Abstract
The structural genes expressing type 1 fimbriae in Escherichia coli alternate between expressed (phase ON) and non-expressed (phase OFF) states due to inversion of the 314 bp fimS genetic switch. The FimB tyrosine integrase inverts fimS by site-specific recombination, alternately connecting and disconnecting the fim operon, encoding the fimbrial subunit protein and its associated secretion and adhesin factors, to and from its transcriptional promoter within fimS. Site-specific recombination by the FimB recombinase becomes biased towards phase ON as DNA supercoiling is relaxed, a condition that occurs when bacteria approach the stationary phase of the growth cycle. This effect can be mimicked in exponential phase cultures by inhibiting the negative DNA supercoiling activity of DNA gyrase. We report that this bias towards phase ON depends on the presence of the Fis nucleoid-associated protein. We mapped the Fis binding to a site within the invertible fimS switch by DNase I footprinting. Disruption of this binding site by base substitution mutagenesis abolishes both Fis binding and the ability of the mutated switch to sustain its phase ON bias when DNA is relaxed, even in bacteria that produce the Fis protein. In addition, the Fis binding site overlaps one of the sites used by the Lrp protein, a known directionality determinant of fimS inversion that also contributes to phase ON bias. The Fis–Lrp relationship at fimS is reminiscent of that between Fis and Xis when promoting DNA relaxation-dependent excision of bacteriophage λ from the E. coli chromosome. However, unlike the co-binding mechanism used by Fis and Xis at λ attR, the Fis–Lrp relationship at fimS involves competitive binding. We discuss these findings in the context of the link between fimS inversion biasing and the physiological state of the bacterium.
Keywords: DNA supercoiling, Fis, nucleoid-associated protein, nucleoprotein complexes, type 1 fimbriae
Introduction
Type 1 fimbriae (or pili) are surface appendages found on members of the Enterobacteriaceae [1]. They are virulence factors in pathogenic strains [2–4] and contribute to biofilm formation in the host [5–7] and in the external environment [8]. Fimbriae are up to 2 µm in length, similar to the length of an Escherichia coli cell, and 7 nm in diameter. Each of the fimbriae has a helical structure composed of repeated copies of the FimA subunit protein; the FimH adhesin is located at the tip, where it is responsible for binding to mannose [1]. Mannose-sensitive agglutination of red blood cells is a diagnostic test for the presence of type 1 fimbriae [1, 9–11].
The production of type 1 fimbriae is subject to phase variation, with fimbriate and afimbriate cells coexisting in the same population [9, 12]. This behaviour has been interpreted as a bet-hedging strategy that balances the risks of producing these highly immunogenic fimbriae (detection by the host immune system; the physiological cost of making, exporting and assembling the structures) with the benefits (biofilm-based community living; colonization of a host or another environmental niche) [13–16]. The invertible fimS genetic element is the basis of phase-variable fim operon expression. This 314 bp DNA segment harbours both the promoter for the transcription of the fim operon (Fig. 1a) and a Rho-dependent transcription terminator that influences the stability of the mRNA transcribed from the fimE gene [17, 18]. Inverting fimS connects/disconnects the fim operon to/from its transcription promoter, and connects/disconnects the fimE gene to/from its terminator, affecting FimE production [17].
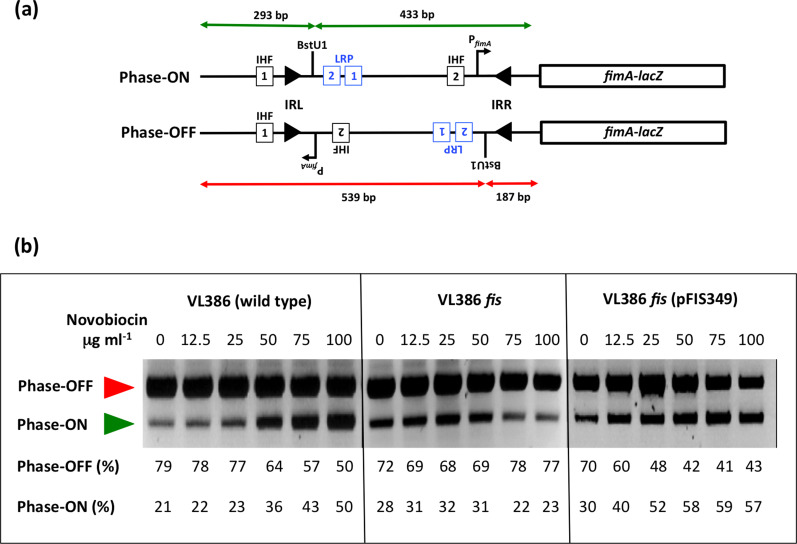
Fig. 1.
The phase OFF-to-phase ON bias of fimS genetic switch inversion in novobiocin-treated cultures is reversed in the absence of the Fis protein. (a) The fimS inversion assay. The fimS genetic element is amplified from the chromosome by PCR and the amplimers are cleaved with the BstUI restriction endonuclease (see Methods). The lengths of the cleavage products are summarized for the ON (green) and OFF (red) orientations of fimS, above and below the drawings, respectively. The angled arrow, labelled P fimA , shows the position and orientation of the transcriptional promoter of the fimA gene. In strain VL386 and its derivatives, the fimA gene is fused to a lacZ reporter gene, allowing phase ON and phase OFF bacterial colonies to be distinguished on MacConkey lactose indicator agar plates. Squares represent the binding sites for IHF (black) and Lrp (blue), respectively. The filled arrowheads represent the left (IRL) and right (IRR) 9 bp inverted repeats that flank fimS. The position of the BstUI restriction endonuclease recognition site between Lrp binding site LRP-2 and IRR is shown. Not to scale. (b) Electrophoresis of the fimS DNA fragments from the wild-type strain (VL386), its fis knockout derivative and the complemented fis mutant, following BstUI digestion of the PCR-amplified fimS genetic element. The red arrowhead indicates the 539 bp phase OFF diagnostic band and the green arrowhead shows the 433 bp diagnostic band. The cultures had been treated with novobiocin at the concentrations given above each gel lane. The intensities of the DNA bands corresponding to the ON and OFF orientations of fimS in each lane were determined by densitometry and are reported as percentages below the lane. The experiment was performed three times and typical data are presented.
Inversion of fimS involves site-specific recombination within the 9 bp inverted repeats that flank the element [19, 20] (Fig. 1a). In pathogenic strains of E. coli, the paralogous, independently acting tyrosine integrases, FimB and FimE, promote inversion [21–24]. These integrases catalyse the recombination reaction using the same chemistry, but their distinct DNA binding preferences at the alternate forms of fimS determine their recombination biases: FimB inverts both the phase ON and phase OFF forms of fimS with equal efficiency, whereas FimE has a strong preference for the phase ON form, biasing FimE-mediated recombination in favour of ON-to-OFF switching [19, 20, 24, 25]. In bacteria producing both FimB and FimE, the ON-to-OFF inversion preference of FimE predominates and inversion of the fimS element is biased strongly towards the OFF orientation [17–19, 25]. Many laboratory strains of E. coli K-12 lack the FimE recombinase due to mutations in the fimE gene; in these strains, fimS inversion depends on the unbiased FimB recombinase alone [26].
FimB-dependent inversion of fimS is sensitive to DNA supercoiling [27–29], a feature that it shares with the Int tyrosine integrase recombinase of bacteriophage λ [30, 31]. Inhibition of type II topoisomerase activity by the drug novobiocin results in a dose-dependent relaxation of negatively supercoiled DNA and concomitant biasing of fimS inversion in favour of the ON orientation [27]. In λ, DNA relaxation favours excision of the prophage from the chromosome while negative DNA supercoiling is required for efficient λ integration [30, 31]. Thus, in both of these tyrosine integrase-mediated site-specific recombination systems, DNA topology exerts differential effects on the directionality of the reaction.
Three nucleoid-associated proteins (NAPs) influence the inversion of fimS: the integration host factor, IHF [32–34]; the leucine-responsive regulatory protein, Lrp [35–38]; and the histone-like nucleoid structuring protein, H-NS [36, 39–41].
IHF is essential for inversion; in its absence the fimS element becomes frozen in either the ON or the OFF orientation, reflecting the switch phase at the moment that IHF was removed from the cell [33, 34]. IHF binds to two sites; the IHF-1 site is adjacent to the left inverted repeat (IRL) of fimS, while site IHF-2 is within fimS (Fig. 1a). Site IHF-2 has an ancillary role in boosting the activity of the fimA promoter [33], while IHF-1 is essential for phase OFF-to-ON orientational bias [36]. IHF acts in concert with Lrp to impose phase ON orientational bias; Lrp binds to two sites, LRP-1 and LRP-2 (Fig. 1a), within fimS [37], and its presence is required for phase ON biasing when DNA is relaxed [38]. Once phase ON bias is established, the H-NS NAP is required to maintain it. This is achieved when H-NS binds to fimS and to the adjacent chromosomal DNA, creating a nucleoprotein 'trap' that maintains fimS in the ON orientation under conditions of relaxed DNA topology [36, 40]. Thus, Lrp acts as a directionality determinant in fimS site-specific recombination, by analogy with the role of the Xis protein during bacteriophage λ excision from the E. coli chromosome, catalysed by the Int tyrosine integrase [42].
Lrp binds cooperatively to DNA [43] at sites matching a degenerate consensus sequence [44], its 8-mer/16-mer oligomeric structure is sensitive to l-leucine [45] and its gene regulatory activities may be indifferent to, stimulated by, or inhibited by l-leucine and other amino acids [43, 46, 47]. The production of Lrp is subject to transcriptional autorepression [10, 48] has a broad impact on gene expression [49] and plays a central part in the adaptation of the bacterium to the stationary phase of the growth cycle [50–52].
In contrast to Lrp, the factor for inversion stimulation, Fis, is produced predominantly in the early exponential phase of growth [53–56]. It influences a wide range of DNA transactions: site-specific recombination [42, 57]; chromosome replication [58–61]; transcription [62–64]; and transposition [65, 66]. The Fis protein influences DNA topology at several levels: it regulates the transcription of topA, the gene that encodes DNA topoisomerase I [67, 68], and also the expression of the gyrA and gyrB genes, encoding the alpha and beta subunits, respectively, of the heterotetrameric DNA gyrase [69, 70]. In addition, Fis acts as a topological buffer to set local DNA topology by constraining plectonemically supercoiled DNA [71, 72]. Negative DNA supercoiling stimulates the transcription of the negatively autoregulated fis gene [73].
Fis is required to maintain the OFF orientation of fimS in the presence of the FimE recombinase [74], suggesting that Fis is another directionality determinant affecting fimS site-specific recombination in the same direction as Lrp. Here, we explore the role of the Fis protein in FimB-mediated fimS inversion by monitoring the inversion preferences of this site-specific recombinase in the presence or absence of Fis and by identifying biochemically, and then disrupting genetically, a binding site for Fis within fimS that is essential for determining the inversion preference of FimB. This Fis binding site substantially overlaps LRP-2, one of the Lrp binding sites in fimS, a situation that is reminiscent of the overlapping binding sites used by Xis and Fis in the attR region of the λ prophage during Int-mediated excision of the bacteriophage from the chromosome [31, 42]. The Fis and Xis proteins bind simultaneously to attR [42]; in contrast, we found that Fis and Lrp bind competitively to the LRP-2 site in fimS.
Methods
Media, growth conditions and genetic techniques
The strains used in these experiments were derivatives of E. coli K-12 (Table 1). Strain XL-1 Blue was used for routine molecular biology and the fimA-lacZ transcriptional fusion strain VL386, and its derivatives, were used for experiments with the fimS genetic switch. The VL386 Δfis::kan knockout mutant was derived by P1vir-mediated transduction [75, 76] using a CSH50 fis::kan mutant lysate. VL386 lrp::cml was also prepared by transduction, using a CSH50 lrp::cml lysate. Complementation of the fis mutation was carried out using plasmid pFIS349, which is a single-copy plasmid based on the mini-F origin plasmid pZC320 [77]. Bacteria were cultured in lysogeny broth (LB, made from Difco media components) or LB agar (containing agar at 1.5 % w/v) [75]. MacConkey lactose agar plates [75] were used for Lac phenotype determination. Unless otherwise stated, liquid cultures were grown overnight at 37 °C with aeration at 200 r.p.m in an orbital incubator (New Brunswick). Where appropriate, antibiotics (Sigma-Aldrich) were used at the following concentrations: carbenicillin (100 μg ml−1), chloramphenicol (25 μg ml−1) and kanamycin (20 μg ml−1). Plasmid DNA was introduced to bacterial cells by CaCl2 transformation [78] or electroporation using a Bio-Rad Gene Pulser as described in Hanahan, 1983 [79].
Table 1.
Bacterial strains and plasmids
|
Strain/plasmid |
Relevant details |
Reference/source |
|---|---|---|
|
Strains |
||
|
VL386 |
φ(fimA-lacZ)λpL(209)fimE::IS1 |
[9] |
|
VL386recD |
VL386 recD::Tn10 |
[24] |
|
CJD2116 |
VL386 Δfis::kan |
This work |
|
CJD2117 |
VL386 Δlrp::cml |
This work |
|
CJD2119 |
CJD2116 (pFIS349) |
This work |
|
VL386fimS-dist |
VL386 with Fis/LRP-2 binding site disrupted |
This work |
|
XL-1 Blue |
recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F' proAB lacIqZ M15 Tn10 (Tetr)] |
Stratagene |
|
Plasmids |
||
|
pSGS501 |
fimB::kan, fimE::IS1, φ(fimA-lacZ) cloned in pACYC184, switch phase OFF Cmr |
[24] |
|
pFIS349 |
pGS349 containing the Salmonella enterica serovar Typhimurium dusB-fis operon, Apr |
[77] |
|
pLSB124 |
pMAK705 containing wild-type fimB and sacB gene from Bacillus subtilis |
This work |
|
pMAK705 |
Temperature-sensitive allele exchange vector |
[109] |
|
pMCL210 |
Cloning vector, p15A replicon |
[110] |
|
pMMC106 |
pMCL210 cut with NheI and XbaI and religated to delete the lac promoter |
[20] |
|
pMMC108 |
fimS cloned as a 550 bp fragment in the PstI site of pMMC106 |
[20] |
|
pSLD203 |
fimB gene cloned in pUC18 |
[28] |
|
pUC18 |
ColE1 replicon, Apr |
[111] |
Molecular biological techniques
Plasmid DNA was isolated using Qiagen Midi columns or Wizard mini prep columns (Promega). Specific DNA fragments were isolated using an agarose gel extraction kit (Roche Applied Science). Restriction enzyme digests were carried out using enzymes purchased from New England Biolabs by following the manufacturer’s recommended procedure. Plasmid DNA was sequenced using a T7 sequencing system (USB). Plasmid pSGS501 was used as the template with oligonucleotide COL69 as the sequencing primer (Table 2). Automated sequencing was carried out at MWG Biotech. This company also synthesized the oligonucleotides used in this study.
Table 2.
Oligonucleotides
|
Name |
Sequence |
Purpose |
|---|---|---|
|
OL4 |
5′-GACAGAACAACGATTGCCAG-3′ |
PCR switch assay |
|
OL20 |
5′-CCGTAACGCAGACTCATCCTC-3′ |
PCR switch assay |
|
BSFORBIO |
5′-CCACCTCATGCAATATAAAC-3′ |
Probe for gel retardation assays |
|
BSREVBIO |
5′-CCCCCAAAAGATGAAACATTT-3′ |
Probe for gel retardation assays |
|
SpvR11 |
5′-CCAAGCTTCAGTACTGATCTTGCGATACTG-3′ |
Probe for gel retardation assays |
|
SpvR14 |
5′-CCCAAGCTTCAGGTCACCGCCATCCTGTTTTTGC-3′ |
Probe for gel retardation assays |
|
COL-DFP |
5′-GAGAAGAGGTTTGATTTAAC-3′ |
Probe for DNase I footprinting |
|
COL69 |
5′-GAGTTTGACTGCCAACACT-3′ |
Probe for DNase I footprinting and primer for Sanger sequencing |
|
FBSFOR2 |
5′-CAATAGAATATTAAGGGGTTAGCTAAACT- GAAAAAG-3′ |
Mutagenesis of Fis binding site |
|
FBSREV2 |
5′-CTTTTTCAGTTTAGCTAACCCCTTAATATTCTATTG-3′ |
Mutagenesis of Fis binding site |
Inhibition of DNA gyrase with novobiocin
Assays involving the DNA gyrase inhibitor novobiocin (Sigma-Aldrich) were performed as follows: bacteria containing the fimA-lacZ transcriptional fusion were screened for their Lac phenotypes on MacConkey lactose indicator medium as described previously [27]. Distinctly phase ON (red/Lac+) or phase OFF (white/Lac−) colonies were used to inoculate 2 ml LB (lysogeny broth) in test tubes and grown overnight. These were used to inoculate 250 ml flasks containing 25 ml of LB. These cultures were grown aerobically at 200 r.p.m. until they reached an optical density of approximately 0.1 at 600 nm. At this point novobiocin (aqueous stock solution 100 mg ml−1) was added to a final concentration of 0, 12.5, 25, 50, 75, or 100 μg ml−1. Cultures were incubated for a further 20 h before sampling to determine the orientation of the fimS switch in the chromosome.
Determination of fimS orientation on the chromosome
A PCR-based assay was used to determine the orientation of the fimS genetic switch on the E. coli chromosome (Fig. 1a). This method exploited the presence of a unique BstUI restriction site in the fim switch, fimS, which results in products of unequal lengths, depending on the switch orientation [24]. This restriction fragment length dimorphism allowed ON and OFF switches to be distinguished and quantified. Bacterial samples were harvested by boiling 50 µl of culture following overnight incubation at 37 °C. Oligonucleotides OL4 and OL20 (Table 2) were used to amplify the switch region and generate a 726 bp DNA product. DNA amplification used Taq polymerase (New England Biolabs) with the following PCR conditions: denature at 94 °C for 3 min, followed by 30 cycles of 94 °C for 1 min, 58 °C for 1 min and 72 °C for 1 min. This was followed by a final extension time of 10 min at 72 °C. Samples were cooled to 60 °C, 10 units of BstUI were added to each reaction and incubation was continued at 60 °C for 3 h. Digested PCR products were electrophoresed on 2 % agarose gels. Phase OFF populations of bacteria yielded two DNA fragments of 539 and 187 bp in length, whereas phase ON populations gave fragments of 433 and 293 bp. The well-resolved 539 and 433 bp DNA fragments were used to compute the relative quantities of ON and OFF switches in the bacterial population: QUANTITY ONE image analysis software was used to measure approximate proportions of the resultant fragments (Fig. 1b). The PCR-based DNA inversion assays were performed in triplicate and typical data are shown.
Analysing protein binding to DNA by electrophoretic mobility shift assay
The association of purified Fis or Lrp proteins with the E. coli fim switch was measured using an electrophoretic mobility shift assay (EMSA). A 135 bp probe was amplified by PCR with Pfu polymerase (Stratagene), using the primer pair BSFORBIO and BSREVBIO (Table 2). The S. enterica spvR promoter was amplified as a 157 bp fragment using the primer pair, spvR11 and spvR14 (Table 2) [80], and this was used as a negative control for the Fis binding experiments [63]. The probes were then purified using a PCR clean-up kit (Roche Applied Science). The oligonucleotides had been ordered with 5′ biotinylated ends allowing for subsequent complex detection. Complexes were formed following incubation of amplified probe with increasing concentrations (0–270 nM) of purified His-tagged Fis [69] or 0–220 nM of purified His-tagged Lrp [38] for 15 min as described by the manufacturers of the Electrophoretic Mobility Shift Assay kit (Pierce). Competitive binding of purified Fis and Lrp was tested with Lrp being added in increasing concentrations to DNA that had been prebound with Fis at a constant concentration. Protein–DNA complexes were resolved by electrophoresis through a 7.5% polyacrylamide gel for 2 h at room temperature. The gel was then electrophoretically blotted and developed using the procedure recommended by the manufacturer (Pierce).
DNase I footprinting
A 385 bp fragment encompassing fimS was amplified from pSGS501 with the primers COL69 and COL-DFP (Table 2). The PCR product was purified with a PCR clean-up kit (Roche Applied Science) and end-labelled with [γ-32P]-ATP (Perkin Elmer) using T4 polynucleotide kinase (New England Biolabs). This fragment was then digested for 2 h with MfeI at 37 °C in a reaction volume of 60 µl. The probe was purified by extraction from a 6% polyacrylamide gel, following electrophoresis in TBE buffer. Labelled DNA was eluted in 3 ml of elution buffer [10 mM Tris–HCl pH 8.0, 1 mM EDTA, 300 mM sodium acetate (pH 5.2), 0.2% SDS] at 37 °C for 48 h. The eluted probe was extracted with an equal volume of phenol : chloroform and ethanol precipitated. The DNA pellet was then resuspended in 100 µl of double-distilled water. Two microlitres of labelled probe solution were used in each footprinting experiment. DNA–protein complexes were formed in 50 µl of footprinting buffer (20 mM Tris–HCl pH 7.5, 80 mM NaCl, 1 mM EDTA, 100 µg ml−1 BSA, 10% glycerol and 1 mM DTT) at 37 °C for 30 min. Then 50 µl of 10 mM MgCl2–5 mM CaCl2 were added and incubation continued for a further 10 min. Next, 0.01 U of DNase I (Roche Molecular Biochemicals) was added, and digestion was allowed to proceed for 1 min. The reaction was terminated by the addition of 90 µl of stop solution (200 mM NaCl, 30 mM EDTA pH 8.0, 1% SDS, 100 µg ml−1 tRNA). Samples were extracted once with an equal volume of phenol : chloroform and then precipitated with ethanol and resuspended in 6 µl of gel loading dye. Samples were denatured at 95 °C for 3 min and were subjected to electrophoresis on a 7% urea–polyacrylamide gel alongside DNA sequencing reactions. Dideoxy chain terminator sequencing [81], primed by oligonucleotide COL69 (Table 2), was used to generate the DNA sequence ladder.
Site-directed mutagenesis and allele replacement
Site-directed mutagenesis was performed using the Quikchange II (Stratagene) site-directed mutagenesis kit, according to the manufacturer’s recommendations. The oligonucleotides used to mutate the Fis binding site (FBSFOR2 and FBSREV2) are described in Table 2, and were supplied by MWG Biotech. Plasmid pMMC108 [20] was used as the substrate for the mutagenesis. The method of allele replacement was as described previously [24]. Briefly, the mutated Fis binding site was introduced to the chromosome by cloning an MfeI-SnaBI fragment of fimS, containing the disrupted site into pSGS501, a plasmid containing the cat chloramphenicol resistance gene (Table 1). The resulting plasmid was digested with EcoRV and an 8 kb fragment containing the mutated fimS region was gel extracted. Two micrograms of this fragment were electroporated into strain VL386recD. Loss of plasmid sequences following homologous recombination with the chromosome was confirmed by testing the transformants for chloramphenicol sensitivity. The presence of the disrupted Fis binding site in the chromosomal fimS element was confirmed by PCR amplification followed by DNA sequencing.
Results
Loss of Fis alters the pattern of fimS inversion when DNA gyrase is inhibited
Inversion of fimS by the FimB recombinase becomes biased towards the ON orientation when the introduction of negative supercoils by DNA gyrase is inhibited by novobiocin. Increasing the dose of the gyrase-inhibiting drug exacerbates this effect. The Fis protein is known to preserve negative supercoils at a local level by binding to DNA [71, 72]. We investigated the impact of eliminating Fis protein production on the inversion of fimS by FimB, using as the wild-type E. coli strain VL386, and CJD2116, an isogenic fis knockout mutant (Table 1), treated with increasing concentrations of novobiocin (Fig. 1b).
In the wild-type, treatment with incrementally increasing concentrations of novobiocin was accompanied by a progressive accumulation in the bacterial population of fimS switches in the ON orientation, in agreement with previous data [27–29, 36, 38]. In the fis mutant, this effect was not observed: as the concentration of the drug increased, the switch orientation remained close to a constant ratio of 30% ON and 70% OFF (Fig. 1b). The introduction of a functional copy of fis on a single-copy plasmid (strain CJD2119, Table 1) restored the biased OFF-to-ON switching that is characteristic of the wild-type (Fig. 1b). These data implicated Fis as a contributing factor in biasing FimB-mediated fimS switching when DNA gyrase activity is inhibited by novobiocin. We decided to investigate the relationship between Fis and fimS in more detail at the molecular level.
Characterization of a Fis binding site within fimS
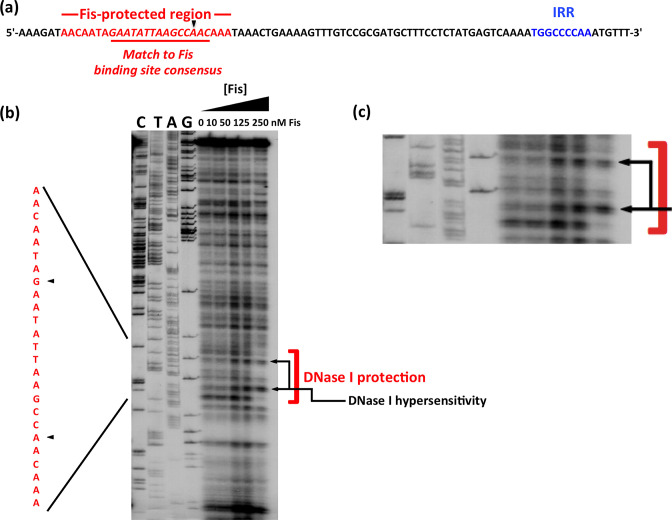
Although the consensus sequence of the Fis binding site in DNA is degenerate, it has a number of features that are highly conserved among high-affinity sites [82, 83]. These allowed us to identify, by inspection, a potential binding site for Fis within the fimS element, located 50 bp from the 9 bp inverted repeat that forms the right-hand boundary of the switch when in the OFF orientation (Fig. 2a). We then used DNase I footprinting to map the binding site of purified E. coli Fis protein on the fimS genetic element in vitro. The site that was protected by Fis from DNase I digestion corresponded to the DNA sequence that matched with the consensus for high-affinity Fis binding sites (Fig. 2b, c). The DNase I footprint consisted of bases that were protected by Fis and bases that became hypersensitive to digestion in the presence of the NAP. The latter are commonly found in sites occupied by DNA-binding proteins that bend DNA, a known property of Fis [58, 82–84].
Fig. 2.
Identifying a binding site for Fis within fimS. (a) The DNA sequence of the right end of fimS in phase OFF, showing the location of the right inverted repeat, IRR (blue), and a sequence matching the consensus for Fis binding sites (red). (b) DNase I footprinting was performed with purified Fis protein and a DNA fragment corresponding to the right end of phase OFF fimS. Concentrations of Fis are given above each lane. The products of dideoxy chain-terminator nucleotide sequencing reactions, carried out with the same DNA fragment, are shown in the lanes labelled C, T, A and G. The assay revealed a region in which Fis protected the DNA from DNase I digestion, with two nucleotides exhibiting hypersensitivity to the enzyme. The protected region is highlighted with a red bracket and black arrows indicate the two hypersensitive bases. Black arrowheads point to the regions of hypersensitivity in the sequence shown on the left in red; this sequence corresponds to that shown in red in (a). This part of the image is reproduced in an enlarged format in (c).
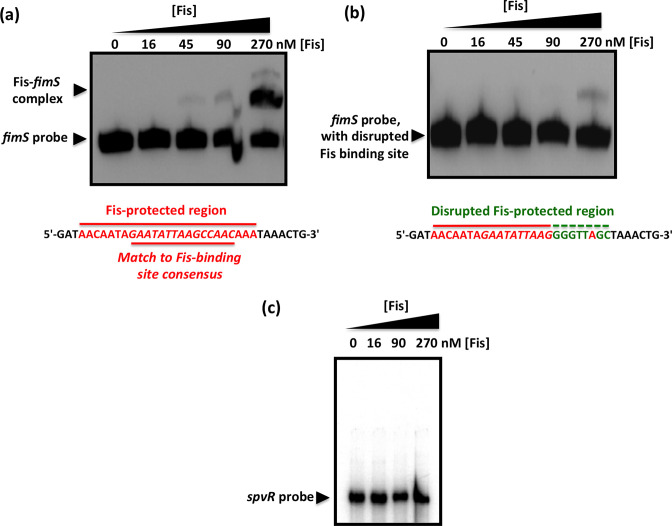
Further evidence of Fis binding to fimS came from an electrophoretic mobility shift assay. At 90 nM Fis, the protein formed a complex that was consistent with the occupation of a single binding site (Fig. 3a). The Fis binding site within fimS was subjected to base substitution mutagenesis to alter its DNA sequence without altering its length. Changes were made to eight contiguous bases, destroying the match to the consensus sequence for high-affinity Fis binding sites. The modified DNA element could not bind Fis at a protein concentration of 90 nM, and only a weak interaction was detected at 270 nM (Fig. 3b) that was likely due to the known tolerance of Fis for mismatches to its binding site consensus sequence [82]. Taken together, the DNase I footprinting data and the EMSA results show that Fis binds to the fimS genetic switch at a site that is located 50 bp from the inverted repeat boundary at the fimA promoter-distal end of fimS. The spvR promoter region from Salmonella enterica serovar Typhimurium, that does not bind Fis [63], was used as a negative control. This DNA sequence did not form a complex with Fis, at this or a higher concentration of the protein (Fig. 3c).
Fig. 3.
Mutation of the Fis binding site abrogates the Fis-mediated electrophoretic mobility shift of fimS DNA. (a) EMSA showing the gel mobility shift of a labelled 135 bp fragment of fimS DNA that includes the Fis binding site, highlighted in red below the gel. (b) Disruption of the Fis binding site by substituting the bases, shown in green below the gel, of the wild-type binding site sequence (red) almost completely abrogated the ability of Fis to alter the electrophoretic mobility of this DNA fragment. (c) A control EMSA using a 157 bp DNA fragment corresponding to the spvR promoter region from S. Typhimurium. This DNA fragment is known not to bind Fis [63]. Purified Fis failed to alter the electrophoretic mobility of the spvR DNA fragment at the same protein concentrations used in (a). The concentrations of purified Fis used in the experiments are given above each gel lane; black arrowheads indicate the bands corresponding to the unbound DNA probes and in (a), the Fis–fimS complex.
The Fis binding site is crucial for fimS inversion preferences
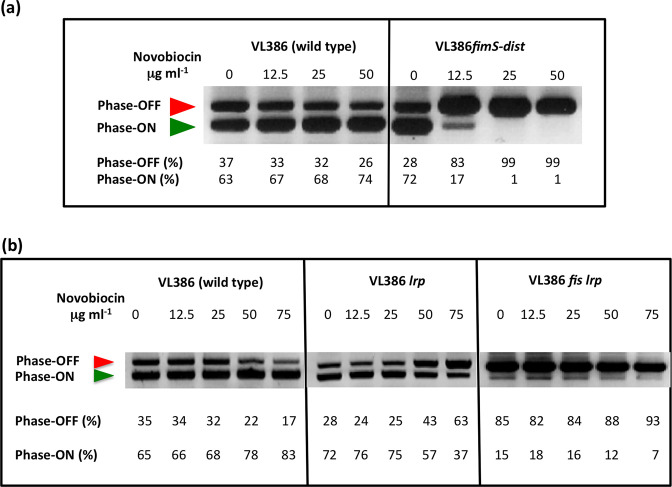
The derivative of fimS with the 8 bp substitution mutation in the Fis binding site was transferred to the E. coli chromosome by homologous recombination. The mutant strain and the wild-type were treated with increasing concentrations of novobiocin and the orientation of fimS was monitored by PCR (Fig. 4a). In the wild-type, the fimS element adopted a dose-dependent preference for the phase ON orientation with novobiocin treatment; in the mutant with the disrupted Fis binding site, fimS adopted a novobiocin-dependent preference for the OFF orientation, the opposite to the situation seen in the wild-type (Fig. 4a). Not only had the direction of the inversion bias been reversed compared to the wild-type, the response to novobiocin occurred at the lowest concentration of the drug (12.5 μg ml−1). These results demonstrated that the Fis binding site plays a pivotal role in determining both the direction of the DNA inversion response and the sensitivity of fimS inversion to DNA gyrase inhibition. Inspection of the Fis binding site’s location suggested that it overlapped Lrp binding site LRP-2 (see below) and the base substitution mutations might also have impaired Lrp binding to that site. For this reason, the strains used in the fimS orientation assay contained a plasmid pUC18 derivative, pSLD203, over-expressing the FimB recombinase (Table 1). This is an established way to allow fimS inversion to continue in strains deficient in co-factor production/binding without affecting the response of fimS recombination to DNA relaxation [24, 28, 36, 38].
Fig. 4.
Loss of Fis binding and loss of Fis production biases fimS towards phase OFF. (a) Electrophoresis of the fimS DNA fragments from the wild-type strain (VL386) and from its derivative (VL386fimS-dist) in which the Fis binding site in fimS is disrupted, following BstUI digestion of the PCR-amplified fimS genetic element. (b) Electrophoresis of the fimS DNA fragments from the wild-type strain (VL386), its lrp knockout mutant derivative and the derivative with knockout mutations in both the lrp and fis genes, following BstUI digestion of the PCR-amplified fimS genetic element. The red arrowhead indicates the 539 bp phase OFF diagnostic band and the green arrowhead shows the 433 bp ON diagnostic band. In (a) and (b), the cultures have been treated with novobiocin at the concentrations given above each gel lane. The intensities of the DNA bands in each lane corresponding to the ON and OFF orientations of fimS in each lane were determined by densitometry and are reported as percentages below the lane. The experiment was performed three times and typical data are presented.
The fimS Fis binding site substantially overlaps a binding site for Lrp
The fimS DNA sequence that is protected from DNase I digestion by Fis overlaps the previously characterized LRP-2 binding site used by the leucine-responsive regulatory protein, Lrp. This Lrp site helps to determine the inversion bias of fimS [36, 38]. We first studied the fimS inversion pattern in lrp and lrp fis knockout mutants with increasing concentrations of novobiocin, compared with the wild-type pattern (Fig. 4b). The wild-type culture followed the usual pattern, with fimS becoming progressively biased towards the phase ON orientation as the novobiocin concentration increased. The lrp mutant (CJD2117, Table 1) became mildly biased towards phase OFF, in agreement with previous findings; full phase OFF biasing requires the disruption of both LRP-1 and LRP-2 [38]. In the lrp fis double mutant, the switch was already biased towards phase OFF before drug treatment and became almost wholly phase OFF as novobiocin was introduced (Fig. 4b).
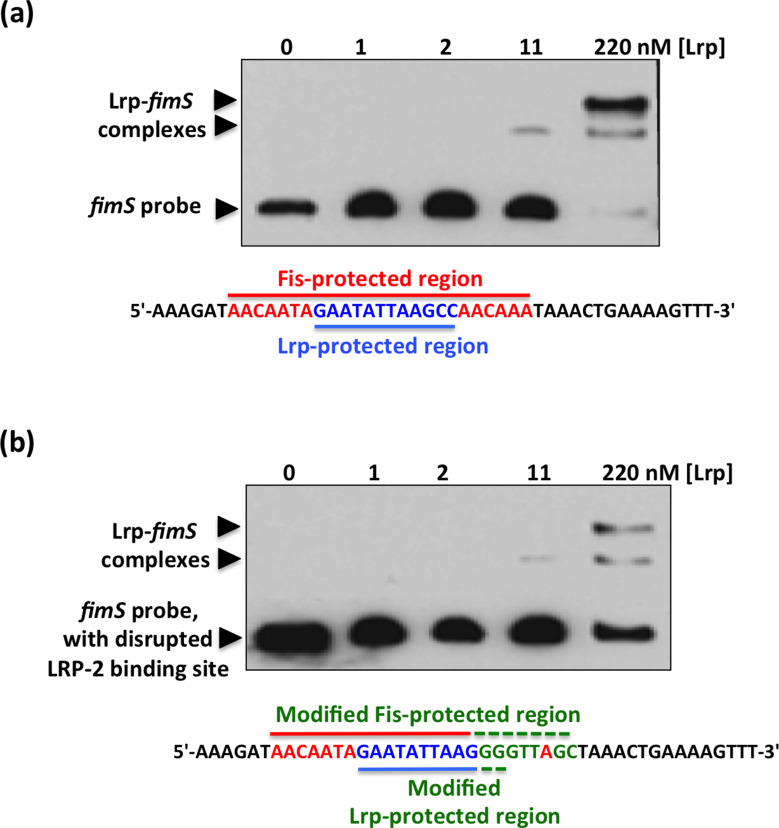
We next investigated the effect of the base substitutions that abrogated Fis binding to fimS on the binding of Lrp to the invertible switch. These sequence changes had only affected 2 bp of the Lrp-protected region at the LRP-2 site (Fig. 5). The 135 bp fimS probe used in the Fis EMSA contains both the LRP-1 and LRP-2 sites. Binding of Lrp to fimS produces a number of complexes that depend on the occupancy of the LRP-1 and LRP-2 sites, individually and collectively [37, 38]. Data from EMSA experiments using the fimS probe, with and without the Fis binding site mutation, showed that formation of the most electrophoretically retarded fimS–Lrp complex was reduced at the highest concentration of purified Lrp (Fig. 5). These data were consistent with the previously described effect of disrupting the LRP-2 site on LRP–DNA complex formation at fimS [38] and with the LRP-2 site also being targeted by the Fis protein.
Fig. 5.
EMSA showing Lrp binding to fimS with an intact or a disrupted Fis binding site. (a) Purified Lrp protein was incubated at the concentrations shown with a PCR-generated 135 bp DNA fragment from fimS that contained an intact Fis binding site. The sequence of the region protected from DNase I digestion by Fis is shown in red and the Lrp binding site (LRP-2) is shown in blue. (b) The EMSA was repeated using the fimS derivative with the disrupted Fis binding site. The base substitutions are shown below the gel in green, together with the unchanged bases from the Fis binding site (red) and the LRP-2 site (blue). In both (a) and (b), arrowheads show the positions of bands corresponding to the unbound DNA probe and the Lrp–fimS complexes.
Lrp displaces Fis from the fimS genetic switch
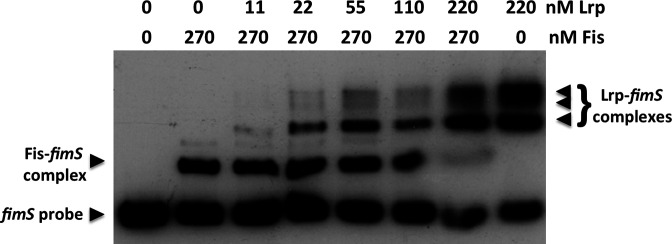
The data obtained thus far show that the Lrp and Fis proteins both target the LRP-2 binding site in fimS (Fig. 4a, b). Fis is available in high concentration at the onset of exponential growth, before becoming rapidly diluted by cell division as the bacteria in the culture expand in numbers. Since Fis and Lrp both influence fimS inversion in the same direction, we hypothesized that Fis might be replaced by Lrp at the LRP-2 site when Fis concentration declines as the exponential phase progresses. It is also possible that Lrp might actively displace Fis through competition for the same binding site. Therefore, we next assessed the ability of Lrp to displace Fis from LRP-2 in a competitive EMSA. Here, the fimS DNA was preloaded with purified Fis at a constant concentration and purified Lrp was added at increasing concentrations (Fig. 6). Lrp and Fis complexes with fimS could co-exist at intermediate concentrations of Lrp (22 to 110 nM), presumably indicating occupation of the LRP-1 site by Lrp and of LRP-2 site by Fis, but at the highest concentrations of Lrp, the Fis–fimS complex was only weakly detected, presumably because the LRP-2 site was now occupied by Lrp on most fimS copies in the reaction (Fig. 6). The EMSA competition showed a specific Fis–fimS complex and Lrp–fimS complexes; we did not detect evidence of a Fis-plus-Lrp complex with fimS, in which Lrp occupied both the LRP-1 and LRP-2 sites with co-binding of Fis and Lrp to LRP-2. Thus, the binding pattern of Fis and Lrp at fimS differed from the pattern seen with Fis and Xis at the λ attR site, where both Fis and Xis act as directionality determinants in λ excision from the chromosome and both bind to overlapping sites in the DNA simultaneously (Fig. 7) [31, 42, 85].
Fig. 6.
Displacement of Fis from fimS by the Lrp protein. Either 0 or 270 nM of purified Fis protein was prebound to a 135 bp fragment of fimS DNA containing the LRP-1, LRP-2 binding sites and the Fis binding site. Increasing concentrations of purified Lrp protein were added to the Fis–fimS complex and resolved by electrophoresis. Control lanes containing fimS DNA with no protein, with just Fis, or with just Lrp were included as controls. Arrowheads indicate the unbound fimS probe, the Fis–fimS complex and the Lrp–fimS complexes. At 220 nM, Lrp almost completely displaced prebound Fis from fimS.
Fig. 7.
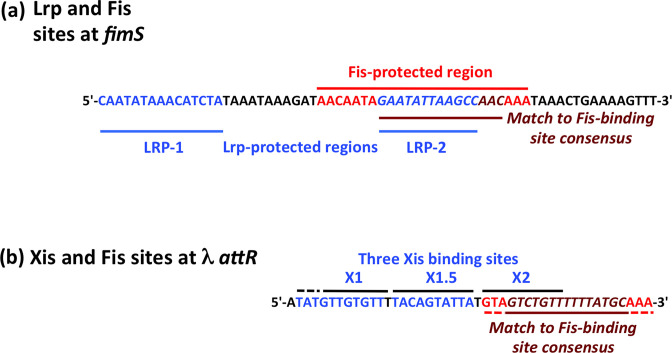
The Fis binding regions in fimS and in the bacteriophage λ prophage. (a) A summary of the DNA sequences of the LRP-1 and LRP-2 binding sites (blue) in fimS, showing that the LRP-2 (blue italics) site is nested completely within the Fis binding site (red/crimson). (b) The three binding sites for the λ Xis excisionase, X1, X1.5 and X2 (blue) are shown. The Fis site (red/crimson) overlaps the X2 site completely. Despite the superficial similarity of the nested relationships of the LRP-2 and Fis sites in fimS, and the X2 and Fis sites in λ, the interactions between the proteins in each system differ: in fimS, Fis competes with Lrp for access to the Fis binding site, whereas Fis and Xis bind cooperatively to the phage DNA in the λ prophage.
Discussion
Our data reveal a delicate interplay between DNA supercoiling/relaxation, Lrp and Fis in determining the directionality of the FimB-mediated site-specific recombination reaction in E. coli . The fimS switch (Fig. 1a) becomes progressively biased towards the ON orientation following novobiocin-induced inhibition of DNA gyrase, the topoisomerase that introduces negative supercoils into DNA (Fig. 1b) [27–29, 36, 38]. In an lrp knockout mutant, DNA relaxation results in a reversal of fimS inversion outcomes in favour of the OFF, rather than the ON, orientation [36, 38]. Inactivation of Fis production in the lrp knockout mutant produces an even stronger preference for the ON orientation, one that is achieved even in the absence of novobiocin, but which becomes much more pronounced as concentrations of the drug increase (Fig. 4). The relationship between Fis and Lrp at fimS has similarities to the relationship between Fis and the Xis directionality determinant at attR in bacteriophage λ excision (Fig. 7).
Int-mediated excisive recombination of bacteriophage λ is enhanced when DNA supercoiling levels are low, whereas integrative recombination requires negative supercoiling of the phage DNA [30, 85]. The phage-encoded Xis architectural protein stimulates excision by a factor of 106 while simultaneously inhibiting reintegration of the phage [86, 87]. Xis binds to the X1, X1.5 and X2 sites in the attR arm of the λ prophage to form a microfilament [88], with site X2 overlapping a binding site for Fis, the F site [89] (Fig. 7). Initially it was thought that Fis substituted for Xis at site X2, allowing excision to proceed under conditions where Xis was limiting [31]. It is now understood that Xis occupies all three of its binding sites, with Fis binding simultaneously to its F site (Fig. 7), producing a nucleoprotein complex with a DNA conformation that is optimal for excisive recombination [42, 88, 90]. The role Fis plays in recruiting Xis does not seem to involve protein–protein contact, but is achieved through DNA allostery [91]. While Xis imposes a preference for excision on the λ prophage, the Fis protein has been reported to stimulate integration as well as excision [57], especially in the absence of Xis [92].
Thus, the λ excision complex differs from the fimS OFF to ON inversion complex in that Xis and Fis bind together to overlapping sites in attR, while Lrp and Fis bind competitively to overlapping sites in fimS (Fig. 6). Despite this distinction, the two systems share a preference for relaxed DNA to facilitate a direction-specific recombination reaction; a dependence on tyrosine integrase recombinases to catalyse the reaction; and a requirement for IHF to occupy two sites in the DNA to organize a recombination substrate with an appropriate architecture (Figs 1a and 7).
Int differs from the FimB and FimE recombinases in that it makes contact with the DNA through both its amino-terminal and its carboxyl-terminal domains (NTD and CTD, respectively) at up to nine sites [31]. The smaller fimbrial recombinases lack the corresponding NTD and only make contact with fimS at four sites, two flanking each of the 9 bp inverted repeats, IRL and IRR, that form the boundaries of fimS [20, 23]. DNA cleavage and ligation take place within these inverted fimS repeats [20] while Int cleaves and religates the λ prophage within the 7 bp inverted repeats that are flanked by Int-CTD binding sites in attL and attR [31]. In λ site-specific recombination, Fis can stimulate both integration and excision; in fimS, Fis plays a role as a directionality co-determinant with Lrp, favouring the ON-to-OFF reaction.
DNA relaxation is a feature of stationary phase cultures [93, 94] and is a condition that biases fimS towards the ON phase [27–29, 36, 38]. This bias requires both Fis and Lrp (Fig. 4b). Loss of either protein introduces an alternative bias towards the OFF phase as DNA relaxes [36, 38]; loss of both proteins results in fimS being maintained in the OFF phase in almost all bacteria in the population (Fig. 4b).
The competitive relationship described here for Fis and Lrp at fimS is reminiscent of the competition between the Dam methylase and Lrp for access to overlapping sites in the regulatory region of pap, the operon that encodes Pap pili in uropathogenic strains of E. coli [95, 96]. The fim and pap operons engage in regulatory crosstalk via PapB-mediated repression of fim operon transcription [97–99]. Although DNA recombination does not contribute to the operation of the phase-variable pap switch, the outcome of the Dam/Lrp competition determines whether the pap operon will or will not be transcribed. Lrp accumulates in stationary phase cultures growing in rich media [100], while Dam concentrations decline under those same growth conditions [101]. It has been suggested that the shift in the Dam/Lrp balance in favour of Lrp facilitates a shift in Pap production from the ON to the OFF phase [96]. These features of pap gene regulation by Dam and Lrp mirror those described here for fim gene regulation by Fis and Lrp. Like Dam, Fis is produced in decreasing amounts as stationary phase approaches, while the production of Lrp increases [100]. In the early exponential phase, the abundant Fis protein collaborates with the less abundant Lrp to bias fimS towards the OFF phase, expanding the number of afimbriate, planktonic bacteria in the population. As exponential growth gives way to growth stasis, the Fis concentration declines sharply and Lrp replaces it at the LRP-2 site in fimS, if necessary by competitive displacement.
Biasing the fimS switch towards the OFF orientation when Fis is abundant and DNA is negatively supercoiled links fim switch inversion preferences to bacterial physiology. The Fis protein is maximally abundant at the beginning of the exponential phase of growth and is almost undetectable by the onset of stationary phase [53–56]. As Fis levels decline, Lrp is available to replace it at fimS, prolonging the bias towards phase OFF as long as the DNA remains negatively supercoiled. However, stationary phase is a period of reduced metabolic flux, producing a reduced [ATP]/[ADP] ratio that is unfavourable for the negative DNA supercoiling activity of DNA gyrase [94, 102–105]. At this stage of the growth cycle, DNA becomes relaxed [93, 94, 106], a condition that biases the fimS switch to the ON phase in the presence of Lrp [27–29, 36, 38]. Removal of Lrp from fimS reverses the inversion bias back towards the OFF phase [38].
The increased representation of fimbriate bacteria in the population of late-exponential phase/stationary phase cells promotes bacterial attachment to abiotic and biological surfaces, with an associated production of biofilm [29, 107, 108]. The resulting transition from a planktonic to a community-based attached lifestyle within the protective shield of a biofilm enhances the survival chances of the bacterial population during a period of unfavourable environmental conditions. Overall, our findings describe a molecular mechanism by which the bet-hedging strategy represented by the stochastic inversion of fimS is suspended in favour of the more deterministic outcome of ensuring that type 1 fimbriae are produced by a majority of bacteria in the population [13]. Fis and Lrp are required, in association with DNA relaxation, for the implementation of this deterministic strategy.
Funding information
This work was supported by Wellcome Trust Project Grant 061796 and Science Foundation Ireland Principal Investigator Award 13/IA/1875.
Acknowledgements
We are grateful to Stephen G.J. Smith for insightful discussions.
Author contributions
Conceptualization: C.J.D. Data analysis: C.C., M.C.B., C.J.D. Funding acquisition: C.J.D. Investigation: C.C., M.C.B., C.J.D. Methodology: C.C. Project administration: C.J.D. Resources: C.J.D. Supervision: C.J.D. Writing – original draft: C.J.D. Writing – review and editing: C.C., M.C.B., C.J.D.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Spaulding CN, Schreiber HL, Zheng W, Dodson KW, Hazen JE, et al. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. Elife. 2018;7:e31662. doi: 10.7554/eLife.31662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connell I, Agace W, Klemm P, Schembri M, Mărild S, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores-Mireles AL, Walker JN, Caparon MG, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch RA, Burland V, Plunkett G, 3rd, Redford P, Roesch P, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli . Proc Natl Acad Sci U S A. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 6.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci USA. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Marshall JS. Mechanics of biofilms formed of bacteria with fimbriae appendages. PLoS One. 2020;15:e0243280. doi: 10.1371/journal.pone.0243280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham JM, Freitag CS, Clements JR, Eisenstein BI. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli . Proc Natl Acad Sci U S A. 1985;82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland KA, Dorman CJ. Autoregulated expression of the gene coding for the leucine-responsive protein, Lrp, a global regulator in Salmonella enterica serovar Typhimurium. Microbiology. 2008;154:2008–2016. doi: 10.1099/mic.0.2008/018358-0. [DOI] [PubMed] [Google Scholar]
- 11.McFarland KA, Lucchini S, Hinton JCD, Dorman CJ. The leucine-responsive regulatory protein, Lrp, activates transcription of the fim operon in Salmonella enterica serovar typhimurium via the fimZ regulatory gene. J Bacteriol. 2008;190:602–612. doi: 10.1128/JB.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenstein BI. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981;214:337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- 13.Dorman CJ. Mechanistic insights from molecular microbiology into the production of immunological and neuronal diversity. Mol Microbiol. 2022;55:26–33. doi: 10.1111/mmi.14997. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Hall AB, Arthur TD, Plichta DR, Covington CT, et al. Invertible promoters mediate bacterial phase variation, antibiotic resistance, and host adaptation in the gut. Science. 2019;363:181–187. doi: 10.1126/science.aau5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez-Romero MA, Casadesús J. Waddington’s landscapes in the bacterial world. Front Microbiol. 2021;12:685080. doi: 10.3389/fmicb.2021.685080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamora M, Ziegler CA, Freddolino PL, Wolfe AJ. A thermosensitive, phase-variable epigenetic switch: pap revisited. Microbiol Mol Biol Rev. 2020;84:e00030-17. doi: 10.1128/MMBR.00030-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinde P, Deighan P, Dorman CJ. Characterization of the detachable Rho-dependent transcription terminator of the fimE gene in Escherichia coli K-12. J Bacteriol. 2005;187:8256–8266. doi: 10.1128/JB.187.24.8256-8266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joyce SA, Dorman CJ. A Rho-dependent phase-variable transcription terminator controls expression of the FimE recombinase in Escherichia coli . Mol Microbiol. 2002;45:1107–1117. doi: 10.1046/j.1365-2958.2002.03081.x. [DOI] [PubMed] [Google Scholar]
- 19.Gally DL, Leathart J, Blomfield IC. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol Microbiol. 1996;21:725–738. doi: 10.1046/j.1365-2958.1996.311388.x. [DOI] [PubMed] [Google Scholar]
- 20.McCusker MP, Turner EC, Dorman CJ. DNA sequence heterogeneity in site-specific recombinase binding sites determines the directionality of fim genetic switch DNA inversion in Escherichia coli K-12. Mol Microbiol. 2008;67:171–187. doi: 10.1111/j.1365-2958.2007.06037.x. [DOI] [PubMed] [Google Scholar]
- 21.Holden N, Blomfield IC, Uhlin B-E, Totsika M, Kulasekara DH, et al. Comparative analysis of FimB and FimE recombinase activity. Microbiology. 2007;153:4138–4149. doi: 10.1099/mic.0.2007/010363-0. [DOI] [PubMed] [Google Scholar]
- 22.Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli . EMBO J. 1986;5:1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulasekara HD, Blomfield IC. The molecular basis for the specificity of fimE in the phase variation of type 1 fimbriae of Escherichia coli K-12. Mol Microbiol. 1999;31:1171–1181. doi: 10.1046/j.1365-2958.1999.01257.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith SGJ, Dorman CJ. Functional analysis of the FimE integrase of Escherichia coli K-12: isolation of mutant derivatives with altered DNA inversion preferences. Mol Microbiol. 1999;34:965–979. doi: 10.1046/j.1365-2958.1999.01657.x. [DOI] [PubMed] [Google Scholar]
- 25.McClain MS, Blomfield IC, Eisenstein BI. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli . J Bacteriol. 1991;173:5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blomfield IC, McClain MS, Princ JA, Calie PJ, Eisenstein BI. Type 1 fimbriation and fimE mutants of Escherichia coli K-12. J Bacteriol. 1991;173:5298–5307. doi: 10.1128/jb.173.17.5298-5307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dove SL, Dorman CJ. The site-specific recombination system regulating expression of the type 1 fimbrial subunit gene of Escherichia coli is sensitive to changes in DNA supercoiling. Mol Microbiol. 1994;14:975–988. doi: 10.1111/j.1365-2958.1994.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 28.Dove SL, Dorman CJ. Multicopy fimB gene expression in Escherichia coli: binding to inverted repeats in vivo, effect on fimA gene transcription and DNA inversion. Mol Microbiol. 1996;21:1161–1173. doi: 10.1046/j.1365-2958.1996.681434.x. [DOI] [PubMed] [Google Scholar]
- 29.Müller CM, Aberg A, Straseviçiene J, Emody L, Uhlin BE, et al. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5:e1000303. doi: 10.1371/journal.ppat.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crisona NJ, Weinberg RL, Peter BJ, Sumners DW, Cozzarelli NR. The topological mechanism of phage lambda integrase. J Mol Biol. 1999;289:747–775. doi: 10.1006/jmbi.1999.2771. [DOI] [PubMed] [Google Scholar]
- 31.Landy A. The λ integrase site-specific recombination pathway. Microbiol Spectr. 2015;3:MDNA3–0051. doi: 10.1128/microbiolspec.MDNA3-0051-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blomfield IC, Kulasekara DH, Eisenstein BI.Integration host factor stimulates both FimB- and FimE-mediated site-specific DNA inversion that controls phase variation of type 1 fimbriae expression in Escherichia coli .Mol Microbiol 199723705–717. 10.1046/j.1365-2958.1997.2241615.x [DOI] [PubMed] [Google Scholar]
- 33.Dorman CJ, Higgins CF. Fimbrial phase variation in Escherichia coli: dependence on integration host factor and homologies with other site-specific recombinases. J Bacteriol. 1987;169:3840–3843. doi: 10.1128/jb.169.8.3840-3843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenstein BI, Sweet DS, Vaughn V, Friedman DI. Integration host factor is required for the DNA inversion that controls phase variation in Escherichia coli . Proc Natl Acad Sci U S A. 1987;84:6506–6510. doi: 10.1073/pnas.84.18.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blomfield IC, Calie PJ, Eberhardt KJ, McClain MS, Eisenstein BI. Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J Bacteriol. 1993;175:27–36. doi: 10.1128/jb.175.1.27-36.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran CP, Dorman CJ. DNA relaxation-dependent phase biasing of the fim genetic switch in Escherichia coli depends on the interplay of H-NS, IHF and LRP. Mol Microbiol. 2009;74:1071–1082. doi: 10.1111/j.1365-2958.2009.06919.x. [DOI] [PubMed] [Google Scholar]
- 37.Gally DL, Rucker TJ, Blomfield IC. The leucine-responsive regulatory protein binds to the fim switch to control phase variation of type 1 fimbrial expression in Escherichia coli K-12. J Bacteriol. 1994;176:5665–5672. doi: 10.1128/jb.176.18.5665-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly A, Conway C, Ó Cróinín T, Smith SGJ, Dorman CJ. DNA Supercoiling and the Lrp Protein Determine the Directionality of fim Switch DNA Inversion in Escherichia coli K-12. J Bacteriol. 2006;188:5356–5363. doi: 10.1128/JB.00344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins CF, Dorman CJ, Stirling DA, Waddell L, Booth IR, et al. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli . Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 40.O’Gara JP, Dorman CJ. Effects of local transcription and H-NS on inversion of the fim switch of Escherichia coli . Mol Microbiol. 2000;36:457–466. doi: 10.1046/j.1365-2958.2000.01864.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawula TH, Orndorff PE. Rapid site-specific DNA inversion in Escherichia coli mutants lacking the histonelike protein H-NS. J Bacteriol. 1991;173:4116–4123. doi: 10.1128/jb.173.13.4116-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papagiannis CV, Sam MD, Abbani MA, Yoo D, Cascio D, et al. Fis targets assembly of the Xis nucleoprotein filament to promote excisive recombination by phage lambda. J Mol Biol. 2007;367:328–343. doi: 10.1016/j.jmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S, Iannolo M, Calvo JM. Cooperative binding of the leucine-responsive regulatory protein (Lrp) to DNA. J Mol Biol. 2005;345:251–264. doi: 10.1016/j.jmb.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 44.Cui Y, Wang Q, Stormo GD, Calvo JM. A consensus sequence for binding of Lrp to DNA. J Bacteriol. 1995;177:4872–4880. doi: 10.1128/jb.177.17.4872-4880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Calvo JM. Leucine-induced dissociation of Escherichia coli Lrp hexadecamers to octamers. J Mol Biol. 2002;318:1031–1042. doi: 10.1016/S0022-2836(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 46.Calvo JM, Matthews RG. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli . Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegler CA, Freddolino PL. The leucine-responsive regulatory proteins/feast-famine regulatory proteins: an ancient and complex class of transcriptional regulators in bacteria and archaea. Crit Rev Biochem Mol Biol. 2021;56:373–400. doi: 10.1080/10409238.2021.1925215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Wu J, Friedberg D, Plakto J, Calvo JM. Regulation of the Escherichia coli lrp gene. J Bacteriol. 1994;176:1831–1839. doi: 10.1128/jb.176.7.1831-1839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho B-K, Barrett CL, Knight EM, Park YS, Palsson BØ. Genome-scale reconstruction of the Lrp regulatory network in Escherichia coli . Proc Natl Acad Sci U S A. 2008;105:19462–19467. doi: 10.1073/pnas.0807227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroner GM, Wolfe MB, Freddolino PL. Escherichia coli Lrp regulates one-third of the genome via direct, cooperative, and indirect routes. J Bacteriol. 2019;201:e00411-18. doi: 10.1128/JB.00411-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tani TH, Khodursky A, Blumenthal RM, Brown PO, Matthews RG. Adaptation to famine: a family of stationary-phase genes revealed by microarray analysis. Proc Natl Acad Sci U S A. 2002;99:13471–13476. doi: 10.1073/pnas.212510999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zinser ER, Kolter R. Prolonged stationary-phase incubation selects for lrp mutations in Escherichia coli K-12. J Bacteriol. 2000;182:4361–4365. doi: 10.1128/JB.182.15.4361-4365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ball CA, Osuna R, Ferguson KC, Johnson RC. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli . J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogue MM, Mogre A, Beckett MC, Thomson NR, Dorman CJ. Network rewiring: physiological consequences of reciprocally exchanging the physical locations and growth-phase-dependent expression patterns of the Salmonella fis and dps Genes. mBio. 2020;11:e0218–20. doi: 10.1128/mBio.02128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O Cróinín T, Dorman CJ. Expression of the Fis protein is sustained in late-exponential- and stationary-phase cultures of Salmonella enterica serovar Typhimurium grown in the absence of aeration. Mol Microbiol. 2007;66:237–251. doi: 10.1111/j.1365-2958.2007.05916.x. [DOI] [PubMed] [Google Scholar]
- 56.Osuna R, Lienau D, Hughes KT, Johnson RC. Sequence, regulation, and functions of fis in Salmonella typhimurium . J Bacteriol. 1995;177:2021–2032. doi: 10.1128/jb.177.8.2021-2032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esposito D, Gerard GF. The Escherichia coli Fis protein stimulates bacteriophage lambda integrative recombination in vitro. J Bacteriol. 2003;185:3076–3080. doi: 10.1128/JB.185.10.3076-3080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gille H, Egan JB, Roth A, Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli . Nucleic Acids Res. 1991;19:4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grimwade JE, Leonard AC. Blocking, bending, and binding: regulation of initiation of chromosome replication during the Escherichia coli cell cycle by transcriptional modulators that interact with origin DNA. Front Microbiol. 2021;12:732270. doi: 10.3389/fmicb.2021.732270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue Y, Tanaka H, Kasho K, Fujimitsu K, Oshima T, et al. Chromosomal location of the DnaA-reactivating sequence DARS2 is important to regulate timely initiation of DNA replication in Escherichia coli . Genes Cells. 2016;21:1015–1023. doi: 10.1111/gtc.12395. [DOI] [PubMed] [Google Scholar]
- 61.Ryan VT, Grimwade JE, Camara JE, Crooke E, Leonard AC. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol Microbiol. 2004;51:1347–1359. doi: 10.1046/j.1365-2958.2003.03906.x. [DOI] [PubMed] [Google Scholar]
- 62.Karambelkar S, Swapna G, Nagaraja V. Silencing of toxic gene expression by Fis. Nucleic Acids Res. 2012;40:4358–4367. doi: 10.1093/nar/gks037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly A, Goldberg MD, Carroll RK, Danino V, Hinton JCD, et al. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology. 2004;150:2037–2053. doi: 10.1099/mic.0.27209-0. [DOI] [PubMed] [Google Scholar]
- 64.O Cróinín T, Carroll RK, Kelly A, Dorman CJ. Roles for DNA supercoiling and the Fis protein in modulating expression of virulence genes during intracellular growth of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2006;62:869–882. doi: 10.1111/j.1365-2958.2006.05416.x. [DOI] [PubMed] [Google Scholar]
- 65.Bétermier M, Lefrère V, Koch C, Alazard R, Chandler M. The Escherichia coli protein, Fis: specific binding to the ends of phage Mu DNA and modulation of phage growth. Mol Microbiol. 1989;3:459–468. doi: 10.1111/j.1365-2958.1989.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 66.Weinreich MD, Reznikoff WS. Fis plays a role in Tn5 and IS50 transposition. J Bacteriol. 1992;174:4530–4537. doi: 10.1128/jb.174.14.4530-4537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cameron ADS, Stoebel DM, Dorman CJ. DNA supercoiling is differentially regulated by environmental factors and FIS in Escherichia coli and Salmonella enterica . Mol Microbiol. 2011;80:85–101. doi: 10.1111/j.1365-2958.2011.07560.x. [DOI] [PubMed] [Google Scholar]
- 68.Weinstein-Fischer D, Altuvia S. Differential regulation of Escherichia coli topoisomerase I by Fis. Mol Microbiol. 2007;63:1131–1144. doi: 10.1111/j.1365-2958.2006.05569.x. [DOI] [PubMed] [Google Scholar]
- 69.Keane OM, Dorman CJ. The gyr genes of Salmonella enterica serovar Typhimurium are repressed by the factor for inversion stimulation, Fis. Mol Gen Genomics. 2003;270:56–65. doi: 10.1007/s00438-003-0896-1. [DOI] [PubMed] [Google Scholar]
- 70.Schneider R, Travers A, Kutateladze T, Muskhelishvili G. A DNA architectural protein couples cellular physiology and DNA topology in Escherichia coli . Mol Microbiol. 1999;34:953–964. doi: 10.1046/j.1365-2958.1999.01656.x. [DOI] [PubMed] [Google Scholar]
- 71.Muskhelishvili G, Travers A. Transcription factor as a topological homeostat. Front Biosci. 2003;8:d279–85. doi: 10.2741/969. [DOI] [PubMed] [Google Scholar]
- 72.Rochman M, Aviv M, Glaser G, Muskhelishvili G. Promoter protection by a transcription factor acting as a local topological homeostat. EMBO Rep. 2002;3:355–360. doi: 10.1093/embo-reports/kvf067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider R, Travers A, Muskhelishvili G. The expression of the Escherichia coli fis gene is strongly dependent on the superhelical density of DNA. Mol Microbiol. 2000;38:167–175. doi: 10.1046/j.1365-2958.2000.02129.x. [DOI] [PubMed] [Google Scholar]
- 74.Saldaña-Ahuactzi Z, Soria-Bustos J, Martínez-Santos VI, Yañez-Santos JA, Martínez-Laguna Y, et al. The Fis nucleoid protein negatively regulates the phase variation fimS switch of the type 1 pilus operon in enteropathogenic Escherichia coli . Front Microbiol. 2022;13:882563. doi: 10.3389/fmicb.2022.882563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller JH. Short Course in Bacterial Genetics. New York: Cold Spring Harbor; 1992. [Google Scholar]
- 76.Sternberg NL, Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium . Methods Enzymol. 1991;204:18–43. doi: 10.1016/0076-6879(91)04004-8. [DOI] [PubMed] [Google Scholar]
- 77.Wilson RL, Libby SJ, Freet AM, Boddicker JD, Fahlen TF, et al. Fis, a DNA nucleoid-associated protein, is involved in Salmonella typhimurium SPI-1 invasion gene expression. Mol Microbiol. 2001;39:79–88. doi: 10.1046/j.1365-2958.2001.02192.x. [DOI] [PubMed] [Google Scholar]
- 78.Sambrook J, Russell D. Molecular Cloning: A Laboratory Guide. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 79.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 80.Marshall DG, Sheehan BJ, Dorman CJ. A role for the leucine-responsive regulatory protein and integration host factor in the regulation of the Salmonella plasmid virulence (spv) locus in Salmonella typhimurium . Mol Microbiol. 1999;34:134–145. doi: 10.1046/j.1365-2958.1999.01587.x. [DOI] [PubMed] [Google Scholar]
- 81.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hancock SP, Stella S, Cascio D, Johnson RC. DNA sequence determinants controlling affinity, stability and shape of DNA complexes bound by the nucleoid protein Fis. PLoS One. 2016;11:e0150189. doi: 10.1371/journal.pone.0150189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stella S, Cascio D, Johnson RC. The shape of the DNA minor groove directs binding by the DNA-bending protein Fis. Genes Dev. 2010;24:814–826. doi: 10.1101/gad.1900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gosink KK, Ross W, Leirmo S, Osuna R, Finkel SE, et al. DNA binding and bending are necessary but not sufficient for Fis-dependent activation of rrnB P1. J Bacteriol. 1993;175:1580–1589. doi: 10.1128/jb.175.6.1580-1589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seah NE, Warren D, Tong W, Laxmikanthan G, Van Duyne GD, et al. Nucleoprotein architectures regulating the directionality of viral integration and excision. Proc Natl Acad Sci U S A. 2014;111:12372–12377. doi: 10.1073/pnas.1413019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abremski K, Gottesman S. Purification of the bacteriophage lambda xis gene product required for lambda excisive recombination. J Biol Chem. 1982;257:9658–9662. [PubMed] [Google Scholar]
- 87.Nash HA. Integrative recombination of bacteriophage lambda DNA in vitro. Proc Natl Acad Sci U S A. 1975;72:1072–1076. doi: 10.1073/pnas.72.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbani MA, Papagiannis CV, Sam MD, Cascio D, Johnson RC, et al. Structure of the cooperative Xis-DNA complex reveals a micronucleoprotein filament that regulates phage lambda intasome assembly. Proc Natl Acad Sci U S A. 2007;104:2109–2114. doi: 10.1073/pnas.0607820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson JF, Moitoso de Vargas L, Koch C, Kahmann R, Landy A. Cellular factors couple recombination with growth phase: characterization of a new component in the lambda site-specific recombination pathway. Cell. 1987;50:901–908. doi: 10.1016/0092-8674(87)90516-2. [DOI] [PubMed] [Google Scholar]
- 90.Dorman CJ, Schumacher MA, Bush MJ, Brennan RG, Buttner MJ. When is a transcription factor a NAP? Curr Opin Microbiol. 2020;55:26–33. doi: 10.1016/j.mib.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hancock SP, Cascio D, Johnson RC. Cooperative DNA binding by proteins through DNA shape complementarity. Nucleic Acids Res. 2019;47:8874–8887. doi: 10.1093/nar/gkz642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ball CA, Johnson RC. Multiple effects of Fis on integration and the control of lysogeny in phage lambda. J Bacteriol. 1991;173:4032–4038. doi: 10.1128/jb.173.13.4032-4038.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bordes P, Conter A, Morales V, Bouvier J, Kolb A, et al. DNA supercoiling contributes to disconnect sigmaS accumulation from sigmaS-dependent transcription in Escherichia coli . Mol Microbiol. 2003;48:561–571. doi: 10.1046/j.1365-2958.2003.03461.x. [DOI] [PubMed] [Google Scholar]
- 94.Conter A, Menchon C, Gutierrez C. Role of DNA supercoiling and rpoS sigma factor in the osmotic and growth phase-dependent induction of the gene osmE of Escherichia coli K12. J Mol Biol. 1997;273:75–83. doi: 10.1006/jmbi.1997.1308. [DOI] [PubMed] [Google Scholar]
- 95.Hernday AD, Braaten BA, Low DA. The mechanism by which DNA adenine methylase and PapI activate the pap epigenetic switch. Mol Cell. 2003;12:947–957. doi: 10.1016/s1097-2765(03)00383-6. [DOI] [PubMed] [Google Scholar]
- 96.Peterson SN, Reich NO. Competitive Lrp and Dam assembly at the pap regulatory region: implications for mechanisms of epigenetic regulation. J Mol Biol. 2008;383:92–105. doi: 10.1016/j.jmb.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 97.Holden NJ, Uhlin BE, Gally DL. PapB paralogues and their effect on the phase variation of type 1 fimbriae in Escherichia coli . Mol Microbiol. 2001;42:319–330. doi: 10.1046/j.1365-2958.2001.02656.x. [DOI] [PubMed] [Google Scholar]
- 98.Lindberg S, Xia Y, Sondén B, Göransson M, Hacker J, et al. Regulatory Interactions among adhesin gene systems of uropathogenic Escherichia coli . Infect Immun. 2008;76:771–780. doi: 10.1128/IAI.01010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia Y, Gally D, Forsman-Semb K, Uhlin BE. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 2000;19:1450–1457. doi: 10.1093/emboj/19.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Landgraf JR, Wu J, Calvo JM. Effects of nutrition and growth rate on Lrp levels in Escherichia coli . J Bacteriol. 1996;178:6930–6936. doi: 10.1128/jb.178.23.6930-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rasmussen LJ, Marinus MG, Løbner-Olesen A. Novel growth rate control of dam gene expression in Escherichia coli . Mol Microbiol. 1994;12:631–638. doi: 10.1111/j.1365-2958.1994.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 102.Hsieh LS, Burger RM, Drlica K. Bacterial DNA supercoiling and [ATP]/[ADP]. Changes associated with a transition to anaerobic growth. J Mol Biol. 1991;219:443–450. doi: 10.1016/0022-2836(91)90185-9. [DOI] [PubMed] [Google Scholar]
- 103.Hsieh LS, Rouviere-Yaniv J, Drlica K. Bacterial DNA supercoiling and [ATP]/[ADP] ratio: changes associated with salt shock. J Bacteriol. 1991;173:3914–3917. doi: 10.1128/jb.173.12.3914-3917.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Workum M, van Dooren SJ, Oldenburg N, Molenaar D, Jensen PR, et al. DNA supercoiling depends on the phosphorylation potential in Escherichia coli . Mol Microbiol. 1996;20:351–360. doi: 10.1111/j.1365-2958.1996.tb02622.x. [DOI] [PubMed] [Google Scholar]
- 105.Westerhoff HV, van Workum M. Control of DNA structure and gene expression. Biomed Biochim Acta. 1990;49:839–853. [PubMed] [Google Scholar]
- 106.Kusano S, Ding Q, Fujita N, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase Eσ70 and Eσ38 holoenzymes. Journal of Biological Chemistry. 1996;271:1998–2004. doi: 10.1074/jbc.271.4.1998. [DOI] [PubMed] [Google Scholar]
- 107.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 108.Schembri MA, Hjerrild L, Gjermansen M, Klemm P. Differential expression of the Escherichia coli autoaggregation factor antigen 43. J Bacteriol. 2003;185:2236–2242. doi: 10.1128/JB.185.7.2236-2242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blomfield IC, Vaughn V, Rest RF, Eisenstein BI. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 110.Nakano Y, Yoshida Y, Yamashita Y, Koga T. Construction of a series of pACYC-derived plasmid vectors. Gene. 1995;162:157–158. doi: 10.1016/0378-1119(95)00320-6. [DOI] [PubMed] [Google Scholar]
- 111.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]