Abstract
Background.
It is unclear how the efficacy of tezepelumab, approved for the treatment of type 2 (“T2”) high and low asthma, compares to the efficacy of other biologics for T2-high asthma.
Objective:
To conduct an indirect comparison of tezepelumab to dupilumab, benralizumab, and mepolizumab in the treatment of eosinophilic asthma.
Methods.
We conducted a systematic review and Bayesian network meta-analyses. We identified randomized controlled trials (RCTs) indexed in PubMed, Embase, or CENTRAL between January 1, 2000, and August 12, 2022. Outcomes included exacerbation rates, prebronchodilator Forced Expiratory Volume (FEV1), and the Asthma Control Questionnaire (ACQ). (PROSPERO CRD42021232084)
Results.
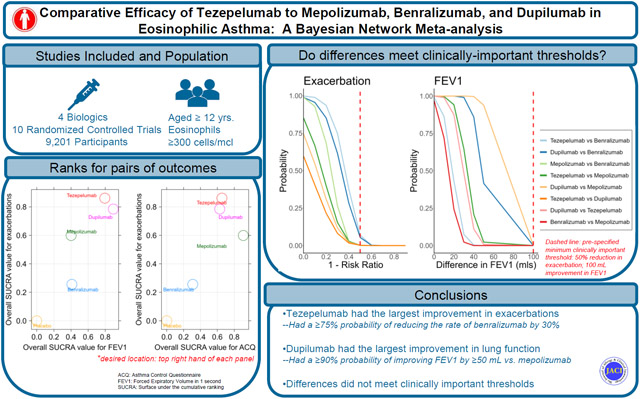
Ten RCTs (n = 9201) met eligibility. Tezepelumab (relative risk 0.63, 95% credible intervals [CI] 0.46-0.86) was associated with significantly lower exacerbation rates than benralizumab; and larger improvements in FEV1 compared to mepolizumab (mean difference, MD, 66, CI −33 to 170) and benralizumab (MD, 62, CI −22 to 150), though the CI crossed the null value of 0. Mepolizumab improved ACQ the most but was not significantly different from tezepelumab (tezepelumab vs. mepolizumab, MD, 0.14, CI −0.10 to 0.38). For efficacy by clinically important thresholds, tezepelumab, mepolizumab, and dupilumab achieved a >99% probability of reducing exacerbation rates by ≥50% compared to placebo, but benralizumab had only a 66% probability of doing so. Tezepelumab and dupilumab had a probability of 1.00 of improving prebronchodilator FEV1 by ≥100 mL above placebo. Compared to mepolizumab, dupilumab had >90% chance for improving FEV1 by ≥50 mL, but none of the differences between biologics exceeded 100 mL.
Conclusions.
In individuals with eosinophilic asthma, tezepelumab and dupilumab were associated with greater improvements in exacerbation rates and lung function than benralizumab or mepolizumab, although below clinical thresholds.
Keywords: Asthma, eosinophilic, tezepelumab, dupilumab, mepolizumab, benralizumab, network meta-analysis, Bayesian, monoclonal antibody, comparative effectiveness
Graphical Abstract
Capsule summary:
A Bayesian network meta-analyses found that tezepelumab and dupilumab were associated with greater improvements in asthma exacerbation and lung function than benralizumab or mepolizumab.
INTRODUCTION
Patients with moderate to severe asthma account for most asthma-related morbidity and mortality.1,2 In many of these individuals, asthma remains uncontrolled despite the use of inhaled corticosteroids.3 For such patients, treatment with monoclonal antibodies can improve asthma control and reduce morbidity and mortality. Mepolizumab (Nucala)4 and benralizumab (Fasenra),5 which target the interleukin-5 signaling pathway, and the anti-interleukin 4 receptor alpha antagonist, dupilumab (Dupixent), have been approved by the US Food and Drug Administration (FDA) for the treatment of eosinophilic asthma, a type 2 (Ύ2’) high phenotype.6 However, in December 2021, the FDA also approved tezepelumab (Tezspire), a novel monoclonal antibody against the epithelial-derived cytokine, thymic stromal lymphopoietin (TSLP), for the treatment of both T2 and non-T2 asthma.7
While many individuals may be suitable candidates for multiple T2 biologics or Tezepelumab there are no head-to-head trials comparing these therapies; we8 and others9,10 have performed indirect treatment comparisons, but these have not included tezepelumab. Furthermore, most indirect treatment comparisons have utilized frequentist statistics and tended to focus on statistically significant, rather than clinically significant, differences between treatments.11
We used a Bayesian network meta-analyses (NMA) framework to compare the efficacy of tezepelumab to dupilumab, benralizumab, and mepolizumab. In addition to allowing for the simultaneous comparison of all the products, rather than pairwise comparisons alone, the NMA framework also allowed for a probability-based ranking of product efficacy and an evaluation of the probability of achieving an effect equal to or exceeding the Minimal Clinically Important Difference (MCID).8
METHODS
Eligibility criteria
We built upon our prior analysis examining the comparative effectiveness of dupilumab, benralizumab and mepolizumab for the treatment of asthma.8 To do so, two of the authors (TN and AA) updated the literature review of peer-reviewed articles, including phase 2b and 3 articles of tezepelumab, indexed in PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from January 1, 2000, through August 12, 2022. Given that there is evidence of the effect modification of the therapeutic effects of these biologics by serum eosinophil count, we focused on patients with serum eosinophil counts ≥300 cells/μL. The study was conducted following the recommendations of the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement and its extension for NMA.12 The protocol was registered a priori. (PROSPERO CRD42021232084)
Efficacy outcomes and Data collection
The primary efficacy outcomes were clinically significant exacerbations, prebronchodilator forced expiratory volume (FEV1), and the Asthma Control Questionnaire (ACQ) score. Two of us (TN and AA) independently extracted data on the study design, baseline characteristics, interventions, outcomes, and on the risk of bias.
Statistical analysis
We fitted Bayesian fixed effects network meta-analysis models.13 We estimated rate ratios for exacerbation rate, and mean differences for continuous outcomes (FEV1 and ACQ). We calculated the associated 95% credible intervals using the Markov chain Monte Carlo algorithms. As there were no head-to-head RCTs included, all data were from indirect treatment comparisons, thus we were unable to assess statistical inconsistency.14
To compare and rank biologics, we calculated rank probabilities using the Surface Under the Cumulative Ranking (SUCRA) score and plotted the cumulative probability that the index biologic would lead to the largest improvements, second-largest, third largest, and least improvement in an outcome. Furthermore, given the multiple domains of these outcomes, we plotted correlograms of ranks to evaluate which biologic would rank the best in improving pairs of outcomes, such as improving both exacerbation rate and lung function. We assessed publication bias by computing each study effect size against the standard error and plotted funnel plots to assess asymmetry visually.
To evaluate if differences met a clinically important threshold, we sought for previously validated minimal clinically important difference (MCID) for each outcome and evaluated the probability that the difference between two biologics met varying thresholds up to the MCID. For ACQ, we used the validated MCID of a reduction of ≥0.5.15 For exacerbation rates and FEV1, there are currently no validated MCID in individuals with asthma but there is expert consensus for improvements in FEV1.16 We defined the MCID for exacerbation as a reduction of at least 50% in annualized exacerbation rate and for FEV1, as an increase of 100 mL.
Sensitivity analyses
In sensitivity analyses, first, we evaluated how the risk of bias designation of a study influenced results by excluding studies that were rated as having at least “some bias”. Second, we evaluated the impact of using data from pooled dosage results if the study population met the eligibility criteria. Thus, because the original PATHWAY study17 had subdivided patients using the eosinophil threshold of 250 cells/μL, we used data from a post hoc analyses of PATHWAY which reported exacerbation outcomes for the subgroup of patients with eosinophil ≥300 cells/μL pooled across all intervention arms.18 Thirdly, we included data from the DREAM study which had pooled results across all mepolizumab arms and evaluated the impact of this on the ranks of the biologics in improving prebronchodilator FEV1 and ACQ.
RESULTS
Study selection and network
We screened 165 studies, of which 16were deemed potentially eligible for inclusion. Of these, 2 were ultimately included;17,19 of the remaining fourteen, two (CASCADE and UPSTREAM)20,21 were excluded after full-text screening due to lack of results stratified by eosinophil level, and one (SOURCE)22 was excluded because of its enrollment of individuals on maintenance oral corticosteroids (Fig E1 and Table E1). The two included studies, PATHWAY and NAVIGATOR, were combined with the eight studies included in our prior analysis;8 thus, this NMA included 10 randomized placebo controlled clinical trials representing 9201 patients. Three each compared mepolizumab23-25 and benralizumab26-28 to placebo, and 2 each compared tezepelumab17,19 and dupilumab29,30 to placebo (Table 1).
TABLE 1.
Characteristics of included trials
| Trial | Intervention | Size of study population |
Efficacy outcomes of interest |
Baseline blood eosinophil requirement |
Study follow-up period (weeks) |
|---|---|---|---|---|---|
| PATHWAY | Tezepelumab | 550 | Exacerbation rate, prebronchodilator FEV1, ACQ-6 score, AQLQ score | No required minimum | 52 |
| NAVIGATOR | Tezepelumab | 1059 | Exacerbation rate, prebronchodilator FEV1, ACQ-6 score, AQLQ score, ASD score | No required minimum | 52 |
| MENSA | Mepolizumab | 576 | Exacerbation rate, prebronchodilator FEV1, SGRQ score, ACQ-5 score | ≥150 cells/μL at screening or ≥300 cells/μL in previous year | 32 |
| MUSCA | Mepolizumab | 556 | SGRQ score, prebronchodilator FEV1, ACQ-5 score | ≥150 cells/μL at screening or ≥300 cells/μL in previous year | 24 |
| DREAM | Mepolizumab | 616 | Exacerbation rate, prebronchodilator FEV1, ACQ-6 score, AQLQ score | ≥300 cells/μL at screening or in previous year | 52 |
| QUEST | Dupilumab | 1902 | Exacerbation rate, prebronchodilator FEV1 | No required minimum | 52 |
| PHASE 2B | Dupilumab | 776 | Exacerbation rate, prebronchodilator FEV1, ACQ-5 score, AQLQ score | No required minimum | 24 |
| SIROCCO | Benralizumab | 1204 | Exacerbation rate, prebronchodilator FEV1, ACQ-6 score, AQLQ score | No required minimum | 48 |
| CALIMA | Benralizumab | 1306 | Exacerbation rate, prebronchodilator FEV1, ACQ-6 score, AQLQ score | No required minimum | 56 |
| ANDHI | Benralizumab | 656 | Exacerbation rate, prebronchodilator FEV1, SGRQ score, ACQ-6 score, CGI-C, PGI-C, SNOT-22, PSIA | ≥300 cells/μL at screening or ≥150 cells/μL with ≥1 of the following: maintenance oral corticosteroid therapy, nasal polyposis, ≥3 exacerbations in previous year, FVC <65% predicted, or age ≥18 years at asthma diagnosis | 24 |
All studies were placebo controlled.
ANDHI, Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab; AQLQ, Asthma Quality of Life Questionnaire; ASD, Asthma Symptom Diary; CALIMA, benralizumab, an anti–IL-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma; CGI-C, Clinical Global Impression of Change; DREAM, mepolizumab for sever eosinophilic asthma; MENSA, mepolizumab treatment in patients with severe eosinophilic asthma; MUSCA, efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma; NAVIGATOR, tezepelumab in adults and adolescents with severe, uncontrolled asthma; PATHWAY, tezepelumab in adults with uncontrolled asthma; PGI-C, Patient Global Impression of Change; PHASE 2B, dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2-agonist: a randomized double-blind placebo-controlled pivotal phase 2b dose-ranging trial; PSIA, Predominant Symptom and Impairment Assessment; QUEST (aka LIBERTY ASTHMA QUEST), dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma; SGRQ, St George’s Respiratory Questionnaire; SIROCCO, efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonist; SNOT-22, Sinonasal Outcome Test 22.
For PATHWAY, we were able to extract the data for the intention-to-treat population by eosinophil thresholds of ≥250 cells/μL from the original study. Outcomes in this subgroup of patients were noted to be comparable to the subgroup with eosinophils ≥300 cells.17 Data for exacerbation outcome in the group with ≥300 cells/μL from PATHWAY was also available from a follow-on study.18 However, that study pooled outcomes across the 3 doses of the intervention arms in PATHWAY (70 mg every 4 weeks, 210 mg every 4 weeks, and 280 mg every 2 weeks, all administered subcutaneously). Given that the 70 mg every 4 weeks and 280 mg every 2 weeks are not approved by FDA, we did not include this study in the primary analyses. However, we evaluated the impact of including these pooled results in sensitivity analyses.
Study and patient characteristics
The median age of study participants across the studies was 49.6 years, and 62% on average were women (Table E2). Caucasians accounted for 62-92 of the population. In the tezepelumab study, NAVIGATOR, which reported proportion of patients with allergic rhinitis and nasal polyposis, 69% of patients had allergic rhinitis compared to 49% in the MENSA-mepolizumab study, 54% in the benralizumab studies, and 67% in the dupilumab studies. Sixteen percent of the participants in NAVIGATOR had nasal polyposis compared to 15% in the mepolizumab studies, 19.6% in the dupilumab studies, and 17% in the benralizumab studies. Study follow-up ranged from 24 to 56 weeks. Risk of bias was noted as “some concerns” for 5 trials with missing data and 2 trials with data reported from post hoc analyses (Table E3).
NMA of efficacy outcomes
The prior NMA had shown that mepolizumab, benralizumab, and dupilumab were significantly better than placebo in reducing exacerbations.8 Tezepelumab was also significantly better than placebo in reducing exacerbations: tezepelumab (risk ratio [RR], 0.31; 95% credible interval [CI], 0.23 to 0.40); improving FEV1: tezepelumab (MD, 210; CI 150 to 280); and in reducing ACQ: tezepelumab (MD −0.49; CI, −0.66 to −0.33) (Fig E2). There was no difference between tezepelumab and dupilumab.
Tezepelumab was significantly better than benralizumab in reducing exacerbation (RR, 0.63; CI 0.46 to 0.86), and led to greater improvements FEV1 compared to mepolizumab and benralizumab although the confidence intervals of these differences in FEV1 crossed the null value of 0 (Fig E2). Mepolizumab was associated with the largest improvement in the ACQ compared to placebo but was not significantly different from tezepelumab. All fitted models converged well. There was no strong evidence of publication bias. (Fig E3-E5). Table 2 demonstrates the summary of results and the certainty in evidence using GRADE criteria, which ranged from very low to moderate.
TABLE II.
Summary of results of efficacy outcomes with GRADE criteria for certainty of evidence
| Tezepelumab | Dupilumab | Benralizumab | Mepolizumaba | |
|---|---|---|---|---|
| Exacerbation: rate ratio, RR (95% CI) | ||||
| Tezepelumab | 1.00 | 1.05 (0.69-1.61)*** | 1.59 (1.17-2.16)*** | 1.20 (0.85-1.68)** |
| FEV1: mean difference in mL (95% CI)b | ||||
| Tezepelumab | 0.00 | 20 (−78 to 120)** | −62 (−150 to 22)** | −66 (−170 to 33)* |
| ACQ: mean difference (95% CI)c | ||||
| Tezepelumab | 0.00 | 0.01 (−0.37 to 0.39)** | 0.17 (−0.02 to 0.37)** | −0.14 (−0.38 to 0.10)* |
Certainty was scored as ****high, ***moderate, **low, *very low. Tezepelumab is the reference group (1.00).
For GRADE, all comparisons were rated as “high” at the starting points as all included studies were RCTs. and then the certainty of evidence was downgraded based on the risk of bias, inconsistency, intransitivity, heterogeneity, imprecision, and publication bias. All comparisons were downgraded by 1 for inconsistency from the potential bias introduced by indirect comparison. Studies with bias originated from missing outcome data was not considered a reason to downgrade because of the relatively balanced dropout rate between study arms.
Mepolizumab comparisons of exacerbation rates and ACQ score were downgraded due to selection of results bias from the MUSCA and MENSA studies, respectively. Mepolizumab comparisons for FEV1 and ACQ score were downgraded by 1 additional point for inconsistency due to the larger differences in placebo effect compared to other mAb trials.
All FEV1 and ACQ score comparisons were downgraded by 1 point because the wide confidence intervals and the relatively small effect sizes.
Ranking of efficacy of monoclonal antibodies
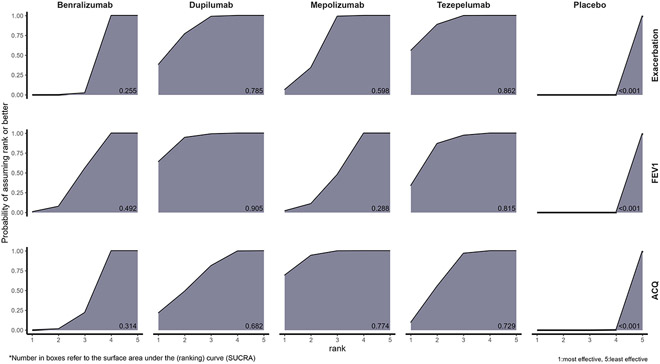
Figure 1 displays the cumulative ranking probability plots along with SUCRA value for all efficacy outcomes. Tezepelumab had the highest SUCRA value (i.e., most effective) for the exacerbation rate reduction with an 89% probability of having the highest or second-best rank in reducing exacerbations. Dupilumab, mepolizumab and benralizumab had a 38%, 6% and <1% chance to being ranked best for the exacerbation rate reduction, respectively. Dupilumab had the highest SUCRA value for the improvement in lung function with a 95% probability of having the highest or second-best rank in improving lung function. Tezepelumab, mepolizumab and benralizumab had a 34%, 2% and <1% chance to being ranked best for improving lung function, respectively. For ACQ, mepolizumab ranked best with a 94% probability of having the best or second-best rank in ACQ improvement. Dupilumab, tezepelumab and benralizumab had a 21%, 9%, and <1% chance to being ranked best for improving ACQ score, respectively.
FIG 1.
Cumulative ranking probability plots with the surface under cumulative ranking (SUCRA) for the efficacy outcomes, including exacerbation, prebronchodilator FEV1, and ACQ. Each plot displayed the cumulative ranking probabilities of each treatment’s being the best (1), second-best (2), third best (3), fourth-best (4), or worst (5) for each efficacy outcome. The best overall treatment would be the treatment with its area under the curve closet to the entire area of the graph shaped like a rectangle which reported as SUCRA value at the bottom right corner ranged from 0 to 1. The higher SUCRA indicates the better ranking. For example, tezepelumab was best treatment in reducing exacerbations with the highest SUCRA at 0.862, while dupilumab was the best for improving FEV1 with SUCRA at 0.905, and mepolizumab ranked highest for improving ACQ with SUCRA at 0.774.
Comparing efficacy on pairs of outcomes
We evaluated the optimal biologic for each pair of efficacy outcomes. Dupilumab and tezepelumab had the greatest benefits in improving both exacerbation rate and FEV1 followed by mepolizumab and benralizumab (Fig 2A). To improve both exacerbation rate and ACQ, tezepelumab ranked the highest, followed by dupilumab and mepolizumab, while benralizumab ranked the least (Fig 2B). For improvement in FEV1 and ACQ score, dupilumab had the highest rank followed by tezepelumab, with mepolizumab and benralizumab ranking the lowest. (Fig 2C).
FIG 2.
Scatterplots of the overall SUCRA score (ranking probability) of improving pairs of efficacy outcomes. (A) Exacerbation rate reduction versus prebronchodilator FEV1 improvement. (B) Exacerbation rate reduction versus ACQ improvement. (C) Prebronchodilator FEV1 improvement versus ACQ improvement.
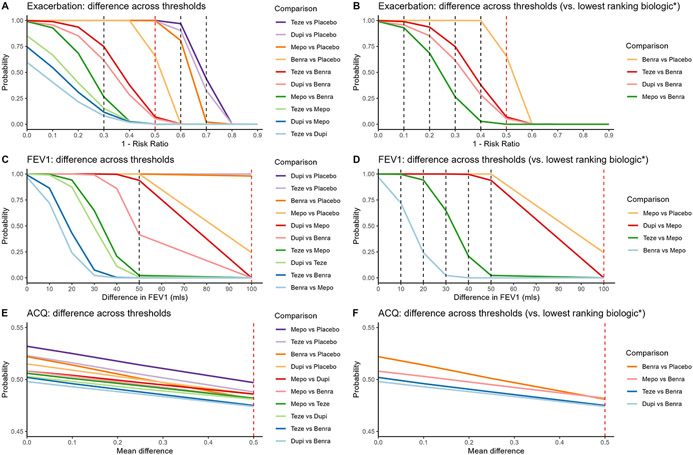
Relative efficacy by varying thresholds up to MCID
Figure 3 demonstrates relative efficacy by varying threshold for each outcome for all comparisons and for comparison of biologics versus lowest ranking biologic. Given the data, all 4 biologics had a probability of 1 in improving the exacerbation rate by 30% or more (rate ratio, ≤0.70) compared to placebo (Fig 3A). In improving the exacerbation rate by 50% or more (rate ratio, ≤0.50) compared to placebo, tezepelumab still had a probability of 1, but this probability decreased to 0.999, 0.996, and 0.658 for dupilumab, mepolizumab, and benralizumab, respectively. For the probability of exacerbation rate reduction by 60% or more (rate ratio, ≤0.40) compared to placebo, tezepelumab had 97% chance followed by dupilumab, 90%, and mepolizumab, 81%. However, benralizumab had a <1% probability of improving exacerbation rate by 60% or more given the data from the included trials. For threshold of rate ratio ≤0.30 or less, all 4 biologics had a probability less than 0.5.
FIG 3.
Relative efficacy by varying thresholds. Each vertical line indicates a threshold of differences between comparisons: the dashed red line indicates the MCID and the black line indicates other thresholds. (A) Exacerbation rate reduction for all comparisons. (B) Exacerbation rate reduction comparing biologics with the lowest ranking biologic, and the lowest ranking biologic with placebo. (C) Prebronchodilator FEV1 improvement for all comparisons. (D) Prebronchodilator FEV1 improvement comparing biologics with the lowest ranking biologic, and the lowest ranking biologic with placebo. (E) ACQ improvement for all comparisons. (F) ACQ improvement comparing biologics with the lowest ranking biologic, and the lowest ranking biologic with placebo. *Also show lowest ranking biologic vs placebo.
Compared to benralizumab, which ranked the least in exacerbation rate reduction, the other 3 biologics had >0.9 probabilities of an exacerbation rate reduction by 10% or more (rate ratio, ≤0.90) (Fig 3B). This decreased to 0.936, 0.852, 0.685 for tezepelumab, dupilumab, and mepolizumab, respectively, fora reduction of ≤20% (rate ratio, <0.80) compared to benralizumab. While tezepelumab and dupilumab still had a probability of 0.748 and 0.615, respectively, mepolizumab had a lower than 0.5 probability of exacerbation improvement by ≥30% (rate ratio, ≤0.7) compared to benralizumab. For threshold of rate ratio ≤0.60 or less compared to benralizumab, none of biologics had a probability more than 0.5.
All 4 biologics had a probability of 1 in improving FEV1 by 50 mL or more compared to placebo (Fig 3C). Dupilumab and tezepelumab had a probability of 1 of improving FEV1 by ≥100 mL above placebo, benralizumab had a probability of 0.981 and mepolizumab had a probability of 0.243. Compared to mepolizumab, dupilumab had a probability of 1 in improving FEV1 by 10 mL or more while tezepelumab and benralizumab had a probability of 0.997 and 0.720, respectively (Fig 3D). While dupilumab and tezepelumab still had a probability of 1 and 0.942, respectively, in improving FEV1 by 20 mL over mepolizumab’s effect, benralizumab had a probability of <0.5. While gradually decreasing, dupilumab maintained more than 90% chance for FEV1 improvement by ≥50 mL compared to mepolizumab. For tezepelumab, the probability decreased to 0.647, 0.208, and 0.023 for improving FEV1 by ≥30, ≥40, and ≥50 mL, respectively. Dupilumab, tezepelumab, and benralizumab had a probability of 0 for improving FEV1 by ≥100 mL in comparison to mepolizumab.
For ACQ, though mepolizumab ranked highest in improving ACQ, all 4 biologics had a probability of ~0.50 in improving ACQ compared to placebo and compared to the other biologics (Fig 3E and 3F).
Sensitivity analyses
The results of sensitivity analyses, including excluding studies with some risk of bias, were consistent with the primary analyses (Table E4). In further sensitivity analyses, for tezepelumab, the PATHWAY data obtained from the pooled tezepelumab arm in group with eosinophil ≥300 cells/μL was used instead of original data for exacerbation (Table E5). For mepolizumab, the DREAM data obtained from the pooled mepolizumab arm was included for prebronchodilator FEV1 and ACQ. For both sensitivity analyses, the efficacy outcomes comparing biologics with placebo were consistent with primary analyses. The rank for each monoclonal antibody for all efficacy outcomes for sensitivity analyses that included pooled intervention data were also consistent with the primary analyses.
DISCUSSION
In this network meta-analyses using ten randomized placebo-controlled trials comparing tezepelumab to mepolizumab, benralizumab, and dupilumab for the treatment of eosinophilic asthma, in comparison to benralizumab, tezepelumab and dupilumab significantly improved the exacerbation rate with a moderate level of certainty, and that mepolizumab demonstrated significant ACQ reduction compared to benralizumab, but with a very low level of certainty. By overall SUCRA ranking, we found that tezepelumab ranked best for exacerbation rate reduction, dupilumab for FEV1 improvement, and mepolizumab for ACQ reduction. Sensitivity analyses with the inclusion of pooled treatment arm provided similar results both in the effect sizes and ranking.
Interestingly, many of the findings that we describe were lower than the MCIDs that have been established or suggested for therapies targeting moderate to severe asthma.15,16 The concept of clinical relevance is however important in interpreting these biologic’s ranks and differences. Patients and their providers need to consider these differences as they seek to optimize therapeutic benefits. For exacerbation rate, benralizumab had the lowest probability of halving the exacerbation rate of placebo. While tezepelumab and dupilumab had significantly better exacerbation rates in comparison to benralizumab, these differences did not reach the MCID of exacerbation rates at a reduction of at least 50% in annualized exacerbation rate. Similarly, for FEV1, although dupilumab and tezepelumab met the MCID improvement of 100 mL when compared to placebo, they did not meet this threshold when compared to mepolizumab or benralizumab. However, comparing dupilumab, which ranked first in FEV1 improvement, to mepolizumab, which ranked last, dupilumab had significantly higher probability of improving FEV1 by half of the MCID threshold. Whether this difference of 50 mL between dupilumab and mepolizumab is clinically relevant however requires more research on MCIDs. Although, one might argue that these MCIDs are strict and lofty goals and that any benefit above and beyond another therapy should be considered a benefit especially in patients who might have very low FEV1 at baseline.
This study provides evidence of higher efficacy for tezepelumab and dupilumab compared to benralizumab and mepolizumab in eosinophilic asthma. The latter comparisons are consistent with prior network meta-analyses.8 Superior efficacy of tezepelumab and dupilumab may be related to their broader spectrum of action compared to mepolizumab and benralizumab which target the IL5 pathway. TSLP is an upstream cytokine and its blockade by tezepelumab prevents the downstream inflammatory cascade including the promotion of Th2 polarization,31-33 and support of type-2 innate lymphoid cells (ILC2) survival.34 Dupilumab exerts broader T2 effects by preventing IL-4 stimulation of Th2 cells and IL-4/IL-13 induced B-cell class switching to IgE.35 Dupilumab’s greater effect on lung function might also be related to its blocking IL-13 mediated airway hyperresponsiveness and remodeling including goblet cell and smooth muscle hyperplasia.36-38 Importantly, the main driving mechanisms of asthma in these study participants may have been related to pathways that are blocked by dupilumab and the alarmin-targeting tezepelumab leading to greater response to these therapies.39 This is in line with emerging evidence, mostly from post-hoc analyses of randomized trials, that patients with T2 asthma can be further subclassified into two groups. These include a high-fractional exhaled nitric oxide (FeNO) group characterized by elevated airway cytokines and alarmins with expected response to alarmin-targeted therapies and IL13 blockade.40,41 A second group with improved response to anti-IL5 therapies are characterized by high IL5 and circulating eosinophils in the peripheral blood and corticosteroid-responsiveness.40,41 However, further research should evaluate the predictive and prognostic effects of this T2 subclassification.
We incorporated minimal clinically important difference (MCID) into this Bayesian NMA to quantify the magnitude of relative efficacy thereby evaluating clinical relevance. We used previously validated MCIDs or MCID noted by expert consensus. For exacerbation, only tezepelumab, dupilumab, and mepolizumab had a probability of about 1 in reducing the exacerbation rate by the MCID of ≥ 50% when compared to placebo. While this study and prior studies have shown benralizumab to be significantly effective in reducing exacerbation rates compared to placebo, although to a lesser degree than other biologics, this study further shows that one-third of patients included in the benralizumab studies may not achieve halving of their pre-index exacerbation rate compared to placebo.10,42 Tezepelumab, dupilumab, and mepolizumab demonstrated more than a 90% probability of having an exacerbation rate ratio of ≤0.9 in comparison to benralizumab, and tezepelumab had ~75% probability of having a rate ratio of ≤0.70 in comparison to benralizumab. The difference between tezepelumab and benralizumab in exacerbation rate improvement may however be clinically insignificant since the MCID for exacerbation is not well established at this time. Thus, more research on the right asthma outcomes and the optimal MCID thresholds are needed.
For prebronchodilator FEV1, all 4 biologics had a probability of 1 for improving FEV1 by at least 50 mL, half of the MCID, compared to placebo. Based on this indirect comparison, none of the biologics would improve FEV1 by up to or greater than the MCID in comparison to another biologic. Dupilumab however had a high probability of improving FEV1 by half of the MCID compared to mepolizumab. The higher efficacy of dupilumab and tezepelumab on prebronchodilator FEV1 improvement might be explained by the dual inhibition of IL-4 and IL-13 by dupilumab,43 and inhibition of TSLP by tezepelumab which prevents subsequent production and release of IL-4, IL-5, and IL-13.17,20 These results might suggest that blockage of IL 13 is important for achieving improvements in lung function in patients with asthma given IL-13’s role in airway mucus production and airway remodeling.44 However, studies from trials of IL13-blocking agents have shown inconsistent to modest results.45 Further studies into if blockade of IL13 in conjunction with blockade of another T2 cytokine is important in harnessing the lung function benefits of anti-IL3 is needed. For comparisons of the anti-IL5 agents, the near-complete depletion of eosinophils via IL-5 receptor blockage might be a plausible explanation for better FEV1 improvement of benralizumab compared to mepolizumab although the difference was minimal.46
Interestingly, for ACQ, the results demonstrated a probability around 0.5 at all thresholds and for all pairs of comparison including to placebo. This indicates a mere coin flip for any of 4 biologics to reduce ACQ in asthma patients with eosinophils ≥300 cells/μL. Moreover, unexplained superior ACQ improvement of mepolizumab despite poor efficacy in improving lung function observed in this meta-analysis raises the concern that ACQ might not capture important improvements based on pathophysiology. One possible explanation was that included RCTs used either ACQ-5 or ACQ-6 which does not include the objective question on FEV1 accounting for the weak-to-moderate correlation between these ACQ versions and FEV1,47,48 in spite of the moderate-to-strong correlation between ACQ-7 and FEV1.49 However, the ACQ is an important outcome, albeit subjective, because it captures a patient’s assessment of their own asthma control. It is also possible that based on the ACQ’s design, an improvement in a specific question might influence the overall score without a significant improvement in asthma control overall or that the ACQ is capturing improvement in a domain which is not captured by exacerbation rates and/or lung function. Thus, additional work is needed to evaluate the appropriateness of the ACQ in the evaluation of the efficacy of biologics.
Our study has limitations. First, we could not evaluate statistical inconsistency between direct and indirect comparisons given that no direct head-to-head trials of these biologics exist. This however highlights the importance of indirect comparisons. Second, the certainty of the evidence was very low to moderate. Though, this was partly due to our assessing each comparison with a ‘moderate’ level of certainty at best given the lack of head-to-head trials. Thirdly, we did not have individual participant level data and differences in the study population may have influenced some of these results. Relatedly, the mepolizumab studies had relatively small placebo effects compared to the placebo effects in the other monoclonal antibody trials. To improve the homogeneity between studies, we had limited to patients with eosinophils ≥300 cells/mcL, and to account for these differences in placebo effects, we downgraded the certainty of evidence in pairwise comparisons including mepolizumab. Finally, PATHWAY used a different cut-off for eosinophil counts at ≥250 cells/μL instead of ≥300 cells/μL which might lead to an inaccurate estimation of tezepelumab’s efficacy. However, prior studies have shown that tezepelumab is more effective in those with higher eosinophil counts19,22 and that for PATHWAY results from patients ≥250 cells and ≥300 cells per mcL were similar.17 Furthermore, we conducted a sensitivity analysis including pooled treatment data from post hoc analyses of PATHWAY which showed results in subgroup with eosinophils of ≥300 cells/μL. The results were consistent with the main analyses.
CONCLUSION
In patients with eosinophilic asthma and eosinophil count ≥300 cells/μL, tezepelumab and dupilumab had better efficacy outcomes compared to benralizumab and mepolizumab. However, many of the statistically significant differences were below the MCID for exacerbation rate and prebronchodilator FEV1.
Supplementary Material
Clinical implications:
In patients with eosinophilic asthma, tezepelumab, dupilumab, and mepolizumab may be preferred over benralizumab for improving exacerbations; and dupilumab or tezepelumab over mepolizumab in improving lung function.
Funding:
Dr. Akenroye is supported by the NIH/NIMHD K99/R00 MOSAIC (K99MD015767) and the Brigham and Women’s Hospital Minority Faculty Career Development Award (MFCDA).
Abbreviations used
- ACQ
Asthma Control Questionnaire
- CENTRAL
Cochrane Central Register of Controlled Trials
- CI
Credible interval
- FDA
US Food and Drug Administration
- FEV1
Forced Expiratory Volume
- GRADE
Grades of Recommendation Assessment, Development and Evaluation
- MCID
Minimal clinically important difference
- MD
Mean difference
- NMA
Network meta-analysis
- PRISMA
Preferred Reporting Items of Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- RR
Risk ratio
- SUCRA
Surface Under Cumulative Ranking
- T2
Type 2
- TSLP
Thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Alexander is past Chair and a current member of FDA’s Peripheral and Central Nervous System Advisory Committee; is a co-founding Principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. All other authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004/06/05 ed. 2004. May;23(5):723–9. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy. 2007/02/15 ed. 2007. Feb;62(2):126–33. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018. Dec;34(12):2075–88. [DOI] [PubMed] [Google Scholar]
- 4.US Food & Drug Administration. Novel Drug Approvals for 2015 [Internet]. 2015. [cited 2022 Sep 5]. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015
- 5.US Food & Drug Administration. Novel Drug Approvals for 2017 [Internet]. 2017. [cited 2022 Sep 5]. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017
- 6.US Food & Drug Administration. New Drug Therapy Approvals 2019 [Internet]. 2019. [cited 2022 Sep 5]. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2019
- 7.US Food & Drug Administration. FDA approves maintenance treatment for severe asthma [Internet]. 2021. [cited 2022 Sep 5]. Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-maintenance-treatment-severe-asthma
- 8.Akenroye A, Lassiter G, Jackson JW, Keet C, Segal J, Alexander GC, et al. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. J Allergy Clin Immunol. 20220627th ed. 2022. Jun 27; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018. Nov;52(5):1801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J Allergy Clin Immunol. 20180908th ed. 2019. Jan;143(1):190–200.e20. [DOI] [PubMed] [Google Scholar]
- 11.Menzies-Gow A, Steenkamp J, Singh S, Erhardt W, Rowell J, Rane P, et al. Tezepelumab compared with other biologics for the treatment of severe asthma: a systematic review and indirect treatment comparison. J Med Econ. 2022. Dec;25(1):679–90. [DOI] [PubMed] [Google Scholar]
- 12.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015. Jun 2;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 13.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 20120823rd ed. 2012. Dec;3(4):285–99. [DOI] [PubMed] [Google Scholar]
- 14.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 20071009th ed. 2008. Jun;17(3):279–301. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 20041126th ed. 2005. May;99(5):553–8. [DOI] [PubMed] [Google Scholar]
- 16.Bonini M, Di Paolo M, Bagnasco D, Baiardini I, Braido F, Caminati M, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev. 20200603rd ed. 2020. Jun 30;29(156). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med. 2017. Sep 7;377(10):936–46. [DOI] [PubMed] [Google Scholar]
- 18.Corren J, Pham TH, Garcia Gil E, Sałapa K, Ren P, Parnes JR, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. 2022. Jun;77(6):1786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med. 2021. May 13;384(19):1800–9. [DOI] [PubMed] [Google Scholar]
- 20.Diver S, Khalfaoui L, Emson C, Wenzel SE, Menzies-Gow A, Wechsler ME, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 20210710th ed. 2021. Nov;9(11):1299–312. [DOI] [PubMed] [Google Scholar]
- 21.Sverrild A, Hansen S, Hvidtfeldt M, Clausson CM, Cozzolino O, Cerps S, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J. 2022. Jan;59(1):2101296. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler ME, Menzies-Gow A, Brightling CE, Kuna P, Korn S, Welte T, et al. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir Med. 2022. Jul;10(7):650–60. [DOI] [PubMed] [Google Scholar]
- 23.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014/09/10 ed. 2014. Sep 25;371 (13):1198–207. [DOI] [PubMed] [Google Scholar]
- 24.Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. [DOI] [PubMed] [Google Scholar]
- 25.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. [DOI] [PubMed] [Google Scholar]
- 26.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–27. [DOI] [PubMed] [Google Scholar]
- 27.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. [DOI] [PubMed] [Google Scholar]
- 28.Harrison TW, Chanez P, Menzella F, Canonica GW, Louis R, Cosio BG, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med. 2021. Mar;9(3):260–74. [DOI] [PubMed] [Google Scholar]
- 29.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018/05/22 ed. 2018. Jun 28;378(26):2486–96. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima S, Kabata H, Kabashima K, Asano K. Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses. Allergol Int. 2020. Apr;69(2):197–203. [DOI] [PubMed] [Google Scholar]
- 32.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007. Feb 1;178(3):1396–404. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005. Nov 7;202(9):1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018. Nov;286(1):37–52. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016. Jan;15(1):35–50. [DOI] [PubMed] [Google Scholar]
- 36.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002. Aug;8(8):885–9. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008. Nov; 118(11):3546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular Targets for Biological Therapies of Severe Asthma. Front Immunol. 2020;11:603312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couillard S, Pavord ID, Heaney LG, Petousi N, Hinks TSC. Sub-stratification of type-2 high airway disease for therapeutic decision-making: A “bomb” (blood eosinophils) meets “magnet” (FeNO) framework. Respirology. 2022. Aug;27(8):573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrimanker R, Keene O, Hynes G, Wenzel S, Yancey S, Pavord ID. Prognostic and Predictive Value of Blood Eosinophil Count, Fractional Exhaled Nitric Oxide, and Their Combination in Severe Asthma: A Post Hoc Analysis. Am J Respir Crit Care Med. 2019. Nov 15;200(10):1308–12. [DOI] [PubMed] [Google Scholar]
- 41.Couillard S, Pavord ID, Heaney LG, Petousi N, Hinks TSC. Sub-stratification of type-2 high airway disease for therapeutic decision-making: A “bomb” (blood eosinophils) meets “magnet” (FeNO) framework. Respirology. 2022. Aug;27(8):573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramonell RP, Iftikhar IH. Effect of Anti-IL5, Anti-IL5R, Anti-IL13 Therapy on Asthma Exacerbations: A Network Meta-analysis. Lung. 20200101st ed. 2020. Feb;198(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavord ID, Siddiqui S, Papi A, Corren J, Sher LD, Bardin P, et al. Dupilumab Efficacy in Patients Stratified by Baseline Treatment Intensity and Lung Function. J Asthma Allergy. 20201216th ed. 2020;13:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018. Mar 1;128(3):997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair P, O’Byrne PM. The interleukin-13 paradox in asthma: effective biology, ineffective biologicals. Eur Respir J. 2019. Feb;53(2):1802250. [DOI] [PubMed] [Google Scholar]
- 46.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 20170921st ed. 2017. Sep 21;9(9):Cd010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan PW, Ghushchyan VH, Marvel J, Barrett YC, Fuhlbrigge AL. Association Between Pulmonary Function and Asthma Symptoms. J Allergy Clin Immunol Pract. 2019. Oct;7(7):2319–25. [DOI] [PubMed] [Google Scholar]
- 48.Picado C, Badiola C, Perulero N, Sastre J, Olaguibel JM, López Viña A, et al. Validation of the Spanish version of the Asthma Control Questionnaire. Clin Ther. 2008. Oct;30(10):1918–31. [DOI] [PubMed] [Google Scholar]
- 49.Silkoff PE, Strambu I, Laviolette M, Singh D, FitzGerald JM, Lam S, et al. Asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) longitudinal profiling study. Respir Res. 2015. Nov 17;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.