Abstract
Background:
There are bi-directional relationships between sleep disturbances and obesity, both of which are prevalent in patients with heart failure with preserved ejection fraction (HFpEF). However, little is known about the sleep-obesity association in HFpEF.
Objectives:
To determine associations of multidimensional sleep health, night movement, sleep fragmentation, and sleep-disordered breathing (SDB) risk with overall and regional adiposity in HFpEF patients.
Methods:
Men and women with HFpEF (n = 49) were assessed via 14-day actigraphy, Pittsburgh Sleep Quality Index, and Epworth Sleepiness Scale to derive multidimensional sleep health. SDB risk was assessed via Berlin Questionnaire. Body composition was measured using anthropometry; MRI quantification of epicardial, abdominal, liver, and thigh adipose tissue was performed in a subsample (n = 22). Spearman correlation (rs) and linear regression analyses (b coefficient) were used to estimate bivariate and age-adjusted associations.
Results:
Multidimensional sleep health was inversely associated with BMI (rs = −0.50, p < .001; unadjusted: β = −4.00, 95%CI: −5.87, −2.13; age-adjusted: β = −2.48, 95%CI: −4.65, −0.30), thigh subcutaneous adipose tissue (rs = −0.50, p = .018; unadjusted: β = −36.95, 95%CI: −67.31, −6.59), and thigh intermuscular fat (age-adjusted: β = −0.24, 95%CI: −0.48, −0.01). Night movement and sleep fragmentation were associated with greater intermuscular thigh and lower liver fat. High SDB risk was associated with a higher visceral-to-subcutaneous ratio of abdominal adiposity and lower thigh adiposity.
Conclusions:
Adverse multidimensional sleep health is associated with higher adiposity measures in HFpEF patients. Further studies are needed to determine whether intervening on sleep could ameliorate excess adiposity or whether weight loss could improve sleep quality in HFpEF.
Keywords: Multidimensional sleep health, Obesity, Adiposity, Heart failure with preserved ejection fraction
Introduction
Approximately half of the six million adults with heart failure (HF) in the United States have HF with preserved ejection fraction (HFpEF),1 a condition with a 30-day readmission rate of 24%2 and a 5-year mortality rate of 75%.3 The prevalence of HFpEF is increasing due to an aging population, improved survival rates following cardiac events, and coinciding epidemics of diabetes mellitus, hypertension, and obesity.1 With a projected annual cost of $69.8 billion for HF care in the United States in 2030,4 significant efforts are being made to reduce HF risk and improve HF management. This is particularly true for HFpEF, which has few effective therapies at this time for reducing mortality.5,6
Obesity is an independent risk factor for HF and may be more influential in the development of HFpEF than HF with reduced ejection fraction (HFrEF).7 Additionally, individuals with obesity of class II or higher (body mass index ≥ 35 kg/m2) may have a distinct obese-HFpEF pheno-type with unique pathophysiology.8 Intentional weight loss in HFpEF patients has been associated with improved New York Heart Association (NYHA) functional class, decreased left ventricle mass and wall thickness, improved diastolic function, and reduced number of HF hospitalizations.9–11 Thus, weight loss may be an important approach to improve prognosis among HFpEF patients with obesity.
Numerous sleep disturbances, such as insomnia, short sleep duration, and sleep-disordered breathing (SDB), are increasingly recognized as contributors to and/or consequences of obesity.12 Approximately 23–73% of HF patients report insomnia symptoms (e.g., poor sleep quality or continuity)13 and 55–82% of HFpEF patients are diagnosed with SDB.14
Despite the growing recognition that obesity may play an important role in HFpEF pathophysiology and that sleep disturbances are prevalent in HFpEF, there is a dearth of research on the sleep-adiposity association in HF. To our knowledge, only one study has examined the association between sleep and adiposity in HF, finding a positive association between SDB and epicardial adipose tissue in HFrEF patients.15 There is a need to elucidate the relationship between sleep and adiposity, particularly regional adiposity, among individuals with HFpEF given the strong association between obesity and this HF phenotype. Such information may serve to identify novel intervention targets in HFpEF patients with the potential to improve the rates of morbidity and mortality. Thus, the primary aim of this study was to determine the associations of multidimensional sleep health with overall and regional adiposity among patients with stable HFpEF. Our secondary aims were to determine associations of additional measures of sleep (i.e., night movement index, sleep fragmentation index, and SDB risk) with overall and regional adiposity, as these sleep disturbances are not captured by the multidimensional sleep score. We hypothesized that poorer sleep (i.e., lower multidimensional sleep health, greater night movement, greater sleep fragmentation, and high SDB risk) would be associated with greater overall and regional adiposity.
Methods
Study design, setting, and participants
Participants for this study were recruited from a cohort enrolled in a prospective American Heart Association Go Red for Women Strategically Focused Research Network study on HFpEF at Johns Hopkins University and Northwestern University. Though sponsored by Go Red for Women, this study did include male participants. Participants of the parent study met the following inclusion criteria:≥ 21 years of age, negative pregnancy test in individuals of childbearing potential, left ventricular ejection fraction (LVEF) ≥ 45% within the prior 6 months on echocardiography, treatment with diuretics for ≥30 days prior to enrollment, and HF symptoms at time of enrollment (i.e., NYHA class II-IV). Participants were excluded from the parent study if they met any of the following criteria: LVEF < 45% on any prior echocardiogram; systolic blood pressure < 100 mmHg; hemoglobin < 9.0 g/dL; estimated glomerular filtration rate < 30 mL/min/1.73 m2; hemodynamically significant arrhythmias within the prior 4 weeks; history of acute coronary syndrome, cardiac surgery, or percutaneous coronary intervention within the prior 3 months; any of the following cardiac diagnoses: active myocarditis, hyper-trophic cardiomyopathy, restrictive cardiomyopathy (including amyloid, sarcoid, or hemochromatosis), moderate or greater regurgitant valvular disease, any valvular stenosis, constrictive pericarditis, complex congenital heart disease, or pulmonary arterial hypertension; current use of IV inotropic medication or mechanical circulatory support; currently hospitalized for HF; inability to adhere to the planned study procedures; or currently pregnant or nursing. All participants enrolled in the parent study were approached for participation in the sleep and magnetic resonance imaging (MRI) ancillary studies.
Of the 157 enrolled in the parent study, 49 consented to the sleep ancillary study where actigraphy and sleep questionnaire assessments were administered, 22 of whom agreed to undergo the MRI scan (Fig. 1). The institutional review boards of Johns Hopkins and Northwestern Universities approved this study and provided ethical oversight. All participants provided written informed consent.
Fig. 1.
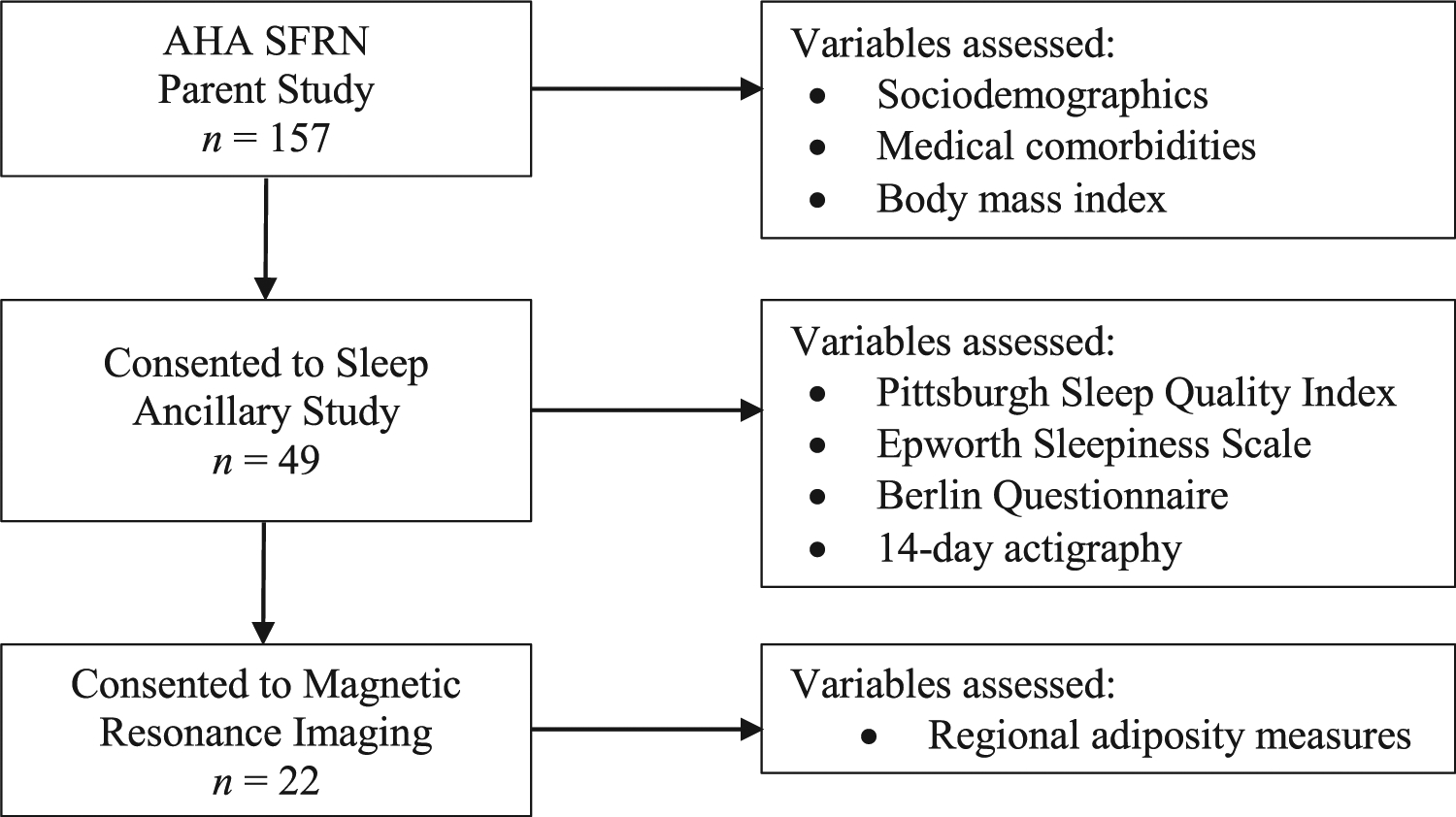
Analytic sample flowchart and variable assessments by study portion.
Participants in the present study included those from the parent study that further consented to completion of the sleep ancillary study (including sleep questionnaires and actigraphy), and magnetic resonance imaging scan. AHA SFRN = American Heart Association Strategically Focused Research Network.
Measures and procedures
As part of the parent study, participants completed questionnaires for sociodemographics, medical comorbidities, and functional capacity; performed a 6 min walk test, resting echocardiograms, and bicycle stress echocardiograms. History of tobacco use, medications, and medical comorbidities, including hypertension, diabetes, coronary artery disease, and atrial fibrillation were abstracted from clinical documentation and verified with patients at the initial study visit. As part of the sleep ancillary study, participants completed sleep-related questionnaires and, for those consenting to actigraphy, were provided with, and instructed on wearing an accelerometer while simultaneously completing sleep diaries.
Sleep variables
Sleep was assessed objectively using an accelerometer (Actigraph GT3X) worn on the non-dominant wrist with concurrent completion of a sleep diary over a 14-day period. Actigraphy data were scored in one-minute epochs with bedtime and waketimes provided by sleep diaries. Sleep was also assessed subjectively using the Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale, and Berlin Questionnaire, which span the measurement of sleep quality, excessive daytime sleepiness, and risk of SDB, respectively.
Multidimensional sleep health is based on the Ru-SATED frame-work, which includes six domains of sleep health: sleep timing, regularity of sleep timing, sleep quality, alertness, sleep efficiency, and sleep duration.16 We computed the sleep health score using both objective and subjective assessments. Sleep timing and regularity were derived from actigraphy data and defined as the mean sleep midpoint and the standard deviation from the sleep midpoint, respectively. Sleep quality was derived from the Pittsburgh Sleep Quality Index global score. Alertness was derived from the Epworth Sleepiness Scale total score. Self-report global/total scores were chosen over potential actigraphy-derived sleep parameters as these measures are widely accepted for their respective sleep domains and more accurately capture the subjective experience of sleep quality and daytime alertness, respectively. Sleep efficiency was derived from actigraphy data and calculated as the total sleep time divided by total time in bed multiplied by 100. Sleep duration was derived from actigraphy data and was defined by the total sleep time in minutes. Actigraphy-derived definitions reflect the conventionally used or empirically derived cut-offs for each domain.17 Each domain was categorized (Poor = 0, Good = 1) using the definitions displayed in Table 2. Scores were then summed across domains to calculate the multidimensional sleep health score. Scores range from 0 to 6, with higher scores representing better sleep.
Table 2.
Multidimensional sleep health definitions and frequencies.
| Domain | Assessment | Definition | Good, n (%) | Poor, n (%) |
|---|---|---|---|---|
| Sleep timing | Actigraphy | Good: average sleep midpoint before 4:00AM Poor: average sleep midpoint after 4:00AM |
41 (83.7) | 8 (16.3) |
| Regularity of sleep timing | Actigraphy | Good: sleep midpoint standard deviation <65 min Poor: sleep midpoint standard deviation ≥65 min |
27 (55.1) | 22 (44.9) |
| Sleep quality | PSQI | Good: global score ≤5 Poor: global score >5 |
12 (24.5) | 37 (75.5) |
| Alertness | ESS | Good: total score ≤10 Poor: total score >10 |
33 (67.4) | 16 (32.7) |
| Sleep efficiency | Actigraphy | Good: ≥85% Poor: <85% |
20 (40.8) | 29 (59.2) |
| Sleep duration | Actigraphy | Good: total sleep time ≥420 min (7 h) Poor: total sleep time <420 min |
11 (22.5) | 38 (77.6) |
Note. Data presented as frequency (percentage).
ESS=Epworth Sleepiness Scale; PSQI=Pittsburgh Sleep Quality Index.
The night movement index and sleep fragmentation index were derived from actigraphy data and modeled as continuous variables. The night movement index is an indicator of movement during time in bed and the sleep fragmentation index is an indicator of nocturnal awakenings during sleep. SDB risk was derived from the Berlin Questionnaire, which categorizes individuals into high or low risk based on three categories of sleep apnea risk, including severity of snoring, excessive daytime sleepiness, and history of either hypertension or obesity. In addition to SDB risk, we assessed self-reported obstructive sleep apnea (OSA) diagnosis, possession of a continuous positive airway pressure (CPAP) machine, and adherence to CPAP as defined by the US Center for Medicare and Medicaid Services criteria of ≥70% of nights with ≥4 h/night of CPAP use. Given that OSA is under-diagnosed,18 we include SDB risk defined by the Berlin Questionnaire in statistical analyses as this assessment will capture those with known OSA and potential unknown cases.
Adiposity variables
Overall adiposity was determined at the first study visit of the parent study by body mass index (BMI) calculated as weight in kg divided by height in m2. In the subset that underwent MRI, participants were scanned in the supine position using 1.5-Tesla whole body MRI systems (Espree or Aera, Siemens, Erlangen, Germany). For the determination of regional adiposity, measurements were taken of the following fat depots: epicardial (right ventricular epicardial adipose tissue [RV EAT], atrioventricular groove epicardial adipose tissue [AV groove EAT]), abdominal (abdominal visceral adipose tissue [VAT], abdominal subcutaneous adipose tissue [SAT], and abdominal VAT/SAT ratio), liver (liver fat fraction [FF]), and thigh (thigh SAT, thigh intermuscular adipose tissue [IMAT], thigh IMAT FF, thigh intermuscular fat [interMF], thigh intramuscular fat [intraMF]) regions using a custom-made MATLAB tool. Thigh adipose tissue regions were quantified using a 3D multi-echo Dixon acquisition in trans-verse orientation superior to the inferior head of the femur as previously described. Another custom-made MATLAB tool was used to determine thigh SAT, thigh IMAT, thigh IMAT FF, thigh interMF, and thigh intraMF.
MRI protocol detail, blinded analysis, and representative images have been previously published.19 Briefly, steady-state free precession CINE imaging techniques were used for cardiac adipose tissue quantification. Tissue thickness (EAT) was quantified during end-diastole (OsiriX Version 9.0, Pixmeo SARL, Geneva, Switzerland). Abdominal adipose tissue images were taken as axial sections every 3 cm from T10 to the sacral vertebra. Abdominal SAT and VAT were determined using a single slice at the L4 level. A custom-made MATLAB tool (MathWorks, Natick, MA, USA) using a semi-automated algorithm was used to segment SAT and VAT compartments of abdominal adiposity. For liver fat quantification, MRI-based proton-density fat fraction (PDFF) was used as this technique has been shown to be an accurate method with high reproducibility and intra/interobserver agreement.20,21 Regions of interest (ROIs) measuring 5cm2 were drawn over the liver in the MRI-PDFF maps on 3 separate axial slices and averaged, avoiding blood vessels, the gallbladder, or other discrete lesions (Fig. 2). PDFF is expressed as a percentage as a measure of degree of liver steatosis.22,23
Fig. 2.
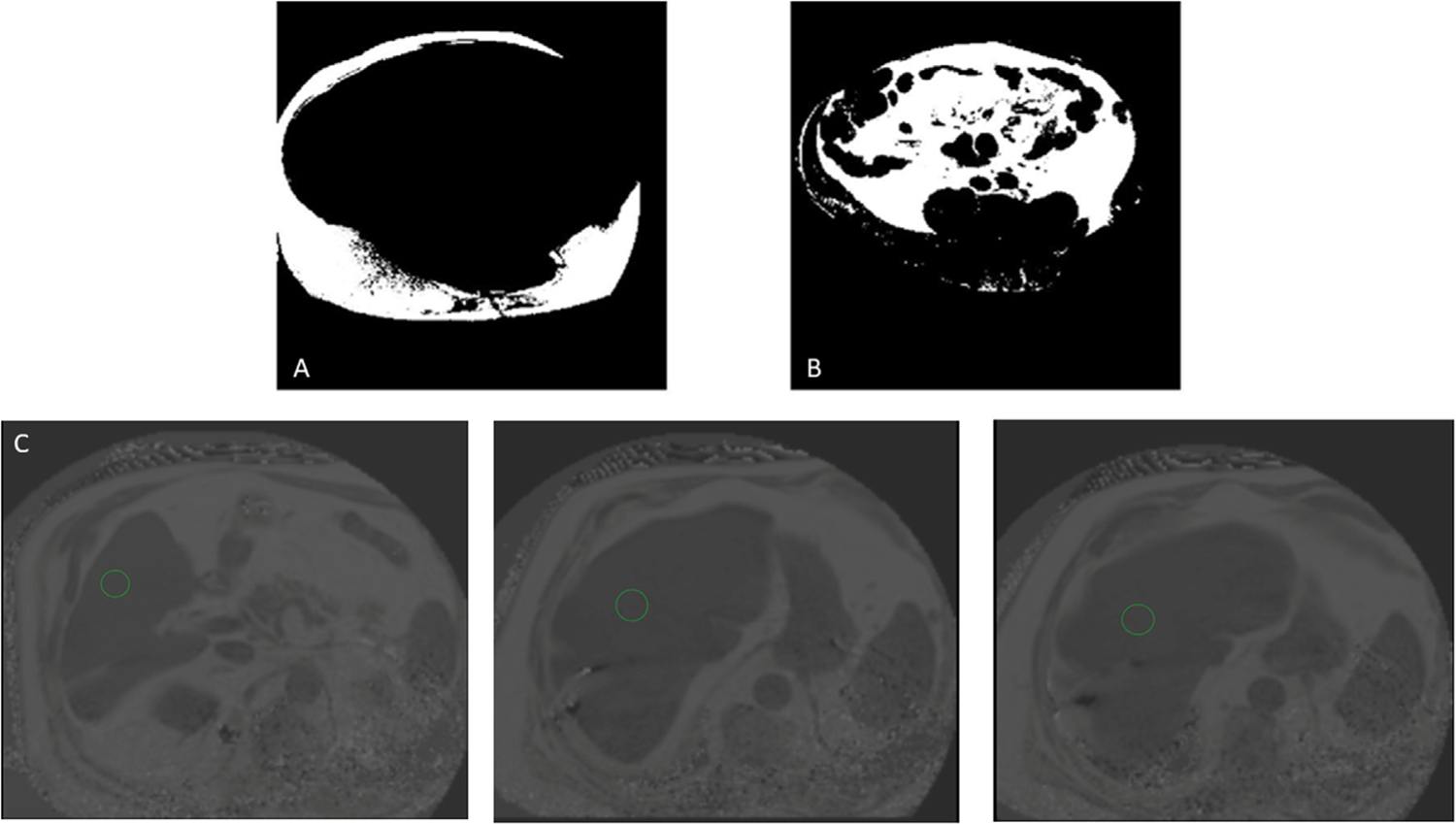
Magnetic resonance imaging of abdominal and liver adiposity in a participant with HFpEF.
Abdominal fat was sectioned into its subcutaneous (A) and visceral (B) components using a semi-automated MATLAB tool. (C) Liver fat fraction was estimated by averaging the magnetic resonance imaging proton-density fat fraction across three axial slices for the region of interest circled in green. HFpEF = heart failure with preserved ejection fraction.
Statistical analysis
Participant characteristics were summarized as mean (standard deviation) or median (interquartile range) for continuous variables and frequency (percentage) for categorical variables. Spearman correlation and linear regression analyses were performed to examine the associations of the four sleep variables (multidimensional sleep health, night movement index, sleep fragmentation index, and high SDB risk) with overall and regional adiposity variables with statistical significance set at p < .05. For the linear regression analyses, we constructed two models (unadjusted and age-adjusted) for each sleep (independent variable) and adiposity (dependent variable) measure pair. Our small sample size limited us from performing analyses stratified by or further adjusted for sex. The following variables were log-transformed due to skew and were presented as exponentiated β coefficients and 95% confidence intervals (CI) in the Results section and Tables 3 and 4: night movement index, sleep fragmentation index, RV EAT, AV groove EAT, liver FF, abdominal VAT, thigh IMAT, and thigh interMF. We present β coefficients and 95% CIs in log-units in the Supplemental Tables 1 and 2. Statistical significance for 95% CIs was set to exclude 0 for non-exponentiated variables and 1.00 for exponentiated variables. All analyses were performed using SAS v 9.4 (SAS Institute, Cary, NC).
Table 3.
Correlation and linear regression analyses examining associations of multidimensional sleep health with overall and regional adiposity.
| Correlation | Unadjusted | Age-Adjusted | ||||
|---|---|---|---|---|---|---|
| r s | p-value | β | 95% CI | β | 95% CI | |
| BMI, kg/m2 | −0.50 | <0.001 | −4.00 | −5.87, −2.13 | −2.48 | −4.65, −0.30 |
| RV EAT, mm | −0.23 | .300 | 0.96 | 0.84, 1.10 | 0.89 | 0.76, 1.05 |
| AV groove EAT, mm | −0.36 | .105 | 0.93 | 0.85, 1.02 | 0.92 | 0.82, 1.03 |
| Abdominal VAT, cm2 | −0.31 | .172 | 0.89 | 0.77, 1.04 | 0.93 | 0.77, 1.13 |
| Abdominal SAT, cm2 | −0.32 | .174 | −42.43 | −97.55, 12.70 | 23.32 | −26.52, 73.15 |
| Abdominal VAT/SAT ratio | 0.09 | .709 | 0.01 | −0.05, 0.07 | −0.04 | −0.11, 0.03 |
| Liver FF,% | −0.25 | .275 | 0.87 | 0.74, 1.04 | 0.89 | 0.72, 1.09 |
| Thigh SAT, cm2 | −0.50 | .018 | −36.95 | −67.31, −6.59 | −20.79 | −55.83, 14.24 |
| Thigh IMAT, cm2 | −0.25 | .287 | 0.94 | 0.74, 1.20 | 1.08 | 0.81, 1.43 |
| Thigh IMAT FF,% | 0.08 | .728 | 0.97 | −2.52, 4.46 | 1.40 | −2.88, 5.68 |
| Thigh interMF, cm2 | −0.24 | .381 | 0.93 | 0.78, 1.12 | 0.78 | 0.62, 0.99 |
| Thigh intraMF, cm2 | −0.36 | .129 | −2.32 | −4.81, 0.18 | −1.83 | −5.14, 1.48 |
Note. Results for the following variables were log-transformed and are presented as exponentiated beta coefficients and 95% CIs: RV EAT, AV groove EAT, abdominal VAT, liver FF, thigh IMAT, and thigh interMF. Bold indicates statistical significance using a threshold of 0 for 95% CIs of non-exponentiated variables and 1.00 for 95% CIs of exponentiated variables.
rs =Spearman’s rho coefficient; β =beta coefficient; AV=atrioventricular; BMI=body mass index; CI=confidence interval; EAT=epicardial adipose tissue; FF=fat fraction; IMAT=intermuscular adipose tissue; interMF=intermuscular fat; intraMF=intramuscular fat; RV=right ventricular; SAT=subcutaneous adipose tissue; VAT=visceral adipose tissue.
Table 4.
Correlation and linear regression analyses examining associations of night movement index, sleep fragmentation index, and high SDB risk with overall and regional adiposity.
| Correlation | Unadjusted | Age-Adjusted | ||||
|---|---|---|---|---|---|---|
| r s | p-value | β | 95% CI | β | 95% CI | |
| Night movementindex | ||||||
| BMI, kg/m2 | 0.25 | .087 | 5.67 | 0.07, 11.26 | 2.33 | −2.89, 7.55 |
| RV EAT, mm | −0.21 | .358 | 0.77 | 0.54, 1.11 | 0.79 | 0.53, 1.17 |
| AV groove EAT, mm | −0.01 | .953 | 1.01 | 0.79, 1.30 | 0.98 | 0.75, 1.28 |
| Abdominal VAT, cm2 | 0.01 | .978 | 1.05 | 0.66, 1.66 | 0.89 | 0.55, 1.45 |
| Abdominal SAT, cm2 | 0.20 | .398 | 111.64 | −44.50, 267.77 | −1.83 | −127.86, 124.20 |
| Abdominal VAT/SAT ratio | −0.34 | .139 | −0.09 | −0.26, 0.07 | −0.03 | −0.19, 0.14 |
| Liver FF,% | −0.35 | .116 | 0.67 | 0.42, 1.06 | 0.53 | 0.34, 0.84 |
| Thigh SAT, cm2 | 0.23 | .299 | 47.32 | −44.57, 139.21 | −0.97 | −91.74, 89.81 |
| Thigh IMAT, cm2 | 0.39 | .094 | 1.64 | 0.87, 3.11 | 1.40 | 0.70, 2.81 |
| Thigh IMAT FF,% | 0.09 | .708 | 3.92 | −5.63, 13.47 | 5.01 | −5.60, 15.61 |
| Thigh interMF, cm2 | 0.38 | .152 | 1.69 | 0.94, 3.05 | 1.99 | 1.11, 3.59 |
| Thigh intraMF, cm2 | 0.27 | .262 | 4.62 | −4.03, 13.27 | 3.18 | −5.42, 11.78 |
| Sleep fragmentation index | ||||||
| BMI, kg/m2 | 0.26 | .066 | 5.43 | −0.80, 11.65 | 1.74 | −4.01, 7.50 |
| RV EAT, mm | −0.25 | .271 | 0.72 | 0.49, 1.04 | 0.73 | 0.49, 1.08 |
| AV groove EAT, mm | 0.04 | .851 | 1.06 | 0.82, 1.37 | 1.04 | 0.79, 1.37 |
| Abdominal VAT, cm2 | 0.05 | .814 | 1.06 | 0.65, 1.73 | 0.94 | 0.57, 1.54 |
| Abdominal SAT, cm2 | 0.08 | .748 | 81.97 | −88.35, 252.29 | −15.23 | −144.02, 113.56 |
| Abdominal VAT/SAT ratio | −0.22 | .349 | −0.05 | −0.23, 0.13 | 0.01 | −0.16, 0.18 |
| Liver FF,% | −0.46 | .035 | 0.61 | 0.38, 0.99 | 0.51 | 0.32, 0.80 |
| Thigh SAT, cm2 | 0.13 | .566 | 37.47 | −60.61, 135.55 | −4.13 | −96.40, 88.14 |
| Thigh IMAT, cm2 | 0.36 | .123 | 1.68 | 0.86, 3.29 | 1.46 | 0.73, 2.93 |
| Thigh IMAT FF,% | 0.16 | .500 | 4.62 | −5.37, 14.61 | 5.39 | −5.26, 16.04 |
| Thigh interMF, cm2 | 0.40 | .121 | 1.72 | 0.94, 3.14 | 1.88 | 1.04, 3.40 |
| Thigh intraMF, cm2 | 0.20 | .420 | 3.96 | −5.00, 12.92 | 3.00 | −5.65, 11.65 |
| High SDB risk | ||||||
| BMI, kg/m2 | 0.24 | .098 | 6.56 | −1.57, 14.69 | 3.64 | −3.64, 10.83 |
| RV EAT, mm | 0.00 | 1.00 | 0.90 | 0.57, 1.44 | 0.99 | 0.58, 1.68 |
| AV groove EAT, mm | 0.11 | .635 | 1.18 | 0.88, 1.59 | 1.18 | 0.84, 1.67 |
| Abdominal VAT, cm2 | 0.24 | .294 | 1.55 | 0.92, 2.59 | 1.38 | 0.76, 2.50 |
| Abdominal SAT, cm2 | 0.28 | .233 | 150.00 | −58.50, 358.40 | −91.36 | −275.56, 92.83 |
| Abdominal VAT/SAT ratio | 0.13 | .575 | 0.09 | −0.13, 0.31 | 0.34 | 0.14, 0.54 |
| Liver FF,% | −0.02 | .931 | 1.12 | 0.61, 2.05 | 0.94 | 0.47, 1.87 |
| Thigh SAT, cm2 | 0.11 | .621 | 35.83 | −78.99, 150.64 | −42.46 | −157.17, 72.25 |
| Thigh IMAT, cm2 | −0.26 | .268 | 0.62 | 0.28, 1.39 | 0.34 | 0.16, 0.75 |
| Thigh IMAT FF,% | −0.52 | .016 | −14.37 | −24.53, −4.22 | −19.05 | −30.10, −7.99 |
| Thigh interMF, cm2 | −0.34 | .191 | 0.62 | 0.36, 1.08 | 0.59 | 0.30, 1.14 |
| Thigh intraMF, cm2 | −0.24 | .331 | −5.19 | −14.14, 3.76 | −11.74 | −20.48, −3.00 |
Note. Results for the following variables were log-transformed and are presented as exponentiated beta coefficients and 95% CIs: RV EAT, AV groove EAT, abdominal VAT, liver FF, thigh IMAT, and thigh interMF. Bold indicates statistical significance using a threshold of 0 for 95% CIs of non-exponentiated variables and 1.00 for 95% CIs of exponentiated variables.
rs =Spearman’s rho coefficient; β =beta coefficient; AV=atrioventricular; BMI=body mass index; EAT=epicardial adipose tissue; FF=fat fraction; IMAT=intermuscular adipose tissue; interMF=intermuscular fat; intraMF=intramuscular fat; RV=right ventricular; SAT=subcutaneous adipose tissue; SDB = sleep-disordered breathing; VAT=visceral adipose tissue.
Results
Participant characteristics
In our sample of 49 participants with HFpEF, the mean age was 65±11 years, with a majority being women (74%) and over one third Black (41%) (Table 1). As expected, there was a high prevalence of comorbidities related to HFpEF risk, including obesity (80%), hypertension (94%), diabetes (53%), and diagnosed OSA (70%). There were no observed differences between those that did and did not consent to the MRI assessment (Supplemental Table 3).
Table 1.
Participant characteristics (N = 49).
| Age, years, mean (SD) | 64.8 (11.4) |
|---|---|
| Female, n (%) | 36 (73.5) |
| Black race, n (%) | 20 (40.8) |
| Obesity, n (%) | 39 (79.6) |
| History of tobacco use, n (%) | 23 (46.9) |
| Hypertension, n (%) | 46 (93.9) |
| Systolic blood pressure, mmHg, mean (SD) | 159.8 (24.5) |
| Diastolic blood pressure, mmHg, mean (SD) | 90.9 (15.8) |
| Heart rate, beats per minute, mean (SD) | 103.5 (16.4) |
| Total cholesterol, mg/dL, mean (SD) | 157.4 (36.3) |
| HDL cholesterol, mg/dL, mean (SD) | 48.7 (13.2) |
| LDL cholesterol, mg/dL, mean (SD) | 83.4 (33.1) |
| Triglycerides, mg/dL, mean (SD) | 131.0 (55.9) |
| Diabetes, n (%) | 26 (53.1) |
| Hemoglobin A1c,%, mean (SD) | 6.8 (1.7) |
| Coronary artery disease, n (%) | 10 (20.41) |
| Atrial fibrillation, n (%) | 12 (24.5) |
| Obstructive sleep apnea, n (%) | 33 (70.2) |
| Possession of CPAP, n (%) | 31 (64.6) |
| Adherence to CPAPa, n (%) | 20 (64.5) |
| eGFR, mL/min/1.73 m2, mean (SD) | 57.9 (21.1) |
| NYHA class, n (%) | |
| 1 | 0 (0) |
| 2 | 24 (51.1) |
| 3 | 22 (46.8) |
| 4 | 1 (2.1) |
| HF admissions in past last year, n (%) | |
| 0 | 12 (38.7) |
| 1 | 15 (48.4) |
| 2 | 3 (9.7) |
| 3 | 1 (3.2) |
| Multidimensional sleep score, mean (SD) | 2.9 (1.4) |
| Night movement index, mean (SD) | 24.2 (13.8) |
| Sleep fragmentation index, mean (SD) | 38.8 (18.8) |
| Berlin Questionnaire, high risk, n (%) | 42 (85.7) |
Note. Continuous variables are presented as mean (standard deviation) and categorical variables are presented as frequency (percentage).
CPAP = continuous positive airway pressure; eGFR=estimated glomerular filtration rate; HDL=high-density lipoprotein cholesterol; LDL=low-density lipoprotein cholesterol; NYHA=New York Heart Association; SD=standard deviation.
Defined by US Center for Medicare and Medicaid Services criteria of 70% of nights with 4 h/night of CPAP use. Denominator only includes those that endorsed possession of CPAP.
The sample medians for each adiposity variable are summarized in the Supplemental Table 4. The mean BMI was 41.4 kg/m2, with 59% in the Obesity class III or IV range. The mean multidimensional sleep health score was 3, with only three of the six domains of sleep health classifying ≥ 50% of participants as “Good” (sleep timing, regularity of sleep timing, and alertness) (Table 2).
Sleep and adiposity
Multidimensional sleep health was inversely associated with BMI (age-adjusted: β = −2.48, 95% CI: −4.65, −0.30) and thigh interMF (age-adjusted: exponentiated β = 0.78, 95% CI: 0.62, 0.99) (Table 3). In other words, every unit higher (i.e., better) in sleep health was associated with a 2.5-unit lower BMI and a 22% lower thigh interMF after adjusting for age.
The nighttime movement index was positively associated with thigh interMF (age-adjusted: exponentiated β = 1.99, 95% CI: 1.11, 3.59), and inversely associated with liver FF (age-adjusted: exponentiated β = 0.53, 95% CI: 0.34, 0.84) (Table 4). That is, every unit higher on night movement index was associated with nearly 100% greater thigh interMF and 47% lower liver FF after controlling for age.
The sleep fragmentation index showed a similar pattern of results as the nighttime movement index, with a positive association with thigh interMF (age-adjusted: exponentiated β =1.88, 95% CI: 1.04, 3.4) and an inverse association with liver FF (age-adjusted exponentiated β = 0.51, 95% CI: 0.32, 0.8) (Table 4). In other words, every unit higher in sleep fragmentation was associated with 88% greater thigh interMF and 49% lower liver FF.
High SDB risk was positively associated with abdominal VAT/SAT ratio (age-adjusted: β = 0.34, 95% CI: 0.14, 0.54), and inversely associated with thigh IMAT (age-adjusted: exponentiated β = 0.34, 95% CI: 0.16, 0.75), thigh IMAT FF (age-adjusted: β = −19.05, 95% CI: 30.10, −7.99), and thigh intraMF (age-adjusted: β = −11.74, 95% CI: −20.48, −3.00) (Table 4).
Discussion
In our study of HFpEF patients, we generally found that poorer sleep was cross-sectionally associated with greater overall and/or regional adipose tissue depots. For our primary aim, we found that multidimensional sleep health was inversely associated with overall adiposity and regional adiposity in the thigh, specifically subcutaneous adipose tissue, and intermuscular fat of the thigh. That is, poorer multidimensional sleep health was associated with greater overall and thigh adiposity. For our secondary aims, we found that the night movement index, sleep fragmentation index, and high SDB risk were differentially associated with some regional adiposity measures in expected and unexpected directions. For nighttime movement and sleep fragmentation, greater disturbances in these domains were expectedly associated with greater intermuscular fat of the thigh and unexpectedly associated with lower fat deposits in the liver. Likewise, we found that high SDB risk was expectedly associated with a higher ratio of visceral to subcutaneous fat in the abdomen and unexpectedly associated with lower adipose tissue and fat of the thigh. While these results are based on unadjusted and age-adjusted analyses, they provide preliminary evidence for an association between sleep and adiposity in HFpEF patients. Further studies are warranted to examine the potential bi-directional association between sleep and adiposity in this population. Such work will inform our understanding of whether we should target sleep health to improve weight status and/or target weight to improve sleep health in patients with HFpEF.
The plausibility of the sleep-adiposity association is supported by evidence from general population research, which has mainly focused on short sleep duration and SDB risk. Recently, Covassin and colleagues have demonstrated that 14-day experimental sleep restriction in healthy, non-obese individuals results in greater increases in weight and computed tomographic-measured subcutaneous and visceral adipose tissue in the abdomen compared to controls.24 Mechanisms underlying sleep dysregulation and adiposity may include: (1) increased food intake and decreased free-living physical activity leading to a positive energy balance; (2) dysregulated appetite hormones (e.g., leptin); and (3) increased brain activity in response to food stimuli in regions implicated in reward and cognitive control.25,26 To our knowledge, there have not been specific studies examining sleep-adiposity mechanisms among individuals with HF, though mechanisms identified by general population research likely still apply. In fact, positive energy balance may be exacerbated by symptoms of HF (e.g., fatigue, decreased activity, and decreased exercise tolerance).
In both the general and HF populations, the utility of sleep as an intervention target for weight management, or vice versa, is an understudied area. Preliminary evidence in non-HF populations suggests that sleep duration extension is associated with attenuation of weight gain and improvement of mechanisms of the sleep-weight association, including increased daytime activity, decreased energy intake, and reductions in sweet and salty appetite cravings.27,28 Furthermore, sleep disturbances have been noted to influence the efficacy of behavioral weight loss interventions in non-HF samples, with blunted weight loss observed among those with poor sleep quality or quantity29–32 and those concurrently undergoing experimentally-induced sleep restriction,33,34 and greater weight loss among those with higher overall sleep health.35 Such evidence supports intervening on sleep prior to intervening on weight using behavioral approaches, though it is unclear whether this would also hold for weight management via pharmacologic options (e.g., Glucagon-like peptide-1 receptor agonists36). Other research suggests surgical weight loss (i.e., gastric bypass surgery) improves SDB37 and behavioral weight loss interventions may improve other sleep disturbances,38 giving evidence that intervening on weight prior to sleep may also be a viable approach. Overall, it’s likely that comorbid sleep disturbances may be an important consideration in weight management but are understudied among HF patients with obesity.
The reason for our unexpected findings for night movement and sleep fragmentation with liver fat and for high SDB risk with thigh fat is unclear. Sleep fragmentation and high SDB risk may serve as proxies for OSA. Thus, our findings of an inverse association of sleep fragmentation and a null association of high SDB risk with liver fat contrasts the growing evidence in humans and animal models linking OSA, particularly intermittent hypoxia, with hepatic steatosis.39 One explanation for our unexpected findings may be methodological limitations. For example, these unexpected results may reflect type 1 error and/or unstable parameter estimates due to a small sample size for analyses of regional adiposity outcomes. It may also be the case that these null findings are influenced by a high CPAP adherence (64%) in our sample, which may bias results towards the null. Importantly, these measures are also individual sleep parameters that do not capture the correlated and multidimensional nature of sleep.40 Isolated examination of sleep parameters, while informative, may increase the likelihood of incomplete conclusions about sleep and its relationship to health outcomes.
To our knowledge, this is the first study to examine sleep health or sleep disturbances and their association with overall and regional adiposity among individuals with HFpEF. A cross-sectional study by Parisi and colleagues15 examined SDB and epicardial adipose tissue in participants with HFrEF, finding that the presence and severity of SDB based on polysomnography was associated with epicardial adipose tissue thickness. We did not observe an extension of this finding between high risk of SDB based on the Berlin Questionnaire and either right ventricular or atrioventricular groove epicardial adipose tissue in HFpEF patients. However, we did not objectively measure SDB severity in this study.
Given this overall paucity of research on sleep and adiposity in HF despite evidence of obesity’s contributions to HFpEF and the bidirectional relationship between sleep and obesity, there is a significant need to further elucidate the role of sleep health and sleep disturbances in weight status in the HF population. Epicardial adipose tissue has been associated with adverse changes in myocardial function and the release of proinflammatory cytokines.23 Liver fat has been associated with myocardial remodeling and dysfunction.20 Furthermore, specific regions of adipose tissue may strongly influence exercise tolerance and severity of HF symptoms in HFpEF. Abnormalities in thigh skeletal muscle tissue and abdominal adipose tissue have been associated with reduced exercise capacity19,41,42 and abnormalities in the skeletal muscle composition of the thigh have been associated with higher NYHA class.19 Future endeavors would benefit from continued attention to the location of adiposity as there is increasing support for the role of regional adiposity in HFpEF pathophysiology.8,43
In addition to our study’s strengths (classification of HF pheno-type, use of a multidimensional sleep score, comprehensive assessment of regional adiposity), there are several limitations. First, as mentioned above, the sample size was small, particularly for our MRI subset (n = 22). This prevented the statistical adjustment of other potential confounders and could have negatively impacted our external validity and power to detect statistically significant associations. Our SDB risk analyses, in particular, should be interpreted with caution as they used a small comparison group (only seven participants classified as low risk of having SDB) and did not examine whether adjustment for CPAP adherence (64% of those reporting possession of CPAP) influenced the results. Furthermore, with our limited sample size, we chose to focus on effect sizes with confidence intervals rather than p-values corrected for multiple comparisons. Second, the cross-sectional design precludes examination of the directionality or causality of the association between sleep and adiposity. Future longitudinal studies are needed to examine whether sleep is independently and prospectively associated with adiposity or other important prognostic markers in HFpEF patients to assess its utility as an intervention target for cardiometabolic health. It is also plausible that greater adiposity may result in greater sleep disturbances, which has been observed in longitudinal studies examining obesity and the risk of incident insomnia44 and development of sleep-disordered breathing.45 Third, due to our modest sample size, we were unable to examine sex differences, an important future research question given the sex differences observed in sleep disturbance prevalence,46 adipose tissue distribution and biology,47 and cardiovascular pathophysiology (e.g., structure and function).48
In summary, poorer sleep is potentially related to greater adiposity in HFpEF patients. Future research is needed to further elucidate the relationship between sleep and adiposity and to determine whether sleep is a potential candidate for individual or adjunctive interventions for individuals with obesity and HFpEF.
Supplementary Material
Funding
Funding was provided by the American Heart Association Strategic Collaborative Grant Award No: 18SCG34320007 and Strategically Focused Research Network Center Grant Award Nos: 16SFRN27880000 and 16SFRN28780016. Dr. Polanka acknowledges support by NIH/NHLBI (T32HL007779-28). Dr. Hays is partially supported by NIH/NHLBI (R01HL147660). Dr. St-Onge is partially supported by NIH/NHLBI (R35HL155670). Dr. Shah is supported by NIH/NHLBI (U54HL160273 R01HL107577, R01HL140731, R01HL149423). Dr. Mathews acknowledges support by the Johns Hopkins Clinician Scientist Award.
Declaration of Competing Interest
Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boeh-ringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics. All other authors do not have any conflicts of interest to disclose related to the present submission.
Abbreviation list
- BMI
body mass index
- CPAP
continuous positive airway pressure
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- NYHA
New York Heart Association
- OSA
obstructive sleep apnea
- SDB
sleep-disordered breathing
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hrtlng.2022.12.005.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.Goyal P, Loop M, Chen L, et al. Causes and temporal patterns of 30-day readmission among older adults hospitalized with heart failure with preserved or reduced ejection fraction. J Am Heart Assoc. 2018;7: e007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 7.Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143: e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao VN, Fudim M, Mentz RJ, Michos ED, Felker GM. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero Funes D, Gutierrez Blanco D, Botero-Fonnegra C, et al. Bariatric surgery decreases the number of future hospital admissions for diastolic heart failure in subjects with severe obesity: a retrospective analysis of the US National Inpatient Sample database. Surg Obes Relat Dis. 2022;18:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikhalkova D, Holman SR, Jiang H, et al. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity. 2018;26:284–290. (Silver Spring). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muscogiuri G, Barrea L, Annunziata G, et al. Obesity and sleep disturbance: the chicken or the egg? Crit Rev Food Sci Nutr. 2019;59:2158–2165. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Yamamoto K. Sleep disorder and heart failure with preserved ejection fraction. Heart Fail Clin. 2021;17:369–376. [DOI] [PubMed] [Google Scholar]
- 14.Khattak HK, Hayat F, Pamboukian SV, Hahn HS, Schwartz BP, Stein PK. obstructive sleep apnea in heart failure: review of prevalence, treatment with continuous positive airway pressure, and prognosis. Tex Heart Inst J. 2018;45:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parisi V, Paolillo S, Rengo G, et al. Sleep-disordered breathing and epicardial adipose tissue in patients with heart failure. Nutr Metab Cardiovasc Dis. NMCD 2018;28:126–132. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brindle RC, Yu L, Buysse DJ, Hall MH. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: results from the Midlife in the United States (MIDUS) study. Sleep. 2019;42:zsz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foldvary-Schaefer N, Kaw R, Collop N, et al. Prevalence of undetected sleep apnea in patients undergoing cardiovascular surgery and impact on postoperative outcomes. J Clin Sleep Med. 2015;11:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying W, Sharma K, Yanek LR, et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8:2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding J, Visser M, Kritchevsky SB, et al. The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hyper-tens. 2004;17:971–976. [DOI] [PubMed] [Google Scholar]
- 21.VanWagner LB, Wilcox JE, Ning H, et al. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA study. J Am Heart Assoc. 2020;9: e014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flüchter S, Haghi D, Dinter D, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity. 2007;15:870–878. (Silver Spring). [DOI] [PubMed] [Google Scholar]
- 23.Packer M Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. [DOI] [PubMed] [Google Scholar]
- 24.Covassin N, Singh P, McCrady-Spitzer SK, et al. Effects of experimental sleep restriction on energy intake, energy expenditure, and visceral obesity. J Am Coll Cardiol. 2022;79:1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Onge M-P, Shechter A Sleep disturbances, body fat distribution, food intake and/or energy expenditure: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu B, Shi C, Park CG, Zhao X, Reutrakul S. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2019;45:18–30. [DOI] [PubMed] [Google Scholar]
- 27.St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017;18(Suppl 1):34–39. [DOI] [PubMed] [Google Scholar]
- 28.Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Effect of sleep extension on objectively assessed energy intake among adults with overweight in real-life settings: a randomized clinical trial. JAMA Intern Med. 2022;182:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson CA, Morrow KL, Flatt SW, et al. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity. 2012;20:1419–1425. (Silver Spring). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaput J-P, Tremblay A Sleeping habits predict the magnitude of fat loss in adults exposed to moderate caloric restriction. Obes Facts. 2012;5:561–566. [DOI] [PubMed] [Google Scholar]
- 31.Elder CR, Gullion CM, Funk KL, Debar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes. 2012;36:86–92. (London). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawamoto R, Nozaki T, Furukawa T, et al. Higher sleep fragmentation predicts a lower magnitude of weight loss in overweight and obese women participating in a weight-loss intervention. Nutr Diabetes. 2014;4:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Sparks JR, Bowyer KP, Youngstedt SD. Influence of sleep restriction on weight loss outcomes associated with caloric restriction. Sleep. 2018. 41: 10.1093/sleep/zsy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med.. 2010;153:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes. 2021;45:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. [DOI] [PubMed] [Google Scholar]
- 37.Ashrafian H, Toma T, Rowland SP, et al. Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses. Obes Surg. 2015;25:1239–1250. [DOI] [PubMed] [Google Scholar]
- 38.Ross KM, Graham Thomas J, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y, Liang Y, Mak JCW, Ip MSM. Obstructive sleep apnea, intermittent hypoxia and non-alcoholic fatty liver disease. Sleep Med. 2022;95:16–28. 10.1016/j.sleep.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Chung J, Goodman M, Huang T, Bertisch S, Redline S. Multidimensional sleep health in a diverse, aging adult cohort: concepts, advances, and implications for research and intervention. Sleep Health. 2021;7:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haykowsky MJ, Nicklas BJ, Brubaker PH, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. J Am Coll Cardiol Heart Fail. 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200–203. [DOI] [PubMed] [Google Scholar]
- 44.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leinum CJ, Dopp JM, Morgan BJ. Sleep-disordered breathing and obesity: patho-physiology, complications, and treatment. Nutr Clin Pract. 2009;24:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker FC, Yu ksel D, de Zambotti M, Attarian H, Viola-Saltzman M. Sex differences in sleep. Sleep Disorders in Women: A Guide to Practical Management. Cham. Springer International Publishing; 2020:55–64. 10.1007/978-3-030-40842-8_5. Available at. [DOI] [Google Scholar]
- 47.Chang E, Varghese M, Singer K. Gender and sex differences in adipose tissue. Curr Diabetes Rep. 2018;18. 69 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


