Abstract
Background and Objective
Esophageal diverticulum (ED) is a relatively rare condition, characterized by high etio- and pathophysiological versatility, with an uncommon clinical impact, consequently requiring a complete and complex diagnostic evaluation, so that the therapeutic decision is “appropriate” to a specific case. The aim of the paper is, therefore, a reassessment of the diagnostic possibilities underlying the establishment of the therapeutic protocol and the available therapeutic resources, making a review of the literature, and a non-statistical retrospective analysis of cases hospitalized and operated in a tertiary center.
Methods
Thus, classical investigations (upper digestive endoscopy, barium swallow) need to be correlated with complex, manometric, and imaging evaluations with direct implications in therapeutic management. Moreover, in the absence of a precise etiology, the operative indication needs to be established sparingly, with the imposition of the identification and interception of the pathophysiological mechanisms through the therapeutic gesture.
Key Content and Findings
The identification of the pathophysiological mechanisms is mandatory for the management of diverticular disease, the result obtained—restoring swallowing and comfort/good quality of life in the postoperative period—is directly related to the chosen therapeutic procedure. In addition, management appears to be a difficult goal in the context of the low incidence of ED but also of the results that emphasize important differences in the reports in the medical literature. Although ED is a benign condition, surgical techniques are demanding, impacted by significant morbidity and mortality. The causes of these results are multiple: possible localizations anywhere in the esophagus, diverticulum size/volume from a few millimeters to an impressive one, over 10–12 cm, metabolic impact in direct relation to the alteration swallowing, numerous diverticular complications but, perhaps most importantly, alteration of the quality of the diverticular wall by inflammatory phenomena, with an impact on the quality of the suture.
Conclusions
The accumulation of cases in a tertiary profile center, with volume/hospital, respectively volume/surgeon + gastroenterologist could be a solution in improving the results. One consequence would be the identification of alternative solutions to open surgical techniques, a series of minimally invasive or endoscopic variants can refine these results.
Keywords: Esophageal diverticula (Zenker, Rokitansky, Killian-Jamieson); diagnostic; management approaches
Introduction
Esophageal diverticulum (ED) is a rare condition that involves herniation of the esophageal mucosa or even the entire parietal structure outside the esophageal wall (1,2). The clinical impact is variable, being dependent on the diverticular dimensions and site, the distal ones being the most “symptomatic”. However, EDs are often oligo- or asymptomatic, occasionally being discovered as a result of investigations of the upper digestive tract. The clinical feature is dysphagia associated with regurgitation and, with increasing size, secondary phenomena of compression of adjacent structures (3,4).
With the technological progression a series of new diagnostic and therapeutic possibilities have appeared in this pathology. Can we perform a surgical resection without a complete lesion picture? The argument that the etiopathogenic identification is secondary, however the resection being the only valid therapeutic resource can justify an incomplete diagnostic picture? Esophageal manometry appears to be mandatory in the case of epiphrenic diverticula in combination with the diagnosis and a pH-metric evaluation seems to become absolutely necessary to complete or modify the therapeutic act. Another question is related to the indication for surgery or any other therapeutic option, even abstention being a medical decision. A number of alternatives to surgery have developed in the last 2 decades and their introduction appears to be timid due essentially to the low number of cases. What validates one indication or another: the size of the ED, the clinical impact, the occurrence of complications, etc.? And if we opt for a therapy, what do we consider to choose one technique or another? The high rate of postoperative fistula validates the need to identify new therapeutic resources. A number of questions arise and will be addressed. The present paper is based on the literature review and our experience regarding this pathology and not the detailed analysis of our cases. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-861/rc).
Methods (see Table 1 and Table S1)
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 07.01.2022 |
| Databases and other sources searched | Embase (Excerpta Medica Database), PubMed Central (PMC), Cochrane Library, MEDLINE Complete (EBSCO) |
| Search terms used (including MeSH and free text search terms and filters) | Search strategy (see Table S1) |
| Timeframe | 2000–2022 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: meta-analyzes; trials studies; clinical trials & updates of clinical trials; reviews; case presentations; original articles; only studies/papers/journals written in English. Exclusion criteria: unpublished data from abstracts contained in volumes from various congresses or conferences; papers that were not in English |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | DP performed the search in the databases according to the presented criteria. If a study appears relevant by at least one reviewer—DP and AC—the full-text article has been retrieved and checked. The selection of full-text articles was made by two reviewers independently DP and AC. Assessing content validity required subjective judgment from the reviewers. The citation number was an important selection criterion. Differences were discussed and if consensus could not be reached between the two reviewers, we requested the consultation and recommendation of a third reviewer (SC). The reference list from each selected article was screened for additional relevant information |
The article is based on the analysis of the data considered relevant for the chosen topic from the studies and articles published in the last 20 years by “St.Mary” General and Esophageal Surgery Clinic, as well as on articles identified in Embase (Excerpta Medica Database), PubMed Central (PMC), Cochrane Library, MEDLINE Complete (EBSCO) starting with 2000. Trials were sought and used as well as data from updates of studies, case presentations, original articles or reviews regarding esophageal diverticular pathology. For a sensitive search strategy the terms used in search engines were: “esophageal diverticulum”, “types of the esophageal diverticulum”, “diagnosis of the esophageal diverticulum”, and “treatment of esophageal diverticulum”. The article focused on data that have been updated in terms of types of esophageal diverticula with characteristics in terms of topography, etiology and pathogenic mechanisms, clinic and the possibilities of diagnosis and treatment. Only these studies and papers were considered eligible, thus being taken into account in the elaboration of this article.
Two authors (AC and DP) selected the articles considered relevant, preferring peer-reviewed articles from highly ranked journals written in English. The decision to select an item was made by agreement of the two. A number of 190 reviews were identified for the period 2000–2022, which included the keywords used in the database search, 13 meta-analyzes, 19 clinical trials, 1 single randomized controlled trial, no clinical trial and 700 case reports.
The reference list from each selected article was screened for additional relevant information. We excluded unpublished data from abstracts contained in volumes from various congresses or conferences, as we excluded papers that were not in English.
Esophageal diverticula classification
In practice, a number of criteria are used in the classification of ED, each having a value and impact on therapeutic management. From the anatomical-clinical point of view, they are divided into true EDs (which combine in the stratigraphy of the diverticular wall all the layers of the esophageal wall) and false EDs (in which the diverticular pouch consists only of the mucosa and submucosa). From the etiopathogenic point of view, they are divided into pulsion ED (secondary to an esophageal motility disorder with relative hyper pressure of an area of low esophageal parietal resistance) and traction ED (secondary to an area of periesophageal inflammation that adheres and eccentrically tracts the esophageal wall) (5,6). Several mechanisms of ED have been described. Pulsion ED is formed under conditions of inadequate relaxation of either the upper esophageal sphincter (UES) or the lower esophageal sphincter (LES), causing an increase of intraluminal pressure resulting in a herniation of the esophageal wall in an area of low parietal resistance (7,8). The phenomenon is associated with various forms of esophageal motility disorders, among which the most visible is achalasia (9,10). In the case of traction ED, the etiological mechanism involves adhesion and traction on the esophageal wall in the presence of a mediastinal inflammatory focus, resulting in the formation of a diverticular pouch. It is apparently common in patients with tuberculosis, although in the United States, the most common cause is histoplasmosis (11). A relationship between reflux disease and ED formation is not currently mentioned, although a number of papers raise questions about this possible synergy (12). Recently, the etiologically so-called classic classification of diverticular disease has been supplemented with a new category—iatrogenic diverticulum. This is a consequence of the development of peroral endoscopic myotomy (POEM)-type endoscopic therapy in the treatment of achalasia (late complication of the procedure), a new class of diverticula that have an etiopathogenic iatrogenic substrate. The relatively minor clinical impact and extremely low statistics explain why, so far, they have not required special attention.
Topographically, ED can be located at the cervical level, mid-esophageal, epiphrenic or, the most “unfortunate” variant from a therapeutic point of view, the diffuse type (diffuse intramural pseudodiverticulosis). There is a correlation between the topography, the anatomical-clinical criterion, and the etiopathogenic one, specifically: (I) cervical EDs are considered false diverticula, they are usually located in the hypopharynx, in the area of low resistance known as Killian’s triangle (Zenker and Killian-Jamieson diverticula are described at this level) (13-15), (II) midesophageal EDs are usually true, traction diverticula, secondary to mediastinal inflammatory processes (classically, traction diverticula are described at this level, but pulsion diverticula can also be found), (III) epiphrenic EDs are also pulsion diverticula, secondary to a disorder of esophageal motility and are expressed in the last 10 cm of the distal esophagus (in addition to the pulsion diverticula, iatrogenic diverticula, secondary to endoscopic therapeutic techniques for achalasia, POEM, were also described at this level) (16,17) (Table 2).
Table 2. The correlation between the topography, the anatomo-clinical criteria and the etiopathogenic categorization of esophageal diverticula (ED).
| Topography | Anatomo-clinical criteria | Etiopathogeny |
|---|---|---|
| Cervical: Zenker/Killian-Jamieson | False—only mucosa and submucosa | Pulsion mechanism—disorders of esophageal motility |
| Thoracic: Rokitansky | True—all the layers of the esophageal wall | Traction mechanism—secondary to mediastinal inflammatory processes (note: pulsion diverticula can also be found) |
| Epiphrenic | False—only mucosa and submucosa | Pulsion mechanism—disorder of esophageal motility (note: iatrogenic diverticulum) |
In a review of the patients diagnosed with ED for the period 2000–2020 in our department which were treated by open, minimally invasive or endoscopic approach we found using the anatomical location of the diverticulum as criteria that 57 cases were pharyngeal-esophageal ED (Zenker type), 13 cases were midthoracic, and 36 cases were epiphrenic. Coincidentally or not, this distribution of the anatomical segments is not fully in line with the statistics stated in the literature that report a higher ratio of pharyngeal-esophageal location. We excluded from the statistics the cases that did not have a therapeutic indication—124 patients—regardless of the cause (asymptomatic, significant comorbidities, etc.). Open surgery was preferred especially in the cervical location, 28 cases versus 29 treated by endoscopic approach (endoscopic diverticulectomy with a stapler) while in the thoracic or abdominal location a minimally invasive approach was especially preferred—invasive, thoracoscopic, laparoscopic or, endoscopic (POEM)—see Table 3. Open surgery was indicated for complicated diverticula or in the early stages of the study (the 2000s).
Table 3. Number of cases by location site of the ED at the level of the esophagus and the type of approach (only for the cases with therapeutic indication).
| ED | Number of cases (N=106) | Therapeutic management |
|---|---|---|
| Cervical | 57 | Open surgery 28, endoscopic 29 |
| Midthoracic | 13 | Open 7, thoracoscopic 6 |
| Epiphrenic | 36 | POEM 6, open surgery 18, laparoscopic 12 |
ED, esophageal diverticula; POEM, peroral endoscopic myotomy.
The presentation of our cases has no other purpose than our certification in approaching this subject.
All images presented (contrast radiology, upper digestive endoscopy, manometry) are part of the clinic’s collection. Each of these represented a particular situation, both in terms of the clinic and metabolic impact, of lesion complications, requiring consequently a personalized therapy, surgical, endoscopic, or even abstinence.
Epidemiology, etio- and pathophysiology of the ED typology
Esophageal diverticula are present in less than 1% of the population (18). They can be present at any age but are typically diagnosed in the elderly, more often in men than in women. They are seen in about 1–3% of patients with dysphagia. In radiological studies, they appear with an incidence of 0.06–4% (19,20).
Several “typologies” of ED are most commonly encountered in medical practice.
Faced with this uncommon pathophysiological picture, dependent on the types of esophageal diverticula ED (anatomopathological type, etiological mechanism, topographic location), several questions naturally arise: what could be the initiating mechanism, why in identical conditions not all patients have the disease, what are the conditions that make possible the evolution towards the establishment of an ED, etc.
Zenker’s diverticulum
It is the typical cervical diverticulum, having as a pathophysiological substrate the pulsion mechanism. From an anatomical point of view, it usually originates on the posterior midline at the junction between the hypopharynx and the cervical esophagus, at the level of a triangular area bordered superiorly by the constrictor muscles of the pharynx and inferiorly by the cricopharyngeal muscle. The incidence of Zenker diverticulum is higher in northern Europe than in the south. It is relatively common in the United States, Canada, and Australia, and very rare in Japan and Indonesia. A study in the UK estimates an incidence of 2 cases per 100,000 population/year (21).
Increased intra-pharyngeal intraluminal pressure causes the hernia of a diverticular sac between the cricopharyngeal muscle and the lower pharyngeal sphincter, an anatomically weak region known as the Killian’s triangle (Figure 1) (22). The increase of intrapharyngeal pressure as an act of swallowing is a physiological one. The phenomenon of increased intrapharyngeal pressure as an act of swallowing is a physiological one. In the conditions of an esopharyngeal motility disorder, there is a dissynergy between the pharyngeal contraction with a propulsive purpose and the esophageal relaxation with the role of taking over the food bowl (lack of esophageal relaxation). Therefore, there will be an abnormal increase in intraluminal pressure that will be directed to the weak area of Killian’s trigone, leading to the formation of the diverticulum.
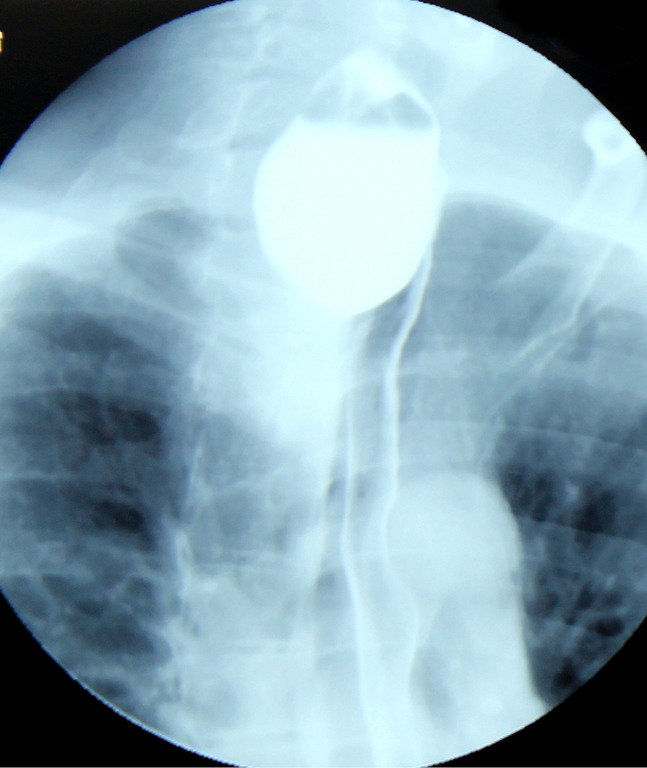
Figure 1.
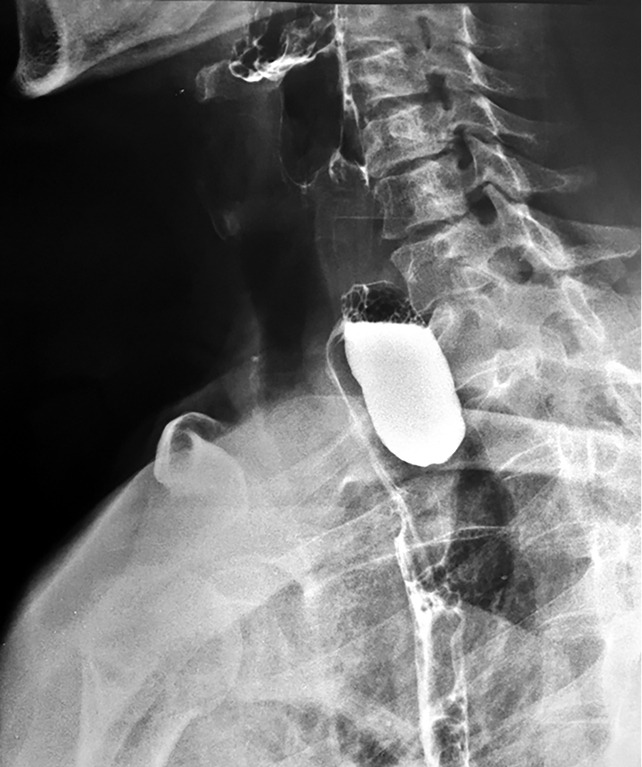
Barium swallow. Voluminous Zenker diverticulum (10 cm length, 4 cm width) with significant retention, plunging through the upper opening, with a mass effect on the esophagus that appears displaced laterally, with secondary dysphagia.
An attempt was made to determine a correlation between the morphological features of the Killian triangle and the individual anthropometric data. A prospective study based on the dissection of the hypopharynx in 47 corpses showed the existence of this area in 60% of men and 34% of women. The average height of this area was 7 mm for men and 4 mm for women, and the base was 16 and 12 mm, respectively. The dimensions of this triangle were obviously correlated with the height of the individual as well as with the length of the pharynx. These data may explain the higher incidence of Zenker diverticulum in men and the different incidences depending on the geographical area (23).
Killian-Jamieson diverticula
They are known as anterolateral cervical diverticula. They may be bilateral because they develop in other weak parietal areas of the pharyngoesophageal junction. It is located below the transverse portion of the cricopharyngeal muscle and has an inverted triangular shape with the base upwards. The base of the triangle is composed of the lower edge of the cricopharyngeal muscle and the soft tissues that cover the lamina of the cricoid cartilage. The lateral margin is represented by the longitudinal portion of the cricopharyngeal muscle, while the medial edge is formed by the longitudinal layer of the esophageal muscular body in the proximity of the cricoid insertion.
Midthoracic traction diverticulum (Rokitansky diverticulum)
Classically, the medioesophageal diverticula are called Rokitansky diverticula, accounting for less than 30% of esophageal diverticula. Although studied for over 200 years, it remains a controversial topic (Figure 2). Most of the time, they are a random discovery and, consequently, because they do not have significant clinical signs (less than a third complain of dysphagia) and the therapeutic approach is a difficult one, surgical abstinence tends to be a general opinion (13). They are formed by the traction of a mediastinal inflammatory process that adheres to the esophageal wall making a “true” diverticular sac. Usually, the tip of the diverticulum is located at a higher level, rarely reaches a large size, or is retentive. The association with pulmonary tuberculosis is typical (24).
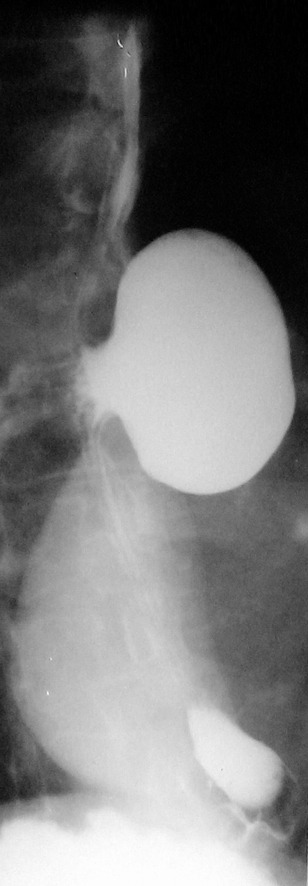
Figure 2.

Barium swallow. Retention of the barium tracer in a giant midthoracic diverticulum approximately 15 cm in diameter, with lateral displacement of the esophagus.
The pulsion mechanism cannot be ignored, although in some studies it has been described in only 27% of patients with medial thoracic ED (16). Moreover, the identification of motility disorders in most of these patients raises speculation regarding the intricacy of a pulsion mechanism including for this location. Motility disorders such as achalasia, nutcracker esophagus, segmental esophageal spasm in the middle third, and nonspecific motility disorders (NSMD) are recorded in 92% of the patients (Figure 3) (16,25).
Figure 3.
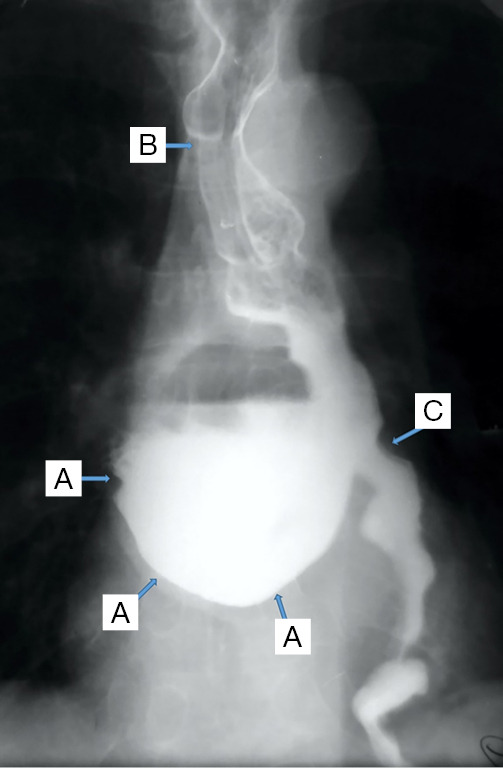
Barium swallow. The barium tracer fixed at the level of a giant midthoracic 15 cm in diameter diverticulum (A), with the association of an esophageal motility disorder [secondary (B) and tertiary (C) contraction waves are highlighted].
Epiphrenic diverticulum
Epiphrenic diverticula are also pulsion diverticula, which are estimated to have a frequency of 1/500,000/year, accounting for less than 10% of all esophageal diverticula (26,27). The incidence varies depending on the study and region, is between 0.015 and 2%, with a slight predominance in males and a maximum frequency between the 6th and 7th decade of life (28). Somewhere between 0.06–4% are discovered incidentally, radiologically (Figure 4). Positively, the right esophageal wall is more susceptible to the appearance of a diverticular sac (about 70%), the presence of the heart on the left side, through the “screen” effect, favoring the contralateral development. Interestingly, spontaneous esophageal ruptures occur most frequently on the left side. In 15% of situations, they have multiple locations (29). A recent study (30) showed that in over 75% of cases, the epiphrenic diverticulum is associated with other esophageal motility disorders (achalazia in aprox 60% cases). Thus, performing high-resolution manometry (HRM) is mandatory before performing a therapeutic technique. Cancer, especially squamous cell carcinoma, is found in 0.6% of these patients, especially men (83%). Cases are described for diverticula over 5 cm in elderly patients (mean age 68 years) (20,31).
Figure 4.
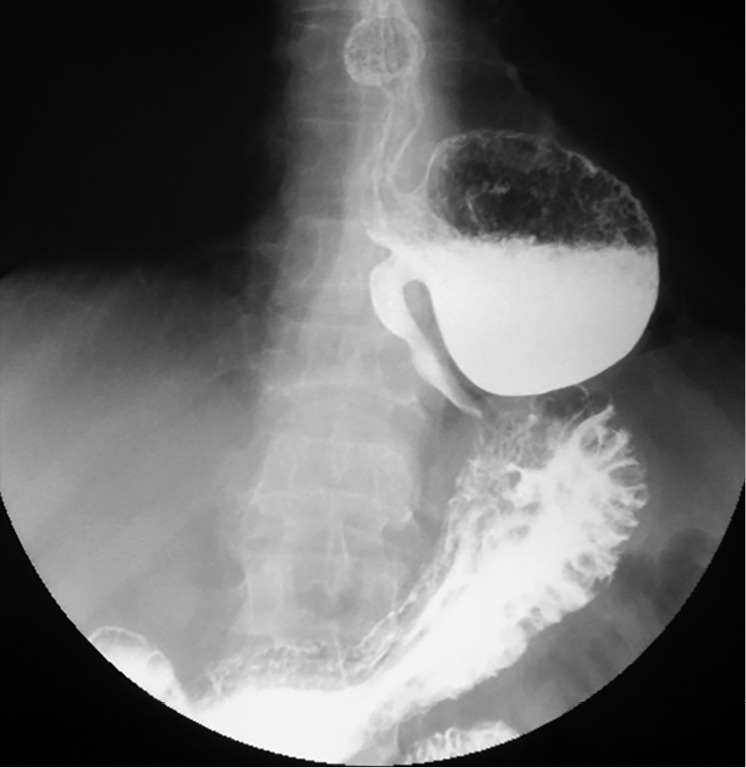
Contrast radiology that highlights a retentive, voluminous epiphrenic diverticulum (8–10 cm in diameter). The diverticulum is located above the diaphragm, with pulmonary and right atrium compression.
Asymptomatic patients are treated conservatively. With this approach, the risk of complications or developing specific symptoms is less than 10% (32). The disappearance of symptoms after surgical treatment is almost 90%, especially for the approach by diverticulectomy associated with myotomy (33). Without myotomy, the recurrence rate is 20% and the risk of leakage is 24%. Reflux after myotomy is described in 48% of cases without the antireflux technique, compared to 9.5% after association with Dor technique (28,29).
Clinical appearance
In most cases, patients are asymptomatic and may remain undiagnosed without the onset of hesitant swallowing, which may be difficult. The typical symptom is dysphagia, the alteration of swallowing being dependent on the diverticular dimensions, its location, and the more or less severe association of diffuse disorders of esophageal motility (34). A number of associated manifestations are described such as regurgitation, weight loss, and a degree of metabolic impact. The clinical picture of some complications of ED includes the most common such as cough caused by food retention in the diverticular sac with the appearance of aspiration phenomena, even aspiration pneumonia, especially through episodes of nocturnal regurgitation, hemorrhages with the diverticular site, esophagitis due to intradiverticular food fermentation, etc.
For the cervical location, in the early stages, patients usually complain of an unpleasant globus sensation, especially after the ingestion of solid and dry food. Over time, the feeling of incomplete swallowing inadvertently leads to the reflex of repeated ingestion and then the urgent need to drink fluids to satisfy the swallowing impasse. This symptomatology is usually tolerated by patients, especially the elderly, for months or years, without seeking medical advice; it is common for a patient diagnosed with a Zenker diverticulum to describe a long history of swallowing difficulties (35).
Over time, the dysphagia worsens due to the fact that the diverticulum grows in size, becomes plunging, and descends along the cervical esophagus; extrinsically obstructs the digestive transit, impairing oral nutrition to malnutrition. There are signs of food stasis, typical of the presence of the diverticular sac, which also progresses slowly over time; exceptionally, the impossibility of swallowing by acute food impaction is cited as the first sign of disease (36). One of the early signs of food stasis in a pharyngeal-ED is the appearance of hydro aerial noises when ingesting fluids. In general, it is considered that at a size over 5 cm, EDs become retentive. Particularly, for epihrenic diverticula, there is no correlation between size and retentive character.
Sialorrhea follows, in response to the difficulty of evacuating the digestive contents downstream of the pharyngoesophageal tract (35,36).
After the formation of a retentive pharyngeal-ED, spontaneous regurgitations begin (with undigested foods, ingested hours ago, interrupting the feeding); patients begin to have foetor oris (foul breath, due to the decomposition of food stasis under the action of bacterial flora). Occasionally, cervical stasis becomes visible through the asymmetry of the cervical regions caused by a fluctuating tumescence, with hydro aerial noises on palpation (Boyce’s sign) located in the lower part of the area of the sternocleidomastoid muscle; some patients usually cause their diverticular sac to be emptied by manual compression on the cervical swelling to cause regurgitation, rumination, and then the resumption of swallowing (37).
For diverticula of the esophageal body, pulmonary complications are described between 24–45%, and in 25% of cases, it is the only manifestation of the disease (38,39).
For epiphrenic diverticulum, the percentage of symptomatic patients varies between 37% and 63% (40). Fasano et al. conclude that all patients with epiphrenic diverticulum over 5 cm have symptoms due to the presence of diverticulum compared to only 41% of those with diverticulum below 5 cm (41). The diagnosis of esophageal cancer is usually preceded by a history of at least 15 years of symptoms for achalasia and 10 years for epiphrenic diverticulum (42).
Paraclinical evaluation
Paraclinical investigations need to be performed systematically, considering several objectives that can be considered standard, such as identification of the diverticular sac, relationship with surrounding structures, topography, retention, and evaluation of the diverticulum mucosa, quality of esophageal motility. For a complete exploration, in the case of bulky diverticula, good preparation is also required for emptying the diverticular sac of food debris, which can mask a number of intradiverticular complications (esophagitis, hemorrhage, neoplasia).
Simple radiology appears outdated, but we believe that the potential data provided by it should not be minimized. It should be performed in both anterior and posterior-anterior and oblique incidences to correctly identify anatomical relationships. Bulky diverticula are evident in simple thoraco-pulmonary exploration, being highlighted by the level of retention, respectively the air-fluid level. It is important to note that epiphrenic diverticula develop preferentially to the right of the esophagus and should be differentiated from a type II hiatal hernia (which usually occurs behind the heart shadow).
Barium swallow, available in most medical services, makes the diagnosis of ED easy. It aims at identifying the diverticular sac, its topography, its retentive character, the relationship with the esophageal body, as well as the dimensions, most frequently related to the parameters of the vertebral bodies (Figures 5,6). It can also identify details of intradiverticular contents as well as the characteristics of esophageal motility. Contrast-enhanced esophageal radiological examination provides 95% diagnostic acuity (43).
Figure 5.
Voluminous Zenker diverticulum, plunging through the upper thoracic opening in the thorax, with retentive character and with a mass effect on the esophagus (barium swallow).
Figure 6.
Midthoracic diverticulum-large communication with the esophageal lumen (barium swallow), with good passage of contrast substance, slightly retentive.
Regarding the size of the diverticular sac (see Table 4), the van Overbeeck classification for cervical diverticula (44) is accurate and easy to use in practice. Verdonck and Morton classify the lesion into small diverticulum for those under 2 cm, medium to 2–4 cm, and bulky over 4 cm (10).
Table 4. Classification of esophageal diverticula regarding the size of the diverticular sac van Overbeek (44) and Verdonck and Morton (10) classification.
| Grade (size of the diverticular sac) | van Overbeek classification | Verdonck and Morton classification |
|---|---|---|
| Grade I | The maximum diameter of the height of a thoracic vertebra | Diverticulum smaller than 2 cm |
| Grade II | Maximum diameter 1–3 thoracic vertebral bodies | Diverticulum between 2–4 cm |
| Grade III | Maximum diameter over 3 thoracic vertebrae | Diverticulum over 4 cm |
In the case of Killian-Jamieson diverticula, on serographies, and especially by cineradiography, during swallowing the barium substance, two completely distinct appearances can be found as radiological image and significance, the difference being the size of communication with the esophageal lumen. A first appearance is a diverticular image with a wide opening to the lumen, which is usually deeper than narrow-neck diverticula. It increases in size during swallowing due to the increase in intrapharyngeal pressure, so that the ballooning appearance tends to be erased by flattening, thus, after the passage of the peristaltic wave, it returns to its original dimensions. Usually, these diverticular images are not retentive, although cineradiography shows that the barium substance is temporarily retained. It is believed that, despite the weakness of the Killian-Jamieson area, there are still contractile muscle elements in the wall, indirectly highlighted by participation in peristalsis.
In this way, diverticular images with wide communication with the lumen should be viewed as diverticular sacs rather than diverticula themselves (45), reflecting a weakness of the pharyngo-esophageal junction at this level, which is otherwise unchanged anatomically.
In contrast, diverticular images with narrow communication with the esophageal lumen form a neck and are retentive, although they are small in volume; they do not contract and do not empty with swallowing. This suggests pseudodiverticulization by herniation of the mucosa and submucosa (46); they are easy to highlight radiologically even without cineradiography, being unmasked by the barium retention as additional images located outside the digestive contour, which persists after swallowing.
The frequent association of gastroesophageal reflux disease in patients with Zenker’s diverticulum is already certain (47,48). The incidence of this association is currently considered double compared to patients with Killian-Jamieson diverticulum (49).
In the case of diffuse esophageal intramural pseudodiverticulosis (EIP), the diverticula are extremely numerous but small in size, even millimeters (1–4 mm.). The diverticulum neck is extremely short and narrow (1 mm), it can be obliterated during peristaltic contraction and the pouches have a bulbous appearance and almost all are buried at the same depth corresponding to the thickness of the esophageal wall. The exact pathogenesis of diffuse intramural esophageal diverticulosis is unclear and controversial. These diverticula or pseudodiverticula are composed of pathologically dilated submucosal glands with surrounding inflammatory cells, so it is postulated that inflammation plays an essential role in their pathogenesis. As a result of the presence of Candida in some cases and its absence in others, it is suggested that esophageal candidiasis could be the cause or the result of EIP. Overall, the radiological appearance of “bunches of grapes” attached to the esophageal axis appears (50,51).
The diverticula are often grouped in a single region of the thoracic esophagus, which appears slightly stenotic. The additional images (diverticular sacs) appear both at the level of the stenotic aspect and above it; sometimes they are present on the entire overlying esophagus. A second characteristic appearance is the radiological evidence of a diffuse esophageal spasm. Thus, intramural diverticulosis is associated with either a partially stenotic area, diffuse hypertonia, or both simultaneously (52,53).
The peristaltic deficiency is better highlighted by video/cineradiography and is consistent with the following findings: diffuse or segmental hyperperistalsis; irregular and non-propulsive contractions indicating neuromuscular incoordination; violent propulsive contractions or, conversely, segmented and prolonged tonics that mimic a stenotic area (Figure 7) (54).
Figure 7.
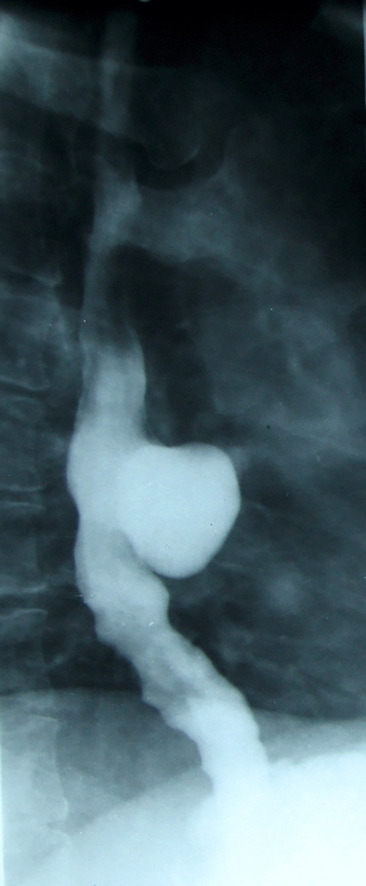
Association between a midthoracic diverticulum and esophageal motility disorder-segmental hyperperistalsis with irregular and non-propulsive contractions (barium swallow).
In the case of epiphrenic diverticula, in addition to the anatomical presence of the diverticular sac, it provides the first indications of the associated motility disorder, which is an important starting point if the patient is asymptomatic. Radiology can also diagnose other lesions (e.g., cancer, distal stenosis, hiatal hernia, Figure 8), and possible bronchopulmonary complications, especially common in elderly patients with peristaltic deficits and aspiration phenomena. Irregularities of esophageal stenosis in the proximity of an ED or the progressive reduction over time, on successive examinations, of the volume of a known diverticulum raise high suspicions of malignancy (55,56).
Figure 8.
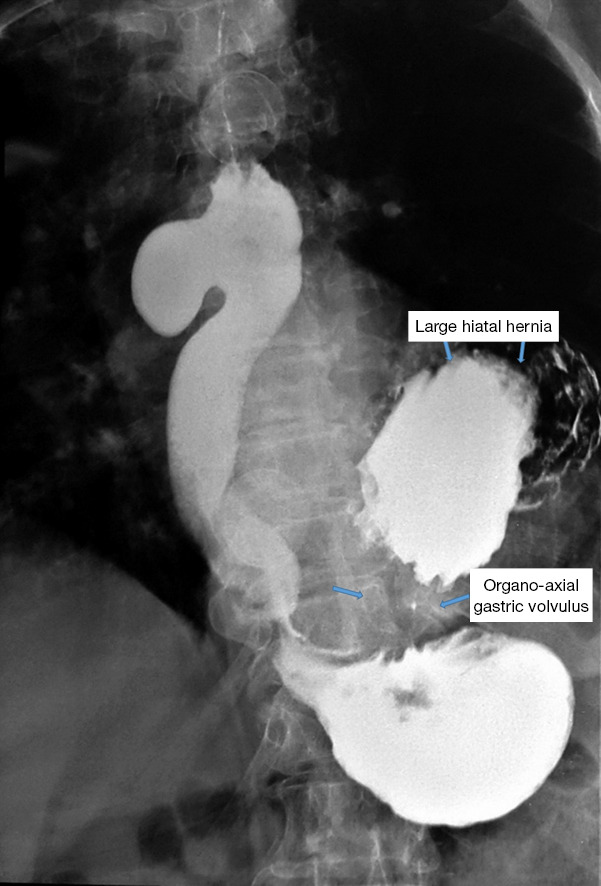
Pathological association of an epiphrenic ED with a large hiatal rolling hernia, approx. 1/2 of the stomach fixed in the chest and with a degree of organo-axial gastric volvulus. ED, esophageal diverticula.
Endoscopic examination [esophagogastroduodenoscopy (EGD)] should be performed under sedation by a certified endoscopist. When suspecting an ED, regardless of its anatomical location, it is advisable to perform a radiological examination of the upper digestive tract through barium swallow before the endoscopy. EGD provides useful information to focus attention on the lesion, through morphological data (location and size of the diverticular orifice, retentive character, etc.). For a complete exploration of the diverticular sac (depth assessment, assessment of inflammatory aspects even neoplasia), it is recommended to clean food debris by washing, and avoiding aspiration (which can clog the working channel of the endoscope). Otherwise, it is preferable to resume exploration after 1–2 days of fasting for solids and to wash the diverticular sac using a nasogastric tube (Figures 9,10).
Figure 9.
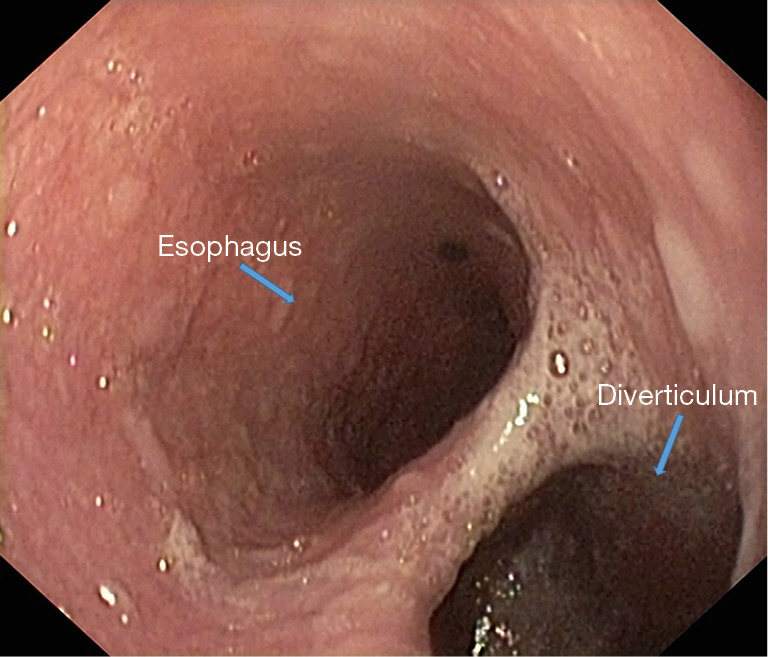
Endoscopic examination-the presence of an important mediothoracic esophageal diverticulum (“double barrel shotgun” aspect).
Figure 10.
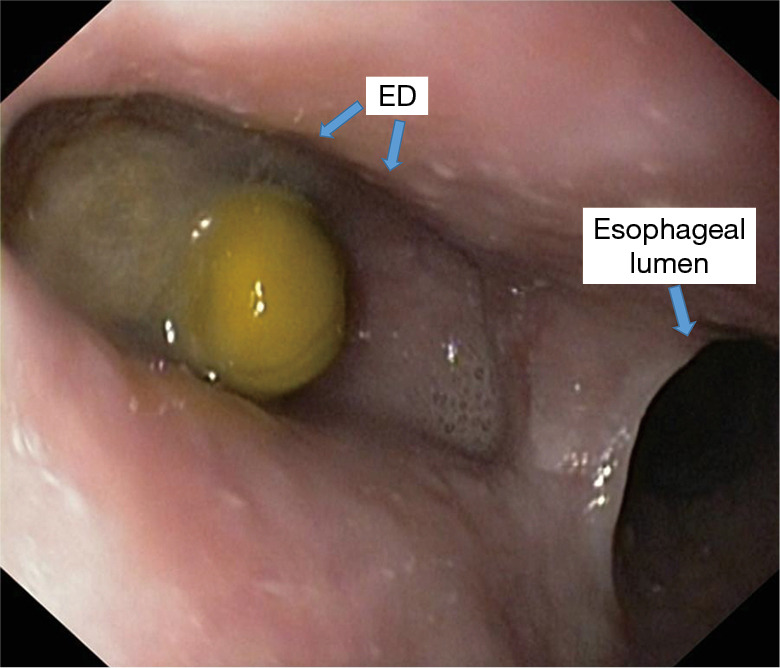
Endoscopic examination-retentive mid-thoracic diverticulum.
In practice, routine endoscopic exploration of the upper digestive tract is performed under minimal sedation, with or without antispasmodic medication; it is an option related to individual indication and tolerance. For pharyngoesophageal diverticula, endoscopic exploration under deep sedation is preferable because exploration of the pharyngoesophageal junction is difficult for the patient to tolerate. The medication facilitates exploration but can impair endoscopic information on native motor behavior. It is common to quickly engage the endoscope to the duodenal level, and most morphological and functional data will be obtained when the endoscope is withdrawn.
The communication of a small pharyngeal-esophageal pouch with the esophageal lumen is eccentric and it may be neglected by an inexperienced endoscopist, both when engaging and withdrawing the endoscope. At the other extreme, in the case of large diverticula, the endoscope tends to engage in the diverticular sac that replaces the esophageal axis; in this case, for the orientation, it is indicated to withdraw the endoscope above the septum that separates the diverticular cavity from the esophageal lumen. The two can be differentiated by the fact that the esophageal lumen is free, while the diverticular cavity invariably has salivary or food stasis.
The increased incidence of gastroesophageal reflux disease in patients with Zenker’s diverticulum has long been known. This association is currently incompletely elucidated. On the one hand, non-specific changes in esophageal motility are assumed to be common pillars, on the other hand, it is believed that, in theory, gastric acid reflux can rise to the pharyngeal level, causing mucosal injury in the Killian triangle and hypertrophy of the cricopharyngeal muscle. Despite the difficulties in documenting the mechanisms of this association, it may be justified to perform esophageal pH-metrics in patients with Zenker diverticulum. Moreover, there are authors who investigate in particular the possible presence of the diverticular sac cervical in patients with endoscopic reflux esophagitis (57).
Endoscopic findings in lateral pharyngeal-esophageal diverticula (Killian-Jamieson) are similar to small Zenker diverticula. The small size of the communication opening with the lumen, as well as the fact that it is lateral and not coaxial, “in the double-barrel shotgun” appearance with the axis of the esophageal lumen, makes the entering in the diverticulum less possible. Sometimes the only useful information, apart from the presence of a diverticular expansion at the level of the pharyngoesophageal junction, refers to the presence or absence of retentive character, by finding the food stasis. For endoscopic exploration, for the differential diagnosis with the Zenker diverticulum, it is very useful to corroborate the radiological image in oblique incidence with the endoscopic appearance, to orient the communication orifice towards the real anatomical position. The Zenker diverticulum also develops in the anterolateral direction during the descent, but the orifice communicating with the lumen remains on the posterior midline.
In the case of diffuse intramural pseudodiverticulosis, small diverticular orifices can be seen, located on a relatively concentric stenotic esophageal segment, covered by hyperemic, normal mucosa or with lesions specific to reflux esophagitis. Muhletaler et al. state that diverticular orifice can be viewed on endoscopy in only 20% of cases (58). For epiphrenic diverticula, endoscopy provides morphological details complementary to the barium swallow.
In general, unlike pharyngeal-esophageal diverticula, there are no technical difficulties; the esophageal lumen and the diverticular orifice are present, “in the double-barrel shotgun” appearance and the real esophageal lumen is always easy to identify. Sometimes the differential diagnosis of gastric hernia is difficult. The identification of the landmarks represented by the imprint of the diaphragmatic pillars can be illusory if the oesophageal-gastric junction is aspirated in the thorax, the real landmark is the transition zone between the esophageal mucosa (pale pink) and the cardiac mucosa (reddish). Endoscopy is the only method to highlight the source and etiology of bleeding in case of hemorrhagic complications. The identification of mucosal lesions makes endobiopsy mandatory to rule out a malignant lesion. Advanced proliferative lesions developed in the diverticular sac require repeated biopsy samples, as food stasis and tumor necrobiosis can impair endobioptic harvesting, leading to false-negative results. Any suspicious endoscopic image justifies further investigation, regardless of the biopsy result (59,60). Endoscopy is formally contraindicated if a local inflammatory complication is suspected with or without the potential for septic dissemination (e.g., acute diverticulitis, perforations, fistulas, etc.), due to the presence of diverticulum.
Ultrasound examination
It is rarely used, although it can bring a lot of clarification in the case of a pharyngeal pouch. The Killian-Jamieson diverticulum may be confused with a thyroid nodule if the sonographer is not informed by modified local anatomy (61). Small air bubbles inside the diverticulum can mimic the microcalcifications described in thyroid cancer (especially papillary carcinoma). To avoid invasive investigations such as biopsy punctures or even untimely surgeries, certain ultrasound features may be helpful in raising suspicion of the Killian-Jamieson diverticulum (62). Ultrasound changes in the dynamics during water swallowing and monitoring the relationship with the esophagus are very useful. Positioning the lesion on the right side of the esophagus does not rule out the possibility of the presence of the Killian-Jamieson diverticulum (63). A careful study can highlight 3 layers of the lesion walls (characteristic of the esophageal wall) (64). This aspect is essential in differentiating from a thyroid node. If the diverticular neck is small or if the contents of the diverticulum do not shield the posterior wall, the Killian-Jamieson diverticulum appears with a continuous hypoechoic wall, similar to a thyroid nodule. In this situation, it is necessary to obtain information regarding the relationship with the neighboring structures, obtained in dynamics. The ultrasound aspect of the diverticulum inside changes in relation to the swallowing or local compression performed by the ultrasound probe. These maneuvers cause changes in size, shape, and echogenicity, all signs of indirect communication with the esophagus. There are few reports of ultrasound changes caused by the Killian-Jamieson diverticulum, the vast majority of which are case studies (65,66).
Computed tomography (CT) targets the size of the diverticular sac and its relationship to the esophageal body and surrounding structures (Figure 11). Intrasacular details can be distinguished by the use of oral contrast, while information regarding the intramural or perisacular inflammatory process can be obtained by intravenous contrast. The investigation is essential in assessing the suspicion of fistulization or perforation with peridiverticular abscess, a situation in which barium swallow and especially endoscopic exploration are to be avoided.
Figure 11.

CT image of the cervical sagittal plane. Large Zenker diverticulum, located above the thoracic opening, with a thickened wall (blue arrows), with the retention of the contrast substance administered orally. CT, computed tomography.
In the case of epiphrenic diverticula, CT is indicated for selected cases, such as suspected proliferative mass associated with the diverticulum or to complete the investigation in case of locoregional septic complications (e.g., local suppurations). Otherwise, the typical pulsion diverticulum appears as a formation well-delimited by the surrounding structures, with a thin wall (consisting of an inner and an outer layer), presenting a hydro-aerial image that communicates with the esophageal lumen. The appearance of irregular, zonal thickening of the diverticulum wall may be suggestive of cancer (Figure 12) (67). In the case of diffuse intramural pseudodiverticulosis, inflammation and fibrosis of the submucosa can be seen. Another aspect is the diffuse thickening of the esophageal wall, with the loss of its stratigraphy, associated with the presence of small intramural gas collections (68).
Figure 12.

CT scan cross section. Significant thickening of the diverticular wall, with the appearance of an extraesophageal infiltration (blue arrows), with the suspicion of a neoplastic degeneration (confirmed malignancy by endoscopic biopsy). CT, computed tomography.
3D CT images can provide details on the topography of the diverticular sac, especially in terms of relationships with surrounding structures (Figure 13).
Figure 13.

3D tomographic reconstruction of the thoracic esophagus. A midthoracic diverticulum (blue arrows) and its direct relationship with aortic artery.
Magnetic resonance imaging (MRI). It is not a routine examination for esophageal conditions due to its low availability and technical limitations. The vicinity of viscera with air content (lungs, trachea, stomach, and even the esophagus itself) significantly affects the accuracy of the images. Respiratory and cardiac movements bring additional challenges. On the other hand, exploration is non-radiant, non-invasive and brings precious details to soft tissues (69).
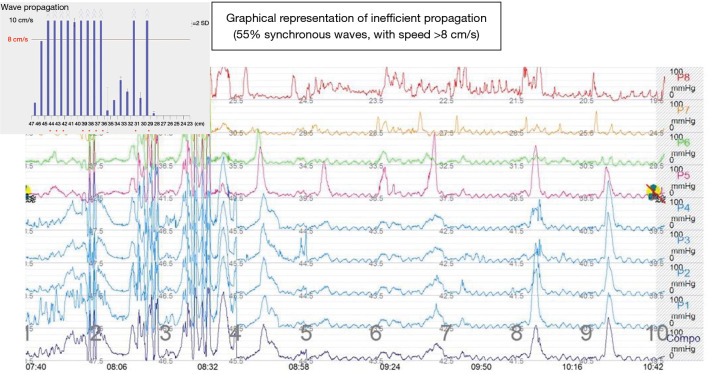
Esophageal manometry quantifies esophageal motor activity with objective identification of possible motility disorders, frequently associated with diverticular disease (Figure 14).
Figure 14.
Conventional esophageal manometry-motility disorder type diffuse esophageal spasm (>30% synchronous waves, waves over 180 mmHg).
In the case of epiphrenic diverticula, exploration is essential to characterize the functional deficit associated with the diverticulum, as well as to establish an adequate correlation between symptomatology and the presence of the diverticulum. Currently, the technique of classical stationary manometry (pull-through) is replaced by computerized ambulatory manometric monitoring, performed during the circadian course (24 hours), a method that more accurately reflects the spectrum of esophageal motility in the daily reality of the patient. The manometric examination must address both the esophageal body and the LES (distal sphincter region, even if the cardia is ascensioned into the chest under the effect of axial gastric hernia). The reason is the frequent association of distal esophageal motility deficits with those of the LES. Several studies (16,34) highlight the impairment of manometric results (false-negative interpretations) due to technical deficiencies, respectively the inability to place manometric catheters (transducers) through the gastro-esophageal junction, due to their involvement in the epiphrenic diverticular sac. Thus, endoscopic guidance of catheters (transducers) through the gastro-esophageal junction is recommended (70).
Stationary manometry is frequently used for the intraoperative identification of the esophageal or cardiac segment affected by the motor deficit, in order to reduce the length of the myotomy to that of the manometric defect. Limited or extended esophageal-cardial myotomy may be adjusted manometrically during surgery to limit impairment of the competence of the LES and esophageal peristalsis. There is a fragile balance between the mandatory to remove the functional obstruction (by myotomy) and the restoration of competence for esophagogastric junction (EGJ) (by fundoplication).
Manometry is the benchmark for evaluating surgical therapeutic efficacy, by comparing pre- and postoperative aspects with symptomatic satisfaction (practically, functional evaluation of the case) (41).
HRM
The enhanced spatial resolution and color-coded topography plots provided by HRM and esophageal pressure topography have improved the ability to evaluate the esophageal motor function and pressurization patterns (71). HRM evaluation reports a lower percentage of association with achalasia, explained by the possibility of optimal positioning and evaluation of the sensor in the LES (33). Unfortunately, it remains a demanding investigation, especially from the perspective of accessibility.
Esophageal pH monitoring
The gold standard for differential diagnosis, specifically for esophageal motility disorders is esophageal pH monitoring. Although no causal relationship has been documented between reflux disease and esophageal diverticular disease, there is a risk of misinterpretation of the symptoms of a reflux disease with known symptoms caused by the presence of an esophageal diverticular pouch. Gastroesophageal reflux disease must be correctly diagnosed (manometric and pH-metric sphincter incompetence, endobioptic evidence) to counteract the side effects of esophageal-cardio-myotomy by applying an appropriate antireflux technique to both the distal sphincter deficits and the peristaltic esophagus. The pH monitoring of the cardiac competence, corroborated with the manometric one, assists in establishing the operative strategy (72).
Treatment
In principle, the direct relationship between the presence of the diverticular sac and the clinical features but also the perspective of specific complications, are essential in establishing therapeutic tactics, which can vary widely, from abstention and monitoring to aggressive techniques, with morbidity and even mortality difficult to neglect.
The treatment protocol has two major objectives:
Approaching the diverticular sac, in order to solve the specific diverticular condition;
Subdiverticular myotomy, to solve the etiopathogenic deficit underlying diverticular condition.
Depending on the topography, these goals are achieved by different techniques: open surgery, minimally invasive surgery, or endoscopic approaches. In choosing the optimal technique, the attending physician must identify how to effectively achieve the two goals versus the risk-benefit balance.
Cervical diverticula
Zenker’s diverticulum
Several techniques are available for the treatment of Zenker diverticulum (73). The approach of the diverticular sac involves diverticulectomy, diverticulopexy or invagination (inversion) which is associated with subdiverticular myotomy. This may be sufficient as the only surgical procedure in the case of small diverticula. The commonly used approach is left anterolateral cervicotomy. Open surgery is often not so simple, but benefits after surgery are achieved in 94–100% of cases (20). Because EDs are frequently diagnosed in the 7th and 8th decades of life, endoscopic minimally invasive techniques have rapidly advanced in recent years.
Endoscopic treatment generally involves either diverticulectomy using devices developed for endoscopic dissection or the POEM technique (74). The septal wall is dissected using a rigid endoscope with a CO2 laser or endostapler, while a flexible endoscope uses devices such as a stag beetle knife and clutch cutter knife.
Two approaches to dissection have been reported: direct incision of the septal wall along with the mucosa using the rigid or flexible endoscope (75), or myotomy of the exposed septal wall using the POEM technique with a flexible endoscope diverticular peroral endoscopic myotomy (D-POEM) (76). For endoscopic techniques, the morbidity is 8.7% and the mortality is 0.2% (31). For open surgery, the morbidity is 10.5% and the mortality is 0.6% (20).
Obviously, endoscopic techniques present a lower risk for nerve damage and wound infection due to the minimally invasive endolumenal character (77). Moreover, the cost of hospitalization is lower for endoscopic techniques than for open surgery (78). The recurrence rate of symptoms varies between 0–35% (79-81). Bonavina et al. analyzed a large group of patients treated with transoral stapling (181 patients) and open surgery (116 patients) finding similar results for both techniques (82). Kamal et al. showed the same results for open and transoral techniques (83). Even minimally invasive endoscopic techniques are not exempt from incidents. Complications are mainly fistula, in up to 15% of cases, and paralysis of the vocal cords in 1–15% of cases. Other complications include chest pain, cervical abscess, mediastinitis, and dental damage (84).
Killian-Jamieson diverticula
Unlike the Zenker diverticulum, which has a tendency to increase in volume and drive into the thoracic cavity, developing diverticular complications on its own, the lateral pharyngoesophageal rarely reaches sufficient size to develop complications through food retention. Therapy is rarely indicated for the elimination of stasis (by diverticulectomy) but especially for symptomatic suffering, and dysphagia (by myotomy).
The surgery presents special risks due to the high incidence of the intraoperative injury of the inferior laryngeal nerve; it is highlighting during operation is mandatory but, even so, simple dissection to avoid incidental injury may be followed by phonation disorders in the postoperative period.
Classically, open surgery is considered safer than endoscopic diverticulectomy because minimally invasive endoscopic techniques do not allow visualization of the region and secondary diathermic nerve damage is difficult to control. Recently, however, favorable results have been reported through endoscopic interventions, using various technical devices to allow lateral viewing even with a flexible axial endoscope with an axial view. The principle of diverticulotomy is the same as in the case of the Zenker diverticulum: to cut the muscular bridge that separates the diverticulum cavity from the esophageal one.
Midthoracic esophageal diverticula
Traction diverticula
The incidentally radiological finding of a traction diverticulum does not usually indicate therapy. Traction diverticula with clear etiopathogenesis (without radiological or especially manometric abnormalities) that suggest or demonstrate the participation of a drive mechanism are rarely indicated for surgery. Most have a large base, so they are not retentive. Occasionally, if they become retentive or have an endoscopic appearance of diverticulitis, they may require thoracotomy or better thoracoscopy. Diverticulectomy is sufficient; if peridiverticulitis is not an impediment to dissection, the suspension may be performed on the prevertebral fascia (which is technically facilitated by the anatomical proximity to the spine).
The inflammatory nature of the traction diverticulum is evident if local complications occur as a result of inflammatory necrosis (perforative or fistular), which completely changes the prognosis and strategy. Spontaneous free perforations followed by acute mediastinitis (requiring emergency thoracic drainage) are extremely rare compared to pulsion epiphrenic diverticula; on the other hand, although currently exceptional due to the decreased incidence of tuberculosis, there are still cases of eso-respiratory fistulization, with trachea or the main bronchi, especially the left one.
Benign esophago-bronchial fistula due to a traction diverticulum is exceptional. It must be clinically suspected when an irritating cough after swallowing appears; the patient is exposed to repeated septic pulmonary complications. In addition to radiological examination of the esophageal transit with a contrast medium (preferably a water-soluble substance, although the morphological detail is lower than using barium suspension), the examination should be completed by chest CT and bronchoscopy. The indication for surgery is compulsory. The operation is usually performed by thoracotomy, although thoracoscopic solutions are also cited. The operation consists in dissecting the fistulous tract and obturating it, preferably by mechanical suture transection; In order to prevent a recurrence, the anatomical structures in the proximity of the esophagus and the bronchial wall are interposed, unaffected by inflammation (pleural or fascial flap, transposition of pediculated intercostal muscles).
The improvement of the symptoms can be obtained after simple resections, but the incidence of complications is high with 9–25% fistulas, 33% pneumonias. Mortality can reach up to 33% (85). Although theoretically, an endoscopic approach is possible, the POEM technique has not been described for this type of diverticulum, certainly due to the rarity of this pathology.
The discovery is often accidental and the frequent non-retentive character often supports a non-surgical treatment.
Pulsion diverticula
In the case of pulsion midthoracic diverticula, strictly diverticular interventions are no longer sufficient to resolve the underlying symptoms and pathology. It is necessary to associate with extra mucosal myotomy, which is performed on the contralateral esophageal wall. Its length depends on the type and extent of dyskinesia. For common cases, caused by NSMD, a segmental myotomy extended proximally and distally to the diverticulum by 3–4 cm is sufficient; some surgeons limit the length of the myotomy strictly to the length of the aberration described manometrically. In the case of diffuse esophageal spasms or nutcracker esophagus (high-amplitude esophageal contractions), as well as those in which the dysfunction also affects the LES, the length of the myotomy should be adjusted accordingly.
Endoscopic treatment through flexible endoscopy and POEM is used successfully for this type of diverticulum (86-88).
Epiphrenic esophageal diverticula
The indications for treatment are an increase in the size of the diverticulum, the presence of specific symptoms, and various complications such as malignancy. Myotomy of the LES and an anti-reflux technique to prevent gastroesophageal reflux need to be considered (75). Traditionally, the approach is by left thoracotomy due to the anatomical features of the distal esophagus. It is associated with an incidence of fistulas of up to 21% and death of 0–11% (89). Varghese et al. report the results in a group of 35 patients operated on for epiphrenic diverticula between 1976 and 2005 with symptom relief of 76% while 21% required regular dilated treatment. The authors recorded 2 cases of fistula (6%) and one death (3%). They stated that “these data should serve as a benchmark against which newer surgical techniques can be measured” (90).
The use of the thoracic approach by thoracoscopy brings advantages to the technique. Currently, the left thoracoscopic approach is the optimal option. Even in the hands of an experienced team, the risk of fistula rises to 21% with a mortality rate of 0–11% (91). In 1998, Rosati et al. reported the first transhiatal laparoscopic approach for diverticulectomy with myotomy and Dor fundoplication in 4 patients with epiphrenic diverticulum. No patient had postoperative complications and all had good functional results (92). In 2009, Melman et al. reported the results of 13 patients operated on using the technique of Rosati et al. They recorded a case of fistula that required thoracotomy. After 14 months of monitoring, it reported good results in 85% of patients (93). The Dor technique is the most widely used after diverticulectomy and laparoscopic myotomy. The use of a Nissen fundoplication raises the incidence of the esophageal fistula to 23% (94). Long-term results are satisfactory (7,14,15). The laparoscopic approach (diverticulectomy, myotomy, and Dor fundoplication) leads to the disappearance of symptoms in 85–100% of cases. The complication rate remains high. Fistulas are described between 9–23%, pulmonary complications 8–10% with a mortality ranging from 0–7%. These results do not appear to differ from the thoracoscopic approach (89). Mortality is not negligible, being up to 1.6%, with higher morbidity for thoracoscopy than for laparoscopy (95).
In 2010, Inoue et al. (96) reported the first series of achalasia patients treated with POEM, an innovative technique for endoscopic esophageal myotomy. Subsequently, the technique (adapted and modified) was used in the treatment of epiphrenic diverticula-salvage POEM (s-POEM), with myotomy performed on the esophageal wall contralateral to the diverticulum (97). Alternatively, D-POEM is proposed, which involves a septotomy of the diverticulum using the POEM technique, with statistics reporting high efficiency (98).
Iatrogenic diverticulum
The development and adaptation of endoscopic minimally invasive techniques such as POEM have led to the emergence of a new pathological entity—the iatrogenic diverticulum, without being considered an important issue for this technique (74,99-101). The right esophageal wall is anatomically predisposed to diverticulum formation. Moreover, motility disorders of the esophageal body after POEM exert too much force on the weak distal esophageal wall. As a consequence, the need for caution in choosing the POEM technique in patients with motility disorders such as jackhammer esophagus (hypercontractile esophagus) or type II (with panesophageal pressurization) and III (with spastic pressurization in the distal esophagus) achalasia. Consequently, HRM is essential before the procedure (102). Depending on the data provided, it is possible to opt for a myotomy extended on the esophageal body or to perform the myotomy on the posterior wall in order not to further weaken the right esophageal wall (74).
Non-therapeutic option (wait and see)
Many authors argue that surgical treatment is not necessary in asymptomatic patients, those with small diverticula, patients with comorbidities, and those with a good response to dilation techniques (100,101). There are no studies on the evolution of patients with unoperated pharyngeal esophageal diverticula.
Klaus et al. re-evaluated a series of 6 patients with epiphrenic diverticula with conservative treatment, finding similar results with the group of operated patients, but in the group treated conservatively, the symptomatic patients benefited from dilated treatment (103). Zaninotto et al. compared the results of conservative and surgical treatment of epiphrenic diverticulum with no improvement in symptoms only in the conservatively treated group (104). Castrucci et al. monitored 13 inoperable patients with esophageal diverticula (midthoracic and epiphrenic) over a period of 64 months. He did not report any complications or worsening of symptoms. Radiologically, there were no changes in size except for one case that increased the diverticular sac from 2 to 4 cm over a 2-year period (105).
Discussion
Diverticular pathology has become a surgical focus in the last two centuries, but there are still uncertainties about the etiopathogenic substrate. Over time, the goal of suppressing the esophageal sac (to resolve the pathogenic effect through food retention, or regional complications) has been replaced by the perception of resolving the pathogenic substrate that underlies the anatomical entity, even before the onset of secondary complications.
If the diagnosis is easy by imaging the diverticular sac, the determination of the cause of dysphagia remains to be established in each case. It is difficult to quantify how much the size of the diverticular sac and esophageal motility disorders (the etiopathogenic substrate of the disease) participate in the onset of swallowing disorders.
The appearance of diverticular pathology is increasingly associated with and proven to be caused by disorders of esophageal (peristaltic) motility, which are polymorphic. Even now, the determinants of multiple esophageal motility deficiencies, mostly considered idiopathic, are unknown. Things get even more complicated when deficits involve the border areas of the esophagus (pharyngoesophageal junction and gastroesophageal junction), which regulate food transit through two sphincteric areas, still vaguely defined as anatomical reality but functionally defined: the UES, cricopharyngeal and LES, cardial.
The surgical interest is clear, both by the possibly debilitating secondary symptoms and by the possible complications (suppurations, perforations, fistulas).
Although it is an eminently benign condition, neoplastic degeneration is reported. The incidence of diverticular cancer is 0.3–7% for the pharyngeal-esophageal, and 1.8% for midthoracic and 0.6% for epiphrenic localization (20).
However, we do not find in the literature a modulation of the technique based on the quantification of motility disorders as a substrate for the appearance of the diverticular sac (although the progress of manometric investigations is obvious). On the other hand, the approach of the diverticular sac (the clinical epiphenomenon of the disease) benefits from less and less aggressive techniques (as the technical progress of the endoscopic devices). In this sense, the interest of going through the learning curve and replacing the classic surgical techniques (even minimally invasive) is obvious. The penetration of these techniques in the surgical world is obviously dependent on the results obtained (debated permanently and transparently in the literature) but also on the dependence on pretentious devices, on the learning curve difficult to follow but certainly on the inertia of the surgical teams (which had years of experience for open techniques).
Conclusions
Diverticular pathology of the esophagus is rare. At the same time, it is a complex one, having a very varied clinical-pathological significance. This is because the appearance of esophageal diverticula or diverticular sacs (single, multiple, or even numerous, e.g., esophageal diverticulosis) can occur during life at various times, with different pathological consequences depending on the location of the lesions, depending on the etiopathogenesis.
The surgical treatment of esophageal diverticular pathology has undergone a continuous evolution over the years, from the initial empirical excision treatment to the current etiopathogenic treatment, as we understand the mechanisms of the appearance of the diverticular sac. In order to improve the therapeutic results, two directions of interest can be noted: on the one hand, the development of therapeutic techniques, from open to minimally invasive, on the other hand deepening the investigation of etiopathogenic mechanisms and the way of intercepting them by therapeutic maneuvers (74,106).
Finally, the benchmark of the effectiveness of the therapeutic protocol is the functional results, which need to be evaluated periodically, due to the possibility that they may change over time.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-861/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-861/coif). The authors have no conflicts of interest to declare.
References
- 1.Hussain T, Maurer JT, Lang S, et al. Pathophysiology, diagnosis and treatment of Zenker’s diverticulum. HNO 2017;65:167-76. 10.1007/s00106-016-0302-z [DOI] [PubMed] [Google Scholar]
- 2.Wang ZM, Zhang SC, Teng X. Esophageal diverticulum serves as a unique cause of bronchoesophageal fistula in children: A case report. Medicine (Baltimore) 2017;96:e9492. 10.1097/MD.0000000000009492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonbare DJ. Pulsion Diverticulum of the Oesophagus: More than just an Out Pouch. Indian J Surg 2015;77:44-8. 10.1007/s12262-013-0955-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little RE, Bock JM. Pharyngoesophageal diverticuli: diagnosis, incidence and management. Curr Opin Otolaryngol Head Neck Surg 2016;24:500-4. 10.1097/MOO.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 5.Thomas ML, Anthony AA, Fosh BG, et al. Oesophageal diverticula. Br J Surg 2001;88:629-42. 10.1046/j.1365-2168.2001.01733.x [DOI] [PubMed] [Google Scholar]
- 6.Law R, Katzka DA, Baron TH. Zenker’s Diverticulum. Clin Gastroenterol Hepatol 2014;12:1773-82; quiz e111-2. 10.1016/j.cgh.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 7.Ishaq S, Siau K, Lee M, et al. Zenker’s Diverticulum: Can Protocolised Measurements with Barium SWALLOW Predict Severity and Treatment Outcomes? The “Zen-Rad” Study. Dysphagia 2021;36:393-401. 10.1007/s00455-020-10148-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khullar OV, Shroff SR, Sakaria SS, et al. Midesophageal Pulsion Diverticulum Resulting From Hypercontractile (Jackhammer) Esophagus. Ann Thorac Surg 2017;103:e127-9. 10.1016/j.athoracsur.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 9.Tedesco P, Fisichella PM, Way LW, et al. Cause and treatment of epiphrenic diverticula. Am J Surg 2005;190:891-4. 10.1016/j.amjsurg.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 10.Verdonck J, Morton RP. Systematic review on treatment of Zenker’s diverticulum. Eur Arch Otorhinolaryngol 2015;272:3095-107. 10.1007/s00405-014-3267-0 [DOI] [PubMed] [Google Scholar]
- 11.do Nascimento FA, Lemme EM, Costa MM. Esophageal diverticula: pathogenesis, clinical aspects, and natural history. Dysphagia 2006;21:198-205. 10.1007/s00455-006-9028-5 [DOI] [PubMed] [Google Scholar]
- 12.Kishi K, Kusunoki R, Fujishiro H, et al. Mid-esophageal Diverticular Bleeding in a Patient with Kyphosis. Intern Med 2019;58:3239-42. 10.2169/internalmedicine.2951-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan DSY, Foliaki A, Lewis WG, et al. Systematic Review and Meta-analysis of SurgicalTreatment of Non-Zenker’s Oesophageal Diverticula. J Gastrointest Surg 2017;21:1067-75. 10.1007/s11605-017-3368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiwaku H, Inoue H, Onimaru M, et al. Multicenter collaborative retrospective evaluation of peroral endoscopic myotomy for esophageal achalasia: analysis of data from more than 1300 patients at eight facilities in Japan. Surg Endosc 2020;34:464-8. 10.1007/s00464-019-06833-8 [DOI] [PubMed] [Google Scholar]
- 15.Nehra D, Lord RV, DeMeester TR, et al. Physiologic basis for the treatment of epiphrenic diverticulum. Ann Surg 2002;235:346-54. 10.1097/00000658-200203000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen SP, Jones CA, Hoffman MR, et al. Pressure abnormalities in patients with Zenker’s diverticulum using pharyngeal high-resolution manometry. Laryngoscope Investig Otolaryngol 2020;5:708-17. 10.1002/lio2.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiq MA, Sood S, Strachan D. Pharyngeal pouch (Zenker’s diverticulum). Postgrad Med J 2001;77:506-11. 10.1136/pmj.77.910.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen DL, Krutouz A, Bermont A, et al. Trends in the presentation of patients with esophageal diverticula in the era of endoscopy. Eur Surg 2021;53:215-21. [Google Scholar]
- 19.Repici A, Pagano N, Fumagalli U, et al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011;24:235-9. 10.1111/j.1442-2050.2010.01143.x [DOI] [PubMed] [Google Scholar]
- 20.Herbella FA, Patti MG. Modern pathophysiology and treatment of esophageal diverticula. Langenbecks Arch Surg 2012;397:29-35. 10.1007/s00423-011-0843-2 [DOI] [PubMed] [Google Scholar]
- 21.Leong SC, Wilkie MD, Webb CJ. Endoscopic stapling of Zenker’s diverticulum: establishing national baselines for auditing clinical outcomes in the United Kingdom. Eur Arch Otorhinolaryngol 2012;269:1877-84. 10.1007/s00405-012-1945-3 [DOI] [PubMed] [Google Scholar]
- 22.Le Mouel JP, Fumery M. Zenker’s Diverticulum. N Engl J Med 2017;377:e31. 10.1056/NEJMicm1701620 [DOI] [PubMed] [Google Scholar]
- 23.Anagiotos A, Preuss SF, Koebke J. Morphometric and anthropometric analysis of Killian’s triangle. Laryngoscope 2010;120:1082-8. 10.1002/lary.20886 [DOI] [PubMed] [Google Scholar]
- 24.Kaman L, Kundel B, Sinha SK, et al. True epiphrenic diverticulum of esophagus secondary to tubercular adenitis. Indian J Gastroenterol 2003;22:65-6. [PubMed] [Google Scholar]
- 25.Schima W, Scharitzer M, Eisenhuber E, et al. Esophagus: Radiologic Evaluation of Esophageal Function. In: Ekberg O. editor. Dysphagia. Medical Radiology. Springer, Cham., 2017. Available online: https://doi.org/ 10.1007/174_2017_135 [DOI] [Google Scholar]
- 26.Abdollahimohammad A, Masinaeinezhad N, Firouzkouhi M. Epiphrenic esophageal diverticula. J Res Med Sci 2014;19:795-7. [PMC free article] [PubMed] [Google Scholar]
- 27.Shahawy S, Janisiewicz AM, Annino D, et al. A comparative study of outcomes for endoscopic diverticulotomy versus external diverticulectomy. Otolaryngol Head Neck Surg 2014;151:646-51. 10.1177/0194599814541920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delliturri A, Wiesel O, Shaw J, et al. A Narrative Review of update in per oral endoscopic myotomy (POEM) and endoscopic esophageal surgery. Ann Transl Med 2021;9:909. 10.21037/atm-20-5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisichella PM, Jalilvand A, Dobrowolsky A. Achalasia and epiphrenic diverticulum. World J Surg 2015;39:1614-9. 10.1007/s00268-015-2950-7 [DOI] [PubMed] [Google Scholar]
- 30.Gockel I, Rabe SM, Niebisch S. Before and After Esophageal Surgery: Which Information from the Functional Laboratory Is Needed? Visc Med 2018;34:116-21. 10.1159/000486556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi AR, Chon NR, Youn YH, et al. Esophageal cancer in esophageal diverticula associated with achalasia. Clin Endosc 2015;48:70-3. 10.5946/ce.2015.48.1.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao AM, Arnold MR, Schlosser KA, et al. Epiphrenic Diverticulum: 20-Year Single-Institution Experience. Am Surg 2018;84:1159-63. [PubMed] [Google Scholar]
- 33.Carlson DA, Gluskin AB, Mogni B, et al. Esophageal diverticula are associated with propagating peristalsis: a study utilizing high-resolution manometry. Neurogastroenterol Motil 2016;28:392-8. 10.1111/nmo.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barajas-Gamboa JS, Kroh M. Diverticulum: Workup and Evaluation. In: Zundel N, Melvin WS, Patti MG, et al. editors. Benign Esophageal Disease. Springer, Cham., 2021. Available online: https://doi.org/ 10.1007/978-3-030-51489-1_14 [DOI] [Google Scholar]
- 35.Wagh MS, Draganov PV. How to Approach a Patient with a Zenker’s Diverticulum. Gastroenterology 2021;160:10-4. 10.1053/j.gastro.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 36.Howell RJ, Giliberto JP, Harmon J, et al. Open Versus Endoscopic Surgery of Zenker’s Diverticula: A Systematic Review and Meta-analysis. Dysphagia 2019;34:930-8. 10.1007/s00455-019-09994-9 [DOI] [PubMed] [Google Scholar]
- 37.Jain D, Sharma A, Shah M, et al. Efficacy and safety of flexible endoscopic management of Zenker’s diverticulum. J Clin Gastroenterol 2018;52:369-85. 10.1097/MCG.0000000000000977 [DOI] [PubMed] [Google Scholar]
- 38.Costamagna G, Iacopini F, Bizzotto A, et al. Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker’s diverticulum. Gastrointest Endosc 2016;83:765-73. 10.1016/j.gie.2015.08.044 [DOI] [PubMed] [Google Scholar]
- 39.Palanivelu C, Rangarajan M, Maheshkumaar GS, et al. Minimally invasive surgery combined with peroperative endoscopy for symptomatic middle and lower esophageal diverticula: a single institute’s experience. Surg Laparosc Endosc Percutan Tech 2008;18:133-8. 10.1097/SLE.0b013e31815acb97 [DOI] [PubMed] [Google Scholar]
- 40.Johnson CM, Postma GN. Zenker diverticulum-which surgical approach is superior? JAMA Otolaryngol Head Neck Surg 2016;142:401-3. 10.1001/jamaoto.2015.3892 [DOI] [PubMed] [Google Scholar]
- 41.Fasano NC, Levine MS, Rubesin SE, et al. Epiphrenic diverticulum: clinical and radiographic findings in 27 patients. Dysphagia 2003;18:9-15. 10.1007/s00455-002-0075-2 [DOI] [PubMed] [Google Scholar]
- 42.Leeuwenburgh I, Scholten P, Alderliesten J, et al. Long-term esopha¬geal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol 2010;105:2144-9. 10.1038/ajg.2010.263 [DOI] [PubMed] [Google Scholar]
- 43.Bagheri R, Maddah G, Mashhadi MR, et al. Esophageal diverticula: Analysis of 25 cases. Asian Cardiovasc Thorac Ann 2014;22:583-7. 10.1177/0218492313515251 [DOI] [PubMed] [Google Scholar]
- 44.van Overbeek JJ. Pathogenesis and methods of treatment of Zenker’s diverticulum. Ann Otol Rhinol Laryngol 2003;112:583-93. 10.1177/000348940311200703 [DOI] [PubMed] [Google Scholar]
- 45.Tao TY, Menias CO, Herman TE, et al. Easier to swallow: pictorial review of structural findings of the pharynx at barium pharyngography. Radiographics 2013;33:e189-208. 10.1148/rg.337125153 [DOI] [PubMed] [Google Scholar]
- 46.Levine MS, Rubesin SE. Radiology of the pharynx and esophagus. The Esophagus 2021:97-147. [Google Scholar]
- 47.Rubesin SE, Levine MS. Pharyngeal manifestations of gastroesophageal reflux disease. Abdom Radiol (NY) 2018;43:1294-305. 10.1007/s00261-018-1471-x [DOI] [PubMed] [Google Scholar]
- 48.Mortimer AM, Corrigan A, Roach H, et al. Fluoroscopic evaluation of the association between Zenker’s diverticulum and oesophageal dysfunction: a case-control trial. European Congress of Radiology-ECR 2010, 2010. Available online: https://dx.doi.org/ 10.1594/ecr2010/C-3315 [DOI] [Google Scholar]
- 49.Caso R, Chang H, Marshall MB. Evolving options in management of minimally invasive diverticular disease: a single surgeon’s experience and review of the literature. J Laparoendosc Adv Surg Tech A 2019;29:780-4. 10.1089/lap.2018.0711 [DOI] [PubMed] [Google Scholar]
- 50.Shintaku M, Shintaku M, Torii I. Development of Epidermoid Metaplasia of the Mucosa in Association with Esophageal Intramural Pseudodiverticulosis and Candidiasis. Case Rep Gastroenterol 2021;15:709-14. 10.1159/000518023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alameri A, Sharman T. Esophageal Intramural Pseudodiverticulosis. 2022 Feb 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 52.Bechtler M, Vollmer H, Vetter S, et al. Long-term follow-up after dilation in symptomatic esophageal intramural pseudodiverticulosis: an observational study in 22 cases. Endoscopy 2014;46:795-7. 10.1055/s-0034-1377304 [DOI] [PubMed] [Google Scholar]
- 53.Lee DW, Kim JH, Lee YM, et al. A Rare Coincidence of Esophageal Intramural Pseudodiverticulosis with Esophageal Web. The Korean Journal of Helicobacter and Upper Gastrointestinal Research 2015;15:196-9. [Google Scholar]
- 54.Halm U, Lamberts R, Knigge I, et al. Esophageal intramural pseudodiverticulosis: endoscopic diagnosis and therapy. Dis Esophagus 2014;27:230-4. 10.1111/dote.12104 [DOI] [PubMed] [Google Scholar]
- 55.Westcott CJ, O’Connor S, Preiss JE, et al. Myotomy-First Approach to Epiphrenic Esophageal Diverticula. J Laparoendosc Adv Surg Tech A 2019;29:726-9. 10.1089/lap.2019.0239 [DOI] [PubMed] [Google Scholar]
- 56.Lai ST, Hsu CP. Carcinoma arising from an epiphrenic diverticulum: a frequently misdiagnosed disease. Ann Thorac Cardiovasc Surg 2007;13:110-3. [PubMed] [Google Scholar]
- 57.Kwak JY, Kim EK. Sonographic findings of Zenker diverticula. J Ultrasound Med 2006;25:639-42. 10.7863/jum.2006.25.5.639 [DOI] [PubMed] [Google Scholar]
- 58.Muhletaler CA, Lams PM, Johnson AC. Occurrence of oesophageal intramural pseudodiverticulosis in patients with pre-existing benign oesophageal stricture. Br J Radiol 1980;53:299-303. 10.1259/0007-1285-53-628-299 [DOI] [PubMed] [Google Scholar]
- 59.Shebrain S. Evaluation of Esophageal Diverticula. In: Grams J, Perry K, Tavakkoli A. editors. The SAGES Manual of Foregut Surgery. Springer, Cham., 2019. Available online: https://doi.org/ 10.1007/978-3-319-96122-4_43 [DOI] [Google Scholar]
- 60.Strong AT, Ponsky JL. Esophageal Diverticula. In: Zundel N, Melvin WS, Patti MG, et al. editors. Benign Esophageal Disease. Springer, Cham., 2021. Available online: https://doi.org/ 10.1007/978-3-030-51489-1_15 [DOI] [Google Scholar]
- 61.Chen X, Liu JF, Gu CJ, et al. Ultrasonographic characteristics of Killian-Jamieson diverticula. J Clin Ultrasound 2021;49:527-32. 10.1002/jcu.23011 [DOI] [PubMed] [Google Scholar]
- 62.Kim HK, Lee JI, Jang HW, et al. Characteristics of Killian-Jamieson diverticula mimicking a thyroid nodule. Head Neck 2012;34:599-603. 10.1002/hed.21575 [DOI] [PubMed] [Google Scholar]
- 63.Kim DC, Hwang JJ, Lee WS, et al. Surgical treatment of killian-jamieson diverticulum. Korean J Thorac Cardiovasc Surg 2012;45:272-4. 10.5090/kjtcs.2012.45.4.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeFriend DE, Dubbins PA. Sonographic demonstration of a pharyngoesophageal diverticulum. J Clin Ultrasound 2000;28:485-7. [DOI] [PubMed] [Google Scholar]
- 65.Ota K, Onoe M, Oka M, et al. Killian-Jamieson diverticulum mimicking a thyroid nodule: A case report. J Gen Fam Med 2019;20:62-4. 10.1002/jgf2.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart KE, Smith DRK, Woolley SL. Simultaneously occurring Zenker’s diverticulum and Killian-Jamieson diverticulum: case report and literature review. J Laryngol Otol 2017;131:661-6. 10.1017/S0022215117001268 [DOI] [PubMed] [Google Scholar]
- 67.Fernando HC, Luketich JD, Samphire J, et al. Minimally invasive operation for esophageal diverticula. Ann Thorac Surg 2005;80:2076-80. 10.1016/j.athoracsur.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 68.Ba-Ssalamah A, Fueger BJ, Schima W. Cross-Sectional Imaging of the Oesophagus Using CT and PET/Techniques. In: Ekberg O. editor. Dysphagia. Medical Radiology. Berlin, Heidelberg: Springer, 2012. Available online: https://doi.org/ 10.1007/174_2012_656 [DOI] [Google Scholar]
- 69.Scharitzer M, Pokieser P. What is the role of radiological testing of lower esophageal sphincter function? Ann N Y Acad Sci 2016;1380:67-77. 10.1111/nyas.13181 [DOI] [PubMed] [Google Scholar]
- 70.Mantsopoulos K, Psychogios G, Karatzanis A, et al. Clinical relevance and prognostic value of radiographic findings in Zenker’s diverticulum. Eur Arch Otorhinolaryngol 2014;271:583-8. 10.1007/s00405-013-2562-5 [DOI] [PubMed] [Google Scholar]
- 71.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. 10.1111/nmo.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morales-Divo C, Jecker P, Lippert B, et al. Extraesophageal reflux in patients suffering from Zenker’s diverticulum. HNO 2007;55:546-50. 10.1007/s00106-006-1469-5 [DOI] [PubMed] [Google Scholar]
- 73.Yuan Y, Zhao YF, Hu Y, et al. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013;30:207-18. 10.1159/000351433 [DOI] [PubMed] [Google Scholar]
- 74.Sato H, Takeuchi M, Hashimoto S, et al. Esophageal diverticulum: New perspectives in the era of minimally invasive endoscopic treatment. World J Gastroenterol 2019;25:1457-64. 10.3748/wjg.v25.i12.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato H, Takeuchi M, Terai S. Gastrointestinal: Endoscopic diverticulectomy for the treatment of Zenker’s diverticulum with a unique “tip”: A first case report in Japan. J Gastroenterol Hepatol 2019;34:1272. 10.1111/jgh.14599 [DOI] [PubMed] [Google Scholar]
- 76.Ishaq S, Sultan H, Siau K, et al. New and emerging techniques for endoscopic treatment of Zenker’s diverticulum: State-of-the-art review. Dig Endosc 2018;30:449-60. 10.1111/den.13035 [DOI] [PubMed] [Google Scholar]
- 77.Chang CY, Payyapilli RJ, Scher RL. Endoscopic staple diverticulostomy for Zenker’s diverticulum: review of literature and experience in 159 consecutive cases. Laryngoscope 2003;113:957-65. 10.1097/00005537-200306000-00009 [DOI] [PubMed] [Google Scholar]
- 78.Naunheim M, Merati AL, Weissbrod PA. Open Surgery for Zenker Diverticulum. Management of Zenker and Hypopharyngeal Diverticula. Springer, Cham, 2018:39-55. [Google Scholar]
- 79.Al Ghamdi SS, Farha J, Moran RA, et al. Zenker’s peroral endoscopic myotomy, or flexible or rigid septotomy for Zenker’s diverticulum: a multicenter retrospective comparison. Endoscopy 2022;54:345-51. [DOI] [PubMed] [Google Scholar]
- 80.Counter PR, Hilton ML, Baldwin DL. Long-term follow-up of endoscopic stapled diverticulotomy. Ann R Coll Surg Engl 2002;84:89-92. [PMC free article] [PubMed] [Google Scholar]
- 81.Vogelsang A, Preiss C, Neuhaus H, et al. Endotherapy of Zenker’s diverticulum using the needle-knife technique: long-term follow-up. Endoscopy 2007;39:131-6. 10.1055/s-2006-944657 [DOI] [PubMed] [Google Scholar]
- 82.Bonavina L, Aiolfi A, Scolari F, et al. Long-term outcome and quality of life after transoral stapling for Zenker diverticulum. World J Gastroenterol 2015;21:1167-72. 10.3748/wjg.v21.i4.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamal F, Khan MA, Lee-Smith W, et al. Peroral Endoscopic Myotomy Is a Safe and Feasible Option in Management of Esophageal Diverticula: Systematic Review and Meta-Analysis. Dig Dis Sci 2021;66:3242-9. 10.1007/s10620-020-06678-5 [DOI] [PubMed] [Google Scholar]
- 84.Diez Redondo P, Nunez Rodriguez H, de Benito SM, et al. Endoscopic treatment of Zenker’s diverticulum with Ligasure: simple, safe and effective. Endosc Int Open 2019;7:E203-8. 10.1055/a-0809-4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albers DV, Kondo A, Bernardo WM, et al. Endoscopic versus surgical approach in the treatment of Zenker’s diverticulum: systematic review and meta-analysis. Endosc Int Open 2016;4:E678-86. 10.1055/s-0042-106203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miutescu BP, Khan S, Mony S, et al. Role of Peroral Endoscopic Myotomy (POEM) in the Management of Esophageal Diverticula. Clin Endosc 2020;53:646-51. 10.5946/ce.2020.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basile P, Gonzalez JM, Le Mouel JP, et al. Per-oral endoscopic myotomy with septotomy for the treatment of distal esophageal diverticula (D-POEM). Surg Endosc 2020;34:2321-5. 10.1007/s00464-019-07354-0 [DOI] [PubMed] [Google Scholar]
- 88.Yang J, Zeng X, Yuan X, et al. An international study on the use of peroral endoscopic myotomy (POEM) in the management of esophageal diverticula: the first multicenter D-POEM experience. Endoscopy 2019;51:346-9. 10.1055/a-0759-1428 [DOI] [PubMed] [Google Scholar]
- 89.Li LY, Yang YT, Qu CM, et al. Endoscopic needle-knife treatment for symptomatic esophageal Zenker’s diverticulum: a meta-analysis and systematic review. J Dig Dis 2018;19:204-14. 10.1111/1751-2980.12588 [DOI] [PubMed] [Google Scholar]
- 90.Varghese TK, Jr, Marshall B, Chang AC, et al. Surgical treatment of epiphrenic diverticula: a 30-year experience. Ann Thorac Surg 2007;84:1801-9; discussion 1801-9. 10.1016/j.athoracsur.2007.06.057 [DOI] [PubMed] [Google Scholar]
- 91.Soares R, Herbella FA, Prachand VN, et al. Epiphrenic diverticulum of the esophagus. From pathophysiology to treatment. J Gastrointest Surg 2010;14:2009-15. 10.1007/s11605-010-1216-9 [DOI] [PubMed] [Google Scholar]
- 92.Rosati R, Fumagalli U, Bona S, et al. Diverticulectomy, myotomy, and fundoplication through laparoscopy: a new option to treat epiphrenic esophageal diverticula? Ann Surg 1998;227:174-8. 10.1097/00000658-199802000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melman L, Quinlan J, Robertson B, et al. Esophageal manometric characteristics and outcomes for laparoscopic esophageal diverticulectomy, myotomy, and partial fundoplication for epiphrenic diverticula. Surg Endosc 2009;23:1337-41. 10.1007/s00464-008-0165-9 [DOI] [PubMed] [Google Scholar]
- 94.Del Genio A, Rossetti G, Maffetton V, et al. Laparoscopic approach in the treatment of epiphrenic diverticula: long-term results. Surg Endosc 2004;18:741-5. 10.1007/s00464-003-9044-6 [DOI] [PubMed] [Google Scholar]
- 95.Rosati R, Fumagalli U, Elmore U, et al. Long-term results of minimally invasive surgery for symptomatic epiphrenic diverticulum. Am J Surg 2011;201:132-5. 10.1016/j.amjsurg.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 96.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. 10.1055/s-0029-1244080 [DOI] [PubMed] [Google Scholar]
- 97.Sato H, Sato Y, Takeuchi M, et al. Salvage peroral endoscopic myotomy for esophageal diverticulum. Endoscopy 2015;47 Suppl 1 UCTN:E14-5. [DOI] [PubMed]
- 98.Talukdar R, Inoue H, Nageshwar Reddy D. Efficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysis. Surg Endosc 2015;29:3030-46. 10.1007/s00464-014-4040-6 [DOI] [PubMed] [Google Scholar]
- 99.Akintoye E, Kumar N, Obaitan I, et al. Peroral endoscopic myotomy: a meta-analysis. Endoscopy 2016;48:1059-68. 10.1055/s-0042-114426 [DOI] [PubMed] [Google Scholar]
- 100.Triggs JR, Krause AJ, Carlson DA, et al. Blown-out myotomy: an adverse event of laparoscopic Heller myotomy and peroral endoscopic myotomy for achalasia. Gastrointest Endosc 2021;93:861-868.e1. 10.1016/j.gie.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halder S, Acharya S, Kou W, et al. Myotomy technique and esophageal contractility impact blown-out myotomy formation in achalasia: an in silico investigation. Am J Physiol Gastrointest Liver Physiol 2022;322:G500-12. 10.1152/ajpgi.00281.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halder S, Acharya S, Kou W, et al. Mechanics informed fluoroscopy of esophageal transport. Biomech Model Mechanobiol 2021;20:925-40. 10.1007/s10237-021-01420-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klaus A, Hinder RA, Swain J, et al. Management of epiphrenic diverticula. J Gastrointest Surg 2003;7:906-11. 10.1007/s11605-003-0038-4 [DOI] [PubMed] [Google Scholar]
- 104.Zaninotto G, Portale G, Costantini M, et al. Long-term outcome of operated and unoperated epiphrenic diverticula. J Gastrointest Surg 2008;12:1485-90. 10.1007/s11605-008-0570-3 [DOI] [PubMed] [Google Scholar]
- 105.Castrucci G, Porziella V, Granone PL, et al. Tailored surgery for esophageal body diverticula. Eur J Cardiothorac Surg 1998;14:380-7. 10.1016/s1010-7940(98)00201-2 [DOI] [PubMed] [Google Scholar]
- 106.Constantinoiu S, Constantin A, Predescu D, et al. Surgical treatment of esophageal diverticula. Chirurgia (Bucur) 2011;106:37-43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as