Significance
Tfh cells are indispensable for effective humoral immunity against infection but detrimental in autoimmune diseases. We demonstrate that LXR, an oxysterol sensor previously known to integrate metabolic and inflammatory signaling, acts as a critical negative regulator of Tfh cell differentiation. LXR deficiency selectively and cell-intrinsically increases Tfh cell differentiation in immunization and viral infection models. Mechanistically, LXR suppresses TCF-1, a transcription factor required for the early Tfh lineage program, by regulating both TCR-mediated and Wnt/β-catenin-mediated inactivation of GSK3β. Since LXR also suppresses human Tfh cell differentiation, our findings accentuate the possibility of targeting LXR to treat Tfh cell-related diseases in humans.
Keywords: liver X receptor, Tfh cells, TCF-1, GSK3β, humoral immunity
Abstract
Liver X receptor (LXR) is a critical regulator of cholesterol homeostasis that inhibits T cell receptor (TCR)-induced proliferation by altering intracellular sterol metabolism. However, the mechanisms by which LXR regulates helper T cell subset differentiation remain unclear. Here, we demonstrate that LXR is a crucial negative regulator of follicular helper T (Tfh) cells in vivo. Both mixed bone marrow chimera and antigen-specific T cell adoptive cotransfer studies show a specific increase in Tfh cells among LXRβ-deficient CD4+ T cell population in response to immunization and lymphocytic choriomeningitis mammarenavirus (LCMV) infection. Mechanistically, LXRβ-deficient Tfh cells express augmented levels of T cell factor 1 (TCF-1) but comparable levels of Bcl6, CXCR5, and PD-1 in comparison with those of LXRβ-sufficient Tfh cells. Loss of LXRβ confers inactivation of GSK3β induced by either AKT/Extracellular signal-regulated kinase (ERK) activation or Wnt/β-catenin pathway, leading to elevated TCF-1 expression in CD4+ T cells. Conversely, ligation of LXR represses TCF-1 expression and Tfh cell differentiation in both murine and human CD4+ T cells. LXR agonist significantly diminishes Tfh cells and the levels of antigen-specific IgG upon immunization. These findings unveil a cell-intrinsic regulatory function of LXR in Tfh cell differentiation via the GSK3β-TCF1 pathway, which may serve as a promising target for pharmacological intervention in Tfh-mediated diseases.
Upon antigenic stimulation, antigen-specific T cells undergo clonal expansion to increase the proportion of antigen-specific T cells in the secondary lymphoid organs (1). After multiple rounds of division, activated T cells lose proliferative potential as a negative feedback mechanism (2). During the clonal expansion, activated T cells reprogram their bioenergetic metabolism to meet the high demands for substrates required for the clonal expansion (3). Anabolic reprogramming of lipid metabolism is necessary for activated T cells to support their cellular growth (4). In particular, cholesterol biosynthesis is up-regulated in the proliferating T cells as it is an essential component of cellular membranes (5). Cholesterol constitutes about 30% of cell membranes and plays an important role in maintaining the fluidity of the membrane (6). T cells are activated by forming an immunological synapse with antigen-presenting cells, during which cholesterol-rich lipid rafts are reorganized (7). In addition, since various receptors and signaling molecules exist in the cell membrane of T cells, cholesterol can regulate receptor signaling through membrane receptor localization and conformational change (8). For instance, hypercholesterol enhances TCR signaling and Treg cell formation (9, 10). Therefore, dynamic changes in cholesterol biosynthesis and metabolism occur during T cell activation and differentiation.
Homeostasis of intracellular cholesterol is regulated by the reciprocal activation of sterol regulatory element-binding proteins and LXRs (11). There exist two isoforms of LXR; LXRα is expressed in metabolically active tissues including the liver and adipose tissues, while LXRβ is ubiquitously expressed. LXRs function as sensors of oxysterols and sterol intermediates that are activated in response to elevated intracellular cholesterol levels. Once activated, LXR/Retionid X receptor (RXR) heterodimers induce the expression of an array of genes involved in cholesterol absorption and efflux as well as fatty acid metabolism (11). In addition to their function in lipid metabolism, LXRs modulate the differentiation and functions of diverse immune cells. For instance, LXRs suppress proinflammatory gene expression in macrophages and dendritic cells by suppressing nuclear factor kappa B activity (12, 13). LXR-deficient mice develop lupus-like phenotypes due to defective clearance of apoptotic bodies by macrophages (14). LXRβ-deficient T cells show increased proliferation upon TCR stimulation in vitro as well as in response to viral infection in vivo (15). More recently, it has been demonstrated that LXR in developing thymocytes reduces negative selection by limiting lipid rafts-mediated induction of Bim (16). Moreover, mice with T cell-specific LXRβ deficiency harbor defective Treg cells and develop fatal autoimmune diseases (17). These studies collectively demonstrate an essential regulatory role of LXRs in T cell development, T cell activation upon antigen-recognition, and the function of regulatory T cells. However, the cell-intrinsic role of LXRs in diverse helper T cell subsets remains unclear.
In the present study, we aimed to investigate the role of LXRs on the differentiation and function of helper T cell subsets in vivo. While LXRβ-deficient T cells showed enhanced Th1 and Th17 differentiation in vitro, we did not observe any evident differences in the frequency of these helper T cell subsets between the wild-type (WT) and LXRβ-deficient CD4+ T cell population in a mixed bone marrow (BM) chimera in vivo. Instead, we observed a significant increase in the frequency of Tfh cells in the LXRβ-deficient CD4+ T cell population in the same mixed BM chimera, indicating a selective and cell-intrinsic regulation of Tfh cell differentiation by LXRβ. Mechanistic studies revealed that LXR inhibited the GSK3β-TCF1 signaling pathway in murine and human CD4+ T cells. These findings identify LXRβ as a negative regulator of TCF-1 during the differentiation of Tfh cells.
Results
LXRβ Regulates the Development and Activation of CD4+ T Cells.
To investigate the role of LXRs in CD4+ T cells, we first compared the levels of intracellular lipids in various CD4+ T cells. BODIPY staining showed a higher level of intracellular lipids in CD44hi effector/memory CD4+ T cells than naïve ones (SI Appendix, Fig. S1A). Among genes involved in cholesterol synthesis, levels of Pmvk, Fdft1, Sqle, Nsdhl, and Dhcr7 were higher in the former (SI Appendix, Fig. S1B). Similarly, levels of Sult2b1a and Cyp27a1, genes involved in cholesterol metabolism, were significantly higher in the effector/memory CD4+ T cells (SI Appendix, Fig. S1C). The levels of Nr1h2 (encoding LXRβ) and Rxrb (encoding RXRβ) were higher than those of Nr1h3 (encoding LXRα) and Rxra (encoding RXRα), respectively, in both naïve and effector/memory CD4+ T cells (SI Appendix, Fig. S1D), suggesting that LXRβ and RXRβ are dominant isoforms in CD4+ T cells. The levels of Nr1h2 and Rxrb were diminished in effector/memory CD4+ T cells. We next determined the kinetics of transcripts encoding enzymes involved in the cholesterol synthesis pathway in CD4+ T cells after stimulation with anti-CD3 and anti-CD28. Consistent with a previous study (15), activation of CD4+ T cells augmented the expression of genes related to cholesterol synthesis in a time-dependent manner with a concomitant increase in intracellular lipids (SI Appendix, Fig. S1 E and F). The levels of Sult2b1a, and Cyp11a1 increased rapidly by 4 h after stimulation (SI Appendix, Fig. S1G), while those of Nr1h3, Srebf2, and Rxrb were all slightly decreased by 24 h (SI Appendix, Fig. S1H).
To determine the role of LXRβ in the T cell development, we analyzed thymocytes and found that Nr1h2−/− mice harbored a slightly elevated number of thymocytes and an increased frequency of CD4+CD8+ population with a concomitant decrease in the CD4+CD8− and CD4−CD8+ population, all of which are consistent with a previous study (16) (SI Appendix, Fig. S2 A and B). The frequency of Foxp3+ cells within the CD4+CD8− population was also slightly reduced in the Nr1h2−/− mice (SI Appendix, Fig. S2C). Nr1h2−/− mice exhibited a decrease in the frequency of CD4+ T cells in the secondary lymphoid organs, at least in part, due to an impaired thymic T cell development and output (16). In addition, we observed a general increment of the effector/memory population within CD4+ T cells (SI Appendix, Fig. S2D). Likewise, activated Nr1h2−/− CD4+ T cells via anti-CD3 and anti-CD28 displayed a more robust proliferation than those of WT, associated with an increased expression of IL-2 and CD25 (SI Appendix, Fig. S3 A and B). These observations demonstrate that diverse subsets of helper T cells as well as regulatory T cells are differentially regulated in the Nr1h2−/− mice at a steady state, and that LXRβ controls the TCR-mediated activation and division of CD4+ T cells.
LXRβ Suppresses Tfh Cell Differentiation in a Cell-Intrinsic Manner.
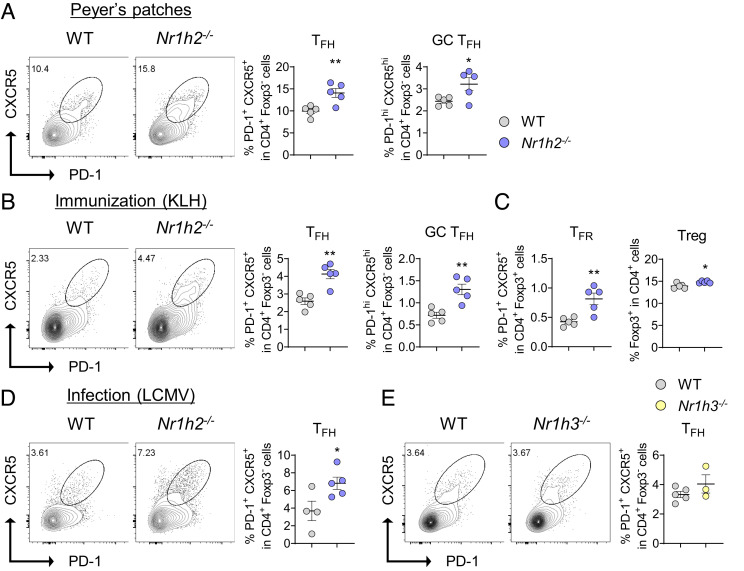
To address whether LXRβ impacts the differentiation of CD4+ T cells in vitro, naïve CD4+ T cells were cultured under Th1-, Th2-, Th17-, and Treg-skewing conditions, respectively. We found that the frequencies of Th1 and Th17 cells were higher in Nr1h2−/− T cells than in the WT ones, while those of Th2 and Treg cells were comparable (SI Appendix, Fig. S3 C–F). To investigate whether LXRβ impacts the differentiation of naïve CD4+ T cells into effector subsets of helper T cells in vivo, we analyzed Peyer’s patches where spontaneous germinal center (GC) reaction occurs in naive mice. Among CD44hiCD4+ T cells, the frequency of Th1 cells and Th17 cells was higher and lower, respectively, in the Nr1h2−/− mice compared with those in the WT mice (SI Appendix, Fig. S4A). Unlike the thymocytes, we observed comparable frequencies of Foxp3+ Treg cells between the two groups (SI Appendix, Fig. S4B). Of note, the frequency of PD-1+CXCR5+ Tfh cells and PD-1hiCXCR5hi GC Tfh were found to be significantly higher in Peyer’s patches of the Nr1h2−/− mice (Fig. 1A).
Fig. 1.
LXRβ deficiency leads to enhanced Tfh cell differentiation in vivo. (A) Analysis of Tfh cell population in Peyer’s patch at steady state. Representative Fluorescence-activated cell sorting (FACS) plots of PD-1+CXCR5+ total Tfh within CD4+Foxp3− cells, and the frequencies of total Tfh and PD-1hiCXCR5hi GC Tfh cell frequencies (n = 5). (B and C) WT and Nr1h2−/− mice were immunized with KLH in CFA and draining LNs were analyzed on day 8 (n = 5). (B) Representative FACS plots and frequencies of Tfh (PD-1+CXCR5+) cells within CD4+Foxp3− cells. Tfh and GC Tfh cell frequencies. (C) Tfr and Treg cell frequencies. (D) WT and Nr1h2−/− mice were infected with 2 × 105 plaque-forming units (pfu) of LCMV-Armstrong, and spleens were analyzed on day 8. Representative FACS plots and frequency of Tfh cells (n = 4 to 5). (E) WT and Nr1h3−/− mice were immunized with KLH in CFA and draining LNs were analyzed on day 8 (n = 3 to 5). Representative FACS plots of Tfh cells and frequencies. Data are representative of at least two independent experiments. Quantification plots show the mean ± SEM; *P < 0.05 and **P < 0.01.
Next, we compared Th subsets in the draining lymph nodes (LNs) between the WT and the Nr1h2−/− mice after immunization with keyhole limpet hemocyanin (KLH) emulsified in Freund′s Adjuvant, Complete (CFA). Frequencies of Th1 and Th17 cells were comparable (SI Appendix, Fig. S4C); however, we observed a significant increase in PD-1+CXCR5+Foxp3− Tfh cells, particularly the PD-1hiCXCR5hi GC Tfh population, in the Nr1h2−/− mice (Fig. 1B). We also observed a similar increase in the PD-1+CXCR5+Foxp3+ Tfr cells (Fig. 1C). Upon immunization with NP-Ovalbumin (OVA) emulsified in CFA, Nr1h2−/− mice showed a significantly increased frequency of GL7+ B220+ GC B cells and had a slight but significant increase in the serum levels of high-affinity NP7-specific IgG in comparison with WT mice, while the levels of global affinity NP29-specific IgG were comparable between the two groups (SI Appendix, Fig. S4 D and E ). To test if LXRβ also impacts Tfh cell responses generated upon viral infection, we employed the LCMV-Armstrong infection model. We observed a significant increase in Tfh cell among CD4+ T population in the spleen of Nr1h2−/− mice (Fig. 1D). The frequency of Th1 cell was increased in the Nr1h2−/− mice, while that of Treg cell was comparable (SI Appendix, Fig. S4 F and G). To determine whether LXRα also impacts Tfh cell responses in vivo, we immunized the WT and Nr1h3−/− mice with KLH emulsified in CFA and observed a comparable Tfh cell population between the two groups, indicating that LXRβ, but not LXRα, represses Tfh cell responses in vivo (Fig. 1E).
To determine LXRβ’s role in the Tfh cells’ ability to promote B cells in vitro, we flow-sorted either the WT or Nr1h2−/− Tfh cells and cocultured them with WT naïve B cells in the presence of anti-IgM and anti-CD3 for 7 d. We observed comparable levels of IgG and IgG1 in the supernatant (SI Appendix, Fig. S4H). Thus, LXRβ-deficient Tfh cells were as efficient as WT Tfh cells in stimulating B cells to produce immunoglobulin.
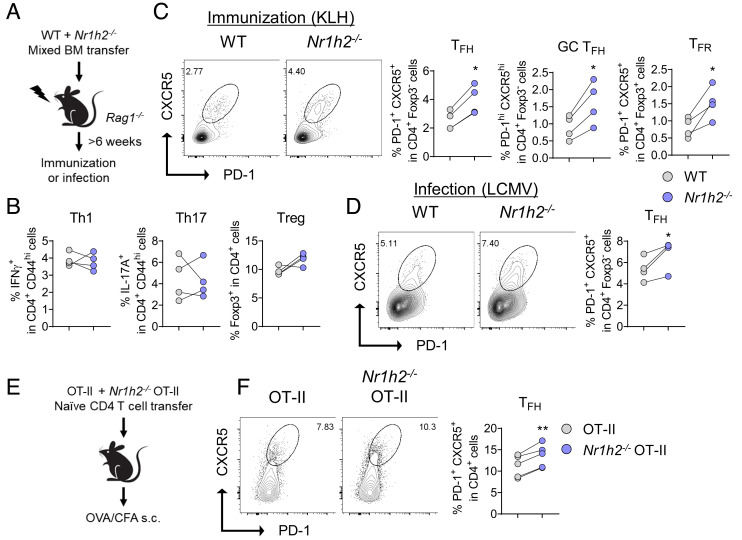
To interrogate the cell-intrinsic role of LXRβ in helper T cell responses in vivo, we generated mixed BM chimera by transferring 1:1 mixture of WT (CD45.1) and Nr1h2−/− (CD45.2) BM cells into the BM-ablated Rag1−/− recipients. These recipients were subsequently immunized with KLH emulsified in CFA, and Th subsets in the draining LNs were analyzed (Fig. 2A). The frequencies of Th1, Th17, and Treg cells were indistinguishable between the WT and Nr1h2−/− compartments within the chimeras (Fig. 2B). By contrast, the frequencies of Tfh, GC Tfh, and Tfr cells were all significantly higher in the Nr1h2−/− compartments of the same recipients (Fig. 2C). Furthermore, we observed a significant increase in Tfh cells in the Nr1h2−/− compartment within the mixed BM chimeras infected with LCMV-Armstrong, indicating a cell-intrinsic inhibitory role of LXRβ in the differentiation of Tfh cells in vivo (Fig. 2D). Unlike that of Tfh cells, the frequency of GC B cells was indistinguishable between the WT and Nr1h2−/− compartment (SI Appendix, Fig. S4I). To further confirm cell-intrinsic regulation of LXRβ in antigen-specific CD4+ T cell responses in vivo, we cotransferred WT OT-II (CD45.1+CD45.2+) and Nr1h2−/− OT-II T cells (CD45.2+) into CD45.1+congenic mice before immunizing the recipients with OVA emulsified in CFA (Fig. 2E). Consistent with the observations in mixed BM chimeras, we observed a significant increase in Tfh cells within the Nr1h2−/− OT-II T cell population in comparison with the WT counterpart (Fig. 2F). Together, these findings demonstrate that LXRβ negatively regulates Tfh cell differentiation in vivo in a cell-intrinsic manner.
Fig. 2.
LXRβ inhibits Tfh cell differentiation in a cell-intrinsic manner. (A–D) BM chimeric mice were generated by adoptive transfer of 1:1 mixture of WT and Nr1h2−/− BM into irradiated Rag1−/− mice. After the reconstitution period, the chimeras were either s.c. immunized with KLH in CFA (B and C) or infected with LCMV (D) before flow cytometric analysis (n = 4). (A) Scheme of the mixed BM chimera experiments. (B) Th1, Th17, and Treg cell frequencies. (C) Representative FACS plots of Tfh cells, and frequencies of Tfh, GC Tfh, and Tfr cells in the draining LNs. (D) Representative FACS plots and frequency of Tfh cells. (E and F) B6.SJL (CD45.1+) mice were i.v. transferred with mixture of WT (CD45.1/2+) and Nr1h2−/− (CD45.2+) OT-II T cells. The recipients were immunized with OVA in CFA and the draining LNs were analyzed on day 8 (n = 5). (E) Schematic representation of the OT-II cotransfer experiments. (F) Representative FACS plots and frequency of Tfh cells. Data are representative of at least two independent experiments. Quantification plots show the mean ± SEM; *P < 0.05, and **P < 0.01.
LXRβ Represses GSK3β Phosphorylation to Down-Regulate TCF-1 in CD4+ T Cells.
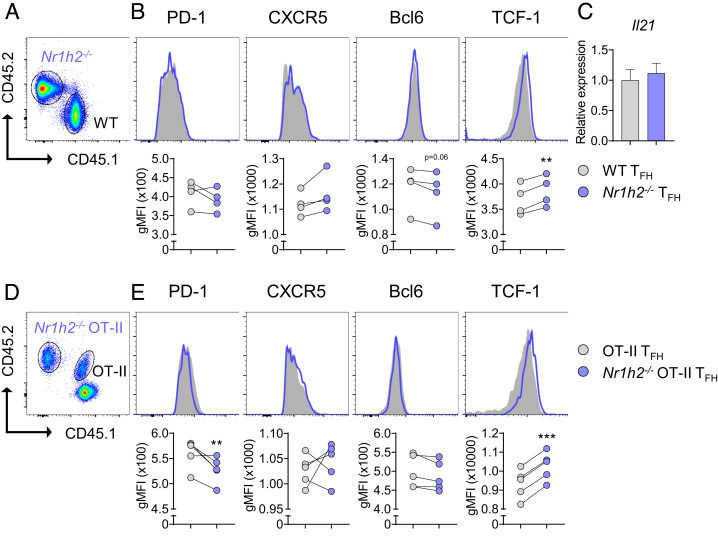
We next sought to dissect the mechanism through which LXRβ suppresses Tfh cell differentiation. CXCR5, PD-1, Bcl6, and TCF-1 represent Tfh cell signature molecules. When we compared the expression of these molecules in the mixed BM chimeras described in Fig. 2A, the levels of CXCR5, PD-1, and Bcl6 did not differ between WT and Nr1h2−/− Tfh cells (Fig. 3 A and B). By contrast, the expression of TCF-1 was higher in Nr1h2−/− Tfh cells than that in the WT (Fig. 3B). Il21 level in the WT Tfh cells and Nr1h2−/− Tfh cells was tantamount (Fig. 3C). Consistently, we also observed higher TCF-1 in Nr1h2−/− OT-II Tfh cells than WT OT-II Tfh cells in a cotransfer study, as depicted in Fig. 3 D and E. Unlike the mixed BM chimera study, the level of PD-1 was slightly lower in Nr1h2−/− OT-II Tfh cells. These findings prompted us to hypothesize that LXRβ inhibits Tfh cell differentiation via TCF-1 repression.
Fig. 3.
LXRβ negatively regulates TCF-1 in Tfh cells. (A and B) BM chimera mice were generated and immunized with KLH in CFA as in Fig. 2A (n = 4). (A) A representative FACS plot of CD45.1+ WT cells and CD45.2+ Nr1h2−/− cells in the draining LNs. (B) Geometric mean fluorescence intensities (gMFIs) of PD-1, CXCR5, Bcl6, and TCF-1 in gated Tfh cells in WT and Nr1h2−/− Tfh cells. (C) Tfh cells from WT and Nr1h2−/− mice were flow-sorted and the relative expression of Il21 was analyzed by qRT-PCR (n = 4). (D and E) B6.SJL (CD45.1+) mice were i.v. transferred with mixture of WT (CD45.1/2+) and Nr1h2−/− (CD45.2+) OT-II T cells, followed by s.c. immunization with OVA in CFA and subsequent flow cytometric analysis in the draining LNs (n = 5). (D) Representative FACS plot of CD45.1+CD45.2+ WT and CD45.2+ Nr1h2−/− OT-II T cells. (E) gMFIs of PD-1, CXCR5, Bcl6, and TCF-1 in gated Tfh cells in the indicated Tfh cells (n = 5). Data are representative of three independent experiments. Quantification plots show the mean ± SEM; **P < 0.01, and ***P < 0.001.
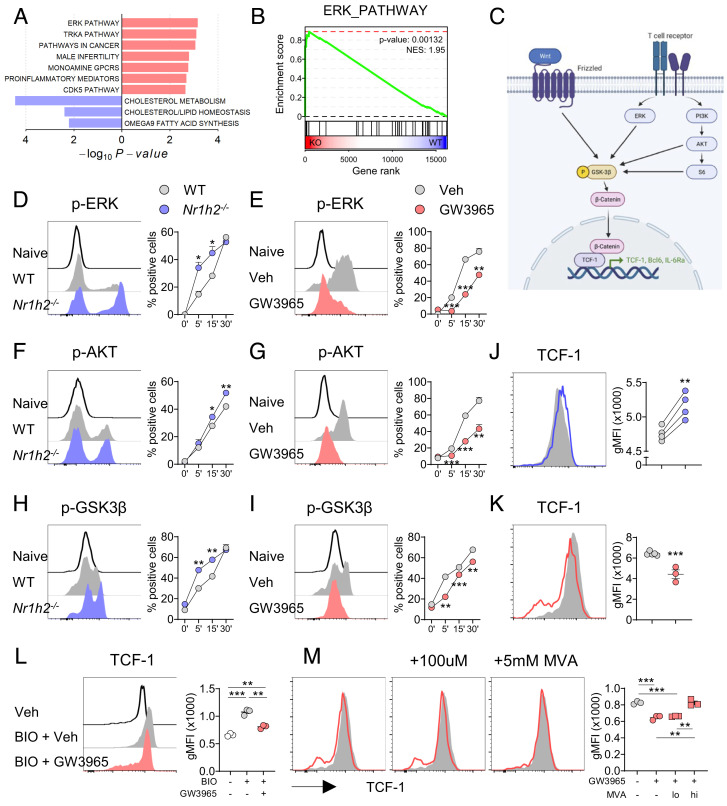
To probe the mechanistic details by which LXRβ controls TCF-1 in Tfh cells in vivo in an unbiased way, we subjected flow-sorted Tfh cells isolated from LCMV-infected Nr1h2−/− mice and WT mice to RNA-sequencing analysis. At a setting of fold change >1.5, and false discovery rate <0.1, 101 genes were up-regulated, and 34 genes were down-regulated in the LXRβ-deficient Tfh cells. As expected, Nr1h2 and well-known LXR target genes, Ldlr, Mylip, and Abcg1 were down-regulated in Nr1h2−/− Tfh cells. Of note, we found that among the up-regulated differentially expressed genes (DEGs) were genes previously known to be positively associated with the Tfh cell lineage program, such as Spp1, C3, Vdr, and Fosl, and among the down-regulated DEGs were genes known to be negatively associated with the Tfh cell lineage program, such as Vamp7, and Dock7 (SI Appendix, Fig. S5 A and B) (18, 19). In addition, gene set enrichment analysis (GSEA) revealed that Nr1h2−/− Tfh cells were enriched for genes involved in the BCL6-high Tfh program, indicating that GC Tfh transcriptome is up-regulated in the absence of LXRβ (SI Appendix, Fig. S5C). In addition, ERK pathway as well as PI3K-AKT signaling pathway was enriched in Nr1h2−/− Tfh cells (Fig. 4 A and B and SI Appendix, Fig. S5D). Indeed, when stimulated with anti-CD3 and anti-CD28, Nr1h2−/− CD4+ T cells showed higher levels of phosphorylated forms of ERK and AKT than WT CD4+ T cells (Fig. 4 D and E). Conversely, addition of an LXR agonist, GW3965, significantly suppressed the phosphorylation of ERK as well as AKT in CD4+ T cells (Fig. 4 F and G). Since AKT induces the activation of mTORC1 pathway, we tested whether downstream molecules of mTORC1 are regulated by LXRβ in CD4+ T cells. We observed an enhanced level of phospho-S6 in the Nr1h2−/− CD4+ T cells, and a delayed phosphorylation of S6 by GW3965 treatment (SI Appendix, Fig. S5 E and F). Thus, LXRβ controls the activation of AKT-mTORC1 and ERK in CD4+ T cells after TCR stimulation.
Fig. 4.
LXR inhibits PI3K/AKT, ERK, and Wnt/β-catenin signaling to repress TCF-1 in CD4+ T cells. (A and B) Tfh cells from the spleens of LCMV-Armstrong-infected WT and Nr1h2−/− mice were subjected to bulk RNA-seq analysis. (A) Top enriched or down-regulated pathways. (B) GSEA of ERK_PATHWAY (C) Schematic view of TCR and Wnt signaling pathway in T cells. (D–K) WT or Nr1h2−/− naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of vehicle or GW3965, and the levels of indicated molecules were determined at each time point (n = 3 to 4). (D and F) Representative histograms of p-ERK expression, and frequencies of p-ERK+ cells. (E and G) Representative histograms of p-AKT expression, and frequencies of p-AKT+ cells. (H and I) Representative histograms of p-GSK3β expression, and frequencies of p-GSK3β+ cells. (J and K) WT or Nr1h2−/- naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of vehicle or GW3965 and analyzed for the expression of TCF-1 (n = 3 to 5). (L) Naïve CD4+ T cells were cultured with BIO in the presence of vehicle or GW3965 and gMFIs of TCF-1 were examined (n = 3). (M) Naïve CD4+ T cells were cultured with GW3965 in the presence of vehicle or mevalonate and gMFIs of TCF-1 were examined (n = 4). Data are representative of three independent experiments. Quantification plots show the mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001.
As illustrated in Fig. 4C, activation of ERK, PI3K/AKT, and Wnt commonly induces the phosphorylation of GSK3β, leading to the release and translocation of β-catenin to the nucleus. TCF-1 contains β-catenin-binding domain, and TCF-1/β-catenin complex facilitates early differentiation of Tfh cell by inhibiting Prdm1 (encoding Blimp1) and Il2ra, and by antagonizing Bcl6-mediated autoinhibitory effect in developing Tfh cells (20, 21). Moreover, inhibition of GSK3β kinase activity increases stability and nuclear translocation of β-catenin and up-regulates TCF-1 (22, 23). Thus, we hypothesized that LXRβ represses TCF-1 expression in helper T cells by regulating GSK3β. Indeed, the level of phospho-GSK3β was significantly increased in the Nr1h2−/− CD4+ T cells but was remarkably diminished by GW3965 treatment in the WT CD4+ T cells (Fig. 4 H and I). Thus, LXRβ negatively regulates the phosphorylation of GSK3β in CD4+ T cells.
Naïve and recently activated CD4+ T cells maintain high levels of TCF-1; however, activated T cells down-regulate TCF-1 after four to five divisions (24). To determine whether LXRβ regulates TCR-mediated downregulation of TCF-1, we measured TCF-1 levels after stimulating naïve CD4+ T cells with anti-CD3 and anti-CD28 and found a significantly higher level of TCF-1 in Nr1h2−/− T cells than that in WT T cells (Fig. 4J). Conversely, the addition of an LXR agonist significantly decreased TCF-1 in activated CD4+ T cells (Fig. 4K). Despite the observed differences in the level of TCF-1, we found no evident difference in the transcript levels of Tcf7 (encoding TCF-1) between WT and Nr1h2−/− T cells, nor between vehicle- and GW3965-treated CD4+ T cells (SI Appendix, Fig. S5 G–I), suggesting a posttranscriptional/translational regulation of TCF-1 by LXR. TCF-1 and LEF-1 contain a conserved high-mobility-group DNA-binding domain, and coordinate Tfh cell differentiation (20). Unlike TCF-1, however, the levels of LEF-1 were comparable between WT and Nr1h2−/− CD4+ T cells as well as between vehicle- and GW3965-treated CD4+ T cells (SI Appendix, Fig. S5 J and K ).
We next asked whether LXRβ-mediated downregulation of phospho-GSK3β accounts for the increased TCF-1 observed in LXRβ-deficient T cells. As expected, treatment with a Wnt/β-catenin agonist BIO increased TCF-1 expression in WT CD4+ T cells. The addition of an LXR agonist, however, almost completely abolished the upregulation of TCF-1 induced by BIO (SI Appendix, Fig. S5L). To investigate whether LXR would also regulate TCF-1 independently of TCR stimulation, naïve CD4+ T cells were treated with BIO without TCR stimulation. Likewise, GW3965 canceled TCF-1 upregulation induced by BIO (Fig. 4L). The same trend was observed when BIO and GW3965 were treated 1 d after TCR stimulation or under IL-2 stimulation conditions (SI Appendix, Fig. S5 M and N). We next addressed if the observed GW3965-mediated downregulation of TCF-1 was mainly due to antiproliferative effect of the agonist, and found that GW3965 significantly down-regulated TCF-1 expression in activated CD4+ T cells regardless of the division (SI Appendix, Fig. S5O). Collectively, these findings indicate that LXR inhibits not only TCR-induced activation of PI3K/AKT and ERK but also Wnt/β-catenin signaling to down-regulate TCF-1 expression in CD4+ T cells.
LXR activation is known to promote cholesterol efflux by up-regulating the ATP-binding cassette (ABC) transporters ABCA1 and ABCG1 (25). Accordingly, GW3965 treatment not only increased the expression of Abca1 and Abcg1 mRNA but also decreased the levels of intracellular cholesterol in CD4+ T cells (SI Appendix, Fig. S5 P and Q). Since LXR activation down-regulates intracellular sterol level, we questioned whether the inhibitory effect of LXR can be restored by mevalonate, a precursor for cholesterol and oxysterols (15). Of note, higher concentration of mevalonate (5 mM) almost completely abolished the GW3965-induced downregulation of TCF-1, while lower concentration of mevalonate (100 μM) failed to do so (Fig. 4M). Given that the lower concentration of mevalonate is known to be sufficient for nonsteroidal modifications while insufficient to drive sterol synthesis (15), this result suggests that LXR inhibits TCF-1 expression, at least in part, by lowering intracellular sterol in CD4+ T cells.
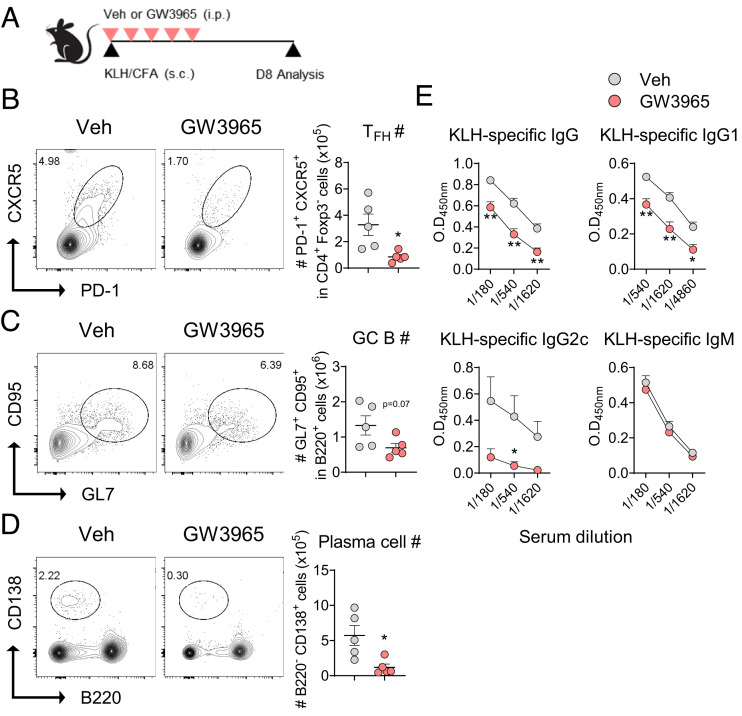
Activation of LXR Attenuates GC Reactions and Antigen-Specific IgG Production.
The observed regulatory role of LXRβ on TCF-1 expression and Tfh cell differentiation proposed that LXR agonism could attenuate GC reactions and subsequent immunoglobulin production in vivo. To address this point, we again employed a mouse model of immunization with KLH emulsified in CFA, and analyzed Tfh, GL7+B220+ GC B cells, and CD138+B220− plasma cells in the draining LNs as well as the levels of KLH-specific Igs in the serum (Fig. 5A). As depicted in Fig. 5 B–D, we observed a significant reduction in the frequencies and numbers of Tfh cells, GC B cells, and plasma cells in the draining LNs of GW3965-treated mice compared with vehicle-treated control mice. As a consequence, the serum levels of KLH-specific IgG, particularly IgG2c, were also significantly reduced in the former, while that of IgM remained comparable between the two groups (Fig. 5E). To study the definitive function of T cell-specific LXR activation, we stimulated naïve OT-II T cells under Tfh-like skewing condition in the presence of GW3965 or vehicle and transferred them into congenic mice before immunizing the recipients with NP-OVA emulsified in CFA (SI Appendix, Fig. S6A) (18). CTV-dilution assay showed that pretreatment of GW3965 during the Tfh-like cell differentiation in vitro did not affect the antigen-driven proliferative potential of the resultant OT-II T cells (SI Appendix, Fig. S6B). GW3965-treated OT-II cells were defective in their ability to become Tfh cells in vivo and were much less effective in inducing GC B cells, and early plasmablasts (SI Appendix, Fig. S6 C and D). Consequently, the levels of NP7-specific IgG in the serum, particularly IgG2b and IgG2c, were also significantly diminished in the recipients of GW3965-treated Tfh-like cells (SI Appendix, Fig. S6E). Thus, treatment with an LXR agonist significantly dampened Tfh cell differentiation from naïve precursors, which led to diminished GC reactions and subsequent T cell-dependent IgG production in vivo.
Fig. 5.
LXR activation diminishes GC response in vivo. (A–D) Groups of mice were s.c. immunized with KLH emulsified in CFA and i.p. injected with vehicle or GW3965. Sera and lymphoid cells from the draining LNs were analyzed at day 8 (n = 5). (A) Scheme of the experiment. Representative FACS plots and absolute numbers (#) of Tfh cells (B), GC B cells (C), and plasma cells (D). (E) Levels of KLH-specific IgG, IgG1, IgG2c, and IgM in the serum. Data are representative of two independent experiments. Quantification plots show the mean ± SEM; *P < 0.05, and **P < 0.01.
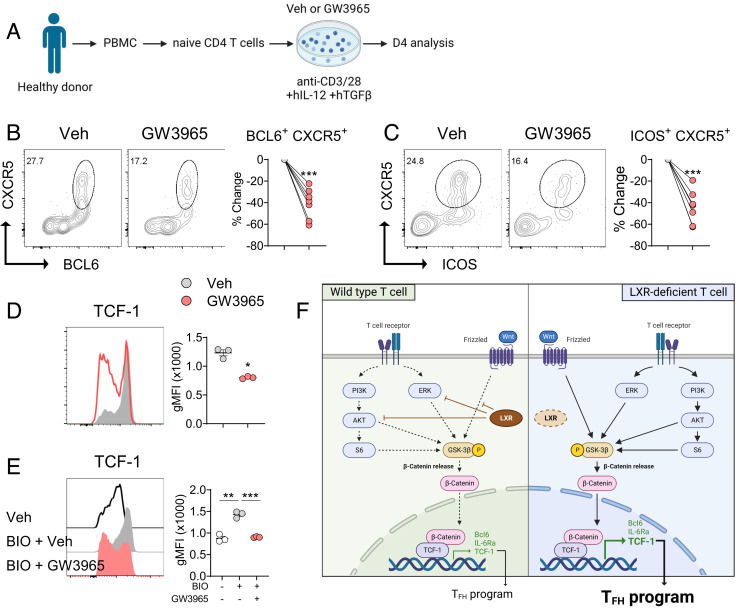
LXR Controls the Differentiation of Human Tfh Cell and TCF-1 Expression.
Finally, we sought to determine the effects of LXR agonism on the differentiation of human Tfh cells. To this end, we labeled flow-sorted naïve CD4+ T cells from healthy donors with vital dye CFSE before stimulating them under Tfh-skewing condition in the presence of IL-12 and TGF-β (Fig. 6A) (26, 27). Consistent with the data observed in multiple murine models, treatment with the LXR agonist significantly diminished the frequencies of BCL6+CXCR5+ and ICOS+CXCR5+ cells (Fig. 6 B and C). CFSE-dilution analysis showed that GW3965 treatment induced a similar significant reduction in the frequency of BCL6+CXCR5+ population in both undivided and 3 to 4 times divided CD4+ T cells in this experimental setting (SI Appendix, Fig. S7 A). To determine whether LXR also controls TCF-1 expression in human CD4+ T cells, we stimulated naïve CD4+ T cells with anti-CD3 and anti-CD28 and found that GW3965 treatment remarkably down-regulated TCF-1 levels (Fig. 6D). Consistently, the levels of TCF-1 expression were all significantly diminished by GW3965 regardless of the degree of T cell division (SI Appendix, Fig. S7 B). Moreover, while TCF-1 expression was increased by BIO, GW3965 almost completely abolished the upregulation of TCF-1 induced by BIO in human CD4+ T cells (Fig. 6E). Together with the results obtained in the murine models, these results strongly suggest that LXR inhibits the differentiation of Tfh cells by repressing the expression of TCF-1 in human CD4+ T cells.
Fig. 6.
LXR inhibits TCF-1 expression and Tfh cell differentiation in human CD4+ T cells. (A–C) Flow-sorted human naive CD4+ T cells were cultured under Tfh-skewing conditions before being analyzed by flow cytometry (n = 7). (A) Scheme of the experiment. (B and C) Percentage changes in BCL6+CXCR5+ cells (B) or ICOS+CXCR5+ cells (C) by GW3965 treatment. Each symbol represents an individual donor. (D and E) Human naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 (D) or with BIO (E) in the presence of vehicle or GW3965 and analyzed for the expression of TCF-1 (n = 3). (F) Working model on the regulatory role of LXR during Tfh cell differentiation. Data are representative of three independent experiments. Quantification plots show the mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
In the present study, we investigated the cell-intrinsic role of LXRβ in helper T cell subsets in vivo. Our findings unveil a crucial regulatory role of LXRβ in the Tfh cell lineage program by demonstrating that i) the frequency of Tfh cells, but not the other subsets of helper T cells, was increased in the LXRβ-deficient population in the mixed BM chimera and OT-II cotransfer studies, ii) LXRβ-deficient Tfh cells expressed higher levels of TCF-1 than WT Tfh cells, iii) activation of LXR down-regulated TCF-1 expression in murine and human CD4+ T cells, iv) treatment with an LXR agonist suppressed GC reactions and high-affinity IgG production in response to protein immunization, and v) LXR activation inhibited human Tfh cell differentiation regardless of the degree of T cell division. Based on these findings, we propose that LXRβ is a cell-autonomous negative regulator of Tfh cell differentiation through the repression of TCF-1.
Several studies have demonstrated a regulatory role of LXR in humoral immunity and antibody-mediated autoimmunity. For instance, LXRαβ-deficient mice spontaneously develop lupus-like phenotypes by the age of 10 mo, and treatment with an LXR agonist ameliorates lupus symptoms in lupus-prone mice (14). Defective clearance of apoptotic cells has been proposed as a cause of lupus-like phenotype in the LXRαβ-deficient mice. Similarly, treatment with LXR agonists induces preventive and therapeutic effects in animal models of collagen-induced arthritis (28, 29). Our recent study also showed that downregulation of LXR in dendritic cells leads to enhanced Tfh cell differentiation and subsequent humoral immunity in atherogenic mice (30). These studies have proposed that LXR negatively regulates humoral immunity by modulating the function of macrophages and dendritic cells. In addition to these immunoregulatory roles of LXR in innate immune cells, the present study revealed a T cell-intrinsic role of LXRβ in controlling Tfh cell differentiation by employing mixed BM chimeras and OT-II T cell cotransfer studies. The present study also proposes an additional cellular mechanism as uncontrolled autoreactive Tfh cell differentiation might contribute to autoantibody production in the LXRαβ-deficient mice. Thus, LXR acts as a critical checkpoint in macrophages, dendritic cells, and T cells to prevent unnecessary humoral immune responses.
Among Tfh cell signature molecules, LXRβ-deficient CD4+ T cells expressed increased levels of TCF-1 in multiple in vivo and in vitro experimental settings. How TCF-1 is regulated in T cells has been obscure. CD4+ T cells are known to down-regulate TCF-1 after a few divisions upon TCR stimulation; however, LXRβ-deficient CD4+ T cells expressed higher TCF-1 despite their enhanced proliferative potential, suggesting that LXRβ actively represses TCF-1 in activated T cells. Supporting this notion, we observed that GW3965 suppressed TCF-1 expression regardless of the degree division in murine and human CD4+ T cells. TCF-1 was initially discovered as a critical regulator of T cell development in the thymus and has been actively studied as an essential factor required for the stemness and memory response in T cells (31). More recent studies uncovered that TCF-1, together with LEF-1, plays a crucial role during the early differentiation of Tfh cells and Tfr cells by upregulating Bcl6, Il6ra, Il6st, and Icos (20, 32–35). Unlike TCF-1, LXR appeared to have little role in regulating LEF-1 expression in CD4+ T cells. TCF-1-binding factors include β-catenin, Bcl6, EZH2, and Foxp3. The transcriptional activity of TCF-1 is regulated by β-catenin, which can be released after the phosphorylation of GSK3β (36). Phosphorylation of GSK3β is triggered by PI3K/AKT and ERK pathway downstream of TCR signaling as well as by Wnt signaling. Our present study demonstrates that LXR controls TCF-1 expression via two different mechanisms. First, LXR suppresses phosphorylation of GSK3β by inhibiting PI3K/AKT and ERK activation in activated CD4+ T cells. This mechanism is supported by the enriched genes associated with PI3K-AKT signaling pathway in Nr1h2−/− Tfh cells in our GSEA analysis as well as increased phospho-AKT and phospho-ERK in Nr1h2−/− CD4+ T cells upon TCR stimulation. Second, LXR suppresses Wnt/β-catenin pathway in a TCR-independent manner, since we observed that LXR activation almost completely abolished the upregulation of TCF-1 induced by a GSK3β inhibitor in resting CD4+ T cells. These two mechanisms likely cooperate in CD4+ T cells to fine-tune the activity of TCF-1 by regulating the translocation of β-catenin (Fig. 6F). Two isoforms of TCF-1 exist; unlike the long isoform, the short form of TCF-1 does not have a β-catenin-binding domain but has a binding domain for Bcl6 and Foxp3. Thus, LXR may control TCF-1 independently of β-catenin, which will be an interesting subject for future studies. The present study also suggests that LXR regulates TCF-1 expression through posttranscriptional/translational modification in T cells. There are several known mechanisms for posttranslational modification of TCF-1 (37). For instance, NLK and NLK-associated ring finger protein regulates the phosphorylation and ubiquitination of TCF-1 in human embryonic kidney cells (38, 39). Moreover, CK-1 has been shown to phosphorylate TCF-1 in mouse myoblast cells (40). Further studies will clarify the detailed mechanism by which LXR represses the expression of TCF-1 in CD4+ T cells. In addition, TCF-1 is known to be required for maintaining stemness of T cells and regulating exhaustion, particularly in CD8+ T cells (41). It would be interesting to investigate whether LXR is involved in the stemness and exhaustion of CD8+ T cells via controlling TCF-1.
Previous studies showed that LXR activation has been shown to inhibit Th17 cell responses and suppress experimental autoimmune encephalomyelitis (EAE) in vivo (42, 43). While we also observed that Nr1h2−/− T cells showed enhanced Th17 cell differentiation in vitro, our immunization and infection models showed little difference in Th17 and Th1 cells between WT and Nr1h2−/− T cell compartments in mixed BM chimera studies, suggesting that the inhibitory role of LXR in Th17 cell responses could be through modulating innate immune cells including dendritic cells. LXR has been recently reported to prevent negative selection of developing thymocytes by reducing lipid rafts (16). Moreover, T cell-specific LXRβ-deficient mice exhibit impaired Treg cell functionality (17). This study also showed that Treg-specific deletion of LXRβ, even loss of a single copy of Nr1h2 (Foxp3creNr1h2 fl/+), results in fatal autoimmunity by 4 wk of age, similar to that observed in Scurfy mice. We observed a slight decrease in the thymic Treg cells, but a normal frequency of Treg cells in the secondary lymphoid organs in Nr1h2−/− mice. We also did not observe any fatal autoimmune symptoms in Nr1h2−/− mice by 6 mo of age. Moreover, our mixed BM chimera study revealed no difference in the frequency of Foxp3+ Treg cells between the WT and Nr1h2−/− T cell compartments in the periphery, except for a slight increase in the CXCR5+Foxp3+ Tfr cells in Nr1h2−/− mice. These findings strongly suggest that LXRβ is dispensable in Treg cell homeostasis, but suppresses differentiation of Tfr cells in a TCF-1–dependent manner (44). Similar to the present study, LXRαβ-deficient mice showed no defect in Foxp3+ Treg cells (14). Further studies are needed to illuminate the precise role of LXR in regulating the development and function of Foxp3+ Treg cells.
In summary, the present study outlines a previously unrecognized role for LXRβ in controlling Tfh cell differentiation via repression of TCF-1. Development of LXR synthetic agonists with selective induction of cholesterol efflux without inducing hypertriglyceridemia is an attractive area of therapeutic intervention, particularly for metabolic diseases. Our findings suggest that LXR could be also an attractive target for the treatment of autoimmune diseases caused by aberrant Tfh cell responses such as Pemphigus vulgaris, Sjogren’s syndrome, and systemic lupus erythematosus (45). Conversely, since LXR activation can hamper humoral immunity against infections, LXR agonists in clinical settings should be given with caution to prevent the risk of infection.
Materials and Methods
A full description of the Materials and Methods is available in SI Appendix.
Ethics Statement.
All animal experiments were performed according to protocols approved by Institutional Animal Care and Use Committees of Seoul National University (SNU 211025-7-2, SNU 200831-9-3, SNU 191210-3-11). Collection of human blood samples from healthy volunteers and subsequent experimental procedures were reviewed and approved by the Seoul National University Institutional Review Board (approval number: 2203/003-003), and all participants provided informed consent.
Animal Models.
For immunization studies, mice were subcutaneously immunized with 50 µg KLH (Sigma-Aldrich) or 100 µg NP-OVA (Biosearch Technologies) emulsified in CFA (Sigma-Aldrich), and lymphoid cells from the draining LNs were stained and analyzed by flow cytometry on day 8 to 10. For viral infection experiments, mice were intraperitoneally injected with LCMV-Armstrong (2 × 105 pfu), and lymphoid cells in the spleen were analyzed on day 8. In some experiments, mice were intraperitoneally treated with either Dimethyl sulfoxide (DMSO) vehicle or 20 mg/kg GW3965 (Tocris).
For BM chimera studies, 8- to 10-wk-old sublethally-irradiated Rag1−/− mice (9 Gy; X-RAD IR160, Precision X-Ray, USA) were i.v. injected 1:1 mixture of WT and Nr1h2−/− BM cells before 6 wk of reconstitution. The recipients were s.c. immunized with KLH in CFA or infected with LCMV, and lymphoid cells from the draining LNs or spleen were analyzed as indicated (46).
For OT-II T cell cotransfer studies, 1:1 mixture of flow-sorted WT and Nr1h2−/− naïve OT-II T cells (2 × 106 cells) were i.v. transferred into sex-matched B6.SJL congenic recipient mice. One day later, the recipients were s.c. injected with 100 µg OVA (Sigma-Aldrich) emulsified in CFA and lymphoid cells from the draining LNs were analyzed on day 8.
CD4+ T Cell Stimulation In Vitro.
CD4+ T cells from the spleen and peripheral LNs were positively selected with CD4 microbeads (L3T4; Miltenyi Biotec), and naive CD4+ T cells were further sorted as CD4+CD25−CD62LhighCD44low cells with the FACSAria III cell sorter (Becton, Dickinson and Company (BD) Biosciences) and stimulated with plate-coated anti-CD3 (1 μg/mL, 145-2C11: BioXCell) and soluble anti-CD28 (1 μg/mL, 37.51; BioXCell) for 3 d in RPMI 1640 (Sigma-Aldrich) supplemented with 10% FBS (Sigma-Aldrich). To measure TCR-mediate protein phosphorylation, flow-sorted naïve CD4+ T cells were stimulated with plate-coated anti-CD3 and anti-CD28 (2 μg/mL) before being analyzed at indicated time points. In some experiments, cells were treated with either DMSO vehicle or 1 µM GW3965 (Tocris), 1 µM BIO (Sigma-Aldrich), or mevalonic acid (Sigma-Aldrich).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Drs. Won-Il Jeong (Korea Advanced Institute of Science and Technology, Korea), Sang Geon Kim (Dongguk University, Korea) for providing Nr1h3−/− and Nr1h2−/− mice, respectively, and the entire Chung laboratory members for discussion and suggestions. This work was supported by the research grants Leader Research Program (2020R1A3B207889011 to Y.C.) and and Basic Science Research Program (NRF-2022R1A6A1A03046247 to Y.C.) from the National Research Foundation of Korea. J.K. is a recipient of Global PhD Fellowship Program (2019H1A2A1074484 to J.K.) from the National Research Foundation of Korea. Some images were created with BioRender.com.
Author contributions
J.K. and Y.C. designed research; J.K., J.-E.L., G.C., H.C., D.K., M.J.P., Y.S.G., and K.-S.S. performed research; C.-Y.K. and S.-K.K. contributed new reagents/analytic tools; J.K., H.L., and Y.C. analyzed data; and J.K. and Y.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
The RNAseq data have been deposited to the Gene Expression Omnibus with accession number GSE224303. All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Mayer A., Zhang Y., Perelson A. S., Wingreen N. S., Regulation of T cell expansion by antigen presentation dynamics. Proc. Natl. Acad. Sci. U.S.A. 116, 5914–5919 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au-Yeung B. B., et al. , A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 111, E3679–E3688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox C. J., Hammerman P. S., Thompson C. B., Fuel feeds function: Energy metabolism and the T-cell response. Nat. Rev. Immunol. 5, 844–852 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Buck M. D., Sowell R. T., Kaech S. M., Pearce E. L., Metabolic instruction of immunity. Cell 169, 570–586 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J., Jiang L.-Y., Yang H., Song B.-L., Intracellular cholesterol transport by sterol transfer proteins at membrane contact sites. Trends Biochem. Sci. 44, 273–292 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zhang J., et al. , Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 114, E6072–E6078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dustin M. L., The immunological synapse. Cancer Immunol. Res. 2, 1023–1033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W., Shi X., Xu C., Regulation of T cell signalling by membrane lipids. Nat. Rev. Immunol. 16, 690–701 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Mailer R. K. W., Gisterå A., Polyzos K. A., Ketelhuth D. F. J., Hansson G. K., Hypercholesterolemia induces differentiation of regulatory T cells in the liver. Circ. Res. 120, 1740–1753 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Cheng H.-Y., et al. , Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Invest. 126, 3236–3246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B., Tontonoz P., Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 14, 452–463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P., Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Kiss M., Czimmerer Z., Nagy L., The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J. Allergy Clin. Immunol. 132, 264–286 (2013). [DOI] [PubMed] [Google Scholar]
- 14.A-Gonzalez N., et al. , Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bensinger S. J., et al. , LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan C. T., et al. , Liver X receptors are required for thymic resilience and T cell output. J. Exp. Med. 217, e20200318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaels A. J., Campbell C., Bou-Puerto R., Rudensky A. Y., Nuclear receptor LXRβ controls fitness and functionality of activated T cells. J. Exp. Med. 218, e20201311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva R. I., et al. , Generation of T follicular helper cells is mediated by Interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., et al. , Genome-wide analysis identifies Bcl6-controlled regulatory networks during T follicular helper cell differentiation. Cell Rep. 14, 1735–1747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi Y. S., et al. , LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 16, 980–990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J., et al. , Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat. Immunol. 21, 777–789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen J., et al. , Pharmacological suppression of glycogen synthase kinase-3 reactivates HIV-1 from latency via activating Wnt/β-catenin/TCF1 axis in CD4+ T cells. Emerg. Microbes Infect. 11, 391–405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perdomo-Celis F., et al. , Reprogramming dysfunctional CD8+ T cells to promote properties associated with natural HIV control. J. Clin. Invest. 132, e157549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nish S. A., et al. , CD4+ T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J. Exp. Med. 214, 39–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkateswaran A., et al. , Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc. Natl. Acad. Sci. U.S.A. 97, 12097–12102 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt N., et al. , The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 15, 856–865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locci M., et al. , Activin A programs the differentiation of human TFH cells. Nat. Immunol. 17, 976–984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park M.-C., Kwon Y.-J., Chung S.-J., Park Y.-B., Lee S.-K., Liver X receptor agonist prevents the evolution of collagen-induced arthritis in mice. Rheumatology 49, 882–890 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Chintalacharuvu S. R., Sandusky G. E., Burris T. P., Burmer G. C., Nagpal S., Liver X receptor is a therapeutic target in collagen-induced arthritis. Arthritis Rheum. 56, 1365–1367 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Ryu H., et al. , Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol. 19, 583–593 (2018). [DOI] [PubMed] [Google Scholar]
- 31.van de Wetering M., Oosterwegel M., Dooijes D., Clevers H., Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. The EMBO J. 10, 123–132 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L., et al. , The transcription factor TCF-1 initiates the differentiation of TFH cells during acute viral infection. Nature Immunology 16, 991–999 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Wu T., et al. , TCF1 is required for the T follicular helper cell response to viral infection. Cell Rep. 12, 2099–2110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi J., Crotty S., Bcl6-mediated transcriptional regulation of follicular helper T cells (TFH). Trends Immunol. 42, 336–349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crotty S., T follicular helper cell biology: A decade of discovery and diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li F., et al. , TFH cells depend on Tcf1-intrinsic HDAC activity to suppress CTLA4 and guard B-cell help function. Proc. Natl. Acad. Sci. U.S.A. 118, e2014562118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao C., Xiao G., Hu J., Regulation of Wnt/β-catenin signaling by posttranslational modifications. Cell Biosci. 4, 13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishitani T., et al. , The TAK1–NLK–MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature 399, 798–802 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Yamada M., et al. , NARF, an nemo-like kinase (NLK)-associated Ring Finger Protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF) *. J. Biol. Chem. 281, 20749–20760 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Wang S., Jones K. A., CK2 controls the recruitment of wnt regulators to target genes in vivo. Curr. Biol. 16, 2239–2244 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Escobar G., Mangani D., Anderson A. C., T cell factor 1: A master regulator of the T cell response in disease. Sci. Immunol. 5, eabb9726 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui G., et al. , Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J. Clin. Invest. 121, 658–670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J., Wagoner G., Douglas J. C., Drew P. D., Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J. Leukoc. Biol. 86, 401–409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang B.-H., et al. , TCF1 and LEF1 control Treg competitive survival and Tfr development to prevent autoimmune diseases. Cell Reports 27, 3629–3645.e3626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gensous N., et al. , T follicular helper cells in autoimmune disorders. Front. Immunol. 9, 1637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuen D.-S., et al. , Critical regulation of follicular helper T cell differentiation and function by Ga13 signaling. Proc. Natl. Acad. Sci. U.S.A. 118, e2108376118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The RNAseq data have been deposited to the Gene Expression Omnibus with accession number GSE224303. All study data are included in the article and/or SI Appendix.