Abstract
Background/Aims
There have been little research on the cancer risks of patients with Peutz-Jeghers syndrome (PJS) in Korea. We aimed to investigate the clinical features of PJS patients and their cancer incidence rate.
Methods
Patients with PJS from nine medical centers were enrolled. In those patients diagnosed with cancer, data obtained included the date of cancer diagnosis, the tumor location, and the cancer stage. The cumulative risks of gastrointestinal cancers and extra-gastrointestinal cancers were calculated using the Kaplan-Meier method.
Results
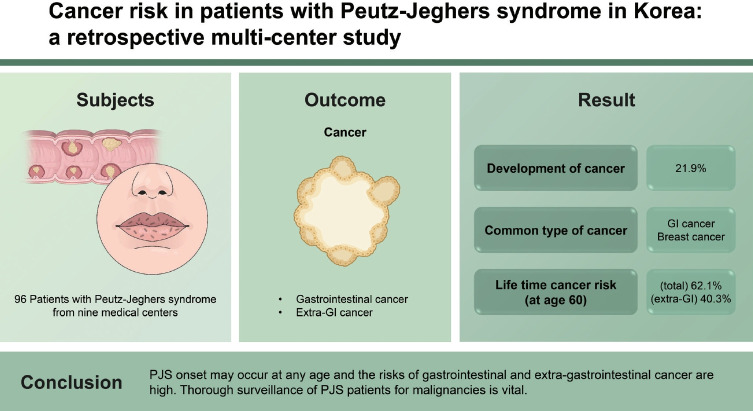
A total of 96 PJS patients were included. The median age at diagnosis of PJS was 23.4 years. Cancer developed in 21 of the 96 patients (21.9%). The age of PJS diagnosis was widely distributed (0.9 to 72.4 years). The most common cancers were gastrointestinal cancer (n = 12) followed by breast cancer (n = 6). The cumulative lifetime cancer risk was calculated to be 62.1% at age 60. The cumulative lifetime gastrointestinal cancer risk was 47.1% at age 70. The cumulative lifetime extra-gastrointestinal cancer risk was 40.3% at age 60.
Conclusions
PJS onset may occur at any age and the risks of gastrointestinal and extra-gastrointestinal cancer are high. Thorough surveillance of PJS patients for malignancies is vital.
Keywords: Neoplasms, Peutz-Jeghers syndrome, Surveillance
Graphical abstract
INTRODUCTION
Peutz-Jeghers syndrome (PJS) is a rare autosomal-dominant inherited polyposis syndrome associated with mucocutaneous pigmentation and multiple gastrointestinal (GI) polyps in the digestive tract [1]. These polyps may result in GI bleeding, anemia, and abdominal pain due to obstruction or intussusception [1]. Although there is debate regarding the carcinogenic mechanism in PJS, it is known that these patients have an increased risk of both GI and extra-GI malignancies. The cancer risks of PJS patients have been reported in various studies [1–11]; resulting in various surveillance recommendations. However, the supporting evidence is limited due to the rarity of PJS [1,2,12–15]. The incidence of PJS has been estimated at 1:50,000 to 1:200,000 live births [16]. In Asia, studies on the cancer risks of PJS patients have been conducted in Japan and China [5,17,18], but there have been few Korean studies on the subject [19].
In this study, we aimed to investigate the clinical features, cancer incidence rate, and the origins of malignancies in PJS patients in Korea.
METHODS
Study population
PJS patients from nine referral medical centers were enrolled from January 1, 2002, to May 31, 2020. Demographic and clinical data were collected, including age, sex, family history of PJS, intussusception, surgery, follow-up date, and cancer diagnosis. These data were retrospectively obtained from electronic medical records. The data were fully anonymized. A clinical diagnosis of PJS was made when any one of the following was present: (1) Two or more histologically confirmed Peutz-Jeghers (PJ) polyps; (2) Any number of PJ polyps and a family history of PJS; (3) Characteristic mucocutaneous pigmentation and a family history of PJS; (4) Any number of PJS polyps and characteristic mucocutaneous pigmentation [1].
For patients diagnosed with cancer, additional data obtained included the date of cancer diagnosis, the original tumor site, the cancer stage, and the histopathology report. When more than one cancer was detected, data for all tumors were collected. Carcinoma in situ (CIS) was considered a malignant tumor. Gastric, small intestinal, colorectal, pancreatic, and biliary cancers were all classed as GI cancers [3,5,6]. Squamous cell carcinoma of the oral cavity was classed as an extra-GI cancer. We also analyzed the frequency of intussusception and surgical resection due to intussusception.
This study was approved by the Institutional Review Board of each participating hospital (Boramae Medical Center, Number 30-2020-089; Samsung Medical Center, 2020-10-087-002; Hallym University Dongtan Sacred Heart Hospital, 2020-07-014-002; etc.). Written informed consent was waived by the Institutional Review Board.
Statistical analysis
Continuous variables were expressed as either the median or the mean ± standard deviation. Categorical variables were expressed as numbers and percentages. The cumulative risks of cancer, GI cancer, and extra-GI cancer were calculated using the Kaplan-Meier method. A comparison of the cumulative cancer risks was performed using a log-rank test. In cases of multiple cancers, only the first cancer was included in the calculation of the cumulative cancer risk. In patients with two GI cancers or two extra-GI cancers, only the first cancer was included in the analysis of the cumulative GI cancer risk or cumulative extra-GI cancer risk. The second cancer was included in these latter calculations when the first and second cancers were GI and extra-GI, in either order [3]. The multivariable Cox proportional hazards model was used to identify factors associated with cancer development in PJS patients. p values < 0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS version 26 (IBM Corp., Armonk, NY, USA) statistical software.
RESULTS
Basic characteristics of the study population
A total of 96 PJS patients from nine referral medical centers were included in this study. These included 48 males (50.0%) and 48 females (50.0%). The median age at the final follow-up was 34.7 years. The median age at diagnosis of PJS was 23.4 years. Among the 65 patients with family history information available, 22 (33.8%) had a positive family history of PJS. Serine/threonine kinase 11 (STK11) mutation analyses were performed in 39 patients and detected in 25 (64.1%). Cancer developed in 21 of the 96 PJS patients (21.9%). GI cancers developed in 10 patients (10.4%) and extra-GI cancers in 13 patients (13.5%). The median age of cancer diagnosis was 42.5 years. The median age of GI cancer diagnosis was 48.6 years. The median age of extra-GI cancer diagnosis was 38.0 years (Table 1). Among the 96 PJS patients, 50 (52.1%) had been admitted with intussusception, and 11 (11.5%) had suffered more than one occurrence of intussusception. Forty-six patients (47.9%) underwent laparotomy due to intussusception, and eight (8.3%) underwent two laparotomies (Table 2). The age distribution of our patients at PJS diagnosis is shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients with Peutz-Jeghers syndrome (n = 96)
| Characteristic | Value |
|---|---|
| Age at the end of follow-up, yr | 34.7 (10.5–81.2) |
| Follow-up duration, yr | 6.2 (0–22.5) |
| Age of PJS diagnosis, yr | 23.4 (0.9–72.4) |
| < 10 | 11 (11.5) |
| 10–20 | 29 (30.2) |
| 20–30 | 14 (14.6) |
| 30–40 | 20 (20.8) |
| 40–50 | 9 (9.4) |
| 50–60 | 8 (8.3) |
| 60–70 | 4 (4.2) |
| > 70 | 1 (1.0) |
| Sex | |
| Male | 48 (50.0) |
| Female | 48 (50.0) |
| Family history of PJS | |
| Positive | 22 (22.9) |
| Negative | 43 (44.8) |
| Unknown | 31 (32.3) |
| STK11 mutation | |
| Positive | 25 (26.0) |
| Negative | 14 (14.6) |
| Unchecked | 57 (59.4) |
| Cancer | 21 (21.9) |
| Age of cancer diagnosis, yr | 42.5 (15.5–59.7) |
| GI cancer | 10 (10.4) |
| Age of GI cancer diagnosis, yr | 48.6 (16.2–66.6) |
| Extra-GI cancer | 13 (13.5) |
| Age of extra-GI cancer diagnosis, yr | 38.0 (15.5–59.7) |
Values are presented as median (range) or number (%).
PJS, Peutz-Jeghers syndrome; STK11, serine/threonine kinase 11; GI, gastrointestinal.
Table 2.
Frequencies of hospital admission and laparotomy due to intussusception in Peutz-Jeghers syndrome patients
| Variable | No. (%) |
|---|---|
| Number of admissions | |
| 0 | 46 (47.9) |
| 1 | 39 (40.6) |
| 2 | 9 (9.4) |
| 3 | 1 (1.0) |
| 4 | 1 (1.0) |
| Number of laparotomies | |
| 0 | 50 (52.1) |
| 1 | 38 (39.6) |
| 2 | 8 (8.3) |
Cancer spectrum and cumulative cancer risk
Cancer distribution by site and gender are shown in Table 3. Double primary cancers occurred in seven of the 21 patients (33.3%) who developed cancer. The most common cancers were GI cancers (n = 12) followed by breast cancer (n = 6), uterine cervical cancer (n = 4), and thyroid cancer (n = 2). Among the GI cancers, pancreatic cancer was the most common (n = 4) followed by colorectal cancer (n = 3), and small bowel cancer (n = 3). The median ages at which each cancer type was diagnosed are shown in Table 3. In our study, cases of gastric cancer, cholangiocarcinoma, testicular germ cell tumor, prostate cancer, oral cavity squamous cell carcinoma, and acute lymphocytic leukemia were identified (Table 3).
Table 3.
Cancer types in patients with Peutz-Jeghers syndrome
| Site | Male | Female | No. of cases | Median age at diagnosis, yr | Age range, yr |
|---|---|---|---|---|---|
| Gastrointestinal cancers | 12 | ||||
| Colorectal cancer | 2 | 1 | 3 | 59.2 | 56.9–66.6 |
| Small bowel cancer | 2 | 1 | 3 | 16.7 | 16.2–46.8 |
| Gastric cancer | 1 | 1 | 30.0 | 30.0 | |
| Pancreatic cancer | 2 | 2 | 4 | 51.0 | 43.6–67.6 |
| Cholangiocarcinoma | 1 | 1 | 63.4 | 63.4 | |
| Extra-gastrointestinal cancers | 16 | ||||
| Breast cancer | 6 | 6 | 39.7 | 23.0–45.2 | |
| Cervical cancer | 4 | 4 | 40.2 | 31.0–57.6 | |
| Thyroid cancer | 2 | 2 | 41.4 | 38.0–44.7 | |
| Testicular germ cell tumor | 1 | 1 | 52.6 | 52.6 | |
| Prostate cancer | 1 | 1 | 59.7 | 59.7 | |
| Oral cavity cancer | 1 | 1 | 34.3 | 34.3 | |
| Leukemia | 1 | 1 | 15.5 | 15.5 | |
| Total no. of cases | 11 | 17 | 28 | ||
| Total no. of patients | 9 | 12 | 21 |
Cumulative cancer risk, by age, was 3.3% at age 20, 6.5% at age 30, 16.6% at age 40, 30.2% at age 50, and 62.1% at age 60 (Table 4, Fig. 1A). There was no difference in cumulative cancer risk between genders (p = 0.618) (Fig. 1B).
Table 4.
Cumulative incidence rates, by age, of cancer in patients with Peutz-Jeghers syndrome
| Age, yr | Cumulative cancer risk, % | ||
|---|---|---|---|
| All cancers | Gastrointestinal cancers | Extra-gastro-intestinal cancers | |
| 20 | 3.3 | 2.2 | 1.1 |
| 30 | 6.5 | 3.9 | 2.6 |
| 40 | 16.6 | 3.9 | 12.9 |
| 50 | 30.2 | 10.6 | 20.8 |
| 60 | 62.1 | 33.8 | 40.3 |
| 70 | 62.1 | 47.1 | 40.3 |
| 80 | 62.1 | 47.1 | 40.3 |
Figure 1.
Cumulative incidence rates of cancer. (A) Cumulative incidence rates of cancer in total patients with Peutz-Jeghers synsdome. (B) Comparision analysis by gender. There was no difference in cumulative cancer risk between male and female patients.
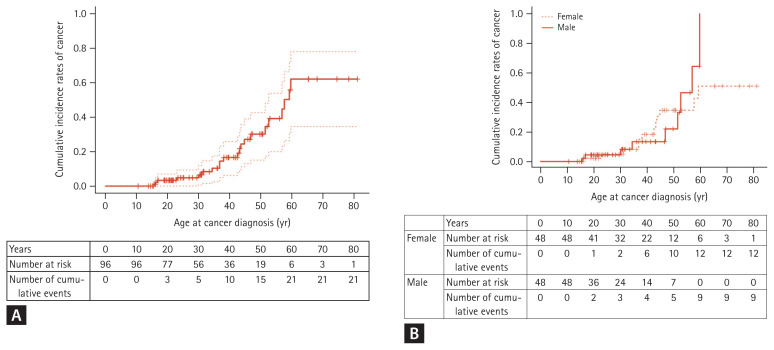
Cumulative GI cancer risk, by age, was 2.2% at age 20, 3.9% at ages 30 and 40, 10.6% at age 50, 33.8% at age 60, and 47.1% at age 70 (Table 4, Fig. 2A). There was no difference in cumulative GI cancer risk between genders (p = 0.142) (Fig. 2B).
Figure 2.
Cumulative incidence rates of gastrointestinal cancer. (A) Cumulative incidence rates of gastrointestinal cancer in total patients with Peutz-Jeghers syndrome. (B) Comparison analysis by gender. There is no difference between male and female patients.
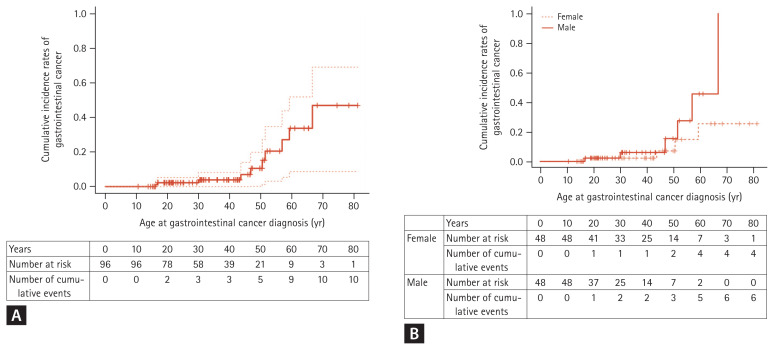
Cumulative extra-GI cancer risk, by age, was 1.1% at age 20, 2.6% at age 30, 12.9% at age 40, 20.8% at age 50, and 40.3% at age 60 (Table 4, Fig. 3A). There was no difference in cumulative extra-GI cancer risk between genders (p = 0.538) (Fig. 3B).
Figure 3.
Cumulative incidence rates of extragastrointestinal cancer. (A) Cumulative incidence rates of extragastrointestinal cancers in total patients with Peutz-Jeghers syndrome (B) Comparison analysis by gender. There is no difference between male and female patients.
In the Cox regression analysis, STK11 mutation, sex, intussusception, and family history of PJS were not significantly associated with cancer development in PJS patients (Supplementary Table 1).
DISCUSSION
To date, this is the largest multi-center study of the clinical characteristics and cancer risks of PJS patients in Korea. A previous study has been conducted in Korea with PJS patients but the sample size was small (n = 30) and the analysis was limited [19]. The current study comprised 96 PJS patients in Korea.
In our study, the median age for PJS diagnosis was 23.4 years, slightly less than the 29 years reported in a Danish study [20]. In our sample, 11.5% were diagnosed with PJS before the age of 10, 41.7% before the age of 20, 13.5% after the age of 50, and 5.2% after the age of 60. The age range for PJS diagnosis was diffuse. This wide age distribution is consistent with the findings of a previous study [20]. Therefore, we need to suspect PJS when PJ polyps or mucocutaneous pigmentation are found in patients of any age.
A family history of PJS was identified in 33.8% of the 65 subjects with available family history information. Although the proportion of subjects with a family history of PJS in our study was lower than the 48% to 51% found in previous studies [19,20], we identified that a considerable portion of PJS patients as de novo cases. About half (47.9%) of the PJS patients in our sample underwent at least one laparotomy due to intussusception, which was lower than the 67% reported in the Danish study [20].
In our study, cancer developed in 21.9% of the sample, which is within the 16% to 28% range of previous studies [5,20]. GI cancers developed in 10.4%, the same result as that of a previous study in China [5]. The median age of cancer diagnosis was 42.5 years, within the range of 41 to 45 years seen in previous research [3,5,20]. A previous study in Korea reported a mean age of cancer diagnosis of 36 years [19]. In our study, cancer incidence rapidly increased after age 30, and the cumulative cancer incidence rate at age 60 was 62.1%, slightly below the 67% seen in a study by Mehenni et al. [7]. There have been various studies on the cumulative cancer risks in PJS patients (Table 5) [3–11], but reports on the cancer risk of PJS patients in Asia are rare. A Japanese study reported a cumulative cancer risk at age 70 of 83%, which is significantly higher than the 62.1% of our study. However, the Japanese study was a comprehensive review of case reports and retrospective cohort studies of patients with PJS reported in Japan, thus was not free from publication bias [17]. In a 2017 Chinese study, the cumulative cancer risk at age 60 was 55%, slightly below the 62.1% in our study [5]. In our study, GI cancer incidence rapidly increased after age 40, and the cumulative GI cancer risk at age 70 was 47.1%, similar to the 42% seen in the Chinese study [5]. In the study performed in Japan, the cumulative GI cancer risk at age 70 was 59%, which was higher than our 47.1%.
Table 5.
Cumulative lifetime cancer risks of patients with Peutz-Jeghers syndrome
| Site | Cumulative risk, % | Reference |
|---|---|---|
| Any cancer | 37–93 | [3–10] |
| GI cancer | 42–66 | [3,5–9] |
| By site | ||
| Stomach | 29 | [4] |
| Small bowel | 13 | [4] |
| Colorectal | 12–39 | [4–6,8] |
| Pancreatic | 11–55 | [4,6,8,11] |
| Breast | 24–54 | [4,6,8,9] |
| Uterine | 9 | [4] |
| Ovarian | 21 | [4] |
| Testicular | 9 | [4] |
GI, gastrointestinal.
Among the 21 cancer patients in our study, seven (33%) had double primary malignancies, which is slightly higher than the result of the Danish study, in which three of 12 (25%) had double malignancies [20]. Since double primary malignancies develop in a considerable portion of PJS patients, it is important to be cognizant of the possibility of a second malignancy in PJS patients already diagnosed with cancer.
Interestingly, among the GI cancer patients in our study, pancreatic cancer was the most common. This was also seen in a study by Resta et al. [8] In a previous study of PJS patients in Korea, the most common malignancy was small bowel cancer [19]. In most other studies, colorectal cancer was the most common GI cancer in PJS patients [3,5,6,9,17]. Giardiello et al. [4] found a cumulative risk of colorectal cancer higher than that of small bowel cancer in PJS patients, but the relative risk of small bowel cancer was higher than that of colorectal cancer. In our study and a previous study in Korea, the incidence of colorectal cancer was lower than that of other studies. This is likely due to the preventative effects of colonoscopic polypectomy, but further research is needed. Enteroscopic polypectomy of small intestinal polyps is known to reduce polyp-related complications, including intussusception [21,22], but it is not known whether enteroscopic polypectomy reduces the risk of cancer of the small intestine.
A study by van Lier et al. [3] found female PJS patients to have a higher risk of cancer than males. This appears to have been due to the additional risks of gynecological cancers and breast cancer [3]. However, in our study, there was no difference in the overall incidence rate of cancer between males and females. A Chinese study found that females had more extra-GI cancers than males [5]. In female Japanese patients, the risk of gynecological cancer and breast cancer was found to be higher than the risk of GI cancers. Gynecological cancers and breast cancer tend to develop earlier than GI cancers in female PJS patients [17]. Although the incidence rate of extra-GI cancers in the current study was not significantly different between sexes, we observed earlier development of female extra-GI cancers. We also found that patients were diagnosed with breast cancer, cervical cancer, and thyroid cancer at younger ages than those with other cancer types. The median ages of breast, uterine cervical, and thyroid cancer diagnosis were all around 40 years. All patients with breast or thyroid cancer were diagnosed before the age of 50. Among our GI cancers, patients with small bowel cancer and gastric cancer were diagnosed relatively early, also before the age of 50. In the previous studies, the average age of the small bowel cancer diagnosis was 37 to 42 years and the average age of diagnosis of the gastric cancer was 30 to 40 years [2,4]. These findings of our study and previous studies support the need for early initiation of GI tract surveillance in PJS patients [15].
There is some debate over the malignant potential of polyps in PJS. Latchford and Phillips [23] evaluated over 1,000 PJS polyps and identified only six cases of atypia and no adenomatous foci or malignant changes. Jansen et al. [24] suggest that PJS polyps do not have malignant potential. In contrast, a hamartoma-adenoma-carcinoma sequence has been suggested [25]. Studies reporting the development of malignancies in hamartomatous polyps support this hypothesis [20,26]. In our study, a case of a hamartomatous polyp with multifocal adenoma and CIS transformation was identified, supporting the hamartoma-adenoma-carcinoma sequence hypothesis. However, since the malignant potential of PJS polyps has not been established, it is not clear whether endoscopic polypectomy can prevent cancer or reduce cancer risk [20,27]. Further studies are needed to clarify these issues. However, cancer-related risks aside, endoscopies are still necessary to detect and remove polyps due to the risks of other future complications such as intussusception or obstruction. Polypectomy of small bowel polyps prevents the need for emergency laparotomies [1,27,28].
In our study, four cases of pancreatic cancer were diagnosed. Previous research has found the lifetime risk of pancreatic cancer in PJS patients to be 11% to 55% [4,6,8,11]. Korsse et al. [11] reported a median age of pancreatic cancer diagnosis in PJS patients of 54 years. In the latter study, two subjects developed pancreatic cancer at a relatively young age (35 and 36 years) and the authors propose that surveillance for pancreatic cancer should begin at age 30 [11]. In our study, the median age of pancreatic cancer diagnosis was 51 years and the youngest diagnosis of pancreatic cancer was 43.6 years.
We found the most common locations for malignancies were GI, followed by the breast, uterine cervix, and thyroid. Thus, GI cancer and breast cancer were the most common in our PJS patients, which is consistent with previous findings [3,6,8]. The median age of breast cancer diagnosis in our study was 39.7 years. Our sample included six breast cancer cases. After excluding one of these that was diagnosed at another hospital, four of the remaining five cases were ductal CIS. This suggests that surveillance could enable the early detection of breast cancer in PJS patients. The youngest of our breast cancer cases was 23-year-old. A previous study included a case of breast cancer diagnosed in a woman aged only 19 [4]. These findings suggest the need for active surveillance for breast cancer in female PJS patients from the age of 20 or earlier. Cervical cancer was the second most common malignancy in female patients of our PJS cohort. The youngest patient diagnosed with cervical cancer was 31-year-old. Japanese studies have reported cervical cancer as the most common in female PJS patients [17,18]; while a Chinese study found ovarian and cervical cancer to be more common than breast cancer [5]. We postulate that the greater incidence of breast cancer in our study may have been because many breast cancer cases were detected early due to breast cancer surveillance.
Only a few previous studies have reported thyroid cancer in patients with PJS [17,29,30]. In our study, there were two cases of thyroid cancer and it was found to be the most common after GI, breast, and cervical cancer. The median age of thyroid cancer diagnosis was 41.4 years. Both cases were papillary thyroid carcinoma. The youngest of the two thyroid cancer patients was 38-year-old. However, the few previous studies that have reported thyroid cancer in PJS patients have identified cases diagnosed in their early twenties suggesting the need for early initiation of thyroid cancer surveillance [29,30]. However, there is no evidence that early diagnosis can significantly improve survival with this type of malignancy [17].
Although the evidence available to inform PJS cancer surveillance programs aimed at reducing morbidity and mortality is limited, the high cancer risks justify such surveillance, nonetheless. Various guidelines have been published [1,2,12–15], and most advise regular surveillance of the GI tract, breasts, and uterus. We would suggest that the utility of pancreatic and thyroid surveillance also requires investigation (Table 6).
Table 6.
Surveillance recommendations for Peutz-Jeghers syndrome patients (adapted from ESGE guideline 2019)
| Modality | Starting age | Surveillance interval | Treatment indication |
|---|---|---|---|
| Stomach | Baseline: 8 years | Baseline: If polyps found, every 1–3 years | Elective polypectomy |
| Esophagogastroduodenoscopy | Routine: 18 years | Routine: every 1–3 years | |
| Small bowel | 8 years | Every 1–3 years | Elective polypectomy for polyps > 15–20 mm |
| Capsule endoscopy or MRI | Preferably using device-assisted enteroscopy | ||
| Colon | Baseline: 8 years | Baseline: if polyps found, every 1–3 years | Elective polypectomy |
| Colonoscopy | Routine: 18 years | Routine: every 1–3 years |
Adapted from van Leerdam et al. [15], with permission from Georg Thieme Verlag KG.
ESGE, European Society of Gastrointestinal Endoscopy; MRI, magnetic resonance imaging.
Our study had some limitations. First, this was a retrospective study and not free from selection bias. However, all PJS patients from multiple institutions were systematically enrolled based on the PJS diagnostic criteria established for this study. Second, the average follow-up period in each hospital was relatively short and medical histories, including previous cancer diagnoses, relied on the patient’s statement. Since PJS is a very rare disease, patients will be well aware of their diseases. And it seems that errors in the memory of cancer development are difficult to occur. Third, our study population was of Asian ethnicity only, and cannot be generalized to other ethnicities. Despite these limitations, our study is the largest Korean multi-center study of cancer risks in PJS patients. It is hoped that our findings will facilitate the creation of surveillance guidelines for Asian PJS patients.
In conclusion, PJS may be diagnosed at any age, and the risk of GI and extra-GI cancers is high. Cancer diagnoses in PJS patients, including small bowel, stomach, breast, and thyroid cancers, often occur at a relatively young age. Thus, thorough surveillance for GI and extra-GI malignancies is necessary and should be stipulated in PJS surveillance guidelines. Previously, there has been insufficient evidence for the development of such guidelines in Korea. It is hoped that the addition of the results of the present study to the existing body of evidence will be sufficient to enable the development of domestic surveillance guidelines for PJS patients.
KEY MESSAGE
1. Peutz-Jeghers syndrome may be diagnosed at any age.
2. The risks of gastrointestinal and extra-gastrointestinal cancer are high in patients with Peutz-Jeghers syndrome.
3. Thorough surveillance for gastrointestinal and extra-gastrointestinal malignancies is necessary in patients with Peutz-Jeghers syndrome.
Acknowledgments
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: RS-2020-KD000235).
The authors would like to thank Sohee Oh, PhD of the Medical Research Collaborating Center in Seoul National University Boramae Medical Center for statistical advice.
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Information
REFERENCES
- 1.Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975–986. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 2.van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 3.van Lier MG, Westerman AM, Wagner A, et al. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut. 2011;60:141–147. doi: 10.1136/gut.2010.223750. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 5.Chen HY, Jin XW, Li BR, et al. Cancer risk in patients with Peutz-Jeghers syndrome: a retrospective cohort study of 336 cases. Tumour Biol. 2017;39:1010428317705131. doi: 10.1177/1010428317705131. [DOI] [PubMed] [Google Scholar]
- 6.Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 7.Mehenni H, Resta N, Park JG, Miyaki M, Guanti G, Costanza MC. Cancer risks in LKB1 germline mutation carriers. Gut. 2006;55:984–990. doi: 10.1136/gut.2005.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Resta N, Pierannunzio D, Lenato GM, et al. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: results of an Italian multicenter study. Dig Liver Dis. 2013;45:606–611. doi: 10.1016/j.dld.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Lim W, Olschwang S, Keller JJ, et al. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004;126:1788–1794. doi: 10.1053/j.gastro.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Lim W, Hearle N, Shah B, et al. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer. 2003;89:308–313. doi: 10.1038/sj.bjc.6601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korsse SE, Harinck F, van Lier MG, et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: a large cohort study and implications for surveillance. J Med Genet. 2013;50:59–64. doi: 10.1136/jmedgenet-2012-101277. [DOI] [PubMed] [Google Scholar]
- 12.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 13.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner A, Aretz S, Auranen A, et al. The management of Peutz-Jeghers syndrome: European Hereditary Tumour Group (EHTG) Guideline. J Clin Med. 2021;10:473. doi: 10.3390/jcm10030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Leerdam ME, Roos VH, van Hooft JE, et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;51:877–895. doi: 10.1055/a-0965-0605. [DOI] [PubMed] [Google Scholar]
- 16.Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol. 2006;4:408–415. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Ishida H, Tajima Y, Gonda T, Kumamoto K, Ishibashi K, Iwama T. Update on our investigation of malignant tumors associated with Peutz-Jeghers syndrome in Japan. Surg Today. 2016;46:1231–1242. doi: 10.1007/s00595-015-1296-y. [DOI] [PubMed] [Google Scholar]
- 18.Iwamuro M, Toyokawa T, Moritou Y, et al. Peutz-Jeghers syndrome and cancer: a retrospective study in 14 Japanese patients with Peutz-Jeghers syndrome. Nihon Shokakibyo Gakkai Zasshi. 2019;116:1015–1021. doi: 10.11405/nisshoshi.116.1015. [DOI] [PubMed] [Google Scholar]
- 19.Choi HS, Park YJ, Youk EG, et al. Clinical characteristics of Peutz-Jeghers syndrome in Korean polyposis patients. Int J Colorectal Dis. 2000;15:35–38. doi: 10.1007/s003840050005. [DOI] [PubMed] [Google Scholar]
- 20.Jelsig AM, Qvist N, Sunde L, et al. Disease pattern in Danish patients with Peutz-Jeghers syndrome. Int J Colorectal Dis. 2016;31:997–1004. doi: 10.1007/s00384-016-2560-3. [DOI] [PubMed] [Google Scholar]
- 21.Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352–376. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 22.Kim ER. Roles of capsule endoscopy and device-assisted enteroscopy in the diagnosis and treatment of small-bowel tumors. Clin Endosc. 2020;53:410–416. doi: 10.5946/ce.2020.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latchford AR, Phillips RK. Gastrointestinal polyps and cancer in Peutz-Jeghers syndrome: clinical aspects. Fam Cancer. 2011;10:455–461. doi: 10.1007/s10689-011-9442-1. [DOI] [PubMed] [Google Scholar]
- 24.Jansen M, de Leng WW, Baas AF, et al. Mucosal prolapse in the pathogenesis of Peutz-Jeghers polyposis. Gut. 2006;55:1–5. doi: 10.1136/gut.2005.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosman FT. The hamartoma-adenoma-carcinoma sequence. J Pathol. 1999;188:1–2. doi: 10.1002/(SICI)1096-9896(199905)188:1<1::AID-PATH327>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Bouraoui S, Azouz H, Kechrid H, et al. Peutz-Jeghers’ syndrome with malignant development in a hamartomatous polyp: report of one case and review of the literature. Gastroenterol Clin Biol. 2008;32:250–254. doi: 10.1016/j.gcb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Latchford AR, Neale K, Phillips RK, Clark SK. Peutz-Jeghers syndrome: intriguing suggestion of gastrointestinal cancer prevention from surveillance. Dis Colon Rectum. 2011;54:1547–1551. doi: 10.1097/DCR.0b013e318233a11f. [DOI] [PubMed] [Google Scholar]
- 28.Chen TH, Lin WP, Su MY, et al. Balloon-assisted enteroscopy with prophylactic polypectomy for Peutz-Jeghers syndrome: experience in Taiwan. Dig Dis Sci. 2011;56:1472–1475. doi: 10.1007/s10620-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 29.Triggiani V, Guastamacchia E, Renzulli G, et al. Papillary thyroid carcinoma in Peutz-Jeghers syndrome. Thyroid. 2011;21:1273–1277. doi: 10.1089/thy.2011.0063. [DOI] [PubMed] [Google Scholar]
- 30.Zirilli L, Benatti P, Romano S, et al. Differentiated thyroid carcinoma (DTC) in a young woman with Peutz-Jeghers syndrome: are these two conditions associated? Exp Clin Endocrinol Diabetes. 2009;117:234–239. doi: 10.1055/s-0028-1102920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.