Abstract
Empyema thoracis is a collection of pus in the pleural space associated with pleural fibrin deposition. Treatment involves systemic antimicrobials, pleural drainage, intrapleural enzymes and sometimes decortication. Our case is a 57‐year‐old gentleman who developed chronic mucormycosis (Cunninghamella sp.) and bacterial (Enterococcus sp.) empyema in a high‐risk post‐lobectomy space in the setting of a non‐expandable lung following non‐tuberculous mycobacterial (NTM) infection. The patient did not tolerate antimicrobial therapy for progressive pulmonary NTM infection, and required lobectomy, complicated by polymicrobial empyema. He did not respond to systemic treatment and long‐term intercostal catheter drainage and therefore intrapleural taurolidine‐citrate, and enzyme therapy was used to help eradicate infection. Intrapleural antifungals and taurolidine‐citrate in combination with long‐term antifungal therapy may help eradicate infection in patients with fungal empyemas. Further studies investigating the safety of taurolidine‐citrate in pleural catheters are needed.
Keywords: empyema, pleural disease, respiratory infections (non‐tuberculous), thoracic surgery
We present a 57‐year‐old gentleman who developed chronic mucormycosis (Cunninghamella sp.) and bacterial (Enterococcus sp.) empyema in a high‐risk post‐lobectomy space in the setting of a non‐expandable lung following non‐tuberculous mycobacterial infection. This was successfully treated with a combination of systemic and intrapleural therapies.
INTRODUCTION
Empyema is a collection of pus in the pleural space associated with pleural organization and scarring of pleural membranes. Empyema most commonly occurs secondary to severe or untreated pneumonia and has a high mortality. Treatment involves antimicrobials, pleural drainage, intrapleural enzymes and in some cases surgery. 1 , 2
We present a 57‐year‐old gentleman who developed chronic mucormycosis (Cunninghamella sp.) and bacterial (Enterococcus sp.) empyema in a high‐risk post‐lobectomy space in the setting of a non‐expandable lung following non‐tuberculous mycobacterial infection. This was successfully treated with a combination of systemic and intrapleural therapies.
CASE REPORT
A 57‐year‐old, male truck‐driver presented to hospital in May 2019 with a 1 month history of productive cough, right‐sided chest pain, fevers, sweats and weight loss. Past history included severe COPD and gastro‐oesophageal reflux disease with no known immunocompromise.
After empirical treatment for community acquired pneumonia, sputum testing cultured non‐tuberculosis mycobacteria (NTM), Mycobacterium xenopi. Computerized Topography (CT) imaging confirmed right upper lobe consolidation with bullous changes without cavitation. Triple therapy (rifampicin, ethambutol, moxifloxacin) was commenced in July 2019, and was poorly tolerated due to severe nausea and liver enzyme elevation. A bronchoscopy was performed in September 2019 and M. xenopi was re‐confirmed on cultures. Significant weight loss ensued due to poor dietary intake. Despite attempts to substitute isoniazid for rifampicin, all therapy had to be paused by clinicians in November 2019.
He had clinical and radiological progression with worsening productive cough and chest radiograph showing right upper lobe cavitation (Figure 1A). Sputum samples continued to culture M. xenopi. Following multidisciplinary team discussions, NTM therapy was reintroduced, in stepwise approach, from November 2019 to February 2020 (initially ethambutol, then clarithromycin and rifampicin). Rifampicin was again not tolerated thus substituted for rifabutin.
FIGURE 1.
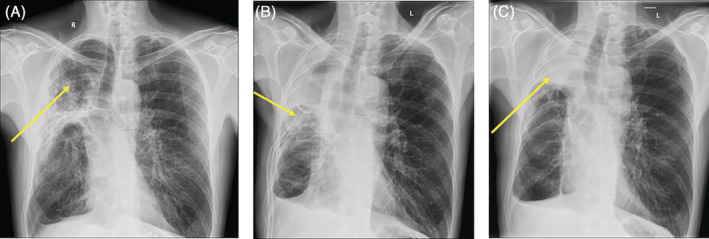
(A) July 2019: Chest radiograph performed prior to commencement of non‐mycobacterial therapy. Extensive fibrocavitatory lung disease in right upper and mid zones as indicated. Associated right upper lobe consolidation. (B) August 2020: Chest radiograph post‐right upper lobectomy and middle lobe wedge resection with de Pezzer catheter in situ as indicated. (C) September 2021: Chest radiograph post de Pezzer catheter removal. Almost complete opacification of the apex of the right hemithorax with small air fluid level. There is no reexpansion of the right lung
Due to progressive lung destruction (Figure 2A) and intolerance of medical management he underwent a right upper lobectomy and right middle lobe wedge resection in June 2020, complicated by intra‐operative fungal contamination from the resected lobe (Cunninghamella bertholletiae), cultured post‐operatively. An empyema developed requiring video‐assisted thoracoscopic surgery (VATS) decortication, an indwelling de Pezzer catheter was left in situ in July 2020 (Figure 1B). He received NTM treatment (16 months ethambutol and 18 months moxifloxacin), Cunninghamella bertholletiae treatment (4 weeks intravenous (IV) liposomal amphotericin, long term posaconazole and terbinafine), and antibacterial cover (6 weeks IV meropenem).
FIGURE 2.
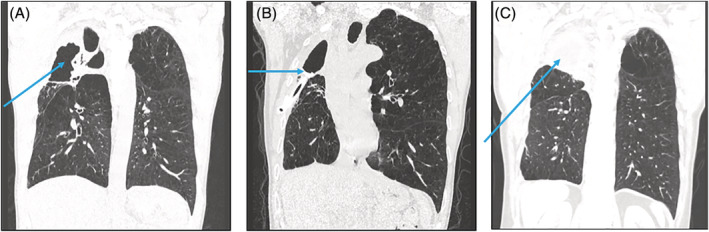
(A) May 2020 Preoperative High resolution CT chest coronal view showing cavitation fibrosis and volume loss of right upper lobe. (B) December 2020 Postoperative high resolution CT chest with de Pezzer drain in situ (as marked). Loculated pleural thickening/collection at right upper apex. (C) September 2022 CT chest, repeat scan at 6 months follow up, de Pezzer drain removed and antimicrobials ceased. Right apical collection (arrow) remains stable
He was admitted to hospital in November 2020 with high catheter output with pus despite ongoing antimicrobial therapy. Pleural ultrasound showed a septated right pleural effusion, pleural fluid cultured Enterococcus faecium and Fusarium sp.
Intrapleural therapy was commenced: (i) Taurolidine 1%‐citrate 4% to decontaminate the long‐term catheter, instilled for 1 h then aspirated (7.5 mL OD for 3 days, the volume used was approximately equal to the catheter volume); (ii) concurrent enzyme therapy (10 mg tPA and 5 mg DNAse BD, for 3 days), as per MIST2 trial protocol 1 ; then (iii) intrapleural standard amphotericin B was given (5 mg in 50 mL 5% dextrose, OD for 5 days, catheter clamped for 1 h after instillation). Treatments were mostly well tolerated, but on one occasion pleuritic chest pain occurred when the catheter was unclamped and may have occurred due to inadvertent aspiration of taurolidine‐citrate into the pleural space, in the context of negative intrapleural pressure with non‐expandable lung. No other complications were observed. He was discharged on 4 weeks of IV vancomycin for Enterococcus faecium.
He improved clinically following this admission with improving inflammatory markers and catheter output; repeat pleural cultures were negative. The intercostal catheter was removed, when drain output became minimal in July 2021 (12 months post‐insertion) (Figure 1C). Immunodeficiency screen was unremarkable. NTM eradication therapy was ceased in January 2022, antifungal therapy was ceased in March 2022. He remains stable at outpatient review 9 months after completion of therapy with no clinical or radiological evidence of disease recurrence (Figure 2C).
DISCUSSION
This case demonstrates a complex empyema occurring post‐lobectomy for aggressive NTM disease in a COPD patient. Post‐lobectomy infections are notoriously difficult to eradicate as the non‐expandable lung creates a space that cannot be closed, leaving a protected, warm space without blood supply, with limited immune defence or antimicrobial penetration. NTM are ubiquitous saprophytic organisms found in water, soil and vegetation and can cause lung cavitation. M. xenopi is a slow growing NTM, most commonly detected in patients with respiratory disease, particularly COPD, which has a higher treatment failure. 3 Treatment involves long‐term combination therapy, which can cause intolerable side‐effects. Incomplete, medical management in this case led to destructive lung disease necessitating surgical resection. 3
Concurrent fungal and pulmonary NTM infection is not uncommon and may be related to fungal colonization following long‐term antibiotic therapy. 4 Cunninghamella sp. are ubiquitous organisms found in soil, water, and in air and cause mucormycosis, an invasive fungal infection which can affect the entire thoracic cavity. 5 Mucormycosis occurs in immunocompromised and occasionally immunocompetent hosts and has a high mortality rate. Intrapleural amphotericin has been used in case reports as an adjunct therapy in the management of fungal empyema but is yet to be incorporated into guidelines. 4
Taurolidine is a non‐antibiotic antimicrobial, anti‐biofilm agent used as a lock‐device in indwelling catheters. Taurolidine‐citrate has antifungal activity and has been used to treat fungal pleural infection and maintain catheter patency. 4
Intrapleural taurolidine‐citrate, antifungals and enzymes in combination with long‐term antimicrobials helped eradicate this life‐threatening polymicrobial empyema. Further studies of intrapleural antimicrobial therapy are required.
AUTHOR CONTRIBUTIONS
Charlotte Wigston drafted the manuscript. Emily Woolnough, Ohide Otome, Lucas Sanders, and Edward Fysh edited the manuscript. All authors have read and approved the final version for publication
FUNDING INFORMATION
Edward Fysh rreceived research funding from the National Health and Medical Research Council and the Raine Foundation.
CONFLICT OF INTEREST STATEMENT
None declared
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENTS
This case was presented at the Australasian Society for Infectious Diseases Annual Scientific Meeting 2022.
Wigston C, Woolnough E, Otome O, Sanders L, Fysh E. Eradication of post‐lobectomy mucormycosis and bacterial empyema with intrapleural antimicrobial therapy in a patient with surgically resected Mycobacterium xenopi (non‐tuberculous Mycobacteria) pulmonary infection. Respirology Case Reports. 2023;11:e01101. 10.1002/rcr2.1101
Associate Editor: Coenraad F Koegelenberg
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365(6):518–26. [DOI] [PubMed] [Google Scholar]
- 2. Shen KR, Bribriesco A, Crabtree T, Denlinger C, Eby J, Eiken P, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017;153(6):e129–46. [DOI] [PubMed] [Google Scholar]
- 3. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270–7. [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl_1):S16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


