Abstract
Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) channel that perturb anion transport across the epithelia of the airways and other organs. To treat cystic fibrosis, strategies that target mutant CFTR have been developed such as correctors that rescue folding and enhance transfer of CFTR to the apical membrane, and potentiators that increase CFTR channel activity. While there has been tremendous progress in development and approval of CFTR therapeutics for the most common (F508del) and several other CFTR mutations, around 10-20% of people with cystic fibrosis have rare mutations that are still without an effective treatment. In the current decade, there was an impressive evolution of patient-derived cell models for precision medicine. In cystic fibrosis, these models have played a crucial role in characterizing the molecular defects in CFTR mutants and identifying compounds that target these defects. Cells from nasal, bronchial, and rectal epithelia are most suitable to evaluate treatments that target CFTR. In vitro assays using cultures grown at an air-liquid interface or as organoids and spheroids allow the diagnosis of the CFTR defect and assessment of potential treatment strategies. An overview of currently established cell culture models and assays for personalized medicine approaches in cystic fibrosis will be provided in this review. These models allow theratyping of rare CFTR mutations with available modulator compounds to predict clinical efficacy. Besides evaluation of individual personalized responses to CFTR therapeutics, patient-derived culture models are valuable for testing responses to developmental treatments such as novel RNA- and DNA-based therapies.
Keywords: CFTR, modulator, primary human epithelial cells, spheroid, organoid, theratyping
Introduction
Cystic fibrosis (CF) is a life-threatening autosomal recessive genetic disorder caused by dysfunction in CF transmembrane conductance regulator (CFTR)-mediated transport of Cl− and HCO3− [1]. To date, more than 2000 CFTR variants have been identified, and many of these cause CF. Based on their effect on the protein function, they are grouped into at least seven classes of mutations characterized by complete absence of mRNA or protein synthesis, protein misfolding, impairment of channel gating or conductance, and diminished amounts of CFTR protein levels or stability [2]. Even though highly effective modulator treatments (HEMT) have been available for the treatment of CF for a decade, capable of correcting the basic defect of the CFTR protein, in Europe about 20% and in the US about 10% of people with CF (PwCF) are not eligible for available modulator therapy. One of the main reasons for this exclusion is that many of these PwCF carry rare or ultra-rare CFTR mutations whose responsiveness to modulators is unknown, having often been excluded from clinical trials due to the reduced number of PwCF carrying these specific mutations. Some PwCF bear mutations that do not produce any CFTR protein (i.e. nonsense mutations, deletions, and insertions). The matching of therapies to specific mutations is called theratyping. However, not all PwCF eligible for modulator treatment may respond to them, due to the action of unknown mutations, mutations in modifier genes, and/or epigenetic modifications. Thus, even individuals with identical CFTR mutations may respond differently depending on their demographics, environmental influences, and individuals’ genetic background. In this context, personalized medicine approaches utilizing advanced models based on patient-derived tissues to study and cure CF with the potential to tailor therapy for a specific patient are needed. 2D and 3D patient-derived cell models (PDCM) are considered the most reliable disease models available since they mimic the in vivo ion channel pathophysiology. These culture models include the genetic background of the PwCF and allow a personalized approach to predict the effect of mutations and therapies and to build a biobank for further analyses. In this review, the state-of-the-art approaches in defining the pathogenicity of CFTR variants and their responsiveness to CFTR modulators are presented. This review will focus mainly on the following PDCM for personalized therapy: nasal and bronchial cells models, and rectal organoids.
CF Therapeutics
Currently available HEMT include correctors that rescue F508del folding and augment transfer to the apical membrane, and potentiators that increase CFTR channel activity, permitting successful treatment of the basic CF protein defect [3]. The first approved (2012) HEMT was the potentiator ivacaftor (VX-770), which improves CFTR function in gating mutants [4,5]. Recent development of a triple combination treatment (Trikafta, Kaftrio) consisting of two corrector compounds, [tezacaftor (VX-661) and elexacaftor (VX-445)] together with ivacaftor resulted in significant improvement of clinical responses [6-8]. Trikafta was initially approved by the U.S. Food and Drug Administration (FDA) in 2019 and Kaftrio by the European Medicines Agency (EMA) in 2020 for the treatment of PwCF who had at least one copy of the F508del mutation. Subsequently, in the US, Trikafta was approved for additional mutations and is now available for 178 mutations (see https://www.cff.org/sites/default/files/2021-10/Trikafta-Approved-Mutations-January-2021.pdf). The development of these HEMT opened the way for the treatment of the basic CF protein defect. Several additional CFTR protein modulators as well as RNA and DNA therapeutics are currently under development in the CF pipeline [9-13]. There are however some concerns about mutations that were included in the list of approved mutations for treatment with Trikafta by the FDA [14], since those considerations were at least partially based on results obtained in FRT cells that do not contain the genomic background of CFTR (only cDNA based constructs that do not include the introns of CFTR). These include c.523A>G that was predicted to produce I175V, but instead causes a splicing defect that leads to a deletion of a portion of the CFTR protein (aa175-193) and R1162L that does not appear to cause CF [14,15]. In light of these concerns, and to test the already available and newly designed CF therapies in a more personalized way, PDCM are now the state-of-the-art models. The roles of three main PDCM for CF research, drug development, and personalized testing are discussed below.
Human Nasal Epithelial Cultures
Human nasal epithelial (HNE) cells are easily collected by a non-invasive brushing procedure of both nostrils. Using a soft interdental or endocervical brush, under direct visualization and without anesthesia, the cells are scraped from the middle part of the inferior turbinate [16]. This anatomical structure of the nasal cavity is characterized by the presence of a pseudostratified airway epithelium that shares most of the anatomical and physiological characteristics of airway epithelium of the remaining part of the upper and lower respiratory tract (Figure 1A). The brushed cells, mainly ciliated, goblet, basal stem cells, and rare ionocytes, recapitulate the distribution of the CFTR protein as cells of bronchial origin [17,18]. Once collected, the HNE cells can be used directly for mRNA analysis, assessing the effect of putative splicing mutation [19] or in general to evaluate the effect of silent or deep intronic mutations on the mRNA maturation and stability. Most importantly, the HNE cells can be expanded and cultured in air-liquid interface (ALI) conditions to obtain a pseudostratified epithelium for functional CFTR evaluation. Indeed, the ALI-cultured HNE cells (derived from PwCF with rare mutations) have been successfully used for the evaluation of the responsiveness to CFTR modulators [17,20-28]. The ALI culture can be achieved in different ways. In one method, the HNE cells are collected and expanded for maximum two passages and then seeded onto the microporous membrane supports until they reach complete confluence in submerged conditions. Then the cells are maintained at ALI and fed with a differentiation medium on the basolateral side only. Usually after 20-30 days in ALI conditions the HNE cultures are highly differentiated and used to evaluate the effect of CFTR modulators by Ussing chamber short-circuit current recording experiments (Figure 1B). As an alternative method, to expand the number of available HNE cells, conditionally reprogrammed cultures (CRC) can be created using a cell culture technique that allows cells to be maintained at higher passage number and thus efficiently enhance the supplies of cells available for measurements that are conducted similarly as with primary HNE cells [27]. Furthermore, patient-derived nasal cells can also be cultured in suspension on plates that prevent attachment, creating spheroids with CFTR channels on the outer surface [29] or alternatively in Matrigel matrix, creating spheroids with luminally oriented CFTR [30]. Upon rescue of mutant CFTR with modulators, ion and fluid transport will cause spheroids to shrink or swell in these models, respectively, which can be quantitated to evaluate CFTR function and the effect of the modulators (Figure 1B).
Figure 1. Human CF models for personalized medicine.
A. Illustrations of cell collection for patient-derived models. B. Cartoon depicting culturing and standard assays for nasal, bronchial, and GI/rectal 2D and 3D patient-derived cell models (PDCM).
Human Bronchial Epithelial Cultures
HBE cells are either derived by a bronchial brush (Figure 1B), which is more invasive than a nasal brush or are harvested from explant lungs of PwCF. It is notable that due to better treatment options for PwCF such as HEMT, the number of CF explant lungs has recently declined [31]. HBE models can support the characterization of novel molecular abnormalities caused by rare mutations of CFTR in individual PwCF [32,33]. These in vitro data can be correlated with in vivo clinical presentation, allowing a more precise physiological summary of the effects of each CFTR mutation on CFTR function and subsequent theratyping of rare CFTR mutations [34].
HBE grown at ALI have served as the gold standard for testing CFTR modulators in US [4,8,35], which has resulted in original approval of HEMT, Kalydeco and Trikafta for treatment of PwCF with G551D and F508del mutations. Similar to HNE cells, HBE cells are cultured at ALI as primary cells [36] or grown as CRC to obtain higher passage numbers for testing (Figure 1B) [37,38]. ALI-cultured HBE cultures have been widely used as a platform for theratyping and to characterize new CFTR mutations of uncertain significance to guide therapy optimization [34]. Assays to evaluate CFTR function range from electrophysiological evaluation by transepithelial short-circuit currents, evaluation of CFTR molecular size that shows proper processing, and other measurements such as airway-surface liquid hydration and mucociliary clearance [39].
Seeding of HBE cells in Matrigel or a similar matrix results in spheroids, in which CFTR activation at the apical membrane (facing the lumen) drives fluid toward the lumen followed by spheroid swelling (Figure 1B), as seen with nasal spheroids. This assay allows quantitation of CFTR therapeutics and in addition spheroids can be utilized to examine CF pathophysiology, including aberrant mucus properties and ciliary beat. [34,40-43]. As an alternative to luminally oriented CFTR, spheroids may develop without Matrigel in media with outside-orientated CFTR, which may be later embedded in a matrix to facilitate analyses [44]. As a result of opposite-directed ion and fluid flow, these spheroids shrink when CFTR is activated.
Advanced human airway organ-on-a-chip models are being developed that incorporate fluid flow and include other cell types besides HBE cells such as lung endothelium, alveolar cells, and immune cells [45-49]. While CF airway chip models may represent the physiology of the human airways in vivo, further development may be necessary before they are broadly accepted as a robust model that allows for drug testing.
Human Gastrointestinal Epithelial Cultures
Adult intestinal stem cells were shown to grow into closed 3D structures with protruding buds and a luminal compartment [50]. These 3D structures named organoids are produced from the stem cells present in the base of crypts isolated from rectal mucosa samples that can be collected by suction or forceps biopsy (Figure 1A). While gastrointestinal (GI) biopsies can be isolated from various parts of the GI tract, rectal tissues are most widely used for personalized testing as the isolation procedure is less invasive. The rectal biopsy procedure is painless and feasible to perform at all ages (even in neonates or infants) in every hospital and there is the possibility of remote analysis in a specialized laboratory since the samples can easily be transported for processing [51]. The crypts isolated from the rectal biopsies can be cultured in extracellular matrix and using a multi-component media that allows fast proliferation of the stem cells present in the crypts as 3D structures; the so-called rectal organoids. As the main channel expressed in these rectal ‘organoids’ is the CFTR protein, a specific assay called ‘Forskolin Induced Swelling’ or FIS assay allows measurement of CFTR function [52] (Figure 1B). Activation of ion transport through the CFTR protein by forskolin induces water movements causing the 3D structures to swell, which is a CFTR-dependent response. Compared to the heterologous cells, which are frequently used as models to study CFTR function, this patient-derived primary cell model has the advantage of faithfully reproducing the subjects’ genetic background. In addition, organoid cultures can be bio-banked and expanded indefinitely and the FIS assays in these organoids allow the prediction of the response to CFTR modulators [53,54] (Figure 1B).
The FIS protocol was fully standardized between three independent laboratories during the HIT-CF European project [55], in order to help fulfill one objective of this project that is to obtain EMA validation of the rectal organoid assays as biomarker of CFTR function in PwCF. If this validation is obtained, this patient-derived CFTR assay may become the most advanced in the regulatory track in Europe [56].
In addition to measuring CFTR function and allowing assessment of CFTR modulators, rectal organoids, can also be used to discriminate between PwCF and non-CF individuals. At baseline (without addition of any compound to enhance CFTR function) the morphology of the rectal organoids depends on whether CFTR is functional or not. Organoids from subjects without CF are rounder (more spherical) and show a liquid-filled lumen due to the activity of CFTR. This can be distinguished from organoids of PwCF that do not have a lumen, presenting a more irregular shape (Figure 2, CF Diagnosis). A novel method to quantify these morphologic differences in the organoids has developed and named ROMA for rectal organoids morphology analysis [57] (Figure 1B). Differences between CF and non-CF rectal organoids have already been reported in the first publication: the usage of the FIS assay to analyze CFTR function [52]. However, with ROMA, it was possible to do a semi-automated analysis to quantify the morphological differences using the fluorescence intensity ratio (IR) after calcein staining and circularity index (CI). Using this assay in a group of 22 organoids from subjects without CF and 167 organoids from PwCF, it was found that these two parameters perfectly differentiated subjects with classic CF versus those without CF [57], thus ROMA may help with accurate diagnosis of CF. Currently, the assay is being tested in borderline CF cases to check the applicability of this assay to guide with diagnosis of more complex cases. Furthermore, ROMA may also be used to predict efficacy of CF therapies.
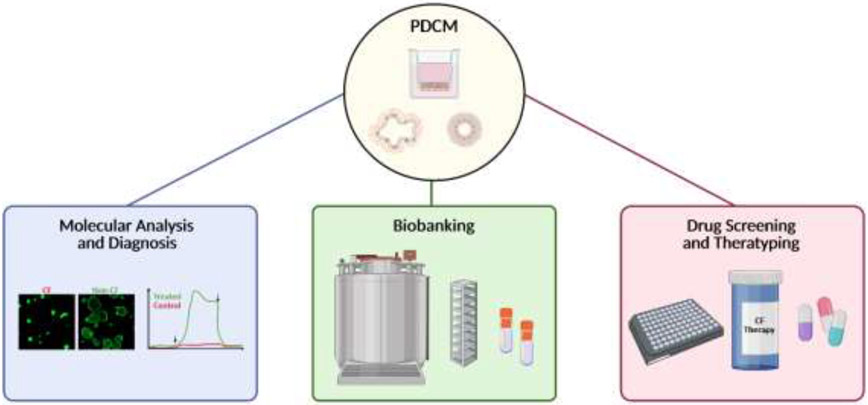
Figure 2. Cartoon depicting suitability of PDCM.
Discussed cell models are suitable for biobanking, diagnosis, obtaining information on molecular defects (CFTR or modifier genes) and therapeutic testing and drug screening. Created with BioRender.com.
In addition to using the 3D organoid structures to study CFTR function and response to modulators, the rectal organoids can also be cultured as polarized 2D cultures on permeable plastic supports and used to measure transepithelial currents in Ussing chambers [58,59], similarly to HNE and HBE (see above sections and Figure 1B).
Conclusions
Treatments targeting mutant CFTR and enhancing its function have become available over the last decade. Even though the FDA approval of highly effective CFTR modulators was partially built on FRT cell line data, it has become obvious that primary epithelial tissues are more reliable than cell lines to evaluate patients’ responses [14,60-64]. One example of these discrepancies is the mutation G970R, that in FRT was shown to benefit from ivacaftor but in a clinical trial no benefit was observed [31] and a study using organoids showed no rescue with ivacaftor in organoids of patients with the G970R mutation, which was found to induce alternative splicing [65]. Nasal, bronchial, and rectal epithelia have proven most useful in analyzing PwCF CFTR function and responses to therapeutics, and thus can support personalized medicine for PwCF. Each model offers its own advantages (Table 1) and has been utilized broadly to assess personalized responses. This review was based on the work presented by the authors in the ECFS Basic Science Conference 2022 and thus did not focus on CF epithelial models derived from human induced pluripotent stem cells (iPSC) or on organ-on-a-chip models that may have great potential for further CF disease modeling and personalized testing.
Table 1.
Most utilized PDCM for personalized medicine for cystic fibrosis.
| Model | Source | Key Feature | Culture | Readout | Application |
|---|---|---|---|---|---|
| HNE | Nasal Brush or Scrape | Non-invasive isolation procedure. Represents main features of the airway epithelium. Permits testing of responses to CFTR therapeutics. |
ALI culture | Isc, Genetic analysis, Theratyping | Common |
| Spheroid | Swelling or shrinking, Therapyping | Less common, but more and more established | |||
| HBE | Bronchial Brush, Explant Lungs | Invasive isolation procedure. Represents airway epithelium. Well-established assays for testing of responses to CFTR therapeutics. |
ALI culture | Isc, Genetic analysis, Theratyping | Very common |
| Spheroid | Swelling or shrinking, Therapyping | Less common | |||
| Human GI Epithelium | GI/Rectal Biopsy | Represents GI tract. Increasingly used for personalized testing of responses to CFTR therapeutics. Organoid assays have HTS capability. High Levels of CFTR protein in rectal biopsy tissues facilitate testing of efficacy of CFTR therapeutics. ICM with tissues requires instant analysis without excessive storage times (Isc measurements of polarized 2D cultures on permeable supports may overcome this limitation). |
Tissue | ICM | Less widespread but accepted |
| Planar culture on permeable support | Isc, Genetic analysis | Less common | |||
| Organoid | Swelling, ROMA Genetic analysis, Therapyping | Very common in Europe |
Indicated are the main source, key feature, culture model, most common readouts, and the prevalence of application of 2D and 3D patient-derived cell models (PDCM). Short-circuit current (Isc), high-throughput screening (HTS), intestinal current measurement (ICM), rectal organoid morphology analysis (ROMA).
Mechanistic insights into modulator actions and their improvements will not only be applicable for CFTR-targeting treatments but may also be relevant to enhance newly developed RNA and gene therapies and read-though therapeutics that may be utilized in combination with CFTR modulators. Thus, the presented models and assays may have immediate translational relevance in providing methods and knowledge for accurate elucidation of CF drug effects, which will allow optimal therapies for all PwCF.
Overall, PDCM like HNE, HBE, and rectal organoids support assessing in vitro drug efficacy to potentially predict in vivo therapeutic responsiveness and therefore are paving the way to personalized medicine for PwCF. Furthermore, these described models may also be applicable to identify treatment for other human diseases where CFTR function is impaired [66,67].
Highlights.
Primary human epithelial tissues are more reliable than cell lines to evaluate cystic fibrosis therapies.
Nasal, bronchial, and rectal epithelia have proven most useful in analyzing CFTR function and responses to cystic fibrosis therapeutics.
2D and 3D patient-derived cell models are considered the most reliable disease models since they mimic the in vivo ion channel pathophysiology.
Personalized medicine approaches utilize advanced models based on patient-derived tissues to tailor therapies for specific patients to cure cystic fibrosis.
Acknowledgements
We thank the members of the authors’ research teams for establishing and conducting related research on human epithelial models. Funding related to research described in this review was provided to by Belgian CF patient Association and the Fund Alphonse Jean Forton from the King Baudouin Foundation (2020-J1810150-217924), KU Leuven internal funds C2 project (3M210333) and Cystic Fibrosis Foundation research grant (CFF 001713G220) to the Leuven CF organoids research lab (ASR), Italian Cystic Fibrosis Research Foundation grant (FFC#7/2013, FFC#1/2020 and FFC#9/2020) to FA, and by the Cystic Fibrosis Foundation (GENTZS18P0, GENTZS19I0, BOUCHE19R0) and the National Institutes of Health (P30DK065988) to MG.
Footnotes
Conflict of Interest Statement
ASR and FA declare no conflict of interest. MG is an inventor of US patent No. 11357758 (Epithelial cell spheroids and methods of making and using the same).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riordan JR; Rommens JM; Kerem B; Alon N; Rozmahel R; Grzelczak Z; Zielenski J; Lok S; Plavsic N; Chou JL; et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [DOI] [PubMed] [Google Scholar]
- 2.De Boeck K; Amaral MD Progress in therapies for cystic fibrosis. Lancet Respir Med 2016, 4, 662–674, doi: 10.1016/S2213-2600(16)00023-0. [DOI] [PubMed] [Google Scholar]
- 3.Gentzsch M; Mall MA Ion Channel Modulators in Cystic Fibrosis. Chest 2018, 154, 383–393, doi: 10.1016/j.chest.2018.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Goor F; Hadida S; Grootenhuis PD; Burton B; Cao D; Neuberger T; Turnbull A; Singh A; Joubran J; Hazlewood A; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 18825–18830, doi:0904709106 [pii]; 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey BW; Davies J; McElvaney NG; Tullis E; Bell SC; Drevinek P; Griese M; McKone EF; Wainwright CE; Konstan MW; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med 2011, 365, 1663–1672, doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heijerman HGM; McKone EF; Downey DG; Van Braeckel E; Rowe SM; Tullis E; Mall MA; Welter JJ; Ramsey BW; McKee CM; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948, doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton PG; Mall MA; Drevinek P; Lands LC; McKone EF; Polineni D; Ramsey BW; Taylor-Cousar JL; Tullis E; Vermeulen F; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med 2019, 381, 1809–1819, doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating D; Marigowda G; Burr L; Daines C; Mall MA; McKone EF; Ramsey BW; Rowe SM; Sass LA; Tullis E; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med 2018, 379, 1612–1620, doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghelani DP; Schneider-Futschik EK Emerging Cystic Fibrosis Transmembrane Conductance Regulator Modulators as New Drugs for Cystic Fibrosis: A Portrait of in Vitro Pharmacology and Clinical Translation. ACS Pharmacol Transl Sci 2020, 3, 4–10, doi: 10.1021/acsptsci.9b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spano V; Montalbano A; Carbone A; Scudieri P; Galietta LJV; Barraja P An overview on chemical structures as DeltaF508-CFTR correctors. Eur. J. Med. Chem 2019, 180, 430–448, doi: 10.1016/j.ejmech.2019.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Lopes-Pacheco M; Pedemonte N; Veit G Discovery of CFTR modulators for the treatment of cystic fibrosis. Expert Opin Drug Discov 2021, 16, 897–913, doi: 10.1080/17460441.2021.1912732. [DOI] [PubMed] [Google Scholar]
- 12.Spano V; Venturini A; Genovese M; Barreca M; Raimondi MV; Montalbano A; Galietta LJV; Barraja P Current development of CFTR potentiators in the last decade. Eur. J. Med. Chem 2020, 204, 112631, doi: 10.1016/j.ejmech.2020.112631. [DOI] [PubMed] [Google Scholar]
- 13.Cystic Fibrosis Foundation Drug Development Pipeline, https://www.cff.org/Trials/Pipeline. 2022.
- 14.Raraigh KS; Lewis MH; Collaco JM; Corey M; Penland CM; Stephenson AL; Rommens JM; Castellani C; Cutting GR Caution advised in the use of CFTR modulator treatment for individuals harboring specific CFTR variants. J Cyst Fibros 2022, doi: 10.1016/j.jcf.2022.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Joynt AT; Evans TA; Pellicore MJ; Davis-Marcisak EF; Aksit MA; Eastman AC; Patel SU; Paul KC; Osorio DL; Bowling AD; et al. Evaluation of both exonic and intronic variants for effects on RNA splicing allows for accurate assessment of the effectiveness of precision therapies. PLoS Genet. 2020, 16, e1009100, doi: 10.1371/journal.pgen.1009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Lullo AM; Scorza M; Amato F; Comegna M; Raia V; Maiuri L; Ilardi G; Cantone E; Castaldo G; lengo M An "ex vivo model" contributing to the diagnosis and evaluation of new drugs in cystic fibrosis. Acta Otorhinolaryngol. Ital 2017, 37, 207–213, doi: 10.14639/0392-100X-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewington JJ; Filbrandt ET; LaRosa FJ 3rd; Moncivaiz JD; Ostmann AJ; Strecker LM; Clancy JP Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight 2018, 3, doi: 10.1172/jci.insight.99385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scudieri P; Musante I; Venturini A; Guidone D; Genovese M; Cresta F; Caci E; Palleschi A; Poeta M; Santamaria F; et al. Ionocytes and CFTR Chloride Channel Expression in Normal and Cystic Fibrosis Nasal and Bronchial Epithelial Cells. Cells 2020, 9, doi: 10.3390/cells9092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amato F; Scudieri P; Musante I; Tomati V; Caci E; Comegna M; Maietta S; Manzoni F; Di Lullo AM; De Wachter E; et al. Two CFTR mutations within codon 970 differently impact on the chloride channel functionality. Hum. Mutat 2019, 40, 742–748, doi: 10.1002/humu.23741. [DOI] [PubMed] [Google Scholar]
- 20.McCravy MS; Quinney NL; Cholon DM; Boyles SE; Jensen TJ; Aleksandrov AA; Donaldson SH; Noone PG; Gentzsch M Personalised medicine for non-classic cystic fibrosis resulting from rare CFTR mutations. Eur. Respir. J 2020, 56, doi: 10.1183/13993003.00062-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terlizzi V; Amato F; Castellani C; Ferrari B; Galietta LJV; Castaldo G; Taccetti G Ex vivo model predicted in vivo efficacy of CFTR modulator therapy in a child with rare genotype. Mol Genet Genomic Med 2021, 9, e1656, doi: 10.1002/mgg3.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terlizzi V; Colangelo C; Marsicovetere G; D'Andria M; Francalanci M; Innocenti D; Masi E; Avarello A; Taccetti G; Amato F; et al. Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor Therapy in Three Subjects with the Cystic Fibrosis Genotype Phe508del/Unknown and Advanced Lung Disease. Genes (Basel) 2021, 12, doi: 10.3390/genes12081178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kmit A; Marson FAL; Pereira SV; Vinagre AM; Leite GS; Servidoni MF; Ribeiro JD; Ribeiro AF; Bertuzzo CS; Amaral MD Extent of rescue of F508del-CFTR function by VX-809 and VX-770 in human nasal epithelial cells correlates with SNP rs7512462 in SLC26A9 gene in F508del/F508del Cystic Fibrosis patients. Biochim Biophys Acta Mol Basis Dis 2019, 1865, 1323–1331, doi: 10.1016/j.bbadis.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Pranke IM; Hatton A; Simonin J; Jais JP; Le Pimpec-Barthes F; Carsin A; Bonnette P; Fayon M; Stremler-Le Bel N; Grenet D; et al. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Scientific reports 2017, 7, 7375, doi: 10.1038/s41598-017-07504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laselva O; Bartlett C; Popa A; Ouyang H; Gunawardena TNA; Gonska T; Moraes TJ; Bear CE Emerging preclinical modulators developed for F508del-CFTR have the potential to be effective for ORKAMBI resistant processing mutants. J Cyst Fibros 2021, 20, 106–119, doi: 10.1016/j.jcf.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Pedemonte N Nasal epithelial cells as a gold-standard predictive model for personalized medicine in cystic fibrosis. J Physiol 2022, doi: 10.1113/JP282586. [DOI] [PubMed] [Google Scholar]
- 27.Sette G; Lo Cicero S; Blacona G; Pierandrei S; Bruno SM; Salvati V; Castelli G; Falchi M; Fabrizzi B; Cimino G; et al. Theratyping cystic fibrosis in vitro in ALI culture and organoid models generated from patient-derived nasal epithelial conditionally reprogrammed stem cells. Eur. Respir. J 2021, 58, doi: 10.1183/13993003.00908-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comegna M; Terlizzi V; Salvatore D; Colangelo C; Di Lullo AM; Zollo I; Taccetti G; Castaldo G; Amato F Elexacaftor-Tezacaftor-Ivacaftor Therapy for Cystic Fibrosis Patients with The F508del/Unknown Genotype. Antibiotics (Basel) 2021, 10, doi: 10.3390/antibiotics10070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guimbellot JS; Leach JM; Chaudhry IG; Quinney NL; Boyles SE; Chua M; Aban I; Jaspers I; Gentzsch M Nasospheroids permit measurements of CFTR-dependent fluid transport. JCI Insight 2017, 2, doi: 10.1172/jci.insight.95734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brewington JJ; Filbrandt ET; LaRosa FJ 3rd; Ostmann AJ; Strecker LM; Szczesniak RD; Clancy JP Detection of CFTR function and modulation in primary human nasal cell spheroids. J Cyst Fibros 2018, 17, 26–33, doi: 10.1016/j.jcf.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes D Jr.; Dani A; Guzman-Gomez A; Zafar F; Morales DLS; Ziady AG Changing racial and ethnic differences for lung transplantation in cystic fibrosis. Pediatr. Transplant 2022, e14404, doi: 10.1111/petr.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentzsch M; Ren HY; Houck SA; Quinney NL; Cholon DM; Sopha P; Chaudhry IG; Das J; Dokholyan NV; Randell SH; et al. Restoration of R117H CFTR folding and function in human airway cells through combination treatment with VX-809 and VX-770. Am. J. Physiol. Lung Cell Mol. Physiol 2016, 311, L550–559, doi: 10.1152/ajplung.00186.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabusap CM; Wang W; McNicholas CM; Chung WJ; Fu L; Wen H; Mazur M; Kirk KL; Collawn JF; Hong JS; et al. Analysis of cystic fibrosis-associated P67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight 2016, 1, doi: 10.1172/jci.insight.86581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy JP; Cotton CU; Donaldson SH; Solomon GM; VanDevanter DR; Boyle MP; Gentzsch M; Nick JA; Illek B; Wallenburg JC; et al. CFTR modulator theratyping: Current status, gaps and future directions. J Cyst Fibros 2019, 18, 22–34, doi: 10.1016/j.jcf.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Goor F; Hadida S; Grootenhuis PD; Burton B; Stack JH; Straley KS; Decker CJ; Miller M; McCartney J; Olson ER; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011, 108, 18843–18848, doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randell SH; Fulcher ML; O'Neal W; Olsen JC Primary epithelial cell models for cystic fibrosis research. Methods Mol. Biol 2011, 742, 285–310, doi: 10.1007/978-1-61779-120-8_18. [DOI] [PubMed] [Google Scholar]
- 37.Gentzsch M; Boyles SE; Cheluvaraju C; Chaudhry IG; Quinney NL; Cho C; Dang H; Liu X; Schlegel R; Randell SH Pharmacological Rescue of Conditionally Reprogrammed Cystic Fibrosis Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol 2017, 56, 568–574, doi: 10.1165/rcmb.2016-0276MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rayner RE; Wellmerling J; Osman W; Honesty S; Alfaro M; Peeples ME; Cormet-Boyaka E In vitro 3D culture lung model from expanded primary cystic fibrosis human airway cells. J Cyst Fibros 2020, 19, 752–761, doi: 10.1016/j.jcf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison CB; Shaffer KM; Araba KC; Markovetz MR; Wykoff JA; Quinney NL; Hao S; Delion MF; Flen AL; Morton LC; et al. Treatment of cystic fibrosis airway cells with CFTR modulators reverses aberrant mucus properties via hydration. Eur. Respir. J 2022, 59, doi: 10.1183/13993003.00185-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachs N; Papaspyropoulos A; Zomer-van Ommen DD; Heo I; Bottinger L; Klay D; Weeber F; Huelsz-Prince G; Iakobachvili N; Amatngalim GD; et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019, 38, doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholon DM; Gentzsch M Recent progress in translational cystic fibrosis research using precision medicine strategies. J Cyst Fibros 2018, 17, S52–S60, doi: 10.1016/j.jcf.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cholon DM; Gentzsch M Established and novel human translational models to advance cystic fibrosis research, drug discovery, and optimize CFTR-targeting therapeutics. Curr. Opin. Pharmacol 2022, 64, 102210, doi: 10.1016/j.coph.2022.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awatade NT; Uliyakina I; Farinha CM; Clarke LA; Mendes K; Sole A; Pastor J; Ramos MM; Amaral MD Measurements of Functional Responses in Human Primary Lung Cells as a Basis for Personalized Therapy for Cystic Fibrosis. EBioMedicine 2015, 2, 147–153, doi: 10.1016/j.ebiom.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boecking CA; Walentek P; Zlock LT; Sun DI; Wolters PJ; Ishikawa H; Jin BJ; Haggie PM; Marshall WF; Verkman AS; et al. A simple method to generate human airway epithelial organoids with externally-oriented apical membranes. Am J Physiol Lung Cell Mol Physiol 2022, doi: 10.1152/ajplung.00536.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plebani R; Potla R; Soong M; Bai H; Izadifar Z; Jiang A; Travis RN; Belgur C; Dinis A; Cartwright MJ; et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip: Cystic fibrosis airway chip. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society 2021, doi: 10.1016/j.jcf.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 46.van Riet S; van Schadewijk A; Khedoe P; Limpens R; Barcena M; Stolk J; Hiemstra PS; van der Does AM Organoid-based expansion of patient-derived primary alveolar type 2 cells for establishment of alveolus epithelial Lung-Chip cultures. Am. J. Physiol. Lung Cell Mol. Physiol 2022, 322, L526–L538, doi: 10.1152/ajplung.00153.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis I; Shrestha J; Paudel KR; Hansbro PM; Warkiani ME; Saha SC Recent advances in lung-on-a-chip models. Drug Discov Today 2022, doi: 10.1016/j.drudis.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Baptista D; Moreira Teixeira L; Barata D; Tahmasebi Birgani Z; King J; van Riet S; Pasman T; Poot AA; Stamatialis D; Rottier RJ; et al. 3D Lung-on-Chip Model Based on Biomimetically Microcurved Culture Membranes. ACS Biomater Sci Eng 2022, 8, 2684–2699, doi: 10.1021/acsbiomaterials.1c01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konar D; Devarasetty M; Yildiz DV; Atala A; Murphy SV Lung-On-A-Chip Technologies for Disease Modeling and Drug Development. Biomed Eng Comput Biol 2016, 7, 17–27, doi: 10.4137/BECB.S34252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato T; Clevers H Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 2013, 340, 1190–1194, doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 51.de Poel E; Lefferts JW; Beekman JM Intestinal organoids for Cystic Fibrosis research. J Cyst Fibros 2020, 19 Suppl 1, S60–S64, doi: 10.1016/j.jcf.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Dekkers JF; Wiegerinck CL; de Jonge HR; Bronsveld I; Janssens HM; de Winter-de Groot KM; Brandsma AM; de Jong NW; Bijvelds MJ; Scholte BJ; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med 2013, 19, 939–945, doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 53.Ramalho AS; Furstova E; Vonk AM; Ferrante M; Verfaillie C; Dupont L; Boon M; Proesmans M; Beekman JM; Sarouk I; et al. Correction of CFTR function in intestinal organoids to guide treatment of cystic fibrosis. Eur. Respir. J 2021, 57, doi: 10.1183/13993003.02426-2019. [DOI] [PubMed] [Google Scholar]
- 54.Berkers G; van Mourik P; Vonk AM; Kruisselbrink E; Dekkers JF; de Winter-de Groot KM; Arets HGM; Marck-van der Wilt REP; Dijkema JS; Vanderschuren MM; et al. Rectal Organoids Enable Personalized Treatment of Cystic Fibrosis. Cell Rep. 2019, 26, 1701–1708 e1703, doi: 10.1016/j.celrep.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 55.Vonk AM; van Mourik P; Ramalho AS; Silva IAL; Statia M; Kruisselbrink E; Suen SWF; Dekkers JF; Vleggaar FP; Houwen RHJ; et al. Protocol for Application, Standardization and Validation of the Forskolin-Induced Swelling Assay in Cystic Fibrosis Human Colon Organoids. STAR Protoc 2020, 1, 100019, doi: 10.1016/j.xpro.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Commission, E. Personalised Treatment For Cystic Fibrosis Patients With Ultra-rare CFTR Mutations, https://cordis.europa.eu/proiect/id/755021/reporting. HIT-CF 2022. [Google Scholar]
- 57.Cuyx S; Ramalho AS; Corthout N; Fieuws S; Fürstová E; Arnauts K; Ferrante M; Verfaillie C; Munck S; Boon M; et al. Rectal organoid morphology analysis (ROMA) as a promising diagnostic tool in cystic fibrosis. Thorax 2021, 76, 1146–1149, doi: 10.1136/thoraxjnl-2020-216368. [DOI] [PubMed] [Google Scholar]
- 58.Zomer-van Ommen DD; de Poel E; Kruisselbrink E; Oppelaar H; Vonk AM; Janssens HM; van der Ent CK; Hagemeijer MC; Beekman JM Comparison of ex vivo and in vitro intestinal cystic fibrosis models to measure CFTR-dependent ion channel activity. J Cyst Fibros 2018, 17, 316–324, doi: 10.1016/j.jcf.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Ciciriello F; Bijvelds MJC; Alghisi F; Meijsen KF; Cristiani L; Sorio C; Melotti P; Fiocchi AG; Lucidi V; De Jonge HR Theratyping of the Rare CFTR Variants E193K and R334W in Rectal Organoid-Derived Epithelial Monolayers. J Pers Med 2022, 12, doi: 10.3390/jpm12040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedemonte N; Tomati V; Sondo E; Galietta LJ Influence of cell background on pharmacological rescue of mutant CFTR. Am. J. Physiol. Cell Physiol 2010, 298, C866–874, doi:ajpcell.00404.2009 [pii]; 10.1152/ajpcell.00404.2009. [DOI] [PubMed] [Google Scholar]
- 61.Rowe SM; Pyle LC; Jurkevante A; Varga K; Collawn J; Sloane PA; Woodworth B; Mazur M; Fulton J; Fan L; et al. DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers. Pulm. Pharmacol. Ther 2010, 23, 268–278, doi:S1094-5539(10)00017-9 [pii]; 10.1016/j.pupt.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostedgaard LS; Rogers CS; Dong Q; Randak CO; Vermeer DW; Rokhlina T; Karp PH; Welsh MJ Processing and function of CFTR-DeltaF508 are species-dependent. Proc Natl Acad Sci U S A 2007, 104, 15370–15375, doi: 10.1073/pnas.0706974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bebok Z; Collawn JF; Wakefield J; Parker W; Li Y; Varga K; Sorscher EJ; Clancy JP Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o-airway epithelial monolayers. J. Physiol 2005, 569, 601–615, doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sondo E; Cresta F; Pastorino C; Tomati V; Capurro V; Pesce E; Lena M; lacomino M; Baffico AM; Coviello D; et al. The L467F-F508del Complex Allele Hampers Pharmacological Rescue of Mutant CFTR by Elexacaftor/Tezacaftor/Ivacaftor in Cystic Fibrosis Patients: The Value of the Ex Vivo Nasal Epithelial Model to Address Non-Responders to CFTR-Modulating Drugs. Int. J. Mol. Sci 2022, 23, doi: 10.3390/ijms23063175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fidler MC; Buckley A; Sullivan JC; Statia M; Boj SF; Vries RGJ; Munck A; Higgins M; Moretto Zita M; Negulescu P; et al. G970R-CFTR Mutation (c.2908G>C) Results Predominantly in a Splicing Defect. Clin. Transl. Sci 2021, 14, 656–663, doi: 10.1111/cts.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saferali A; Qiao D; Kim W; Raraigh K; Levy H; Diaz AA; Cutting GR; Cho MH; Hersh CP; Medicine, N.T.i.P. CFTR variants are associated with chronic bronchitis in smokers. Eur. Respir. J 2022, doi: 10.1183/13993003.01994-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crespo-Lessmann A; Bernal S; Del Rio E; Rojas E; Martinez-Rivera C; Marina N; Pallares-Sanmartin A; Pascual S; Garcia-Rivero JL; Padilla-Galo A; et al. Association of the CFTR gene with asthma and airway mucus hypersecretion. PLoS One 2021, 16, e0251881, doi: 10.1371/journal.pone.0251881. [DOI] [PMC free article] [PubMed] [Google Scholar]