Abstract
Importance:
Uterine fibroids and endometriosis are two of the leading causes of morbidity amongst reproductive aged women. There are significant racial disparities in disease prevalence, incidence, age of onset, and treatment profile in fibroids. The data on endometriosis are less clear.
Objective:
To conduct a systematic review of racial disparities in prevalence of uterine fibroids and endometriosis in the US and summarize the literature on these two highly prevalent benign gynecological conditions using a framework that explicitly incorporates and acknowledges the social, structural and political contexts as a root cause of racial disparities between Black and White women.
Evidence Review:
A systematic review regarding racial disparities in prevalence of fibroids and endometriosis was conducted separately. Two separate searches in PubMed to identify relevant original research manuscripts and prior systematic reviews regarding racial disparities in uterine fibroids and endometriosis using standardized search terms. In addition, we conducted a structured literature search to provide social, structural and political context of the disparities.
Findings:
Systematic review of the literature indicated that prevalence of uterine fibroids was consistently higher in Black women compared to White women with the magnitude of the difference varying depending on population and case definition. Prevalence of endometriosis varied considerably depending on the base population and case definition but was consistently lower among Black versus White women. As a result of the social, structural, and political context in the United States, Black women disproportionately experience a range of exposures across the life course that may contribute to their increased uterine fibroid incidence, prevalence, and severity of uterine fibroids. However, data suggests no racial difference in the incidence of endometriosis. Nevertheless, Black women with fibroids or endometriosis experience worse clinical and surgical outcomes then their White counterparts.
Conclusion and relevance:
Racial disparities in uterine fibroids and endometriosis can be linked with differential exposures to suspected etiologic agents, lack of adequate access to health care, including highly skilled gynecologic surgeons, and bias and discrimination within the health care system. Eliminating these racial disparities will require solutions that address root causes of health disparities through policy, education and programs to ensure that all patients receive culturally- and structurally competent care.
Keywords: fibroids, endometriosis, racial disparities, racism, structural determinants, allostatic load
Capsule:
Fibroids and endometriosis are leading causes of gynecological morbidity and are associated with significant racial disparities. The social, structural and political contexts that contribute to those disparities are summarized here.
INTRODUCTION
Healthy People 2030 defines health disparities as “a particular type of health difference that is closely linked with economic, social or environmental disadvantage,” which are the result of the social, structural, and political context (1–6). Specifically, the social, structural, and political contexts in combination with an individual’s identities shape the exposures they experience across the life-course, which in turn influence their disease characteristics and experiences with the health care system [Figure 1] (1–6). Historically, health disparities were discussed in the context of “health equality”. While health equality is an idealized goal, it presumes that individuals from different groups are starting from the same place, ignoring the role of the social, structural, and political context, and emphasizing genetics, and individual behaviors and risk factors. In contrast, health equity scholarship recognizes the important contextual factors that shape individual’s lives, choices, and opportunities in ways that privilege some groups while disadvantaging others (1–6). These inequities in lived experience result in health disparities across the life course through embodiment, or the biologic manifestation of the social conditions in which an individual or population lives (7, 8). Embodiment is a multi-level process that can take many forms including alterations in gene expression or physical characteristics, which contribute to an individual’s disease risk. A key mechanism for embodiment is allostatic load, defined as the interaction of body systems with the cumulative burden of chronic stress and events across the life-course (9, 10). One way in which allostatic load may manifest with respect to health is through a process termed “weathering,” which refers to the early deterioration of health due to cumulative socioeconomic disadvantage (11, 12). Health equity frameworks illuminate the need for interventions that move beyond the individual level and address institutional and structural factors to change the context that shapes people’s health and health outcomes through embodiment.
Figure 1:
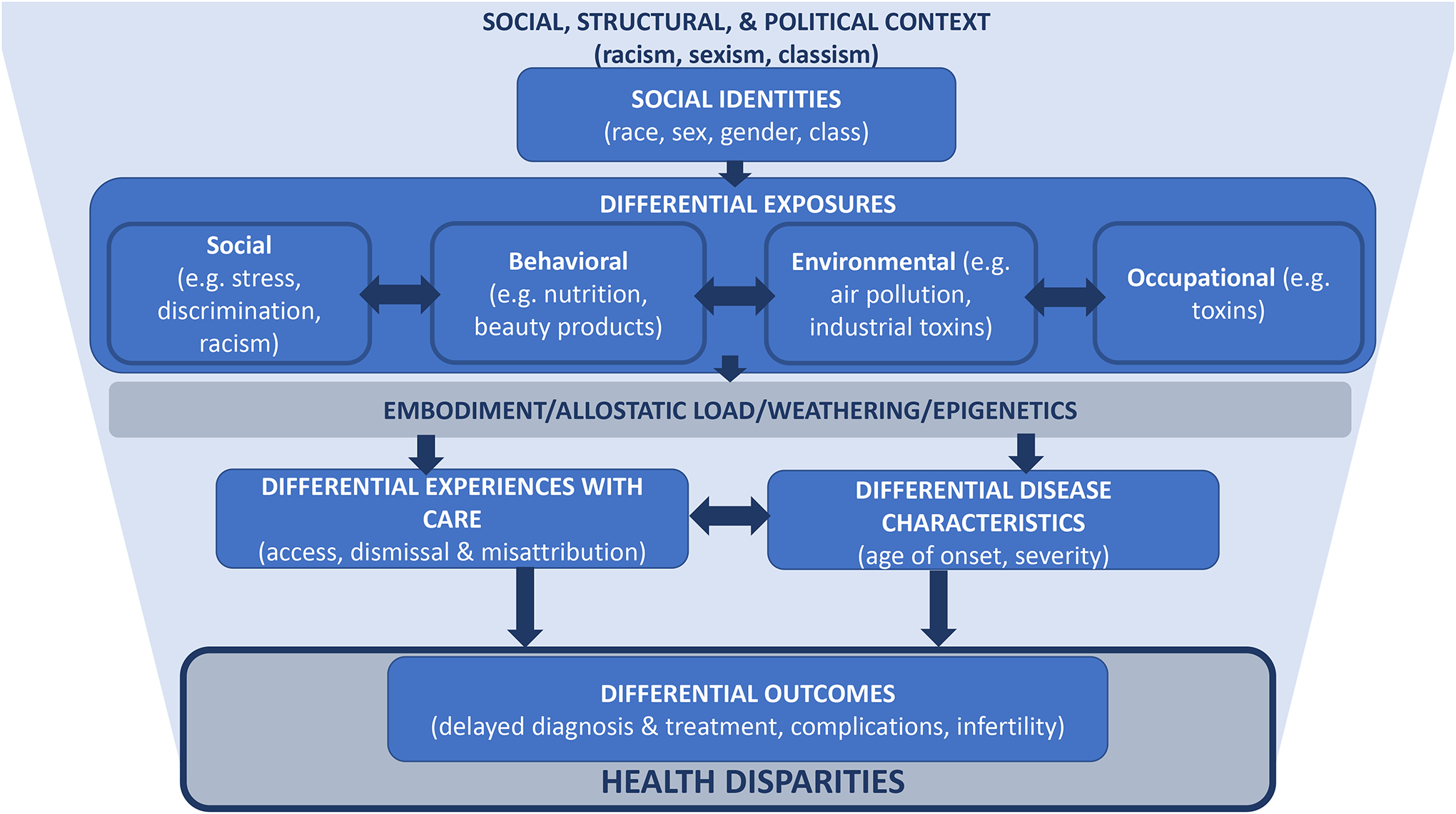
Conceptual model for the social, structural, and political determinants of racial health disparities in uterine fibroids and endometriosis
Measuring and more importantly addressing health disparities can provide a means of assessing progress towards health equity (5). While there are many types of health disparities, racial disparities are amongst the most documented in the US, including persistent disparities in reproductive health outcomes, particularly between Black and White women (13). Within the scope of reproductive health, Black/White disparities in pregnancy and cancers are most significantly researched yet disparities in benign gynecological conditions are relatively understudied (13–15). The purpose of this review is to summarize the literature regarding Black/White health disparities in two prevalent benign gynecological conditions: uterine fibroids and endometriosis. Using a framework that explicitly incorporates and acknowledges social, structural and political determinants of health, we will review key drivers of disparity in these two common gynecologic conditions.
METHODS
To systematically review racial disparities in prevalence of uterine fibroids and endometriosis we conducted two separate searches of PubMed using the search terms: “(fibroids) AND (race) AND (prevalence) AND (United States)” and “(endometriosis) AND (race) AND (prevalence) AND (United States)” on January 12, 2023. These searches were limited to articles published between January 1, 1995, and October 1, 2022. We used the PubMed filters to limit the search to articles dealing with human species, female sex, and that were in English. Articles from the resulting lists were retained if they summarized findings from a US population, were original research, and included estimates of population prevalence for uterine fibroids or endometriosis among Black and White, and were not limited by a specific other condition or disease. We also reviewed reference lists of included articles and identified reviews.
To summarize the literature regarding disparities in uterine fibroids and endometriosis and the role of social, structural and political context we conducted two separate searches in PubMed to identify relevant original research manuscripts and prior systematic reviews regarding racial disparities in uterine fibroids and endometriosis using standardized search terms for articles published between January 1, 1995, and October 1, 2022 (search conducted on October 4, 2022). The search terms for uterine fibroids were “(uterine fibroids) AND (disparities)” “(uterine fibroids) AND (race)” “(uterine fibroids) AND (ethnicity).” Similarly, search terms for endometriosis were “(endometriosis) AND (disparities)” “(endometriosis) AND (race)” “(endometriosis) AND (ethnicity).” The resulting article lists were scanned for relevance based on title and abstract. Reference lists of review articles were also searched for additional relevant manuscripts.
RESULTS
Uterine Fibroids
Uterine leiomyomata (commonly called uterine fibroids) are non-cancerous smooth muscle tumors of clonal origin that are common among individuals with a uterus. Approximately 70% of individuals with a uterus will have uterine fibroids by age 50 (16). While uterine fibroids can be asymptomatic, 25–50% of those with uterine fibroids experience symptoms such as heavy bleeding, bulk symptoms, or pain which can negatively impact quality of life (17, 18). Uterine fibroids have also been linked to increased risk of infertility and recurrent pregnancy loss, preterm birth, placenta previa, placental abruption, and cesarean section (19). While the number of conservative options for treatment have increased over time, hysterectomy remains the only definitive treatment for uterine fibroids and continues to be one of the most common treatments (20). When surgery is deemed necessary, minimally invasive approaches are preferred due to shorter recovery time and lower risk of complications compared with an open abdominal approach (21, 22). Current estimates indicate that in the US costs associated with uterine fibroids are approximately $34.4 billion per year (23). Compared with White women, Black women are disproportionately impacted by uterine fibroids (16, 24–26). While other racial disparities in uterine fibroids may exist, the majority of the literature focuses exclusively on Black/White disparities and these will be the focus of this review (27).
Our systematic review of the literature regarding racial disparities in prevalence identified three manuscripts meeting our criteria [Figure 2]. Two involved ultrasound determination of presence of uterine fibroids, one of these was limited to younger asymptomatic women and one included a random sample of women regardless of age or symptom status (16, 28). A third study relied on electronic medical records and ICD9 codes to identify uterine fibroid cases (29). In all studies Black women had higher prevalence of uterine fibroids compared with White women, though the difference in prevalence varied depending on the population and case definition (16, 28, 29). Among those 18–30 years old with no fibroids symptoms 26% of Black women and 7% of White women had ultrasound evidence of uterine fibroids (28). Among those 35–49 years old sampled regardless of symptoms or clinical diagnosis approximately 80% of Black women and 70% of white women had evidence of uterine fibroids by ultrasound (16). Using electronic records, the prevalence of diagnosed uterine fibroids among Black and White women 18–65 years old was 18.5% and 10.3% respectively (29).
Figure 2:
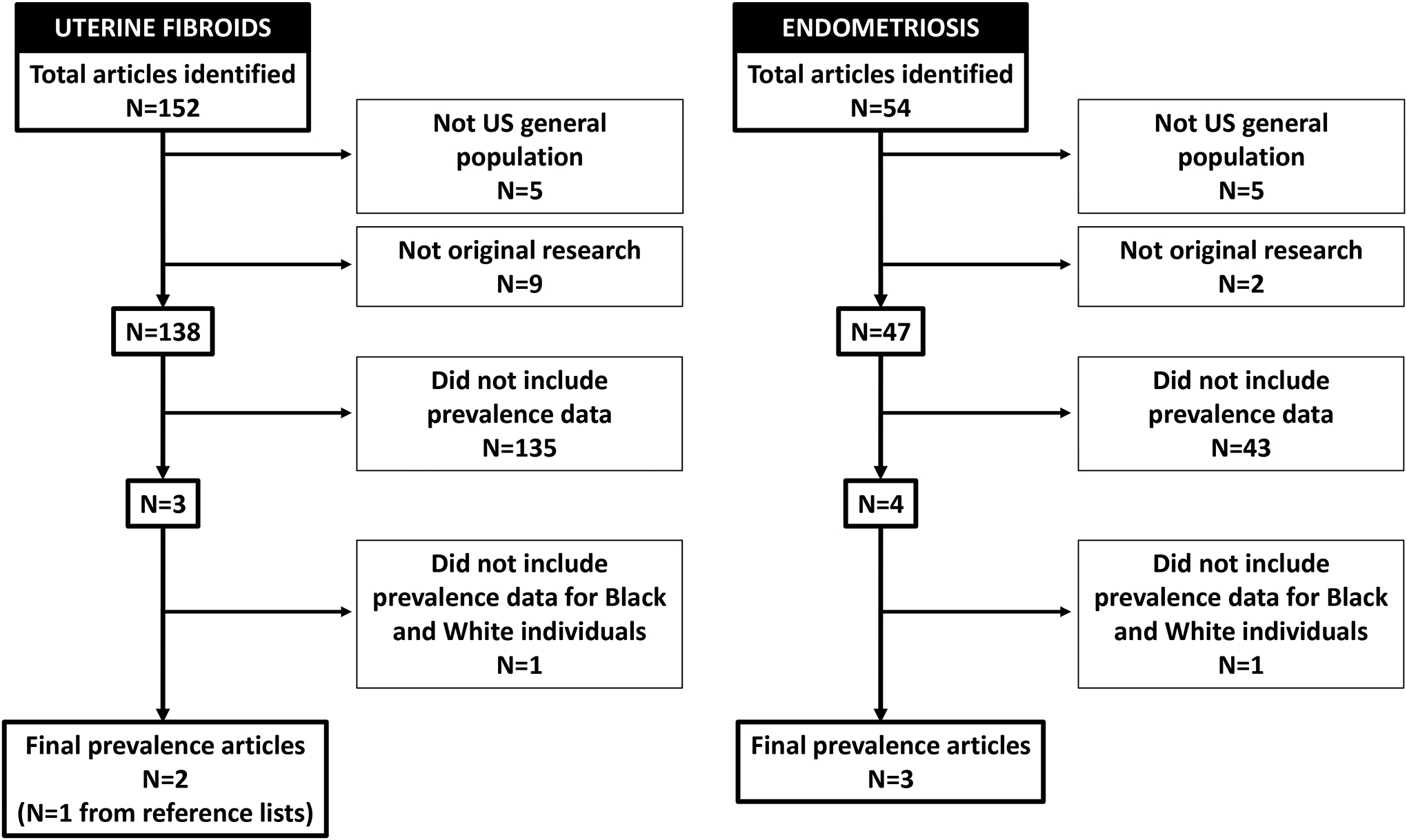
Flow chart for sequential article exclusion for the systematic review of racial disparities in prevalence of uterine fibroids and endometriosis.
Both myometrial mass and morphology of smooth muscle cells are altered in uterine fibroids, and it is well established that both estrogen and progesterone play key roles in promoting fibroid growth (30). Notably, racial differences are reported at the gene expression level among those with fibroids. This includes the expression and intensities of several proteins compared between fibroids from African Americans and other racial groups (31). Pan et. al. found that of 1,470 genes identified that were differentially expressed in fibroids, 177 genes were overexpressed and 91 genes were under-expressed among fibroids from African Americans versus those from White individuals (32). There is also some evidence of differences in micro-RNA expression in fibroid tissue obtained from African American versus White individuals (33, 34). Other potential pathways that are implicated in fibroid etiology and might provide some insight into racial disparities in uterine fibroids include polymorphisms of genes involved in estrogen synthesis, variation in the expression of retinoic acid nuclear receptors, and aromatase inhibitors (30).
The differences in gene expression, as well as racial disparities in prevalence, may reflect that Black women disproportionately experience a wide range of exposures that are also associated with increased risk for uterine fibroids across the life-course reflecting their positionality at the intersection of racism and sexism (Figure 1) (35–37). These include social exposures such as stress and interpersonal racism (10, 15); behavioral factors such as poor diet, lower levels of physical activity, vitamin D deficiency, and use of certain beauty products (e.g. hair straighteners) (38–41); and a wide range of environmental and occupational exposures including persistent organic pollutants (POPs), endocrine disrupting compounds, and air pollution (42, 43).
In terms of social exposures, a 2019 meta-analysis of observational studies concluded that chronic psychological stress was associated with risk of uterine fibroids (highest versus lowest category of chronic stress: ORpooled 1.24 95% CI 1.15, 1.34) (44). Similarly, moderate daily stress and anger squelching are reported to be associated with increased risk of fibroids among Black women (45). Another study which followed a cohort of more than 22,000 Black women found that higher exposure to perceived racism was associated with an increased risk of fibroids among US-born Black women (highest versus lowest quartile of everyday racism IIR 1.27, 95% CI 1.14, 1.43) (46). Increased risk of fibroids and more severe fibroid symptoms are also associated with adverse childhood experiences, which may be more prevalent among Black versus White women depending on the instrument used and population sampled (47–52).
While stress and childhood adversity may influence fibroid risk through activation of inflammatory and other biologic pathways, it may also increase fibroid risk indirectly by leading to behavioral coping mechanisms such as alcohol use, poor diet and lack of physical activity (47, 53–58). The Black Women’s Health Study found that increased consumption of beer was associated with increased risk of uterine fibroids in a dose dependent manner (55). Additionally, various indicators of poor diet are reported to increase risk of fibroids including increased intake of fatty acids of animal origin and lower intake of fruits and vegetables (54, 59). Lower levels of physical activity were also found to be associated with increased risk of fibroids with Baird et al reporting that relative to those in the lowest category of physical activity those in the highest had 40% lower odds of developing uterine fibroids (OR 0.6, 95% CI 0.4–0.9) (58). Findings regarding tobacco use and caffeine intake are inconsistent (55, 60, 61). Historically, pelvic infection was thought to play a role in fibroid incidence, but recent evidence does not support this. Non-Hispanic Black women have higher incidence and prevalence of STIs than non-Hispanic White women in the US, though this is largely explained by partner networks and concurrency rather than individual behaviors (62). Importantly, multiple studies failed to find any association between infection with bacterial vaginosis, herpes simplex virus type 2, or chlamydia trachomatis and increased risk of fibroid development or growth (63–65).
Pressure to conform to predominantly white standards of beauty has increased the likelihood of Black women using products such as hair straighteners and relaxers, which contain chemicals that are associated with increased risk of uterine fibroids (4, 66–68). Black consumers in the US purchase hair relaxers and straighteners at much higher rates than White consumers, and compared with White women, Black women are more likely to use a greater number and variety of products (41, 69). Use of these products frequently begins at ages as young as four to eight years old (70). Notably, Wise et al found that ever versus never use of hair relaxers among Black women was associated with 17% higher incidence of uterine fibroids (IIR 1.17, 95% CI 1.06, 1.30), with positive trends identified for association of fibroid risk with frequency and duration of use (66).
In addition to toxins contained within beauty products, Black women are disproportionately exposed to a wide range of environmental and occupational exposures across the life-course including heavy metals, persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDC) which are associated with increased risk of fibroids(42, 43). Several studies have noted a relationship between fibroids and environmental contaminant exposures. Diethylstilbestrol (DES), which is classified as an EDC, exposure in the prenatal period has been associated with development of fibroids with Baird et al reporting that those exposed to DES prenatally had greater than two-fold increased odds of fibroids and Mahalingaiah reporting a 12% higher incidence associated with DES exposure (71, 72). Additionally, exposure to phthalates has been associated with fibroid development (42). One study found that exposure to phthalates was universal in a cohort of pre-menopausal women who had fibroids, however, Black women with fibroids had significantly higher levels of specific phthalates compared to White and Hispanic women (73). Due to historical and current racial segregation and discriminatory patterns of zoning, Black women are more likely than White women to be exposed to high levels of air pollution (74, 75). Multiple studies suggest that chronic exposure to air pollution is associated with a higher risk of fibroids (76, 77). For example, Lin et al reported an 11% increase in odds of fibroids associated with a 10mug/m(3) increase in PM2.5 was (OR 1.11, 95% CI 1.07, 1.14), and an 8% increase in odds of fibroids per 10ppb increase in O3 (OR 1.08, 95% CI 1.04, 1.11) (76).
A potential consequence of the cumulative disproportionate exposures to childhood adversity, stress, and certain environmental exposures may be the greater likelihood of early menarche among Black versus White women (78–81). Early menarche is thought to potentially increase the risk of uterine fibroids through increased exposure to menstrual cycles across the life-course (61). Indeed, Edwards et al found that not only was there an inverse relationship between age at menarche with each 1-year increase in age at menarche associated with a 13% lower risk of fibroids (RR 0.87, 95% CI 0.82, 0.91), but that early menarche was associated with increased likelihood of multiple fibroids (82). These findings were consistent with earlier findings by Wise et al (83).
The same structural determinants that may lead to higher prevalence and severity of fibroids among Black women also influence their ability to access high-quality, patient-centered fibroid care as demonstrated by a small but growing number of qualitative studies. Black women frequently experience biased and discriminatory treatment in the context of seeking care for fibroids. One qualitative study conducted semi-structured interviews with 37 Black women who were planning surgical management of their fibroids. Several themes emerged including poor patient-provider interactions that left women feeling unsatisfied with their care (84). Patients often felt that their concerns were dismissed, leading to delayed diagnosis of fibroids, and that they did not receive empathy/compassion from their healthcare providers, further compromising the therapeutic relationship (84). Another qualitative study found that women of color with fibroids reported negative interactions with health care providers related to racism. These experiences led to feelings of distrust and skepticism, negatively impacting their care (85).
Negative experiences with care seeking, combined with healthcare system and societal access barriers may result in greater likelihood of delayed or foregone care for Black women with uterine fibroids and worse clinical outcomes (86, 87). Despite higher overall population rates of hysterectomy, recent evidence highlights the fact that Black women with uterine fibroids are more interested in and more likely than White women to schedule a uterine preserving surgery (e.g. myomectomy) when more conservative care is presented as an option (27, 88–91). This preference for uterine preserving treatments may reflect a combination of cultural values, delayed childbearing, and/or mistrust of the medical system due to the historical and present-day mistreatment and coercive reproductive health care practices towards Black women, including forced sterilization (15, 84, 85, 92–94). Therefore, if not offered treatment options that are uterine preserving, or if they believe that they will not be offered such treatments, Black women may be more likely than White women to delay or forego treatment and the sequalae thereof (84). Additionally, evidence indicates that newer surgical techniques, including minimally invasive approaches such as robotic assisted surgeries, may diffuse more slowly among Black patient populations due to the under resourcing of predominantly Black serving hospitals, greater reliance on Medicaid or Medicare, and limited access to high-volume surgeons (87, 95–99). Thus, if Black women do ultimately undergo hysterectomy, they may have larger fibroids than White women, a greater number of prior surgeries, and be less likely to have their surgery in a hospital with capacity for minimally invasive hysterectomy (87, 100). These factors may at least partially explain why Black women are reported to have twice the odds of White women of having an open abdominal versus minimally invasive hysterectomy (101, 102). The Black/White disparity in minimally invasive hysterectomy persists even in the context of an enhanced access system such as the Veterans Health Administration supporting the idea that access alone cannot create health equity (103, 104). Regardless of surgical route, Black women undergoing surgery for uterine fibroids have higher rates of surgical complications, longer surgery times, and greater likelihood of hospital readmission compared with White women (101, 102).
Endometriosis
Endometriosis is a chronic inflammatory gynecologic disease that is characterized by the growth of endometrial glands and stroma in areas outside of the uterus (105, 106). The gold standard for diagnosis has historically been tissue pathology which requires surgery. Women with endometriosis exhibit a range of symptoms including pelvic pain, dyspareunia, dyschezia and infertility (105). Many women with endometriosis have been shown to experience negative effects on quality of life in a variety of domains—sexual functioning, ability to participate in daily and social activities, work and educational productivity and mental wellbeing. On an individual level, endometriosis is associated with 6.3 hour per week productivity loss and higher annual health care costs of approximately $16,753 per patient (107, 108). The annual economic cost of endometriosis was estimated at $69.4 billion in a 2009 study(109).
Our systematic review of the literature regarding racial disparities in prevalence of endometriosis among Black and White individuals in the US included three manuscripts, all of which used different study populations and case-definitions including an electronic health record based study which relied on ICD-9s, a patient survey that used self-reported prior diagnosis, and surgical population based study that used post-operative notes and pathology [Figure 2] (110–112). In the two non-surgical based population studies Black individuals had similar to slightly lower prevalence of endometriosis (Black 0.7–4% versus White 0.9–7%) (110, 111). In the one study that used a surgical population (e.g. those undergoing laparoscopy or laparotomy) overall prevalence of endometriosis was higher and the difference in diagnosis was substantially larger (Black 12.5% versus White 67%) (112).
Given the racialized disparities in access to healthcare in the US, and the need for surgical diagnosis of endometriosis, it is difficult to establish prevalence and incidence rates and determine if there are racial disparities in these through reliance on administrative data or population-based surveys. Additionally, the recognition and diagnostic description of endometriosis in 1921 as a gynecologic condition was heavily influenced by gender, class, racial biases and cultural politics of the time. Early theories of the etiology of endometriosis suggested that it was a consequence of contraceptive use and delayed childbearing (113, 114). Thus, it was frequently assumed to be a disease predominantly of middle-class White women and this idea was popularized in many medical texts and popular culture leading to misattribution of symptoms and misdiagnosis or delayed diagnosis among Black women (115, 116). This meant that clinicians were less likely to consider endometriosis in their differential diagnosis for Black women experiencing pelvic pain and other symptoms consistent with the condition. For example, Chatman et al found that 20% of Black women who had a laparoscopy as part of a diagnostic workup for pelvic pain had endometriosis, and approximately 38% of these had inaccurately been diagnosed with pelvic inflammatory disease prior to the surgery (117). Therefore, despite evidence from the 1950s, 60s, and 70s indicating no difference in incidence of endometriosis among White and Black women among private patients, prevalence studies continue to find that White women are more likely than Black women to be diagnosed with endometriosis (117–120).
While the etiology of endometriosis is still poorly understood, some of the same factors that are known to be associated with increased risk of fibroids are also putatively associated with increased risk of endometriosis. As described in the previous section, Black women experience these exposures at greater frequency and severity than their White peers. For example, relative to White women, Black women have higher likelihood of exposure to air pollution, endocrine disrupting chemicals, and heavy metals all of which are associated with an increased risk of endometriosis (42, 121). Additionally, earlier menarche is associated with increased risk of endometriosis and occurs more frequently among Black women possibly due to differential exposures to environmental factors (81, 106). Nevertheless, studies report that Black women appear to have similar incidence of endometriosis, if not lower, than White women (118, 119). This inconsistency may be due to a combination of diagnostic bias and disparities in access to health care, as well as potential differences in disease presentation as endometriosis is a heterogenous condition with multiple and complex etiology(118). At least one study suggests that Black women may be more likely than White women to have uterine implants, which are otherwise considered rare (122). However, it is unclear how or if this difference in disease would impact diagnosis and more recent findings indicate no difference in location of endometriosis lesions by race or ethnicity among patients undergoing surgery for endometriosis (123, 124). Current qualitative research efforts seek to understand how access to health care as well as bias and discrimination shape experiences of endometriosis and result in different pathways to diagnosis and treatment among racially and ethnically diverse samples (125). This is important not just due to persistent diagnostic bias on the part of clinicians, but also because the sentinel symptom of endometriosis is pelvic pain and presentation of pelvic pain, and its components are heavily influenced by psychosocial context.
Ultimately, differential experiences with seeking care and diagnosis and limited access to quality health care may also lead to racial disparities in treatment and treatment outcomes. At least two recent studies indicate that relative to White women, Black women undergoing surgery for endometriosis are more likely to have surgical complications even after adjusting for surgical approach and individual patient factors. Movilla et al estimated that Black women had 64% higher odds (95% CI 1.10–2.45) of major complications from hysterectomy for endometriosis relative to White women(124). Similarly, Orlando et al found that among those having any surgery for endometriosis relative to White women, Black women had 71% higher odds of any complications in the 30 days post-surgery (95% CI 1.39–2.10) (123). Both studies included adjustment for surgical route (e.g. open abdominal versus minimally invasive) as Black patients undergoing gynecologic surgery for benign conditions are less likely than White patients to have a minimally invasive route, which is associated with increased risk of surgical complications (25, 126).
CONCLUSIONS
Black/White racial disparities in uterine fibroids and endometriosis exist and persist. For uterine fibroids there are ongoing racial disparities in prevalence, symptom severity, treatment, and outcomes. In the case of endometriosis, it is unclear whether racial disparities in incidence or prevalence exist. However, consistent findings indicate that Black/White racial disparities in diagnosis, treatment and outcomes for endometriosis still occur. While the limited racial disparity research in uterine fibroids and endometriosis is largely focused on differences in tumor biology, symptom presentation, and treatment choice, we know that root causes of racial health disparities are much more complex than genes, individual health behaviors, and access to care. For example, gene expression is impacted by allostatic load which is driven by the chronic daily stressors of racism, sexism, classism, homophobia, and many other marginalizing harmful factors. Additionally, health behaviors and health decisions are not simply individual choices, but are driven by our jobs, incomes, where we live, what language we speak, and how safe we feel, all of which are similarly shaped by the interaction of our identities within a specific social, structural, and political context. We propose a model, informed by scholarship on health equity, Black feminism, and social epidemiology that incorporates these contextual factors as root causes and determinants of racial disparities in uterine fibroids and endometriosis. This model suggests that findings regarding racial disparities in these gynecologic conditions, must be interpreted within the context of these root causes and determinants if we are to make meaningful strides towards health equity. Further, our findings highlight the need to address structural causes of health disparities through policy, education and programs, ensuring that all patients receive culturally- and structurally-competent care (127), and investing in development of novel diagnostics and treatments and equitable access to these technologies.
Funding Statement:
This work was supported in part by R01MD011570 (EEM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: E.E.M. has served as a consultant for Myovant Sciences and Pfizer. The views expressed herein are those of the author(s) and do not necessarily reflect the official policy of the Department of the Army, Department of Defense, or the US Government. The authors have no other disclosures.
REFERENCES
- 1.Crear-Perry J, Correa-de-Araujo R, Lewis Johnson T, McLemore MR, Neilson E, Wallace M. Social and Structural Determinants of Health Inequities in Maternal Health. J Womens Health (Larchmt). 2021;30(2):230–5. Epub 2020/11/13. doi: 10.1089/jwh.2020.8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diderichsen F, Evans T, Whitehead M. The social basis of disparitie sin health. In: Evans T, Whitehead M, Diderichsen F, Bhuiya A, Wirth M, editors. Challenging Inequities in Health. New York, YY: Oxford University Press; 2001. [Google Scholar]
- 3.Hofrichter R, Bhatia R. Tackling Health Inequities Through Public Health Practice: Theory to Action: Oxford University Press; 2010. [Google Scholar]
- 4.Zota AR, VanNoy BN. Integrating Intersectionality Into the Exposome Paradigm: A Novel Approach to Racial Inequities in Uterine Fibroids. Am J Public Health. 2021;111(1):104–9. Epub 2020/11/20. doi: 10.2105/AJPH.2020.305979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braveman P What are health disparities and health equity? We need to be clear. Public Health Rep. 2014;129 Suppl 2:5–8. Epub 2014/01/05. doi: 10.1177/00333549141291S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health Equity in Healthy People 2030: US Department of Health and Human Services; [cited 2022. 10/15/2022]. Available from: https://health.gov/healthypeople/priority-areas/health-equity-healthy-people-2030#:~:text=Healthy%20People%202030%20defines%20a,%2C%20and%2For%20environmental%20disadvantage.
- 7.Krieger N Embodiment: a conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59(5):350–5. Epub 2005/04/16. doi: 10.1136/jech.2004.024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39(7):887–903. Epub 1994/10/01. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 9.Guidi J, Lucente M, Sonino N, Fava GA. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom. 2021;90(1):11–27. Epub 2020/08/18. doi: 10.1159/000510696. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–101. Epub 1993/09/27. [PubMed] [Google Scholar]
- 11.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–21. Epub 1992/01/01. [PubMed] [Google Scholar]
- 12.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–33. Epub 2005/12/29. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichelberger KY, Doll K, Ekpo GE, Zerden ML. Black Lives Matter: Claiming a Space for Evidence-Based Outrage in Obstetrics and Gynecology. Am J Public Health. 2016;106(10):1771–2. doi: 10.2105/AJPH.2016.303313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.In: Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington (DC) 2003. [PubMed] [Google Scholar]
- 15.Prather C, Fuller TR, Jeffries WLt, Marshall KJ, Howell AV, Belyue-Umole A, et al. Racism, African American Women, and Their Sexual and Reproductive Health: A Review of Historical and Contemporary Evidence and Implications for Health Equity. Health Equity. 2018;2(1):249–59. doi: 10.1089/heq.2017.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–7. [DOI] [PubMed] [Google Scholar]
- 17.Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149(1):3–9. Epub 2020/01/22. doi: 10.1002/ijgo.13102. [DOI] [PubMed] [Google Scholar]
- 18.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043. Epub 2016/06/24. doi: 10.1038/nrdp.2016.43. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho LM, Assis WA, Spagnuolo-Souza A, Reis FM. Uterine Fibroids and Pregnancy: How Do They Affect Each Other? Reprod Sci. 2022;29(8):2145–51. Epub 2021/06/19. doi: 10.1007/s43032-021-00656-6. [DOI] [PubMed] [Google Scholar]
- 20.American College of O, Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 Pt 1):387–400. Epub 2008/08/02. doi: 10.1097/AOG.0b013e318183fbab. [DOI] [PubMed] [Google Scholar]
- 21.Committee on Gynecologic P Committee Opinion No 701: Choosing the Route of Hysterectomy for Benign Disease. Obstet Gynecol. 2017;129(6):e155–e9. doi: 10.1097/AOG.0000000000002112. [DOI] [PubMed] [Google Scholar]
- 22.Nieboer TE, Johnson N, Lethaby A, Tavender E, Curr E, Garry R, et al. Surgical approach to hysterectomy for benign gynaecological disease. The Cochrane database of systematic reviews. 2009(3):CD003677. Epub 2009/07/10. doi: 10.1002/14651858.CD003677.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211 e1–9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210(3):194–9. doi: 10.1016/j.ajog.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. 2010;202(6):514–21. Epub 2010/05/01. doi: 10.1016/j.ajog.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in Fibroid Incidence, Prognosis, and Management. Obstet Gynecol Clin North Am. 2017;44(1):81–94. doi: 10.1016/j.ogc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. J Womens Health (Larchmt). 2018;27(11):1359–67. doi: 10.1089/jwh.2018.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh EE, Ekpo GE, Cardozo ER, Brocks M, Dune T, Cohen LS. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18–30 years old): a pilot study. Fertil Steril. 2013;99(7):1951–7. Epub 2013/03/19. doi: 10.1016/j.fertnstert.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu O, Scholes D, Schulze-Rath R, Grafton J, Hansen K, Reed SD. A US population-based study of uterine fibroid diagnosis incidence, trends, and prevalence: 2005 through 2014. Am J Obstet Gynecol. 2018;219(6):591 e1–e8. Epub 2018/10/07. doi: 10.1016/j.ajog.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31(5):370–9. Epub 2013/08/13. doi: 10.1055/s-0033-1348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei JJ, Chiriboga L, Arslan AA, Melamed J, Yee H, Mittal K. Ethnic differences in expression of the dysregulated proteins in uterine leiomyomata. Hum Reprod. 2006;21(1):57–67. Epub 2005/09/21. doi: 10.1093/humrep/dei309. [DOI] [PubMed] [Google Scholar]
- 32.Pan Q, Luo X, Chegini N. Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians. Reprod Biol Endocrinol. 2007;5:34. Epub 2007/08/25. doi: 10.1186/1477-7827-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer. 2012;19(4):541–56. Epub 2012/06/12. doi: 10.1530/ERC-12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46(4):336–47. Epub 2007/01/24. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 35.Crenshaw K Mapping the Margins: Intersectionality, Identity Politics, and Violence against Women of Color. Stanford Law Review. 1991;43:1241–99. doi: 10.2307/1229039. [DOI] [Google Scholar]
- 36.Essed P Understanding Everyday Racism: An Interdisciplinary Theory: Sage Publishing; 1991. [Google Scholar]
- 37.Lewis J, Mendenhall R, Harwood S, Browne Huntt M. Coping with Gendered Racial Microaggressions among Black Women College Students. Journal of African American Studies. 2013;17(1):51–73. doi: 10.1007/s12111-012-9219-0. [DOI] [Google Scholar]
- 38.Hernandez DC, Reesor LM, Murillo R. Food insecurity and adult overweight/obesity: Gender and race/ethnic disparities. Appetite. 2017;117:373–8. Epub 2017/07/26. doi: 10.1016/j.appet.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Bild DE, Jacobs DR Jr., Sidney S, Haskell WL, Anderssen N, Oberman A. Physical activity in young black and white women. The CARDIA Study. Ann Epidemiol. 1993;3(6):636–44. Epub 1993/11/01. doi: 10.1016/1047-2797(93)90087-k. [DOI] [PubMed] [Google Scholar]
- 40.Weishaar T, Rajan S, Keller B. Probability of Vitamin D Deficiency by Body Weight and Race/Ethnicity. J Am Board Fam Med. 2016;29(2):226–32. Epub 2016/03/10. doi: 10.3122/jabfm.2016.02.150251. [DOI] [PubMed] [Google Scholar]
- 41.James-Todd T, Senie R, Terry MB. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immigr Minor Health. 2012;14(3):506–11. Epub 2011/06/01. doi: 10.1007/s10903-011-9482-5. [DOI] [PubMed] [Google Scholar]
- 42.Rumph JT, Stephens VR, Martin JL, Brown LK, Thomas PL, Cooley A, et al. Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health. Int J Environ Res Public Health. 2022;19(3). Epub 2022/02/16. doi: 10.3390/ijerph19031257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo B, Kravitz-Wirtz N, Sass V, Crowder K, Teixeira S, Takeuchi DT. Residential Segregation and Racial/Ethnic Disparities in Ambient Air Pollution. Race Soc Probl. 2019;11(1):60–7. Epub 2019/08/24. doi: 10.1007/s12552-018-9254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin H, Lin Z, Vasquez E, Xu L. The association between chronic psychological stress and uterine fibroids risk: A meta-analysis of observational studies. Stress Health. 2019;35(5):585–94. Epub 2019/08/28. doi: 10.1002/smi.2895. [DOI] [PubMed] [Google Scholar]
- 45.Vines AI, Nguyen TTX, Ta M, Esserman D, Baird DD. Self-Reported Daily Stress, Squelching of Anger and the Management of Daily Stress and the Prevalence of Uterine Leiomyomata: The Ultrasound Screening Study. Stress Health. 2011;27(3):e188–e94. Epub 2011/08/01. doi: 10.1002/smi.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise LA, Palmer JR, Cozier YC, Hunt MO, Stewart EA, Rosenberg L. Perceived racial discrimination and risk of uterine leiomyomata. Epidemiology. 2007;18(6):747–57. doi: 10.1097/EDE.0b013e3181567e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise LA, Palmer JR, Rosenberg L. Lifetime abuse victimization and risk of uterine leiomyomata in black women. Am J Obstet Gynecol. 2013;208(4):272 e1–e13. Epub 2013/01/09. doi: 10.1016/j.ajog.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zota AR, Chu MT, Marfori CQ, Khati NJ, Al-Hendy A, Taggart T. Adverse childhood experiences and health-related quality of life among women undergoing hysterectomy for uterine leiomyoma. Am J Obstet Gynecol. 2022;227(2):351–3 e5. Epub 2022/04/25. doi: 10.1016/j.ajog.2022.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boynton-Jarrett R, Rich-Edwards JW, Jun HJ, Hibert EN, Wright RJ. Abuse in childhood and risk of uterine leiomyoma: the role of emotional support in biologic resilience. Epidemiology. 2011;22(1):6–14. Epub 2010/11/12. doi: 10.1097/EDE.0b013e3181ffb172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mersky JP, Choi C, Plummer Lee C, Janczewski CE. Disparities in adverse childhood experiences by race/ethnicity, gender, and economic status: Intersectional analysis of a nationally representative sample. Child Abuse Negl. 2021;117:105066. Epub 2021/04/13. doi: 10.1016/j.chiabu.2021.105066. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Monnat SM. Racial/ethnic differences in clusters of adverse childhood experiences and associations with adolescent mental health. SSM Popul Health. 2022;17:100997. Epub 2022/01/06. doi: 10.1016/j.ssmph.2021.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maguire-Jack K, Lanier P, Lombardi B. Investigating racial differences in clusters of adverse childhood experiences. Am J Orthopsychiatry. 2020;90(1):106–14. Epub 2019/03/01. doi: 10.1037/ort0000405. [DOI] [PubMed] [Google Scholar]
- 53.Wise LA, Radin RG, Kumanyika SK, Ruiz-Narvaez EA, Palmer JR, Rosenberg L. Prospective study of dietary fat and risk of uterine leiomyomata. Am J Clin Nutr. 2014;99(5):1105–16. doi: 10.3945/ajcn.113.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wise LA, Radin RG, Palmer JR, Kumanyika SK, Boggs DA, Rosenberg L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr. 2011;94(6):1620–31. doi: 10.3945/ajcn.111.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum Reprod. 2004;19(8):1746–54. Epub 2004/06/26. doi: 10.1093/humrep/deh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Andaloussi A, Chaudhry Z, Al-Hendy A, Ismail N. Uterine Fibroids: Bridging Genomic Defects and Chronic Inflammation. Semin Reprod Med. 2017;35(6):494–8. Epub 2017/11/04. doi: 10.1055/s-0037-1607240. [DOI] [PubMed] [Google Scholar]
- 57.AlAshqar A, Reschke L, Kirschen GW, Borahay MA. Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapiesdagger. Biol Reprod. 2021;105(1):7–31. Epub 2021/03/20. doi: 10.1093/biolre/ioab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Association of physical activity with development of uterine leiomyoma. Am J Epidemiol. 2007;165(2):157–63. Epub 2006/11/09. doi: 10.1093/aje/kwj363. [DOI] [PubMed] [Google Scholar]
- 59.Brasky TM, Bethea TN, Wesselink AK, Wegienka GR, Baird DD, Wise LA. Dietary Fat Intake and Risk of Uterine Leiomyomata: A Prospective Ultrasound Study. Am J Epidemiol. 2020;189(12):1538–46. Epub 2020/06/20. doi: 10.1093/aje/kwaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiaffarino F, Parazzini F, La Vecchia C, Chatenoud L, Di Cintio E, Marsico S. Diet and uterine myomas. Obstet Gynecol. 1999;94(3):395–8. Epub 1999/09/03. doi: 10.1016/s0029-7844(99)00305-1. [DOI] [PubMed] [Google Scholar]
- 61.Sparic R, Mirkovic L, Malvasi A, Tinelli A. Epidemiology of Uterine Myomas: A Review. Int J Fertil Steril. 2016;9(4):424–35. Epub 2016/03/18. doi: 10.22074/ijfs.2015.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton DT, Morris M. The racial disparities in STI in the U.S.: Concurrency, STI prevalence, and heterogeneity in partner selection. Epidemics. 2015;11:56–61. Epub 2015/05/17. doi: 10.1016/j.epidem.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore KR, Harmon QE, Zhao S, Taylor BD, Baird DD. Bacterial Vaginosis and Prospective Ultrasound Measures of Uterine Fibroid Incidence and Growth. Epidemiology. 2022;33(3):415–21. Epub 2022/01/25. doi: 10.1097/EDE.0000000000001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore KR, Harmon QE, Baird DD. Herpes Simplex Virus Type 2 Seroprevalence and Incidence and Growth of Ultrasound-Diagnosed Uterine Fibroids in a Large Population of Young African-American Women. Am J Epidemiol. 2021;190(10):2158–62. Epub 2021/05/27. doi: 10.1093/aje/kwab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore KR, Smith JS, Cole SR, Dittmer DP, Schoenbach VJ, Baird DD. Chlamydia trachomatis Seroprevalence and Ultrasound-Diagnosed Uterine Fibroids in a Large Population of Young African-American Women. Am J Epidemiol. 2018;187(2):278–86. Epub 2017/06/24. doi: 10.1093/aje/kwx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wise LA, Palmer JR, Reich D, Cozier YC, Rosenberg L. Hair relaxer use and risk of uterine leiomyomata in African-American women. Am J Epidemiol. 2012;175(5):432–40. doi: 10.1093/aje/kwr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaston SA, James-Todd T, Harmon Q, Taylor KW, Baird D, Jackson CL. Chemical/straightening and other hair product usage during childhood, adolescence, and adulthood among African-American women: potential implications for health. J Expo Sci Environ Epidemiol. 2020;30(1):86–96. Epub 2019/10/24. doi: 10.1038/s41370-019-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217(4):418 e1–e6. Epub 2017/08/20. doi: 10.1016/j.ajog.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bryant T Refinery29. 2016. [cited 2023 01/04/2023]. Available from: https://www.refinery29.com/en-us/2016/02/103964/black-hair-care-makeup-business.
- 70.Strachan DD, Okereke U. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2012;66(1):157–8; author reply 8–9. Epub 2011/12/20. doi: 10.1016/j.jaad.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 71.Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20(1):81–4. Epub 2005/04/06. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Mahalingaiah S, Hart JE, Wise LA, Terry KL, Boynton-Jarrett R, Missmer SA. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses’ Health Study II. Am J Epidemiol. 2014;179(2):186–91. Epub 2013/10/22. doi: 10.1093/aje/kwt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zota AR, Geller RJ, Calafat AM, Marfori CQ, Baccarelli AA, Moawad GN. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111(1):112–21. Epub 2018/11/19. doi: 10.1016/j.fertnstert.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J, Clark LP, Bechle MJ, Hajat A, Kim SY, Robinson AL, et al. Disparities in Air Pollution Exposure in the United States by Race/Ethnicity and Income, 1990–2010. Environ Health Perspect. 2021;129(12):127005. Epub 2021/12/16. doi: 10.1289/EHP8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park YM, Kwan MP. Understanding Racial Disparities in Exposure to Traffic-Related Air Pollution: Considering the Spatiotemporal Dynamics of Population Distribution. Int J Environ Res Public Health. 2020;17(3). Epub 2020/02/07. doi: 10.3390/ijerph17030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin CY, Wang CM, Chen ML, Hwang BF. The effects of exposure to air pollution on the development of uterine fibroids. Int J Hyg Environ Health. 2019;222(3):549–55. Epub 2019/03/02. doi: 10.1016/j.ijheh.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Mahalingaiah S, Hart JE, Laden F, Terry KL, Boynton-Jarrett R, Aschengrau A, et al. Air pollution and risk of uterine leiomyomata. Epidemiology. 2014;25(5):682–8. Epub 2014/05/13. doi: 10.1097/EDE.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wise LA, Palmer JR, Rothman EF, Rosenberg L. Childhood abuse and early menarche: findings from the black women’s health study. Am J Public Health. 2009;99 Suppl 2(Suppl 2):S460-6. Epub 2009/05/16. doi: 10.2105/AJPH.2008.149005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braithwaite D, Moore DH, Lustig RH, Epel ES, Ong KK, Rehkopf DH, et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 2009;20(5):713–20. Epub 2008/12/25. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- 80.D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR, Sandler DP. Prenatal and infant exposures and age at menarche. Epidemiology. 2013;24(2):277–84. Epub 2013/01/26. doi: 10.1097/EDE.0b013e31828062b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111(1):110–3. [DOI] [PubMed] [Google Scholar]
- 82.Velez Edwards DR, Baird DD, Hartmann KE. Association of age at menarche with increasing number of fibroids in a cohort of women who underwent standardized ultrasound assessment. Am J Epidemiol. 2013;178(3):426–33. Epub 2013/07/03. doi: 10.1093/aje/kws585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159(2):113–23. Epub 2004/01/14. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.VanNoy BN, Bowleg L, Marfori C, Moawad G, Zota AR. Black Women’s Psychosocial Experiences with Seeking Surgical Treatment for Uterine Fibroids: Implications for Clinical Practice. Womens Health Issues. 2021;31(3):263–70. Epub 2021/02/22. doi: 10.1016/j.whi.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orellana M, Riggan KA, K DS, Stewart EA, Venable S, Balls-Berry JE, et al. Perceptions of Ethnoracial Factors in the Management and Treatment of Uterine Fibroids. J Racial Ethn Health Disparities. 2022;9(4):1184–91. Epub 2021/05/21. doi: 10.1007/s40615-021-01059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sengoba KS, Ghant MS, Okeigwe I, Mendoza G, Marsh EE. Racial/Ethnic Differences in Women’s Experiences with Symptomatic Uterine Fibroids: a Qualitative Assessment. J Racial Ethn Health Disparities. 2017;4(2):178–83. Epub 2016/04/14. doi: 10.1007/s40615-016-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Price JT, Zimmerman LD, Koelper NC, Sammel MD, Lee S, Butts SF. Social determinants of access to minimally invasive hysterectomy: reevaluating the relationship between race and route of hysterectomy for benign disease. Am J Obstet Gynecol. 2017;217(5):572 e1–e10. Epub 2017/08/09. doi: 10.1016/j.ajog.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 88.Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt). 2013;22(10):807–16. Epub 2013/09/17. doi: 10.1089/jwh.2013.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wegienka G, Stewart EA, Nicholson WK, Zhang S, Li F, Thomas L, et al. Black Women Are More Likely Than White Women to Schedule a Uterine-Sparing Treatment for Leiomyomas. J Womens Health (Larchmt). 2021;30(3):355–66. Epub 2021/02/02. doi: 10.1089/jwh.2020.8634. [DOI] [PubMed] [Google Scholar]
- 90.Wechter ME, Stewart EA, Myers ER, Kho RM, Wu JM. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205(5):492 e1–5. doi: 10.1016/j.ajog.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–55. Epub 1994/04/01. [DOI] [PubMed] [Google Scholar]
- 92.Owens DC. Medical Bondage: Race, Gender, and the Origins of American Gynecology. Georgia: University of Georgia Press; 2017. [Google Scholar]
- 93.Roberts D Killing the Black Body: Race, Reproduction, and the meaning of Liberty. New York, New York: Pantheon Books; 1997. [Google Scholar]
- 94.Prather C, Fuller TR, Marshall KJ, Jeffries WLt. The Impact of Racism on the Sexual and Reproductive Health of African American Women. J Womens Health (Larchmt). 2016;25(7):664–71. doi: 10.1089/jwh.2015.5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehta A, Xu T, Hutfless S, Makary MA, Sinno AK, Tanner EJ 3rd, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216(5):497 e1–e10. Epub 2016/12/31. doi: 10.1016/j.ajog.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abenhaim HA, Azziz R, Hu J, Bartolucci A, Tulandi T. Socioeconomic and racial predictors of undergoing laparoscopic hysterectomy for selected benign diseases: analysis of 341487 hysterectomies. J Minim Invasive Gynecol. 2008;15(1):11–5. Epub 2008/02/12. doi: 10.1016/j.jmig.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 97.Robinson WR, Cheng MM, Howard AG, Carpenter WR, Brewster WR, Doll KM. For U.S. Black women, shift of hysterectomy to outpatient settings may have lagged behind White women: a claims-based analysis, 2011–2013. BMC Health Serv Res. 2017;17(1):526. doi: 10.1186/s12913-017-2471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knisely A, Huang Y, Melamed A, Gockley A, Tergas AI, St Clair CM, et al. Disparities in Access to High-Volume Surgeons Within High-Volume Hospitals for Hysterectomy. Obstet Gynecol. 2021;138(2):208–17. Epub 2021/07/09. doi: 10.1097/AOG.0000000000004456. [DOI] [PubMed] [Google Scholar]
- 99.Alexander AL, Strohl AE, Rieder S, Holl J, Barber EL. Examining Disparities in Route of Surgery and Postoperative Complications in Black Race and Hysterectomy. Obstet Gynecol. 2019;133(1):6–12. Epub 2018/12/12. doi: 10.1097/AOG.0000000000002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carey C, Katon J, Bossick AS, Gray K, Doll K, Christy A, et al. Uterine Weight as a Modifier of Black/White Racial Disparities in Minimally Invasive Hysterectomy Among Veterans with Fibroids in the Veterans Health Administration. Health Equity. 2022;6(1). Epub 16 December 2022. doi: 10.1089/heq.2022.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ko JS, Suh CH, Huang H, Zhuo H, Harmanli O, Zhang Y. Association of Race/Ethnicity with Surgical Route and Perioperative Outcomes of Hysterectomy for Leiomyomas. J Minim Invasive Gynecol. 2021;28(7):1403–10 e2. Epub 2020/11/27. doi: 10.1016/j.jmig.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 102.Stentz NC, Cooney LG, Sammel MD, Shah DK. Association of Patient Race With Surgical Practice and Perioperative Morbidity After Myomectomy. Obstet Gynecol. 2018;132(2):291–7. Epub 2018/07/12. doi: 10.1097/AOG.0000000000002738. [DOI] [PubMed] [Google Scholar]
- 103.Callegari LS, Katon JG, Gray KE, Doll K, Pauk S, Lynch KE, et al. Associations between Race/Ethnicity, Uterine Fibroids, and Minimally Invasive Hysterectomy in the VA Healthcare System. Womens Health Issues. 2019;29(1):48–55. Epub 2018/10/09. doi: 10.1016/j.whi.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Katon JG, Bossick AS, Doll KM, Fortney J, Gray KE, Hebert P, et al. Contributors to Racial Disparities in Minimally Invasive Hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57(12):930–6. Epub 2019/11/16. doi: 10.1097/MLR.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 105.Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–56. Epub 2020/03/27. doi: 10.1056/NEJMra1810764. [DOI] [PubMed] [Google Scholar]
- 106.Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol. 2017;209:3–7. Epub 2016/05/25. doi: 10.1016/j.ejogrb.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 107.Soliman AM, Coyne KS, Gries KS, Castelli-Haley J, Snabes MC, Surrey ES. The Effect of Endometriosis Symptoms on Absenteeism and Presenteeism in the Workplace and at Home. J Manag Care Spec Pharm. 2017;23(7):745–54. Epub 2017/06/27. doi: 10.18553/jmcp.2017.23.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soliman AM, Surrey E, Bonafede M, Nelson JK, Castelli-Haley J. Real-World Evaluation of Direct and Indirect Economic Burden Among Endometriosis Patients in the United States. Adv Ther. 2018;35(3):408–23. Epub 2018/02/17. doi: 10.1007/s12325-018-0667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–9. Epub 2012/03/17. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 110.Christ JP, Yu O, Schulze-Rath R, Grafton J, Hansen K, Reed SD. Incidence, prevalence, and trends in endometriosis diagnosis: a United States population-based study from 2006 to 2015. Am J Obstet Gynecol. 2021;225(5):500 e1–e9. Epub 2021/06/21. doi: 10.1016/j.ajog.2021.06.067. [DOI] [PubMed] [Google Scholar]
- 111.Fuldeore MJ, Soliman AM. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol Obstet Invest. 2017;82(5):453–61. Epub 2016/11/08. doi: 10.1159/000452660. [DOI] [PubMed] [Google Scholar]
- 112.Hemmert R, Schliep KC, Willis S, Peterson CM, Louis GB, Allen-Brady K, et al. Modifiable life style factors and risk for incident endometriosis. Paediatr Perinat Epidemiol. 2019;33(1):19–25. Epub 2018/10/12. doi: 10.1111/ppe.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brzezinski A, Koren Z, Kedar S. Contribution to the problem of the etiology of endometriosis. Isr Med J. 1962;21:111–7. Epub 1962/05/01. [PubMed] [Google Scholar]
- 114.Bougie O, Healey J, Singh SS. Behind the times: revisiting endometriosis and race. Am J Obstet Gynecol. 2019;221(1):35 e1–e5. Epub 2019/02/10. doi: 10.1016/j.ajog.2019.01.238. [DOI] [PubMed] [Google Scholar]
- 115.Novak E, Jones G, Novak E. Endometriosis. In: Novak E, editor. Textbook of Gynecology. 6 ed. Baltimore MD: Williams and Wilkin; 1961. p. 247–50. [Google Scholar]
- 116.Kistner R. Endometriosis. In: Kistner R, editor. Gynecology; Principles and Practice. 2 ed. Chicago IL: Mosby; 1971. p. 432–56. [Google Scholar]
- 117.Chatman DL. Endometriosis in the black woman. Am J Obstet Gynecol. 1976;125(7):987–9. Epub 1976/08/01. doi: 10.1016/0002-9378(76)90502-0. [DOI] [PubMed] [Google Scholar]
- 118.Bougie O, Yap MI, Sikora L, Flaxman T, Singh S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG. 2019;126(9):1104–15. Epub 2019/03/26. doi: 10.1111/1471-0528.15692. [DOI] [PubMed] [Google Scholar]
- 119.Lloyd FP. Endometriosis in the Negro Woman: A Five Year Study. Am J Obstet Gynecol. 1964;89:468–9. Epub 1964/06/15. doi: 10.1016/0002-9378(64)90549-6. [DOI] [PubMed] [Google Scholar]
- 120.Cavanagh WV. Fertility in the etiology of endometriosis. Am J Obstet Gynecol. 1951;61(3):539–47. Epub 1951/03/01. doi: 10.1016/0002-9378(51)91399-3. [DOI] [PubMed] [Google Scholar]
- 121.Wesselink AK, Bethea TN, McClean M, Weuve J, Williams PL, Hauser R, et al. Predictors of plasma polychlorinated biphenyl concentrations among reproductive-aged black women. Int J Hyg Environ Health. 2019;222(7):1001–10. Epub 2019/07/10. doi: 10.1016/j.ijheh.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kyama CM, Mwenda JM, Machoki J, Mihalyi A, Simsa P, Chai DC, et al. Endometriosis in African women. Womens Health (Lond). 2007;3(5):629–35. Epub 2007/09/01. doi: 10.2217/17455057.3.5.629. [DOI] [PubMed] [Google Scholar]
- 123.Orlando MS, Luna Russo MA, Richards EG, King CR, Park AJ, Bradley LD, et al. Racial and ethnic disparities in surgical care for endometriosis across the United States. Am J Obstet Gynecol. 2022;226(6):824 e1–e11. Epub 2022/02/02. doi: 10.1016/j.ajog.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 124.Movilla P, van Reesema L, Andrews B, Gaughan T, Loring M, Bhakta A, et al. Impact of Race and Ethnicity on Perioperative Outcomes During Hysterectomy for Endometriosis. J Minim Invasive Gynecol. 2022. Epub 2022/09/22. doi: 10.1016/j.jmig.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Cromeens MG, Thoyre S, Carey ET, Knafl K, Robinson WR. Inquiry into women’s pathways to diagnosis of endometriosis: A qualitative study protocol. J Adv Nurs. 2021;77(2):1017–26. Epub 2020/10/28. doi: 10.1111/jan.14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pollack LM, Olsen MA, Gehlert SJ, Chang SH, Lowder JL. Racial/Ethnic Disparities/Differences in Hysterectomy Route in Women Likely Eligible for Minimally Invasive Surgery. J Minim Invasive Gynecol. 2020;27(5):1167–77 e2. Epub 2019/09/14. doi: 10.1016/j.jmig.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Downey MM, Gomez AM. Structural Competency and Reproductive Health. AMA J Ethics. 2018;20(3):211–23. Epub 2018/03/16. doi: 10.1001/journalofethics.2018.20.3.peer1-1803. [DOI] [PubMed] [Google Scholar]


