Abstract
The tumor microenvironment (TME) in liver cancers such as hepatocellular cancer (HCC) consists of a complex milieu of liver tissue-resident cells, infiltrated immune cells, and secreted factors that collectively serve to promote tumor growth and progression. Intercellular crosstalk contributes to tissue homeostasis, and perturbations during injury, inflammation and tumorigenesis that are important for tumor progression. Extracellular vesicle (EV) mediated transfer of a payload of RNA molecules that serve as an intercellular signaling is an important contributor to tissue homeostasis within the TME. Several types of RNA have been implicated in EV mediated signaling. Biological processes that can be modulated by EV RNA signaling within the liver include tumor growth, invasion, and metastasis, angiogenesis, and modulation of the immune cell activities. This mini review describes the liver TME, and the biological effects of EV RNA mediated signaling within the liver to highlight the role of EV RNA in intercellular communication.
Keywords: Extracellular vesicles, Liver cancer, RNA therapeutics, Extracellular RNA, microRNA
1. Introduction
Primary liver cancers are complicated tumors often arising in the context of persistent injury and inflammation, and dynamic immune responses within a complex tissue environment. The tumor microenvironment (TME) associated with primary liver cancers such as hepatocellular cancer (HCC) and cholangiocarcinoma is a critical determinant of tumor growth and spread [1, 2], The liver TME reflects the cellular composition of the underlying liver tissue, comprising of several types of cells within an intricate stromal milieu, and macromolecular extracellular matrix (ECM) components such as glycosaminoglycans and proteoglycans [3]. The TME includes cells found within normal liver tissue such as hepatic epithelia, portal fibroblasts, hepatic stellate cells (HSC), endothelial cells (ECs), and immune cells. In addition, tumor-associated cells that can facilitate tumor progression such as cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), or that can suppress tumor growth such as CD8+ cytotoxic T cells and T helper cells [4] are also present within the TME (Figure 1).
Figure 1: Cellular component of the tumor microenvironment in the liver.
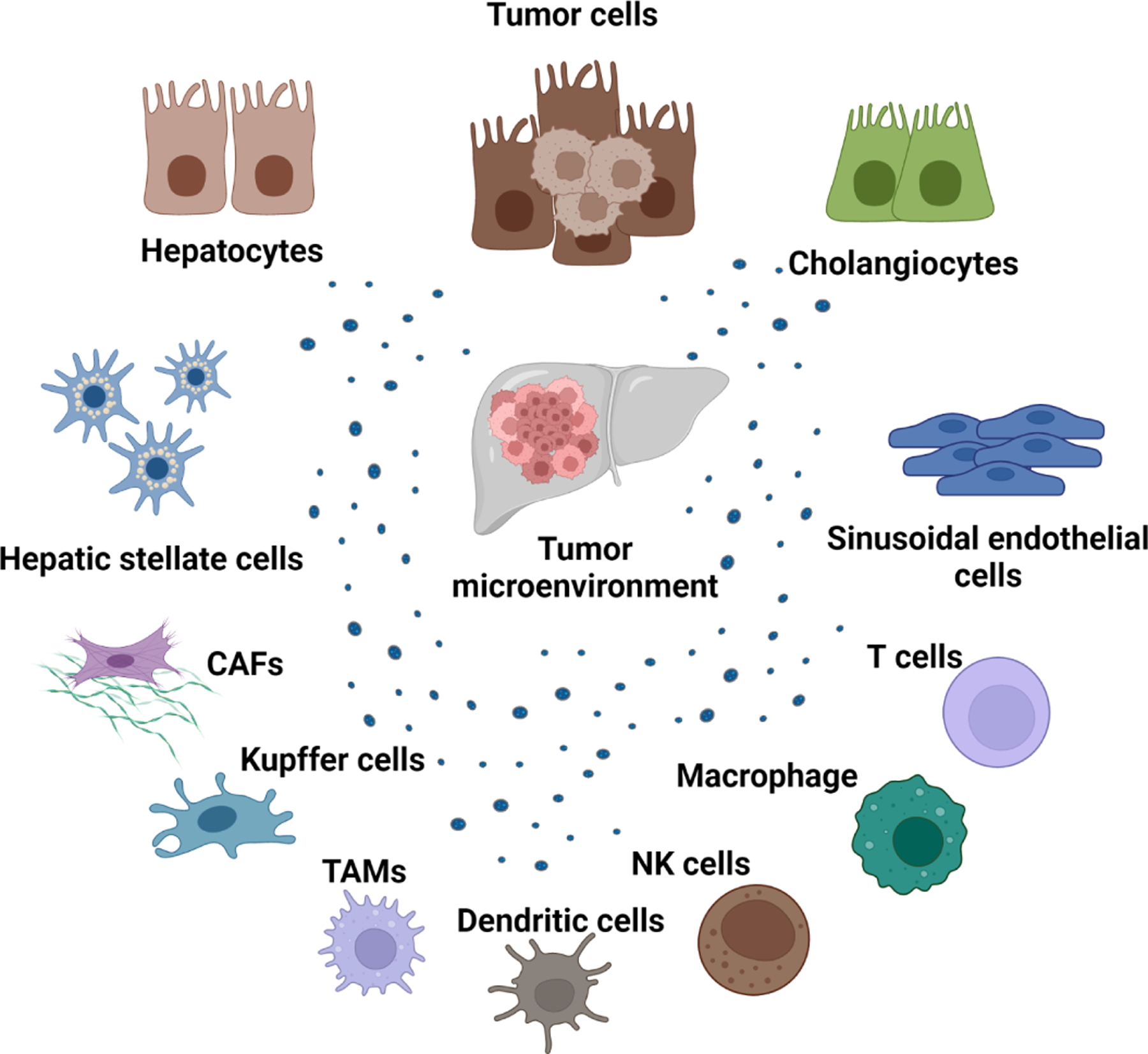
Several different types of cells are present within the tumor microenvironment. These include normal hepatic epithelia, tumor cells, immune cells, fibroblasts, and endothelial cells. Extracellular vesicles can be released from all cell types can modulate cellular processes in recipient cells after their uptake. Abbreviations: CAF, cancer-associated fibroblast; NK cells, natural killer cells; TAM; tumor-associated macrophages; TME, tumor microenvironment. Created with Biorender.com.
Intercellular communication within the tissue environment is not only required for tissue homeostasis and function but has also been implicated in tumor progression and metastasis. [5, 6]. Indeed, intercellular interactions between tumor and other cells in the TME are crucial factors of tumor growth [7]. As such, developing a comprehensive understanding of the signaling mediators of intercellular communication within the TME will be valuable for developing advanced diagnostics or novel therapeutics for liver cancer. Intercellular signaling may involve expression of surface proteins, cytoplasmic transfer of cellular constituents, the release and uptake of extracellular vesicles (EVs), or extracellular secretion or release of RNA or soluble proteins such as cytokines, chemokines, inflammatory signal mediators, and hormones [6]. EV-mediated transfer of bioactive cargoes such as nucleic acids, proteins, and lipids is being increasingly recognized. RNA is a versatile molecule with the ability to influence gene expression in target cells; hence, it is a potent mediator of intercellular signaling that can result in long-lasting changes in recipient cells. In this mini-review, we will emphasize the role of extracellular RNA as a signaling mediator contributing to intercellular communication within the liver TME [8].
2. Extracellular vesicles: Mediators of RNA-based intercellular signaling
Within the tissue microenvironment, EV mediated signaling involves many different cellular contributors and physiological processes in normal liver, or pathobiological processes during tissue injury or malignancy (Figure 2). Within the tumor microenvironment, EV mediated signaling can contribute to tumor growth and spread by promoting epithelial-to-mesenchymal transition, angiogenesis, inducing immune evasion and drug resistance, and activating HSCs to cancer associated fibroblast. Each of the cellular elements within the TME can modulate processes and contribute to tumor growth. For example, activated HSC can promote tumor vascularization [9]. Because tumor vasculature is disorganized and dysfunctional, blood flow is compromised and contributes to a hypoxic and acidic microenvironment [10]. CAFs have been found to suppress natural killer cell (NK cell) activation, enabling HCC growth. CAFs also promote tumor growth by remodeling the TME. ECs can secrete biologically active proteins such as interleukin (IL) IL-6, IL-8, monocyte chemoattractant protein-1, and growth-regulated protein alpha, which can promote tumor progression, and can enhance the potential for metastasis [11]. As HCC frequently arises within the context of a persistent inflammatory state, the interplay between non-immune cells and tissue-resident or infiltrating immune cells such as tumor-infiltrating lymphocytes, regulatory T cells, exhausted T cells, and antigen-presenting cells within the TME are central to tumor progression [1]. Alterations in the matrix components of the liver TME can also impact tumor cell growth, adhesion, migration, survival, and angiogenesis [12]. Thus, intercellular signaling between components of the TME can individually or collectively contribute to tumor behavior. EV such as exosomes can be released by cells can contribute to intercellular signaling. The mechanism by which exosomes are generated and released by cells are being gradually elucidated [13, 14]. The release of EVs and their subsequent uptake by recipient cells with delivery of a bioactive payload such as RNA provides a pathway for dynamic cell-directed environmental regulation. Indeed, RNA content in EVs has been shown to play an important role in cell-cell signaling. The lipid membrane of EVs protects the EV RNA from degradation by extracellular RNases and allows them to retain their structural integrity and function. The RNA population within EVs is size-limited and consists of intact small non-coding RNA (ncRNA) such as microRNA (miRNA), long ncRNA (lncRNA), and circular RNA (circRNA), or fragments of larger protein-coding mRNAs [15].
Figure 2: Extracellular vehicles (EVs) mediated intercellular signalling in liver tissue injury and malignancy:
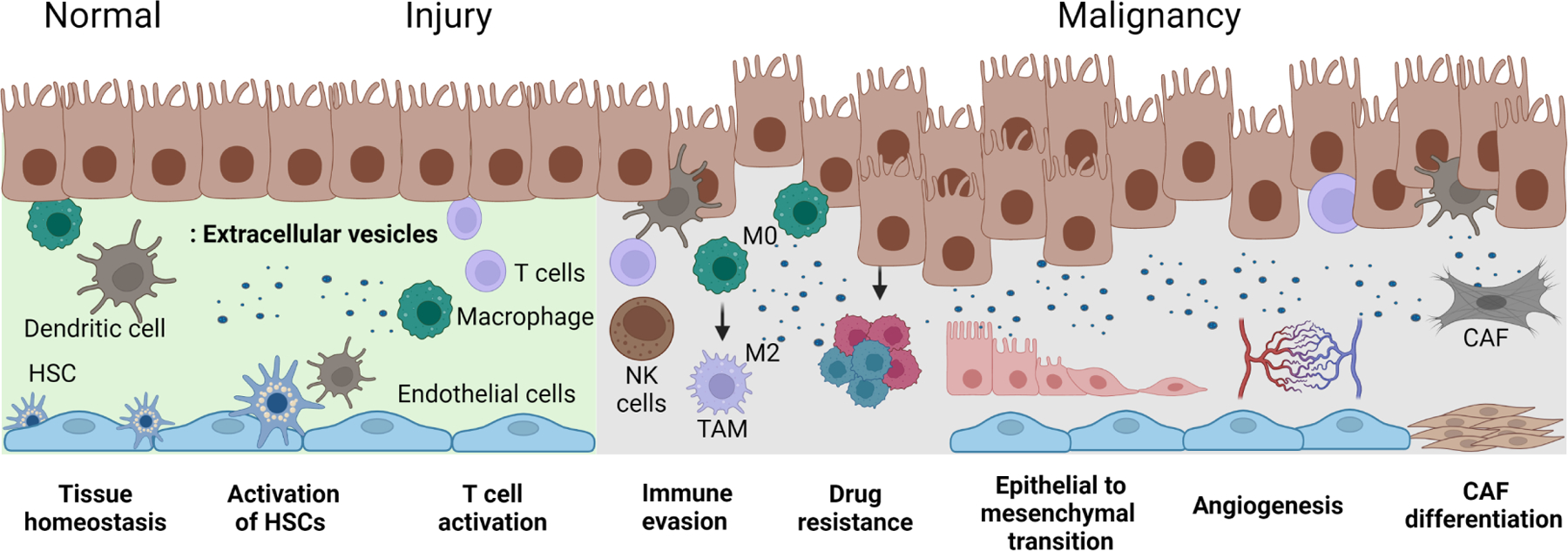
This figure illustrates cellular contributors and physiological or pathobiological processes involving EV mediated signaling within the tissue microenvironment, from left to right, in normal liver, during liver injury and inflammation, and in liver cancers. EV mediated signaling can have diverse effects in maintaining normal tissue homeostasis and in tissue responses to injury and inflammation, contributing to activation of hepatic stellate cells and immune cells leading to inflammation and fibrosis. Within the tumor microenvironment, EV mediated signaling can contribute to tumor growth and spread by promoting epithelial-to-mesenchymal transition, angiogenesis, inducing immune evasion and drug resistance, and activating HSCs to cancer associated fibroblast. Abbreviations: CAF: cancer-associated fibroblast; HSCs, hepatic stellate cells; M0, non-activated or resting macrophages; M2, polarized anti-inflammatory macrophage; NK cells, Natural killer cells. Created with Biorender.com.
2.1. miRNA.
EV-mediated transfer of miRNA has been recognized in several contexts within the TME. Transfer of miRNA within HepG2 EVs promoted the differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) into fibroblasts, supporting the epithelial-to-mesenchymal transition (EMT), and promote invasion, and migration of liver cancer cells. In a study by Wei et al., tumor cell-derived EVs deliver miR-181d-5p into BMSCs and target cytokine signaling 3 to activate the FAK/Src pathway, causing them to acquire fibroblastic characteristics [16]. The effects on cancer cells were reversed by downregulation of miR-181d-5p in BMSCs. Similarly, EVs originating from HepG2 and Huh7 cells can shape the surrounding TME by delivering miR-21, miR-221, and miR-151 to transform HSCs into cancer-associated HSCs. Remarkably, these activated HSCs can also release EVs that can increase liver cancer cell invasion and metastasis via protein kinase B (AKT)/extracellular signal-regulated kinase (ERK) signaling [17]. Furthermore, EV-mediated transfer of miR-126-3p from HSCs to HepG2 cells promoted tumor cell motility, invasion, and growth of multicellular tumor spheroids [18]. In other studies, EVs released from induced M2 polarized macrophages could deliver miR-15b into HL-7702 cells HCC cells, thereby promoting liver cancer growth and metastasis via inhibiting the tumor suppressor kinase 1 pathway [19]. EVs derived from liver cancer cells can also modulate angiogenic signaling. Hep3B cells communicated pro-angiogenic signals through the release of EV containing miR-584-5p and their transfer into vascular ECs, stimulating them to proliferate and migrate via modulation of the phosphoenolpyruvate carboxykinase 1 (PCK1)/nuclear factor E2-related factor 2 axis, which in turn enhanced tumor formation and angiogenesis by suppressing PCK1 expression [20].
2.2. lncRNA.
Similar to EV-miRNAs, lncRNA within EVs can mediate signaling and crosstalk between cancer cells and other cells within the TME such as MSCs, CAFs, TAM, ECs, and NK cells [21]. Kogure et al. identified enrichment of several ultraconserved lncRNA within EVs derived from Hep3B and PLC/PRF/5 HCC cells. Amongst these, TUC339 was increased in EV derived from both cell types and verified to promote cancer cell proliferation. Moreover, EV-mediated transfer of TUC339 implicated in the transformation of adjacent cells towards malignant phenotypes [22]. The lncRNA-prostate androgen-regulated transcript 1 was elevated in EV derived from Huh7 and HCCLM3 HCC cells and promoted proliferation, metastasis, and modulation of the miR-372-3p/TLR4 axis to polarize macrophages to the M2 phenotype [23]. In other studies, EV-based lncRNA reprogramming regulators were reported to influence cancer cell chemoresistance to sorafenib and doxorubicin [24].
2.3. circRNA.
EV-based circRNA-mediated signaling has also been reported within the liver TME. By delivering circRNA-deubiquitination (circRNA-DB), which targets miR-34a, a negative regulator of ubiquitin-specific protease 7 and cyclin A2, EVs from adipocytes can undermine cyclin A2-dependent cell cycle arrest and enhance cancer cell progression [25]. Exosomes from highly metastatic LM3 HCC cells can deliver circRNA-prostaglandin reductase 1 (circRNA-PTGR1) that targets miR-449a in HepG2 and 97L cells, further demonstrating the potential of EV-circRNA in modulating TME. Moreover, miR-449a can regulate the proto-oncogene mesenchymal-epithelial transition factor (MET) in HCC [26]. Intriguingly, EV-packed circRNA-PTGR1 can boost the metastatic potential of adjacent cells in TME. Similarly, highly metastatic 97h cells release exosomal circRNA-100338, which can promote the pro-angiogenic potential of adjacent human umbilical vein endothelial cells (HUVECs) and enhances metastases of HCC cells that have a lower metastatic potential [27].
2.4. DNA, lipids, and proteins.
The signaling potential and roles of biologically active molecules other than RNA within EV and their contributions to the onset, maintenance or progression of liver cancers remain to be determined. However, emerging studies suggest that EV DNA and protein-mediated signaling could participate in tumor growth [28, 29]. For example, EVs harboring oxidized mitochondrial DNA could activate oncogenic pathways to promote HCC. Mitochondrial aldehyde dehydrogenase 2 deficient hepatocytes treated with carbon tetrachloride and ethanol release oxidized mitochondrial DNA via their EVs and can promote cancer progression via the activation of C-Jun N-terminal kinase (JNK), signal transducer and activator of transcription 3, B cell lymphoma 2, and transcriptional coactivator with PDZ-binding motif (TAZ) pathways [28]. Furthermore, colorectal cancer (CRC) cell-derived exosomes are rich in HSPC111 protein, which can promote CRC cell metastasis to liver by activating CAFs and regulating CXCL5 signaling [29]. Unlike EV-based RNA signaling, the potential involvement of EV-mediated transfer of DNA, protein, or lipids in cell-cell communication has not been widely studied and remains to be established.
3. Mechanism of RNA incorporation into EVs
EVs released by tumor cells have emerged as a channel for RNA transfer from one cell to another, hence sustaining the TME [20]. Within EVs, the RNA content varies based on the subcellular source and pathological status of the donor cells [30]. During the synthesis of an EV, the endosomal sorting complexes of the donor cell package a heterogeneous pool of RNAs into the EV. Furthermore, enforced cellular expression of miRNA in donor cells can increase its concentration in EV and has been used experimentally to study the effects of EV-RNA [31]. Some reports have indicated that EVs can express a broader range of miRNA than observed within cells of origin [32]. Thus, while the RNA expression profile of distinct cell types is partly reflected within their budding EVs, the RNA content within EVs may differ from their parental cells, supporting the presence of mechanisms for preferential incorporation of RNA into vesicles [33, 34]. Despite evidence that EV-RNA cargo selection is dysregulated in pathological conditions, the mechanisms determining selective inclusion of EVs or specific RNA sorting into EVs are still unknown. Several mechanistic pathways of RNA sorting into EVs have been proposed and include pathways that involve RNA binding proteins (RBPs), lipids, RNA-induced silencing complex (RISC), or 3’ miRNA sequence-dependent mechanisms. Amongst these, RBP-dependent RNA trafficking has been verified, but additional evidence is required for the other processes.
3.1. RNA binding proteins: A major shuttle for RNA into EVs.
RBPs are proteins that have one or more RNA binding domains and that can regulate function of the bound RNA [35]. Several RBPs such as heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), Y-box binding protein 1 (YBX1), fragile X mental retardation 1, synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP) [36], major vault protein (MVP) [37] and human antigen R (HuR) [38] have been implicated in controlled incorporation of RNA into EVs.
Villarroya-Beltri et al. reported that hnRNPA2B1 has a 3’ end GGAG motif binding domain that guides miR-575, miR-451, miR-125a, and miR-198 into human primary T cell-derived exosomes [39], whereas, another study indicated that a membranous protein caveolin-1 (CAV1) is essential for hnRNPA2B1 to recognize AGG and UAG motifs on miR-17 and miR-93 for their selective inclusion into shedding microvesicles [40]. This RBP can influence EV packaging of lncRNA such as H19 [41] to promote drug resistance in non-small cell lung carcinoma [42] and lncRNA LNMAT2 to promote lymphatic metastasis in bladder cancer [43]. On the other hand, an anti-export mechanism has been reported whereby hnRNPA2B1 can negatively regulate the sorting of exosomal miR-503 into ECs [44]. Similarly, the RBP YBX1 is involved in RNA sorting into EV and potentially recognizes 5′-CTGATTGGCCAA of Y-box motif, single-stranded motif GGGG as well as either single- or double-stranded variants of the motifs CACC and CATC. YBX1 can actively regulate the inclusion of not only miR-223, but also tRNA, Y RNA, and Vault RNA into HEK-293-derived EVs [45, 46]. In a distinctive mechanism involving condensation with P-bodies, YBX1 selectively recruits miR-223 into exosomes from a pool of RNA [47]. Under hypoxic conditions, it preferentially transfers miR-133 into EVs produced from ECs [48].
Several other RBPs such as HuR, argonaute RISC catalytic component 2 (AGO2), SYNCRIP, and MVPs have been implicated in RNA incorporation into EVs. HuR and AGO2 were discovered to bind competitively with miR-122 in starved hepatoma cells, thereby facilitating its delivery into nascent multivesicular bodies [38]. AGO2 itself can modulate inclusion of miR-452, miR-150 and -142-3p in EVs of HEK293 [46]. In addition, SYNCRIP has been shown to shift miRNAs such as miR-690 and miR-6981-5p into EV of non-tumorigenic hepatocytes; however, the precise protein binding motif sequences are unknown [49]. Additional research is required to uncover MVP-binding motifs on RNAs. MALDI-TOF mass spectrophotometry has uncovered a connection between miR-193a and MVPs during miRNA sorting in exosomes in a study in which MVP-mediated enhanced encapsulation of miR-193a fuelled colon cancer growth and metastasis [50].
3.2. EV membrane lipid: An attractant for RNA.
Lipids may play a role in RNA sorting mechanisms in a variety of ways. EWI motif-containing protein 2 (EWI2) is an RBP that regulates the abundance of miR-3934-5p in prostate cancer cells and their EVs [51]. The cytoplasmic tail of EWI-2 interacts with negatively charged phosphatidylinositol phosphates but not with other membrane incorporated lipids [52].
Lipid rafts in EV membranes have been implicated in the sorting of proteins into multivesicular bodies [53], but their significance in the sorting of RNA remains largely unknown. Interestingly, lipid rafts occurring on the EV membrane have affinity for RNA aptamers with a CCCU motif which facilitates their interaction with the lipid nanodomains of a vesicle [54]. [55]. A role for neutral sphingomyelinase 2 (nSMase2) in the secretion of miRNA is suggested by studies showing that inhibition of nSMase2 reduced the amount of miRNA within the exosomes of HEK293 cells, whereas overexpression has the opposite effect [56]. Knockdown of nSMase2 can suppress the expression of the EV membrane protein Alix [57], which has been implicated in AGO2-mediated miRNA trafficking into EVs [58]. Furthermore, nSMase2 orchestrates the trafficking of angiogenic miRNAs into the EV of metastatic breast cancer cells via a ceramide-dependent route, and can thereby dictate the EC niche within the TME to impact on tumor growth [59]. However, it is uncertain if nSMase2 aids in the sorting of RNA or simply increases the release of exosomes, given that ceramide and Alix are primarily responsible for the over-secretion of EVs.
3.3. RISC complex: A carrier of small RNAs.
Other than the RBPs, components of the machinery for processing short RNA, such as RISC, participate in sorting their associated RNA into EVs [60]. Exportin 5, a transporter of pre-miRNA and RBPs across the nuclear envelope, actively binds to ADP-ribosylation factor 6 for the trafficking of pre-miRNA and miRNAs into shedding vesicles by activating casein kinase 2 [61]. The incorporation of RBP-RNA complexes into EV was aided by microtubules [62]. Additionally, the presence of pre-miRNA processing machinery within the vesicles has been demonstrated.
3.4. RNA sequence: cis-acting regulating elements targeting RNA to EVs.
In-depth analyses of EV content have demonstrated the enrichment of specific sequences in RNAs, particularly in 3’ UTR regions. This has prompted the search for specific sequence motifs linked with the sorting of RNA from the cell to vesicles [63]. In line with this, ab initio computational analysis has identified comprehensive motif sequences in EV-RNA, with ACCAGCCU, CAGUGAGC, and UAAUCCCA motifs on RNAs identified as cis-acting elements that target RNA into exosomes [64]. Bolukbasi et al. reported a 25-nucleotide “zipcode” region in the 3’ UTR of glioblastoma-derived EV-enriched mRNA, including the core sequence CTGCC and a binding site for miR-1289. Direct binding of miR-1289 to this region resulted in a significant enrichment of mRNA bearing the zipcode motif into EVs [65]. Other studies have revealed a 12-nucleotide motif that is abundantly prevalent in hepatocytes’ EV-RNA. The addition of the motif sequence to a luciferase mRNA reporter enhanced the incorporation of luciferase into EVs [66]. In addition, the location of miRNAs can be determined by the addition of non-templated terminal nucleotides during maturation. For example, 3’-adenylated miRNAs were retained in B-cells, but 3’-uridylation facilitated their sorting into vesicles [67].
4. Biological effects of EV-based RNA signaling
As efficient biological cargo shuttles, EVs play a crucial role in mediating the intercellular crosstalk within the liver TME. Mounting evidence suggest that the horizontal transfer of EV-associated RNA cargoes can contribute to several different processes involved in the development, maintenance, progression, and metastasis of HCC (Figure 3). Horizontal transfer of EV-associated RNA payloads can promote angiogenesis, modify the stromal populations within the liver TME, induce chemoresistance, and boost the metastatic potential of HCC cells (Table 1). Many of the EV-related RNAs investigated in pre-clinical models were also found to be concentrated in the serum EV of HCC patients, and some of these RNAs were associated with shorter overall survival [68–70]. This evidence demonstrates the therapeutic potential of targeting EV-mediated RNA signaling to halt the progression of the disease.
Figure 3: Biological effects of EV based RNA involved in signaling in the liver tumor microenvironment.
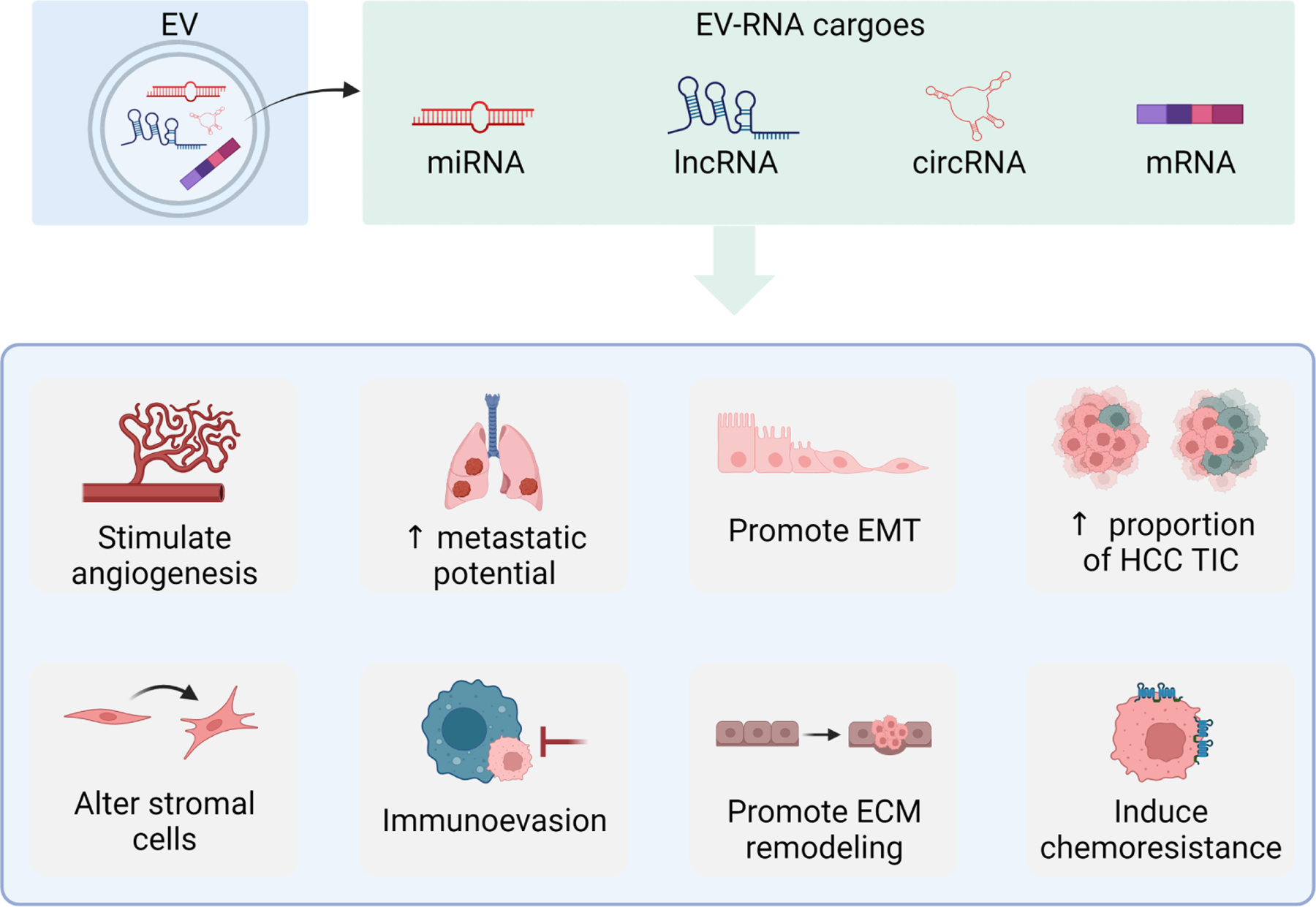
Hepatocellular cancer (HCC) cell-derived EV can contain several different types of RNA cargoes, such as microRNAs (miRNA), long non-coding RNAs (lncRNA), circular RNA (circRNA) and messenger RNAs (mRNA). Intercellular signaling within the liver TME with transfer of biologically active EV-associated RNA can contribute to diverse effects, supporting tumor cell growth, promoting the progression and metastasis of disease, stimulating tumor angiogenesis and modulating the immune cell activities. Created with Biorender.com.
Table 1.
Biological effects of EV-RNA signaling in HCC
| Biological effect | EV source | RNA cargo | Biological Effects | Reference |
|---|---|---|---|---|
| Induction of tumor initiating cells (TIC) | HCCLM3 and HepG2 cells EV | miR-429 | Induced cisplatin resistance, promoted HCC proliferation & colony formation | [71] |
| Promoting angiogenesis | HepG2 HCC cells | miR-378b | Increased HCC tumor burden, accelerated tumor invasion, and boosted angiogenic activity in the TME of HepG2-tumor bearing animals. | [72] |
| SMMC-7721 and HepG2 HCC cells | miR-1290 | Increased HUVEC angiogenic activity | [69] | |
| HCCLM1 and MHCC-97H HCC cells | Linc-00161 | Repressed endogenous miR-590-3p and upregulated MMP-3, MMP-9, and VEGFA in HUVEC cells. | [68] | |
| HCC cell-EV (MHCC97H and Huh7 cells) | miR-103 | Enhanced the endothelial permeability in HUVEC cells | [70] | |
| HCC patient serum and QGY-7703 HCC cells | miR-210 | Enhanced tubulogenesis by suppressing SMAD4 and STAT6 in recipient HUVEC | [73] | |
| CD90+ Huh7 cells | lncRNAH19 | Increased H19-dependent angiogenic activity of recipient HUVEC cells. | [74] | |
| Inducing chemoresistance | HepG2 | Linc-ROR | Reduced chemotherapy-induced cell death | [24] |
| HCCLM3 and HepG2 cells | miR-429 | Suppressed the tumor-suppressor gene RBBP4 and conferred cisplatin chemoresistance. | [71] | |
| Bel/5-FU and Bel7402 cells | miR-32-5p | Conferred a drug-resistant phenotype by inhibiting PTEN and activating the PI3K/Akt signaling cascade. | [76] | |
| HepG2 cells | Linc-VLDLR | Boosted endogenous linc-VLDLR expression and promoted chemoresistance; increased expression of ABCG2 | [75] | |
| Promotion of tumor progression & metastasis | HepG2,Hep3B, SMMC7721 and MHCC97H | miRNA-155 | Increased recipient cell growth by decreasing PTEN expression and activating PI3K-Akt cascade | [104] |
| Hep3B | 11miRNA | Increased HCC cell colony formation. | [105] | |
| MHCC97L, HKCI-8, and HCKI-C3 | mRNA encoding Cav1, Cav2 and S100A4 | Enhanced the endogenous expression of CAV1 and CAV2 in MIHA non-malignant hepatocytes. Transformed MIHA cell behavior by promoting cell migration and boosting MMP-2 and MMP-9 | [80] | |
| HepG2/ST, Hep3B, PLC/PRF/5 cells and EV | LncRNATUC339 | Promoted HCC cell proliferation and colony formation | [22] | |
| HCCLM3 and HepG2 cells | miR-429 | Boosted EpCAM+ tumor starting cells, Hep3B proliferation, and colony formation. | [71] | |
| HCC patient serum | lncRNA Fal1 | Suppressed miR-1236 expression and d AFP and ZEB1 expression. Increased HCC cell proliferation, colony formation, migration, and invasion, and promoted EMT | [77] | |
| SMMC-7721 and Hep3B cells and HCC patient serum | miR-21 and miR-10b | Acidic conditioned HCC-EV treatment boosted the proliferation, motility, and invasion of HCC recipient cells and promoted EMT | [78] | |
| Huh7 and MHCC97H cells | miR-1273f | Hypoxic conditioned cell EV increased normoxic recipient cell proliferation, migration, and invasion and induced EMT in recipient cells. | [79] | |
| Altering the stromal population | CSQT-2 and HCC-LM3 cells | miR-1247-3p | Promoted MRC5 lung fibroblast activation by suppressing B4GALT3 and activating the NF-kB signaling. Metastatic HCC-EV pre-treated MRC5 cells increased spheroid formation, motility, and chemoresistance HCC SMMC-7721 cells. | [81] |
| Huh7 cells, HepG2 cells and activated LX2 cells | miR-151, miR-21 and miR-221 | Hypoxia-conditioned Huh7-EV increased LX2 migration, invasion, and proliferation via transfer of three oncomiRs. Hypoxia-activated LX2-EV had a similar effect on Huh7 cells. Boosted cell proliferation, migration, and invasion via miR-151, miR-21, and miR-221 and stimulated AKT and ERK in recipient cells. | [17] | |
| Modulating immune cell activities | SNU-387 and SNU-423 | Let-7i-5p | Suppressed TSP1 in recipient cells, promoting immune cell evasion. | [83] |
Abbreviations: ABCG2, ATP-binding cassette sub-family G member 2; AFP, alpha fetal protein; Akt, protein kinase B; B4GALNT3, beta-1,4-galactosyltransferase 3; Cav, caveolin; ECM, extracellular matrix; EpCAM, epithelial cellular adhesion molecule; EMT, epithelial to mesenchymal transition; Erk, extracellular signal-regulated kinase; HCC, hepatocellular carcinoma; HUVEC, human umbilical vein endothelial cells; MMP, matrix metalloprotease; PI3L, phosphatidylinositol 3-kinase; PTEN, phosphatase and TENsin homolog; RBBP4, retinoblastoma binding protein; SMAD, mothers against decapentaplegic homolog 4; STAT, signal transducer and activator of transcription; TME, tumor microenvironment; TSP1, thrombospondin 1; VEGF, vascular endothelial growth factor; VLDLR, very low density lipoprotein receptor; ZEB, zinc finger E-box binding homeobox
4.1. Induction of tumor-initiating cell phenotype.
Recently, a population of tumor-initiating cells (TICs) has been found in HCC, and it is believed that these cells play essential roles in the formation and progression of HCC. These TICs resemble pluripotent stem cells in that they are capable of self-renewal and display stem cell surface markers such as CD133 and epithelial cell adhesion molecule (EpCAM). HCC TICs contribute to chemoresistance and mediate disease progression and metastasis by virtue of their unique features. The expression of miR-429 has been reported to be colocalized with TIC-containing regions of the TME. MicroRNA-429 is reported to play a vital role in the propagation and maintenance of the liver TIC population, by repressing the expression of a known tumor suppressor gene, retinoblastoma protein binding protein 4. Li et al. revealed that HCC-EV could mediate horizontal transfer of miR-429 to HCC cells and that HCC-EV-associated miR-429 increased the number of EpCAM+ TIC, enhanced colony formation and boosted chemoresistance in HCC recipient cells [71].
4.2. Stimulating angiogenesis.
A growing body of evidence suggests that HCC-EVs can promote tumor cell- EC interaction and boost the angiogenic activities of ECs within the liver TME [68–70, 72–74]. Multiple payloads of ncRNA have been shown to contribute to the pro-angiogenic activity by HCC-EV. By inhibiting the expression of TGFB receptor 3 in recipient ECs, miR-378b within HCC-EV demonstrated oncogenic and angiogenic actions. HCC-EV administration stimulated tumor EC proliferation, migration, and invasion in HCC-bearing mice in a miR-378b-dependent manner [72]. The EV-associated linc-00161 elicited similar effects, by acting as a miRNA sponge to modulate the miR-590-3p/ROCK2 axis [68]. The horizontal transfer of HCC-EV-RNA cargo could stimulate angiogenesis by repressing the endogenous expression of an anti-angiogenic factor [69]. These findings suggest that the pro-angiogenic effects elicited by HCC-EV-mediated RNA signaling are attributed to the horizontal transfer of several ncRNA species.
Disruption of the vascular architecture within the hepatic TME also contributes to the growth and dissemination of HCC. It has been found that miR-103-3p induces hyperpermeability of ECs both in vitro and in vivo. The levels of miR-103-3p were greatly enriched in EVs derived from two metastatic HCC cell lines, MHCC97 and QGY-7703 compared to those from non-metastatic HepG2 cells. Administration of EV from malignant HCC cells markedly enhanced the endothelial permeability of HUVECs via the transfer of EV-associated miR-103-3p. In HUVEC recipients, miR-103-3p repressed the expression of three notable endothelial adhesion molecules, vascular endothelial cadherin, phosphoprotein of 120 kDa, and zonula occludens protein 1. The EV-mediated hyperpermeability was concomitant with an increase in the transendothelial invasion of the metastatic HCC cells, QGY-7703 cells [70].
4.3. Inducing drug resistance.
Through the transfer of RNA cargo such as miR-429, miR-32-5p, linc-ROR and linc-VLDLR, HCC-derived EVs have been shown to influence responsiveness to therapy in HCC [24, 71, 75, 76]. These ncRNA species are selectively enriched within HCC-EVs and the intravesicular levels of the latter two ncRNAs are increased in response to TGF-β or chemotherapeutic treatment of the parental cells, respectively. The observed chemoresistance phenotype is partially attributable to EV-mediated modulation of drug efflux. Takahashi et al. reported transfer of EV-associated linc-VLDLR could increase the expression of the ATP-binding cassette transporter superfamily protein, ABCG2, a recognized drug exporter protein, within recipient cells [75]. The horizontal transfer of miR-32-5p has also been related to EV-mediated chemoresistance. This ncRNA is abundant in EVs produced from multidrug-resistant Bel/5-FU cells and the administration of these EVs could promote chemoresistance in previously sensitive Bel7402 cells by inhibiting endogenous phosphatase and tensin homolog expression and consequently activating the phosphatidylinositol-3-kinase and AKT(PI3K/AKT) signaling cascade.
4.4. Tumor progression and metastasis.
HCC cells undergo EMT prior to acquiring metastatic potential. EVs have a significant role in mediating the growth and metastasis of HCC as several studies have shown that HCC-EVs can trigger EMT in recipient cells by transferring RNA payloads [77–79]. The EV-associated lncRNA, focally amplified lncRNA on chromosome 1 (Fal1), was found to stimulate EMT reprogramming in recipient cells as indicated by an increase in the expression of vimentin and a decrease in E-cadherin. Consequently, the migratory and invasion activities of the HCC cells treated with EV were significantly boosted. In recipient cells, EV-associated Fal1 acted as a sponge for miR-1236, thereby easing the repression of two factors associated with hepatocarcinogenesis, alpha feto protein and zinc metal E-box-binding homeobox 1. Several environmental stressors, including hypoxia and low pH, increased the quantities of EMT-inducing ncRNAs within HCC-EV cells [78, 79]. Following EMT, HCC tumor cells can spread with extrahepatic metastases. Tian et al. reported that EVs recovered from acidic cultured HCC augmented the number of metastatic lung nodules in HCC-bearing mice. Acidic conditioning of SMMC-7721 and Hep3B HCC cells increased endogenous and intravesicular expression of miR-21 and miR-10b in a hypoxia inducible-factor (HIF) -1 and HIF-2-dependent manner. Using pH-sensitive nanoparticles to target intratumoral miR-21 and miR-10b dramatically decreased the frequency of metastatic lung nodules and tumor burden [78]. In conclusion, this study demonstrates the therapeutic potential of addressing HCC EV-mediated crosstalk.
ECM remodeling is a highly dynamic process that can enhance hepatocarcinogenesis and contribute to the progression and metastasis of HCC when it is improperly regulated. The horizontal transfer of HCC-EV-associated RNA cargo has been shown to mediate ECM remodeling, indicated by an increased secretion of the matrix metalloproteases, MMP-2, and MMP-9 [68, 80]. In a study by He et al., EV derived from the metastatic HCC cell lines, MHCC97L and HKCI-8, promoted immortalized human hepatocyte (HH) invasion and migration and enhanced the secretion of MMP-2 and -9, via the horizontal transfer of the mRNA and proteins encoding CAV1, CAV2 and S100A4. Once internalized, the EV-associated cargo activated the PI3K/AKT, and mitogen-activated protein kinase signaling cascades in HH recipients.
4.5. Altering the stromal population.
HCC-EV can modulate the activities of CAFs in the liver TME. In comparison to EV isolated from non-metastatic HCC cells (HepG2 and MHCC-97L cells), metastatic cell-derived HCC-EV (CSQT-2 and HCC-LM3 cells) promoted the activation of MRC5 embryonic lung fibroblasts and upregulated the endogenous expression of several pro-inflammatory markers. Once activated, the MRC5 cells closely resembled CAFs. In HCC-tumor-bearing mice, administration of EV from metastatic HCC cells promoted fibroblast activation in the TME and increased the number of metastatic lung nodules. These metastatic HCC-EVs exhibited enriched intravesicular expression of miR-1247-3p, which contributed to the increase in pro-inflammatory gene expression in recipient MRC5 cells. The HCC-EV-associated miR-1247-3p induced CAF activation by repressing the expression of β-1,4-galactosyltransferases III (B4GALT3), a negative regulator of NF-kβ signaling [81]. Indeed, CAF isolated from the livers of HCC patients with invasive disease exhibited low expression of B4GALT3. Moreover, the intravesicular levels of miR-1247-3p in serum EV were higher in HCC patients with lung metastasis than in those without metastatic disease [81].
In the liver, activated HSC works in concert with HCC CAF to remodel the liver ECM architecture and modulate the TME in support of HCC progression. The activated HSC also engage in bidirectional crosstalk with HCC cells to further promote tumor progression. Li et al, reported that HSC-HCC intercellular crosstalk was mediated in part through the exchange of EV [17, 82]. Treatment with hypoxia-conditioned Huh7-EV increased the expression of α-smooth muscle actin and vimentin in LX2 recipients, indicating that HCC-EV treatment-induced the activation of and promoted EMT in HSCs. The EV treatment induced effects were attributed to the horizontal transfer of miR-151, miR-21, and miR-221. Interestingly, these oncomiRs were also enriched in the activated hypoxic-conditioned LX2-EV and were responsible for mediating the LX2-EV pro-tumorigenic effects in recipient Huh7 cells [17].
4.6. Immunomodulation.
It has been observed that EV-mediated crosstalk modulates the immune cell activity within the liver TME. Treatment with HCC-EV promoted immune cell evasion of recipient HCC cells, via the horizontal transfer of let-7i-5p. The HCC-EV-associated let-7i-5p repressed the endogenous expression of thrombospondin-1 (THBS1), which effectively reduced the secretion of soluble THBS1 (TSP1). TSP1 competes with signal regulatory protein (SIRP) for binding to CD47 on the surface of macrophages. As a result, the TSP1-CD47 interaction suppresses the SIRP “don’t eat me” signal to enhance macrophage phagocytosis. By decreasing TSP1 production, HCC-EV therapy facilitated macrophage phagocytosis evasion in HCC recipient cells [83]. HCC-EV-RNA signaling can also promote NK cell dysfunction and immune evasion [84–86]. Horizontal transfer of HCC-EV-associated miR-92b impaired NK cell suppressive activity by repressing the endogenous expression of the activation marker CD69 on recipient NK cells in vitro and in vivo [84]. Other RNA within EV such as circUHRF1 can also contribute to NK cell dysfunction in HCC. HCC-EV-circUHRF1 reduced NK cell tumor infiltration, impaired NK cell function and conferred resistance to PD-L1 therapy in HCC tumor bearing animals. circUHRF1 served as a miR-449c5p sponge to negatively regulate the miR-449c-5p/T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) axis [85]. HCC-EV-RNAs can also modulate TAMs and induce T cell exhaustion. Thus, HCC-EV-miR-146a-5p induced macrophage polarization to a CD206+PD-L1+CD80+ M2 phenotype, and these HCC-EV educated macrophages induced CD3+ T cell dysfunction by upregulating the expression of several inhibitory receptors on co-cultured T cells [86].
Depending on the profile of their cargo, immune cell derived EVs can elicit pro- or anti-tumor immune responses. EVs derived from some immune cells such as NK cells and dendritic cells (DCs), have been reported to halt the progression of HCC tumors. Given their prevalence in the liver, NK cells represent a major cellular effector of an antitumor response in HCC. Recent evidence clearly shows that NK cell-EVs contain several of the same cytolytic granules as their parental cells and therefore are capable of inducing cytotoxicity in HCC cells. Notably, these NK cell-EVs exhibit minimal cytotoxicity towards THLE-2 normal liver cells [87]. DCs can indirectly contribute to an antitumor immune response by activating intratumoral CD8+ T cells. EVs isolated from tumor-EV-pulsed DCs retain the surface expression of major histocompatibility complex I and II proteins, and upon phagocytosis by recipient DCs, can function in antigen presentation [88]. The combination of sorafenib and EVs isolated from Hepa1-6 HCC-EV-pulsed immature DCs increased cytotoxic T cells and decreased intratumoral regulatory T cells (Tregs), but did not improve survival, likely due to cytotoxic T cell exhaustion. The addition of a PD-1 antibody improved survival outcomes and increased the number of cytotoxic T cells and reduced the proportion of Tregs [89]. This finding highlights an important consideration: although immune cell-EVs are capable of eliciting an anti-tumor immune response, the immunosuppressive nature of the liver TME likely polarizes intratumoral immune cells, and consequently EVs derived from these cells, towards an immunosuppressive or exhausted phenotype.
5. EV mediated RNA therapeutics for liver cancers.
Based on the biological roles of EV-based RNA signaling, there is interest in therapeutically modifying EV RNA to influence target cell or immune system behavior, or to use EVs as delivery vehicles for RNA-based therapeutics. EVs can be created for therapeutic uses by genetically modifying their source cells to modify their RNA composition or by introducing therapeutic RNA such as miRNA, mRNA, lncRNA, circRNA, ASO or other RNA into their cargo to accomplish desired effects in target cells after uptake. Several preclinical studies have reported on EV based delivery of RNA to modulate gene expression in recipient cells in vitro and in vivo (Table 2). Their success has led to a phase I study of ASO-loaded EVS for advanced HCC. However, in many studies, EV have been administered intratumorally and further development of the utility of EV-RNA for therapeutics is necessary prior to clinical translation. In particular, studies to demonstrate effective delivery to liver cancers, selective uptake by liver cancer cells, and robust functional effects after cellular uptake of the RNA therapeutic are necessary to further advance translational development of EV mediated RNA therapeutics for liver cancer.
Table 2:
Preclinical in vivo studies of EV mediated RNA therapeutics for HCC
| Identity of EV-RNA | Target HCC cells | Source of EV | Effects of EV-RNA | Reference |
|---|---|---|---|---|
| miR-221 ASO | HepG2, Hep3B and PLC/PRF/5 cells | MNV loaded with miR-221 ASO | Induced apoptosis in HepG2 recipient cells. In vivo, EV + doxorubicin reduced tumor burden and metastasis in orthotopic PLC/PRF/5 HCC bearing mice | [106] |
| lncRNA C5orf66-AS1 | Huh7 and Hep3B cells | BM-MSC-EV with endogenous C5orf66-AS1 | EV increased intratumoral C5orf66-AS1 and DUSP1, decreased miR-127-3p, suppressed phosphorylation of ERK and reduced cell proliferation in the tumors of HCC bearing mice | [107] |
| SENP3-EIF4A1 lncRNA | Huh7 & Hep3B cells | HL-7702 normal human liver cells transfected with a SENP3-EIF4A1 overexpression plasmid | The EV-associated SENP3-EIF4A1 acted as a miRNA sponge to modulate the miR-9-5p/ZFP36 axis. Reduced cell proliferation, abrogated colony formation and migration of HCC recipient cells. Intratumoral injection of EV reduced tumor volume and restored the intratumoral expression of ZFP36 in Huh7 tumor bearing mice. | [108] |
| circ-0051443 | Huh7 & Hep3b cells | HL-7702 cells (normal liver cells) transfected with circ-0051443 | EV-circ-0051443 acts as a miRNA sponge to modulate the miR-331-3p/BAK1 axis. Reduced HCC cell proliferation, induced cell cycle arrest and apoptosis. Restored BAK1 expression in HCC recipient cells, and abrogated tumor growth in HCC bearing mice. | [109] |
| miR-335 | MHCC97H cells | LX2 hepatic stellate cell EV loaded with miR-335 mimic | Reduced HCC tumor spheroid invasion. Reduced tumor cell proliferation, enhanced apoptosis, and decreased tumor growth in vivo after intratumoral administration | [110] |
| miR-451, miR-223, miR-31, miR-24, miR-214 | HepG2 cells and primary HCC cells | HLSC -EV, with endogenous miRNAs | Inhibited proliferation and induced apoptosis Synergistic cytotoxic effect in combination with doxorubicin or vincristine. Downregulated MDR1 expression in recipient HCC cells (miR-451) HLSC-EV from DICER knockdown cells impaired tumor suppressor activity | [111] |
| miR-122 | HepG2 & Huh7 cells | EV with enriched miR-122 generated from AMSC transfected with a miR-122 expression plasmid. | Suppressed expression of ADAM10, CCNG1 and IGF1R, in HepG2 recipient cells and in the tumor tissue of HCC tumor bearing mice. Enhanced HepG2 cell sensitivity to sorafenib and in vitro and in vivo in HCC tumor bearing mice. | [112] |
| miR-199a | PLC/PRF/5 cells | EV with enriched miR-199a generated from AMSC were transduced with miR-199a lentiviral vector. | Restored expression of miR-199a in recipient HCC cells. Enhanced doxorubicin induced cytotoxicity in HCC cells via inhibition of the mTOR pathway. Combination treatment reduced tumor growth and restored the intratumoral expression of miR-199a in HCC tumor bearing mice | [113] |
| miR-15a | Hep3B & Huh7 cells | EV with enriched miR-15a generated from primary human BM-MSC transfected with a miR-15a mimic | Reduced the proliferation, migration and invasion, and induced apoptosis of HCC recipient cells. HCC tumor bearing mice generated with BM-MSC-EV-miR-15a pretreated Hep3B cells exhibited lower tumor burden, reduced intratumoral expression of MMP2, MMP9 and SALL4. Modulated the miR-15a/SALL4 axis in recipient HCC cells | [114] |
| miR-26a mimic | Hep3B, Huh7 & HepG2 cells | GPC3 targeting miR-26a loaded EV generated from hUC-MSC transduced with an anti-GPC3 scFv-Lamp2b lentivirus and electroporated with miR-26a mimic | Restored endogenous expression of miR-26a in recipient Huh7 & Hep3B HCC cells. Inhibited Hep3B and Huh7 HCC cell proliferation, and impaired colony formation and migration. Intratumoral injection of EV reduced tumor volume in HepG2 HCC bearing mice | [115] |
| β-catenin siRNA | c-MET and mutant beta-catenin induced HCC in vivo | MNV loaded with β-catenin siRNA | EV reduced tumor growth in HCC bearing mice and enhanced the anti-tumor effects of anti-PD-L1 Ab treatment. | [116] |
| β-catenin siRNA | LCSC & Hep3B cells | MNV with EpCAM targeting RNA aptamer, loaded with β-catenin siRNA | EV reduced endogenous expression of β-catenin and decreased cell viability following uptake by EpCAM positive cells. EV treatment reduced tumor burden, decreased intratumoral expression of β-catenin and impaired HCC cell proliferation in LCSC and Hep3B tumor bearing mice | [117] |
| Multiplex siRNA to GPX4 and DHODH | HepG2 cells | EV with SP94 targeting generated from engineered HEK293T cells with a SP94-Lamb2-RRM fusion protein transfected with a multi-targeting siRNA. | Increased intracellular ROS and lipid peroxidation levels, and sensitized HepG2 cells to sorafenib. Enhanced sorafenib-induced ferroptotic cell death. EV + sorafenib reduced tumor growth, increased survival and reduced expression of ferroptosis inhibitors, GPX4 and DHODH, in recipient HepG2 cells in orthotopic HepG2 tumors in vivo | [118] |
Abbreviations: Ab, antibody ADAM, a disintegrin and metalloprotease; AS1, anti-sense RNA 1; ASO, antisense oligonucleotide; AMSC, adipose tissue derived mesenchymal stem cells; BAK1, Bcl2 antagonist/killer 1; BM-MSC, bone-marrow derived MSC; casp, caspase; CCNG1, cyclin G1; CDK2, cyclindependent kinase 2; DHODH, dihydroorotate dehydrogenase; E2F2, E2F transcription factor 2; EV, extracellular vesicle; ERK, extracellular signal-regulated kinase; EV, extracellular vesicles; GPC3, glypican 3; GPX4, glutathione peroxidase 4; HCC, hepatocellular cancer HCV-E2, hepatitis C virus E2 envelope glycoprotein; HLSC, human adult liver stem cells; HSC, hepatic stellate cells; hUC-MSC, human umbilical cord blood derived MSC; IGF1R, insulin-like growth factor receptor 1; MDR1, multidrug resistance mutation 1; MMP, matrix metalloprotease; mTOR, mammalian target of rapamycin; MSC, mesenchymal stem cells; PAX2, paired box gene 2; RAB14, Ras-related protein 14; ROS, reactive oxygen species; SALL4, spalt-like transcription factor; SENP3-EIF4A1, SUMO specific peptidase 3-eukaryotic translation initiation factor 4A1; SP1, transcription factor specificity protein 1.
6. Other perspectives on EV-based RNA signaling
The functional activity of EV-derived miRNA in recipient cells has been demonstrated. In one study, vectors encoding the 3’ untranslated regions of two known miRNA targets fused to luciferase were transfected into healthy mammary epithelial cells. There was a significant decrease in luciferase activity and in two target genes in these cells after treatment with EV from malignant breast cancer cells, demonstrating that the cancer-EV-associated miRNA could recognize and bind to complementary target sequences within the recipient cells and confirming the functional effectiveness of EV-associated miRNA [90]. These and other studies have confirmed that EV-associated miRNAs are functionally active in recipient cells [90, 91]. However, some researchers have questioned the role of EV as “miRNA shuttles,” as the intravesicular concentration of miRNA species would be insufficient for a significant biological effect in recipient cells [92, 93]. A stoichiometric analysis of biological fluid and cell-derived exosomes (Ex) concluded that there would be fewer than one miRNA copy/EV from all sources [93]. However, ultracentrifugation in these studies can lead to EV with a lower intravesicular RNA concentration compared to other methods for EV isolation [94]. Of greater relevance, however, this argument does not consider the bulk effect of the vast numbers of EVs that may be produced by a single cell, nor refute observations of functional effects following EV uptake by several groups. Indeed, recipient cells would need to internalize multiple EVs to accumulate enough EV-associated miRNA for a functional effect to occur [93, 95].
Interestingly, EVs have recently been identified as sites of cell-independent miRNA biogenesis. Compared with bioactivity of miRNA isolated from days-old EV, miRNA from freshly isolated EV failed to elicit a functional effect in recipient cells [90]. These observations suggest that some of the miRNA species in EV cargo may exist in their precursor forms. Indeed, an increase in expression of six mature miRNAs with a decrease in the levels of their counterpart precursor miRNAs was observed in EV cultured in cell-free conditions for 1–3 days. The RISC loading complex proteins, DICER, TRBP, and AGO2 which are required for miRNA processing were detected within EV [61, 90]. These findings indicate that the EV-associated miRNA cargo may require time to mature and become functionally active.
Intercellular exchange of RNA species can occur via mechanisms other than through EV mediated transfer. Paracrine miRNA signaling can involve Ribonucleoprotein (RNP) complexes where extracellular (ex)-miRNA is bound to a carrier protein, such as Argonaute 2 or high-density lipoprotein [61, 95]. The sequestration of ex-miRNA within RNP complexes, as with EV, offers protection from degradation by circulating nucleases. While both EV and RNP complexes are found in systemic circulation, approximately 90% of circulating miRNA is contained within RNP complexes [96]. At least some miRNA species, however, appear to be preferentially transferred via EV [96]. Although the relative contributions of non-vesicular RNA based signaling is unknown, these mechanisms likely work in concert with EV RNA to mediate inter-cellular signaling within the TME. Other mechanisms involving RNA signaling in the TME could involve juxtacrine transfer through tunneling nanotubes (TNT) or gap junctions. TNT are membranous extensions that provide a direct conduit for the bidirectional exchange of RNA and other cytoplasmic components across cells [97]. The size of RNA within EV is limited with most being smaller than 700 nucleotides and the content comprises of intermediate-sized RNA species—mostly small non-coding RNAs, mRNA fragments, and some full-length mRNA transcripts—ranging in size from 25–4,000 nucleotides, [98, 99]. TNTs, on the other hand, can transport a wider range of RNA species, including full-length mRNA transcripts, and are capable of bidirectional signaling [100]. Despite the differences in their RNA cargo, TNT can work in tandem with EV to mediate intercellular RNA transfer [101]. Gap junction-mediated RNA cargo exchange, like TNT, are limited to cells that are near one another. While RNA can be exchanged between adjacent cells via gap junctions, only miRNA species have been reported to be shuttled through gap junctions, [102, 103].
7. Conclusions
EVs function as carriers for the dynamic intercellular exchange of RNA payloads, which is determined by cellular and environmental factors and contributes to tissue homeostasis. The importance of EV-based RNA transfer to intercellular signaling and their role in the development of liver malignancies are becoming increasingly apparent. EV-mediated RNA signaling within the liver TME has been associated with tumor growth, progression, and treatment responses. Depending on the recipient cells, several diverse processes, such as the regulation of angiogenesis, modulation of immune responses, and the promotion of tumor cell invasion and metastasis, can be impacted. Using EVs as delivery vehicles for RNA-based treatments and manipulating EV RNA to influence the behavior of target cells or the immune system are two examples of how this emerging knowledge could be used to develop new therapeutic approaches for liver cancers. Defining the repertoire of tumor cell-specific EV-RNA signaling mediators, determining the cellular processes by which they are selectively incorporated into vesicles, elucidating the mechanisms underlying the selectivity of target cell uptake, and developing robust methods for manipulating the RNA payloads within EV will all establish the path for EV-RNA signaling-based therapeutic strategies for liver cancers.
Highlights.
Multiple cell types make up the liver tumor microenvironment.
RNA molecules in extracellular vesicles can facilitate cell-to-cell communication in the liver
Extracellular vesicle RNA molecules can affect tumor growth and spread in many ways.
Acknowledgements:
The authors would like to acknowledge helpful discussions and input from the members of the Patel lab.
Funding:
This work was supported by the National Cancer Institute [NIH grant CA217833], by the James C and Sarah K Kennedy Deanship and Alfred D and Audrey M Petersen Professorship at Mayo Clinic to Dr Tushar Patel.
Abbreviations:
- AGO2
argonaute RISC catalytic component 2
- AKT
protein kinase B
- BMSCs
bone marrow-derived mesenchymal stem cells
- CAF
cancer-associated fibroblasts
- circRNA
circulatory RNA
- circUHRF
hsa_circ_0048677 derived from ubiquitin-like with PHD and ring finger domain 1 RNA
- CRC
colorectal cancer
- ECM
extracellular matrix
- ECs
endothelial cells
- EMT
epithelial-to-mesenchymal transition
- ERK
extracellular signaling regulated kinase
- EVs
extracellular vesicles
- FMR1
fragile X mental retardation 1
- HCC
hepatocellular carcinoma
- HH
human hepatocytes
- HIF
hypoxia-inducible factor
- hnRNPA2B1
heterogeneous nuclear ribonucleoprotein A2B1
- HSCs
hepatic stellate cells
- HuR
human antigen R
- HUVECs
human umbilical vascular endothelial cells
- MET
mesenchymal-to-epithelial transition
- miRNA
microRNA
- MVP
major vault protein
- ncRNA
non-coding RNA
- NK cells
natural killer cells
- nSMase2
neutral sphingomyelinase 2
- PCK1
phosphoenolpyruvate kinase 1
- RBP, RNA
binding protein
- RISC, RNA
induced silencing complex
- RNP
ribonucleoprotein
- SIPR
signal regulatory protein
- SYNCRIP
synaptotagmin-binding cytoplasmic RNA-interacting protein
- TAM
tumor-associated macrophages
- THBS1
thrombospondin 1
- TICs
tumor-initiating cells
- TME
tumor microenvironment
- TNTs
tunneling nanotubes
- TSP1
soluble THBS1
- YBX1
Y-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Polidoro MA, et al. , Tumor microenvironment in primary liver tumors: A challenging role of natural killer cells. World J Gastroenterol, 2020. 26(33): p. 4900–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu P, Weaver VM, and Werb Z, The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol, 2012. 196(4): p. 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J, et al. , Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression. Int J Mol Sci, 2020. 21(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, et al. , Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature, 2020. 579(7799): p. 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holle AW, Young JL, and Spatz JP, In vitro cancer cell–ECM interactions inform in vivo cancer treatment. Advanced drug delivery reviews, 2016. 97: p. 270–279. [DOI] [PubMed] [Google Scholar]
- 6.Baghban R, et al. , Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling, 2020. 18(1): p. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominiak A, et al. , Communication in the cancer microenvironment as a target for therapeutic interventions. Cancers, 2020. 12(5): p. 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt JM, Kroemer G, and Zitvogel L, Extracellular vesicles: masters of intercellular communication and potential clinical interventions. The Journal of clinical investigation, 2016. 126(4): p. 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novikova MV, Khromova NV, and Kopnin PB, Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression. Biochemistry (Mosc), 2017. 82(8): p. 861–873. [DOI] [PubMed] [Google Scholar]
- 10.Fukumura D and Jain RK, Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res, 2007. 74(2–3): p. 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia CC, et al. , Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One, 2013. 8(5): p. e63243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iozzo RV and Sanderson RD, Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med, 2011. 15(5): p. 1013–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurung S, et al. , The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal, 2021. 19(1): p. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han QF, et al. , Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer, 2022. 21(1): p. 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KM, et al. , RNA in extracellular vesicles. Wiley Interdisciplinary Reviews: RNA, 2017. 8(4): p. e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei H, et al. , Hepatoma cell-derived extracellular vesicles promote liver cancer metastasis by inducing the differentiation of bone marrow stem cells through microRNA-181d-5p and the FAK/Src pathway. Frontiers in cell and developmental biology, 2021. 9: p. 607001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Li J, et al. , Extracellular vesicles-derived OncomiRs mediate communication between cancer cells and cancer-associated hepatic stellate cells in hepatocellular carcinoma microenvironment. Carcinogenesis, 2020. 41(2): p. 223–234. [DOI] [PubMed] [Google Scholar]
- 18.Moirangthem A, et al. , Extracellular vesicle-mediated miR-126–3p transfer contributes to inter-cellular communication in the liver tumor microenvironment. International journal of oncology, 2022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, et al. , MicroRNA-15b in extracellular vesicles from arsenite-treated macrophages promotes the progression of hepatocellular carcinomas by blocking the LATS1-mediated Hippo pathway. Cancer Letters, 2021. 497: p. 137–153. [DOI] [PubMed] [Google Scholar]
- 20.Shao Z, Pan Q, and Zhang Y, Hepatocellular carcinoma cell-derived extracellular vesicles encapsulated microRNA-584–5p facilitates angiogenesis through PCK1-mediated nuclear factor E2-related factor 2 signaling pathway. The International Journal of Biochemistry & Cell Biology, 2020. 125: p. 105789. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W.l., et al. , Extracellular vesicle long non–coding RNA-mediated crosstalk in the tumor microenvironment: Tiny molecules, huge roles. Cancer Science, 2020. 111(8): p. 2726–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kogure T, et al. , Extracellular vesicle–mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes & cancer, 2013. 4(7–8): p. 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, et al. , Tumor-derived extracellular vesicles containing long noncoding RNA PART1 exert oncogenic effect in hepatocellular carcinoma by polarizing macrophages into M2. Digestive and Liver Disease, 2022. 54(4): p. 543–553. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, et al. , Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS open Bio, 2014. 4: p. 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, et al. , Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene, 2019. 38(15): p. 2844–2859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang G, et al. , Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a–MET pathway. EBioMedicine, 2019. 40: p. 432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X-Y, et al. , Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. Journal of Experimental & Clinical Cancer Research, 2020. 39(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo W, et al. , ALDH2 deficiency promotes alcohol-associated liver cancer by activating oncogenic pathways via oxidized DNA-enriched extracellular vesicles. Journal of hepatology, 2019. 71(5): p. 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, et al. , Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell death & disease, 2022. 13(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costanzi E, et al. , The role of extracellular vesicles as shuttles of RNA and their clinical significance as biomarkers in hepatocellular carcinoma. Genes, 2021. 12(6): p. 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu W, et al. , Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Molecular cancer, 2020. 19(1): p. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. , Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics, proteomics & bioinformatics, 2015. 13(1): p. 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baglio SR, et al. , Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem cell research & therapy, 2015. 6(1): p. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sork H, et al. , Heterogeneity and interplay of the extracellular vesicle small RNA transcriptome and proteome. Scientific reports, 2018. 8(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang W, et al. , Noncoding RNAs from tissue-derived small extracellular vesicles: Roles in diabetes and diabetic complications. Molecular Metabolism, 2022: p. 101453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobor F, et al. , A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nature communications, 2018. 9(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Statello L, et al. , Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PloS one, 2018. 13(4): p. e0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee K, et al. , Reversible HuR-micro RNA binding controls extracellular export of miR-122 and augments stress response. EMBO reports, 2016. 17(8): p. 1184–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villarroya-Beltri C, et al. , Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nature communications, 2013. 4(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H, et al. , Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. Journal of Experimental Medicine, 2019. 216(9): p. 2202–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Grady T, et al. , Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC biology, 2022. 20(1): p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei Y, et al. , Tumor-released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non-small cell lung cancer. Oncology Reports, 2018. 40(6): p. 3438–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, et al. , Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. The Journal of clinical investigation, 2020. 130(1): p. 404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez-Boza J, et al. , hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cellular and Molecular Life Sciences, 2020. 77(21): p. 4413–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shurtleff MJ, et al. , Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. elife, 2016. 5: p. e19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shurtleff MJ, et al. , Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proceedings of the National Academy of Sciences, 2017. 114(43): p. E8987–E8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X-M, Ma L, and Schekman R, Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates. Elife, 2021. 10: p. e71982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin F, et al. , YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Research & Therapy, 2019. 10(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santangelo L, et al. , The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell reports, 2016. 17(3): p. 799–808. [DOI] [PubMed] [Google Scholar]
- 50.Teng Y, et al. , MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nature communications, 2017. 8(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu C, et al. , EWI-2 controls nucleocytoplasmic shuttling of EGFR signaling molecules and miRNA sorting in exosomes to inhibit prostate cancer cell metastasis. Molecular oncology, 2021. 15(5): p. 1543–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He B, et al. , Differential functions of phospholipid binding and palmitoylation of tumour suppressor EWI2/PGRL. Biochemical Journal, 2011. 437(3): p. 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Gassart A, et al. , Lipid raft-associated protein sorting in exosomes. Blood, 2003. 102(13): p. 4336–4344. [DOI] [PubMed] [Google Scholar]
- 54.Janas T, et al. , Binding of RNA aptamers to membrane lipid rafts: Implications for exosomal miRNAs transfer from cancer to immune cells. International journal of molecular sciences, 2020. 21(22): p. 8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mańka R, et al. , Role of RNA motifs in RNA interaction with membrane lipid rafts: implications for therapeutic applications of exosomal RNAs. International journal of molecular sciences, 2021. 22(17): p. 9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kosaka N, et al. , Secretory mechanisms and intercellular transfer of microRNAs in living cells*♦. Journal of Biological Chemistry, 2010. 285(23): p. 17442–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choezom D and Gross JC, Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification. Journal of cell science, 2022. 135(5): p. jcs259324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iavello A, et al. , Role of Alix in miRNA packaging during extracellular vesicle biogenesis. International journal of molecular medicine, 2016. 37(4): p. 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kosaka N, et al. , Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. Journal of Biological Chemistry, 2013. 288(15): p. 10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Datta B, et al. , Intriguing biomedical applications of synthetic and natural cell-derived vesicles: a comparative overview. ACS Applied Bio Materials, 2021. 4(4): p. 2863–2885. [DOI] [PubMed] [Google Scholar]
- 61.Clancy JW, et al. , An ARF6–Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nature cell biology, 2019. 21(7): p. 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Datta B, et al. , Importance of Extracellular Vesicle Derived RNAs as Critical Colorectal Cancer Biomarkers. ACS Bio & Med Chem Au, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abels ER and Breakefield XO, Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cellular and molecular neurobiology, 2016. 36(3): p. 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batagov AO, Kuznetsov VA, and Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. in BMC genomics. 2011. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolukbasi MF, et al. , miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Molecular Therapy-Nucleic Acids, 2012. 1: p. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szostak N, et al. , Sorting signal targeting mRNA into hepatic extracellular vesicles. RNA biology, 2014. 11(7): p. 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koppers-Lalic D, et al. , Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell reports, 2014. 8(6): p. 1649–1658. [DOI] [PubMed] [Google Scholar]
- 68.You LN, et al. , Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther, 2021. 28(6): p. 719–736. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q, et al. , Exosomal MiR-1290 Promotes Angiogenesis of Hepatocellular Carcinoma via Targeting SMEK1. J Oncol, 2021. 2021: p. 6617700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang JH, et al. , Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology, 2018. 68(4): p. 1459–1475. [DOI] [PubMed] [Google Scholar]
- 71.Li L, et al. , Epigenetic modification of MiR-429 promotes liver tumour-initiating cell properties by targeting Rb binding protein 4. Gut, 2015. 64(1): p. 156–67. [DOI] [PubMed] [Google Scholar]
- 72.Chen W, et al. , Hepatocellular carcinoma cells-derived exosomal microRNA-378b enhances hepatocellular carcinoma angiogenesis. Life Sci, 2021. 273: p. 119184. [DOI] [PubMed] [Google Scholar]
- 73.Lin XJ, et al. , Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo. Mol Ther Nucleic Acids, 2018. 11: p. 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conigliaro A, et al. , CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer, 2015. 14: p. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi K, et al. , Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res, 2014. 12(10): p. 1377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu X, et al. , Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res, 2018. 37(1): p. 52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Li B, et al. , LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci, 2018. 197: p. 122–129. [DOI] [PubMed] [Google Scholar]
- 78.Tian XP, et al. , Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics, 2019. 9(7): p. 1965–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu Y, et al. , Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Exp Cell Res, 2019. 385(1): p. 111649. [DOI] [PubMed] [Google Scholar]
- 80.He M, et al. , Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis, 2015. 36(9): p. 1008–18. [DOI] [PubMed] [Google Scholar]
- 81.Fang T, et al. , Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun, 2018. 9(1): p. 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma YN, et al. , Crosstalk between hepatic stellate cells and tumor cells in the development of hepatocellular carcinoma. Chin Med J (Engl), 2021. 134(21): p. 2544–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang HD, et al. , HDAC6 Suppresses Let-7i-5p to Elicit TSP1/CD47-Mediated Anti-Tumorigenesis and Phagocytosis of Hepatocellular Carcinoma. Hepatology, 2019. 70(4): p. 1262–1279. [DOI] [PubMed] [Google Scholar]
- 84.Nakano T, et al. , Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. American Journal of Transplantation, 2019. 19(12): p. 3250–3262. [DOI] [PubMed] [Google Scholar]
- 85.Zhang PF, et al. , Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer, 2020. 19(1): p. 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yin C, et al. , SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology, 2019. 8(7): p. 1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HY, et al. , Delivery of human natural killer cell-derived exosomes for liver cancer therapy: an in vivo study in subcutaneous and orthotopic animal models. Drug Deliv, 2022. 29(1): p. 2897–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nikfarjam S, et al. , Dexosomes as a cell-free vaccine for cancer immunotherapy. J Exp Clin Cancer Res, 2020. 39(1): p. 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi S, et al. , Dendritic Cells Pulsed with Exosomes in Combination with PD-1 Antibody Increase the Efficacy of Sorafenib in Hepatocellular Carcinoma Model. Transl Oncol, 2018. 11(2): p. 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melo SA, et al. , Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell, 2014. 26(5): p. 707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, et al. , Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell, 2010. 39(1): p. 133–44. [DOI] [PubMed] [Google Scholar]
- 92.Albanese M, et al. , MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet, 2021. 17(12): p. e1009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chevillet JR, et al. , Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A, 2014. 111(41): p. 14888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andreu Z, et al. , Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J Extracell Vesicles, 2016. 5: p. 31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mateescu B, et al. , Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles, 2017. 6(1): p. 1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arroyo JD, et al. , Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A, 2011. 108(12): p. 5003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haimovich G, Dasgupta S, and Gerst JE, RNA transfer through tunneling nanotubes. Biochemical Society Transactions, 2020. 49(1): p. 145–160. [DOI] [PubMed] [Google Scholar]
- 98.Batagov AO and Kurochkin IV, Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biol Direct, 2013. 8: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pérez-Boza J, Lion M, and Struman I, Exploring the RNA landscape of endothelial exosomes. Rna, 2018. 24(3): p. 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haimovich G, et al. , Intercellular mRNA trafficking via membrane nanotube-like extensions in mammalian cells. Proc Natl Acad Sci U S A, 2017. 114(46): p. E9873–e9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Connor Y, et al. , Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nat Commun, 2015. 6: p. 8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim PK, et al. , Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res, 2011. 71(5): p. 1550–60. [DOI] [PubMed] [Google Scholar]
- 103.Katakowski M, et al. , Functional microRNA is transferred between glioma cells. Cancer Res, 2010. 70(21): p. 8259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun JF, et al. , Exosome-Mediated MiR-155 Transfer Contributes to Hepatocellular Carcinoma Cell Proliferation by Targeting PTEN. Med Sci Monit Basic Res, 2019. 25: p. 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kogure T, et al. , Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology, 2011. 54(4): p. 1237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.George J, Yan IK, and Patel T, Nanovesicle-mediated delivery of anticancer agents effectively induced cell death and regressed intrahepatic tumors in athymic mice. Lab Invest, 2018. 98(7): p. 895–910. [DOI] [PubMed] [Google Scholar]
- 107.Gu H, et al. , Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Hum Cell, 2021. 34(6): p. 1812–1829. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, et al. , Exosome-transmitted long non-coding RNA SENP3-EIF4A1 suppresses the progression of hepatocellular carcinoma. Aging (Albany NY), 2020. 12(12): p. 11550–11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen W, et al. , Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett, 2020. 475: p. 119–128. [DOI] [PubMed] [Google Scholar]
- 110.Wang F, et al. , Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology, 2018. 67(3): p. 940–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fonsato V, et al. , Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells, 2012. 30(9): p. 1985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lou G, et al. , Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol, 2015. 8: p. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lou G, et al. , MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res, 2020. 39(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma YS, et al. , Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov, 2021. 7(1): p. 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mahati S, et al. , Delivery of miR-26a Using an Exosomes-Based Nanosystem Inhibited Proliferation of Hepatocellular Carcinoma. Front Mol Biosci, 2021. 8: p. 738219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsuda A, et al. , Extracellular Vesicle-Based Therapeutic Targeting of beta-Catenin to Modulate Anticancer Immune Responses in Hepatocellular Cancer. Hepatol Commun, 2019. 3(4): p. 525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishiguro K, et al. , Targeting Liver Cancer Stem Cells Using Engineered Biological Nanoparticles for the Treatment of Hepatocellular Cancer. Hepatol Commun, 2020. 4(2): p. 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X, et al. , Designer Exosomes for Targeted Delivery of a Novel Therapeutic Cargo to Enhance Sorafenib-Mediated Ferroptosis in Hepatocellular Carcinoma. Front Oncol, 2022. 12: p. 898156. [DOI] [PMC free article] [PubMed] [Google Scholar]


