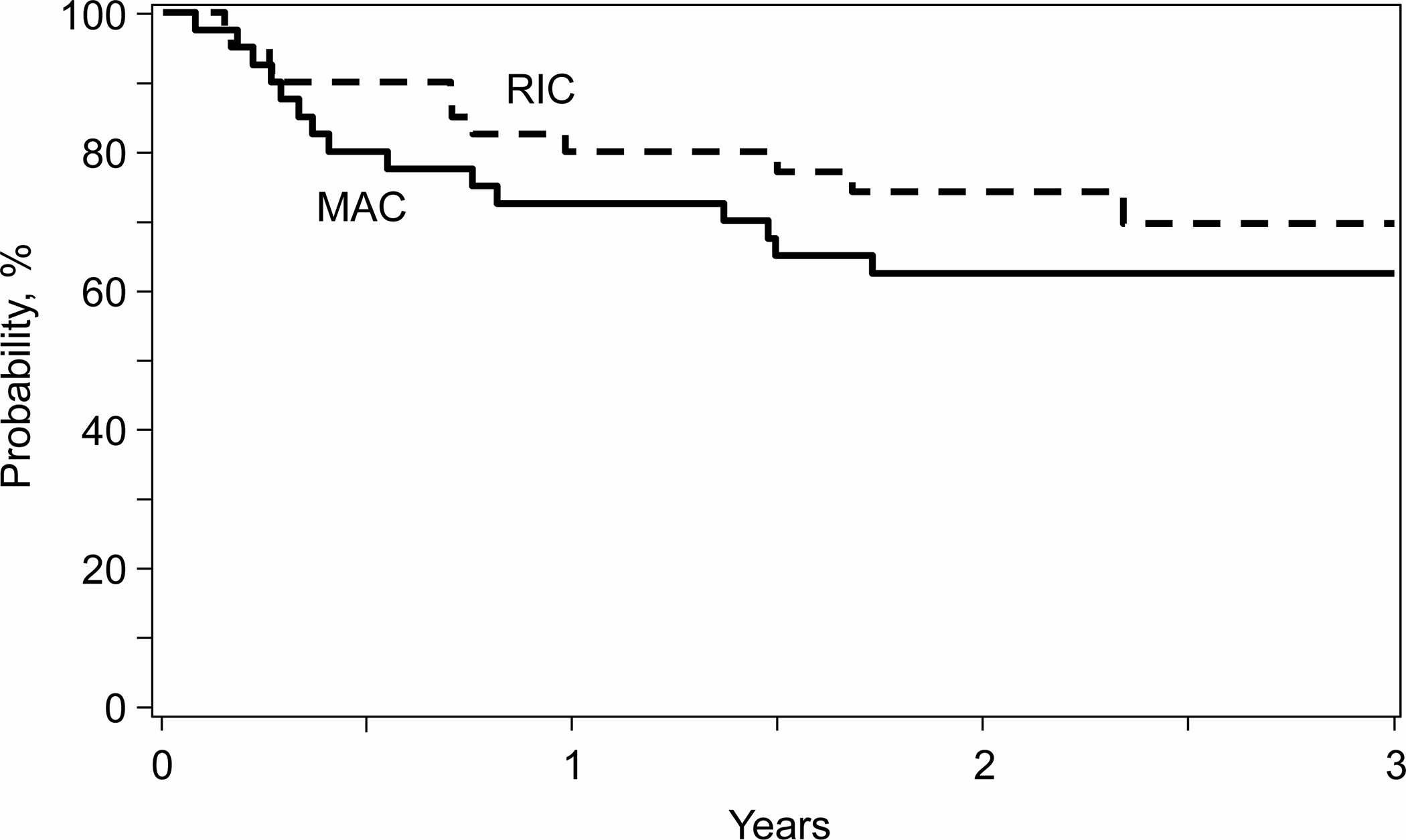
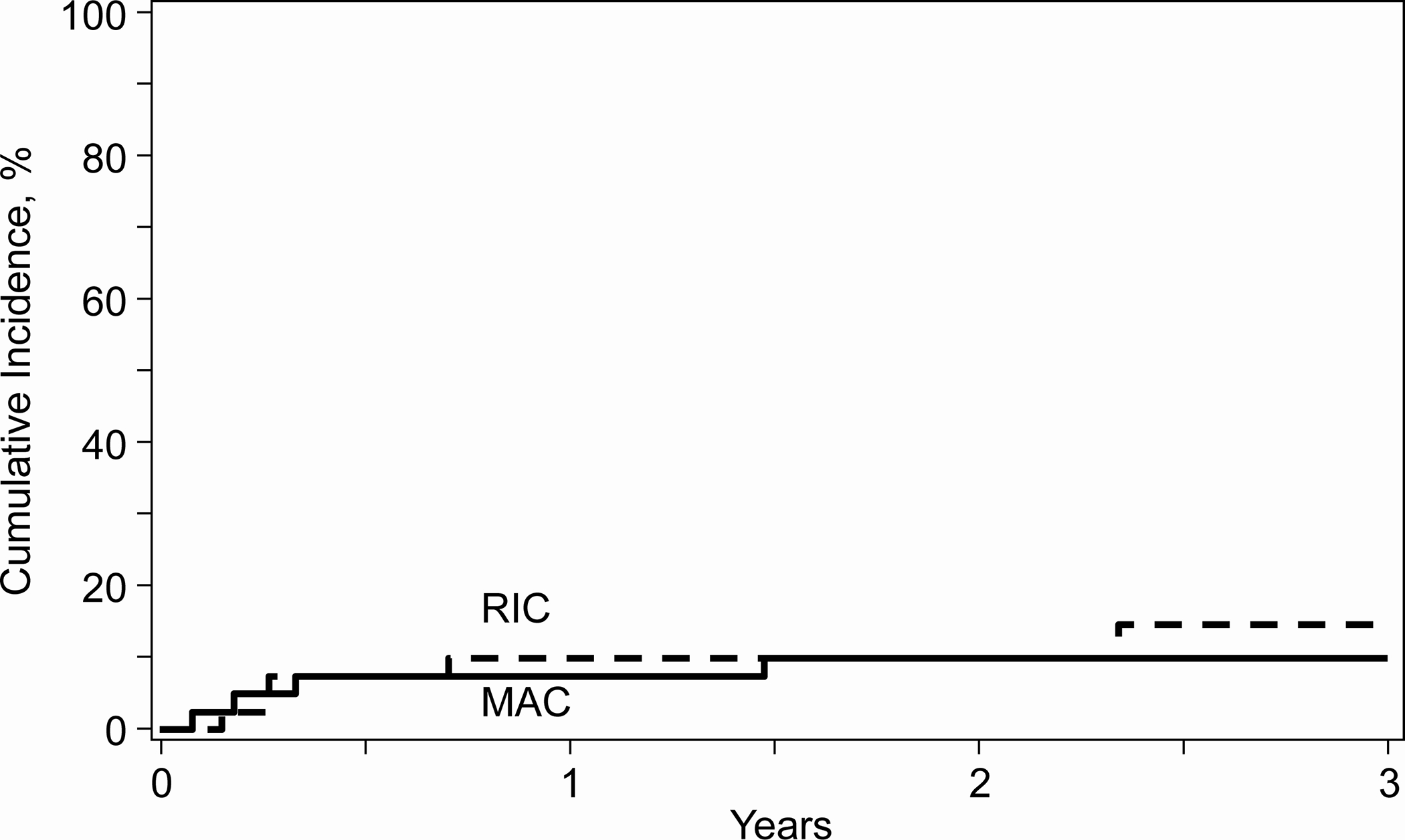
a. 3-year overall survival by conditioning intensity, b. 3-year non-relapse mortality by conditioning intensity. RIC: reduced intensity conditioning, MAC: Myeloablative conditioning
Table 2:
3-year univariate outcomes for clinical trial patients by conditioning intensity
| MAC (N = 40) | RIC (N = 40) | |||
|---|---|---|---|---|
| Outcomes | N | Prob (90% CI) | N | Prob (90% CI) |
|
| ||||
| Overall survival | 40 | 40 | ||
| 1-year | 29 | 72.5 (60.3–83.2)% | 29 | 79.9 (68.6–89.2)% |
| 3-year | 11 | 62.4 (49.5–74.5)% | 11 | 69.6 (55.8–81.8)% |
| Non-relapse mortality | 40 | 40 | ||
| 1-year | 24 | 7.5 (2.1–15.8)% | 26 | 10 (3.6–19.2)% |
| 3-year | 9 | 10 (3.5–19.2)% | 9 | 14.7 (5.7–26.9)% |
| Relapse | 40 | 40 | ||
| 1-year | 24 | 35 (23-48)% | 26 | 20 (10.6–31.5)% |
| 3-year | 9 | 50.5 (36.3–64.7)% | 9 | 29.4 (17.7–42.7)% |
| Progression-free survival | 40 | 40 | ||
| 1-year | 23 | 57.5 (44.5–70)% | 25 | 70 (57.5–81.1)% |
| 3-year | 8 | 39. 5 (26.4–53.4)% | 8 | 55.9 (41.6–69.8)% |
| Chronic GVHD | 40 | 40 | ||
| 1-year | 15 | 37.5 (25.2–50.6)% | 24 | 20 (10.6–31.5)% |
| 3-year | 6 | 37.5 (25.2–50.6)% | 6 | 20 (10.6–31.5)% |
| Severe Chronic GVHD | 40 | 40 | ||
| 1-year | 25 | 12.5 (5.2–22.4)% | 29 | 5 (0.9–12.2)% |
| 3-year | 8 | 12.5 (5.2–22.4)% | 12 | 5 (0.9–12.2)% |
| GVHD-/relapse-free survival (GRFs) | 40 | 40 | ||
| 1-year | 10 | 25 (14.7–37)% | 19 | 55 (42–67.6)% |
| 3-year | 3 | 16.9 (8.2–27.8)% | 5 | 44.3 (30.6–58.5)% |
Relapse
The relapse rate in the RIC strata of 29% at 3 years (23% at 1 year) is similar to published reports of PTCy-based HCT7. Conversely, we report a high rate of relapse of 51% at 3 years (30% at 1 year) in the MAC strata. This stratum had a predominance of patients with acute myeloid leukemia (AML) and >90% of these had an intermediate or high disease risk index (DRI) at HCT, which may be one explanation for the higher incidence in this cohort. The median time to relapse in the MAC patients (N=19) was 7.57 months (range: 1.84–30.33), with those relapsing within 6 months of HCT having a short survival (N=9, 2.14 months (range: 0.69–10.72)). Of note, eight of the 10 patients who relapsed >6 months post-HCT, all with acute leukemia, remain alive at last follow-up (median follow-up post relapse is 11.68 months (range: 3.32–37.17) (Supplemental Table 1). Only one patient received additional cells (donor lymphocytes). Five of the eight patients had GVHD, the onset of which occurred prior to the relapse. These findings are provocative and warrant further investigation, including into the mechanism of relapse and potential differences between donor types8. Besides low numbers, an important limitation of the study is that pre-HCT measurable residual disease (MRD) data were not routinely collected. Another potential contributor to higher relapse is that only BM was allowed as a graft source in this study. While data are not yet available specific to the MMUD setting, we can extrapolate from a recent meta-analysis including several thousand patients receiving a haplo-HCT with PTCy, where a 16% reduction in relapse risk (HR 0.84; p =0.001) was reported with the use of peripheral blood stem cell (PBSC) grafts compared with BM grafts9. Although the meta-analysis also reported a higher rate of GVHD with PBSC compared to BM, the overall low rates of severe cGVHD and lack of late onset cases observed in our study, suggest that a slight increase in GVHD associated with PBSC may be justifiable to allow better disease control long term. In fact, the field has already moved decisively in this direction, with CIBMTR data showing that currently 90% of MMUD transplants using PTCy (as standard of care) are performed using PBSC (data not shown). NMDP/CIBMTR is currently testing the approach of using PBSC prospectively in a study (ACCESS, NCT04904588).
HLA match grade
An open question in the field is the importance of the degree of HLA mismatching in the PTCy setting. Emerging data in the haplo setting suggest that assessing qualitative rather than quantitative effects may be more relevant10. A study of 1434 recipients of haplo HCT showed that the total number of HLA mismatches was not significantly associated with outcomes (confirming previous studies)11,12 rather that individual loci (mis)matches were associated with better outcomes. Specifically, mismatches in the GVHD direction at HLA-DRB1 were associated with decreased relapse (and better DFS when in conjunction with a match at DQB1), and HLA-B leader matching and HLA-DPB1 TCE-nonpermissive mismatching were each associated with improved overall survival13. Although our study was underpowered to detect a true difference related to match grade, as well as to assess mismatches at individual loci, we did not observe a difference in survival dependent on HLA match grade (63% in the 7/8 strata and 71% in the 4–6/8 strata, p=0.733) (Supplemental Figure 1). The incidence of cGVHD was, in fact, higher in the better matched patients (37% vs. 16%), as were the relapse rates (44% vs. 33%). We also analyzed the impact of HLA-DPB1 matching and found no significant differences in any outcome (data not shown). In our study, match grade was equivalent between the strata, suggesting no specific selection bias by conditioning intensity (although donor selection bias by transplant center was noted, data not shown).
This report is limited by the small numbers included in the phase II clinical trial, the registry level data included in the long term follow up (vs. clinical trial intensity/frequency of data) and the exploratory and descriptive nature of some endpoints.
In conclusion, these encouraging longer term outcomes support the use of PTCy-based MMUD HCT to expand access to HCT. In fact, NMDP data already show a 25% global increase in MMUD transplants in 2021–2022 (S. Devine, personal communication), and emerging data suggested outcomes using MMUDs may be superior to haplo in some settings14. Future research should focus on relapse reduction and early relapse detection strategies, with biological correlates thoughtfully included in prospective clinical trials15, as well as improving the precision of donor selection in the HLA mismatched setting.
Supplementary Material
Highlights.
PTCy prophylaxis is associated with encouraging 3-year outcomes in MMUD HCT
No new onset GVHD was reported beyond 1 year
Outcomes were not worse in vases with a higher degree of HLA mismatch
ACKNOWLEDGEMENTS
Supported by The National Marrow Donor Program/Be the Match, the Be the Match Foundation, and the Center for International Blood and Marrow Transplant Research (CIBMTR), which is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research; CIBMTR is also supported by the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Anthem; Astellas Pharma US; Atara Biotherapeutics; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Tscan; Vertex; Xenikos BV.
Footnotes
CONFLICTS OF INTEREST
Dr. Perales reports honoraria from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis.
Dr. Shah reports participation on advisory boards and/or consultancy for Kite Pharma, BMS, TG therapeutics, Miltenyi Biotec, Lilly Oncology, Epizyme, Incyte, Novartis, Seattle Genetics, and Umoja. He has research funding and honoraria from Miltenyi Biotec. In addition, Nirav Shah is on a scientific advisory board for Tundra Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. 2021.
- 2.Kosuri S, Wolff T, Devlin SM, et al. Prospective Evaluation of Unrelated Donor Cord Blood and Haploidentical Donor Access Reveals Graft Availability Varies by Patient Ancestry: Practical Implications for Donor Selection. Biol Blood Marrow Transplant 2017;23(6):965–970. DOI: 10.1016/j.bbmt.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation 2009;88(8):1019–24. DOI: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw BE. Related haploidentical donors are a better choice than matched unrelated donors: Counterpoint. Blood Adv 2017;1(6):401–406. DOI: 10.1182/bloodadvances.2016002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 2021;39(18):1971–1982. DOI: 10.1200/JCO.20.03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–480. [Google Scholar]
- 7.Gooptu M, Romee R, St Martin A, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood 2021;138(3):273–282. DOI: 10.1182/blood.2021011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vago L Clonal evolution and immune evasion in posttransplantation relapses. Hematology Am Soc Hematol Educ Program 2019;2019(1):610–616. DOI: 10.1182/hematology.2019000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcuri LJ, Hamerschlak N, Rocha V, Bonfim C, Kerbauy MN. Outcomes after Haploidentical Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide: A Systematic Review and Meta-Analysis Comparing Myeloablative with Reduced-Intensity Conditioning Regimens and Bone Marrow with Peripheral Blood Stem Cell Grafts. Transplant Cell Ther 2021;27(9):782 e1–782 e7. DOI: 10.1016/j.jtct.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Fleischhauer K Haplo-PtCy: adjusting the HLA barrier. Blood 2022;139(10):1431–1433. DOI: 10.1182/blood.2021014532. [DOI] [PubMed] [Google Scholar]
- 11.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant 2010;16(4):482–9. DOI: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon SR, Aubrey MT, Zhang X, et al. Class II HLA mismatch improves outcomes following haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv 2020;4(20):5311–5321. DOI: 10.1182/bloodadvances.2020003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs EJ, McCurdy SR, Solomon SR, et al. HLA informs risk predictions after haploidentical stem cell transplantation with posttransplantation cyclophosphamide. Blood 2022;139(10):1452–1468. DOI: 10.1182/blood.2021013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battipaglia G, Galimard JE, Labopin M, et al. Post-transplant cyclophosphamide in one-antigen mismatched unrelated donor transplantation versus haploidentical transplantation in acute myeloid leukemia: a study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 2022;57(4):562–571. DOI: 10.1038/s41409-022-01577-x. [DOI] [PubMed] [Google Scholar]
- 15.McCurdy SR, Radojcic V, Tsai HL, et al. Signatures of GVHD and relapse after posttransplant cyclophosphamide revealed by immune profiling and machine learning. Blood 2022;139(4):608–623. DOI: 10.1182/blood.2021013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


