Abstract
Exopolysaccharide (EPS)-producing Bifidobacterium bifidum EPS DA-LAIM was isolated from healthy human feces, the structure of purified EPS from the strain was analyzed, and its prebiotic activity was evaluated. The EPS from B. bifidum EPS DA-LAIM is a glucomannan-type heteropolysaccharide with a molecular weight of 407–1007 kDa, and its structure comprises 2-mannosyl, 6-mannosyl, and 2,6-mannosyl residues. The purified EPS promoted the growth of representative lactic acid bacteria and bifidobacterial strains. Bifidobacterium bifidum EPS DA-LAIM increased nitric oxide production in RAW 264.7 macrophage cells, indicating its immunostimulatory activity. Bifidobacterium bifidum EPS DA-LAIM also exhibited high gastrointestinal tract tolerance, gut adhesion ability, and antioxidant activity. These results suggest that EPS from B. bifidum EPS DA-LAIM is a potentially useful prebiotic material, and B. bifidum EPS DA-LAIM could be applied as a probiotic candidate.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01213-w.
Keywords: Exopolysaccharide, Bifidobacterium bifidum, Prebiotics, Probiotics, Immunostimulatory activity
Introduction
The genus Bifidobacterium includes commensal microorganisms that are commonly found in the human gut (Hidalgo-Cantabrana et al., 2015). Bifidobacteria have been used as probiotics because of their scientific relationship with the host’s health benefits (Hill et al., 2014). Regarding intestinal microbial homeostasis, bifidobacteria exhibit antimicrobial activity against pathogens and inhibit or reduce cancer formation (Coakley et al., 2009).
Exopolysaccharides (EPS) are extracellular layers of carbohydrates found in microorganisms and are polymers attached to the extracellular surface of the cell-forming slime (Hidalgo-Cantabrana et al., 2015). Some bacteria are covered with a layer of polysaccharides called the glycocalyx. Recently, demand for and interest in bacterial-derived EPS have been increasing due to their favorable effects on health issues (Amiri et al., 2019; Nachtigall et al., 2021). EPS production has been linked to the producer microorganism and its host through several physiological pathways (He and Bauer, 2014). EPS produced by bifidobacteria can cope with bacterial toxins and pathogens and have a beneficial effect on the host (Garrigues et al., 2010). EPS is a postbiotic secreted by lactic acid bacteria and bifidobacteria that can interact with host cells by acting as ligands and defend the host by aggregating with pathogens in the intestine (Castro-Bravo et al., 2018). Additionally, oxidative stress protection and anti-cancer properties of EPS have been discovered (Saadat et al., 2019).
EPS can be classified into two types depending on the composition of the repeating unit: homopolysaccharides (HoPS) and heteropolysaccharides (HePS) (Ryan et al., 2015). HoPS and HePS differ in whether they are composed of one type of monosaccharide or more than two types of monosaccharides (Ryan et al., 2015). Although previous studies have focused on EPS from lactic acid bacteria, only a few bifidobacterial polymers have been characterized and purified so far (Ruas-Madiedo et al., 2012). EPS described in bifidobacteria is the HePS type, and its main monosaccharide constituents are glucose, galactose, and rhamnose, which are the same as those identified in EPS produced by lactic acid bacteria (Hidalgo-Cantabrana et al., 2015). It is reported that there are two types of EPS, ropy type and non-ropy type, and EPS produced from the ropy type-producing B. breve provides resistance to acid and bile-salt (Fanning et al. 2012). Ropy type EPS-producing strains produce long filaments or strands due to the high viscosity of EPS when extended with an inoculation loop.
EPS is the polysaccharide produced by microorganisms in the largest amount, and it has the highest commercial potential due to its easy recovery from culture and low purification cost (Nachtigall et al., 2021). Previous studies on EPS have mainly focused on its industrial applications, including emulsification, texturization, sweetening, gelling, and water-binding capacity. However, researchers are currently focusing on the bioactive properties of EPS. Additionally, recent research has shown that EPS can improve health through its immunomodulatory, prebiotic, anti-inflammatory, antibiofilm, and antioxidant effects (Amiri et al., 2019).
In this study, Bifidobacterium bifidum, which produces exopolysaccharides, especially ropy-type EPS, in high yield was isolated from human feces, and its prebiotic and immunostimulatory activities, as well as probiotic properties such as acid tolerance, bile salt tolerance, pancreatin-tolerance, and gut adhesion ability, were evaluated.
Materials and methods
Bacterial strains and medium
Bifidobacterium bifidum EPS DA-LAIM, B. bifidum KCTC 13270BP, B. bifidum DLP1224, Lactobacillus helveticus DLP1181, Lactiplantibacillus plantarum DLP1110, Limosilactobacillus fermentum DLP1406, Bifidobacterium bifidum DLP1224, and B. animalis DLP1267 were cultivated in De Man, Rogosa, and Sharp medium (MRS, Oxoid, Hampshire, UK) (10 g protease peptone No. 3, 10 g beef extract, 5 g yeast extract, 20 g dextrose, 1 g polysorbate 80, 2 g ammonium citrate, 5 g sodium acetate, 0.1 g magnesium sulfate, 0.05 g manganese sulfate, 2 g dipotassium hydrogen phosphate, per liter) or BL medium (MB cell, Seoul, Korea) at 37 °C for 24 h under anaerobic conditions. For EPS production, mMRS medium (MRS medium without glucose) or BL medium was supplemented with sucrose, and the cells were incubated at 37 °C under anaerobic conditions. B. bifidum KCTC 13270BP was purchased from the Korean Collection for Type Cultures (Daejeon, Republic of Korea).
Escherichia coli was cultivated in tryptic soy broth (TSB, BD, Heidelberg, Germany) (17 g tryptone (pancreatic digest of casein), 3 g soytone (peptic digest of soybean), 2.5 g glucose, 5 g sodium chloride, 2.5 g dipotassium phosphate, pH 7.3, per l).
Isolation of Bifidobacterium strains
After collecting feces from healthy Korean volunteers, the feces were serially diluted using sterile 0.85%(w/v) NaCl, and then, 100 μL samples were spread onto a transgalactosylated oligosaccharide-mupirocin (TOS-MUP) selective agar plate (Bifidobacterium selective media)(MB cell, Seoul, Korea) (10 g tryptone, 1 g yeast extract, 3 g monopotassium phosphate, 4.8 g dipotassium phosphate, 3 g ammonium sulfate, 0.2 g magnesium sulfate heptahydrate, 0.5 g l-cysteine-HCl, 15 g sodium propionate, 10 g TOS, per liter), which was incubated at 37 °C for 48 h under anaerobic conditions. Colonies grown on TOS-MUP-selective agar plates were transferred to a new TOS-MUP-selective agar plate for pure culture, and 129 Bifidobacterium sp. candidate strains were selected. The design of this study was reviewed by the Public Institutional Review Board designated by the Ministry of Health and Welfare (IRB approval number: P01-202102-33-002).
Identification of Bifidobacterium strain
Genomic DNA was isolated from Bifidobacterium strains as previously described (Sharma et al., 2020). PCR amplification and nucleotide sequence analysis of the 16S rRNA gene were performed by Macrogen, Inc. (Seoul, Korea). Phylogenetic tree analysis was carried out as previously described (Kwon and Park, 2021).
Production of EPS from B. bifidum EPS DA-LAIM and removal of EPS
To produce EPS, a single colony of B. bifidum EPS DA-LAIM was inoculated into mMRS medium or BL medium supplemented with 2–10%(w/v) sucrose and incubated at 37 °C for 24–96 h under anaerobic conditions. EPS were removed from the cells by centrifugation at 11,000 × g for 20 min, and the cell pellet was washed with 0.85%(w/v) NaCl or PBS three times.
Crystal violet staining of EPS
Bifidobacterium bifidum EPS DA-LAIM was cultured at 37 °C for 96 h under anaerobic conditions in BL medium containing 5%(w/v) sucrose or 5%(w/v) glucose. Crystal violet staining of EPS was performed by exposing heat-fixed cells to crystal violet diluted with a half volume of distilled water for at least 1 min.
Scanning electron microscopy of B. bifidum EPS DA-LAIM
To prepare the EPS-containing B. bifidum EPS DA-LAIM sample, a bacterial colony was inoculated in mMRS broth with 10%(w/v) sucrose and incubated at 37 °C for 48–72 h under anaerobic conditions. To prepare EPS-removed B. bifidum EPS DA-LAIM samples, the culture was centrifuged at 11,000 × g for 20 min, and the pellet was washed three times with phosphate-buffered saline (PBS). The sample was centrifuged at 21,200 × g for 5 min for samples with or without EPS, fixed with 2.5%(v/v) glutaraldehyde for 8 h, and washed twice with 0.1 M phosphate buffer (pH 7.4) for 15 min each. For the second fixation, 1%(w/v) osmium tetroxide solution was used for 1 h and rinsed with 0.1 M phosphate buffer (pH 7.4) for 15 min. The morphology of B. bifidum EPS DA-LAIM was examined using a scanning electron microscope (SEM, H-7600, Hitachi, Tokyo, Japan) installed at Eulji University (Seongnam, Korea).
Purification of EPS
Bifidobacterium bifidum EPS DA-LAIM was cultured in mMRS broth supplemented with 10%(w/v) sucrose at 37 °C for 48–72 h anaerobically. The culture was centrifuged at 11,000 × g at 4 °C for 20 min. Trichloroacetic acid was added to a final concentration of 10%(w/v), and the EPS was precipitated at 4 °C for 20 h. The solution was centrifuged at 11,000 × g at 4 °C for 20 min and filtered using a 0.45-μm SmartPor®-11PVDF syringe filter (Woongki Science Co., Ltd., Seoul, Korea). Two volumes of ice-cold 100% ethanol were added to the filtrate and incubated at 4 °C for 24 h. The precipitate was collected by centrifugation at 4 °C for 20 min at 11,000 × g, dissolved in distilled water, and dialyzed using dialysis-sack (molecular weight cut-off 12–14 kDa, Spectra/Por® membrane, Pre-wetted RC Tubing, Spectrum Laboratories, Inc., Broadwick Street, UK) for 24 h. Dialyzed EPS was then freeze-dried using a vacuum freeze-dryer (SUNILEYELA Co., Ltd., Seongnam, Korea).
Monosaccharide composition analysis by high-performance anion-exchange chromatography
EPS from B. bifidum EPS DA-LAIM was completely hydrolyzed to monosaccharides using 2 M trifluoroacetic acid (TFA) for 2 h. The residual acid was removed by flushing with N2 gas, re-dissolving the dried precipitate in deionized water, and filtering through a 0.45 μm nylon syringe filter (GVS, Indianapolis, IN, USA). The filtered samples were analyzed using a high-performance anion-exchange chromatography (HPAEC) system (Dionex, Sunnyvale, CA, USA). A CarboPac PA-1 anion-exchange column and PA-1 guard column (Thermo Fisher Scientific, Waltham, MA, USA) were used to separate the monosaccharides. Twenty millimolar NaOH was used for isocratic separation of eluent (Hemmerich et al., 1994; Panagiotopoulos et al., 2001), and 120 mM NaOAc in 100 mM NaOH and 200 mM NaOH were used for residual washing and re-equilibrium processes before the next sample injection at 25 °C. Each monosaccharide used to identify the peak retention time was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Molecular weight analysis by high-performance size-exclusion chromatography
EPS from B. bifidum EPS DA-LAIM was dissolved in deionized water to make a 0.2%(w/v) solution, followed by filtering through a 0.45 μm nylon syringe filter (GVS). The filtered sample solution was analyzed using a high-performance size-exclusion chromatography (HPSEC) system (Ultimate 3000, Thermo Fisher Scientific) with a refractive index detector (RID, Thermo Fisher Scientific) at 50 °C. Shodex OHpak SB-804 and SB-802.5 HQ analytical columns (Shodex, Tokyo, Japan) were used for chromatographic separation in a column oven at 50 °C, and filtered deionized water (18 mΩ) was used as the column eluent at 0.4 a flow rate (Alves et al., 2010). A standard curve was constructed using the P-82 pullulan standard (Shodex).
Methylation analysis for glycosidic linkage types by gas-chromatography mass-spectrometer
EPS from the B. bifidum EPS DA-LAIM sample was permethylated using a NaOH/DMSO slurry with methyl iodide and separated into a dichloromethane layer for washing to obtain methyl derivatives. The O-methyl-derivative sample was fully hydrolyzed by 2 M trifluoroacetic acid (TFA), followed by the removal of the residual TFA by adding methanol and flushing with N2 gas. The hydrolyzed residues were dissolved in NH4OH and reduced with NaBD4 to form linear sugars. The remaining hydroxyl groups were acetylated using acetic anhydride at 100 °C for 2.5 h and terminated by the addition of deionized water. Partially methylated alditol acetate (PMAAs) derivatives were separated into dichloromethane layers and dried by flushing with N2 gas. The dried sample was re-dissolved in acetone as the final solvent for gas chromatography-mass spectrometry (GC–MS) analysis. The PMAAs were separated using a GC–MS system (Thermo Scientific ™ ISQ 7000, Waltham, MA, USA) with a TraceGOLD TG-5MS GC column (Thermo Scientific™; 0.25 μm thickness; 0.25 mm ID; 30 m length). The analytical conditions was gradient mode: 50–300 °C at 10 °C/min with He as the carrier gas. Each PMAA peak was identified by referring to the PMAA mass spectral database of the Complex Carbohydrate Research Center (https://glygen.ccrc.uga.edu/ccrc/specdb/ms/pmaa/pframe.html).
Acid-tolerance
The Bifidobacterium strain was cultured in MRS broth supplemented with 2%(w/v) sucrose and incubated at 37 °C for 24 h anaerobically. For both EPS-containing and EPS-free bacterial samples, an MRS medium containing 2%(w/v) sucrose with 1,000 units pepsin/ml (Sigma-Aldrich) at pH 3.0 was used. EPS-containing samples were inoculated at a final concentration of 1%(v/v) in the medium. The EPS-removed bacterial sample was centrifuged at 11,000 × g for 20 min, washed three times with 0.85%(w/v) NaCl, and resuspended in the medium. The samples were serially diluted and spread on MRS agar as a control (0 h). After 2 h incubation at 37 °C anaerobically, the EPS-containing sample and EPS-removed sample were serially diluted using 0.85%(w/v) NaCl and spread onto MRS agar as a sample (2 h). Agar plates were incubated at 37 °C for 48 h, and the viable cells were counted. Acid tolerance was calculated using Eq. (1).
| 1 |
Bile-tolerance
The Bifidobacterium strain was cultured as described in the acid-tolerance section. For both EPS-containing and EPS-free bacterial samples, an MRS medium containing 2%(w/v) sucrose and 0.3%(w/v) Bacto Oxgall (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used. The EPS-containing and EPS-free samples were treated as described in the acid-tolerance section. The samples were serially diluted and spread on MRS agar as a control (0 h). After 3 h incubation at 37 °C anaerobically, the EPS-containing sample and EPS-free sample were serially diluted using 0.85%(w/v) NaCl and spread onto MRS agar as a sample (3 h). Agar plates were incubated at 37 °C for 48 h anaerobically, and the viable cells were counted. Bile tolerance was calculated using Eq. (2).
| 2 |
Pancreatin tolerance
The Bifidobacterium strain was cultured as described in the acid-tolerance section. For both EPS-containing and EPS-free bacterial samples, MRS broth supplemented with 2%(w/v) sucrose and 0.5% pancreatin (Sigma-Aldrich) was used. The EPS-containing and EPS-free samples were treated as described in the bile-tolerance section. Pancreatin-tolerance was calculated using Eq. (3).
| 3 |
DPPH radical scavenging activity
For the assay, a 0.2 mM DPPH solution dissolved in 1 ml ethanol was added to the bacterial cell pellet (5 × 108 CFU) dissolved in 1 ml PBS. The mixed solution was allowed to react at 25 °C for 30 min in the dark, and the reaction solution was centrifuged at 13,500 × g for 3 min at 4 °C. The supernatant was added to each well of a 96-well plate, and the absorbance was measured at 517 nm. Ascorbic acid (0.1 mg/ml) was used as a positive control. The DPPH radical scavenging activity was calculated using Eq. (4).
| 4 |
Gut adhesion ability assay using Caco-2 cells
The Bifidobacterium strain was cultured in 5 ml of MRS medium supplemented with 2%(w/v) sucrose at 37 °C for 24 h. To prepare EPS-containing samples, bacterial cell pellet was obtained by centrifugation at 5,000 × g for 3 min and resuspended in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (Corning) adjusted with pH 6.0. To prepare the EPS-free samples, the cell pellet was prepared by centrifuging 1 ml of the bacterial culture at 11,000 × g for 20 min. The pellet was washed three times with Dulbecco's phosphate buffered saline (DPBS; Welgene, Gyeongsan, Korea) and resuspended in 1 ml Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (Corning) adjusted with pH 6.0. Cultivation of Caco-2 cells and treatment of bacterial cells were performed as described previously (Kwon and Park, 2021). The adhesion ability was calculated using Eq. (5).
| 5 |
Prebiotic index
One hundred microliters of mMRS broth or TSB broth without glucose supplemented with 2%(w/v) EPS was dispensed into a 96-well plate, and the cultures of, Lactobacillus helveticus,, Lactiplantibacillus plantarum, Limosilactobacillus fermentum, B. bifidum, and B. animalis were inoculated at a final concentration of 1%(w/v). For comparative experiments, the same experiment was conducted on mMRS broth or TSB broth without glucose with 2%(w/v) inulin, and the same experiments were conducted using a culture medium without a carbon source or with 2%(v/v) glucose as a control. The prebiotic index was calculated using Eq. (6):
| 6 |
Determination of cytotoxicity on RAW 264.7 cells
The cytotoxicity and proliferation of B. bifidum EPS DA-LAIM cells and EPS in a RAW 264.7 murine macrophage cell line were evaluated by GeoVista. RAW 264.7 cells were seeded into DMEM to make 1 × 105 cells per well and incubated at 37 °C with 5% CO2. RAW 264.7 macrophage cells were treated with B. bifidum EPS DA-LAIM cells at 1.0 × 106 cells and treated with EPS at concentrations of 1, 10, 25, and 50 μg/ml. RAW 264.7 cells were treated with LPS at a final concentration of 1 μg/ml as a positive control. After sample treatment, cells were incubated at 37 °C with 5% CO2 for 24 and 48 h. After 24 and 48 h of sample treatment, cell viability of RAW 264.7 cells was measured using the MTT assay.
Determination of nitric oxide production and expression of cytokines
To measure the nitric oxide (NO) production, RAW 264.7 cells were seeded at 5 × 105 cells per well in a 6-well plate and incubated at 37 °C with 5% CO2 for 20 h, and RAW 264.7 cells were treated with B. bifidum EPS DA-LAIM and EPS with the same method as that used for the determination of cytotoxicity. RAW 264.7 cells treated with 1 μg/ml lipopolysaccharide (LPS) were used as a positive control. NO secretion and the expression of cytokines IL-1β and IL-6 were determined using Griess Reagent System (G2930, Promega, Madison, WI, USA), Mouse IL-1β ELISA Kit (ab100704, Abcam, Cambridge, UK), and Mouse IL-6 ELISA Kit (ab100712, Abcam), respectively.
Whole genome sequencing of Bifidobacterium bifidum EPS DA-LAIM
Whole-genome sequencing of B. bifidum EPS DA-LAIM was performed by CJ Bioscience Inc. (Seoul, Korea) using the Illumina MiSeq platform (Illumina, San Diego, CA, USA). Genomic sequences were screened for the presence of antibiotic resistance genes using ResFinder v.4.1 software and the Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca/), and the resistance gene identifier software RGI v.5.2.0. ResFinder analysis was performed by default, and the RGI analysis criteria were perfect, strict, and loose hits. Virulence genes were retrieved using VirulenceFinder 2.0. An analytical database system was provided by the Center for Genomic Epidemiology (CGE). Homologous sequences to virulence genes of Escherichia coli, Listeria, Staphylococcus aureus, and Enterococcus were screened, and thresholds for percent identity (%ID) and minimum length were set at 90% and 60%, respectively.
Antibiotic susceptibility test
Antibiotic susceptibility was determined using an E-test strip (ETEST®, bioMérieux, Marcy-l'Étoile, France), according to the manufacturer’s instructions. The minimum inhibitory concentration (MIC) of the antibiotics was compared with the cut-off values for each antibiotic, guided by the European Food Safety Authority (EFSA, 2012).
Statistical analysis
All experiments were performed in triplicate, and the results are expressed as the mean ± standard deviation (SD). Statistical significance of the experimental data was verified by one-way analysis of variance (ANOVA) using SPSS 25 (SPSS Inc., Chicago, IL, USA). Dunnett’s test and Duncan’s test were used to determine significant differences between multiple groups at p < 0.05.
Results and discussion
Isolation and identification of B. bifidum EPS DA-LAIM
To screen for EPS-producing bifidobacteria, 129 strains were isolated from human fecal samples. Among them, the strain EPS DA-LAIM was selected for this study because it has the highest EPS production rate and ropiness. Strain EPS DA-LAIM was identified as Bifidobacterium bifidum by phylogenetic tree analysis based on the nucleotide sequence of the 16S rRNA gene (Fig. S1).
Visualization of EPS
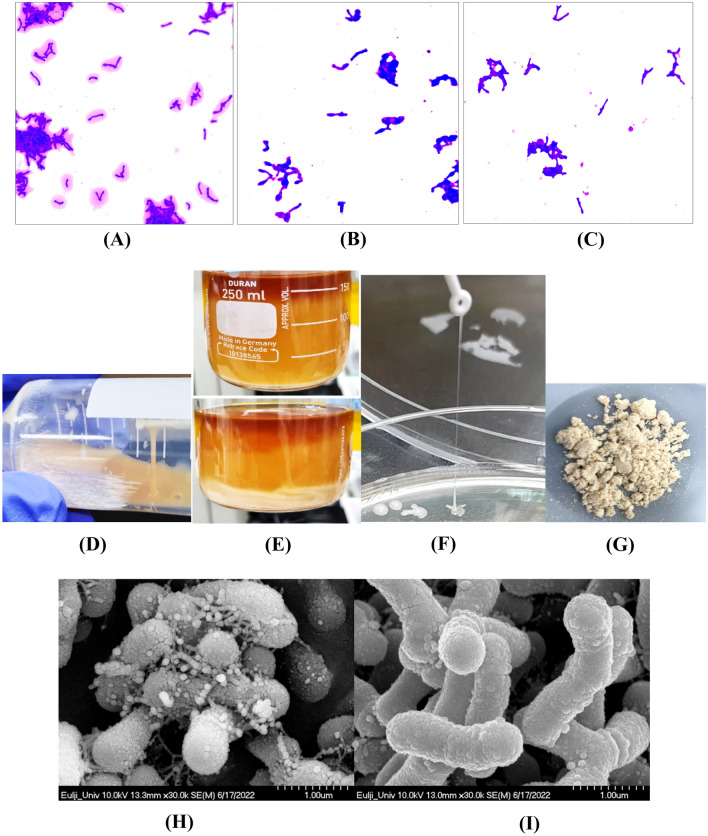
Because the selected B. bifidum EPS DA-LAIM strain produced significant quantities of EPS, the EPS was visible by crystal violet staining. B. bifidum EPS DA-LAIM cells and the EPS around the cells were stained purple with crystal violet, and the stained area was more obvious when the cells were cultured with sucrose supplementation than when cultured with glucose supplementation (Fig. 1A and B). Bifidobacterium bifidum DLP1224, an EPS-non-producing bacterium, was not stained around the cells (Fig. 1C). The culture of B. bifidum EPS DA-LAIM was highly viscous due to the ropy EPS, and three-layer separation of culture medium-EPS-bacteria cells was observed in the culture (Fig. 1D and E).
Fig. 1.
Visualization of EPS produced by B. bifidum EPS DA-LAIM. A Crystal violet staining of B. bifidum EPS DA-LAIM cultured in BL + 5% sucrose; B Crystal violet staining of B. bifidum EPS DA-LAIM cultured in BL + 5% glucose; C Crystal violet staining of B. bifidum DLP1224 cultured in BL + 5% sucrose; D Ropiness of B. bifidum EPS DA-LAIM culture broth; E three-layer separation of culture medium-EPS-bacteria cells in the culture broth F Ropiness of EPS from B. bifidum EPS DA-LAIM colony; G Freeze-dried EPS powder; H Scanning electron microscope’s image of the culture medium of B. bifidum EPS DA-LAIM; I Scanning electron microscope’s image of B. bifidum EPS DA-LAIM treated by centrifugation and washing with PBS (× 10,000)
When the ropiness of the B. bifidum EPS DA-LAIM colony was checked, its length was determined to be 50 mm (Fig. 1F) and the freeze-dried EPS is shown in Fig. 1G. Nakajima et al. (1992) reported that ropy-fermented milk-fed rats showed the highest ratio of high-density lipoprotein cholesterol/total cholesterol, indicating the important role of the extracellular material. When the culture broth of Bifidobacterium bifidum EPS DA-LAIM was observed using a scanning electron microscope, EPS were found to be particles on the cell surface (Fig. 1H). However, EPS was not found around the cells after centrifugation and washing, which is consistent with the results of crystal violet staining (Fig. 1I). Flemming and Wingender (2010) reported that polysaccharides are fine strands attached to the cell surface and form networks, which is similar to the results of this study.
Structural analysis of EPS from B. bifidum EPS DA-LAIM
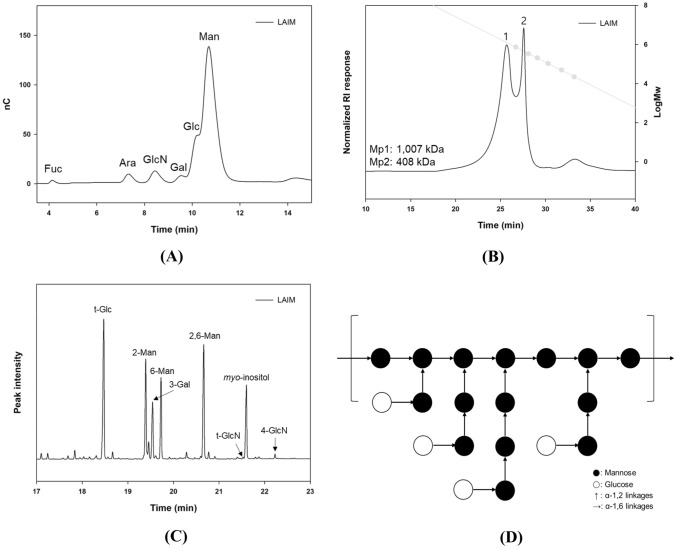
The monosaccharide composition results (Fig. 2A) confirmed that B. bifidum EPS DA-LAIM mainly consisted of glucose and mannose, and small amounts of other monosaccharides (fucose, arabinose, glucosamine, and galactose) were also included in the EPS sample. The amount of mannose was greater than that of glucose, and the molar ratio of glucose to mannose was 0.26:1. EPS from B. bifidum EPS DA-LAIM had two main peaks, described as Mp1 (1007 kDa) and Mp2 (408 kDa), as shown in Fig. 2B. Other molecules in EPS, except for the two main peaks, mostly existed between 408 and 1007 kDa. These results suggest that EPS from B. bifidum EPS DA-LAIM is a glucomannan-type heteropolysaccharide with molecules of 408–1007 kDa. Methylation analysis showed that B. bifidum EPS DA-LAIM predominantly contained 2-mannosyl, 6-mannosyl, and 2,6-mannosyl residues (branching points) in its structure (Fig. 2C). Most glucose residues have a terminal glucose (t-Glc) form, which has a glycosidic linkage via only C1 (anomeric carbon) except other carbons. B. bifidum EPS DA-LAIM has a highly branched structure with an oligo-2-mannosyl side chain on the 6-mannan backbone, similar to the glucomannan structure described in a previous study (Alves et al., 2010). Glucose residues were linked at the end of the mannosyl side chains, and galactose residues were expected to be linked to the mannose backbone or side chains (Daba et al., 2021). Figure 2D describes the proposed structure of EPS from B. bifidum EPS DA-LAIM according to the overall structural analysis results and a previous study.
Fig. 2.
Determination of the structure of EPS from B. bifidum EPS DA-LAIM. A Monosaccharide composition of EPS from B. bifidum EPS DA-LAIM. Fuc, fucose; Ara, arabinose; GlcN, glucosamine; Gal, galactose; Glc, glucose; Man; mannose. B Molecular weight distribution of EPS from B. bifidum EPS DA-LAIM. Grey symbols were pullulan standard from P-20 to P-800. C Glycosyl linkage types of EPS from B.bifidum EPS DA-LAIM identified by mass spectra of PMAAs. Myo-inositol acted as the internal standard. D Schematic illustration of the proposed structure of EPS from B. bifidum EPS DA-LAIM
Other reports show that EPS from B. animalis RH consists of glucose, mannose, galactose, arabinose, fructose, and rhamnose, which have pro-inflammatory effects. EPS from B. longum subsp. longum 35,624 consists of glucose, galactose, galacturonic acid, and 60-deoxy-talose and has an anti-inflammatory effect (Altmann et al., 2016). These EPS were not glucomannan-type and did not share a structure with EPS from B. bifidum EPS DA-LAIM.
Tolerance to Gastrointestinal Environment
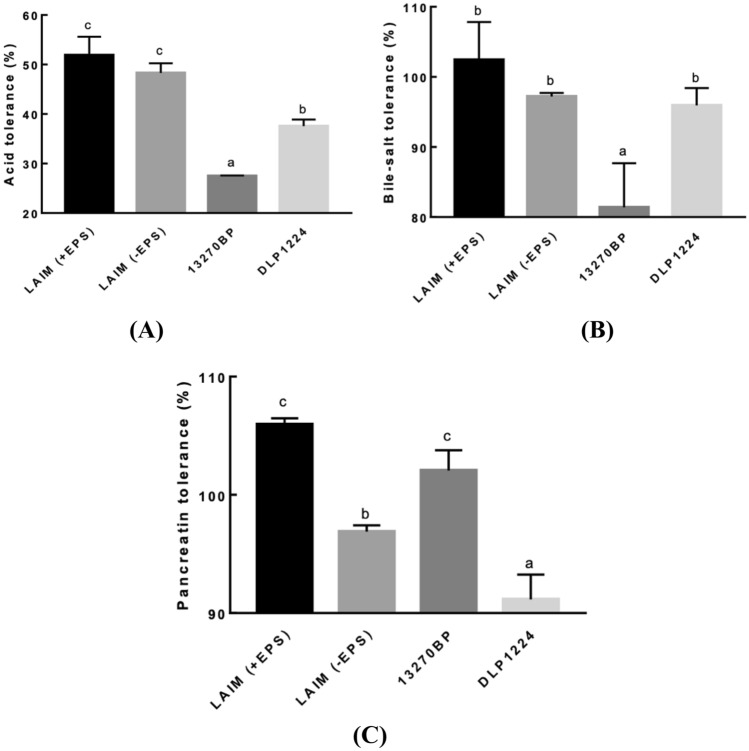
Bifidobacterium bifidum EPS DA-LAIM cells showed higher tolerance to acid, bile salts, and pancreatin in the presence of EPS than in the absence of EPS (Fig. 3, Table S1). The tolerances of B. bifidum EPS DA-LAIM to acid, bile salts and pancreatin were significantly higher than those of B. bifidum DLP1224, an EPS-non-producing strain or those of B. bifidum KCTC 13270BP, an EPS-producing patent strain. This result implies that EPS from B. bifidum EPS DA-LAIM had a protective effect on B. bifidum EPS DA-LAIM cells in the gastrointestinal environment, and its protective effect was higher than those of EPS-producing B. bifidum KCTC 13270BP.
Fig. 3.
Gastrointestinal tract tolerance of Bifidobacterium bifidum strains. A Acid tolerance. B Bile-salt tolerance. C Pancreatin tolerance. LAIM (+ EPS), EPS-containing B. bifidum EPS DA-LAIM culture; LAIM (-EPS), EPS-free B. bifidum EPS DA-LAIM cell pellet; 13270BP, B. bifidum KCTC 13270BP; DLP1224, B. bifidum DLP1224
Tolerance to human gastric transit is an important selection criterion for probiotics. This bacterium may be able to better tolerate stomach acid and bile salts if it has an EPS protective layer (Brink et al., 2006). According to Takahashi et al. (2004), most strains under study showed survival rates of less than 1% at pH 3.0. Serafini et al. (2013) reported that the viabilities of B. bifidum PRL2010 after treatment with pH 3, 0.5% oxgall, and pancreatin were 16.2, 36.2, and 71.7%, respectively. This study indicated that the EPS-producing bifidobacterial strain, B. bifidum EPS DA-LAIM, possesses higher tolerance to acid, bile salt, and pancreatin than EPS-non-producing bifidobacterial strains.
Gut adhesion ability by Caco-2 cell lines
The gut adhesion abilities of B. bifidum EPS DA-LAIM cells with EPS and without EPS were 73.8% and 71.6%, respectively, and EPS slightly promoted the gut adhesion activity (Fig. 4) The gut adhesion abilities of B. bifidum EPS DA-LAIM was similar to that of Lacticaseibacillus rhamnosus GG, representative probiotic strain. Gut adhesion of B. bifidum PRL2010, B. bifidum DSM20215, B. bifidum KCTC337, and B. bifidum KCTC5082 to HT-29 cells was lower than that of B. bifidum EPS DA-LAIM (González-Rodríguez et al., 2012; Serafini et al., 2013) (Table S2).
Fig. 4.
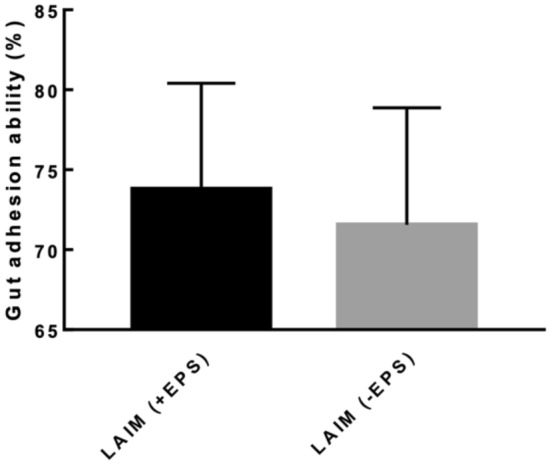
Gut adhesion activity of Bifidobacterium bifidum strains. LAIM (+ EPS), EPS-containing B. bifidum EPS DA-LAIM culture; LAIM (-EPS), EPS-free B. bifidum EPS DA-LAIM cell pellet
It is crucial for probiotic bacteria to adhere to the intestinal epithelium, because it encourages colonization and has immunomodulatory effects (Turchi et al., 2013). The components of bifidobacterial cells, including carbohydrates, extracellular proteins, lipoteichoic acids, and surface proteins, on the outer surface of the cell wall act as adhesive factors (González-Rodríguez et al., 2012).
Immunostimulant effects of EPS-producing B. bifidum EPS DA-LAIM
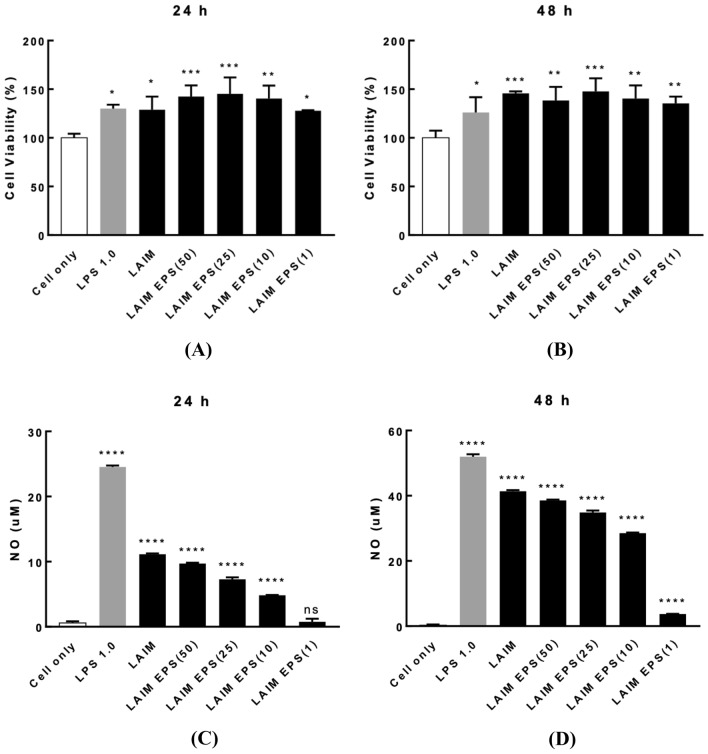
The viability of RAW 264.7 cells by treatment with B. bifidum EPS DA-LAIM cells and EPS from B. bifidum EPS DA-LAIM was higher than 120%, which showed no cytotoxicity of B. bifidum EPS DA-LAIM cells and EPS (Fig. 5A, B). The difference in cell viability between 24 and 48 h of incubation was negligible. Treatment with B. bifidum EPS DA-LAIM cells to RAW 264.7 cells promoted NO production, and it was proportional to the concentration of EPS on both 24 h and 48 h of incubation (Fig. 5C, D). This result indicates that B. bifidum EPS DA-LAIM cells and EPS from B. bifidum EPS DA-LAIM had immunostimulant activity on RAW 264.7 cells, and EPS had a pivotal role in the immunostimulant activity of B. bifidum EPS DA-LAIM. When NO and cytokines are produced, the immune response is boosted in macrophages (Bogdan, 2001).
Fig. 5.
RAW 264.7 cell viability and NO production by treatment of B. bifidum EPS DA-LAIM. A Cell viability by treatment of B. bifidum EPS DA-LAIM on 24 h of incubation. B Cell viability by treatment of B. bifidum EPS DA-LAIM on 48 h of incubation. C NO production by treatment of B. bifidum EPS DA-LAIM on 24 h of incubation. D NO production by treatment of B. bifidum EPS DA-LAIM on 48 h of incubation. Cell only, RAW 264. 7 cells without sample treatment; LPS 1.0, treatment with 1.0 μg/ml LPS; LAIM, B. bifidum EPS DA-LAIM cells at a concentration of 1 × 106/ml were used for treatment; LAIM EPS (50), 50 μg/ml of EPS was used for treatment; LAIM EPS (25), EPS 25 μg/ml of EPS was used for treatment; LAIM EPS (10), 10 μg/ml of EPS was used for treatment; LAIM EPS (1), 1 μg/ml of EPS was used for treatment. Asterisks show significant differences among groups at *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 (n ≥ 3) using one-way ANOVA and Dunnett’s test. ns, not significant
Cytokines are crucial for cell signaling and immune reactions. Interleukin (IL)-6 was expressed in RAW264.7 cells by treatment of B. bifidum EPS DA-LAIM as well as by treatment of EPS (Fig. S2a, b). IL-1β was also expressed by treatment with B. bifidum EPS DA-LAIM as well as by treatment of EPS (Fig. S2c). IL1β and IL-6 are proinflammatory cytokines secreted by mammalian macrophages upon stimulation and activate different types of immune responses (Ayaz, 2018). Because IL1β and IL-6 are regulated by NF-κB, B. bifidum EPS DA-LAIM may enhance NF-κB-mediated innate immunity (Vossenkämper et al., 2010).
DPPH radical scavenging activity
When the antioxidant ability was examined, the DPPH radical scavenging activity of B. bifidum EPS DA-LAIM cells (63.4%) was 1.5 times higher than that of 0.1 mg/ml ascorbic acid (43.2%) (Fig. S3). It is reported that DPPH scavenging activity of B. longum ATCC15708 is 41.6% and L. acidophilus ATCC4356 is 20.8%, which is lower than that of B. bifidum EPS DA-LAIM (Lin and Chang, 2000).
Prebiotic activity
When the prebiotic activity of purified EPS produced from B. bifidum EPS DA-LAIM was assessed using representative probiotic strains, EPS showed a high prebiotic index for all probiotic strains used in this study. The prebiotic index of the purified EPS produced by B. bifidum EPS DA-LAIM was higher than that of inulin, a representative prebiotic carbohydrate (Fig. 6). This result shows that EPS from B. bifidum EPS DA-LAIM had growth-promoting activity on probiotic strains, especially lactic acid bacteria, than inulin, indicating that EPS from B. bifidum EPS DA-LAIM is a promising prebiotic material.
Fig. 6.
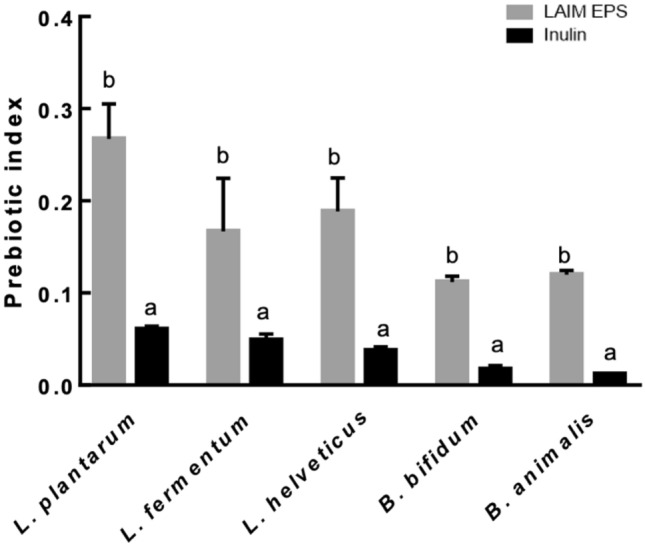
Prebiotic effect of B. bifidum EPS DA-LAIM on probiotic strains. The black bar is the sample treated with inulin, and the gray bar shows the sample treated with EPS from B. bifidum EPS DA-LAIM
Prebiotics are defined as ‘substrates that are selectively utilized by host microorganisms conferring a health benefit’ (Gibson et al., 2017). Previous studies have reported that EPS that reaches the intestinal level can resist gastric and intestinal degradation, with EPS acting as a substrate for bacterial growth in the intestinal tract. At the intestinal level, EPS act as a prebiotic that stimulates the growth of beneficial bacteria and the production of metabolites such as short-chain fatty acids, which play a key role in host health (Korcz et al., 2018).
Antibiotics susceptibility
When the antibiotic susceptibility of B. bifidum EPS DA-LAIM was tested using the E-test for nine antibiotics, the minimum inhibitory concentrations of all antibiotics tested were below the cut-off value, indicating that the sensitivity of B. bifidum EPS DA-LAIM to antibiotics was acceptable (Table S3). Potential probiotics must be safe to consume, and it is crucial to perform a minimal safety evaluation that includes antibiotic resistance to prevent clinical hazards. This result satisfied the criteria for antibiotic resistance in the Probiotic Safety Evaluation Guidelines for Functional Ingredients for Health Functional Food, published by the Korean Ministry of Food and Drug Safety in July 2021.
Analysis of the whole genome sequencing of B. bifidum EPS DA-LAIM
Whole genome sequencing was performed to identify antibiotic resistance (AMR) genes and virulence genes in the genome of B. bifidum EPS DA-LAIM. The whole genome sequence of B. bifidum EPS DA-LAIM consisted of nine contigs with a genome size of 2,236,819 bp, 1798 coding sequences, three rRNA genes, and 52 tRNA genes. The G + C content was 62.6%.
When AMR genes were analyzed using the RGI program, no perfect hits, three strict hits, and 100 loose hits were found. By filtering < 70% identity hits for matching CARD references, the AMR gene was obtained with one loose hit for elfamycin and two strict hits for mupirocin-like antibiotic and rifamycin (Table S4). ResFinder analysis did not identify a matching AMR gene. No virulence genes were found in the B. bifidum EPS DA-LAIM genome when the genome sequence was compared with the sequences of four well-known pathogens. Although elfamycin, mupirocin-like antibiotic, and rifamycin were detected as loose hits and strict hits, these antibiotics were not included in the list of important human and veterinary antibiotics in the EFSA guidelines (ESFA et al., 2018).
In this study, ropy-type EPS-producing B. bifidum EPS DA-LAIM was isolated and the structure of purified EPS from the strain was successfully analyzed. The EPS from B. bifidum EPS DA-LAIM are glucomannan-type heteropolysaccharides. The structure of EPS included mannose subunits linked with ⍺-1,6 glycosidic linkage as a backbone and ⍺-1,2 glycosidic linkages as branches, which is different from that of glucomannan which is generally found in plants, whose backbone consists of β-1,4 glycosidic linkages of mannose. Purified EPS promoted the growth of health-beneficial probiotic strains; therefore, it can be used for the development of prebiotic materials. EPS-producing B. bifidum EPS DA-LAIM showed acid, bile salt, and pancreatin tolerance properties, and EPS itself improved gastrointestinal tract tolerance. Bifidobacterium bifidum EPS DA-LAIM also showed high antioxidant activity, gut adhesion ability, and immunostimulatory activity in macrophages, and its antibiotic susceptibility satisfied the criteria for antibiotic resistance guided by the Korean Ministry of Food and Drug Safety. Because of the gastrointestinal tract tolerance, high gut adhesion ability, immunostimulatory properties, and acceptable antibiotic susceptibility, B. bifidum EPS DA-LAIM is a potential probiotic candidate, and the mechanism of immunostimulatory activity on macrophages must be elucidated.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min Joo Kang, Email: bloemgirl10@gmail.com.
Huijin Jeong, Email: huijin0218@gmail.com.
Suin Kim, Email: Sue030465@gc.gachon.ac.kr.
Jaein Shin, Email: sjaein08@gmail.com.
Youngbo Song, Email: ybsong9482@gmail.com.
Byung-Hoo Lee, Email: blee@gachon.ac.kr.
Hyoung-Geun Park, Email: dongle@donga.co.kr.
Tae-Ho Lee, Email: testtube@donga.co.kr.
Hai-Hua Jiang, Email: jianghh@donga.co.kr.
Young-Sun Han, Email: rndyshan@donga.co.kr.
Bong-Gyeong Lee, Email: lbg@donga.co.kr.
Ho-Jin Lee, Email: hjlee1224@donga.co.kr.
Min-Ju Park, Email: annken@donga.co.kr.
Young-Seo Park, Email: ypark@gachon.ac.kr.
References
- Altmann F, Kosma P, O’Callaghan A, Leahy S, Bottacini F, Molloy E, Plattner S, Schiavi E, Gleinser M, Groeger D. Genome analysis and characterisation of the exopolysaccharide produced by Bifidobacterium longum subsp longum 35624™. PloS One. 2016;11:e0162983. doi: 10.1371/journal.pone.0162983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves EF, Bose SK, Francis RC, Colodette JL, Iakovlev M, Van Heiningen A. Carbohydrate composition of eucalyptus, bagasse and bamboo by a combination of methods. Carbohydrate Polymers. 2010;82:1097–1101. doi: 10.1016/j.carbpol.2010.06.038. [DOI] [Google Scholar]
- Amiri S, Mokarram RR, Khiabani MS, Bari MR, Khaledabad MA. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp lactis BB12: optimization of fermentation variables and characterization of structure and bioactivities. International Journal of Biological Macromolecules. 2019;123:752–765. doi: 10.1016/j.ijbiomac.2018.11.084. [DOI] [PubMed] [Google Scholar]
- Ayaz F. Ruthenium based photosensitizer exerts immunostimulatory and possible adjuvant role on the mammalian macrophages in vitro. Cumhuriyet Science Journal CSJ. 2018;39:991–998. [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nature Immunology. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Brink M, Todorov S, Martin J, Senekal M, Dicks L. The effect of prebiotics on production of antimicrobial compounds, resistance to growth at low pH and in the presence of bile, and adhesion of probiotic cells to intestinal mucus. Journal of Applied Microbiology. 2006;100:813–820. doi: 10.1111/j.1365-2672.2006.02859.x. [DOI] [PubMed] [Google Scholar]
- Castro-Bravo N, Wells JM, Margolles A, Ruas-Madiedo P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Frontiers in Microbiology. 2018;9:2426. doi: 10.3389/fmicb.2018.02426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley M, Banni S, Johnson MC, Mills S, Devery R, Fitzgerald G, Paul Ross R, Stanton C. Inhibitory effect of conjugated α-linolenic acid from bifidobacteria of intestinal origin on SW480 cancer cells. Lipids. 2009;44:249–256. doi: 10.1007/s11745-008-3269-z. [DOI] [PubMed] [Google Scholar]
- Daba GM, Elnahas MO, Elkhateeb WA. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. International Journal of Biological Macromolecules. 2021;173:79–89. doi: 10.1016/j.ijbiomac.2021.01.110. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Additives and Products of Substances used in Animal Feed (FEEDAP) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal. 2012;10:2740. [Google Scholar]
- EFSA Panel on Additives Products or Substances used in Animal Feed (FEEDAP) Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos MDL, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López-Alonso M, Puente SL, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R, Galobart J. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal. 2018;16:e05206. doi: 10.2903/j.efsa.2018.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, O'Connell Motherway M, Shanahan F, Nally K, Dougan G. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proceedings of the National Academy of Sciences. 2012;109:2108–2113. doi: 10.1073/pnas.1115621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H, Wingender J. The biofilm matrix. Nature Review Microbiology. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Garrigues C, Johansen E, Pedersen MB. Complete genome sequence of Bifidobacterium animalis subsp. lactis BB-12, a widely consumed probiotic strain. Journal of Bacteriology. 2010;192:2467–2468. doi: 10.1128/JB.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Hutkins RW, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- González-Rodríguez I, Sánchez B, Ruiz L, Turroni F, Ventura M, Ruas-Madiedo P, Gueimonde M, Margolles A. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Applied and Environmental Microbiology. 2012;78:3992–3998. doi: 10.1128/AEM.08024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Bauer CE. Chemosensory signaling systems that control bacterial survival. Trends in Microbiology. 2014;22:389–398. doi: 10.1016/j.tim.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S, Bertozzi CR, Leffler H, Rosen SD. Identification of the sulfated monosaccharides of GlyCAM-1, an endothelial-derived ligand for L-selectin. Biochemistry. 1994;33:4820–4829. doi: 10.1021/bi00182a010. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Cantabrana C, Sánchez B, Álvarez-Martín P, López P, Martínez-Álvarez N, Delley M, Martí M, Varela E, Suárez A, Antolín M. A single mutation in the gene responsible for the mucoid phenotype of Bifidobacterium animalis subsp lactis confers surface and functional characteristics. Applied and Environmental Microbiology. 2015;81:7960–7968. doi: 10.1128/AEM.02095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Korcz E, Kerényi Z, Varga L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: potential health benefits with special regard to cholesterol-lowering effects. Food & Function. 2018;9:3057–3068. doi: 10.1039/C8FO00118A. [DOI] [PubMed] [Google Scholar]
- Kwon A, Park YS. Immunostimulatory activity of synbiotics using Lactococcus lactis SG-030 and glucooligosaccharides from Weissella cibaria YRK005. Microorganisms. 2021;9:2437. doi: 10.3390/microorganisms9122437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M-Y, Chang F-J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Digestive Diseases and Sciences. 2000;45:1617–1622. doi: 10.1023/A:1005577330695. [DOI] [PubMed] [Google Scholar]
- Nachtigall C, Surber G, Bulla J, Rohm H, Jaros D. Pilot scale isolation of exopolysaccharides from Streptococcus thermophilus DGCC7710: Impact of methodical details on macromolecular properties and technofunctionality. Engineering in Life Sciences. 2021;21:220–232. doi: 10.1002/elsc.202000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Suzuki Y, Hirota T. Cholesterol loweing activity of ropy fermented milk. Journal of Food Science. 1992;57:1327–1329. doi: 10.1111/j.1365-2621.1992.tb06848.x. [DOI] [Google Scholar]
- Panagiotopoulos C, Sempere R, Lafont R, Kerherve P. Sub-ambient temperature effects on the separation of monosaccharides by high-performance anion-exchange chromatography with pulse amperometric detection: Application to marine chemistry. Journal of Chromatography a. 2001;920:13–22. doi: 10.1016/S0021-9673(01)00697-5. [DOI] [PubMed] [Google Scholar]
- Ruas-Madiedo P, Sánchez B, Hidalgo-Cantabrana C, Margolles A, Laws A. Exopolysaccharides from lactic acid bacteria and bifidobacteria. In: Hui YH, Evranuz EO, editors. Handbook of animal based fermented food and beverage technology. Boca Raton: CRC Press Inc; 2012. pp. 125–151. [Google Scholar]
- Ryan P, Ross R, Fitzgerald G, Caplice N, Stanton C. Sugar-coated: exopolysaccharide producing lactic acid bacteria for food and human health applications. Food & Function. 2015;6:679–693. doi: 10.1039/C4FO00529E. [DOI] [PubMed] [Google Scholar]
- Saadat YR, Khosroushahi AY, Gargari BP. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydrate Polymers. 2019;217:79–89. doi: 10.1016/j.carbpol.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Serafini F, Strati F, Ruas-Madiedo P, Turroni F, Foroni E, Duranti S, Milano F, Perotti A, Viappiani A, Guglielmetti S. Evaluation of adhesion properties and antibacterial activities of the infant gut commensal Bifidobacterium bifidum PRL2010. Anaerobe. 2013;21:9–17. doi: 10.1016/j.anaerobe.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kaur J, Lee S, Park YS. Tracking of deliberately inoculated Leuconostoc mesenteroides and Lactobacillus brevis in kimchi. Food Science and Biotechnology. 2020;29:817–824. doi: 10.1007/s10068-019-00719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Xiao J-Z, Miyaji K, Yaeshiima T, Hiramatsu A, Iwatsuki K, Kokubo S, Hosono A. Selection of acid tolerant bifidobacteria and evidence for a low-pH-inducible acid tolerance response in Bifidobacterium longum. Journal of Dairy Research. 2004;71:340–345. doi: 10.1017/S0022029904000251. [DOI] [PubMed] [Google Scholar]
- Turchi B, Mancini S, Fratini F, Pedonese F, Nuvoloni R, Bertelloni F, Ebani VV, Cerri D. Preliminary evaluation of probiotic potential of Lactobacillus plantarum strains isolated from Italian food products. World Journal of Microbiology and Biotechnology. 2013;29:1913–1922. doi: 10.1007/s11274-013-1356-7. [DOI] [PubMed] [Google Scholar]
- Vossenkämper A, Marchès O, Fairclough P, Warnes G, Stagg A, Lindsay J, Evans P, Luong L, Croft N, Naik S, Frankel G, MacDonald T. Inhibition of NF-κB signaling in human dendritic cells by the enteropathogenic Escherichia coli effector protein NleE. Journal of Immunology. 2010;185:4118–4127. doi: 10.4049/jimmunol.1000500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.