Abstract
Background
Up to 48% of ventilated coronavirus disease 2019 (COVID-19) patients develop ventilator-associated pneumonia (VAP) during hospitalization in an ICU. Dysbiotic oral microbiota can colonize the lower respiratory tract and lead to VAP. It is recommended to introduce oral care strategies in the ICU to prevent VAP. In this study, we observed the impact of an oral hygienic protocol with tooth brushing on cultivable oral bacteriota, the incidence of HAI and patient safety among mechanically ventilated COVID-19 patients in an ICU setting.
Methods
In this prospective cohort study, we recruited 56 adult COVID-19 patients who qualified for mechanical ventilation. Patients were divided into 2 groups depending on the oral care procedure: standard and extended oral procedures with tooth brushing. Oral bacteriota samples were taken first within 36 h and after 7 days of intubation. Microorganisms were identified by MALDI/TOF mass spectrometry. bacterial health care-associated infection (HAI) cases were retrospectively analyzed by etiology. A PFGE study was performed for Klebsiella pneumoniae to check for clonal spreading of strains from oral bacteriota samples and HAI cases.
Results
We observed significant dysbiosis and a decrease in cultivable oral bacteriota diversity, with a high frequency of potentially pathogenic species, including Acinetobacter baumannii and K. pneumoniae. The HAI incidence rate was high (55.2/1000 patient-days), most commonly of K. pneumoniae and A. baumannii etiologies, which correlated with the presence of A. baumannii and K. pneumoniae in the oral samples. Strains isolated from VAP cases were the same as oral isolates in 8 cases. The procedure with tooth brushing led to less frequent identification of A. baumannii in oral samples (55.6% vs. 5.3%, p = 0.001); however, it did not decrease the incidence of HAIs.
Conclusions
Dysbiotic oral bacteriota is an important source of respiratory pathogens. The introduction of tooth brushing in oral hygiene protocols in an ICU setting was effective in decreasing the extent of oral bacteriota dysbiosis; however, it did not reduce the risk of HAIs or mortality.
Trial registration: 1072.6120.333.2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-023-01218-y.
Keywords: Oral microbiota, Dysbiosis, COVID-19, Mechanical ventilation, ARDS, VAP, HAI, Ventilator-associated pneumonia, Health care-associated infection
Introduction
Interactions between the human oral microbiota and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are currently being intensively investigated [1]. SARS-CoV-2, similar to other coronaviruses, replicates in the oral cavity [2] and can cause pneumonia. It is estimated that approximately 15–20% of coronavirus disease 2019 (COVID-19) patients require admission to the intensive care unit (ICU) due to acute respiratory distress syndrome (ARDS). This population is at high risk of developing bacterial or fungal coinfections, with previous reports showing a high incidence of Enterobacter spp., Acinetobacter baumannii and Aspergillus spp. [3–5], health care-associated infections (HAIs). Up to 48% of ventilated COVID-19 patients develop ventilator-associated pneumonia (VAP) during hospitalization in an ICU, as reported in a recent meta-analysis [6]. VAP is a known risk factor for poor outcomes in this population of patients [7]; thus, it is important to identify and introduce effective interventions to prevent VAP. It is crucial in our setting, as studies have shown that HAIs are a major concern in Poland, and we lack proper monitoring [8–10].
Dysbiotic oral microbiota has the potential to colonize the lower respiratory tract [11] and lead to severe complications, including VAP. Oral aspiration is a key factor leading to lower respiratory tract infections.
There is a range of actions that can lower the risk of contamination of the lower respiratory tract during intubation and limit the occurrence of VAP [12, 13], but few have been investigated in larger trials. Avoiding intubation is recommended, as it has a major impact on the incidence of VAP; however, in many cases, there is no other treatment option. The modification of endotracheal tube cuff shapes or materials enabled minimization of fluid seepage into the lungs [14, 15]. Proper oral hygiene protocols were proven to limit the incidence of VAP in an ICU setting [16]. Nevertheless, the evidence for specific interventions is lacking or even conflicting.
The latest update of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) guidelines [17] recommended against the use of chlorhexidine as a selective oral decontamination for patients requiring mechanical ventilation, as some studies revealed that exposure to chlorhexidine oral care was associated with an increased risk of death [18].
In a recent Cochrane Library systematic review on oral care strategies for critically ill patients to prevent VAP [19], the authors concluded that antiseptics and tooth brushing may be more effective than standard oral health hygiene strategies in reducing the incidence of VAP and the length of ICU stay.
It is recommended to introduce oral care strategies as part of the medical treatment plan when a patient is admitted to the ICU [20]. Nevertheless, the practice of tooth brushing is not universally incorporated in local oral health protocols. In Poland, there is no clear consensus of the recommended oral health care protocols in ICUs.
The aims of this study were as follows:
Observation of dynamics in cultivable oral bacteriota among mechanically ventilated COVID-19 patients in an ICU setting
Evaluation of the incidence of HAIs and VAP and their association with cultivable oral bacteriota among mechanically ventilated COVID-19 patients in an ICU setting
Assessment of the impact of different oral hygienic procedures on cultivable oral bacteriota, the incidence of HAI and patient safety among mechanically ventilated COVID-19 patients in an ICU setting and approaches to oral care in an ICU setting
Materials and methods
Study design and participants
Recruitment for this prospective cohort study took place in the University Hospital in Krakow, Poland. Patients were offered the opportunity to participate in the study and asked for the signed consent form on admission to the hospital. During hospitalization, 56 of them qualified for intubation and were hospitalized in the temporary intensive care unit (ICU) for COVID-19 patients between 1st September and 31st January 2022.
The inclusion criteria were as follows:
SARS-CoV-2 infection confirmed by real-time reverse transcriptase-polymerase chain reaction (RT‒PCR) assay of nasal and pharyngeal swabs upon hospital admission
Signed consent to participate in the study
Patients admitted to the ICU
Intubation due to COVID-19-related pneumonia and acute respiratory distress syndrome (ARDS) within 36 h preceding study procedures
All medical procedures in the ICU were performed according to the local standards. To prevent leakage of fluids below the laryngeal/tracheal cuff, VBM medical manometers 21 [21] were used. The cuff pressure was measured by the nursing staff 3 times/day, with a normal range between 40 and 60 mmHg.
Demographic and clinical data were gathered from the hospital electronic medical records, including but not limited to age, sex, date of COVID-19 diagnosis, admission to the hospital and ICU, date of intubation, selected comorbidities, pre- and postintubation treatment, including systemic steroids and antibiotics, days of antibiotic treatment (DOT) before and after intubation (the number of Days a patient receives an antibiotic). Selected baseline and maximal laboratory results were also extracted.
Oral health assessment
On admission to the ICU, the patients’ oral health was assessed utilizing the modified Beck Oral Assessment Score (BOAS), consisting of 5 subscales: assessment of lips, mucosa and gingiva, tongue, teeth, and saliva. A higher score reflects dysfunction or tissue injury. BOAS scores range from 5 (no oral dysfunction) to 20 (severe dysfunction). A score greater than 5 is abnormal [22, 23] (Additional file 1: Appendix A1).
Oral cavity sampling methods
Samples were taken two times, first within 36 h of intubation (baseline) and again after 7 days of intubation (follow-up).
In each sampling, four oral habitats were sampled: buccal mucosa, tongue, buccal dental surface and gingival pocket. Every sample was taken by a trained dentist.
Sampling the posterior part of the dorsum of the tongue and buccal mucosa, we used ESwab™. ESwab combines a COPAN-invented flocked swab with 1 mL of liquid amine in a plastic, screw cap tube. Dental plaque was collected from the buccal dental surface side using Tooth Cleanic KerrHawe—KWX-OP-SZ-011, and after collection, the brush was placed in 1 mL of Liquid Amies in a plastic screw cap tube. Three pieces of PerioPaper Strips, designed to absorb or carry 0–1.2 µl of fluid, were used to collect gingival cerficular fluid (GCF) samples. The strips were placed in the gingival pocket for 30–45 s until its surface soaked up. To minimize the risk of preanalytical errors during sample collection, sterile gauze was used to remove excess saliva from the mucosae and dry the dental surfaces, preventing salivary contamination of GCF.
Intervention
Patients were divided into 2 groups depending on the oral care procedure:
Standard oral procedure (SP, cleaning and moisturizing of oral cavity, suction of excess fluid)
Extended oral procedure with tooth brushing (EP, cleaning and moisturizing of the oral cavity, tooth brushing, suction of excess fluid)
The procedures were performed twice daily in each patient. Detailed information on these procedures is included in Additional file 1: Appendix A2. The allocation protocol was based on the patients’ location in the ward. Each room was assigned one type of procedure (standard—even number or extended—uneven number of rooms) that was permanent during the study period. The efficacy of two different procedures for oral care in an ICU setting was assessed.
Microbiological cultures
After the collection of patient samples, they were delivered as soon as possible to the microbiological laboratory for the purpose of microorganism isolation. Banking and identification of bacterial strains from HAIs of all hospitalized patients (samples were collected throughout the duration of the whole hospitalization, not only during the intervention) in the temporary units for COVID-10 patients in the UH were performed in the Department of Microbiology, UH, Krakow. Various diagnostic samples were collected, cultured and assessed for the microbial etiology of infections. In this group, 11 K. pneumoniae strains from HAIs of patients under surveillance (with SP or EP) were used for further analysis: 5 from PNU, 3 from BSI and 3 from UTI cases. One strain from an oral sample of patient 6 was not stored. Strains from oral samples and HAIs were stored in the Department of Microbiology, UH, Krakow, at − 70 °C using Microbank® (Biomaxima, Lublin). Dilutions of samples (from − 1 to − 6) were inoculated on the following media: McConkey (Graso, Biotech), Columbia (Lab-Agar, Biomaxima), Scheadler (Scheadler-Agra, Biomaxima), Bile Esculine Azide (Lab-Agar, Biomaxima), and MRS Agar (Oxoid). Simultaneously, swab samples were inoculated with the qualitative culture method using the same media as in the dilution method. Media were aerobically incubated at 37 °C for 24 h (McConkey, Columbia, Bile Esculine Azide) or anaerobically at 37 °C for 48 h (M.R. S and Scheadler, GENbag Atmosphere Generators, BioMérieux, France). Next, the colonies were counted and phenotypically reported, and the results are presented as CFU/mL (colony forming unit). The microorganisms were identified using MALDI TOF MS mass spectrometry (Vitek MS Home bioMérieux).
Laboratory-confirmed health care-associated infections
The bacterial health care-associated infection (HAI) cases were analyzed retrospectively using definitions from the Healthcare-Associated Infections Surveillance Network (HAI-Net) [24], including bloodstream infections (BSI), pneumonias (PNU), urinary tract infections (UTI) and others. The cases and samples were obtained via passive surveillance defined as identifying and reporting of HAIs by nontrained personnel. In 2020–2022, no active, prospective surveillance and registration of HAIs was carried out in the hospital; therefore, there was no other source of information on HAIs that had not been microbiologically confirmed. LOS was defined as the sum of the days of stay (patient days, pds), including the day of admission and the day of discharge.
Microbiological samples were routinely acquired when there was suspicion of infection by taking blood, bronchoalveolar lavage (BAL) or urine samples. Only laboratory-confirmed cases based on culture growth qualified for the analysis; with multiple samples from the same infection case, only the first isolate from each patient was selected for microbiological analysis, with subsequent patient and HAI cultures excluded.
Multiple logistic regression models were built to identify predictors of HAI of A. baumannii, Enterococcus faecalis and Klebsiella. pneumoniae etiology (3 etiology-specific models) that included the type of oral care procedure applied, identification of A. baumannii/E. faecalis/K. pneumoniae in the baseline oral sampling, baseline BOAS score category and pre-ICU antibiotic use.
Pulsed-field gel electrophoresis (PFGE)
All isolates of Klebsiella pneumoniae were analyzed using the standardized PFGE protocol developed at the Centers for Disease Control and Prevention by the PulseNet program (version for Escherichia coli). Data from previous studies showed that K. pneumoniae strains isolated in Polish hospitals (mainly from ICUs for adults and neonates and pediatric units) often belong to one clone. For this reason, a PFGE study was performed for this species to check for clonal spreading of strains coming from oral bacteriota samples and HAI cases. Genomic DNA was prepared in situ in agarose blocks and was subsequently digested with restriction enzymes: XbaI (25 U per block, Thermo Scientific. The digested products were separated on a CHEF III PFGE system (Bio-Rad, Warsaw, Poland) in 0.5 × Tris-borate-EDTA buffer at 14 °C at 6 V for 22 h with a starting pulse of 2 s and a final pulse of 35 s. GelCompar (Applied Maths, Kortrijk, Belgium) was used for cluster analysis with the unweighted pair group method with an arithmetic mean and the Dice coefficient. Similarity must be > 90% for the pattern to be considered to belong to the same pulsotype. The results of the PFGE analysis are presented for each patient with a number that they were assigned on recruitment (1–56). The term “case” refers to HAI diagnoses.
Ethics statement
The study and its protocol were approved by the Jagiellonian University Bioethics Committee, decision number 1072.6120.333.2020; Dec 7, 2020, and 1072.6120.353.2020. Dec 16, 2020. Written informed consent was obtained from each subject prior to participation.
Statistical analysis
The PS Imago Pro ver. 7.0 was used for all statistical analyses. The normality of the continuous variable distribution was assessed using the Shapiro‒Wilk test. Differences between groups were analyzed with Student’s t test or nonparametric test (Mann–Whitney U test) when appropriate. Paired data were analyzed using the Wilcoxon test. Continuous variables are presented as the arithmetic mean (x̄) ± standard deviation (SD) or as the median with interquartile range (IQR) when the data were not normally distributed. The distribution of categorical variables was described as counts and percentages. Statistical testing was completed to compare categorical variables using an independent sample chi-squared test or Fisher's exact test when appropriate and dependent samples with McNemar’s test. We built a multivariate logistic regression model including an arbitrarily chosen number of predictors. We assured that the multicollinearity assumption was fulfilled. Negelkerke’s index was used as the coefficient of determination, R2. We measured the strength association by the odds ratio (OR) and 95% confidence intervals (CI). Statistical inference was set at p < 0.05.
Results
Demographic and clinical characteristics
The study population included 56 patients admitted to an ICU who required mechanical ventilation due to COVID-19-related ARDS. The mean age of the participants was 66.5 ± 12.7 years, and there were 24 (42.9%) females. The population was obese with a mean BMI of 31.9 ± 5.8. Sixteen (28.6%) were admitted directly from the emergency department, and 40 (71.4%) were transferred from another hospital ward. The mean time from COVID-19 diagnosis to intubation was 6.95 ± 6.62 days. Pre-ICU, systemic steroid therapy was used in 76.9% and antibiotics in 63.5% of patients. The median antibiotic days of therapy (DOT) before intubation was 7 (IQR 3–13.5). The full pre-ICU characteristics of the study population have been previously described elsewhere [25].
In the ICU, steroids and antibiotics were used in 85.7% and 55.4% of patients, respectively, with a median DOT of 8 (IQR 4–14) after intubation. The full baseline and follow-up characteristics of the study participants are presented in Table 1.
Table 1.
Baseline comparison of participants according to procedure
| Characteristics | Standard procedure N = 25 | Extended procedure with tooth brushing N = 31 | p value |
|---|---|---|---|
| Age [years] | 67 (56–73.5) | 67 (62–79) | NS |
| Female [n (%)] | 10 (40.0%) | 14 (45.2%) | NS |
| WHO ordinal scale, on admission to an ICU [43] | 7 (6–9) | 6 (5–9) | NS |
| Baseline BOAS, sum score | 11 (9.5–12.5) | 12 (11.25–14.8) | 0.032 |
| Baseline BOAS category | NS | ||
| 1–10 | 9 (36.0%) | 4 (16.7%) | |
| 11–20 | 16 (64.0%) | 20 (83.3%) | |
| Baseline BOAS, teeth | 3 (2–3) | 3 (2–4) | NS |
| In-hospital pharmacotherapy before intubation [n (%)] | |||
| Steroid therapy | 19 (76.0%) | 21 (77.8%) | NS |
| Antibiotic | 19 (76.0%) | 14 (51.9%) | NS |
| DOT before intubation [days] | 7 (2.8–13.3) | 7 (3–15) | NS |
| Baseline number of genera, all sites | 4 (3–5) | 5 (4–6) | 0.031 |
| Baseline number of species, all sites | 5 (4–6) | 7 (5–9) | 0.006 |
| Baseline number of patients with selected genera/species | |||
| Acinetobacter baumannii | 31.0% | 16.1% | NS |
| Enterococcus faecalis | 52.0% | 29.0% | NS |
| Escherichia coli | 16.0% | 19.4% | NS |
| Klebsiella pneumoniae | 28.0% | 12.9% | NS |
| Lactobacillus spp. | 56.0% | 58.1% | NS |
| Streptococcus spp. | 76.0% | 83.9% | NS |
| Baseline CFU/ml from oral samples | |||
| All bacterial strains | 4.0E + 5 (5.0E + 4–1.5E + 6) | 4.0E + 5 (1.0E + 5–1.4E + 6) | NS |
| All G + strains | 5.0E + 5 (1.5E + 5–1.5E + 6) | 5.0E + 5 (1.5E + 5–1.5E + 6) | NS |
| All G- strains | 2.3E + 4 (2.8E + 3–2.3E + 5) | 2.0E + 5 (2.0E + 4–8.0E + 5) | 0.021 |
| Streptococcus spp. | 7.0E + 5 (1.8E + 5–2.2E + 6) | 4.0E + 5 (1.5E + 5–1.5E + 6) | NS |
| Lactobacillus spp. | 1.0E + 6 (2.0E + 5–2.0E + 6) | 9.0E + 5 (2.0E + 5–2.0E + 6) | NS |
| Klebsiella pneumoniae | 3.0E + 4 (3.0E + 2–5.0E + 4) | 1.5E + 4 (1.6E + 3–1.4E + 5) | NS |
| Acinetobacter baumannii | 1.3E + 4 (2.3E + 3–1.9E + 4) | 1.0E + 4 (1.6E + 3–1.1E + 5) | NS |
| Enterococcus faecalis | 3.0E + 5 (7.0E + 4–1.0E + 6) | 5.0E + 5 (2.1E + 4–5.4E + 6) | NS |
Data are presented as the means (SDs), medians (Q1–Q3) or N [%]
WHO, World Health Organization; ICU, intensive care unit; BOAS, Beck Oral Assessment Scale, DOT, days of antibiotic therapy, HAI, health care-associated infection, CFU, colony forming unit, NS, not significant
Oral cultivable bacteriota composition
Between the baseline and follow-up oral sampling, the number of bacterial genera did not change, but a decrease in the overall number of species from all sites was observed (median 6 vs. 4, p = 0.005). Specifically, the number of species in mucosal swabs, dental surface samples and the tongue were lower at follow-up (medians 3, 4, and 3 vs. 2, 3, and 2; p = 0.008, p = 0.04 and p = 0.17, respectively). There were no differences in the overall CFU counts of all bacterial strains from all sites between baseline and follow-up.
At baseline, the number of patients with E. faecalis, A. baumannii, K. pneumoniae and E. coli in oral samples was high—39.3%, 23.2%, 19.6% and 17.9%, respectively. There was a general decrease in the rate of identification of all species at follow-up, but it remained relatively high for E. faecalis and A. baumannii—both 29.7%.
The complete qualitative and quantitative bacteriota characteristics are presented in Tables 2 and 3.
Table 2.
Follow-up comparison of participants according to procedure
| Characteristics | Standard procedure N = 18* | Extended procedure with tooth brushing N = 19* | p value |
|---|---|---|---|
| Follow-up BOAS, sum score | 11.5 (9–14.5) | 11 (9–13) | NS |
| Baseline BOAS category | NS | ||
| 1–10 | 8 (44.4%) | 6 (40.0%) | |
| 11–20 | 10 (55.6%) | 9 (60.0%) | |
| Follow-up BOAS, teeth | 3 (2–4) | 2 (1–3) | 0.026 |
| Follow-up number of genera, all sites | 4 (2.8–5.3) | 3 (2–4) | NS |
| Follow-up number of species, all sites | 4.5 (4–6.3) | 3 (2–5) | NS |
| Follow-up number of patients with selected genera/species | |||
| Acinetobacter baumannii | 55.6% | 5.3% | 0.001 |
| Enterococcus faecalis | 55.6% | 5.3% | 0.001 |
| Escherichia coli | 11.1% | 5.3% | NS |
| Klebsiella pneumoniae | 16.7% | 15.8% | NS |
| Lactobacillus spp. | 27.8% | 47.4% | NS |
| Streptococcus spp. | 61.1% | 42.1% | NS |
| Follow-up CFU from oral samples | |||
| All bacterial strains | 3.3E + 5 (5.8E + 4–1.1E + 6) | 1.9E + 5 (5.0E + 4–4.8e + 5 | 0.067 |
| All G + strains | 8.0E + 5 (2.0E + 5–1.7E + 6) | 1.4E + 5 (4.3E + 4–3.0E + 5) | < 0.001 |
| All G- strains | 3.0E + 4 (2.6E + 3–3.1E + 5) | 7.5E + 5 (1.1E + 5–1.9E + 6) | 0.004 |
| Streptococcus spp. | 7.5E + 5 (3.0E + 5–1.0E + 6) | 8.5E + 4 (3.5E + 4–5.3E + 5) | 0.008 |
| Lactobacillus spp. | 1.0E + 6 (1.5E + 5–2.0E + 6) | 1.5E + 5 (8.0E + 4–3.0E + 5) | 0.039 |
| Klebsiella pneumoniae | 1.2E + 5 (6.5E + 2–1.1E + 7) | 3.5E + 5 (1.4E + 5–4.8E + 5) | NS |
| Acinetobacter baumannii | 3.5E + 4 (4.8E + 3–3.1E + 5) | 3.0E + 4 (2.0E + 4 – NA) | NS |
| Enterococcus faecalis | NA | NA | NA |
Data are presented as the means (SDs), medians (Q1–Q3) or N [%]
WHO, World Health Organization; ICU, intensive care unit; BOAS, beck oral assessment scale, DOT, days of antibiotic therapy, HAI, health care-associated infection, CFU, colony forming unit, NS, not significant, NA, not available
*Number of patients who survived until the follow-up
Table 3.
Baseline and follow-up characteristics by procedure performed
| Characteristics | Standard procedure | Extended procedure with tooth brushing | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | p value* | Baseline | Follow-up | p value* | |
| BOAS, sum score | 11 (9.5–12.5) | 11.5 (9–14.5) | NS | 12 (11.25–14.8) | 11 (9–13) | NS |
| BOAS category | ||||||
| 1–10 | 9 (36.0%) | 8 (44.4%) | NS | 4 (16.7%) | 6 (40.0%) | NS |
| 11–20 | 16 (64.0%) | 10 (55.6%) | NS | 20 (83.3%) | 9 (60.0%) | NS |
| BOAS, teeth | 3 (2–3) | 2.5 (2–3) | NS | 3 (2–4) | 2 (1–3) | 0.026 |
| Percentage of patients with selected genera/species in oral samples | ||||||
| Acinetobacter baumannii | 31.0% | 55.6% | NS | 16.1% | 5.3% | NS |
| Enterococcus faecalis | 52.0% | 55.6% | NS | 29.0% | 5.3% | NS |
| Escherichia coli | 16.0% | 11.1% | NS | 19.4% | 5.3% | NS |
| Klebsiella pneumoniae | 28.0% | 16.7% | NS | 12.9% | 15.8% | NS |
| Lactobacillus spp. | 56.0% | 27.8% | NS | 58.1% | 47.4% | NS |
| Streptococcus spp. | 76.0% | 61.1% | NS | 83.9% | 42.1% | 0.039 |
Data are presented as the means (SDs), medians (Q1–Q3) or N [%]
BOAS, beck oral assessment scale, NS, not significant
*p value for comparison baseline versus follow-up
Impact of the extended oral care procedure with tooth brushing
The extended procedure with tooth brushing was performed for 55.4% of patients. Oral samples were taken from 56 patients at baseline and 37 (66.1%) 7 days post intubation due to death before the prespecified follow-up sample collection date. The 7-day mortality rate in our study sample was 33.9%.
There were some significant baseline differences between the intervention subgroups, with patients undergoing EP in more severe condition and with worse baseline oral health status (Table 1).
At follow-up, there were no differences in the total BOAS score, mortality rate or hospital infection rate. Patients who had EP performed had significantly lower follow-up BOAS teeth subscale scores after 7 days of observation (median 2 [IQR 1–3] vs. 3 [IQR 2–4], p = 0.026).
EP resulted in a lower occurrence of E. faecalis and A. baumannii compared to the standard procedure after 7 days of intubation (both 55.6% vs. 5.3%, p = 0.001). The overall CFU of all bacterial strains from all sites was lower in the EP group, but it did not reach statistical significance (median 3.3E + 5 [IQR 5.8E + 4–1.1E + 6] vs. 1.9E + 5 [IQR 5.0E + 4 = 4.8E + 5], p = 0.067).
The full comparison of baseline and follow-up characteristics of the study participants depending on the procedure performed is presented in Table 1.
Bacterial health care-associated infections
In total, 52 different HAIs were detected, including 9 BSIs, 21 PNUs, and 22 UTIs. The HAI incidence density was 55.2/1000 pds. A total of 21.4% of patients had > 1 infection during hospitalization. There were no differences in the HAI incidence between the SP and EP groups (71.0% vs. 72.0%, p = 0.932). The most common etiologies for infections included K. pneumoniae (27.5% of patients with HAIs), A. baumannii (25%) and E. faecalis (25%). The polyetiological HAIs were confirmed in 25% of patients. There were no differences in HAI etiology between the oral care procedure groups. A summary of the cases of HAIs is presented in Table 4.
Table 4.
Cases of infections in ventilated patients hospitalized in the ICU
| Standard procedure N = 25*, 436 patient-days | Extended procedure with tooth brushing N = 31*, 525 patient-days | p value | |||
|---|---|---|---|---|---|
| Number of cases | Incidence rate | Number of cases | Incidence rate | ||
| HAIs, any types of infections | 24 | 55.1/1000 pds | 28 | 53.3/1000 pds | NS |
| PNU | 10 | 22.9/1000 pds | 11 | 20.9/1000 pds | NS |
| BSI | 4 | 9.2/1000 pds | 5 | 9.5/1000 pds | NS |
| UTI | 10 | 22.9/1000 pds | 12 | 22.9/1000 pds | NS |
| HAIs etiology | |||||
| Acinetobacter baumannii | 7 | 16.1/1000 pds | 4 | 7.6/1000 pds | NS |
| Enterococcus faecalis | 7 | 16.1/1000 pds | 3 | 5.7/1000 pds | NS |
| Klebsiella pneumoniae | 7 | 16.1/1000 pds | 4 | 7.6/1000 pds | NS |
| Other | 8 | NA | 21 | NA | NA |
ICU, intensive care unit, HAI, health care-associated infection, VAP, ventilator-associated pneumonia, NS, not significant, NA, not available
*Number of patients who were included in the standard/extended procedure, regardless of survival status
Impact of oral cultivable bacteriota composition on health care-associated infections
There were some significant associations between selected species identified in the oral samples and HAIs. A higher incidence of VAP of any etiology was identified in patients with A. baumannii on follow-up sampling (100% vs. 42.9%, p = 0.023). The baseline and follow-up identification of A. baumannii and K. pneumoniae in oral samples resulted in a higher incidence of VAP of the same etiology during hospitalization (baseline: 100.0% vs. 22.2% and 80% vs. 9.1%, p = 0.003 and p = 0.013, respectively; follow-up 100.0% vs. 14.3%, 100.0% vs. 20%, p = 0.005 and p = 0.035).
The baseline and follow-up identification of A. baumannii, K. pneumoniae and E. faecalis in oral samples resulted in a higher incidence of any infection of the same etiology during hospitalization (baseline: 72.7% vs. 6.9%, 70% vs. 13.3%, and 41.2% vs. 13%, p < 0.001, p = 0.002 and p = 0.049, respectively; follow-up 85.7% vs. 4.8%, 80% vs. 17.4%, 50% vs. 5,6%, p < 0.001, p = 0.015 and p = 0.013).
There were no associations between BOAS scores, LOS and patients’ origin and the incidence of infection in the ICU.
In multiple logistic regression models for predictors of A. baumannii-HAI and K. pneumoniae-HAI, the only significant factors increasing the risk of infections were identification of A. baumannii and K. pneumoniae in the baseline oral sampling (ORs 35.1 and 16.2, respectively, Tables 5, 6, 7).
Table 5.
Multiple logistic regression model for predictors of HAI of K. pneumoniae etiology
| p | OR | CI | |
|---|---|---|---|
| EP versus SP | 0.258 | 0.29 | 0.03–2.51 |
| Baseline BOAS category 11–20 versus 0–10 | 0.557 | 1.97 | 0.21–18.90 |
| Antibiotic before intubation (yes vs. no) | 0.866 | 1.21 | 0.14–10.47 |
| K. pneumoniae in baseline oral samples (yes vs. no) | 0.005 | 16.24 | 2.34–112.80 |
N = 32
OR, odds ratio, SP, standard procedure, EP, extended procedure with tooth brushing, BOAS, Beck Oral Assessment Scale
Table 6.
Multiple logistic regression model for predictors of HAI of A. baumannii etiology
| p | OR | CI | |
|---|---|---|---|
| EP versus SP | 0.506 | 0.445 | 0.04–4.82 |
| Baseline BOAS category 11–20 versus 0–10 | 0.656 | 0.580 | 0.05–6.38 |
| Antibiotic before intubation (yes vs. no) | 0.452 | 0.346 | 0.02–5.50 |
| A. baumannii in baseline oral samples (yes vs. no) | 0.012 | 31.96 | 2.13–479.46 |
N = 31
OR, odds ratio, SP, standard procedure, EP, extended procedure with tooth brushing, BOAS, Beck Oral Assessment Scale
Table 7.
Multiple logistic regression model for predictors of HAI of E. faecalis etiology
| p | OR | CI | |
|---|---|---|---|
| EP versus SP | 0.353 | 0.38 | 0.05–2.93 |
| Baseline BOAS category 11–20 versus 0–10 | 0.375 | 0.45 | 0.08–2.61 |
| Antibiotic before intubation (yes vs. no) | 0.730 | 1.44 | 0.18–11.30 |
| E. faecalis in baseline oral samples (yes vs. no) | 0.243 | 2.82 | 0.49–16.08 |
N = 32
OR, odds ratio, SP, standard procedure, EP, extended procedure with tooth brushing, BOAS, Beck Oral Assessment Scale
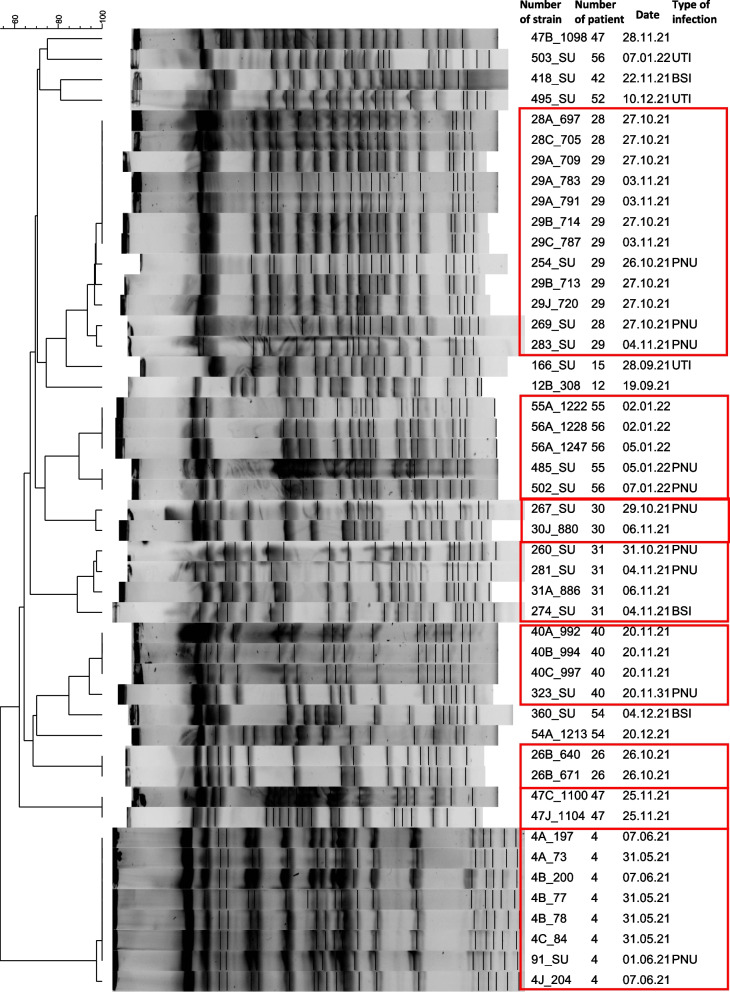
Pulsed-field gel electrophoresis (PFGE, Fig. 1)
Fig. 1.
The results of pulsed-field gel electrophoresis (PFGE). K. pneumoniae strains. The results are presented for each patient with a number that they were assigned on recruitment (1–56). Rows ending in bloodstream infections (BSI), pneumonia (PNU), and urinary tract infections (UTI) represent HAI strains, and rows ending in blank space represent oral samples
The comparison of K. pneumoniae strains isolated from different habitats of the oral cavity showed that in 11 patients, these strains were identical. Only in one patient were two different strains identified in the oral cavity (patient 47).
Strains isolated from all PNU and one BSI case were the same as oral isolates in respective patients in 8 HAI cases (PNU: patients 4, 28, 29, 31 30, 40, 47; BSI: patient 31). In five HAI cases (UTI: patients 15, 52, 56; BSI: patients 42, 54), the strains isolated from HAIs were different from the oral isolates.
Additionally, in two pairs of patients (28 and 29 and 55 and 56), the presence of K. pneumoniae strains belonging to one clone was observed, both in the oral and HAI case strains.
Discussion
In this study, we analyzed the dynamics of oral cultivable bacteriota and its association with HAIs, including VAP, among mechanically ventilated COVID-19 patients hospitalized in an ICU in the 7-day postintubation follow-up period. The oral bacteriota samples from both study periods revealed substantial dysbiosis, both qualitative and quantitative. During hospitalization, we observed a significant decrease in cultivable oral bacteriota diversity, with a high frequency of potentially pathogenic species, including A. baumannii, K. pneumoniae and E. faecalis, and a decreasing frequency of commensal Streptococci spp. strains. The incidence of HAIs was high, and their etiology correlated with the presence of A. baumannii and K. pneumoniae in the oral samples. The implemented procedure, which added tooth brushing to the standard oral care protocol, led to less frequent identification of A. baumannii and E. faecalis in oral samples; however, it was not sufficient for improving overall oral health or decreasing the risk of HAIs and mortality.
Both baseline and follow-up identification rates of selected bacterial species, such as A. baumannii, K. pneumoniae, E. faecalis and E. coli, in samples from the oral cavity were surprisingly high in our study population. During the observation period, those rates decreased but remained substantially high. The presence of A. baumannii and K. pneumoniae in oral samples was associated with HAIs of corresponding etiology. The presence of A. baumannii in oral samples was associated with VAP of the corresponding etiology. This finding is clinically significant, providing further evidence for a direct link between oral dysbiosis and VAP etiology [26]. Notably, for patients hospitalized in an ICU, the process is multifactorial. Salivary dysfunction due to medical procedures or medications and compromised oral care leading to accumulation of biofilm play an important role in the development of oral dysbiosis in this population. Additionally, the potential risk of cross-contamination between patients is listed among factors that can lead to a higher risk of dysbiotic oral bacteria reaching the lower respiratory tract [26]. Thus, providing proper oral hygiene can act as a protective measure against VAP [17, 19]. Using antiseptics, i.e., chlorhexidine, is a contested strategy [19]. Others include mechanical removal of dental biofilm, plaque and debris from the oral cavity to reduce the potentially pathogenic bacterial load and limit the risk of its aspiration to the airways [19].
In our study, we implemented an extended oral hygiene procedure that included tooth brushing as a measure of dental plaque control. The dental plaque of ventilated patients has been reported to present severe dysbiosis, with a high frequency of identification of E. faecalis and E. coli [27]. Oral dysbiosis of COVID-19 patients had been previously investigated and showed a high abundance of Enterococcus spp. and E. coli in oral samples from COVID-19 patients who did not require ICU hospitalization.
In the current study, patients undergoing the extended procedure with tooth brushing achieved better scores for the teeth subscale in the BOAS and, most importantly, had A. baumannii and E. faecalis less frequently identified in oral swabs. Unfortunately, it was not sufficient for improving overall oral health and decreasing the incidence of HAIs and mortality. One study reported similar observations, with lower BOAS scores and plaque index but no reduction in VAP incidence rates [28].
In our opinion, a lower identification rate of A. baumannii, a major cause of VAP in the ICU [29], in oral swabs is a first step toward effective VAP prevention. The fact that in our population it was not followed by a lower incidence of VAP could have resulted from significant dysbiosis and a high baseline number of patients with potentially pathogenic strains identified in the oral samples. Moreover, it is noteworthy that patients undergoing the extended procedure with tooth brushing had worse conditions of the oral cavity at baseline, thus probably limiting the efficacy of the intervention in comparison to patients with standard oral care. Analysis of PFGE results revealed that in some cases, PNU of K. pneumoniae etiology was diagnosed at the start of intubation (patients 4, 30, 40, 28, 29, 55, 56, Fig. 1), or additionally, the patients were subject to horizontal transmission of K. pneumoniae strains pre-ICU (patients 28, 29). In one case (patients 55 and 56 undergoing the standard procedure), PNU was diagnosed in the early postintubation period. Additionally, in one patient (56), UTIs of different K. pneumoniae strain etiologies were diagnosed. Finally, in one patient (31, undergoing the extended procedure with tooth brushing), PNU and BSI of the same K. pneumoniae strain etiology were recorded, suggesting BSI secondary to PNU and/or contamination outside the CVC (or PVC) or catheter hub by contact with hands. This implies that due to the high risk of oral contamination by potentially pathogenic microorganisms and early postintubation dysbiosis of the cultivable oral bacteriota, it may have been too late for the intervention to play a significant role in the prevention of VAP in our setting. It is possible that introducing proper pre-ICU oral care protocols could play some role in the prevention of HAIs in an ICU setting. In this case, patients in a clinical condition enabling proper comprehension and cooperation with the ward staff could take an active role in these measures. Finally, a single oral health care procedure may not be enough to assure good oral health, and such procedures should be bundled. A recent study of introducing oral bundle care in the place of chlorhexidine did not show a clear benefit for ventilated patients in the ICU [30]. Nevertheless, this issue requires further research.
The incidence of HAIs was high, affecting more than 70% of our study population, with bloodstream infections and VAP as the most frequent. The most common etiologies involved K. pneumoniae and A. baumannii.
HAIs in critical care are a major problem, with VAP as one of the most common infections that increases mortality and the length of hospitalization [20]. Multiple species are associated with VAP, especially Pseudomonas aeruginosa, E. coli, K. pneumoniae and Acinetobacter spp. [29]. The prevalence of multidrug resistant strains is high, and severe infections of this etiology are associated with mortality as high as 70% [31, 32].
In the population of COVID-19 patients, similar observations were made, with on average half those admitted to the ICU developing VAP (but some reports showed the rate of VAP to be as high as 86%), and ca. 43% mortality in those who developed VAP [33, 34]. In COVID-19 patients, Enterobacterales were reported to be the most common etiology of VAP (nearly 50%), but with mixed etiologies in up to 39% of patients [35]. The high incidence of VAP in COVID-19 patients is believed to result from a severe dysregulation of the immune system and hyperinflammatory reaction against SARS-CoV-2, leading to organ damage and further impairment of antimicrobial mechanisms [34].
Most previous studies investigating the relationship between SARS-CoV-2 virus, K. pneumoniae and A. baumannii focused mainly on the incidence of multidrug-resistant strains of those species in the ICU setting [36, 37]. One study showed similar results to ours, with COVID-19 increasing the risk of A. baumannii infections [38], with extremely high mortality rates in the elderly population.
There are some limitations to our study. The allocation protocol based on the fixed procedure performed in designated rooms did not assure sufficient control for confounders. Notably, analyses revealed that the patients undergoing the extended oral health care procedure with tooth brushing were in a more severe condition and had worse baseline oral health status. We believe that this may be one of the reasons for the lack of efficacy differences between the two investigated procedures but, importantly, it limited the risk of type I error.
Second, evidence of the dynamics of the oral microbiota among patients in a prone position is lacking. The prone position is a recommended method for mechanically ventilated patients. This affects transpulmonary pressure and achieves better blood oxygenation in patients with respiratory failure [39–42]. It has several advantages: the lungs are less compressed, gas exchange is more efficient, ventilator-induced injury risk is reduced and better drainage of secretions from diseased lungs is observed. However, we did not record the actual time spent in this position; thus, we were not able to ascertain its impact on oral bacteriota.
Conclusions
Patients with a severe course of COVID-19 who are hospitalized in an ICU and require mechanical ventilation are at elevated risk of developing health care-associated infections. The most prevalent etiologies of HAI in this population were E. faecalis, K. pneumoniae and A. baumannii. Dysbiotic oral bacteriota is an important source of these pathogens. The introduction of tooth brushing in oral hygiene protocols in an ICU setting was effective in decreasing the extent of oral bacteriota dysbiosis; however, it did not reduce the risk of HAIs or mortality.
Supplementary Information
Additional file 1. Appendix A1. Beck Oral Assessment Score (BOAS). Appendix A2. Oral care procedures.
Acknowledgements
The authors would like to thank all the patients who participated in this study. We would like to acknowledge the effort of medical staff in our hospital during the COVID-19 pandemic.
Abbreviations
- SD
Standard deviation
- WHO
World Health Organization
- ICU
Intensive care unit
- BOAS
Beck oral assessment scale
- DOT
Days of antibiotic therapy
- HAI
Health care-associated infection
- VAP
Ventilator-associated pneumonia
- CFU
Colony forming unit
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ARDS
Acute respiratory distress syndrome
- ERS
European Respiratory Society
- ESICM
European Society of Intensive Care Medicine
- ESCMID
European Society of Clinical Microbiology and Infectious Diseases
- ALAT
Asociación Latinoamericana del Tórax
- SOFA
Sequential organ failure assessment
- GCF
Gingival crevicular fluid
- PFGE
Pulsed-field gel electrophoresis
- IQR
Interquartile range
- OR
Odds ratio
Author contribution
IGM conceived and designed this study, was responsible for coordination and supervision, performed study procedures, sampled oral niches, and drafted the manuscript. AP participated in the development of the study methodology, performed microbiological analyses and validated the results. MF participated in the development of the study methodology, performed study procedures, sampled oral niches, curated the data, and helped draft the manuscript. MK curated the data, performed formal data analysis, prepared tables and drafted the manuscript. AK participated in the development of the study methodology, performed microbiological analyses and validated the results. PM developed the study methodology, performed study procedures, sampled oral niches, curated the data and helped draft the manuscript. EJM participated in the development of the study methodology, performed microbiological analyses and validated the results. DR participated in the development of the study methodology, performed microbiological analyses and validated the results. AC: performed microbiological analyses, validated the results and prepared figures BZ: was responsible for resources, project coordination and supervision JWM participated in the study design, developed the study methodology, performed microbiological analyses, validated the results and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This publication was supported by the National Center for Research and Development CRACoV-HHS project (Model of multi-specialist hospital and non-hospital care for patients with SARSCoV-2 infection) through the initiative “Support for specialist hospitals in fighting the spread of SARSCoV-2 infection and in treating COVID-19” (contract number SZPITALE-JEDNOIMIENNE/18/2020). The research described was implemented by consortium of the University Hospital in Krakow and the Jagiellonian University Medical College.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to institutional regulations but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study and its protocol were approved by the Jagiellonian University Bioethics Committee, decision numbers 1072.6120.333.2020, Dec 7, 2020, and 1072.6120.353.2020 from 16.12.2020. Written informed consent was obtained from each subject prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/29/2023
A Correction to this paper has been published: 10.1186/s13756-023-01256-6
References
- 1.Soltani S, Zakeri A, Zandi M, et al. The role of bacterial and fungal human respiratory microbiota in COVID-19 patients. Biomed Res Int. 2021 doi: 10.1155/2021/6670798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drozdzik A, Drozdzik M. Oral pathology in COVID-19 and SARS-CoV-2 infection—molecular aspects. Int J Mol Sci. 2022 doi: 10.3390/IJMS23031431/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangel K, Chagas TPG, De-Simone SG. Acinetobacter baumannii infections in times of COVID-19 pandemic. Pathogens. 2021;10(8):1006. doi: 10.3390/PATHOGENS10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiema M, Wlodarczyk A, Wojkowska-Mach J, Garlicki J, Gregorczyk-Maga I. Atypical presentation of Aspergillus niger infection in the oral cavity as a prediction of invasive pulmonary aspergillosis in a patient with COVID-19: case report and literature review. Microorganism. 2022;10(8):1630. doi: 10.3390/MICROORGANISMS10081630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonazzetti C, Morena V, Giacomelli A, et al. Unexpectedly high frequency of Enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49(1):e31. doi: 10.1097/CCM.0000000000004748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Khanna P, Sarkar S. Comparative evaluation of ventilator-associated pneumonia in critically ill COVID- 19 and patients infected with other corona viruses: a systematic review and meta-analysis. Monaldi Arch chest Dis Arch Monaldi per le Mal del torace. 2021 doi: 10.4081/MONALDI.2021.1610. [DOI] [PubMed] [Google Scholar]
- 7.Li HY, Wang HS, Wang YL, Wang J, Huo XC, Zhao Q. Management of ventilator-associated pneumonia: quality assessment of clinical practice guidelines and variations in recommendations on drug therapy for prevention and treatment. Front Pharmacol. 2022;13:903378. doi: 10.3389/FPHAR.2022.903378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deptuła A, Trejnowska E, Dubiel G, Wanke-Rytt M, Deptuła M, Hryniewicz W. Healthcare associated bloodstream infections in Polish hospitals: prevalence, epidemiology and microbiology—summary data from the ECDC point prevalence survey of healthcare associated infections 2012–2015. Eur J Clin Microbiol Infect Dis. 2017;37(3):565–570. doi: 10.1007/S10096-017-3150-1. [DOI] [PubMed] [Google Scholar]
- 9.Kołpa M, Wałaszek M, Różańska A, Wolak Z, Wójkowska-Mach J. Hospital-wide surveillance of healthcare-associated infections as a source of information about specific hospital needs. A 5-year observation in a multiprofile provincial hospital in the South of Poland. Int J Environ Res Public Heal. 2018;15(9):1956. doi: 10.3390/IJERPH15091956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafa E, Wałaszek MZ, Wałaszek MJ, Domański A, Różańska A. The incidence of healthcare-associated infections, their clinical forms, and microbiological agents in intensive care units in Southern Poland in a multicentre study from 2016 to 2019. Int J Environ Res Public Heal. 2021;18(5):2238. doi: 10.3390/IJERPH18052238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglini D, Robba C, Fedele A, et al. The role of dysbiosis in critically ill patients with COVID-19 and acute respiratory distress syndrome. Front Med. 2021;8:671714. doi: 10.3389/FMED.2021.671714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luyt CE, Sahnoun T, Gautier M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020 doi: 10.1186/S13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi: 10.1007/S00134-020-05980-0/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaillette E, Girault C, Brunin G, et al. Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: a multicenter cluster-randomized cross-over controlled trial. Intensive Care Med. 2017;43(11):1562–1571. doi: 10.1007/S00134-017-4736-X. [DOI] [PubMed] [Google Scholar]
- 15.Deem S, Yanez D, Sissons-Ross L, Broeckel JAE, Daniel S, Treggiari M. Randomized pilot trial of two modified endotracheal tubes to prevent ventilator-associated pneumonia. Ann Am Thorac Soc. 2016;13(1):72–80. doi: 10.1513/ANNALSATS.201506-346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Silva Pinto AC, Da Silva BM, Santiago-Junior JF, De Carvalho Sales-Peres SH. Efficiency of different protocols for oral hygiene combined with the use of chlorhexidine in the prevention of ventilator-associated pneumonia. J Bras Pneumol. 2021;47(1):1–8. doi: 10.36416/1806-3756/E20190286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 18.Deschepper M, Waegeman W, Eeckloo K, Vogelaers D, Blot S. Effects of chlorhexidine gluconate oral care on hospital mortality: a hospital-wide, observational cohort study. Intensive Care Med. 2018;44(7):1017–1026. doi: 10.1007/S00134-018-5171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao T, Wu X, Zhang Q, Li C, Worthington HV, Hua F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD008367.PUB4/MEDIA/CDSR/CD008367/IMAGE_N/NCD008367-CMP-005.06.SVG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershonovitch R, Yarom N, Findler M. Preventing ventilator-associated pneumonia in intensive care unit by improved oral care: a review of randomized control trials. SN Compr Clin Med. 2020;2(6):727–733. doi: 10.1007/S42399-020-00319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuff Manometer | VBM Medizintechnik GmbH. Accessed 3 Sept 2022. https://www.vbm-medical.de/en/products/airway-management/cuff-pressure-gauges/cuff-manometer/
- 22.Beck S. Impact of a systematic oral care protocol on stomatitis after chemotherapy. Cancer Nurs. 1979;2(3):185–199. doi: 10.1097/00002820-197906000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ames NJ, Sulima P, Yates JM, et al. Effects of systematic oral care in critically ill patients: a multicenter study. Am J Crit Care. 2011;20(5):e103. doi: 10.4037/AJCC2011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Centre for Disease Prevention and Control, Surveillance of healthcare-associated infections in intensive care units : HAI-Net ICU protocol, version 2.2, European Centre for Disease Prevention and Control, 2017. https://data.europa.eu/doi/10.2900/833186.
- 24.Gregorczyk-Maga I, Fiema M, Kania M, Kędzierska J, Romaniszyn D, Wójkowska-Mach J. Cultivable oral bacteriota dysbiosis in mechanically ventilated COVID-19 patients. Front Microbiol Sec Infect Agents Dis. 2022 doi: 10.3389/fmicb.2022.1013559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao L, Zhang C, Dong J, Zhao L, Li Y, Sun J. Oral microbiome and SARS-CoV-2: beware of lung co-infection. Front Microbiol. 2020;11:1840. doi: 10.3389/FMICB.2020.01840/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands KM, Twigg JA, Lewis MAO, et al. Microbial profiling of dental plaque from mechanically ventilated patients. J Med Microbiol. 2016;65(Pt 2):147. doi: 10.1099/JMM.0.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haghighi A, Shafipour V, Bagheri-Nesami M, GholipourBaradari A, Yazdani CJ. The impact of oral care on oral health status and prevention of ventilator-associated pneumonia in critically ill patients. Aust Crit Care. 2017;30(2):69–73. doi: 10.1016/j.aucc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Rewa O, Muscedere J. Ventilator-associated pneumonia: update on etiology, prevention, and management. Curr Infect Dis Rep. 2011;13(3):287–295. doi: 10.1007/S11908-011-0177-9/TABLES/1. [DOI] [PubMed] [Google Scholar]
- 30.Dale CM, Rose L, Carbone S, et al. Effect of oral chlorhexidine de-adoption and implementation of an oral care bundle on mortality for mechanically ventilated patients in the intensive care unit (CHORAL): a multi-center stepped wedge cluster-randomized controlled trial. Intensive Care Med. 2021;47(11):1295–1302. doi: 10.1007/S00134-021-06475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duszynska W, Litwin A, Rojek S, Szczesny A, Ciasullo A, Gozdzik W. Analysis of <em>Acinetobacter baumannii </em>hospital infections in patients treated at the intensive care unit of the University Hospital, Wroclaw, Poland: a 6-year, single-center, retrospective study. Infect Drug Resist. 2018;11:629–635. doi: 10.2147/IDR.S162232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wałaszek M, Różańska A, Bulanda M, Wojkowska-Mach J, Team PS of HI. Alarming results of nosocomial bloodstream infections surveillance in Polish intensive care units. Przegl Epidemiol. 2018;72(1):33–44. [PubMed]
- 33.Ippolito M, Misseri G, Catalisano G, et al. Ventilator-associated pneumonia in patients with covid-19: a systematic review and meta-analysis. Antibiotics. 2021 doi: 10.3390/ANTIBIOTICS10050545/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care. 2021;25(1):1–11. doi: 10.1186/S13054-021-03460-5/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blonz G, Kouatchet A, Chudeau N, et al. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: a multicenter retrospective study in 188 patients in an un-inundated French region. Crit Care. 2021;25(1):1–12. doi: 10.1186/S13054-021-03493-W/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo A, Gavaruzzi F, Ceccarelli G, et al. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection. 2022;50(1):83. doi: 10.1007/S15010-021-01643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckardt P, Canavan K, Guran R, et al. Containment of a carbapenem-resistant Acinetobacter baumannii complex outbreak in a COVID-19 intensive care unit. Am J Infect Control. 2022;50(5):477–481. doi: 10.1016/J.AJIC.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rayeesa Faheem S, Srinivas T, Sadhana Y. A comparative study of <em>Acinetobacter</em> infections in COVID and non-COVID patients. J Infect Dis Epidemiol. 2022;8(2):250. doi: 10.23937/2474-3658/1510250. [DOI] [Google Scholar]
- 39.Camporota L, Sanderson B, Chiumello D, et al. Prone position in COVID-19 and -COVID-19 acute respiratory distress syndrome: an international multicenter observational comparative study∗. Crit Care Med. 2022;50(4):633–643. doi: 10.1097/CCM.0000000000005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahmani F, Salmasi S, Rezaeifar P. Prone position effects in the treatment of Covid-19 patients. Casp J Intern Med. 2020;11(Suppl 1):580. doi: 10.22088/CJIM.11.0.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattinoni L, Camporota L, Marini JJ. Prone position and COVID-19: mechanisms and effects*. Crit Care Med. 2022;50(5):873. doi: 10.1097/CCM.0000000000005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadaya J, Benharash P. Prone positioning for acute respiratory distress syndrome (ARDS) JAMA. 2020;324(13):1361. doi: 10.1001/JAMA.2020.14901. [DOI] [PubMed] [Google Scholar]
- 43.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. 2020. https://www.who.int/docs/defaultsource/documents/emergencies/minimalcoreoutcomemeasure.pdf. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Appendix A1. Beck Oral Assessment Score (BOAS). Appendix A2. Oral care procedures.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to institutional regulations but are available from the corresponding author upon reasonable request.