Abstract
Background
Previous research has found a link between dietary factors and asthma. However, few studies have analyzed the relationship between dietary factors and asthma using Mendelian randomization. Methods: The IEU Open GWAS project (https://gwas.mrcieu.ac.uk/) was the source of exposure and outcome datasets. The exposure datasets included Alcoholic drinks per week, Alcohol intake frequency, Processed meat intake, Poultry intake, Beef intake, Non-oily fish intake, Oily fish intake, Pork intake, Lamb/mutton intake, Bread intake, Cheese intake, Cooked vegetable intake, Tea intake, Fresh fruit intake, Cereal intake, Salad/raw vegetable intake, Coffee intake, and Dried fruit intake. The weighted median, MR-Egger, and Inverse Variance Weighted methods were used as the main methods of Mendelian randomization analysis. Heterogeneity and pleiotropic analysis were performed to ensure the accuracy of the results.
Results
Alcohol intake frequency (after removing outliers OR: 1.217; 95% CI: 1.048-1.413; p=0.00993) was related to an increased risk of Asthma. Fresh fruit intake (OR: 0.489; 95% CI: 0.320-0.748; p=0.000954) and Dried fruit intake (after removing outliers OR: 0.482; 95% CI: 0.325-0.717; p= 0.000312) were discovered as protective factors. Other dietary intakes found no causal relationship with asthma.
Conclusion
This study found that dried fruit intake and fresh fruit intake were associated with a reduced risk of asthma, and alcohol intake frequency was associated with an increased risk of asthma. This study also found that other factors included in this study were not associated with asthma.
Keywords: Mendelian randomization, dietary intake, asthma, alcohol intake, fruit intake
1. Introduction
As a common chronic disease, asthma is a major health problem worldwide (1). The incidence of asthma has increased rapidly over the past few decades (2–4). The goal of asthma treatment is to minimize both symptoms and the risk of adverse outcomes (5). The increase in asthmatic patients has increased the medical burden worldwide. In Western countries, the financial burden for an individual asthma patient ranges from US$300 to US$1,300 per year (6). Asthma patients in developing countries face both a financial burden and a higher risk of adverse outcomes due to appropriate treatment is deficient. Dietary factors may play an important role in this increase in asthma (7). In adults, the causes of asthma include environmental and lifestyle factors. A previous study has reported the critical role of diet in the development of allergic diseases (8). Dietary factors may be directly related to asthma pathogenesis (9). Knowing whether dietary changes benefit patients with asthma has important implications for both clinicians and patients with asthma. Previous studies have found that alcohol intake (10), fruit intake (11), vegetable intake (12), dairy intake (13), fish intake (14), and meat intake (15) were associated with asthma or asthma symptoms. Mendelian randomization (MR) uses genetic variants as instrumental variables (IVs), which has advantages over other research methods (16). However, there are few MR studies on the causal relationships between dietary factors and asthma. We, therefore, performed this MR analysis to explore the relationships between dietary factors and asthma.
2. Methods
The following basic assumptions constitute the premise of MR analysis. First, IVs must be intensely associated with the exposure factor(s). Second, IVs cannot be directly correlated to the outcome. Third, IVs were not related to any potential confounding factors. The GWAS summary-level data used in this study was issued by the IEU open GWAS project. This project, supported by the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol, collated and analyzed GWAS data from UK Biobank, published articles, and FinnGen biobank. This study was exempt from the approval of the Ethical Review Authority because the data used in this study was public, anonymized, and de-identified.
2.1. Data sources
Diet-Related Exposure factors used in this study included vegetable intake (Salad/raw vegetable intake and Cooked vegetable intake), meat intake (Processed meat intake, Poultry intake, Beef intake, Non-oily fish intake, Oily fish intake, Pork intake, and Lamb/mutton intake), staple food intake (Bread intake and Cereal intake), beverage intake (Alcoholic drinks per week, Alcohol intake frequency, Tea intake, and Coffee intake), fruit intake(Dried fruit intake and Fresh fruit intake), and another food intake (Cheese intake). These GWAS summary-level data were extracted directly or indirectly from UK Biobank by the IEU open GWAS project. The GWAS summary-level data of asthma was extracted from FinnGen biobank by the IEU open GWAS project. We did not use proxy SNPs when finding SNPs from the outcome, mainly because the FinnGen biobank contained enough SNPs (16,380,176 SNPs in the dataset of asthma). More information about the exposure and outcome datasets is presented in Table 1 and Supplementary Table 1 .
Table 1.
Information of the exposures and outcome datasets.
| IEU GWAS id | Exposure or outcome | Identified SNPs | Participants included in analysis | F-statistic |
|---|---|---|---|---|
| ieu-b-73 | Alcoholic drinks per week | 33 | 335394 European-descent individuals | 97.217 |
| ukb-b-5779 | Alcohol intake frequency | 92 | 462346 European-descent individuals | 112.254 |
| ukb-b-6324 | Processed meat intake | 23 | 461981 European-descent individuals | 39.164 |
| ukb-b-8006 | Poultry intake | 7 | 461900 European-descent individuals | 24.621 |
| ukb-b-2862 | Beef intake | 14 | 461053 European-descent individuals | 28.481 |
| ukb-b-17627 | Non-oily fish intake | 11 | 460880 European-descent individuals | 26.780 |
| ukb-b-2209 | Oily fish intake | 60 | 460443 European-descent individuals | 37.644 |
| ukb-b-5640 | Pork intake | 13 | 460162 European-descent individuals | 18.849 |
| ukb-b-14179 | Lamb/mutton intake | 30 | 460006 European-descent individuals | 19.030 |
| ukb-b-11348 | Bread intake | 25 | 452236 European-descent individuals | 38.339 |
| ukb-b-1489 | Cheese intake | 61 | 451486 European-descent individuals | 44.815 |
| ukb-b-8089 | Cooked vegetable intake | 17 | 448651 European-descent individuals | 20.679 |
| ukb-b-6066 | Tea intake | 39 | 447485 European-descent individuals | 63.637 |
| ukb-b-3881 | Fresh fruit intake | 52 | 446462 European-descent individuals | 15.502 |
| ukb-b-15926 | Cereal intake | 38 | 441640 European-descent individuals | 32.723 |
| ukb-b-1996 | Salad / raw vegetable intake | 18 | 435435 European-descent individuals | 17.650 |
| ukb-b-5237 | Coffee intake | 38 | 428860 European-descent individuals | 41.751 |
| ukb-b-16576 | Dried fruit intake | 39 | 421764 European-descent individuals | 25.412 |
| finn-b-J10_ASTHMA | Asthma | NA | 20629 European-descent cases and 135449 European-descent controls | NA |
The information of the exposure and outcome datasets. SNPs, Single-nucleotide polymorphisms. IEU, Integrative Epidemiology Unit; GWAS, Genome-Wide Association Studies. N/A, Not applicable.
2.2. The selection of IVs
In MR analysis, IVs were utilized as mediators between exposure factors and outcomes to explore the causal relationship between exposure and outcomes. IVs are generally genetic variations, among which Single nucleotide polymorphisms (SNPs) are the most commonly used. SNPs associated with dietary factors were extracted from the IEU open GWAS project (https://gwas.mrcieu.ac.uk/). we screened the SNPs intensely related with exposures at the genome-wide significance level (p < 5×10–8), clumping window > 10,000 kb, and the linkage disequilibrium level (r2 < 0.001). More information is shown in Table 1 . The F statistic was used to ensure the strong association between IVs and exposure, The F statistic greater than 10 was generally considered to meet the requirements of strong association (17).
2.3. Statistical analysis
We conducted the inverse variance weighted (IVW) method as the primary method for calculating the causal effect. The IVW model is the method with the strongest ability to detect causation in the two-sample MR analysis (18). We contrasted the consequences of the IVW method with the weighted median and the MR-Egger methods. The weighted median method allows no more than 50% of invalid IVs, and the MR-Egger method allows all IVs to be voided. Therefore, it will be more convincing when the three models are consistent. The heterogeneity of the IVW model was assessed by Cochran’s Q test. Cochran’s Q-test p<0.05 indicated heterogeneity. However, the existence of heterogeneity does not mean that the IVW model is necessarily invalid. The MR-Egger method allows for the existence of non-zero intercepts and was used to detect directional pleiotropy. Leave-one-out analysis was performed to assess whether there was a significant effect on the results after the removal of a single SNP. We use the MR-PRESSO method to detect outliers. Once outliers were found, they will be removed immediately. After removing outliers, the MR analysis will be performed again. All analyses were performed in R software (version 4.2.0) using the TwoSampleMR package (19).
3. Results
The causal relationship between dietary factors and asthma were analyzed using 18 different exposure factors. The amounts of SNPs used in this study ranged from 7 to 92. The F-statistics are all greater than 10 (range: 15.502 to 112.254). The number of European-descent individuals included in the exposures ranged from 335,394 to 462,346. The outcome included 20 629 European-descent asthma cases and 135 449 European-descent controls from the FinnGen biobank, and there was little overlap between the populations involved in exposures and outcome. See Table 1 for more information on exposures and results. The number of SNPs used for different exposures in this study ranged from 7 to 92, and the range after removing outliers was between 5 and 90 (the number of outliers found in different exposures ranged from 0 to 2). See Table 2 for more information. As shown in Table 1 , the F statistic (after removing outliers) ranges from 15.502 to 112.254, which indicates that IVs used in our study satisfies the requirements of strong association with exposures.
Table 2.
The results of Mendelian randomization analyses.
| Exposure | Used SNPs | inverse variance weighted method | Weighted median method | MR-Egger method | Cochrane's Q test | Pleiotropy | MR-PRESSO a | Outliers excluded b | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR(95% CI) | P-value | OR(95% CI) | P-value | OR(95% CI) | P-value | Q | P-value | MR-Egger intercept | se | P-value | Raw | Outliers | outlier-corrected | inverse variance weighted method | Weighted median method | MR-Egger method | Cochrane’s Q test | Pleiotropy | |||||||||||||
| casual estimate | sd | P-value | casual estimate | sd | P-value | OR(95% CI) | P-value | OR(95% CI) | P-value | OR(95% CI) | P-value | Q | P-value | MR-Egger intercept | se | P-value | |||||||||||||||
| ieu-b-73 | Alcoholic drinks per week | 33 | 0.903(0.599-1.359) | 0.624 | 0.651(0.409-1.036) | 0.070 | 0.884(0.341-2.292) | 0.802 | 65.832 | 0.000395 | 0.000386 | 0.00827 | 0.963 | -0.102 | 0.209 | 0.627 | rs28712821;rs28929474 | -0.219 | 0.169 | 0.206 | 0.804(0.577-1.120) | 0.196 | 0.633(0.402-0.996) | 0.0484 | 0.730(0.328-1.625) | 0.448 | 34.783 | 0.251 | 0.00170 | 0.00659 | 0.798 |
| ukb-b-5779 | Alcohol intake frequency | 92 | 1.173(0.999-1.378) | 0.0513 | 1.254(1.033-1.521) | 0.0221 | 1.192(0.722-1.967) | 0.494 | 174.852 | 3.05E-07 | -0.00039 | 0.00607 | 0.949 | 0.160 | 0.082 | 0.0544 | rs28768122;rs11940694 | 0.197 | 0.0763 | 0.0116 | 1.217(1.048-1.413) | 0.00993 | 1.270(1.0462-1.542) | 0.0157 | 1.624(1.010-2.610) | 0.0483 | 139.917 | 0.000464 | -0.00709 | 0.00566 | 0.213 |
| ukb-b-6324 | Processed meat intake | 23 | 1.315(0.797-2.168) | 0.284 | 1.284(0.746-2.208) | 0.367 | 1.020(0.079-13.254) | 0.988 | 39.941 | 0.0109 | 0.00384 | 0.0194 | 0.845 | 0.27300 | 0.255 | 0.295 | rs2029401 | 0.127 | 0.232 | 0.588 | 1.136(0.721-1.790) | 0.583 | 1.272(0.722-2.241) | 0.404 | 1.004(0.104-9.709) | 0.998 | 29.760 | 0.0969 | 0.00188 | 0.0172 | 0.914 |
| ukb-b-8006 | Poultry intake | 7 | 1.101(0.151-8.051) | 0.924 | 1.124(0.280-4.508) | 0.869 | 3.537E-18(4.064E-42-3.078E+06) | 0.212 | 32.126 | 1.54E-05 | 0.436 | 0.304 | 0.211 | 0.0963 | 1.015 | 0.928 | rs1051730;rs9997448 | 0.603 | 0.599 | 0.371 | 1.827(0.565-5.906) | 0.314 | 1.025(0.245-4.290) | 0.973 | 1.449E-14(6.521e-27-0.0322) | 0.116 | 5.256 | 0.262 | 0.354 | 0.158 | 0.111 |
| ukb-b-2862 | Beef intake | 14 | 0.807(0.307-2.124) | 0.664 | 0.462(0.198-1.080) | 0.0748 | 0.0556(0.000156-19.864) | 0.354 | 38.305 | 0.000257 | 0.0339 | 0.0375 | 0.383 | -0.215 | 0.494 | 0.671 | rs62169335;rs429358 | -0.318 | 0.387 | 0.428 | 0.479(0.227-1.012) | 0.0538 | 0.458(0.194-1.080) | 0.0745 | 0.0143(0.000152-1.351) | 0.0971 | 16.712 | 0.117 | 0.0449 | 0.0293 | 0.157 |
| ukb-b-17627 | Non-oily fish intake | 11 | 0.957(0.302-3.029) | 0.940 | 0.681(0.243-1.912) | 0.466 | 0.0187(0.000107-3.267) | 0.165 | 31.48 | 0.000488 | 0.0488 | 0.0320 | 0.161 | -0.0440 | 0.588 | 0.942 | rs56094641 | -0.433 | 0.554 | 0.455 | 0.648(0.219-1.923) | 0.435 | 0.430(0.144-1.285) | 0.131 | 0.0112(0.000155-0.810) | 0.0737 | 22.006 | 0.00886 | 0.0503 | 0.0264 | 0.093 |
| ukb-b-2209 | Oily fish intake | 60 | 0.739(0.501-1.091) | 0.128 | 0.713(0.479-1.062) | 0.0961 | 0.539(0.103-2.816) | 0.467 | 161.092 | 2.06E-11 | 0.00469 | 0.0122 | 0.702 | -0.302 | 0.199 | 0.134 | rs1421085;rs2952140 | -0.287 | 0.166 | 0.0885 | 0.751(0.543-1.038) | 0.0831 | 0.654(0.438- 0.977) | 0.0379 | 0.219(0.0570-0.842) | 0.0311 | 102.784 | 0.000193 | 0.0183 | 0.00991 | 0.0702 |
| ukb-b-5640 | Pork intake | 13 | 1.287(0.429-3.863) | 0.653 | 0.766(0.258-2.275) | 0.631 | 0.297(0.000244-361.855) | 0.744 | 30.325 | 0.00249 | 0.0152 | 0.0371 | 0.690 | 0.252 | 0.561 | 0.661 | rs12721051;rs2387807 | 0.203 | 0.459 | 0.668 | 1.225(0.498-3.013) | 0.658 | 0.755(0.247-2.308) | 0.622 | 1.173(0.000271-5073.120) | 0.971 | 13.455 | 0.199 | 0.000427 | 0.0416 | 0.992 |
| ukb-b-14179 | Lamb/mutton intake | 30 | 1.127(0.564-2.251) | 0.735 | 0.954(0.427-2.132) | 0.908 | 2.627(0.138-49.982) | 0.526 | 65.171 | 0.000135 | -0.00940 | 0.0162 | 0.567 | 0.120 | 0.353 | 0.737 | rs429358 | -0.133 | 0.335 | 0.694 | 0.875(0.454-1.687) | 0.691 | 0.843(0.405-1.755) | 0.648 | 0.303(0.0134-6.819) | 0.458 | 52.012 | 0.00382 | 0.0114 | 0.0167 | 0.499 |
| ukb-b-11348 | Bread intake | 25 | 0.942(0.615-1.442) | 0.784 | 0.884(0.520-1.501) | 0.647 | 1.090(0.146-8.164) | 0.934 | 34.310 | 0.0793 | -0.00212 | 0.0146 | 0.885 | -0.0596 | 0.217 | 0.786 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ukb-b-1489 | Cheese intake | 61 | 0.876(0.678-1.132) | 0.310 | 1.019(0.727-1.427) | 0.914 | 0.513(0.172-1.533) | 0.237 | 89.582 | 0.00795 | 0.00919 | 0.00934 | 0.329 | -0.133 | 0.131 | 0.314 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ukb-b-8089 | Cooked vegetable intake | 17 | 1.230(0.558-2.710) | 0.607 | 0.987(0.403-2.416) | 0.977 | 4.737(0.000627-35769.624) | 0.738 | 29.263 | 0.0222 | -0.0139 | 0.0469 | 0.770 | 0.207 | 0.403 | 0.614 | rs1421085 | -0.0178 | 0.371 | 0.962 | 0.982(0.475-2.031) | 0.962 | 0.648(0.253-1.654) | 0.364 | 3.737(0.00134-10436.562) | 0.750 | 21.594 | 0.119 | -0.0138 | 0.0416 | 0.745 |
| ukb-b-6066 | Tea intake | 39 | 0.827(0.621-1.101) | 0.192 | 0.897(0.641-1.255) | 0.526 | 0.963(0.512-1.809) | 0.907 | 63.357 | 0.00606 | -0.00326 | 0.00614 | 0.598 | -0.190 | 0.146 | 0.200 | rs2279844 | -0.232 | 0.136 | 0.0949 | 0.793(0.608-1.034) | 0.0865 | 0.847(0.601-1.194) | 0.344 | 1.053(0.590-1.877) | 0.863 | 52.559 | 0.0466 | -0.00615 | 0.00569 | 0.287 |
| ukb-b-3881 | Fresh fruit intake | 52 | 0.489(0.320-0.748) | 0.000954 | 0.462(0.255-0.838) | 0.0110 | 0.971(0.229-4.122) | 0.968 | 61.804 | 0.143 | -0.00659 | 0.00678 | 0.336 | -0.7150 | 0.217 | 0.00175 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ukb-b-15926 | Cereal intake | 38 | 0.617(0.404-0.943) | 0.0256 | 0.607(0.373-0.987) | 0.0442 | 0.338(0.0546-2.095) | 0.252 | 72.903 | 0.000389 | 0.00882 | 0.0133 | 0.510 | -0.483 | 0.216 | 0.0317 | rs11940694 | -0.398 | 0.204 | 0.0586 | 0.671(0.450-1.001) | 0.0508 | 0.612(0.367-1.021) | 0.0601 | 0.291(0.0537-1.575) | 0.161 | 61.489 | 0.00511 | 0.0123 | 0.0123 | 0.325 |
| ukb-b-1996 | Salad / raw vegetable intake | 18 | 0.810(0.287-2.284) | 0.691 | 2.264(0.767-6.683) | 0.139 | 16.837(0.155-1824.858) | 0.254 | 41.510 | 0.000789 | -0.0329 | 0.0253 | 0.212 | -0.211 | 0.529 | 0.695 | rs10819082 | 0.0260 | 0.486 | 0.958 | 1.026(0.396-2.660) | 0.957 | 2.276(0.791-6.552) | 0.127 | 9.368(0.125-703.379) | 0.326 | 31.464 | 0.0117 | -0.0242 | 0.0235 | 0.320 |
| ukb-b-5237 | Coffee intake | 38 | 0.947(0.676-1.328) | 0.754 | 0.977(0.642-1.486) | 0.914 | 1.021(0.514-2.031) | 0.952 | 58.495 | 0.0137 | -0.00142 | 0.00575 | 0.807 | -0.0540 | 0.172 | 0.756 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ukb-b-16576 | Dried fruit intake | 39 | 0.521(0.339-0.780) | 0.00287 | 0.478(0.290-0.788) | 0.00382 | 0.174(0.0263-1.153) | 0.0780 | 64.068 | 0.00514 | 0.0137 | 0.0117 | 0.251 | -0.652 | 0.219 | 0.00498 | rs11152349 | -0.729 | 0.202 | 0.000915 | 0.482(0.325-0.717) | 0.000312 | 0.477(0.284-0.801) | 0.00510 | 0.231(0.0398-1.343) | 0.112 | 52.398 | 0.0481 | 0.00923 | 0.0110 | 0.406 |
OR, Odds ratio; SNPs, Single-nucleotide polymorphisms; CI, Confidence interval; NA, Not available;
a: The results of MR-PRESSO are presented in the form of beta values, and there is a conversion relationship between beta values and OR, specifically beta=log(OR).
b:We repeated the Mendelian randomization analysis after removing outliers.
In this study, a total of 3 causalities were identified (p < 0.05 by IVW method). We found that alcohol intake frequency (after removing outliers OR: 1.217; 95% CI: 1.048-1.413; p=0.00993) was related to an increased risk of Asthma. This discovery was further verified by the consequences of the MR-Egger (after removing outliers OR: 1.624; 95% CI: 1.010-2.610; p= 0.0483) and weighted median (after removing outliers OR: 1.270; 95% CI: 1.046-1.542; p=0.0157) model. Fresh fruit intake (OR: 0.489; 95% CI: 0.320-0.748; p=0.000954) and Dried fruit intake (after removing outliers OR: 0.482; 95% CI: 0.325-0.717; p= 0.000312) were discovered as protective factors. And we have reached the same conclusion in the Weighted median model (Fresh fruit intake OR: 0.462; 95% CI: 0.255-0.838; p=0.011; Dried fruit intake OR: 0.477; 95% CI: 0.284-0.801; p=0.0051). However, there were no significant results in the MR-Egger model (P>0.05). Cereal intake showed a positive result before the outliers were not removed, and the positive result disappeared after the outlier was removed (P= 0.0256 VS 0.0508). This study also found that Alcoholic drinks per week (OR: 0.903; 95% CI: 0.599-1.359; p=0.624; Outliers excluded: OR:0.804; 95% CI:0.577-1.120; p=0.196), Processed meat intake (OR: 1.315; 95% CI: 0.797-2.168; p=0.284; Outliers excluded: OR:1.136; 95% CI:0.721-1.790; p=0.583), Poultry intake (OR: 1.101; 95% CI: 0.151-8.051; p= 0.924; Outliers excluded: OR:1.827; 95% CI:0.565-5.906; p=0.314), Beef intake (OR: 0.807; 95% CI: 0.307-2.124; p= 0.664; Outliers excluded: OR:0.479; 95% CI:0.227-1.012; p=0.0538), Non-oily fish intake (OR: 0.957; 95% CI: 0.302-3.029; p= 0.940; Outliers excluded: OR:0.648; 95% CI:0.219-1.923; p=0.435), Oily fish intake (OR: 0.739; 95% CI: 0.501-1.091; p= 0.128; Outliers excluded: OR:0.751; 95% CI:0.543-1.038; p=0.0831), Pork intake (OR: 1.287; 95% CI: 0.429-3.863; p= 0.653; Outliers excluded: OR:1.225; 95% CI:0.498-3.013; p=0.658), and Lamb/mutton intake (OR: 1.127; 95% CI: 0.564-2.251; p= 0.735; Outliers excluded: OR:0.875; 95% CI:0.454-1.687; p=0.691), Bread intake(OR: 0.942; 95% CI: 0.615-1.442; p= 0.784; No outliers), Cheese intake(OR: 0.876; 95% CI: 0.678-1.132; p= 0.310; No outliers), Cooked vegetable intake(OR: 1.230; 95% CI: 0.558-2.710; p= 0.607; Outliers excluded: OR:0.982; 95% CI:0.475-2.031; p= 0.962), Tea intake(OR: 0.827; 95% CI: 0.621-1.101; p= 0.192; Outliers excluded: OR:0.793; 95% CI:0.608-1.034; p=0.0865), Salad/raw vegetable intake(OR: 0.810; 95% CI: 0.287-2.284; p= 0.691; Outliers excluded: OR:1.026; 95% CI:0.396-2.660; p=0.957), and Coffee intake(OR: 0.947; 95% CI: 0.676-1.328; p= 0.754; No outliers) were not associated with asthma either before or after exclusion of outliers. More MR analysis results are in Table 2 . Although heterogeneity was discovered in a considerable number of exposures (Cochrane’s Q test P<0.05), the consequences of the MR-Egger intercept suggested that no directional pleiotropy was discovered ( Table 2 ). Leave-one-out analysis indicated that the causalities of the positive results were very robust ( Figure 1 ). As shown in Table 2 , the results of the MR-PRESSO analysis were greatly consistent with the results of the IVW model (causal relationships were only shown in Alcohol intake frequency, Fresh fruit intake, and Dried fruit intake).
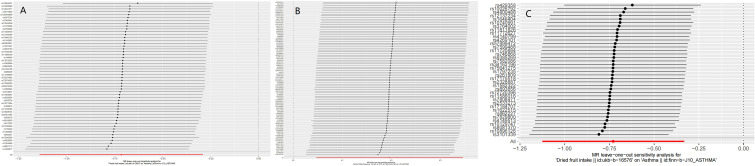
Figure 1.
The results of Leave-one-out analyses (A) Fresh fruit intake (B) Alcohol intake frequency (C) Dried fruit intake.
4. Discussion
The most important finding of this MR analysis is that Alcohol intake frequency, Fresh fruit intake, and Dried fruit intake were associated with asthma. Other findings are also noteworthy. First of all, a causal relationship between Cereal intake and asthma cannot be completely ruled out. In our analysis, if there were any outliers, we repeated the MR analysis and used the new results of the IVW model as a basis for determining whether there was a causal relationship. When we analyzed whether there was a causal relationship between grain intake and asthma, we found no causal relationship after excluding outliers (OR: 0.671; 95% CI: 0.450-1.001; p=0.0508). And a causal relationship was shown in the IVW model (OR: 0.617; 95% CI: 0.404-0.943; p= 0.0256) and the weighted median model (OR: 0.607; 95% CI: 0.373-0.987; p= 0.0442) before excluding outliers. We, therefore, think that the 95% confidence intervals and p-values at critical value have limited convincing power. Secondly, Alcoholic drinks per week, meat intake (Processed meat intake, Poultry intake, Beef intake, Non-oily fish intake, Oily fish intake, Pork intake, and Lamb/mutton intake), Bread intake, Cheese intake, Cooked vegetable intake, Tea intake, Salad/raw vegetable intake, and Coffee intake were not associated with asthma. To our knowledge, there have been many MR studies on the risk or protective factors of asthma (20, 21). However, there are few studies involving meat intake, staple food intake, fruit and vegetable intake, and beverage intake. Asthma imposes a heavy economic burden on the world every year. Asthma and asthma complications also seriously affect the quality of life of patients with asthma. The conclusions of our study can help clinicians to improve their health education for patients with asthma, and encourage patients with asthma to change their eating habits (such as reducing the frequency of alcohol intake and increasing fruit intake). For those at high risk for asthma, adjusting dietary habits also reduces the risk of developing asthma. Therefore, this study has important implications for deepening the understanding of the risk and protective factors of asthma.
Alcohol can affect the human immune system (22, 23). Numerous studies have shown that heavy drinking is associated with higher immunoglobulin E (IgE) levels (24–26). Alcohol consumption is also associated with the development of allergic diseases, such as allergic rhinitis and atopic dermatitis (27, 28). A prospective study found a U-shaped association between alcohol consumption and the risk of asthma, with moderate alcohol consumption having the lowest asthma risk (10). However, Mendelian randomization analysis did not find a causal relationship between alcohol intake and asthma (29, 30). Unlike observational studies, MR studies using genetic variations (primarily SNPs) as instrumental variables are immune to confounding factors and reverse causality. This study found no causality between Alcoholic drinks per week and asthma, however, there was a causal relationship between the frequency of alcohol intake and asthma. We believe that the possible reasons for the differences between MR studies and observational studies in the relationship between alcohol intake and asthma are as follows: First, although possible confounding factors have been adjusted, observational studies may still be affected by other confounding factors. Second, there is indeed a U-shaped association between alcohol intake and asthma, which was not detected by the MR study. Therefore, more observational studies and more ingenious MR studies are needed in the future to further reveal the relationship between alcohol intake and asthma. Our study indicated that there may indeed be a causal relationship between alcohol intake and asthma, and this causal relationship is more about the frequency of alcohol intake than the amount of alcohol intake.
The airway is particularly vulnerable to oxidative damage. In experiments, oxidants can induce many symptoms of asthma by inducing the release of pro-inflammatory mediators including cytokines and chemokines (31). Vegetables and fruits contain many antioxidants. Studies in children showed that asthma symptoms were inversely correlated with fish, vegetable, and fruit intake (14, 32). Several studies in adults have linked high fruit and vegetable intake to a lower risk of asthma (33–36). However, not all studies have shown an association between vegetable intake and asthma. However, not all studies have shown an association between vegetable intake and asthma. A study found no association between vegetable intake and asthma (37). This study used the MR analysis method to find a causal relationship between fruit intake and asthma, but not vegetable intake. There have been many studies on the relationship between fish intake and asthma (38–41). Most of these studies supported an association between fish intake and a reduced risk of asthma (38–41). A Japanese study found that the risk of childhood asthma increased with the frequency of fish intake (42). However, our study showed no causal relationship between fish intake (including Oily fish intake and Non-oily fish intake) and asthma. This study provides powerful new evidence for insights into the relationship between fish intake and asthma. A study from Singapore suggested that a diet rich in meats may increase the risk of cough with phlegm (15). A study from Australia found a positive association between meat/cheese intake, poultry/seafood intake and asthma or hayfever (43). However, this study found no causal relationship between either meat intake or poultry intake and asthma. A study from South Korea found coffee intake had a protective effect on asthma (44). A prospective cohort study from the UK Biobank has found that coffee and tea intake may be protective against asthma (45). This study found coffee and tea intake were not associated with asthma. We must correctly understand the relationship between MR and randomized controlled trials (RCTs). On the one hand, MR can effectively overcome the bias caused by confounding (16). on the other hand, As a powerful supplement to randomized controlled trials, MR is not a substitute for RCTs. Therefore, this conclusion must be viewed with caution.
The mechanisms by which dietary factors affect asthma are not fully understood. Antioxidants and lipids may play an important role (46). Another possible pathway is through the gut microbiome. The intake of different foods can affect the composition of bacteria in the gut, which will affect the metabolism of nutrients (47, 48). A link between changes in the gut microbiome and improvements in airway hyperresponsiveness was found in a mouse model (49).
Although this MR analysis suggested associations between Fresh fruit intake, Dried fruit intake, and alcohol intake and asthma, the MR analysis should be interpreted with more caution. First, the causality found by the MR analysis reflected the effects of long-term exposure to associated factors. Therefore, short-term exposures may not be of clinical significance. Second, another notable problem is that MR cannot distinguish causal relationships between different periods. For example, an MR study found a causality between vitamin D and multiple sclerosis (50). However, this effect was only present in childhood or earlier (51). Third, Univariate MR analyses revealed only overall effects between exposures and outcomes, not direct effects between them. There can be extremely complex mechanisms between exposures and outcomes.
This study has some strengths and limitations. MR uses genetic variation as IVs to infer the causality, which can effectively overcome the bias caused by reverse causality and confounding (16). In order to ensure the accuracy of MR analysis, we performed sensitivity and pleiotropic analysis. We used European populations from different countries in exposures and outcome to avoid unnecessary bias. Of course, the limitations of this study cannot be ignored. First, the F-statistics indicated that the IVs used in this study satisfied the requirement of strong associations with exposure (F-statistics>10). however, a considerable part of the F-statistics is lower than 100, so this may affect the accuracy of the consequences. Second, we cannot further subdivide different types of dietary intake, nor can we distinguish the effects of different dietary combinations. Third, we were unable to conduct a sex-stratified analysis due to the lack of summary-level GWAS data for different sexes.
5. Conclusion
This study found that dried fruit intake and fresh fruit intake were associated with a reduced risk of asthma, and alcohol intake frequency was associated with an increased risk of asthma. This study also found that alcoholic drinks per week, processed meat intake, poultry intake, beef intake, non-oily fish intake, oily fish intake, pork intake, lamb/mutton intake, bread intake, cheese intake, cooked vegetable intake, tea intake, cereal intake, salad/raw vegetable intake, and coffee intake were not associated with asthma.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: All GWAS data used in this study are available in the IEU open GWAS project (https://gwas.mrcieu.ac.uk/).
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent was not provided because All GWAS data used in this study are available in the IEU open GWAS project (https://gwas.mrcieu.ac.uk/). This study was exempt from the approval of the Ethical Review Authority because the data used in this study was public, anonymized, and de-identified.
Author contributions
The study was designed by WY and BH. Statistical analyses were performed by WY, LH, MZ, SS, FW and YY. The manuscript was written by WY and YY. All authors contributed to the interpretation of data and commented on the manuscript. All authors read and approved the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
Special thanks to the IEU open GWAS project developed by The MRC Integrative Epidemiology Unit (IEU) at the University of Bristol. Thank them for extracting relevant GWAS summary-level data from published articles, UK Biobank, and FinnGen biobank.
Funding Statement
Supported by Natural Science Foundation of Gansu Province (21JR1RA118) and Gansu Provincial Youth Science and Technology Fund (21JR1RA107, 18JR3RA305).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1126457/full#supplementary-material
References
- 1. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet (2006) 368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0 [DOI] [PubMed] [Google Scholar]
- 2. Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United states, 2001-2010. In: Vital & health statistics. series 3, analytical and epidemiological studies (2012) (Hyattsville, Maryland: National Center for Health Statistics; ), vol. 2012. . p. 1–58 Available at: https://www.cdc.gov/nchs/data/series/sr_03/sr03_035.pdf. [PubMed] [Google Scholar]
- 3. Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the united states, 2001-2010. In: NCHS data brief (2012(94) (Hyattsville, Maryland: National Center for Health Statistics; ). p. 1–8. [PubMed] [Google Scholar]
- 4. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin immunopathology. (2020) 42(1):5–15. doi: 10.1007/s00281-020-00785-1 [DOI] [PubMed] [Google Scholar]
- 5. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet (2018) 391(10122):783–800. doi: 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 6. Braman SS. The global burden of asthma. Chest (2006) 130(1 Suppl):4s–12s. doi: 10.1378/chest.130.1_suppl.4S [DOI] [PubMed] [Google Scholar]
- 7. Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax (1994) 49(2):171–4. doi: 10.1136/thx.49.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol (2015) 15(5):308–22. doi: 10.1038/nri3830 [DOI] [PubMed] [Google Scholar]
- 9. Guo CH, Liu PJ, Lin KP, Chen PC. Nutritional supplement therapy improves oxidative stress, immune response, pulmonary function, and quality of life in allergic asthma patients: an open-label pilot study. Altern Med Rev J Clin therapeutic. (2012) 17(1):42–56. [PubMed] [Google Scholar]
- 10. Lieberoth S, Backer V, Kyvik KO, Skadhauge LR, Tolstrup JS, Grønbæk M, et al. Intake of alcohol and risk of adult-onset asthma. Respir Med (2012) 106(2):184–8. doi: 10.1016/j.rmed.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 11. Butland BK, Strachan DP, Anderson HR. Fresh fruit intake and asthma symptoms in young British adults: confounding or effect modification by smoking? Eur Respir J (1999) 13(4):744–50. doi: 10.1034/j.1399-3003.1999.13d08.x [DOI] [PubMed] [Google Scholar]
- 12. Seyedrezazadeh E, Moghaddam MP, Ansarin K, Vafa MR, Sharma S, Kolahdooz F. Fruit and vegetable intake and risk of wheezing and asthma: a systematic review and meta-analysis. Nutr Rev (2014) 72(7):411–28. doi: 10.1111/nure.12121 [DOI] [PubMed] [Google Scholar]
- 13. Woods RK, Walters EH, Raven JM, Wolfe R, Ireland PD, Thien FC, et al. Food and nutrient intakes and asthma risk in young adults. Am J Clin Nutr (2003) 78(3):414–21. doi: 10.1093/ajcn/78.3.414 [DOI] [PubMed] [Google Scholar]
- 14. Chatzi L, Torrent M, Romieu I, Garcia-Esteban R, Ferrer C, Vioque J, et al. Diet, wheeze, and atopy in school children in menorca, Spain. Pediatr Allergy Immunol (2007) 18(6):480–5. doi: 10.1111/j.1399-3038.2007.00596.x [DOI] [PubMed] [Google Scholar]
- 15. Butler LM, Koh WP, Lee HP, Tseng M, Yu MC, London SJ. Prospective study of dietary patterns and persistent cough with phlegm among Chinese singaporeans. Am J Respir Crit Care Med (2006) 173(3):264–70. doi: 10.1164/rccm.200506-901OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyko EJ. Observational research–opportunities and limitations. J Diabetes its complications. (2013) 27(6):642–8. doi: 10.1016/j.jdiacomp.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Staiger DO, Stock JH. Instrumental variables regression with weak instruments. Mass., USA: National Bureau of Economic Research Cambridge; (1994). [Google Scholar]
- 18. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife (2018) 7, e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mikkelsen H, Landt EM, Benn M, Nordestgaard BG, Dahl M. Causal risk factors for asthma in mendelian randomization studies: A systematic review and meta-analysis. Clin Trans Allergy (2022) 12(11):e12207. doi: 10.1002/clt2.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skaaby T, Kilpeläinen TO, Mahendran Y, Huang LO, Sallis H, Thuesen BH, et al. Association of milk intake with hay fever, asthma, and lung function: a mendelian randomization analysis. Eur J Epidemiol. (2022) 37(7):713–22. doi: 10.1007/s10654-021-00826-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res Curr Rev (2015) 37(2):185–97. [PMC free article] [PubMed] [Google Scholar]
- 23. Szabo G, Saha B. Alcohol's effect on host defense. Alcohol Res Curr Rev (2015) 37(2):159–70. [PMC free article] [PubMed] [Google Scholar]
- 24. Domínguez-Santalla MJ, Vidal C, Viñuela J, Pérez LF, González-Quintela A. Increased serum IgE in alcoholics: relationship with Th1/Th2 cytokine production by stimulated blood mononuclear cells. Alcoholism Clin Exp Res (2001) 25(8):1198–205. doi: 10.1111/j.1530-0277.2001.tb02336.x [DOI] [PubMed] [Google Scholar]
- 25. González-Quintela A, Vidal C, Gude F, Tomé S, Lojo S, Lorenzo MJ, et al. Increased serum IgE in alcohol abusers. Clin Exp Allergy (1995) 25(8):756–64. doi: 10.1111/j.1365-2222.1995.tb00014.x [DOI] [PubMed] [Google Scholar]
- 26. González-Quintela A, Vidal C, Lojo S, Pérez LF, Otero-Antón E, Gude F, et al. Serum cytokines and increased total serum IgE in alcoholics. Ann allergy Asthma Immunol (1999) 83(1):61–7. doi: 10.1016/S1081-1206(10)63514-4 [DOI] [PubMed] [Google Scholar]
- 27. Bendtsen P, Grønbaek M, Kjaer SK, Munk C, Linneberg A, Tolstrup JS. Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. Clin Exp Allergy (2008) 38(7):1179–85. doi: 10.1111/j.1365-2222.2008.02945.x [DOI] [PubMed] [Google Scholar]
- 28. Linneberg A, Petersen J, Grønbaek M, Benn CS. Alcohol during pregnancy and atopic dermatitis in the offspring. Clin Exp Allergy (2004) 34(11):1678–83. doi: 10.1111/j.1365-2222.2004.02101.x [DOI] [PubMed] [Google Scholar]
- 29. Skaaby T, Kilpeläinen TO, Taylor AE, Mahendran Y, Wong A, Ahluwalia TS, et al. Association of alcohol consumption with allergic disease and asthma: a multi-centre mendelian randomization analysis. Addict (Abingdon England). (2019) 114(2):216–25. doi: 10.1111/add.14438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lomholt FK, Nielsen SF, Nordestgaard BG. High alcohol consumption causes high IgE levels but not high risk of allergic disease. J Allergy Clin Immunol (2016) 138(5):1404–1413.e1413. doi: 10.1016/j.jaci.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 31. Caramori G, Papi A. Oxidants and asthma. Thorax (2004) 59(2):170–3. doi: 10.1136/thorax.2002.002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chatzi L, Apostolaki G, Bibakis I, Skypala I, Bibaki-Liakou V, Tzanakis N, et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax (2007) 62(8):677–83. doi: 10.1136/thx.2006.069419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel BD, Welch AA, Bingham SA, Luben RN, Day NE, Khaw KT, et al. Dietary antioxidants and asthma in adults. Thorax (2006) 61(5):388–93. doi: 10.1136/thx.2004.024935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaheen SO, Sterne JA, Thompson RL, Songhurst CE, Margetts BM, Burney PG. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med (2001) 164(10 Pt 1):1823–8. doi: 10.1164/ajrccm.164.10.2104061 [DOI] [PubMed] [Google Scholar]
- 35. Romieu I, Varraso R, Avenel V, Leynaert B, Kauffmann F, Clavel-Chapelon F. Fruit and vegetable intakes and asthma in the E3N study. Thorax (2006) 61(3):209–15. doi: 10.1136/thx.2004.039123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia V, Arts IC, Sterne JA, Thompson RL, Shaheen SO. Dietary intake of flavonoids and asthma in adults. Eur Respir J (2005) 26(3):449–52. doi: 10.1183/09031936.05.00142104 [DOI] [PubMed] [Google Scholar]
- 37. Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax (2000) 55(9):775–9. doi: 10.1136/thorax.55.9.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laerum BN, Wentzel-Larsen T, Gulsvik A, Omenaas E, Gíslason T, Janson C, et al. Relationship of fish and cod oil intake with adult asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2007) 37(11):1616–23. doi: 10.1111/j.1365-2222.2007.02821.x [DOI] [PubMed] [Google Scholar]
- 39. Miyamoto S, Miyake Y, Sasaki S, Tanaka K, Ohya Y, Matsunaga I, et al. Fat and fish intake and asthma in Japanese women: baseline data from the Osaka maternal and child health study. Int J tuberculosis Lung disease. (2007) 11(1):103–9. [PubMed] [Google Scholar]
- 40. Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med J Australia. (1996) 164(3):137–40. doi: 10.5694/j.1326-5377.1996.tb122010.x [DOI] [PubMed] [Google Scholar]
- 41. Yang H, Xun P, He K. Fish and fish oil intake in relation to risk of asthma: a systematic review and meta-analysis. PloS One (2013) 8(11):e80048. doi: 10.1371/journal.pone.0080048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takemura Y, Sakurai Y, Honjo S, Tokimatsu A, Gibo M, Hara T, et al. The relationship between fish intake and the prevalence of asthma: the tokorozawa childhood asthma and pollinosis study. Prev Med (2002) 34(2):221–5. doi: 10.1006/pmed.2001.0978 [DOI] [PubMed] [Google Scholar]
- 43. Hooper R, Heinrich J, Omenaas E, Sausenthaler S, Garcia-Larsen V, Bakolis I, et al. Dietary patterns and risk of asthma: results from three countries in European community respiratory health survey-II. Br J Nutr (2010) 103(9):1354–65. doi: 10.1017/S0007114509993266 [DOI] [PubMed] [Google Scholar]
- 44. Wee JH, Yoo DM, Byun SH, Song CM, Lee HJ, Park B, et al. Analysis of the relationship between asthma and Coffee/Green Tea/Soda intake. Int J Environ Res Public Health (2020) 17(20):7471. doi: 10.3390/ijerph17207471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin F, Zhu Y, Liang H, Li D, Jing D, Liu H, et al. Association of coffee and tea consumption with the risk of asthma: A prospective cohort study from the UK biobank. Nutrients (2022) 14(19):4039. doi: 10.3390/nu14194039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol (2005) 115(6):1109–17; quiz 1118. doi: 10.1016/j.jaci.2004.12.1139 [DOI] [PubMed] [Google Scholar]
- 47. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature (2011) 474(7351):327–36. doi: 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Sci (New York N.Y.). (2011) 332(6032):970–4. doi: 10.1126/science.1198719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med (2014) 20(2):159–66. doi: 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 50. Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, et al. Vitamin d and risk of multiple sclerosis: A mendelian randomization study. PloS Med (2015) 12(8):e1001866. doi: 10.1371/journal.pmed.1001866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chaudhuri A. Why we should offer routine vitamin d supplementation in pregnancy and childhood to prevent multiple sclerosis. Med hypotheses. (2005) 64(3):608–18. doi: 10.1016/j.mehy.2004.06.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: All GWAS data used in this study are available in the IEU open GWAS project (https://gwas.mrcieu.ac.uk/).