Abstract
Background:
Stressful early-life experiences increase the risk of developing an alcohol use disorder. We had previously found that male C57BL/6J mice reared under limited bedding and nesting (LBN) conditions, a model of early-life adversity, escalate their ethanol intake in limited-access two-bottle choice (2BC) sessions faster than control (CTL)-reared counterparts when exposed to chronic intermittent ethanol (CIE) vapor inhalation. However, the alcohol consumption of female littermates was not affected by LBN or CIE. In the present study, we sought to determine whether this phenotype reflected a general insensitivity of female mice to the influence of early life stress on alcohol responses.
Methods:
In a first experiment, CTL and LBN females with a history of 2BC combined or not with CIE were tested in affective and nociceptive assays during withdrawal. In a second group of CTL and LBN females, ethanol-induced antinociception, sedation, plasma clearance, and c-Fos induction were examined.
Results:
In females withdrawn from chronic 2BC, CIE increased digging, reduced grooming, and increased immobility in the tail suspension test regardless of early-life history. In contrast, LBN rearing lowered mechanical nociceptive thresholds regardless of CIE exposure. In females acutely treated with ethanol, LBN rearing facilitated antinociception and delayed the onset of sedation without influencing ethanol clearance rate or c-Fos induction in the paraventricular nucleus of the hypothalamus, paraventricular nucleus of the thalamus, central nucleus of the amygdala, or auditory cortex.
Conclusion:
CIE withdrawal produced multiple indices of negative affect in C57BL/6J females, suggesting that their motivation to consume alcohol may differ from air-exposed counterparts despite equivalent intake. Contrasted with our previous findings in males, LBN-induced mechanical hyperalgesia in chronic alcohol drinkers was specific to females. Lower nociceptive thresholds combined with increased sensitivity to the acute antinociceptive effect of ethanol may contribute to reinforcing ethanol consumption in LBN females but are not sufficient to escalate their intake.
Keywords: Early-life stress, resilience, vulnerability, hyperkatifeia, pain
Introduction
Stress influences acute sensitivity to alcohol and voluntary alcohol consumption in both humans and rodents (Becker, 2017; Peltier et al., 2019). The effects of stress can be enduring, as exemplified by the life-long consequences of adverse childhood experiences on mental health outcomes and addiction vulnerability, including a higher risk to develop an alcohol use disorder (AUD) (Enoch, 2011). Animal models have been used to investigate the molecular mechanisms that underlie the long-lasting effects of early-life adversity (ELA) on these outcomes (Bath, 2020; Chen & Baram, 2016; Walters & Kosten, 2019).
We recently reported that a combination of limited bedding and nesting (LBN), which results in fragmented and unpredictable maternal care (Rice et al., 2008), and chronic intermittent ethanol vapor inhalation (CIE), which leads to a gradual escalation of voluntary alcohol intake (Becker & Lopez, 2004), could serve as a novel model of AUD vulnerability induced by ELA in C57BL/6J mice, as LBN facilitated CIE-induced alcohol drinking escalation (Okhuarobo et al., 2020a). This phenotype, however, was only observed in males and neither CIE nor LBN influenced the ethanol intake of their female littermates (Okhuarobo et al., 2020a). In the present study, we sought to determine whether this sexual dimorphism reflected a general resilience of female mice to the influence of ELA and alcohol withdrawal.
AUD is associated with affective dysfunction, which serves as a source of negative reinforcement, whereby alcohol is consumed to mitigate negative affect (Koob, 2022). AUD is also associated with enhanced pain sensitivity, which can further fuel the motivation to consume alcohol (Cucinello-Ragland & Edwards, 2021). Accordingly, we reasoned that the absence of alcohol drinking escalation in control and LBN-reared females might be associated with a lack of affective or nociceptive effects of CIE withdrawal in this sex. The females whose ethanol intake was reported in our original publication (Okhuarobo et al., 2020a) were thus subjected to a battery of tests assessing their emotional and nociceptive states during CIE withdrawal. In contrast to our prediction, CIE-withdrawn females displayed multiple indices of negative affect, regardless of their early-life history. Moreover, LBN-reared females exhibited marked hyperalgesia during withdrawal from chronic alcohol drinking, even in the absence of CIE exposure, raising the possibility that alcohol drinking in LBN-reared females might be driven by the relief of hyperalgesia.
While the acute antinociceptive effect of alcohol is thought to motivate alcohol drinking in AUD (Cucinello-Ragland & Edwards, 2021), there is an inverse relationship between sensitivity to the sedative effect of alcohol intoxication and heavy drinking (King et al., 2011; Quinn & Fromme, 2011). Furthermore, different modalities of chronic stress were shown to modulate the sensitivity to alcohol-induced sedation in rodents (Boyce-Rustay et al., 2007; Doremus-Fitzwater et al., 2018; Drugan et al., 1992; Fernández et al., 2016; Jones et al., 1990; Matsumoto et al., 1997; Parker et al., 2008), suggesting that ELA could impact this variable.
In a second experiment, we therefore tested whether LBN rearing might affect alcohol-induced antinociception and sedation in females. Alcohol clearance rate was measured to rule out potential metabolic differences. We also examined the influence of LBN on the cellular response to alcohol by quantifying c-Fos induction in three brain regions known to be sensitive to acute intoxication in C57BL/6J mice (Hitzemann & Hitzemann, 1997; Rhinehart et al., 2020): paraventricular nucleus of the hypothalamus (PVN), paraventricular nucleus of the thalamus (PVT), and central nucleus of the amygdala (CeA). We also noted a strong induction of c-Fos expression by ethanol in deep layers of the auditory cortex (AuC), which we quantified.
In summary, as a follow-up to our previous study, the present study sought to probe the influence of ELA on behavioral and cellular responses to acute and chronic alcohol exposure in C57BL/6J female mice.
Materials and Methods
Animals
Virgin 8-week-old female and male C57BL/6J mice (stock #000664) were obtained from The Jackson Laboratory (Sacramento, CA) and housed in an uncrowded, quiet animal facility room on a 12-hour light/dark cycle with free access to lab chow and water. Beginning on postnatal day (P)75, 2–3 females were paired with each male for breeding. Females were examined daily for evidence of a vaginal plug (confirmation of successful mating, considered to be embryonic day [E]0). Pregnant females were separated on E17, prior to parturition. Dams were checked for parturition every 12 h, and the day of birth was considered P0. LBN was implemented from P2 to P9.
The offspring were transferred from the University of California Irvine (UCI) to The Scripps Research Institute (TSRI) when they were 3.5–5 weeks old. Behavioral testing was conducted in adulthood when the mice were at least 10 weeks old. The mice were first habituated for at least one week to the procedure room in which they were housed and tested. Mice were housed in individual static cages lined with Sani-Chip bedding (Envigo) and provided with ad libitum access to reverse osmosis water and 7012 feed (Envigo).
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of UCI and TSRI.
Early-life manipulation
ELA was induced P2-P9 via an impoverished environment with limited bedding and nesting (referred to as LBN), as described previously (Rice et al., 2008). Briefly, on the morning of P2, litters were adjusted to 5–8 pups if needed. Control (CTL) dams and litters were placed in cages with standard amounts of corn husk bedding (~650 ml) and one square piece of cotton-like nesting material measuring 5 cm × 5 cm. LBN dams and litters were placed in cages fitted with a fine-gauge plastic-coated aluminum mesh platform (mesh dimensions 0.4 × 0.9 cm, catalog no. 57398; McNichols Co.) sitting ~2.5 cm above the cage floor. Bedding was reduced to only sparsely cover the cage floor (~60 ml) and one-half of a square of nesting material was provided. Both groups were completely undisturbed until the morning of P10, at which point all cages were changed to standard cages with ample bedding and nesting material. At P21, pups were weaned and housed with same-sex littermates in groups of 3–5.
Chemicals
Ethanol was obtained from PHARMCO/Greenfield Global; 200-proof ethanol was used for drinking solutions and intraperitoneal (i.p.) administration while 190-proof ethanol was used for vaporization in inhalation chambers. Ethanol (15% v:v) drinking solutions were prepared using reverse osmosis water. Pyrazole was obtained from Sigma-Aldrich and dissolved in either 20% v:v ethanol or saline. Fresh solutions were prepared weekly. For i.p. administration, different volumes of 20% v:v ethanol were injected to yield the appropriate dose of ethanol (e.g., 0.1 mL/10 g body weight for a dose of 1.5 g/kg).
Experiment 1.
This experiment used 14 females from 4 CTL litters and 9 females from 5 LBN litters. These mice were subjected to weeks of limited-access two-bottle choice (2BC) alcohol drinking alternated with weeks of CIE (CTL, n=7; LBN, n=5) or Air (CTL, n=7; LBN, n=4) exposure, for a total of 6 rounds. Their body weights, ethanol intake, and blood alcohol levels (BALs) during CIE were reported in our previous publication (Okhuarobo et al., 2020a). Following withdrawal from CIE/2BC, they were subjected to a series of affective tests (see Fig. 1A for timeline). Mice were then exposed to an additional week of CIE or Air and mechanical nociception was measured 32 h after the last vapor exposure. Two mice (CTL-CIE, n=1; LBN-CIE, n=1) died during this last CIE week. All tests were conducted in the dark phase of the circadian cycle under red lighting.
Figure 1. Affective and nociceptive phenotypes associated with withdrawal from moderate and excessive alcohol drinking in C57BL/6J females raised under control (CTL) or limited bedding and nesting (LBN) conditions (Experiment 1).
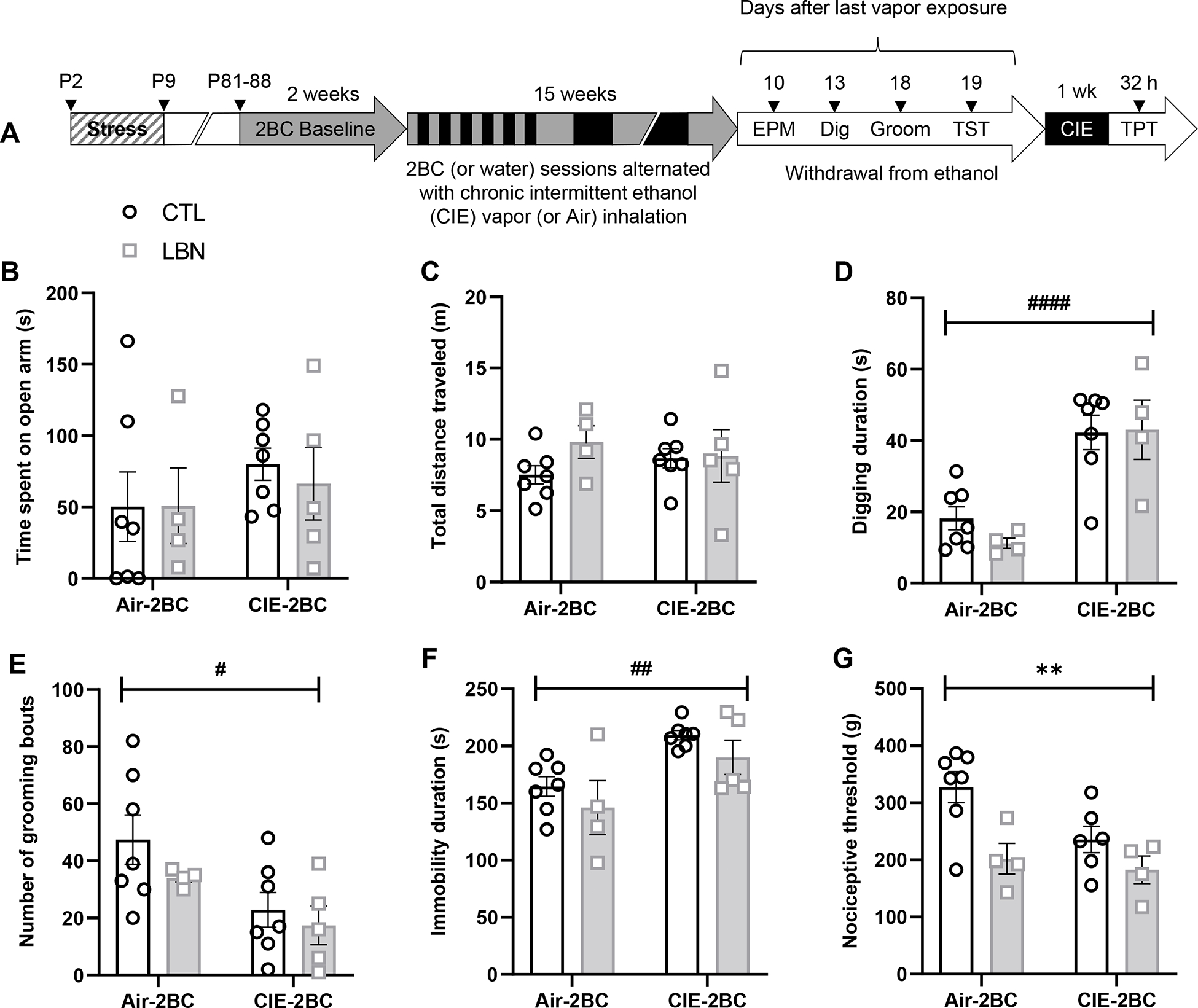
A. Experimental timeline (EPM, elevated plus-maze; TST, tail suspension test; TPT, tail pressure test). B-C. EPM: % time spent on open arms (B) and total distance traveled (C). D. Digging test: digging duration. ####, main effect of CIE, p<0.0001. E. Splash test: number of grooming bouts. #, main effect of CIE, p<0.05. F. TST: immobility duration. ##, main effect of CIE, p<0.01. G. TPT: nociceptive threshold. **, main effect of LBN, p<0.01.
Alcohol exposure
Procedures for alcohol drinking (2BC) and vapor exposure (CIE) are described in detail in our original publication (Okhuarobo et al., 2020a). Briefly, mice were given access to two bottles containing ethanol (15% v:v) and water in their home cage for 2 h starting at the beginning of the dark phase. A single bottle of water was available the rest of the time. For inhalation, ethanol was dripped into a heated flask and an air pump conveyed vaporized ethanol into chambers. The drip rate was adjusted to yield target BALs of 150–250 mg/dL. Mice received an i.p. injection of ethanol (1.5 g/kg) and pyrazole (68 mg/kg) before each 16-h ethanol vapor inhalation session. Control mice (Air-2BC) breathed room air and received pyrazole. CIE weeks consisted of 4 cycles of 16-h intoxication followed by 8-h withdrawal.
Elevated plus maze (EPM) test
The EPM apparatus consisted of a 5 cm × 5 cm central square connected to two opposite open arms and two opposite closed arms. Each arm was 30-cm long and 5-cm wide. The closed arms had 15-cm high walls while the open arms had 3-mm high ledges. The runway was placed on top of a 30-cm high stand. The runway floors were made of matte gray acrylic, all other surfaces were made of clear acrylic. The mouse was placed in the central square of the apparatus, facing a closed arm, and allowed to explore freely for 5 min. The apparatus was wiped with 70% ethanol in between mice. The test was recorded by a camera mounted above the EPM and connected to a computer. The distance travelled, number of entries, and time spent in each area of the EPM were calculated by ANY-maze (Stoelting Co.). The total distance traveled was used as an index of locomotor activity and the time spent in the open arms was used as an index of anxiety-like behavior, as previously described (Komada et al., 2008). The apparatus design (open arms with ledges and closed arms with transparent walls) was meant to encourage exploration of the open arms and facilitate the detection of an anxiogenic-like effect of alcohol withdrawal (Horii & Kawaguchi, 2015).
Digging test
Digging activity was assessed according to Deacon (2006). The mouse was placed in the middle of a standard acrylic mouse cage (with no lid) filled with a 5-cm depth of Sani-Chip bedding (Envigo). The latency to dig, number of digging bouts, and duration of digging were measured using stopwatches and a tally counter for a total duration of 3 min. Bedding was changed between each mouse. Digging is a spontaneous, species-typical behavior performed by wild mice foraging for buried food in their natural habitats and exhibited by laboratory mice placed on a thick layer of bedding substrate. Digging behavior is sensitive to multiple psychotropic drugs (see Deacon, 2006 and references therein) and is robustly increased during withdrawal from CIE (Bloch et al., 2022; Sidhu et al., 2018). One LBN-CIE mouse was identified as an outlier using the Grubbs’ test (Grubbs, 1969) and was therefore excluded from analysis.
Splash test
The splash test was conducted in the home cage using the method of Ducottet et al. (2004). A solution of 10% sucrose was sprayed on the dorsal coat of the mouse using a single squirt from a standard gardening spray bottle in mist position. The latency to groom, the number of grooming bouts and duration of grooming were measured using stopwatches and a tally counter for a total duration of 5 min. Grooming activity is used as an index of self-care that is degraded in mouse models of depressive-like behavior (Yalcin et al., 2005).
Tail suspension test (TST)
This test was used to assess the coping strategy used by mice facing an acute, inescapable stress (Cryan et al., 2005). The tail of the mouse was inserted in a hollow cylinder (3.5-cm length, 1-cm diameter, 1 g) to prevent tail climbing as described by Can et al. (2012). The 2-cm end of a 17-cm piece of tape was adhered to the mouse tail and back to itself, leaving the distal 2–3 mm of the tail protruding out of the tape. The other end of the tape was stuck to a shelf placed 30 cm above the bench, such that the tape hung vertically to the suspension bar. The duration of immobility of each mouse was measured for 6 min. Relative levels of immobility were interpreted in terms of active vs. passive stress-coping strategy (Anyan & Amir, 2018; Commons et al., 2017; Molendijk & de Kloet, 2015).
Tail pressure test (TPT)
Mechanical nociceptive thresholds were assessed by applying pressure on the tail (tail pressure test, TPT) using a digital Randall-Selitto apparatus (Harvard Apparatus), as previously described by Elhabazi and colleagues (2014). The mice were first habituated to enter a restrainer pouch made of woven wire (stainless steel 304L 200 mesh, Shanghai YiKai) over three days. On testing days, the mouse was gently introduced into the restrainer and the distal portion of the tail was positioned under the conical tip of the apparatus. The foot switch was then depressed to apply uniformly increasing pressure onto the tail until the first nociceptive response (struggling or squeaking) occurred. The force (in g) eliciting the nociceptive response was recorded. A cutoff force of 600 g was enforced to prevent tissue damage. The measure was repeated on the medial and proximal parts of the tail of the same mouse, with at least 30 s between each measure. The average of the three measures (distal, medial, proximal) was used for statistical analysis.
Experiment 2.
This experiment used 9 females from 5 CTL litters and 9 females from 6 LBN litters. These mice were tested for antinociception, sedation, plasma clearance, and c-Fos induction following acute administration of ethanol (see Fig. 2A for timeline). All tests were conducted in the light phase of the circadian cycle under white lighting.
Figure 2. Acute behavioral responses to alcohol in C57BL/6J females raised under CTL or LBN conditions (Experiment 2).
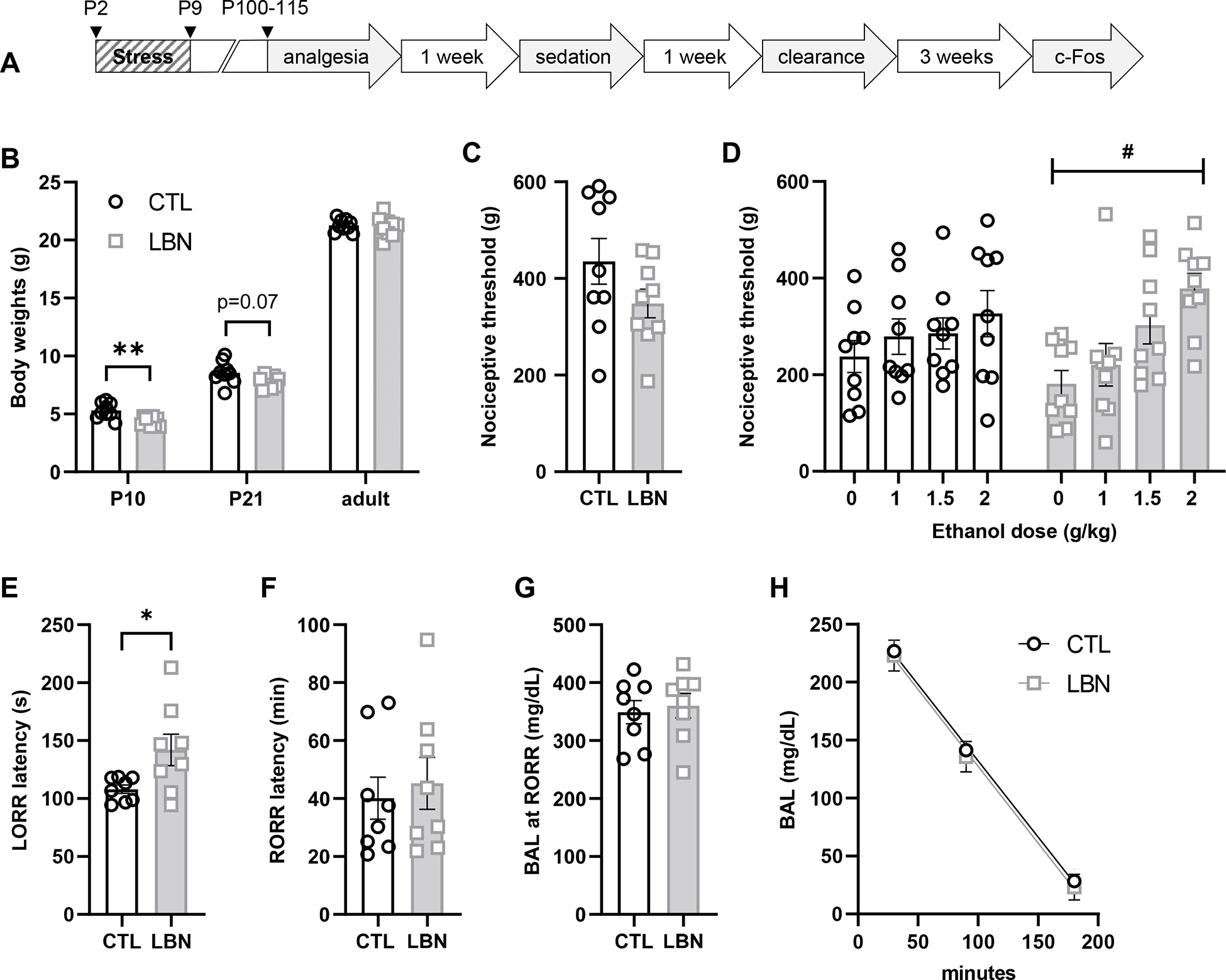
A. Experimental timeline (c-Fos data are presented in Fig. 3). B. Body weights at the end of the early-life manipulation (P10), at weaning (P21) and at the time of behavioral testing (adult). **, effect of LBN, p<0.01. C. Baseline nociceptive thresholds in the tail pressure test. D. Antinociceptive effect of alcohol in the tail pressure test. *, main effect of dose, p<0.05. E-G. Sedative effect of alcohol (3.5 g/kg) in the loss-of-righting-reflex (LORR) assay: latency to LORR (E), latency to regain righting reflex (F), BAL at recovery (G). *, effect of LBN, p<0.05. H. Time-course of alcohol (2 g/kg) clearance from the plasma.
Antinociception
Mechanical nociceptive thresholds were measured in the TPT as described for Experiment 1. Baseline measures were first obtained, and an ethanol dose-response was collected on the following four days according to a within-subject design. Mice received vehicle (saline) and the three test doses (1, 1.5, 2 g/kg) in a counterbalanced order. TPT was conducted 5 min after i.p. injection. We selected this timepoint because the brain concentration of ethanol following i.p. administration in mice reaches its peak within the first 5 minutes (Jamal et al., 2016; Smolen & Smolen, 1989). Moreover, we had previously determined the dose-response of ethanol in C57BL/6J males in this assay and detected a highly significant antinociceptive effect at the 5-min timepoint (Okhuarobo et al., 2020b).
Sedation
Mice were injected i.p. with 3.5 g/kg ethanol. The latencies to lose and regain righting reflex (i.e., ability to return to standing on all four limbs when placed on its back) were recorded. Submandibular blood was collected in an EDTA-coated tube at the time of righting reflex recovery and centrifuged at 13,000 g for 10 min. BALs were measured by gas chromatography and flame ionization detection (Agilent 7820A). One LBN mouse failed to fall asleep and was excluded from analysis.
Ethanol clearance
Mice were injected i.p. with 2 g/kg ethanol. Caudal vein blood was collected 30 min, 90 min, and 180 min following injection using a heparinized capillary tube. Plasma was processed for BAL determination as described above.
c-Fos immunohistochemistry
Mice were injected i.p. with either saline (CTL, n=4; LBN, n=4) or 2 g/kg of ethanol (CTL, n=5; LBN, n=5) 90 min before they were anesthetized with chloral hydrate (35%) and transcardially perfused with cold phosphate-buffered saline (PBS, pH 7.4) followed by 3.7% formaldehyde in phosphate buffer (PB) 0.1 M. Brains were post-fixed in 3.7 % formaldehyde for 4 h at 4°C, cryoprotected in sterile 30% sucrose in PB at 4°C, frozen on dry ice, and stored at -80°C. Coronal 35-μm thick brain sections were sliced with a cryostat, collected in 5 series in PBS containing 0.01% sodium azide, and stored at 4°C. Two brains (CTL-saline, n=1; CTL-ethanol, n=1) had to be excluded from immunohistological processing due to sectioning issues. The sections were rinsed in PBS for 10 min then transferred to a blocking solution for 1 h (0.25% Triton X-100, 5% normal goat serum [NGS], in PBS). The primary anti-c-Fos antibody (rabbit monoclonal, Cell Signaling Technologies #2250) was diluted 1:5000 in PBS, 0.5% Tween 20, 5% NGS, and incubated under slow orbital shaking for 5 days at 4°C. After 6 10-min rinses in PBS, the sections were then incubated with the secondary antibody (AlexaFluor 568-conjugated goat anti-rabbit, Thermo Fisher Scientific A-11011, diluted 1:500 in PBS) at room temperature for 2 h and protected from light from there on. After another 6 10-min rinses in PBS, the sections were then mounted on microscope slides, air dried overnight, coverslipped with Vectashield (HardSet with DAPI, H-1500), and stored at 4°C. Sections were imaged using a Keyence BZX710 fluorescence microscope. Anatomical landmarks were identified at 2x magnification and 10x images were captured for c-Fos signal quantification. For each brain region, the anteroposterior range (mm from bregma) and number of sections analyzed in each mouse were as follows: PVT, −0.5 to −1.6, 5.8 ± 0.5; PVN, −0.7 to −0.9, 2.0 ± 0.; CeA, −0.9 to −1.6, 3.4 ± 0.3; AuC, −1.7 to −2.1, 2.2 ± 0.2 (see Fig. 3A–E for brain section diagrams and representative images).
Figure 3. Acute cellular responses to alcohol in C57BL/6J females raised under CTL or LBN conditions (Experiment 2).
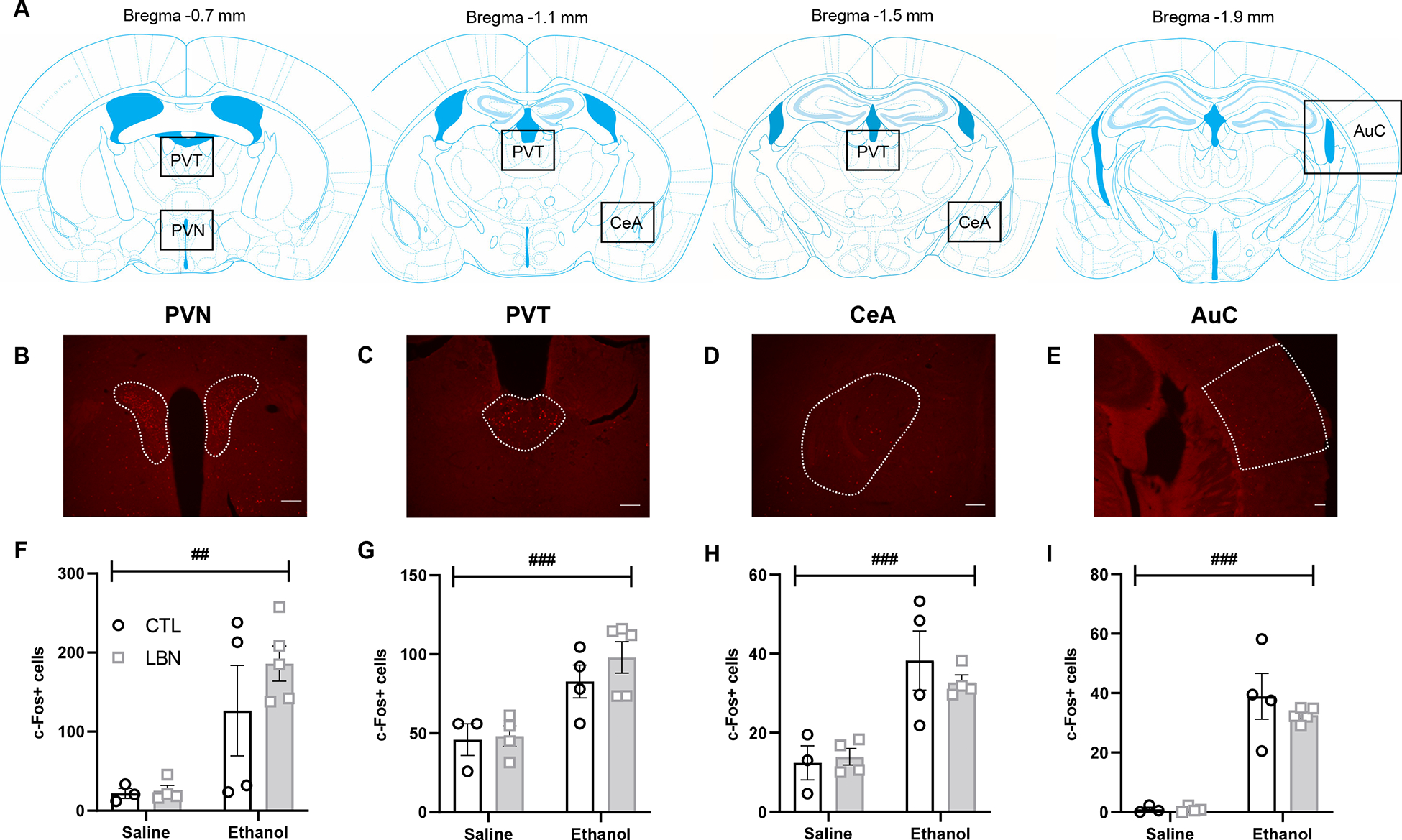
c-Fos-positive nuclei were quantified in the paraventricular nucleus of the hypothalamus (PVN, B and F), paraventricular nucleus of the thalamus (PVT, C and G), central nucleus of the amygdala (CeA, D and H), and auditory cortex (AuC, D and I) following administration of 2 g/kg ethanol. A. Brain section diagrams showing the location of images used for quantification (from Franklin & Paxinos, 2007). B-E. Representative images used for quantification (scale bar, 100 μm). F-I. Number of c-Fos-positive nuclei. ## or ###, main effect of ethanol, p<0.01 or p<0.001.
ImageJ (NIH) was used to manually count c-Fos-positive nuclei within the anatomical boundaries of the PVT, PVN, CeA, and AuC deep layers. For each brain region, counts were averaged across images to yield a single value per animal. One LBN-ethanol mouse was excluded from the CeA counts because too many anteroposterior levels were missing.
Data analysis
Prism 9.3.1 (GraphPad) was used for statistical analysis. In Experiment 1, data were analyzed by two-way ANOVA with LBN and CIE as between-subjects factors. In Experiment 2, antinociception data were analyzed by one-way repeated-measures analysis of variance (ANOVA) with dose as within-subject factor, the Geisser-Greenhouse correction was applied, and the Dunnett test was used for posthoc comparisons. Clearance data were analyzed by simple linear regression. c-Fos counts were analyzed by two-way ANOVA with LBN and treatment as between-subjects factors, and variances between groups were compared using a F-test. Other data were analyzed using unpaired two-tailed t-tests and the Holm-Šídák correction for multiple comparisons was applied for bodyweight analysis. Data are shown as mean ± SEM.
Results
LBN rearing lowers nociceptive thresholds in females withdrawn from chronic alcohol exposure
In Experiment 1, we tested the hypothesis that C57BL/6J females might be insensitive to the effects of LBN and CIE on affective or nociceptive states, which could explain the stability of their voluntary alcohol consumption.
In the EPM, there was no significant effect of LBN (F1,19=0.09, p=0.77), CIE (F1,19= 1.02, p=0.32), or LBN × CIE interaction (F1,19=0.10, p=0.75) on the time spent in the open arms (Fig. 1B). This outcome was not confounded by locomotor alterations as there was also no effect of LBN (F1,19=1.31, p=0.27), CIE (F1,19= 0.007, p=0.93) or LBN × CIE interaction (F1,19=0.98, p=0.3) on the total distance travelled in the EPM (Fig. 1C).
Other measures of affect were altered by CIE but not by LBN. In the digging test, CIE withdrawal significantly increased digging activity (F1,18=31.62, p<0.0001) regardless of early-life history (main effect of LBN: F1,18=0.40, p=0.54; LBN × CIE interaction: F1,18=0.61, p=0.45) (Fig. 1D). In the splash test, CIE withdrawal significantly reduced grooming activity (F1,19=7.76, p=0.012) regardless of early-life history (main effect of LBN: F1,19=1.63, p=0.22; LBN × CIE interaction: F1,19=0.59, p=0.60) (Fig. 1E). In the TST, CIE withdrawal significantly increased immobility duration (F1,19=13.69, p=0.0015) regardless of early-life history (main effect of LBN: F1,19=2.48, p=0.13; LBN × CIE interaction: F1,19=0.002, p=0.97) (Fig. 1F).
In contrast, in the TPT, both LBN rearing (F1,17= 10.65, p=0.0046) and CIE withdrawal (F1,17=4.10, p=0.059) lowered nociceptive thresholds, such that Air-2BC LBN were comparable to CIE-2BC CTL mice and the combination of LBN and CIE did not further exacerbate this phenotype (Fig. 1G). The LBN × CIE interaction did not reach significance (F1,17=1.76, p=0.20).
LBN rearing modulates antinociception and sedation in females acutely exposed to alcohol
Experiment 2 tested the hypothesis that LBN-reared females might be more sensitive to the antinociceptive effect of alcohol given their higher vulnerability to withdrawal-induced hyperalgesia. In this cohort, LBN rearing significantly reduced bodyweights at the end of the manipulation (P10, t16=3.51, adjusted p=0.0087) but this effect dissipated by the time of weaning (P21, t16=1.97, adjusted p=0.13) and was no longer detectable in adulthood, when behavioral testing was conducted (t16=0.32, adjusted p=0.75) (Fig. 2B). This pattern is similar to what we had observed in previous cohorts, including mice from Experiment 1 (Bolton et al., 2022; Okhuarobo et al., 2020a).
In the TPT, baseline nociceptive thresholds tended to be lower in LBN females (t16=1.57, p=0.14; Fig. 2C). In the alcohol dose-response, there was a significant main effect of ethanol dose in LBN mice (F1.9,14.8=5.70, p=0.016) but not in CTL mice (F2.2,17.5=0.98, p=0.40; Fig. 2D). Posthoc comparisons to vehicle identified a significant effect of 2 g/kg ethanol in LBN mice (adjusted p = 0.015); this effect was absent in CTL mice (adjusted p = 0.43).
We next tested whether this differential sensitivity to ethanol-induced antinociception generalized to the sedative effect of ethanol. LBN rearing delayed the loss of righting reflex following the administration of 3.5 g/kg ethanol (t14=2.42, p=0.030, Fig. 2E) but did not impact recovery (latency: t14=0.45, p=0.66, Fig. 2F; BAL: t14=0.39, p=0.70, Fig. 2G).
BALs measured at different time points following administration of 2 g/kg ethanol indicated that LBN had no influence on the rate of ethanol clearance (Fig. 2H). The slopes and Y-intercepts were virtually identical between CTL and LBN mice (slopes: −1.317 vs. −1.326, F1,50=0.003, p=0.95; Y-intercept: 263.8 vs 159.9, F1,51=0.24, p=0.62).
LBN rearing tightens c-Fos induction in females acutely exposed to alcohol
We then sought to determine whether the differential sensitivity of LBN-reared females to ethanol-induced antinociception and sedation correlated with differential cellular activation in ethanol-responsive brain regions. Administration of 2 g/kg ethanol increased the number of c-Fos-positive nuclei in the PVN (F1,12=16.4, p=0.0016, Fig. 3F), PVT (F1,12=19.9, p=0.0008, Fig. 3G), CeA (F1,11=22.9, p=0.0006, Fig. 3H) and AuC (F1,12=76.1, p<0.0001, Fig. 3I) regardless of early-life history. There was no significant main effect of LBN (PVN, F1,12=0.91, p=0.36; PVT, F1,12=0.80, p=0.39; CeA, F1,11=0.18, p=0.68; AuC, F1,12=0.64, p=0.44) or LBN × ethanol interaction (PVN, F1,12=0.74, p=0.41; PVT, F1,12=0.45, p=0.52; CeA: F1,11=0.58, p=0.46; AuC, F1,12=0.61, p=0.45) in either brain region. Despite similar averages, c-Fos counts in ethanol-treated LBN females tended to be less variable than in their CTL counterparts, especially in the CeA and AuC (PVN, F3,4=5.3, p=0.14; PVT, F4,3=1.2, p=0.95; CeA, F3,3=15.3, p=0.051; AuC, F4,3=39.5, p=0.004).
Discussion
Our results show that LBN-reared females with a history of alcohol drinking were as sensitive to the affective consequences of CIE withdrawal as their CTL counterparts, as reflected by increased digging, reduced grooming, and passive coping in response to an inescapable stressor. Furthermore, we found that LBN rearing was associated with mechanical hyperalgesia in females withdrawn from chronic alcohol drinking for 4 weeks. Early withdrawal from alcohol vapor inhalation also produced mechanical hyperalgesia in CTL females, and the combination of LBN and CIE did not lower nociceptive thresholds any further. While mechanical hyperalgesia did not reach significance in alcohol naïve LBN females, alcohol exerted a stronger antinociceptive effect in LBN females than in their CTL counterparts. LBN females were less sensitive to the sedative effect of alcohol at the onset of intoxication but equally sensitive during alcohol elimination. Altogether, these findings indicate that ELA, as modeled by impoverished housing conditions during the early postnatal period, modulates the interaction between alcohol and nociception in adult C57BL/6J females.
The results of Experiment 1 complement the existing literature reporting behavioral disturbances during withdrawal from CIE in C57BL/6J mice, as few of these previous studies included females (see Bloch et al., 2022 for review). The lack of effect of CIE on anxiety-like behavior in the EPM is consistent with previous results obtained in the EPM or light-dark box test in females withdrawn from CIE (Daut et al., 2015; Hartmann et al., 2020) or chronic alcohol drinking (Holleran et al., 2016; Jimenez Chavez et al., 2020; Pang et al., 2013b). In contrast, the increase in digging activity is at odds with results obtained in the marble burying test, where females withdrawn for 6 days from 4 weeks of CIE performed comparably to air-exposed counterparts (Jury et al., 2017). The lower sensitivity of endpoint marble counting compared to direct scoring of digging activity (discussed in Sidhu et al., 2018) or the compounded effect of voluntary drinking in our study could explain this discrepancy. Importantly, we had also observed unaltered EPM behavior and increased digging in CIE-withdrawn CTL males (Okhuarobo et al., 2020a), indicating that sex does not influence these phenotypes.
To the best of our knowledge, the present study is the first to identify reduced grooming and increased TST immobility in CIE-withdrawn C57BL/6J females. These observations are in line with other measures of anhedonia (e.g., sucrose preference) and stress coping (e.g., forced swim test) obtained in C57BL/6J females during protracted abstinence from CIE (Hartmann et al., 2020) or long-term continuous alcohol drinking (Dao et al., 2020; Holleran et al., 2016; Holleran & Winder, 2017; Pang et al., 2013a; Pang et al., 2013b). Interestingly, we had not observed these phenotypes in CIE-2BC vs. Air-2BC CTL males (Okhuarobo et al., 2020a), which suggests that females are more prone to exhibiting signs of depressive-like behavior under comparable alcohol withdrawal and testing conditions. The latter finding parallels the higher vulnerability of C57BL/6J females to depressive-like phenotypes in models of immune challenge or Parkinson’s disease (Schamne et al., 2018; Sens et al., 2017).
The absence of effect of LBN rearing on the EPM behavior of C57BL/6J males (Okhuarobo et al., 2020a) and females (present study) is consistent with previous reports that also failed to detect a change in anxiety-like behavior in LBN-reared male and female C57BL/6N mice (Goodwill et al., 2019) and rats (Davis et al., 2020; Molet et al., 2016). On the other hand, Goodwill et al. (2019) reported reduced sucrose preference and increased behavioral despair selectively in females. We therefore expected LBN Air-2BC females to display reduced grooming or passive stress coping, but these features only emerged in CIE-exposed mice and were not specific to LBN-reared females. The C57BL/6 substrain and the timing of the LBN manipulation (P4-P11 vs. P2-P9) may explain the differential effect of LBN on depressive-like behavior.
Altogether, our data demonstrate that the lack of voluntary alcohol intake escalation in CIE-exposed CTL- and LBN-reared females cannot be explained by their insensitivity to the affective consequences of CIE withdrawal.
Withdrawal from CIE induced significant mechanical hyperalgesia in females with a history of chronic alcohol drinking, which is consistent with what we had observed in males (Okhuarobo et al., 2020a). However, this effect was solely driven by CTL females. The nociceptive thresholds of LBN Air-2BC females were lower than those of CTL Air-2BC mice and similar to those of CTL CIE-2BC, and the combination of LBN and CIE did not exacerbate hyperalgesia. This pattern was unique to females, as LBN rearing did not influence nociceptive thresholds in alcohol-withdrawn males (Okhuarobo et al., 2020a). Based on these data, as for negative affect, the lack of effect of LBN rearing and CIE exposure on the voluntary alcohol consumption of females cannot be explained by a resistance to hyperalgesia. This conclusion is reminiscent of the sex-specific effects of chronic inflammatory pain on alcohol drinking: although male and female C57BL/6J mice treated with Complete Freund’s Adjuvant displayed an equivalent lowering of their mechanical nociceptive thresholds, only males escalated their alcohol intake compared to saline-treated counterparts (Yu et al., 2019). Taken together, these observations indicate that hyperalgesia is not sufficient to increase alcohol consumption in female mice.
A trend for mechanical hyperalgesia in LBN females was also present in Experiment 2 but did not reach significance, suggesting that a history of chronic alcohol drinking is necessary to reveal this effect of LBN. Consistent with this notion, indices of early life adversity were not associated with higher sensitivity to pressure pain in humans, although the potential influence of alcohol consumption on this outcome was not examined (Waller et al., 2020).
In Experiment 2, acute ethanol administration produced significant mechanical antinociception solely in LBN females. A similar observation was made in males selectively bred for high swim stress-induced analgesia and exposed to chronic mild stress in adulthood, whereby stressed mice exhibited lower thermal nociceptive thresholds than their unstressed counterparts, and alcohol-induced antinociception was only detected in stressed mice (Sacharczuk et al., 2009). An important caveat is that nociceptive thresholds were measured at a single time-point following ethanol injection, such that antinociception may have emerged in CTL females at a later timepoint. Furthermore, we did not test enough doses to fit a dose-response curve for ED50 calculation, such that the antinociceptive potency of ethanol cannot be compared between CTL and LBN females. The fact that the antinociceptive effect of ethanol did not reach significance in the CTL females, while it did under identical testing conditions and comparable baseline thresholds in C57BL/6J males (Okhuarobo et al., 2020b) suggests that C57BL/6J females may be less sensitive than males, which is consistent with a recent report (White et al., 2023).
LBN females exhibited a delayed onset of alcohol-induced sedation but recovered at the same time and BAL as their CTL counterparts. This pattern reflects a lower sensitivity restricted to the ascending limb of the BAL time course. Differences in alcohol’s early pharmacokinetics could explain the slower onset of sedation in LBN females. There was no influence of LBN rearing on alcohol clearance rate, but the time course of our BAL analysis did not capture the rise in BAL in the minutes following injection. Alternatively, the signaling mechanisms driving the onset of sedation may be less responsive in LBN females. Compared to this effect of ELA, studies that have examined the effect of chronic or repeated inescapable stress experienced in adulthood on alcohol-induced sedation have produced mixed results. Sleep duration was prolonged in some instances (Boyce-Rustay et al., 2007; Drugan et al., 1992) and shortened in others (Fernández et al., 2016; Jones et al., 1990; Matsumoto et al., 1997), opposite effects were seen in inbred long-sleep vs. short-sleep mice (Parker et al., 2008), and one study reported faster sleep onset (Doremus-Fitzwater et al., 2018). More studies will be needed to evaluate whether the delayed sedation phenotype of LBN females can be generalized to other ELA modalities.
Higher sensitivity to ethanol-induced antinociception and lower sensitivity to ethanol-induced sedation would be predicted to promote alcohol drinking in LBN-reared females, but this was not the case (Okhuarobo et al., 2020a). It is still possible that LBN rearing affects aspects of alcohol drinking that were not captured with the temporal resolution of home cage 2BC sessions (e.g., increased front-loading behavior), especially since the differences in acute sensitivity were measured within the first 5 minutes of alcohol administration.
At the cellular level, the magnitude of c-Fos induction by alcohol in the PVN, PVT, CeA, and AuC was not affected by LBN rearing, but the response to alcohol was more consistent in LBN females than in CTL females. This reduced variability could reflect a higher sensitivity to alcohol, which may have been revealed with lower doses of ethanol. Along these lines, c-Fos induction by forced swim, acute cocaine, or social play was higher in the CeA of LBN-reared rats (Bolton et al., 2018a; Bolton et al., 2018b; Raineki et al., 2012). The CeA plays an important role in the regulation of pain perception and mediates the antinociceptive effects of multiple drugs (see for instance Allen et al., 2021; Neugebauer et al., 2020; Wilson et al., 2019). Ethanol’s ability to activate CeA cells could therefore contribute to the elevation of nociceptive thresholds. Likewise, while the functional significance of ethanol-induced cellular activation in the AuC deserves further investigation, it might also contribute to the antinociceptive effect of ethanol given recent evidence for a role of AuC projections to the thalamus in the control of pain (Zhou et al., 2022).
A major limitation of the present study is that, while we had previously reported the effects of alcohol withdrawal in males (Okhuarobo et al., 2020a), the acute effects of ethanol were solely measured in females. More research will therefore be needed to assess potential sex differences in the influence of LBN rearing on acute sensitivity to ethanol-induced antinociception and sedation.
In conclusion, the main finding of this study was that LBN rearing produced mechanical hyperalgesia in C57BL/6J females withdrawn from chronic alcohol drinking and potentiated the acute antinociceptive effect of alcohol in naïve females. Accordingly, even though the ethanol intake of CTL and LBN females was indistinguishable (Okhuarobo et al., 2020a), it is possible that the motivation driving alcohol drinking differed between the two groups, such that LBN females consumed alcohol to alleviate hyperalgesia. The same speculation can be made for the effect of CIE, since CIE exposure did not alter ethanol intake in CTL or LBN females (Okhuarobo et al., 2020a) but produced multiple indices of negative affect (increased digging, reduced grooming, and passive coping), which may have served as a source of negative reinforcement. Future research will aim to identify the sex-specific mechanisms that underlie altered nociception in mice exposed to ELA and chronic alcohol drinking.
Acknowledgements
We are grateful for the support of the TSRI Alcohol Research Center Animal Models Core, which conducted blood alcohol level analysis for this study.
Funding
This work was supported by National Institutes of Health grants AA026685 (CC), AA027636 (CC), AA006420 (CC), AA027372 (CC, TZB), as well as stipends from University of Benin, Benin City, Nigeria (AO), Kingsefe Pharmacy, Benin City, Nigeria (AO), the Hewitt Foundation for Medical Research (JLB), and the Brain & Behavior Research Foundation (JLB). These funding sources were not involved in study design, data collection, analysis, or interpretation, nor decision to publish.
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to disclose.
CRediT authorship contribution statement
Agbonlahor Okhuarobo: Investigation, Methodology, Formal analysis, Writing – original draft. Maggie Angelo: Investigation, Formal analysis. Jessica L Bolton: Methodology, Investigation. Catherine Lopez: Investigation. Ighodaro Igbe: Supervision. Tallie Z Baram: Conceptualization, Funding acquisition. Candice Contet: Conceptualization, Funding acquisition, Supervision, Formal analysis, Writing – review & editing.
References
- Allen HN, Bobnar HJ & Kolber BJ (2021) Left and right hemispheric lateralization of the amygdala in pain. Prog Neurobiol, 196, 101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyan J & Amir S (2018) Too Depressed to Swim or Too Afraid to Stop? A Reinterpretation of the Forced Swim Test as a Measure of Anxiety-Like Behavior. Neuropsychopharmacology, 43, 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG (2020) Synthesizing Views to Understand Sex Differences in Response to Early Life Adversity. Trends Neurosci, 43, 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2017) Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology, 122, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC & Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res, 28, 1829–38. [DOI] [PubMed] [Google Scholar]
- Bloch S, Holleran KM, Kash TL, Vazey EM, Rinker JA, Lebonville CL, O’Hara K, Lopez MF, Jones SR, Grant KA, Becker HC & Mulholland PJ (2022) Assessing negative affect in mice during abstinence from alcohol drinking: Limitations and future challenges. Alcohol, 100, 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, Yang DZ, Obenaus A & Baram TZ (2018a) Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biol Psychiatry, 83, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, Cross C, Baram TZ & Mahler SV (2018b) Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress, 8, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Short AK, Othy S, Kooiker CL, Shao M, Gunn BG, Beck J, Bai X, Law SM, Savage JC, Lambert JJ, Belelli D, Tremblay ME, Cahalan MD & Baram TZ (2022) Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep, 38, 110600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA & Holmes A (2007) Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav, 91, 77–86. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S & Gould TD (2012) The tail suspension test. J Vis Exp, e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y & Baram TZ (2016) Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology, 41, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA & Ehlinger DG (2017) The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci, 8, 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C & Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev, 29, 571–625. [DOI] [PubMed] [Google Scholar]
- Cucinello-Ragland JA & Edwards S (2021) Neurobiological aspects of pain in the context of alcohol use disorder. Int Rev Neurobiol, 157, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao NC, Suresh Nair M, Magee SN, Moyer JB, Sendao V, Brockway DF & Crowley NA (2020) Forced Abstinence From Alcohol Induces Sex-Specific Depression-Like Behavioral and Neural Adaptations in Somatostatin Neurons in Cortical and Amygdalar Regions. Front Behav Neurosci, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, Camp M & Holmes A (2015) Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addict Biol, 20, 259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LK, Bolton JL, Hanson H & Guarraci FA (2020) Modified limited bedding and nesting is a model of early-life stress that affects reproductive physiology and behavior in female and male Long-Evans rats. Physiol Behav, 224, 113037. [DOI] [PubMed] [Google Scholar]
- Deacon RM (2006) Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc, 1, 122–4. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Paniccia JE, Gano A, Vore AS & Deak T (2018) Differential effects of acute versus chronic stress on ethanol sensitivity: Evidence for interactions on both behavioral and neuroimmune outcomes. Brain Behav Immun, 70, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC, Scher DM, Sarabanchong V, Guglielmi A, Meng I, Chang J, Bloom K, Sylvia S & Holmes P (1992) Controllability and duration of stress alter central nervous system depressant-induced sleep time in rats. Behav Neurosci, 106, 682–9. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Aubert A & Belzung C (2004) Susceptibility to subchronic unpredictable stress is related to individual reactivity to threat stimuli in mice. Behav Brain Res, 155, 291–9. [DOI] [PubMed] [Google Scholar]
- Elhabazi K, Ayachi S, Ilien B & Simonin F (2014) Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities. J Vis Exp, e51264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA (2011) The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl), 214, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández MS, Fabio MC, Miranda-Morales RS, Virgolini MB, De Giovanni LN, Hansen C, Wille-Bille A, Nizhnikov ME, Spear LP & Pautassi RM (2016) Age-related effects of chronic restraint stress on ethanol drinking, ethanol-induced sedation, and on basal and stress-induced anxiety response. Alcohol, 51, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ & Paxinos G (2007) The Mouse Brain in Stereotaxic Coordinates, Compact, Academic Press. [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T & Bath KG (2019) Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology, 44, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs F (1969) Procedures for Detecting Outlying Observations in Samples. Technometrics, 11, 1–21. [Google Scholar]
- Hartmann MC, Haney MM, Smith CG, Kumar V & Rosenwasser AM (2020) Affective Disruption During Forced Ethanol Abstinence in C57BL/6J and C57BL/6NJ Mice. Alcohol Clin Exp Res, 44, 2019–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann B & Hitzemann R (1997) Genetics ethanol and the Fos response: a comparison of the C57BL/6J and DBA/2J inbred mouse strains. Alcohol Clin Exp Res, 21, 1497–507. [PubMed] [Google Scholar]
- Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, Rocco LE, Patel S & Winder DG (2016) Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology, 41, 2062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM & Winder DG (2017) Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav, 16, 8–14. [DOI] [PubMed] [Google Scholar]
- Horii Y & Kawaguchi M (2015) Higher detection sensitivity of anxiolytic effects of diazepam by ledge-free open arm with opaque walled closed arm elevated plus maze in male rats. Behav Brain Res, 294, 131–40. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Tanaka N, Ito A, Takakura A, Kumihashi M & Kinoshita H (2016) Ethanol and Acetaldehyde After Intraperitoneal Administration to Aldh2-Knockout Mice-Reflection in Blood and Brain Levels. Neurochem Res, 41, 1029–34. [DOI] [PubMed] [Google Scholar]
- Jimenez Chavez CL, Coelho MA, Brewin LW, Swauncy I, Tran T, Albanese T, Laguna A, Gabriela I & Szumlinski KK (2020) Incubation of Negative Affect during Protracted Alcohol Withdrawal Is Age-, but Not Sex-Selective. Brain Sci, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, Connell JM & Erwin VG (1990) Isolate housing alters ethanol sensitivity in long-sleep and short-sleep mice. Pharmacol Biochem Behav, 35, 469–72. [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL & Holmes A (2017) Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol, 58, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ & Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry, 68, 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Takao K & Miyakawa T (2008) Elevated plus maze for mice. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2022) Anhedonia, Hyperkatifeia, and Negative Reinforcement in Substance Use Disorders. Curr Top Behav Neurosci, 58, 147–165. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ojima K & Watanabe H (1997) Central corticotropin-releasing factor and benzodiazepine receptor systems are involved in the social isolation stress-induced decrease in ethanol sleep in mice. Brain Res, 753, 318–21. [DOI] [PubMed] [Google Scholar]
- Molendijk ML & de Kloet ER (2015) Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology, 62, 389–91. [DOI] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ & Stern H (2016) Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry, 6, e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E & Porreca F (2020) Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology, 170, 108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhuarobo A, Bolton JL, Igbe I, Zorrilla EP, Baram TZ & Contet C (2020a) A novel mouse model for vulnerability to alcohol dependence induced by early-life adversity. Neurobiol Stress, 13, 100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhuarobo A, Kreifeldt M, Bhattacharyya P, Dopico AM, Roberts AJ, Homanics GE & Contet C (2020b) Ethanol’s action at BK channels accelerates the transition from moderate to excessive alcohol consumption. BioRxiv, 10.1101/2020.10.29.360107. [DOI] [Google Scholar]
- Pang TY, Du X, Catchlove WA, Renoir T, Lawrence AJ & Hannan AJ (2013a) Positive environmental modification of depressive phenotype and abnormal hypothalamic-pituitary-adrenal axis activity in female C57BL/6J mice during abstinence from chronic ethanol consumption. Front Pharmacol, 4, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ & Hannan AJ (2013b) Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur J Neurosci, 37, 1803–10. [DOI] [PubMed] [Google Scholar]
- Parker CC, Ponicsan H, Spencer RL, Holmes A & Johnson TE (2008) Restraint stress and exogenous corticosterone differentially alter sensitivity to the sedative-hypnotic effects of ethanol in inbred long-sleep and inbred short-sleep mice. Alcohol, 42, 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR & McKee SA (2019) Sex differences in stress-related alcohol use. Neurobiol Stress, 10, 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD & Fromme K (2011) Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res, 35, 1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L & Sullivan RM (2012) Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci, 32, 7758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart EM, Waldron M, Kelly-Quigley H, Zellers M, Turco A & Grisel JE (2020) β-Endorphin and sex differentially modulate the response to EtOH in a site-specific manner. Brain Res, 1741, 146845. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR & Baram TZ (2008) A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology, 149, 4892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharczuk M, Juszczak G, Swiergiel AH, Jaszczak K, Lipkowski AW & Sadowski B (2009) Alcohol reverses depressive and pronociceptive effects of chronic stress in mice with enhanced activity of the opioid system. Acta Neurobiol Exp (Wars), 69, 459–68. [DOI] [PubMed] [Google Scholar]
- Schamne MG, Mack JM, Moretti M, Matheus FC, Walz R, Lanfumey L & Prediger RD (2018) The Gender-Biased Effects of Intranasal MPTP Administration on Anhedonic- and Depressive-Like Behaviors in C57BL/6 Mice: the Role of Neurotrophic Factors. Neurotox Res, 34, 808–819. [DOI] [PubMed] [Google Scholar]
- Sens J, Schneider E, Mauch J, Schaffstein A, Mohamed S, Fasoli K, Saurine J, Britzolaki A, Thelen C & Pitychoutis PM (2017) Lipopolysaccharide administration induces sex-dependent behavioural and serotonergic neurochemical signatures in mice. Pharmacol Biochem Behav, 153, 168–181. [DOI] [PubMed] [Google Scholar]
- Sidhu H, Kreifeldt M & Contet C (2018) Affective Disturbances During Withdrawal from Chronic Intermittent Ethanol Inhalation in C57BL/6J and DBA/2J Male Mice. Alcohol Clin Exp Res, 42, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen TN & Smolen A (1989) Blood and brain ethanol concentrations during absorption and distribution in long-sleep and short-sleep mice. Alcohol, 6, 33–8. [DOI] [PubMed] [Google Scholar]
- Waller R, Smith AJ, O’Sullivan PB, Slater H, Sterling M & Straker LM (2020) The association of early life stressors with pain sensitivity and pain experience at 22 years. Pain, 161, 220–229. [DOI] [PubMed] [Google Scholar]
- Walters H & Kosten TA (2019) Early life stress and the propensity to develop addictive behaviors. Int J Dev Neurosci, 78, 156–169. [DOI] [PubMed] [Google Scholar]
- White A, Caillaud M, Carper M, Poklis J, Miles MF & Damaj MI (2023) Thermal antinociceptive responses to alcohol in DBA/2J and C57BL/6J inbred male and female mouse strains. Behav Brain Res, 436, 114087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Martinez Gonzalez S, Sugimura YK & Carrasquillo Y (2019) Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep, 29, 332–346 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Aksu F & Belzung C (2005) Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol, 514, 165–74. [DOI] [PubMed] [Google Scholar]
- Yu W, Hwa LS, Makhijani VH, Besheer J & Kash TL (2019) Chronic inflammatory pain drives alcohol drinking in a sex-dependent manner for C57BL/6J mice. Alcohol, 77, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ye C, Wang H, Mao Y, Zhang W, Liu A, Yang CL, Li T, Hayashi L, Zhao W, Chen L, Liu Y, Tao W & Zhang Z (2022) Sound induces analgesia through corticothalamic circuits. Science, 377, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]


