Abstract
The basis of medical nutrition therapy for patients with LC-FAODs is to provide adequate energy to maintain anabolism and prevent catabolism. In practice, energy needs are estimated based on formulas derived from normal populations but it is unknown if energy expenditure among patients with LC-FAODs is similar to the normal population. We measured resting energy expenditure (REE), total energy expenditure (TEE) and body composition in 31 subjects with LC-FAODs ranging in age from 7 to 64 years. Measured REE was lower than estimated REE by various prediction equations and measured TEE was lower than estimated TEE. It is possible that the lower energy expenditure based on prediction formulas from the normal population is due to differences in body composition; we compared body composition to normal data from the 2017–18 National Health and Nutrition Examination Survey (NHANES). Fat free mass and fat mass was similar between subjects with an LC-FAOD and NHANES normal data suggesting no difference in body composition. We then compared measured REE and TEE to normal published data from the Dietary Reference Intakes (DRI). Measured REE and TEE were significantly lower among subjects with LC-FAODs compared to normal published energy expenditure data. Our results suggests patients with a LC-FAOD exhibit a lower REE and therefore actually have a slightly lower TEE than estimated. Current prediction equations may overestimate energy expenditure of patients with a LC-FAOD.
Introduction:
Long-chain fatty acid oxidation disorders are inherited disorders of the fatty acid oxidation pathway presenting with clinical symptoms such as hypoketotic hypoglycemia in infancy and exercise or stress induced recurrent rhabdomyolysis in adolescence or adulthood, as well as cardiomyopathy at any age.[1] Multiple different disorders have been described in humans but some of the more frequently identified disorders include: carnitine palmitoyltransferase-2 (CPT-2; OMIM#255110, or #600649), very long-chain acylCoA dehydrogenase (VLCAD; OMIM# 201475), trifunctional protein (TFP; OMIM#609015), and isolated long-chain 3-hydroxyacylCoA dehydrogenase (LCHAD; OMIM#609016) deficiencies.[2] They are characterized by a decreased ability to oxidize fatty acids in the mitochondrial fatty acid oxidation pathway and an accumulation of partially oxidized fatty acids in the circulation, measured as long-chain acylcarnitine’s (Figure 1).[3] Episodic metabolic decompensation can occur during periods of negative energy balance when fatty acids become essential for energy production and result in high morbidity and mortality in this patient population.[1, 4]
Figure 1. The mitochondrial long-chain fatty acid oxidation pathway:
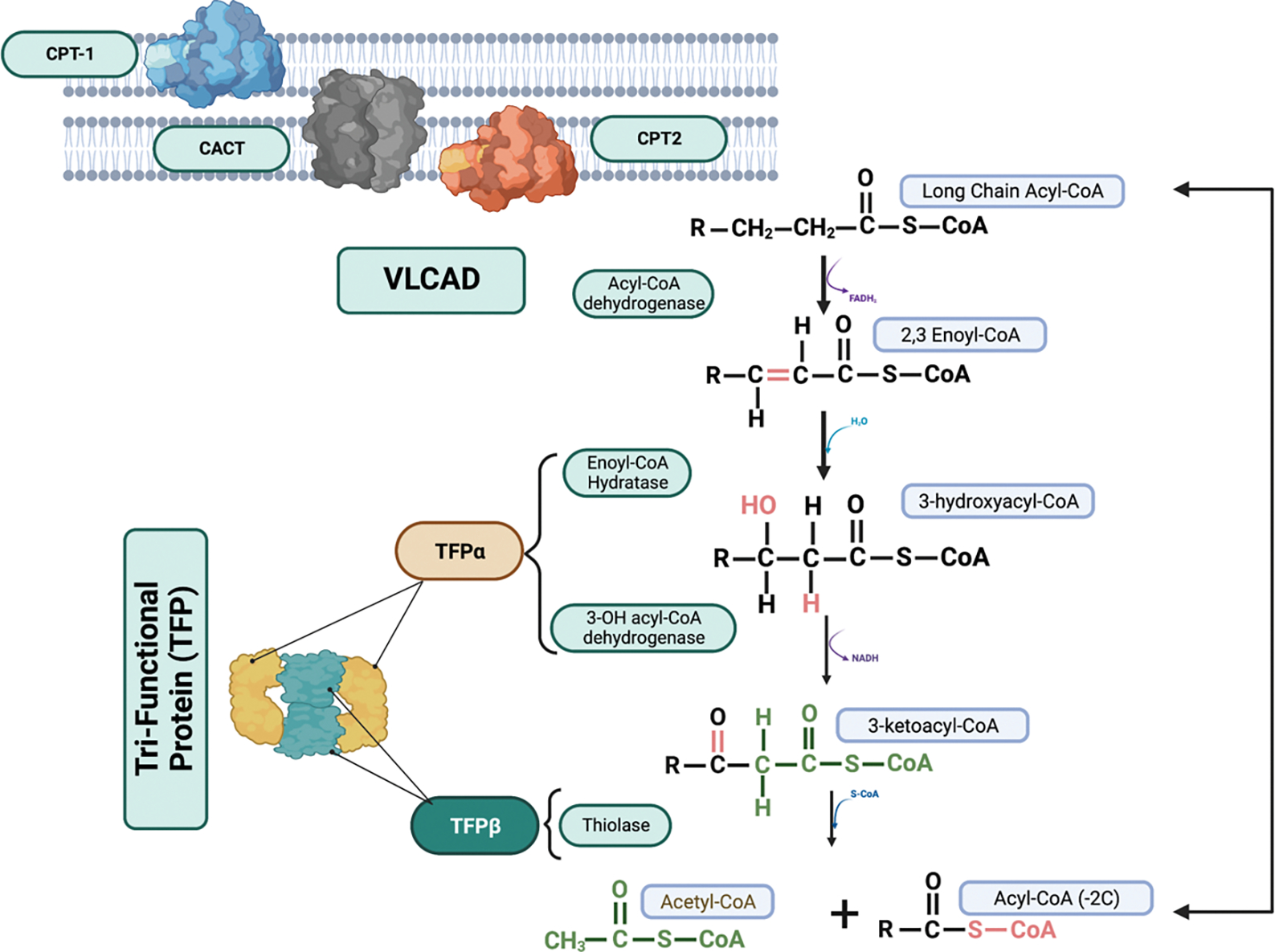
Long-chain fatty acids are imported into the mitochondria via the carnitine palmitoyltransferase shuttle. Once inside the mitochondria, B-oxidation proceeds in a 4-step process catalyzed by very long-chain acyl-CoA dehydrogenase (VLCAD) and trifunctional protein (TFP). Participants in this study had inherited defects in CPT2, VLCAD and TFP, including long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD). CPT1 = carnitine palmitoyltransferase 1, CACT = carnitine acylcarnitine translocase, CPT2 = carnitine palmitoyltransferase 2. Created in BioRender.com
Medical nutrition therapy (MNT) for these disorders is based on maintaining anabolism, while preventing catabolism and the consequent reliance on fat for energy.[5] In practice this is achieved by consuming adequate calories in small frequent meals over the course of the day, limiting periods of fasting and decreasing total dietary energy intake from long-chain fatty acids by consuming a high-carbohydrate, low-fat diet. Patients with LC-FAODs often also supplement their diet with medium chain triglycerides that use different mitochondrial FAO enzymes bypassing the block in the long-chain FAO pathway.[6, 7] If maintaining anabolism is the core tenet of management, estimating energy needs for patients is of high import. The current MNT recommendations suggest patients with LC-FAODs do not need more or less calories than people without a FAOD but rather, adequate total calories at regular intervals. However, the measured energy expenditure and thus energy needs of this population have not been thoroughly explored.
Total energy expenditure (TEE) is comprised of resting energy expenditure, energy expended with physical activity and the thermal effect of food or energy needed to digest and absorb and process nutrients.[8] Resting energy expenditure (REE) accounts for the majority of energy needs (60–70% depending on physical acitivty). It is typically measured first thing in the morning prior to eating or performing any exercise while resting but not sleeping. Physical acitivity is the most variable component of energy expenditure depending on the daily activities of the individual. Thermic effect of food is a small component of TEE, around 10%. The gold standard method to measure TEE in free-living individuals is with doubly-labled water (DLW).
We completed a randomized trial of even versus odd medium chain triglyceride supplements in 32 subjects with a LC-FAOD.[9] During that clinical trial we measured body composition, resting energy expenditure (REE) and total energy expenditure (TEE) on two occasions 4 months apart. In this analysis we explored REE, TEE and body composition among subjects with LC-FAODs compared to available normal data. The goal was to determine if energy expenditure, and thus energy needs in subjects with LC-FAODs is similar to the normal population and what is the best method to estimate energy needs in this group of rare inborn errors of metabolism.
Methods:
Study Design:
This is a secondary analysis of a previously published clinical trial conducted between November 2011 and February 2015.[9] Patients aged ≥ 7 years with a confirmed diagnosis of carnitine palmitoyltransferase-2 (CPT-2; OMIM#255110, or #600649), very long-chain acylCoA dehydrogenase (VLCAD; OMIM# 201475), trifunctional protein (TFP; OMIM#609015) or long-chain 3-hydroxyacylCoA dehydrogenase (LCHAD; OMIM#609016) deficiencies were enrolled. The diagnosis was confirmed by review of medical records documenting at least one significant episode of rhabdomyolysis and at least 2 of the following diagnostic criteria: 1) disease specific elevations of acylcarnitines on a newborn blood spot or in plasma, 2) low enzyme activity in cultured fibroblasts, or 3) one or more known pathogenic mutations. (Table 1). The institutional review board at each study site (Oregon Health & Science University, OHSU, Portland, OR and University of Pittsburgh Medical Center, Pittsburgh, PA) approved the trial. Written informed consent and assent was obtained from all patients and their legal guardians. The trial was registered at clinicaltrials.gov (NCT01379625).
Table 1. Subject Characteristics:
Individual subject characteristics for each participant include age in years, sex (Sex; F= female; M= male), diagnosis (LCHAD/TFP = long-chain hydroxyacylCoA dehydrogenase/trifunctional protein, VLCAD = very long-chain acylCoA dehydrogenase, CPT2 = carnitine palmitoyltransferase 2 deficiencies), mutations (note the change in the respective cDNA of the gene corresponding to the protein listed under diagnosis). Mutations: ? = no 2nd mutation was identified after sequencing all the exons of the gene; for CPT2, common mutations not detected indicates sequencing for the 6 common mutations in CPT2 was completed but complete sequencing of the gene was not performed. Body weight in kilograms (kg), body mass index (BMI; kg/m2), REE=average Resting Energy Expenditure, and TEE=average total energy expenditure measured at baseline and 4 months later in kcal/d, PAL=physical activity level calculated as TEE/REE
| Age (Years) | Sex | Diagnosis | Mutations | Body Wt (kg) | BMI (kg/m2) | REE (kcal/d) | TEE (kcal/d) | PAL |
|---|---|---|---|---|---|---|---|---|
| 7 | M | LCHAD/TFP | c.1528G>C/c.703C>T | 18.6 | 14.2 | 1006 | 1810 | 1.80 |
| 7 | F | VLCAD | c.1619T>C/c.1707_1716dupGACGGGGCC | 26.4 | 17.4 | 1429 | 2470 | 1.73 |
| 8 | F | LCHAD/TFP | c.1528G>C/ ? | 21.9 | 14.9 | 1417 | ||
| 9 | M | LCHAD/TFP | c.1528G>C/ c.1528G>C | 34.3 | 18.7 | 1671 | 2642 | 1.58 |
| 9 | M | CPT2 | c.338C>T/c.340+3A>T | 29.8 | 16.6 | 1400 | 1830 | 1.31 |
| 11 | F | LCHAD/TFP | c.1528G>C/ c.1528G>C | 49.0 | 23.2 | 1341 | 2318 | 1.73 |
| 11 | M | LCHAD/TFP | c.1528G>C/ c.1528G>C | 64.5 | 23.9 | 1578 | 2862 | 1.81 |
| 15 | M | CPT2 | c.338C>T / c.1239_1240delGA /c.1342T>C | 72.8 | 26.4 | 1719 | 1274 | 1.80 |
| 16 | F | CPT2 | c.338C>T/c.1666_1667delTT | 69.3 | 24.7 | 594 | 2481 | 2.09 |
| 16 | M | LCHAD/TFP | c.1150-1 G>T/ c.208T>C | 80.7 | 24.4 | 1246 | 2352 | 1.89 |
| 17 | F | LCHAD/TFP | c.1528G>C/ c.1528G>C | 65.5 | 23.1 | 1492 | 2114 | 1.42 |
| 17 | F | LCHAD/TFP | c.901G>A/? | 44.7 | 18.5 | 1203 | 2344 | 1.95 |
| 19 | M | CPT2 | c.338C>T and c.1666_1667delTT | 91.7 | 29.6 | 834 | 3507 | 2.10 |
| 21 | F | CPT2 | Common mutations not detected | 63.2 | 23.7 | 1393 | 2811 | 2.02 |
| 23 | M | LCHAD/TFP | c.1528G>C/ ? | 65.5 | 22.1 | 1209 | 2127 | 1.76 |
| 24 | F | VLCAD | c.1500_1502delCTT / c.1500_1502delCTT | 54.4 | 21.7 | 1454 | 2519 | 1.73 |
| 24 | F | CPT2 | c.338C>T/? | 91.4 | 32.2 | 1287 | 1906 | 1.48 |
| 24 | F | LCHAD/TFP | c.1528G>C/c.479-482T AGC>AATA | 55.2 | 21.6 | 1133 | ||
| 27 | F | VLCAD | c.898A>C/c.1097G>A | 82.1 | 27.4 | 1488 | 2385 | 1.60 |
| 29 | F | LCHAD/TFP | c.1528G>C/ c.1528G>C | 63.8 | 23.6 | 1401 | 2392 | 1.71 |
| 33 | M | VLCAD | c.1322G>A/ c.1837C>T | 92.1 | 31.3 | 1691 | 3241 | 1.92 |
| 36 | F | VLCAD | No DNA analysis available | 68.1 | 24.2 | 1450 | 1422 | 1.96 |
| 39 | F | CPT2 | No DNA analysis available | 93.8 | 32.7 | 1481 | 1984 | 1.34 |
| 39 | M | VLCAD | c.343delG/c.1244C>T | 88.0 | 27.5 | 1408 | 2116 | 1.50 |
| 39 | F | VLCAD | c.637G>A/ c.1065_1067delCAT | 61.1 | 20.4 | 1237 | 2537 | 2.05 |
| 41 | F | CPT2 | c.338C>U/c.1238_1239delAG | 63.5 | 26.5 | 1144 | 1892 | 1.65 |
| 41 | F | CPT2 | c.338C>T/c.1511C>T | 81.4 | 28.2 | 1362 | 2380 | 1.75 |
| 42 | M | VLCAD | c.637G>A/ c.1065_1067delCAT | 113.0 | 31.5 | 1311 | 2119 | 1.62 |
| 43 | M | VLCAD | c.694G>A/c.1388G>A | 94.8 | 30.2 | 1609 | 2586 | 1.61 |
| 43 | F | CPT2 | c.338C>T/c.1239>1240delGA | 97.1 | 34.5 | 1637 | 3001 | 1.83 |
| 64 | F | CPT2 | c.338C>T/ c.338C>T | 45.1 | 18.5 | 1397 | 2587 | 1.85 |
Randomization:
After baseline assessments, participants were assigned to either triheptanoin (C7) or trioctanoin (C8) supplementation in a 1:1 ratio stratified by diagnosis (CPT2, VLCAD or TFP/LCHAD). In-patient evaluation at baseline prior to randomization and again at study completion was conducted in the respective institutional Clinical and Translational Research Center. Participants were already following a diet low in long-chain fats, supplemented with commercial MCT at baseline. Subjects were instructed to consume a low-long-chain-fat diet and 20% of their estimated total energy needs from the study oil (either C7 or C8) during the 4-month study. Diet intake was assessed by patient recorded 3-day diet records at the beginning and end of the study. Diet records were analyzed with Food Processor nutrient software (ESHA Research, Salem, Oregon).
Body composition:
Body weight (BW), height, and body composition by dual-energy X-Ray absorptiometry (DEXA) were measured after an overnight 10-hour fast.[10]
Energy Expenditure:
Resting energy expenditure (REE) was measured after an overnight fast by indirect calorimetry as previously described. [10, 11] Total energy expenditure (TEE) was measured using a doubly labeled water method (DLW; 2H2O and H218O purchased from Cambridge Isotope Laboratories, Tewksbury, MA).[12] Ingestion of the DLW and subsequent collection of urine samples for analysis took place after returning home from the baseline assessment and prior to returning to the clinical site for end-of-study assessment in order to control for differences in ground water isotope enrichment among the physical locations of the subjects’ homes. Physical activity level (PAL) was calculated as REE/TEE (Table 1).[13]
Prediction equations:
Each subjects basal metabolic rate (BMR) was estimated using a variety of standard prediction equations including Schofield, Harris-Benedict and Mifflin St Jeor (supplemental table 1). Subject estimated TEE was calculated using the DRI estimated energy requirements (EER) equations (supplemental table 2). and the physical activity factor associated with the measured PAL in table 1.
NHANES body composition data:
The body composition and diet intake data from NHANES 2017–2018 was downloaded from https://wwwn.cdc.gov/nchs/nhanes. Data was sorted by age, BMI and sex. All data with the same age in years, same sex (male or female) and same BMI ± 1.0 kg/m2 as one of the subjects with a LC-FAOD was used as the control population for body composition and diet intake. There were 252 controls that met matching inclusion criteria. Body composition was measured by DEXA and diet intake assessed by one 24-hour recall completed at the mobile testing center for each subject.
Energy expenditure control data:
Data published in Appendix I of the Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids was exported to excel and sorted by age, BMI and sex.[14] All data with the same age ± 2.0 years, same sex (male or female) and same BMI ± 1.0 kg/m2 as one of the subjects with an LC-FAOD was used as the control population. Appendix I presents data as Basal Energy Expenditure observed (BEEo) measured by indirect calorimetry and total energy expenditure (TEE) measured by doubly labeled water. There were 192 controls that met matching inclusion criteria.
Statistics:
Primary data are provided in supplementary data tables. Multiple linear regression analysis was performed to determine factors that are related to mREE and mTEE in subjects with an FAOD. Energy expenditure variables were dependent and age, sex, FFM (kg), BMI category and timepoint were independent variables in the model. Paired t-test was used to compare measured Resting Energy Expenditure (mREE) to predicted BMR by various equations and measured Total Energy Expenditure (mTEE) to predicted EER. Body composition (FM, and FFM) of subjects with FAOD and NHANES matched control data (n=252) was compared with a mixed effects linear regression model with age, sex, BW and group (FAOD or control) as fixed effects and subject as a random effect. Macronutrient distribution (percent of kcals from protein, carbohydrate and fat) and kcal/kg were compared by unpaired t-test between subjects with FAOD and controls. REE for the 31 subjects with a LC-FAODs and BEEm for 192 matched controls was fit by a mixed effects linear regression model with age, sex, BW and group (FAOD or control) as fixed effects and subject as a random effect. Similarly, TEE and activity EE for the 29 subjects with a LC-FAODs and for 192 matched controls was fit by a mixed effects linear regression model with age, sex, BW and group (FAOD or control) as fixed effects and subject as a random effect. For all analysis two-sided tests were conducted and p<0.05 was considered statistically significant. Analysis conducted with JMP 17.0 software (Cary, NC) and graphed with Prism 9.0 (Graphpad, San Diego, CA).
Results:
Subjects ranged in age from 7 to 64 years with a diagnosis of CPT2, VLCAD or LCHAD/TFP deficiency (Table1). One subject was excluded because consent for secondary analyses outside of the original project was not provided. There were 19 females and 12 males. Fifty-three percent or 17 subjects had BMI or BMI%tile in the overweight or obese range (BMI>25kg/m2; Table 1). We measured resting energy expenditure (REE) by indirect calorimetry after an overnight fast on two occasions 4 months apart (baseline and 4 months). Body composition was measured by DEXA scan at the same timepoints. Age, fat free mass, and BMI category (healthy, overweight or obese) but not sex or timepoint (baseline and 4 months) were significant predictors of REE suggesting there was no difference between timepoints (Figure 2A). We also measured total energy expenditure (TEE) by doubly labeled water. Our previous analysis found no difference in TEE between subjects randomized to C7 or C8.[9] Two subjects did not return their urine samples during the study; our final sample size was 29 subjects with a LC-FAOD. Age, fat free mass and BMI category, but not sex or timepoint, were significant predictors of TEE (Figure 2B).
Figure 2: Resting and total energy expenditure of 31 subjects with a LC-FAOD by FFM.
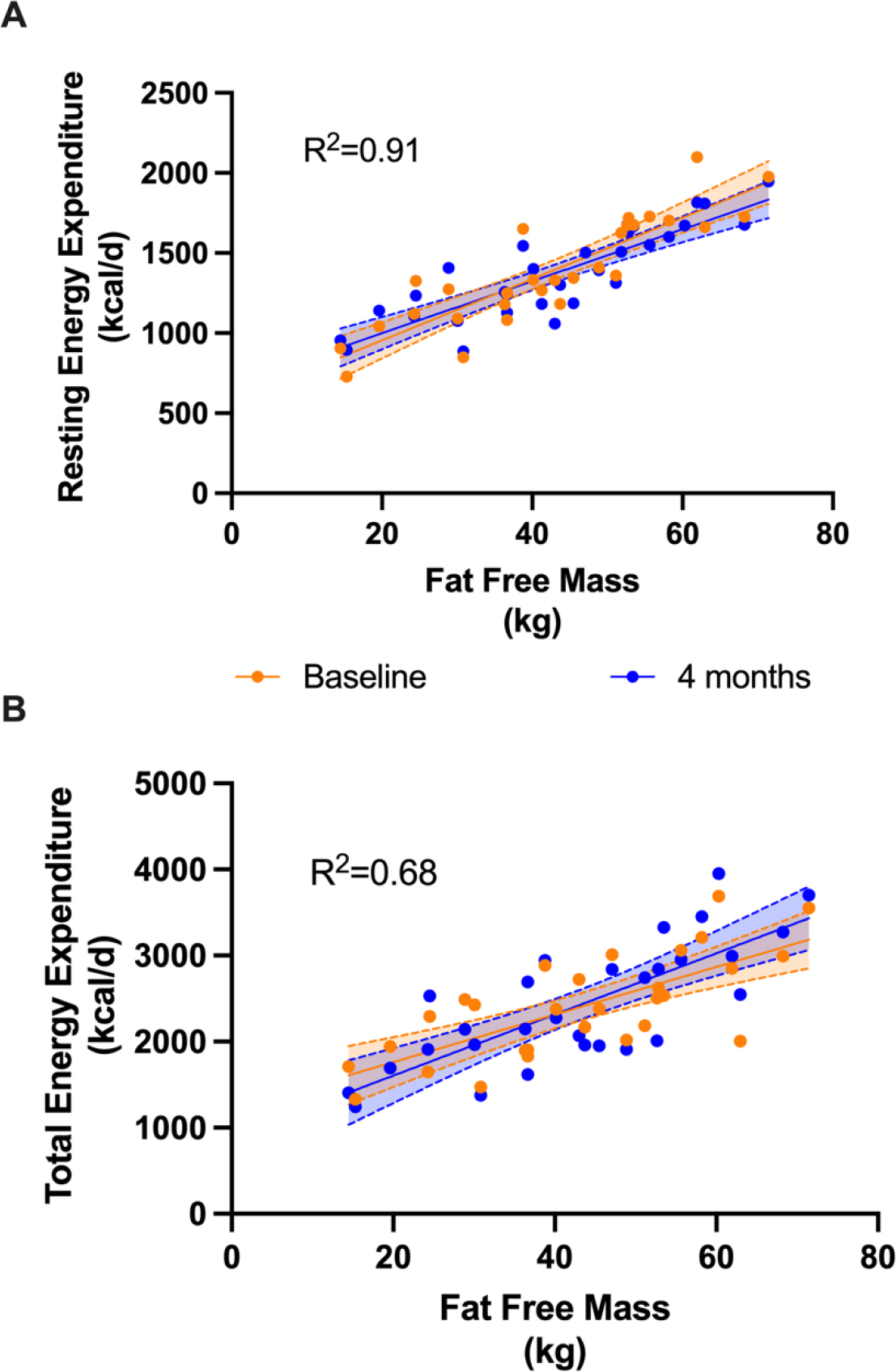
Resting Energy Expenditure (REE) and Total Energy Expenditure (TEE) were measured at baseline (orange) and again after 4 months (blue). The corresponding shading represents the 95% confidence intervals of the measurement. A) The adjusted R2 for the full model is 0.91. BMI category (normal, overweight/obese; p<0.001), age(p<0.001) and FFM(p<0.001) but not sex or timepoint (baseline or 4 months) were significantly related to measured REE. B) The adjusted R2 for the full model is 0.68. Similarly, BMI category (p=0.002), age (p=0.006) and FFM(p<0.001) but not sex or timepoint were significantly related to measured TEE. kcal = kilocalories; kg= kilograms
Next we determined if the measured REE and TEE in patients with a LC-FAOD were similar to predicted based on standard equations. Because timepoint was not a significant factor, we averaged the two REE measures for each subject (Table 1) and compared measured REE (mREE) to predicted Basal Metabolic Rate (BMR) using a variety of prediction equations (Supplemental Table 1). Subjects with an LC-FAOD had lower REE than predicted for all equations (Figure 3). Measured REE was on average 140 kcal/d lower than predicted BMR. We averaged the two measured TEE (mTEE) for each subject (Table 1) and compared to the predicted Estimated Energy Requirement (EER) using the Dietary Reference Intake (DRI) equations specific to BMI category (Supplemental Table 2). Measured TEE was on average 267 kcal/d lower than predicted EER by the DRI equations (Figure 3).
Figure 3: Measured REE (mREE) compared to predicted BMR and measured TEE (mTEE) compared to predicted EER in subjects with a LC-FAOD.
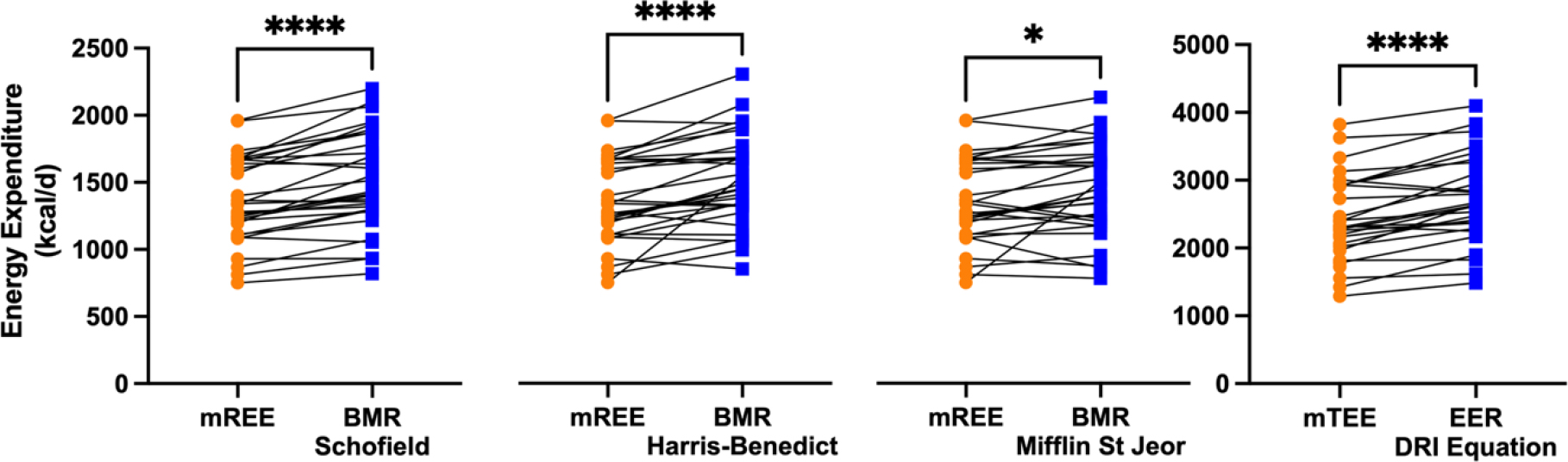
Spaghetti plots illustrate measured REE (mREE) compared to predicted BMR by various equations. Each line is one subject. Measured REE was significantly lower than predicted basal metabolic rate (BMR) by the Schofield, Harris-Benedict and Mifflin St Jeor equations. Measured TEE (mTEE) was lower than Estimated Energy Requirement (EER) by the DRI equations. Paired t-test * p< 0.05, ****p< 0.0001
Measured REE is related to fat free mass (FFM); lower FFM leads to a lower REE. In a previously published small cohort of 9 subjects with LCHADD, we observed lower FFM compared to age, sex and BMI matched controls, but a very small dataset can produce biased results and should be interpreted with caution.[10] It was unknown if in a larger cohort the body composition among patients with LC-FAODs is similar to normal healthy subjects with the same BMI. To address this question, we compared FFM measured by DEXA in our 31 subjects with LC-FAODs with the FFM in the NHANES 2017–18 data. FFM for subjects with LC-FAODs and FFM from an age, sex, and BMI matched sub-sample of the NHANES normal population (n=252) was analyzed by mixed effects linear regression model with age, sex, BW and group (FAOD or control) as fixed effects and subject as a random effect. Sex and BW were significant predictors of FFM but age and group (FAOD and controls) were not significant. FFM (Figure 4A) and Fat Mass (FM; Figure 4B) were similar between controls and subjects with a LC-FAOD suggesting body composition is similar to the normal population.
Figure 4: Similar Fat Free Mass (FFM) and Fat Mass (FM) between subjects with a LC-FAOD and matched controls.
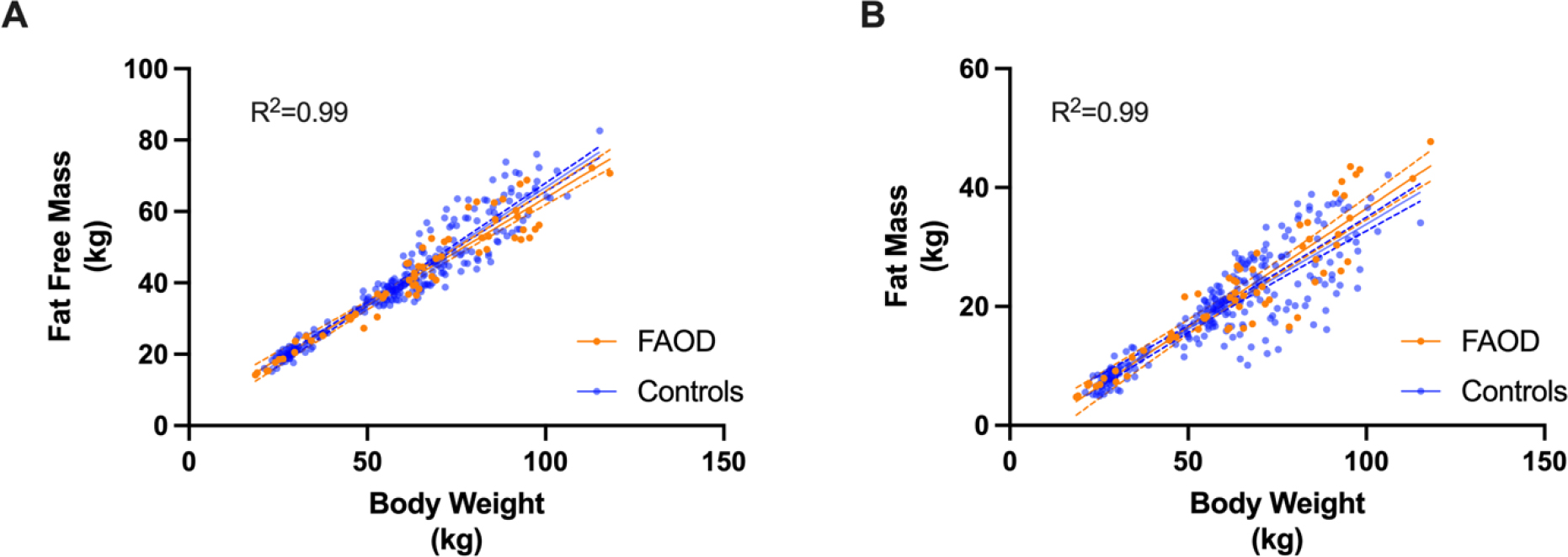
(A) Fat Free Mass and (B) Fat Mass were similar between subjects with an LC-FAOD compared to age, sex and BMI matched NHANES 2017–18 data (n=252). Control subjects are in blue, subjects with a LC-FAOD in orange. The best fit line (solid line) and 95% confidence interval (dotted lines) are illustrated for FAOD (orange) and controls (blue). A) The adjusted R2 for the full model is 0.99. Sex and BW (p<0.0001) but not age (p=0.67) or group (FAOD or control; p=0.08) are related to FFM. B) The adjusted R2 for the full model is 0.99. Sex and BW (p<0.0001) but not age (p=0.85) or group (FAOD or control; p=0.11) are related to FM. kg=kilograms
Subjects with an FAOD were prescribed a low-long-chain-fat diet supplemented with either odd or even medium chain triglyceride. To determine if there was a difference in dietary macronutrient distribution or calorie intake between groups, macronutrient distribution (percent of kcals from protein, carbohydrate and fat, including both medium and long-chain fat) and kcal/kg were compared between subjects with an FAOD and controls. There was no difference in kcal/kg between groups (Fig 5A). Subjects with a LC-FAOD consumed similar % of kcals from protein, more carbohydrate and less total fat compared to controls (Fig 5B). Dietary intake may impact TEE by changing the thermal effect of food (TEF) and diets higher in carbohydrate and medium-chain triglyceride have been associated with higher TEF.[15]
Figure 5: Dietary intake of subjects with a LC-FAOD compared to NHANES controls.
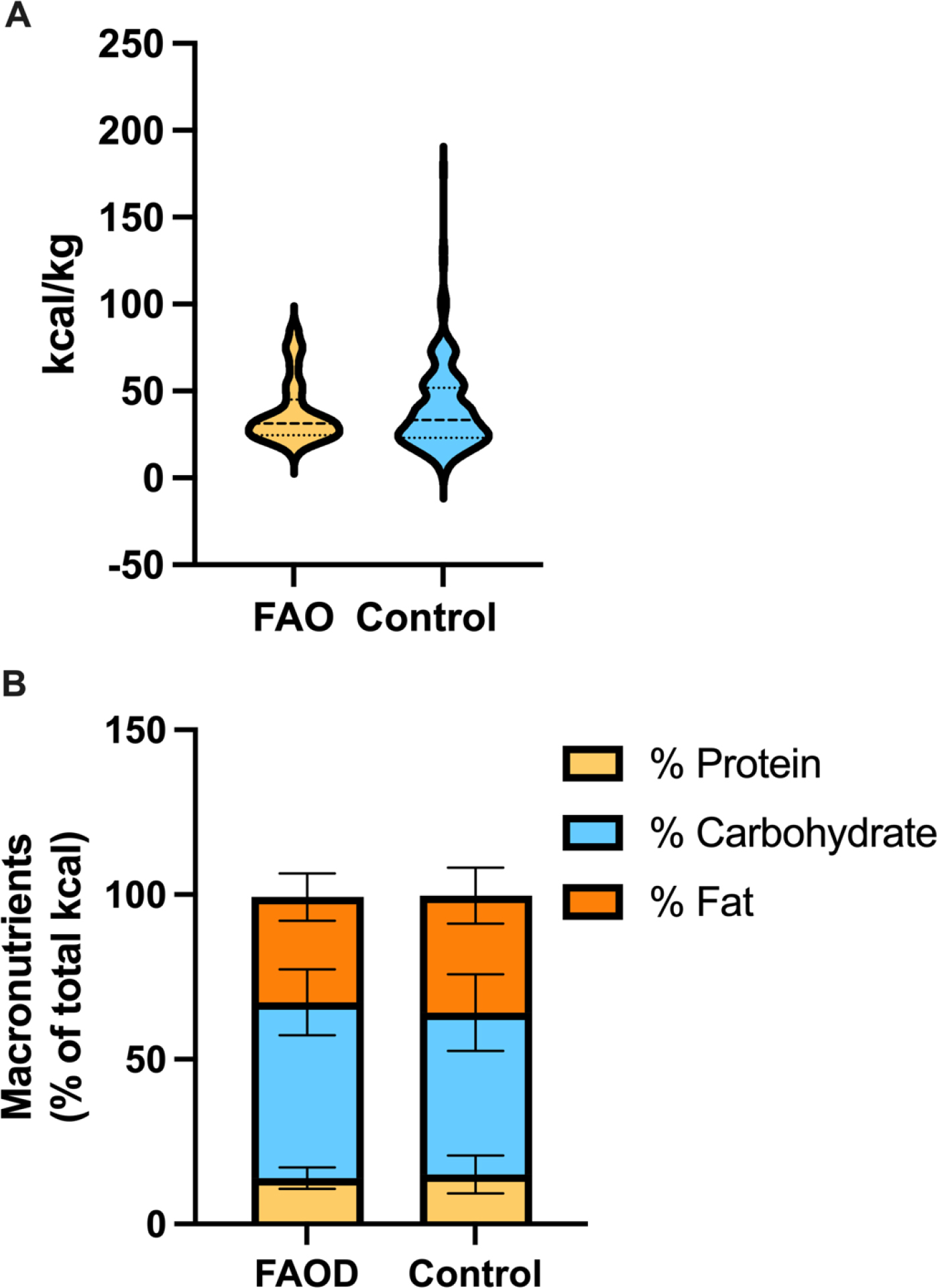
A) Violin plot of kilocalories per kg of body weight (kcal/kg) illustrates similar energy intake between subjects with FAOD (yellow 13.9±3.2) compared to NHANES controls (blue 15.0±5.7). The bold center dotted line is the median for each group, the smaller dotted lines indicate the lower and upper interquartile range. B) The macronutrient distribution is illustrated in the stacked bars. Percent protein was not different (FAOD 13.9±3.2, Control 15.0±5.7; p=0.14), % carbohydrate was higher (FAOD 53.4±10.0, Control 49.2±11.6; p=0.044) and % fat lower (FAOD 31.9±7.2, Control 35.5±8.5; p=0.02) among subjects with a FAOD compared to controls. Data in legend presented as mean±standard deviation of the mean. P-values from unpaired t-tests.
If body composition is similar, why was the measured REE and TEE lower than predicted? To explore these relationships in greater detailed, we compared measured REE in 31 subjects with a LC-FAOD to published observed Basal Energy Expenditure (BEEo) measured by indirect calorimetry in the DRI tables for age, sex and BMI matched normal subjects. REE for the 31 subjects with a LC-FAODs and BEEo for 192 matched controls was fit by a mixed effects linear regression model with age, sex, body weight (BW) and group (FAOD or control) as fixed effects and subject as a random effect. Group, sex and BW were significantly related to REE but age was not a significant factor in this model. The mean difference in REE between subjects with an LC-FAOD and controls was 61 kcal/d which would be almost 500 kcal over a week. This subtle difference is evident in the REE by BW plot (Figure 6A) where subjects with a LC-FAOD are consistently slightly below normal control data. A similar analysis was conducted with TEE. TEE for the 29 subjects with a LC-FAODs and measured TEE for 192 matched controls was analyzed by a mixed effects linear regression model with age, sex, BW and group (FAOD or control) as fixed effects and subject as a random effect. Sex, BW and group were significantly related to TEE. The difference in TEE between groups was 101 kcal/d (p<0.0015). While this is a small daily difference in TEE, over time this could contribute to excessive energy intake and weight gain among subjects carefully balancing their dietary intake to prevent catabolism and metabolic decompensation with negative energy intake (Figure 6B).
Figure 6: Resting Energy Expenditure (REE), Total Energy Expenditure (TEE) and Activity Energy Expenditure (EE) of subjects with a LC-FAOD compared to controls.
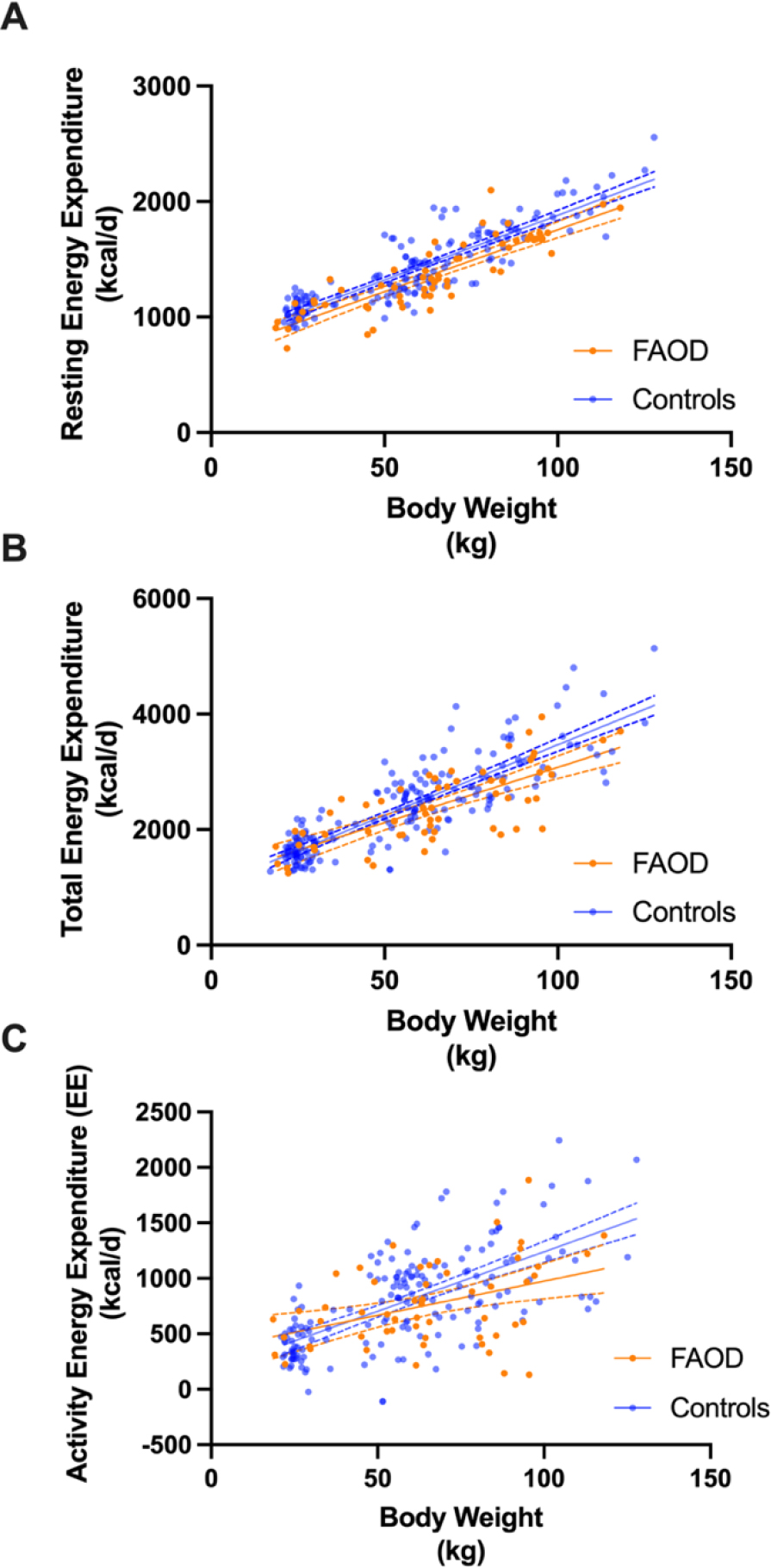
REE (A) and TEE (B) are lower in subjects with a LC-FAOD compared to age, sex and BMI matched published control data (n=192). Control subjects are in blue, subjects with a LC-FAOD in orange. The best fit line (solid line) and 95% confidence interval (dotted lines) are illustrated for FAOD (orange) and controls (blue). A) The adjusted R2 for the model is 0.97. Age, sex, body weight (BW) and group (FAOD or control) were significant effects in the model (p<0.001). There was a 61 ± 10 kcal difference between FAOD and controls. B) The adjusted R2 for the model is 0.94. Sex, BW (p<0.001) and group (p=0.0015) were significant effects in the model. There was a 101 ± 33 kcal difference between FAOD and controls. C) Activity Energy Expenditure was not different between groups. The adjusted R2 for the model is 0.81. BW (p<0.001) was but age (p=0.5) and group (FAOD or control p=0.14) were not significant effects in the model.
Finally, we explored if estimated activity Energy Expenditure (EE) could explain the difference in TEE between subjects with a LC-FAOD compared to controls; i.e., were subjects with a LC-FAOD less physically active? We estimated activity Energy Expenditure by TEE-(TEE × 0.1)-REE for both groups and analyzed the difference by a mixed effects model similar to the models for REE and TEE. Despite more variability in the data, there was no difference in activity EE between subjects and controls (Figure 5C) suggesting activity is similar between groups.
DISCUSSION:
The mainstay of treatment for LC-FAODs is to provide adequate energy to prevent catabolism and avoid periods of prolonged fasting that induce FAO.[5, 16, 17] If maintaining energy balance is critical for anabolism and preventing metabolic decompensation, then estimating energy needs is a key element of developing a MNT treatment plan for patients with LC-FAODs. In this analysis, we considered what is the best approach to estimate energy needs. Like other populations, FFM or BW are strong predictors of REE. Contrary to our previous paired analysis, we observed similar body composition between subjects with a LC-FAOD and NHANES controls by using a much larger sample size. Despite similar body composition, multiple prediction equations overestimated measured REE and TEE. Similarly, subjects with LC-FAOD have a significantly lower REE and TEE compared to DRI published normal data. One potential consequence of lower energy expenditure would be increased prevelance of overweight and obesity due to energy intakes that exceed energy needs. In this cohort, 53% of subjects with a LC-FAOD were overweight or obese, similar to the general US population.[18, 19] We did not observe a higher prevalence of overweight or obesity in our cohort than is typically reported in the general population.
Current clinical guidelines suggest subjects with LC-FAOD do not need more energy than the normal population but, rather, small frequent meals spread across the day to prevent reliance on FAO for energy during periods of fasting.[5, 16, 17] This analysis suggests subjects with an LC-FAOD have lower resting and total energy expenditure than the normal population with a similar body composition. This was a surprising result and the exact reason is unclear. Lower physical activity is one potential explanation for the lower TEE but the estimated Activity Energy Expenditure was not different between groups. Similarly, physical activity levels or PALs were within a normal range typically observed in the population (Table 1). We have not measured physical activity directly with wearable devices in patients so it is possible that some patients with a LC-FAOD have a lower energy expenditure due to low physical activity. However PAL caculated by DLW is often considered the gold standard measurement and this analysis suggests lower physical activity does not account for the differences in TEE between subjects with a LC-FAOD and controls.[13, 20]
Differences in dietary intake can impact the thermal effect of food (TEF) but this contributes a very small amount (approximately 10%) of overall TEE.[15] Subjects with an LC-FAOD consumed similar protein but higher carbohydrate and lower total fat than controls. Much of the fat consumed was in the form of a medium chain triglyceride. Both higher carbohydrate diets and intake of MCT increase TEF suggesting this diet would increase TEE.[15] However, we observed the opposite. It is unlikely differences in dietary intake explains the lower TEE observed.
Likewise, lower REE cannot be explained by lower physical activity or lower TEF. We have consider several different possible explanations. Other heritable genetic factors have been implicated in determining energy expenditure; REE and TEE are similar within families[21] and energy expenditure interclass correlations are greater among monozygotic versus dizygotic twins.[22] It is possible that these factors are different between the subjects with an LC-FAOD and the control population compared here; subjects with an LC-FAOD expressing other genetics factors associated with lower energy expenditure and controls expressing genetic factors associated with higher energy expenditure. However, given the relatively large sample size, this seems improbable.
It is also possible there is a subtle decrease in mitochondrial respiration or mitochondrial number among patients with LC-FAODs compared to the control population. Lower mitochondrial respiration has been observed in African American women compared to age, body weight and BMI matched Caucasian women and was associated with lower RMR.[23] Similarly, lower oxygen consumption has been measured in cultured fibroblasts from patients with VLCAD.[24] One exercise study suggested patients with VLCAD deficiency had more fast-twitch or type 2 muscle fibers and less slow-twitch or type 1 muscle fibers; type 2 muscle fibers have lower mitochondrial content than type 1 muscle fibers.[25] It is possible that muscle of patients with LC-FAOD have either lower mitochondrial respiration or lower mitochondrial number compared to a control population due to adaptive changes in muscle metabolism related to their disorder. This could be tested in muscle biopsy samples from patients with LC-FODs ex vivo.
It is also possible the subtle decrease in REE is an artifact of the technique used; i.e., indirect calorimetry. IC measures oxygen consumption and carbon dioxide production but does not measure heat lost due to anaerobic metabolism.[26] Subjects with LC-FAODs may have a subtle increased reliance on anaerobic metabolism that would not be measured with either IC or DLW.[27, 28] Evidence to suggest there is increased anaerobic metabolism among subjects with LC-FAODs includes frequently increased blood lactate during metabolic decompensation and on metabolomics profiles measured when patients are well.[6, 29, 30] A shift toward fast-twitch fibers as hypothesized above would also suggest increased reliance on anaerobic metabolism as fast-twitch muscle fibers have increased glycolytic metabolism compared to slow-twitch fibers. However, even a modest increase in anaerobic metabolism is unlikely to fully explain the 6 kcal/d lower REE measured among subjects with a LC-FAOD; this caloric deficit would be associated with generation of approximately 0.4 mol of lactate per day.
In conclusion, body composition of subjects with LC-FAODs was similar to normal data from NHANES. Current formulas to estimate REE and TEE based on normal populations overestimate measured REE and TEE in subjects with a LC-FAOD. Our current methods of measuring REE and TEE, although considered the gold standards, may slightly underestimate true energy expended if in fact there is a slight increased reliance on anaerobic metabolism. However, our overall conclusion is that subjects with a LC-FAOD have lower energy expenditure due to a lower resting metabolic rate. Additional studies are needed to confirm or refute the lower REE and TEE measured in subjects with LC-FAODs compared to control data.
Supplementary Material
Funding:
This study was funded by the Food & Drug Administration, Orphan Drug Development Program, Grant FD038950. The authors sincerely thank all of the subjects who participated in this trial. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers UL1TR000128 and UL1TR001857
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Spiekerkoetter U, Mitochondrial fatty acid oxidation disorders: clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening J Inherit Metab Dis 33 (2010) 527–532. [DOI] [PubMed] [Google Scholar]
- [2].Vockley J, Bennett MJ, Gillingham MB, Mitochondiral Fatty Acid Oxisation Disorders, in: Mitchell G, Gibson KM, Ballabio A, Antonkarakis SE, Kinzler KW, Vogelstein B, Beaudet AL, Valle D (Eds.), The Online Metabolic and Molecular Bases of Inherited Disease, The McGraw-Hill Companies, Inc., New York, NY, 2017. [Google Scholar]
- [3].Elizondo G, Matern D, Vockley J, Harding CO, Gillingham MB, Effects of fasting, feeding and exercise on plasma acylcarnitines among subjects with CPT2D, VLCADD and LCHADD/TFPD Mol Genet Metab 131 (2020) 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, Jouvet P, Boutron M, Slama A, Vianey-Saban C, Bonnefont JP, Rabier D, Kamoun P, Brivet M, Recognition and management of fatty acid oxidation defects: a series of 107 patients J Inherit Metab Dis 22 (1999) 488–502. [DOI] [PubMed] [Google Scholar]
- [5].Van Calcar SC, Sowa M, Rohr F, Beazer J, Setlock T, Weihe TU, Pendyal S, Wallace LS, Hansen JG, Stembridge A, Splett P, Singh RH, Nutrition management guideline for very-long chain acyl-CoA dehydrogenase deficiency (VLCAD): An evidence- and consensus-based approach Mol Genet Metab 131 (2020) 23–37. [DOI] [PubMed] [Google Scholar]
- [6].Gillingham M, Van Calcar S, Ney D, Wolff J, Harding C, Dietary management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD). A case report and survey J Inherit Metab Dis 22 (1999) 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gillingham MB, Connor WE, Matern D, Rinaldo P, Burlingame T, Meeuws K, Harding CO, Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency Mol Genet Metab 79 (2003) 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DeLany JP, Measurement of energy expenditure Pediatr Blood Cancer 58 (2012) 129–134. [DOI] [PubMed] [Google Scholar]
- [9].Gillingham MB, Heitner SB, Martin J, Rose S, Goldstein A, El-Gharbawy AH, Deward S, Lasarev MR, Pollaro J, DeLany JP, Burchill LJ, Goodpaster B, Shoemaker J, Matern D, Harding CO, Vockley J, Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial J Inherit Metab Dis 40 (2017) 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gillingham MB, Harding CO, Schoeller DA, Matern D, Purnell JQ, Altered body composition and energy expenditure but normal glucose tolerance among humans with a long-chain fatty acid oxidation disorder Am J Physiol Endocrinol Metab 305 (2013) E1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].DeLany JP, Dube JJ, Standley RA, Distefano G, Goodpaster BH, Stefanovic-Racic M, Coen PM, Toledo FG, Racial differences in peripheral insulin sensitivity and mitochondrial capacity in the absence of obesity J Clin Endocrinol Metab 99 (2014) 4307–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH, High energy expenditure masks low physical activity in obesity Int J Obes (Lond) 37 (2013) 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sharifzadeh M, Bagheri M, Speakman JR, Djafarian K, Comparison of total and activity energy expenditure estimates from physical activity questionnaires and doubly labelled water: a systematic review and meta-analysis The British journal of nutrition 125 (2021) 983–997. [DOI] [PubMed] [Google Scholar]
- [14].Trumbo P, Schlicker S, Yates AA, Poos M, Food TNA Nutrition Board of the Institute of Medicine, Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids J Am Diet Assoc 102 (2002) 1621–1630. [DOI] [PubMed] [Google Scholar]
- [15].Quatela A, Callister R, Patterson A, MacDonald-Wicks L, The Energy Content and Composition of Meals Consumed after an Overnight Fast and Their Effects on Diet Induced Thermogenesis: A Systematic Review, Meta-Analyses and Meta-Regressions Nutrients 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arnold GL, Van Hove J, Freedenberg D, Strauss A, Longo N, Burton B, Garganta C, Ficicioglu C, Cederbaum S, Harding C, Boles RG, Matern D, Chakraborty P, Feigenbaum A, A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency Mol Genet Metab 96 (2009) 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spiekerkoetter U, Lindner M, Santer R, Grotzke M, Baumgartner MR, Boehles H, Das A, Haase C, Hennermann JB, Karall D, de Klerk H, Knerr I, Koch HG, Plecko B, Roschinger W, Schwab KO, Scheible D, Wijburg FA, Zschocke J, Mayatepek E, Wendel U, Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop J Inherit Metab Dis 32 (2009) 498–505. [DOI] [PubMed] [Google Scholar]
- [18].Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL, Trends in Obesity Among Adults in the United States, 2005 to 2014 JAMA 315 (2016) 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ogden CL, Flegal KM, Carroll MD, Johnson CL, Prevalence and trends in overweight among US children and adolescents, 1999–2000 Jama 288 (2002) 1728–1732. [DOI] [PubMed] [Google Scholar]
- [20].Westerterp KR, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, Bedu-Addo K, Blaak EE, Blanc S, Bonomi AG, Bouten CVC, Bovet P, Buchowski MS, Butte NF, Camps S, Close GL, Cooper JA, Das SK, Cooper R, Dugas LR, Ekelund U, Entringer S, Forrester T, Fudge BW, Goris AH, Gurven M, Hambly C, El Hamdouchi A, Hoos MB, Hu S, Joonas N, Joosen AM, Katzmarzyk P, Kempen KP, Kimura M, Kraus WE, Kushner RF, Lambert EV, Leonard WR, Lessan N, Martin CK, Medin AC, Meijer EP, Morehen JC, Morton JP, Neuhouser ML, Nicklas TA, Ojiambo RM, Pietilainen KH, Pitsiladis YP, Plange-Rhule J, Plasqui G, Prentice RL, Rabinovich RA, Racette SB, Raichlen DA, Ravussin E, Reynolds RM, Roberts SB, Schuit AJ, Sjodin AM, Stice E, Urlacher SS, Valenti G, Van Etten LM, Van Mil EA, Wells JCK, Wilson G, Wood BM, Yanovski J, Yoshida T, Zhang X, Murphy-Alford AJ, Loechl CU, Luke AH, Pontzer H, Rood J, Schoeller DA, Wong WW, Speakman JR, g. International Atomic Energy Agency Doubly Labeled Water database, Physical activity and fat-free mass during growth and in later life Am J Clin Nutr 114 (2021) 1583–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bogardus C, Lillioja S, Ravussin E, Abbott W, Zawadzki JK, Young A, Knowler WC, Jacobowitz R, Moll PP, Familial dependence of the resting metabolic rate N Engl J Med 315 (1986) 96–100. [DOI] [PubMed] [Google Scholar]
- [22].Bouchard C, Tremblay A, Nadeau A, Despres JP, Theriault G, Boulay MR, Lortie G, Leblanc C, Fournier G, Genetic effect in resting and exercise metabolic rates Metabolism 38 (1989) 364–370. [DOI] [PubMed] [Google Scholar]
- [23].Toledo FGS, Dube JJ, Goodpaster BH, Stefanovic-Racic M, Coen PM, DeLany JP, Mitochondrial Respiration is Associated with Lower Energy Expenditure and Lower Aerobic Capacity in African American Women Obesity (Silver Spring) 26 (2018) 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seminotti B, Leipnitz G, Karunanidhi A, Kochersperger C, Roginskaya VY, Basu S, Wang Y, Wipf P, Van Houten B, Mohsen AW, Vockley J, Mitochondrial energetics is impaired in very long-chain acyl-CoA dehydrogenase deficiency and can be rescued by treatment with mitochondria-targeted electron scavengers Hum Mol Genet 28 (2019) 928–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Diekman EF, Visser G, Schmitz JP, Nievelstein RA, de Sain-van der Velden M, Wardrop M, Van der Pol WL, Houten SM, van Riel NA, Takken T, Jeneson JA, Altered Energetics of Exercise Explain Risk of Rhabdomyolysis in Very Long-Chain Acyl-CoA Dehydrogenase Deficiency PLoS One 11 (2016) e0147818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kenny GP, Notley SR, Gagnon D, Direct calorimetry: a brief historical review of its use in the study of human metabolism and thermoregulation Eur J Appl Physiol 117 (2017) 1765–1785. [DOI] [PubMed] [Google Scholar]
- [27].Scott CB, Kemp RB, Direct and indirect calorimetry of lactate oxidation: implications for whole-body energy expenditure J Sports Sci 23 (2005) 15–19. [DOI] [PubMed] [Google Scholar]
- [28].Pahud P, Ravussin E, Jequier E, Energy expended during oxygen deficit period of submaximal exercise in man J Appl Physiol Respir Environ Exerc Physiol 48 (1980) 770–775. [DOI] [PubMed] [Google Scholar]
- [29].Tyni T, Palotie A, Viinikka L, Valanne L, Salo MK, von Dobeln U, Jackson S, Wanders R, Venizelos N, Pihko H, Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency with the G1528C mutation: clinical presentation of thirteen patients [see comments] J Pediatr 130 (1997) 67–76. [DOI] [PubMed] [Google Scholar]
- [30].Ventura FV, Ruiter JP, L IJ, de Almeida IT, Wanders RJ, Lactic acidosis in long-chain fatty acid beta-oxidation disorders J Inherit Metab Dis 21 (1998) 645–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


