Abstract
This report provides guidance for Member states who plan to submit applications under the work programme ‘CP‐g‐22‐04.01 Direct grants to Member States' authorities’. The priority pathogens on which the coordinated surveillance under the grant initiative shall focus have been identified in a prioritisation exercise with Member States and ECDC. These are Crimean Congo haemorrhagic fever, echinococcosis, hepatitis E, highly pathogenic avian influenza (HPAI), influenza in swine, Lyme disease, Q‐fever, Rift Valley fever, tick‐borne encephalitis, West Nile fever and Disease X (Disease Y of animals). Surveillance activities (surveillance cards) have been proposed for these agents in this report. Member States should select one or more diseases from the list of priority diseases and then choose surveillance activities from the surveillance cards and modify them where needed, to reflect their national needs and situation. Member States can also design alternative surveillance activities for the priority infectious agents that may better fit the epidemiological situation in their country. Further, this report provides a section on surveillance perspectives that links infectious agents to different hosts, allowing Member States to consider the testing for multiple infectious agents in samples from a single host population, as well as sections providing guidance on surveillance in vectors and wildlife and for Disease X (Disease Y in animals). Member States are encouraged to develop cross‐sectoral collaborations and the report provides guidance on cross‐sectoral collaboration to help them. Finally, there is a roadmap providing an overall description of the steps in the process of developing a surveillance system in order to apply for the grant.
Keywords: surveillance, early detection, One Health, zoonoses, transboundary
Summary
This report provides guidance for Member states who plan to submit applications under the work programme ‘CP‐g‐22‐04.01 Direct grants to Member States' authorities’. The purpose of the direct grant is to facilitate improvement of existing surveillance and establishment of One Health surveillance that will provide animal health and environmental health information to reduce the risk and harm caused by zoonotic diseases. This programme is a first step in the development of one health surveillance capacity in Europe. The scope of this programme is very limited in terms of One Health (see below) as surveillance of humans was excluded, intersectoral collaboration is recommended but not required, and the programme focuses on human health only with no provisions for animal health or ecosystem health. The number of diseases that qualify for the grant was limited to a small number in order to allow the comparison of the performance and value of different surveillance activities for these diseases between applicant Member States. Diseases were selected through a prioritisation process involving the Working Group followed by a consultative workshop with representatives from Member States. The final list included Crimean Congo haemorrhagic fever, echinococcosis, hepatitis E, highly pathogenic avian influenza (HPAI), influenza in swine, Lyme disease, Q‐fever, Rift Valley fever, tick‐borne encephalitis, West Nile fever and Disease X (Disease Y of animals). Once the final selection of diseases was made, consultants with experience in surveillance and animal health created short descriptions of biologically and epidemiologically important information (called Disease Briefs) that is needed to design surveillance activities. The consultants then identified and described one to several individual surveillance activities (called Surveillance Activity Cards) or each selected disease. No surveillance activities in humans, food‐borne and waterborne transmission of pathogens, and antimicrobial resistance/use surveillance have been proposed as these are outside the scope of the grant initiative. The Disease Briefs and Surveillance Cards were then edited for biological soundness and practicality by members of Enetwild and VectorNet. This was followed by a final selection by the working group, based primarily on principles of surveillance, epidemiology and statistics. Member States are instructed to select one or more diseases from the list of selected diseases and then to choose surveillance activities from the surveillance activity cards. The Surveillance Activity Cards are proposals and Member States are free to design alternative surveillance activities for the priority infectious agents that may better fit the epidemiological situation in their country. This report also provides guidance on different approaches to surveillance, that will allow Member States to consider basic principles of surveillance system design and sampling based on the epidemiological situation for the infectious agent in their country. This is followed by descriptions of the Disease Briefs and the Surveillance Activity Cards and how they should be used. There is a section on surveillance perspectives that links infectious agents to different hosts, allowing Member States to consider the testing for multiple infectious agents in samples from a single host population. This is supplemented with a section on how to add value to surveillance activities. There are sections providing guidance on surveillance in vectors and wildlife. Another section provides guidance on surveillance for Disease X (Disease Y in animals). Member States are encouraged to develop cross‐sectoral collaborations and we have provided guidance on cross‐sectoral collaboration to help them. Finally, there is a roadmap providing an overall description of the steps in the process of developing a surveillance system in order to apply for the grant.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The Commission has allocated specific resources for Member States for setting up a coordinated surveillance system under the One Health approach for cross‐border pathogens that threaten the Union. Policy context, objectives and scope are provided in Annex I. The Commission is in need of scientific and technical assistance on developing, and keeping updated, a coordinated surveillance methodology in particular for certain zoonoses in animals and the environment under the One Health approach to be performed by the Member States. As indicated in the work programme for the initiative “CP‐g‐22‐04.01 Direct grants to Member States' authorities”, this coordinated surveillance will contribute to the scaling up of existing surveillance and the establishment of a One Health surveillance that will provide the animal health and environmental side to complement in full synergy the ongoing initiatives on the human side for integrated surveillance. This implies the need to map existing surveillance for zoonoses in animals and the environment and ensure a synergistic and complementary approach in the design of the coordinated surveillance as well as in the implementation phase by the Member States. The results of this surveillance should be collected by EFSA to perform a risk assessment aiming at identifying One Health zoonotic risks for the EU for which surveillance is needed and allowing for an iterative approach which would allow to review the surveillance priorities.
Terms of Reference (ToR)
There is a need to setup an EU coordinated surveillance aimed at identifying One Health risks including emerging and re‐emerging zoonotic diseases based on the background information provided above. In accordance with Article 31 of Regulation (EC) No 178/2002, the Commission asks EFSA for scientific and technical assistance structured in the following way:
A – Design of an EU coordinated surveillance system under the One Health approach for cross‐border zoonotic pathogens that may threaten the Union
Review updated relevant scientific literature available related to surveillance for cross‐border zoonoses in animals and the environment and perform a mapping of the main existing structured and systematic initiatives for surveillance in the EU for zoonoses in animals and the environment.
Assess the main targeted zoonotic risks for the EU based on the current epidemiological situation in the EU, its neighbouring areas and beyond.
- To address the risks identified, recommend options for sustainable surveillance strategies for Member States indicating its relevant objectives and suitable methodologies, in particular as regards target cross‐border pathogens, vectors, scope, sampling methodologies and frequencies, and testing methods taking into account the need for early detection of emerging and re‐emerging zoonoses. Such surveillance strategies need to account for:
- changes in ecosystems and vector (e.g. ticks) distribution,
- domestic animal husbandry practices and interactions with native wildlife,
- human travel and trade patterns and practices,
- possible future, still unknown, emerging zoonotic diseases (“Disease X"),
- avoid duplication with existing initiatives (e.g. foodborne zoonoses, antimicrobial resistance, already co‐funded EU surveillance programmes for brucellosis, tuberculosis or rabies) unless complementarities are required (e.g. avian influenza surveillance programme).
B – Collect Surveillance Data and Identify the Risks
Prepare the data model for collecting the results of the surveillance carried out by Member States.
Provide an interface for and collect the surveillance data from the Member States that implement this initiative (e.g., by web services allowing for automated data transfer from existing databases). Make the surveillance results available in an appropriate way both to Member States and stakeholders (see point C2), and to the public.
Perform a regular risk assessment based on the surveillance data collected which is to be used to review the surveillance priorities and methodologies for the following year(s).
C – Stakeholder Involvement
Involve experts appointed by Member States that joined this initiative to ensure coordination at EU level, notably for points A2, A3 and B1.
Ensure consultation of other relevant stakeholders (from EU institutions / agencies and international organisations).
D – Timeframe
Risk assessment (points A1 andA2) and the surveillance priorities (point A3) for surveillance to be available for January 2023.
Given the iterative nature of this mandate, there is a need, based on the data collected each year (under point B), to review all steps presented in point A on an annual basis since 2023 until the completion of the mandate in 2026.
1.2. Interpretation of the Terms of Reference
This EFSA scientific report addresses ToR A 3 of the mandate and provides options for coordinated surveillance systems under the One Health approach to be set up under the EU4Health programme initiative CP‐g‐22‐04.01. It is providing Member States that intend to apply for a direct grant under that initiative with guidance on how to plan and develop their surveillance systems.
The surveillance options proposed in this report target the diseases and infectious agents that have been identified in the prioritisation exercise carried out under ToR A 2 and described in another scientific report of EFSA (2023). These are Crimean Congo haemorrhagic fever, echinococcosis, hepatitis E, highly pathogenic avian influenza (HPAI), influenza in swine, Lyme disease, Q‐fever, Rift Valley fever, tick‐borne encephalitis and West Nile fever.
The scope of this report and the surveillance activities proposed are focused only on zoonotic pathogens in animals and the environment, excluding surveillance in humans, food‐borne and waterborne transmission of pathogens, antimicrobial resistance/use surveillance, and surveillance for pathogens covered by other EU programmes.
The EU4Health programme initiative and the mandate sometimes refer to ‘pathogens’ and in other parts to ‘zoonoses’ (=diseases). In this report, the term ‘infectious agent’ is used to reflect that an infectious agent can infect a range of species, while causing disease (=being a pathogen) only in some of these.
In this report, when using the term ‘animals’, both vertebrate wildlife and livestock animals are considered, unless specified otherwise. Insect vectors are considered part of the environment components mentioned in the terms of reference, as are soil, surface water, air, effluents from farming establishments, etc.
The One Health High Level Expert Panel (OHHLEP) established by FAO, UNEP, WHO and WOAH has developed the following definition of One Health: ‘One Health is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems. It recognizes the health of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) are closely linked and inter‐dependent’. Initiative CP‐g‐22‐04.01 and the related mandate for EFSA use the term ‘One Health’ when asking for a ‘coordinated surveillance system under the One Health approach for cross‐border pathogens that threaten the Union’. However, the aim is limited to protecting public health through the early detection of emerging and re‐emerging zoonotic pathogens in animals and the environment. In this report, the term ‘One Health’ is used as intended in the mandate, while acknowledging that for proper One Health surveillance in the sense of the quadripartite definition, also protection of animal health and ecosystem health should be part of the systems' objectives. Therefore, these aims were specifically not included in this scientific report.
The importance of cross‐sectoral integration and collaboration are the core of the added value expected from a One Health approach. The surveillance components proposed in this report focus only on specific infectious agents and components; however, guidance and recommendations for cross‐sectoral collaboration and integration of knowledge from different sectors are also provided.
It is important to note that the surveillance activities proposed in this report are not intended to generate data suitable to serve trade purposes, e.g. provide evidence of absence of infectious agents.
2. Data and methodologies
2.1. Data
The data and information collated in reports resulting from a range of preparatory activities have been used for the design of the coordinated surveillance system under the One Health approach.
Briefly, experts contracted under EFSA's Independent Scientific Advice scheme have prepared an overview of the different animal species that can be infected with the selected infectious zoonotic pathogens and collated scientific evidence for the disease and surveillance cards.
The Enetwild consortium has carried out literature reviews on worldwide surveillance systems targeting transboundary zoonotic and emerging diseases within the holistic One‐Health perspective (Enetwild consortium et al., 2022a), and on the main existing structures and systematic/academic initiatives for surveillance in the EU for zoonoses in the environment and the methods for surveillance of pathogens in the environment (Enetwild consortium et al., 2022b), which were consulted by the Working Group (WG) during the assessment process.
The description of the main existing structures and systematic initiatives and academic activities for surveillance in the EU for transboundary, emerging and re‐emerging zoonoses in domestic animals and wildlife (Enetwild consortium et al., 2022c) has been considered by the WG experts in their assessment of the feasibility of different possible surveillance options.
The information presented by the Enetwild consortium on endangered wildlife hosts in Europe for selected pathogens to be targeted by One Health surveillance (Enetwild consortium et al., 2022d) and the recommendations and technical specifications for sustainable surveillance of zoonotic pathogens where wildlife is implicated (Enetwild consortium et al., 2023) have been used for developing the proposals of surveillance options targeting wildlife species.
Data and information on vectors and vector surveillance developed under the VectorNet project (ECDC, 2014; ECDC and EFSA, 2018) have been used by the WG experts for the development of proposals of surveillance options targeting mosquitos and ticks.
2.2. Methodologies
The proposal for the coordinated surveillance system under the One Health approach was developed by EFSA's WG on One Health surveillance, which brings together expertise in the areas of One Health surveillance, animal health surveillance, public health surveillance, livestock and wildlife health, systems thinking, decision‐making for risk management, health economics, public health, and epidemiology. The recruited experts belong to academia, other EU institutions (the European Centre for Disease Prevention and Control, ECDC) and international organisations (FAO). In the area of wildlife and vector surveillance, the WG was supported by hearing experts from the VectorNet project and the Enetwild consortium with specific expertise in these topics.
To respond to ToR A 3, the working group has identified and explained the variables that need to be defined and described to characterise a surveillance system (Section 3.2), applying the concepts outlined in chapter 1.4 of the Terrestrial animal code of the World Organisation for Animal Health (WOAH, 2022), and by Cameron et al. (2020).
Based on the Animal Health Surveillance Reporting Guidelines 1 developed under the RISKSUR project, 2 ‘disease briefs’ and ‘surveillance cards’ have been developed by contractors (Schüpbach et al., 2023; Carminati, 2023; Nigsch, 2023). The WG reviewed and modified these where necessary. The ‘disease briefs’ collate information regarding the priority diseases that is relevant for the identification of surveillance activities that best fit the national context of the countries planning a surveillance activity (Section 3.1). Building on the adaptation of surveillance pyramids developed by Braks et al. (2014), the WG has assembled pyramids for all hosts of each infectious agent covered in this report, to indicate for each priority disease the different surveillance options related to the different infectious agent hosts (Section 3.1). The ‘surveillance cards’ describe specific surveillance activities that the WG proposes for different objectives related to different epidemiological phases for the priority diseases, outlining the surveillance methodology, the sampling methodology and the testing methods (Section 3.2). As requested by the mandate, disease briefs and surveillance cards have also been developed for Disease X. However, as Disease X is considered a severe disease in humans of unknown origin, the working group decided to call a severe disease in animals caused by an unknown pathogen with a potential of causing a severe disease in humans as Disease Y. A dedicated section on the critical aspects of surveillance aiming at early detections of Disease Y has been included in the report (Section 3.3.3).
In addition, the WG has collected guidance for countries to set up or improve cross‐sectoral collaboration and the integration of findings from the different sectors involved in One Health surveillance (Section 3.4.2).
Finally, an explanation and examples on how the sample size and system sensitivity can be calculated for probability‐based sampling of known populations has been added to an annex of the report (Annex A Description of how to use the RiBESS tool for the calculation of the sample size for probability‐based surveillance components).
During the preparation of this report, EFSA has engaged with Member States' experts and consulted ECDC, as required by ToRs C 1 and 2.
3. Assessment results
Approaches to surveillance system design used in this project
There is a set of sequential steps that are common to most operational surveillance systems. The first step is to perform the individual surveillance activities. These activities output information that is used to make decisions about whether an intervention is needed or not. If an intervention is needed it is implemented with the intention of achieving a disease mitigation goal (Figure 1).
Figure 1.
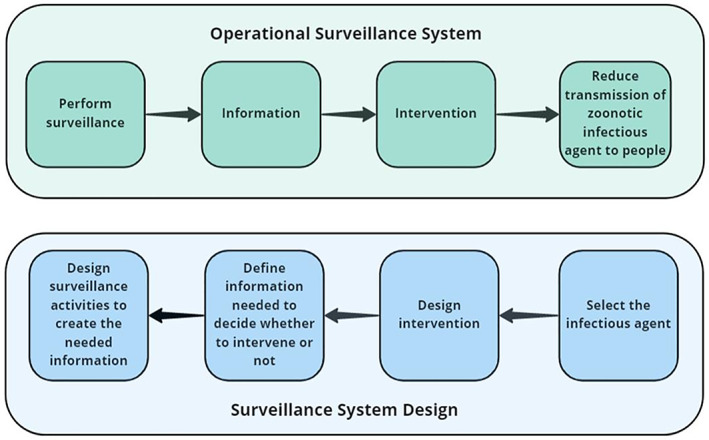
Sequence of steps in an operational surveillance system and the steps involved in the design of a surveillance system
Surveillance system design follows the same steps, but in the opposite direction. The first step is to select an infectious agent that warrants a response if the disease transmission risk from the infectious agent reaches a certain threshold. The second step is to design an appropriate intervention. Since this project is concerned only with the design of surveillance activities, we will not discuss mitigation strategies. The next step is to define the information that is needed to make a decision about whether mitigation is needed or not. In this project, the information needed relates to early warning of an increasing risk of transmission of zoonotic infectious agents to people. The final step is to design the surveillance activities that will be used to create the needed information.
The information needed from a surveillance system will be determined by the infectious agent, the disease it causes and the epidemiological situation in the country. Infectious agents and the disease they cause can be either: (1) known, an infectious agent that is a known pathogen that causes a disease that has been characterised, and (2) unknown, an infectious agent that may or may not be known and that has not previously been reported to cause a disease. There are two epidemiological situations of importance for early detection surveillance: (1) early identification of the presence of the infectious agent or disease in a geographic region or population that was previously free from the pathogen, and (2) early identification of an increase in the risk of transmission of the infectious agent to people in regions where the infectious agent is endemic (seasonally endemic or continuously). We will consider the information needed for each type of infectious agent and each epidemiological situation (Figure 2).
Figure 2.
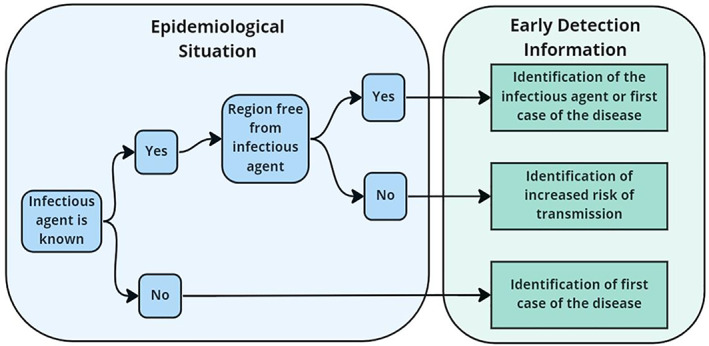
The types of information needed from early detection surveillance based on the epidemiological situation in the region under surveillance
A. Infectious agent is a known infectious agent that causes a known disease
The region is free from the infectious agent
Information that signals an increase in the risk of pathogen transmission to people will include the early detection of the first incident of the disease in animals, seropositivity in animals or the presence of the pathogen in vectors or environmental samples. Since the infectious agent is known, knowledge about the disease can be used to identify surveillance targets (animal or vector species, environmental compartments) and diagnostic tests will likely have been developed and validated for some livestock, wildlife and vectors species. To achieve early warning, surveillance should be continuous. If continuous surveillance is not possible, repeated surveys of the population must be used. Surveys should be repeated at a frequency that is appropriate for the rate at which the pathogen may be transmitted to humans and the severity of the disease in humans.
-
2
The infectious agent is present in the region
In endemic regions, increasing risk of infectious agent transmission to humans can be measured using metrics that signal there may be an increase in the amount of disease or infectious agent the region. Information can include increases in the number of diseased animals, increases in the number of positive tests for the presence of the infectious agent in samples from animal populations or sentinel animals, increases in the number of vectors (mosquito pools or ticks) that are positive for the presence of the infectious agent, or increases in the number of environmental samples that are positive for the presence of the pathogen. To achieve early detection, surveillance should be continuous, especially during high‐risk seasons. If continuous surveillance is not possible, repeated surveys of the population must be used. Surveys should be repeated at a frequency that is appropriate for the rate at which the infectious agent may be transmitted to humans and the severity of the disease in humans.
B. Infectious agent is unknown as a pathogen and the disease it will cause is unknown
Since the infectious agent is unknown as a pathogen, the epidemiology of the disease it will potentially cause including the rate of transmission, and the clinical severity of the disease will be unknown. Diagnostic tests to identify the disease and/or infectious agent will not be available. This makes the surveillance tool box very limited. The most common approach is to use surveillance activities that can rapidly detect an increase in cases of any unusual disease. These include traditional surveillance activities where veterinarians, medics and diagnosticians identify unusual disease cases, and follow them up with intensive diagnostic laboratory and field investigation. To ensure effective traditional surveillance, well‐trained veterinary and medical service and diagnostic laboratory systems are required. Indicator‐based surveillance can also be used for early detection of unknown diseases. It is based on monitoring of indicators of diseases (e.g. clinical syndromes, pharmaceutical sales and many others). Indicator‐based surveillance is non‐specific in that it provides early detection of many different diseases and therefore should be accompanied by well‐funded and trained field or outbreak investigation units that are able to rapidly investigate any signals generated.
Surveillance sampling
The information needed from most surveillance activities focuses on whether there has been a recent change, usually an increase in the amount of the infectious agent in animals, vectors or environmental samples. In a perfect world with unlimited resources, each individual in each population could be tested for the presence of the pathogen. However, resources are never unlimited, and in many cases (wild animals, mosquitos, ticks), it is not possible to access each individual in the population. For this reason, surveillance systems create information by collecting samples from populations of animals, vectors and environmental compartments. There are two approaches to sampling that are commonly used in surveillance (1) probability‐based sampling and (2) non‐probability‐based sampling.
1) Probability‐based sampling
Probability‐based sampling is a method in which a sample is chosen from a larger population using probability theory. Probability sampling requires that each individual in the population has a known and equal probability of being sampled and that individuals are selected by random selection. This means that each individual in the population must be identified and must be accessible for testing if they are selected. This can be achieved in countries that have livestock identification systems where each animal in the population has a unique number that is recorded in a central database. Probability sampling is not possible when all individuals in the population cannot be identified and sampled, such as free‐ranging wildlife populations, mosquito pools, ticks or pets.
There are many advantages of using probability‐based sampling. When it can be achieved, the sample is considered to be a non‐biased sample of the population making the sample prevalence a non‐biased estimate of the population prevalence. Since the sample is probability based, many statistical methods can be used including estimating a sample size to achieve a certain level of confidence in prevalence estimates, and estimating surveillance system sensitivity with a certain level of confidence.
There several disadvantages of probability sampling. It is not continuous. Achieving early detection requires repeated sampling. Repeated sampling can be resource intensive, costly and a burden on animal owners. Probability sampling is very limited in its application as it is seldom possible to collect a probability‐based sample from wildlife and vector populations.
2) Non‐probability‐based sampling
Non‐probability‐based sampling includes all sampling methods that cannot be based on probability theory. In disease surveillance, non‐probability‐based sampling is the most common and widely used form of sampling. It includes sampling of animals submitted to veterinary clinics and diagnostic laboratories, people seen at physicians’ offices and emergency rooms, animals submitted for slaughter, animals submitted for sale at markets, hunter‐killed animals, road‐killed animals, animals found dead, sentinel animal sampling, sampling of vectors, indicator‐based surveillance, many environmental samples and many other sampling methods.
Non‐probability‐based samples are biased samples of the population. They should not be used to estimate population prevalence, since prevalence estimates calculated from these samples will be biased. Since they are not based on probability theory, it is not possible to use statistical methods for estimating sample size or surveillance system sensitivity on non‐probability samples.
Even though non‐probability samples are biased, they are the foundation of most animal disease surveillance systems and they can produce useful information if they are conducted properly and the results interpreted with care. They are most useful for identifying signals that indicate a recent change in the population, such as the introduction of a new infectious agent. For example, testing mosquito pools, which are biased samples of mosquito populations, will not allow for an unbiased estimation of the prevalence of West Nile virus (WNV) in the mosquito population. However, the first positive WNV result from a highly specific PCR test taken from a mosquito pool in a region that was previously free from WNV is a strong signal that the pathogen has arrived in the region. If non‐probability samples are collected with similar sampling intensity and frequency over time, they can be used to identify signals that may represent changing temporal and geographic patterns of disease. For example, hunter‐killed animals have been used to identify signals for change in the geographic range of pathogens such as Echinococcus multilocularis and chronic wasting disease. However, since these samples are biased, signals should be thoroughly investigated with field studies to ensure they are valid.
3.1. Disease briefs
As described above and depicted in Figure 1, the design of surveillance starts with the selection of infectious agent and relies on information about the interaction of the infectious agent with its host populations to design the strategies for information collection.
To guide surveillance design, key information about each of the prioritised pathogens was tabulated following a harmonised structure, building individual ‘disease briefs’. These disease briefs are meant to be living documents, and updated versions can be published over time. All disease briefs are available on EFSA's Knowledge Junction [LINK]. Below, we give an overview of the content of the disease briefs at the time of publishing this report (Table 1).
Table 1.
Disease briefs made available in EFSA's Knowledge Junction
| Vector‐borne diseases | Other prioritised diseases | Unknown diseases |
|---|---|---|
| Crimean Congo haemorrhagic fever (CCHF) | Echinococcosis (Echinococcus granulosus)* | Disease Y of companion animals |
| Lyme borreliosis | Hepatitis E | Disease Y of exotic animals |
| Q‐fever | HPAI | Disease Y of livestock |
| Rift Valley fever (RVF) | Influenza in swine | Disease Y of wildlife |
| Tick‐borne encephalitis (TBE) | ||
| West Nile fever (WNF) |
Note that surveillance for Echinococcus multilocularis is not eligible for funding under the initiative CP‐g‐22‐04.01 of the EU4Health programme, due to the already existing legislative requirements, as clarified by the European Commission.
Each disease brief follows the same general format, with small adaptations as needed to customise specific disease information needs/availability.
Brief description of the infectious agent and the disease
Regulatory status in the EU
Geographical distribution (of autochthonous cases)
Reservoir/main host
Host range/susceptible species (domestic/wild)
Vector‐borne transmission
Environmental transmission
(Main) transmission routes to domestic animals
(Main) transmission routes to humans
Disease indicators, including clinical signs, seroconversion and others.
Indicators relevant for monitoring (Indicator‐based surveillance)
Test systems available for infection/disease detection
Surveillance component options (+ rationale)
Comparison of surveillance component options (main advantages/disadvantages)
Countries which already carry out surveillance for this pathogen
References
The disease briefs contain the main information needed to guide surveillance design for the listed infectious agents, given the specific disease situation in a country/geographical area, and for a given surveillance objective.
We adopt the guidelines proposed in the surveillance design framework first developed by the RISKSUR project, 3 and further refined into the animal health surveillance reporting guidelines 4 (AHSURED).
In this proposed structure, a surveillance system is defined as a collection of various surveillance components which are all aimed at ‘describing health‐hazard occurrence and contributing to the planning, implementation, and evaluation of risk‐mitigation actions’ (Hoinville et al., 2013) for one health‐hazard in particular, and in a defined region. A surveillance system is therefore characterised by:
1. One defined hazard which is targeted by surveillance – a disease or another health threat;
2. The objective of the surveillance system. The following have been identified within the RISKSUR project: case detection, prevalence estimation, demonstrate disease freedom and early detection.
3. The geographical area covered by the surveillance system.
These surveillance systems are designed within a context which includes the specific animal population susceptible to the hazard in the region of interest, and characteristics of the distribution of the hazard (or hazard risk) at the population level, herd level or animal level, which can impact the design of surveillance. Furthermore, the surveillance system should be described with its political and economic context.
Assuming the common objective of early detection, various surveillance components have been suggested in each disease brief. The list is not meant to be exhaustive, and in particular, the provided surveillance components are a deliberately constrained set that matches the expectations of the European Commission for the EU4Health programme initiative CP‐g‐22‐04.01 01 in order to avoid duplications with already existing activities. While humans host populations are indicated in the table, surveillance components in humans are not in the scope of this mandate.
In order to provide an overview of the host populations involved in the transmission cycle of each infectious agent, we adapted the visual approach proposed by Braks et al. (2014), which consists in listing the populations of hosts that interact with a given infectious agent, and visually representing the population progression of the infectious and, if relevant, clinical stages, in order to highlight all possible targets and levels of surveillance. Initially developed for vector‐borne diseases and displayed as pyramids of disease burden, the approach is adapted into a table format here (Figures 3 and 4) to allow cross‐tabulating these populations against the suggested surveillance components, giving an overview of their coverage. These host population and components cross‐tabulations are also shown in the individual disease briefs.
Figure 3.
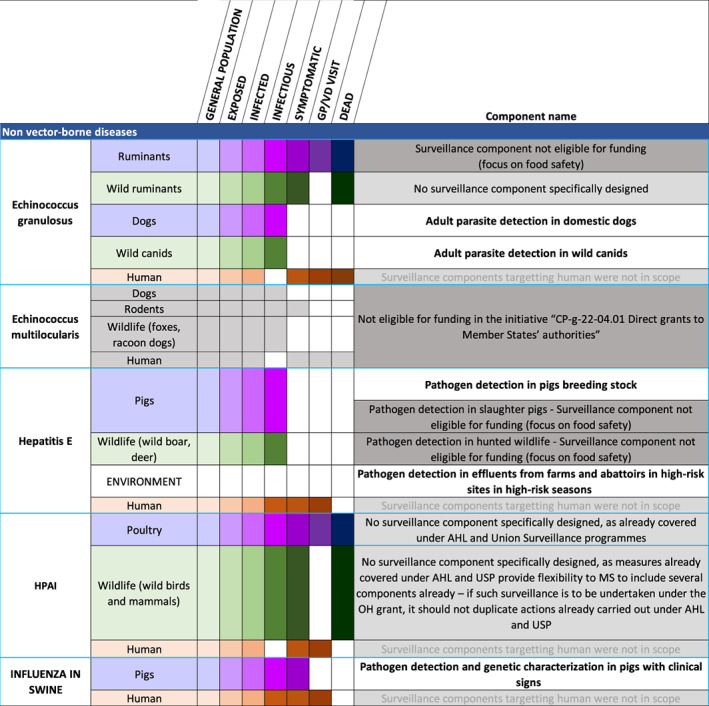
Host populations and suggested surveillance components for each of the prioritised diseases which are not vector‐borne. Domestic populations are shaded in purple, wild populations in green and humans in brown. The shading gradient represents disease progression and indicates on which stages surveillance may focus
Figure 4.
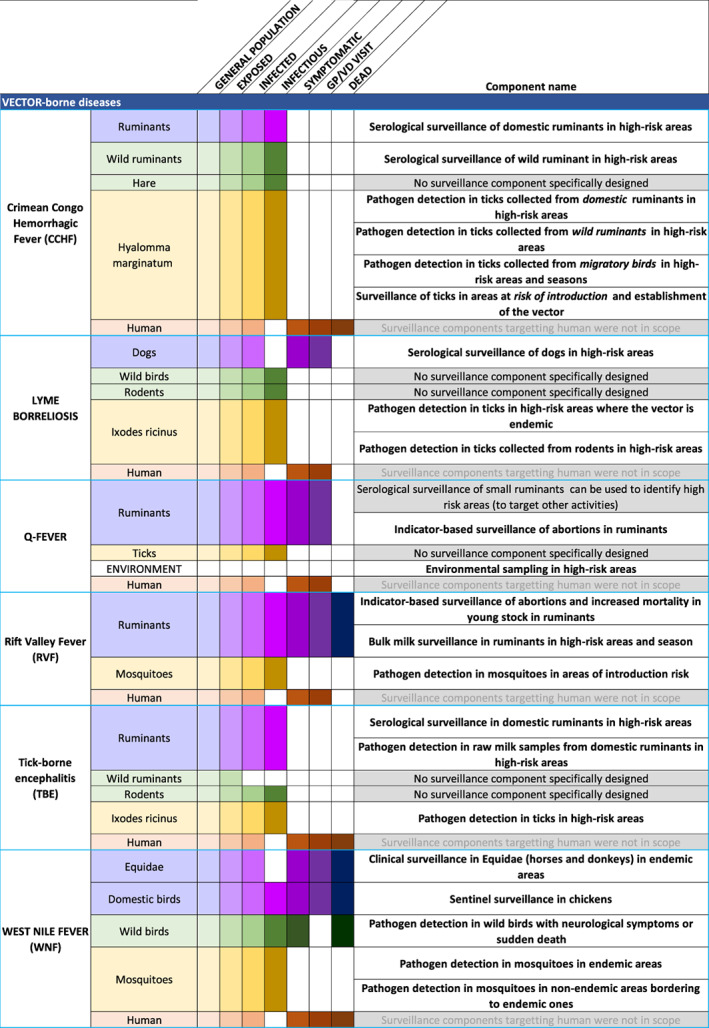
Host populations and suggested surveillance components for the vector‐borne prioritised diseases. Domestic populations are shaded in purple, wild populations in green, vectors in yellow and humans in brown. The shading gradient represents disease progression and indicates on which stages surveillance may focus
There is also a disease brief for Disease Y and surveillance cards focussing on different animal host populations (livestock, companion animals, exotic animals, wildlife) and effluents and wastewater.
In addition, there is a Disease brief ‘template’ – an empty disease brief which can be used to reproduce the methods for diseases prioritised in the future.
Section 3.4.2 describes the information made available for each of the individual components listed in Figures 3 and 4.
3.2. Surveillance cards
For each suggested surveillance component listed in Figures 3, 4, and 5, a detailed surveillance card was made available. As with the disease briefs, these are meant to be living documents, available on EFSA's Knowledge Junction [LINK].
Figure 5.
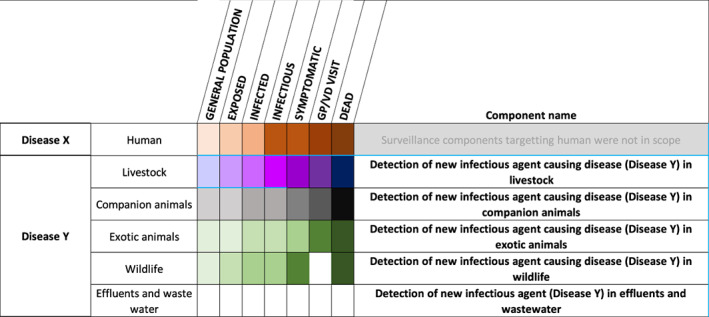
Host populations and suggested surveillance components for Disease Y. Domestic populations are shaded in purple, wild populations in green, vectors in yellow and humans in brown. The shading gradient represents disease progression and indicates on which stages surveillance may focus
The following general structure was followed, with adaptations as needed according to the available information for each infectious agent or relevant for the specific surveillance component.
Surveillance component name: these names were harmonised to be short but descriptive, allowing quick identification of the target of surveillance (disease indicator) and the targets species.
Surveillance aim
Target species and group
Target sector/production type
Geographical area covered
Age group
Sampling point and strategy
Sampling time period
Sampling matrix
Type of disease indicators
Sampling unit
Allocation of animal groups/animals to sampling
Testing protocol/Diagnostic test
Design prevalence (only relevant for probability‐based sampling)
Level of confidence (only relevant for probability‐based sampling)
Contribution of the component to the surveillance system
Other pathogens that could be targeted with this surveillance component
References
Additional comments
Member States are free to choose one or more of the surveillance components suggested. The description of the surveillance components can be modified where necessary to adapt them to the national situation. There is also a surveillance card ‘template’ – an empty surveillance card that countries are encouraged to use to describe, for one of the priority pathogens identified for the EU4Health programme initiative CP‐g‐22‐04.01, a surveillance component that is different from those proposed in this report and does not overlap with already ongoing activities under the AHL or Union surveillance programmes. An overview of information provided by the European Commission of surveillance projects focusing on animals or on the environment can be found in Annex B.
3.3. Surveillance perspectives
Surveillance most commonly targets individual diseases. However, there are synergies between surveillance activities, and surveillance activities for individual diseases targeting one host can be used to detect multiple pathogens. The table below demonstrates some of these synergies by illustrating where multiple infectious agents can be targeted in one surveillance activity (Table 2).
Table 2.
Overview of infectious agents and the hosts that can be targeted for these
| CCHF | TBE | Lyme | RVF | WNF | Q‐fever | HPAI | Influenza in Swine | Hepatitis E | Echinococcus granulosus | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hyalomma spp. | x | x | ||||||||
| Ixodes spp. | x | x | ||||||||
| Other ticks | x | |||||||||
| Aedes spp. | x | x | ||||||||
| Culex spp. | x | x | ||||||||
| Dogs | x | x | ||||||||
| Ruminants | x | x | x | x | x | x* | ||||
| Pigs | x | x | ||||||||
| Horses/Equids | x | |||||||||
| Poultry | x | x | ||||||||
| Wild birds | x | x | x | x | ||||||
| Wild boar | x | x | x | |||||||
| Wild carnivores | x | |||||||||
| Wild ruminants | x | x | ||||||||
| Rodents | x | x | ||||||||
| Lagomorphs | x | x | x | |||||||
| Environment (e.g. water) | x | x |
Surveillance of ruminants for E. granulosus in ruminants outside of scope of the EU4Health programme initiative CP‐g‐22‐04.01.
3.3.1. Surveillance in vectors
Setting up a national surveillance system for vector‐borne diseases and identifying the most suitable target for surveillance is challenging. A framework aiding countries in identifying the most suitable surveillance strategy for vector‐borne diseases in their geographic region and national context has been developed by Braks et al. (2014). The framework uses five different contexts that reflect the current knowledge of the presence (√) or absence (−) of autochthonous human cases, the pathogens and their competent vectors (Table 3). In a one health approach, the former is expanded by considering the presence of absence of autochthonous cases of animals of veterinary importance as well as those of humans (ECDC, 2021). The framework can be applied by countries to assess their current situation for the priority vector‐borne diseases. The overview of host populations and suggested surveillance components provided in this report (Section 3.1) can then be used to select the surveillance component that best fits the national context.
Table 3.
Overview of five contexts and their definitions providing a summarised overview of the epidemiological situation according to the knowledge of the current presence (√) or absence (−) of autochthonous cases, infectious agents and vectors in a country (adapted from ECDC, 2021)
| Context | Status | Definition | Autochthonous human/animal cases | Infectious agent | Vector |
|---|---|---|---|---|---|
| 1a | Local transmission | Autochthonous cases in human and/or animals of veterinary importance occurred every year over the last 5 years. | √ | √ | √ |
| 1b | Local transmission | Autochthonous case(s) in human and/or animals of veterinary importance occurred sporadically, i.e. a single event or multiple events occurring during up to 4 years of local transmission in the last 5 years. | √ | √ | √ |
| 2 | Infectious agent and vector are present | See context 3 and 4 for definitions. | – | √ | √ |
| 3 | Vector is present | A vector is considered present when an arthropod species capable of transmitting a certain vector‐borne infectious agent is indigenous; or when a previously exotic vector species is established. | – | – | √ |
| 4 | Infectious agent is present | An infectious agent is considered to be present when it is circulating among indigenous vectors and non‐human (wildlife) hosts, and also when it is regularly introduced by vectors, reservoir hosts or humans. When an infectious agent circulates in animal populations of veterinary importance (e.g. cattle or pets), this is considered local transmission and therefore 1a or 1b. | – | √ | – |
| 5 | None of the above | No cases occurred, no vectors are present, no infectious agent is present | – | – | – |
In countries where both the infectious agent and vector are currently absent (context 5), the main concern is the early detection of the introduction and establishment of the competent vector(s). However, surveillance activities are not recommended if the establishment of the vector is impossible or highly improbable due to climatic or environmental constraints. This assessment should be repeated regularly, to account for changes in the geographic distribution of the vectors in Europe.
In countries where the infectious agent is regularly introduced although the vector is absent (context 4), an assessment should be made to determine whether the vector can establish itself should it be introduced. In countries where the local climatic and environmental conditions allow the establishment of the vector, surveillance focusing on early detection of ng the introduction of the vector can be considered, focusing on potential ports of entry/high‐risk areas.
In countries in which competent vector populations are established, but no evidence of introduction or circulation of the infectious agent exists so far (context 3), surveillance should focus on early detection of the introduction or circulation of the infectious agent. The surveillance could target the vectors or reservoir or sentinel animal species.
In countries with an established vector population and enzootic circulation of the infectious agent or with frequent introduction of infectious reservoir animals (and humans), that have not yet resulted in autochthonous cases (context 2), surveillance could be considered to delineate the risk areas and periods of circulation of the infectious agent. The surveillance could target the vectors or reservoir or sentinel animal species.
In countries that experience autochthonous human or animal cases every year (context 1a) or infrequently (context 1b), surveillance could be considered to delineate the risk areas and periods of circulation of the infectious agent. The surveillance could target the detection of changes of infection rates of the vectors or reservoir or sentinel animal species. However, to reduce the incidence of the disease, the implementation of control measures for one or more of the three components (disease burden, infectious agent, and vector) and the environment becomes an important element of the surveillance programme.
The VectorNet project of ECDC and EFSA produces regularly updated distribution maps of invasive and native mosquitos 5 as well as the tick species 6 in Europe. These maps can be utilised when determining the context of a particular vector‐borne disease in a country. VectorNet has also developed a range of tools that can assist countries in planning and implementing vector surveillance activities, such as a guide for field sampling methods for a range of vectors 7> and ECDC has developed guidelines for the surveillance of invasive as well as native mosquitos in Europe. 8 , 9 Finally, VectorNet is supplemented with a structured entomological network that is harmonised with other networks within the European public and animal health agencies. This VectorNet Entomological Network (VEN) encompasses 51 countries: EU/EEA (26 + 3), EU Enlargement policy (5) and European Neighborhood Policy partner (15) countries. A strong professional VEN is intended to encourage national governments to ensure sustainable vector surveillance (Braks et al., 2022). These should be involved in the planning and implementation of any surveillance activity targeting vectors.
3.3.2. Surveillance in wildlife
The main recommendations and technical specifications for sustainable coordinated OH surveillance for early detection of the listed zoonotic pathogens where wildlife is implicated are presented in a report of the ENETWILD‐consortium (ENETWILD‐consortium et al., 2023) and summarised below. These include general recommendations for the first steps in sustainable surveillance for zoonotic diseases in wildlife in the EU, and specific recommendations of surveillance aimed at risk based early detection of infectious agents in the main groups of wild animal species.
Regarding the general recommendations:
-
–
a priori, targeted surveillance options for the list of selected pathogens would be the selected options to address these surveillance objectives. This approach indicates that at least different targeted wildlife disease surveillance programmes could be run in parallel. However, it is not practical to have only targeted (disease‐specific, usually based on active surveillance) surveillance programmes for every disease or pathogen and a combination with general surveillance (which usually relies more on passive surveillance) is the best approach. An approach also incorporating strategically general surveillance for these selected pathogens has potential to generate cost effectively information that is needed to improve the current understanding, prevention, and control of certain zoonotic pathogens, but also to provide information about n other pathogens.
-
–
Under the OH approach, interdisciplinary collaboration across stakeholders in human, animal (including wildlife) and environmental health representatives is required at all stages of surveillance (i.e. design, implementation, management and evaluation), if not, the system will be ineffective, less sustainable, and short‐lived.
-
–
Initially, there is a need to select the surveillance components that are more effective to achieve early detection of pathogens and to prioritise data sources, considering the limitations of resources. Different surveillance components may target specific reservoirs of infection or infestation and/or may simply be efficient indicators of risk to humans and domestic animals. Therefore, the different compartments of OH must identify criteria to guide the selection of zoonotic disease surveillance components, which may be adjusted to local epidemiological circumstances.
-
–
Under the OH approach, determining disease emergence, maintenance and risk of transmission in multi‐host communities is recommended, for which we need to focus surveillance on a diverse array of pathogens at once in a number of host community assemblages. This is the so‐called ‘observatory approach’ (e.g. https://wildlifeobservatory.org/), which complements classical approaches which normally are fragmented in terms of target population (rarely entire communities of hosts) and pathogens are addressed, and opportunist spatiotemporal sources of samples/data.
The main specific recommendations of surveillance aimed at risk‐based early detection of zoonotic pathogens in the main wild species groups were:
-
–
Farmlands (particularly outdoor) should be priority areas to be incorporated into sampling strategies for wildlife in relation to most pathogens of the list.
-
–
It is essential to develop best possible initial mapping of pathogen (or threat) presence and distribution, at least for those already present in the EU and nearby countries, for further development of risks‐based surveillance (planning and sampling).
-
–
Currently, we are not ready to produce a complete range of good resolution maps of the spatial distribution of the wildlife/livestock interfaces in Europe, but only in some countries and for some species.
-
–
The interface where direct and indirect contacts of wildlife with pets and humans occurs (outdoor recreational activities, farms, peri‐urban areas, and parks) is a priority target for disease surveillance in most wildlife groups and should be considering during the surveillance planning phase.
-
–
Regarding the risk posed by vectors where infected wildlife animals are present, efforts are needed to map at the finest possible resolution and at large biogeographical scales where hosts and vectors distribution overlaps, and to determine at a local level, the habitat, land uses and features where both vectors and host sampling is recommended. This information will provide a solid background for sustainable vector‐borne zoonotic disease surveillance in the future.
-
–
Therefore, a necessary first step for designing disease surveillance strategies is mapping both abundance and management of wildlife over Europe, using standards for data collection to incorporate wildlife abundance to disease surveillance planning (i.e. as ENETWILD initiative does).
-
–
Wildlife zoonotic disease surveillance should target where direct contact of wildlife with hunters, and consumers of meat are present.
-
–
More evidence is needed on the potential role and practical use of wild species as potential sentinels for early detection of zoonosis; however their inclusion in wildlife zoonotic disease surveillance is recommended.
-
–
Wetlands and breeding grounds habitats/areas, and at larger scale, EU borders and where bird migration paths overlaps, especially where recent outbreaks occurred in neighbour countries are essential risk to be considered in surveillance sampling design.
Furthermore, recent reports by the ENETWILD‐consortium (2022a, b) described and mapped the main existing structures and systematic initiatives, for surveillance of zoonoses (transboundary, emerging and re‐emerging) in domestic animals and wildlife in the EU. The analysis of a questionnaire distributed to MSs about official surveillance programmes (ENETWILD‐consortium et al., 2022c) revealed that the integration between sectors is not generalised, which is a necessary step to develop OH surveillance for multi‐host transboundary zoonotic pathogens. Despite the relevance of wildlife in the prioritised list of infectious agents as hosts, wildlife species were unrepresented in surveillance programmes, particularly mammals, namely rodents and bats, and to a lesser extent, wild ungulates and carnivores. Furthermore, the interconnectedness of the health, ecology and population status of wildlife in regard to the presence and the transmission of microbial agents is a threat to One Health that is currently poorly understood, in the areas, among others, of antimicrobial resistance, food security and zoonoses.
3.3.3. Surveillance for the early detection of yet unknown pathogens (Disease X)
Disease X is a placeholder name that was adopted by the World Health Organization (WHO) in February 2018 on their shortlist of blueprint priority infectious diseases to represent a hypothetical, unknown pathogen that could cause a future epidemic (WHO, 2022). So far, there have been detected 25 families of viruses that can potentially infect humans and an estimated 1.67 million unknown viruses, of which 631,000 to 827,000 could have the capacity to infect human beings (EcoHealth Alliance, 2022). Although the total number of zoonoses is unknown, Taylor et al. (2001) catalogued 1,415 known human pathogens, including 217 viruses and prions, 538 bacteria and rickettsia, 307 fungi, 66 protozoa and 287 helminths, of which 61% were zoonotic.
Human Disease X is characterised to be caused by an infectious agent from a previously unrecognised source (ecosystem, animal, human) with an epidemic spread essentially following two patterns: (1) hit & run (e.g. influenza virus) or (2) hide & persist (e.g. HIV). The challenge in (1) is that it will most likely have fulminant clinical symptoms and spread very fast, while (2) will probably have spread quite far before it is recognised, because there is a delay between infection and clinical manifestation.
Furthermore, there are no diagnostics available for the Disease X during its emergence and no vaccine or treatments. It is expected that the causative agents of Disease X can mutate into other variants. Clearly the spread of COVID‐19 via airports, harbours, borders, postal services, can be considered to be an occurrence of Disease X with pandemic extent. HIV is an earlier example with different epidemiological properties but similar reach.
There is some discussion about the likelihood for causative agents of Disease X to first be detected in humans after it has already been present in animals and performed a host‐change into humans as has occurred with the causative agents of BSE, Q‐fever, Spanish flu, AIDS, Nipah and Ebola when they first emerged. By definition, the next Disease X can only be known to exist after its causative agent has been observed to occur in humans. Given the large number of candidate pathogens, the complexity of processes required for the evolution of human pathogens, and the current level of our scientific knowledge, it is impossible to predict the next Disease X based on finding an infectious agent in animals. At the present time, information created from the surveillance for infectious agents in vertebrate animals or vectors is of little or no help for predicting the next Disease X. However, this does not mean that animal health surveillance is not needed to prevent and understand pandemics that are caused by new infectious agents with Disease X characteristics. Health surveillance that integrates surveillance of humans, animals and the environment will provide more holistic information about the relationships between humans, animal, infectious agents and the environment, which will better inform both infectious disease prevention and mitigation for humans and animals. The interconnectedness of the health of animals and humans is now well accepted. A recent modelling study related increases in malaria cases in people in Central America, to the reduction in amphibians, who are predators of mosquitos, due to the introduction of chytridiomycosis (Springborn et al., 2022).
The appearance of a previously unknown disease in animals (Disease Y) could also be considered. This Disease Y, similar to Disease X, would be caused by a new infectious agent causing (severe?) clinical signs in animals with considerable socio‐economic impacts and a possible risk for transmission to humans. The detection of a possible Disease Y in animals could take place through indicator‐based surveillance that could detect any unusual/increased mortality, abortions or divergent behaviour in animals (wild or domestic). In addition, precision livestock farming (PLF), relying on the automatic monitoring of individual animals, specifically their growth, milk production, behaviour and their physical environment, as well as the detection of diseases, could further provide an early detection system for any sudden and unexpected changes in health and production parameters on livestock farms.
Other existing surveillance systems could be strengthened, such as the further analysis of regular samples submitted for pathology/laboratory testing especially when there has been no conclusive diagnosis established (investigate diagnostic dilemmas in all animals and especially wildlife).
Metagenomics could be applied in places where there is a higher likelihood for a Disease Y to emerge, e.g. densely populated areas with intensive animal‐human contacts.
There is, however, an urgent need to address/reduce the risk factors for emergence and spill‐over. For this, a more systematic investigation on previous infectious disease emergences is needed to identify the dispositions of the socio‐ecological systems that facilitate such events. These include ecological changes (loss of biodiversity, urban encroachment of habitats, climate change), changes in agriculture and food production, movement of infectious agents, vectors and hosts, including via travel and trade. Human behaviour, comorbidities (e.g. diabetes, obesity) and demographical (e.g. ageing) factors as well as microbial changes and adaptation and the importance of wildlife and vectors for the emergence of Disease Y or X need to be considered. In essence, the prevention of Disease Y or X implies One Health as an objective of having healthy people, animals, and ecosystems.
Overall, a well‐functioning ‘care system’ in the animal, human and environmental sectors is a prerequisite to generate the warning signals that can be propagated through surveillance systems. Strengthening the surveillance and response system(s) (including syndromic surveillance, outbreak investigations and trace back) in the animal, human and environmental sectors and the institutionalisation of integrated planning, sampling, analysis and dissemination, essentially through constituent information sharing and collaboration between sectors, will contribute to the early detection and response to the emergence of a Disease X in humans and a Disease Y in animals. In addition, creating awareness and changing behaviours are needed to reduce the risks and further spread of these previously unknown diseases. Essentially, the prevention, surveillance and response require One Health as an approach to be effective.
For meaningful sampling, an intersectoral understanding of the socio‐economic system that gives rise to Diseases X and Y must be developed at various governance levels. This will facilitate the identification of critical indicators relevant to observe the system. The authors suggest that because the need for protein has increased worldwide, a better understanding of the global and local protein flows can provide important clues regarding spill‐over risks. Risk mapping of the determinants of emergence (e.g. human and animal population interfaces, protein fluxes) and considering specific approaches (critical point monitoring and bow‐tie approach) 10 are possible measures to support governance of Disease X. Possible points for environmental sampling for pathogens include various effluents (from farms, slaughterhouses, clinics, markets, airplanes, harbours, caves) to be further considered as well as the rising importance of legal and illegal trade in (exotic) animals and animal products (including via Internet). Also, it must be stated that time and location of emergence and the nature of Disease X and Disease Y are unknown and hence vigilance must be broad. Surveillance for Disease Y could still be oriented at situations where the determinants of emergence/spill‐over represent a high risk, particularly to sensitise stakeholders. Supporting and creating capacity in countries (within and beyond the EU) with weaker surveillance and response systems and a functioning international early warning and response system should not be left unmentioned as in the end ‘No one is safe until everyone is safe’.
As implied above, in Europe as in other parts of the world, marginalised and underserved (human and non‐human) populations are at highest risk of experiencing an unnoticed emergence/ spill‐over and thus undermine an early detection. In contrast, the likelihood of emergence/ spill‐over is likely to be associated to a context with high densities, (resource scarcity), high mobility, high contact rates, risk behaviour, which have different spatial, social and chronological distributions and require an intersectoral and ‐disciplinary understanding of the socio‐ecological system to address prevention effectively.
Innovative combined surveillance and response systems that focus on drivers of emergence should be considered. As an example, methodologies of ‘risk landscaping’, such as the bowtie approach can be used to map drivers, impacts and vulnerabilities of a specific ecosystem, and pinpoint critical monitoring points. The approach was recently used by the Quadripartite alliance of FAO, UNEP, WHO and WOAH 11 to map risks associated with risk categories broad enough to include unknown hazards: epidemic and emerging zoonotic diseases; antimicrobial‐resistant microorganisms; contamination of water and soil from chemical fertilisers/pesticides; and non‐zoonotic animal diseases affecting food security (Calvin et al., 2022).
3.4. Building a coordinated surveillance system for early disease detection
In a One Health framework, it is important to step back from the consolidated approaches, designed to face health problems, in the attempt to embrace them all and realise that they all contribute (or can contribute) in synergy to achieve preparedness and shorten the time needed to react to a certain, sometimes unknown, threat.
In this perspective, every activity aiming, directly or indirectly, at detecting the presence of a given adverse agent (e.g. bacteria, virus, parasites) in a certain context, can provide useful information to understand the overall picture, describing changes or anomalies in the usual patterns (whatever the pattern is about). The One Health perspective should cut across all sectors and science domains to collect all pieces of information and put them together to provide an overall, comprehensive picture.
More concretely, it should be possible to start from the selection of an adverse agent and, based on its characteristics, identify all contexts in which it could be found, e.g. the environment (e.g. water, soil), the animal population (e.g. farmed animals, wildlife), vectors (e.g. mosquitos, ticks). Every piece of information can contribute to the overall knowledge on that specific adverse agent, providing useful information for the management of potential threats.
Overviews of the populations involved in transmission cycle of each infectious agent of relevance in this mandate, as well as the role of environmental surveillance, were provided above in Figures 3 and 4. These can be used to design surveillance systems based on combinations of complementary surveillance components as suggested above (see disease briefs and surveillance cards). In addition to the surveillance design framework and other resources provided in the disease briefs and surveillance cards, Annex A details and exemplifies a stepwise approach for calculating the sample size and sensitivity of a surveillance system applying probability‐based sampling, using the RiBESS tool.
Sampling frequencies
Frequency of sampling will depend on the type of surveillance activity and the biology of the infectious agent. Surveillance aimed at early detection for rapidly spreading infectious agents will ideally need to be conducted continuously. For continuous surveillance, it is not necessary to specify a sampling frequency, as sampling is continuous. For repeated samples aimed at early detection of the introduction of a pathogen, sampling frequency will be dependent on the rate of spread of the pathogen and the potential harm the pathogen can cause in human and animal populations. We have not been able to find any published standards defining the frequency of sampling necessary for the infectious agents chosen for this grant. It would be advantageous to restrict continuous sampling to high‐risk time periods for seasonally endemic infectious agents, therefore we have provided some guidance on sampling time period in the surveillance cards.
3.4.1. Consideration of further added value of surveillance activities
While we cannot design surveillance against infectious disease threats we do not yet know, it is possible to increase the overall surveillance system robustness to the type of risks it is able to monitor, and the type of outbreak signals it is capable of detecting.
Member States that intend to apply for a direct grant under the EU4Health programme initiative CP‐g‐22‐04.01 should use the guidelines in the disease briefs and surveillance cards to design (or strengthen existing) surveillance components that can increase the overall robustness of their surveillance systems.
The host (reservoir and/or susceptible) and vector tables listed above are useful in guiding the design of surveillance which covers a wide range of hosts and potential pathways for disease transmission.
Moreover, during the exercises of prioritisation, the working group identified criteria to consider the overall utility of any surveillance component. These can be used by countries to assess the overall utility of the different components proposed in the surveillance cards, prioritising the implementation of components which are beneficial and constructive, per criteria below.
Beneficial: Is there a benefit from early detection of the emergence or re‐emergence of the infectious agent? Criteria that could be used to assess the benefits include:
Early detection: Can the infectious agent be detected early enough to prevent by isolating, curing culling or otherwise controlling the spread of the infectious agent before it produces large‐scale harm (e.g. because it causes evident clinical signs and/or mortality, because rapid tests are available)?
Early warning: Are there ‘signals’ that indicate an increased risk of the infectious agent spreading to humans? For example, finding antibodies to West Nile Fever virus in sentinel chickens located in city parks before humans become infected.
Broad surveillance benefits: Can other infectious agents be targeted by the surveillance for this pathogen (synergies between different diseases/hosts/geographical locations)?
Contribution to detection of emerging threats: Can the surveillance for this infectious agent increase the chance of detecting Disease X?
Constructive: Does a surveillance system for the infectious agent contribute to increasing surveillance capacity? Criteria that could be used to assess constructiveness include:
-
e
Cross‐sectoral collaboration: Will the surveillance for this infectious agent foster cross‐sectoral collaboration (data exchange and analysis) (e.g. with the human health sector)?
-
f
Multi‐national collaboration: Will the surveillance for this infectious agent foster cross‐country collaboration (e.g. between neighbouring countries)?
-
g
One Health operationalisation: Will the surveillance for this infectious agent improve One Health operationalisation in your country?
-
h
Sustainable surveillance framework: Surveillance for this infectious agent is sustainable and/or contributes to the sustainability of the surveillance framework in the country (e.g. human and laboratory resources) in general.
3.4.2. Cross‐sectoral collaboration and integration of knowledge across sectors
Within the One Health paradigm, surveillance of zoonoses can be understood as an element in the governance cycle during which knowledge integration contains several aspects, namely integrating knowledge about objectives and interests, about perspectives and processes, or knowledge relating to the transformation of the current version of a system towards a more desired version (Hitziger, 2018). A fully integrated governance of zoonotic diseases would thus require co‐producing objectives of surveillance and a sampling plan, joint sampling and analysis, and joint interpretation and evaluation, including stakeholders from the relevant sectors and disciplines and be subject to feedback loops for adaptation over time (Figure 6). This includes systemic thinking, cross‐sectoral planning, transdisciplinarity, a sharing infrastructure, institutionalised adaptation/learning and decentralised and adaptive leadership. However, a fully fledged OH approach may not be practical or desirable in every case, and within this larger picture, the integration of surveillance findings essentially concerns the two steps of data analysis and interpretation. This reduces the exigencies for integration to an effective collaboration. Bordier et al., (2020) provide a more detailed account of the opportunities for cross‐sectoral collaboration along the steps of the surveillance process and propose a table classifying the different degrees of collaboration during different steps of a surveillance process (Table 4). Accordingly, collaboration can be as simple as a single sector comparing results across all relevant health sectors, while in the highest degree surveillance is carried out jointly by a multi‐sectoral working group or body.
Figure 6.
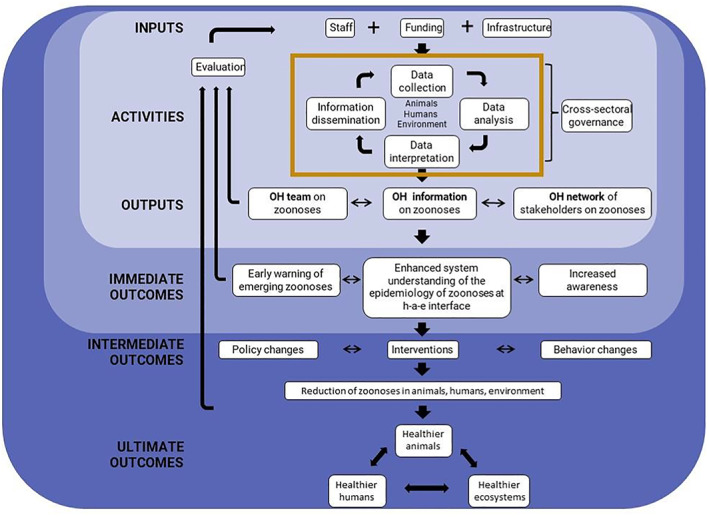
Logical model of integrated governance of zoonoses (adapted from Aenishaenslin et al., 2021)
Table 4.
Surveillance steps and the opportunities for collaboration across different sectors (adapted from Bordier et al., 2020)
| Steps | Degree of collaboration | ||||
|---|---|---|---|---|---|
| Planning | Separate in each sector | by a single sector for all components | Cross‐sectoral consultation, separate plans | By a multi‐sectoral working group | By a multi‐sectoral body |
| Data collection | Separate in each sector | By a single sector for all components | Harmonisation across sectors | Joint activities | By a multi‐sectoral body |
| Data sharing | No exchange | Notification of unusual events | Continuous & institutionalised | ||
| Analysis/ interpretation | Separate in each sector | separate & compared by a single sector | Single sector for all components | Separate analysis and comparison in a joint working group | Joint analysis by a multi‐sectoral body or working group |
| Result dissemination | Separate in each sector | Joint dissemination in sectoral activities | Dissemination by a single sector to all | by a multi‐sectoral working group | By a multi‐sectoral body |
| Response & prioritisation | Separate in each sector | Cross‐sectoral consultation, separate r&p | r&p by a single sector for all | by a multi‐sectoral working group | by a multi‐sectoral body |
| Prevention | Separate in each sector | Cross‐sectoral consultation, separate prevention | Prevention by a single sector for all | by a multi‐sectoral working group | By a multi‐sectoral body |
The ORION project within the One Health European Joint Programme suggests a further step of ‘response and prioritisation’ succeeding the dissemination step (Filter et al. 2021), and one could consider ‘prevention’ as a further opportunity arising from OH surveillance. The ORION project investigated a pilot set of European One Health Surveillance Initiatives (Filter et al., 2021), and illustrated a diversity in the degree of collaboration. Within the restricted responsibilities of a surveillance system, countries should consider the collaboration scenario that optimises surveillance performance, which in turn should be measured according to the objectives. As illustrated in Figure, these objectives may change under the influence of changing policies or other socio‐ecological conditions, which in turn are affected by the information provided by the surveillance component or system. To give an example, a model for early detection of Campylobacter outbreaks in humans in Sweden showed that integration of human cases data into the time‐series of results from slaughterhouse testing (in broiler chickens) did not improve the sensitivity nor the timeliness of detection. The best solution for cross‐sectoral integration, in this case, was the establishment of best‐practices for communication procedures between the veterinary authorities and the public health authorities, with the former sending alert signals to the latter.
For each surveillance component implemented or adapted under the One Health approach, countries should carefully consider the surveillance objective and the performance measures to monitor and evaluate performance. To which degree stakeholders beyond the governance actors are engaged in the process of determining the objectives of zoonosis control and consequently of surveillance is a political decision. Objectives and thresholds for action are the result of the common framing of the challenges by the participating parties. Studies on sustainable development suggest, that a broader engagement facilitates socially, economically and environmentally more acceptable solutions (Shiroyama et al., 2012). The degrees of collaboration across health sectors in each step should then be optimised to achieve the desired performance under the country's specific context – organisational structure of the health sectors, data sharing practices, etc. According to the OHHLEP definition of One Health, all relevant sectors should be considered, notably the sectors of animal health, public health and ecosystem health (One Health High‐Level Expert Panel (OHHLEP) et al., 2022).
3.4.2.1. Available frameworks and tools to guide cross‐sectoral surveillance design and implementation
Within the One Health European Joint Programme (OHEJP), the ORION project created the One Health Surveillance Codex (Filter et al., 2021) to gather resources that can help strengthen specific principles of cross‐sectoral surveillance: collaboration, data sharing, knowledge exchange and dissemination. The Codex was later expanded by the OHEJP project MATRIX into the ‘knowledge integration platform’, 12 which also added a fifth principle of OH surveillance: planning and management.
The OHEJP project MATRIX also developed ‘A Step‐by‐Step guide to creating a One Health Surveillance System from existing surveillance programs’, which is publicly available. 13 The OHEJP project COHESIVE developed the One Health risk analysis system Road Map (OHRAS). 14
The Tripartite alliance of the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO) and the World Organisation for Animal Health (WOAH, founded as OIE) also developed a guide for adoption of One Health surveillance at the country level ‐ Taking a Multisectoral One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries (TZG; WHO et al., 2019). According to the authors' own description, ‘The 2019 TZG provides relevant country ministries and agencies with lessons learned and good practices identified from country‐level experiences in taking OH approaches for preparedness, prevention, detection and response to zoonotic disease threats, and provides guidance on multisectoral communication, coordination, and collaboration. It informs on regional and country‐level OH activities and relevant unisectoral and multisectoral tools available for countries to use’.
To operationalise the recommendations in the TZG, the Tripartite alliance published three complementary tools: the ‘Joint Risk Assessment Operational Tool (JRA OT)’ (WHO et al., 2020); the ‘Surveillance and Information Sharing Operational Tool (SIS OT)’ (WHO et al., 2022a) and the ‘Multisectoral coordination mechanisms operational tool (MCM OT)’ (WHO et al., 2022b).
While some of the frameworks proposed above are focused on general cross‐sectoral collaboration, two tools are specifically aimed at multi‐sectoral infectious disease risk analyses (OHRAS and JRA OT). Increasing capacity of countries to perform rapid risk assessments using a One Health approach will have significant impact on the preparedness and response to (re)emerging zoonotic threats. This will be particularly relevant when infectious disease (re)emergence happens outside a specific country's border, and rapid risk assessments are needed to quickly respond to the threat of transboundary spread.
3.5. Road map for the coordinated surveillance strategies under the One Health approach
The tools provided in this report and those available elsewhere (e.g. the RiBESS tool, see Annex A) should be used by countries in their applications for a direct grant under the EU4Health programme initiative CP‐g‐22‐04.01. One or more of the surveillance components suggested should be chosen and modified where relevant according to the national situation, and/or new surveillance components different from those proposed in this report should be described by completing the ‘surveillance card template’ for one of the priority pathogens identified for the EU4Health programme initiative CP‐g‐22‐04.01. For surveillance components applying probability‐based sampling, the sample size can be calculated using the RiBESS tool. For surveillance components applying non‐probability‐based sampling, the sample size can be defined in the surveillance card. The completed surveillance card(s) should be submitted to HaDEA, together with the other required information (Figure 7).
Figure 7.
Use of the tools presented in this report in the planning, application, implementation and reporting phases of surveillance activities related to the EU4Health programme initiative CP‐g‐22‐04.01
During the implementation phase, the surveillance cards should be used to describe the surveillance activity in detail and be submitted together with the raw data generated by the surveillance activity to EFSA. The already existing EFSA standards such as SIGMA Est and the EFSA Data Collection Framework will be used at that stage. Under the direct grant, Member States have the obligation to report at least once per year, however, EFSA will enable the submission of data at a higher frequency, which will be agreed with Member States in the next project phase. An automatic generation of dashboards and standard reports for the submitted data and information will be developed (Figure 7). The details of this process are related to ToR B and outside the scope of this report. They are subject to another EFSA report that will be developed at a later stage, in close collaboration with Member States and ECDC.
4. Conclusions
This report aims to provide guidance and recommendations for Member States to design surveillance systems for zoonotic diseases under the umbrella of One Health for the EU4Health programme initiative CP‐g‐22‐04.01.
Surveillance systems create information that is used to make decisions aimed at mitigating the impact of diseases on a population. For single diseases, the information is often very straightforward as it is commonly aimed at identifying an increase in the amount of disease or disease risk. The situation is much different for One Health.
Currently One Health is a concept that is still evolving. The overall outcomes expected from One Health as a goal are still being defined and there is currently no unified understanding of the implementation of a One Health approach. This means that at this time the information needed to support decision making generated by One Health Surveillance is also still being defined. This uncertainty combined with the limitations placed on this mandate has resulted in the working group having to adopt a narrow vision of One Health for the project focussing solely on public health concerns. Working within the bounds of these limitations, the working group has done their best to advance surveillance for specific zoonotic pathogens. This report and the grant it supports should not be considered a definitive One Health Surveillance guide, rather it should be regarded as a step in a journey towards reinforcing integrated One Health surveillance systems in Europe.
The tools presented in this report, specifically the disease briefs and surveillance cards, can be used for the development of any surveillance activity that a country would like to set up or enhance under the EU4Health programme initiative CP‐g‐22‐04.01, provided that the activity targets one or more of the infectious agents identified as priority pathogens (EFSA, 2023) or Disease Y and that the information requested in the surveillance card template provided in this report is defined and described for the component.
For some of the diseases prioritised for the coordinated surveillance under the One Health approach, activities are already carried out in Member States under the Animal Health Law and Union Surveillance programmes, such as Echinococcus multilocularis and HPAI. Therefore, no surveillance activities have been proposed for these in this report.
5. Recommendations
While the projects will enhance surveillance in domestic and wild animal populations and the environment including vectors, the main aim is to prevent diseases in humans. This will require good collaboration and communication between the various sectors involved as well as shared objectives and equitable resources in a One Health spirit.
Countries should ensure that their surveillance activities under this grant will be synergistic with other ongoing or planned surveillance activities in areas such as food‐borne pathogens, antimicrobial resistance, particularly, with the initiative CP‐g‐23‐01 (‘Direct grants to Member States' authorities: improving and strengthening national surveillance systems’) and with the initiative CP‐g‐23‐18 (‘Direct grants to Member States' authorities: to enhance, extend and consolidate wastewater surveillance for public health’). They should also build on and be in coordination with the actions carried out by the European Commission on human health surveillance, based on the report on ‘Lessons learnt from COVID‐19 surveillance and other epidemics on integrated and real‐time of surveillance in the EU/EEA’ and on the Joint Action on Integrated surveillance, including the setting – up of human and animal health data integration [under the 2021 work programme (AWP 2021 ‐ CP‐g‐02.1.1)]. Overlaps with already ongoing activities under the AHL or Union surveillance programmes must be avoided.
If a vector‐borne pathogen is considered to be absent from a country, it is important to first establish if known or anticipated competent vectors for that pathogen are present, by using existing data or creating new data through surveillance. When no such vector is present yet, early detection of the invasion of such vector species through surveillance should precede surveillance for the infectious agents in vectors, vertebrate animal host or humans.
Environmental sampling, development of new diagnostic tools and the inclusion of diseases and pathways could be considered to be part of surveillance for Diseases Y and X.
There are a number of candidates for Disease X in humans such as RNA viruses, haemorrhagic fevers and the more recent non‐polio enteroviruses or organisms emerging from an area where the right mix of risk factors promotes the risk for sustained transmission. More research is needed to identify genes, genotypes and phenotypes of animal pathogens that are strongly associated with Disease X phenotypes in people, i.e. causing severe clinical signs in people, and being rapidly transmitted among people.
Abbreviations
- ECDC
European Centre for Disease Prevention and Control
- FAO
Food and Agriculture Organization of the United Nations
- HPAI
highly pathogenic avian influenza
- OHHLEP
One Health High Level Expert Panel
- PLF
precision livestock farming
- ToR
Terms of Reference
- VEN
VectorNet Entomological Network
- WG
Working Group
- WHO
World Health Organization
- WNV
West Nile virus
- WOAH
World Organisation for Animal Health
Glossary
- Early detection
The first detection and characterisation of a damaging agent (be it an infectious pathogen, pest, invasive species, etc.) in an area that was previously unaffected. It is part of disease response.
- Early warning
The identification of a change in the risk (of introduction) of disease. It is part of disease preparedness, allowing preventive measures to be taken.
- Epidemiological detection prevalence
Threshold for detection at level of epidemiological unit
- Epidemiological sensitivity
Probability that the round of examinations performed at epidemiological unit level detects at least one sampling unit, given that the epidemiological unit is (truly) positive (i.e. the prevalence in the epidemiological unit is greater than the Epidemiological Detection Prevalence)
- Epidemiological unit
A group of animals with the same likelihood of exposure to a pathogenic agent. In certain circumstances, the epidemiological unit may be a single animal
- Exam sensitivity
Probability of the examination of returning a positive signal, given that the testing unit is (truly) positive (e.g. diagnostic test, clinical examination).
- Geographical scope
The level at which it is needed to draw conclusions on presence/absence of the disease. Normally geographical areas in surveillance. The smaller, the higher the confidence of being able to detect the ‘first’ case at country level; the greater the effort at epidemiological area level.
- Indicator‐based surveillance
Surveillance based on the monitoring of indicators of disease or pathogen presence, such as for instance number of animals with a specific clinical sign, or number of deaths in a specified unit of time, for a given geographical unit.
- Infectious agent
Agents (bacteria, viruses, fungi, parasites, prions etc) that can infect living organisms (hosts). Infectious agents may or may not harm the hosts they infect. When they harm the host, the harm is called disease, and the agents are called pathogens.
- Matrix
Sampling materials that may contain a pathogen (blood, vectors, water, soil, debris, faeces, air etc).
- Reservoir host
A reservoir host is one in which an infectious agent normally lives and multiplies, and serves as a source of infection to other animals. Reservoirs should be defined in relation to specific ‘target’ populations. ‘Reservoir’ is also used to refer to any substance that is a common source of infection (e.g. water, soil)
- Risk
Product of probability of an event occurring and the expected consequences of that event. Risk = P(event) x Consequence(event). While surveillance delivers data on the probability, the consequences are defined by the context of the infection, e.g. farm size, severity, costs, among others.
- Risk factors and related Relative Risk (RR)
To be defined at any level having an impact on the sample size. NOTE: if the RR value is NOT available, it is NOT possible to go for a risk‐based approach
- Active surveillance
Investigator‐initiated collection of animal health related data using a defined protocol to perform actions that are scheduled in advance. Decisions about whether information is collected, and what information should be collected from which animals is made by the investigator
- Passive surveillance
Observer‐initiated provision of animal health related data (e.g. voluntary notification of suspect disease) or the use of existing data for surveillance. Decisions about whether information is provided, and what information is provided from which animals is made by the data provider
- Population size
Number of epidemiological units in each component.
- Sample sensitivity
Probability that the round of examinations performed at sampling unit level detects at least one testing unit, given that the sample unit is (truly) positive (i.e. the prevalence in the sampling unit is greater than the Sampling Detection Prevalence)
- Sampling detection prevalence
Threshold for detection at sampling level unit
- Sampling unit
Units selected for sampling in surveillance activity
- Sentinel surveillance
The repeated collection of information from the same selected sites or groups of animals (e.g. veterinary practices, laboratories, herds or animals, sentinel animals) to identify changes in the health status of a specified population over time. These sentinels should act as a proxy for the larger population of interest; they may be selected on the basis of risk but can also be selected randomly or on the basis of convenience or compliance
- Surveillance Component
A single surveillance activity (defined by the source of data and the methods used for its collection) used to investigate the occurrence of one or more hazards in a specified population
- Surveillance System or network
A range of surveillance components (and the associated organisational structures) used to investigate the occurrence of a single hazard in a specified population
- Susceptible host
Any species that can be infected with the pathogen in question
- Target population
The total population about which information is required. Ideally, this should be the population at risk
- Testing unit
The unit concretely undergoing the examination/test
- Vector
An arthropod species that can transmit a particular pathogen
- Disease X
A hypothetical, unknown pathogen that could cause a future epidemic in humans
- Disease Y
A previously unknown disease in animals caused by new pathogen causing (severe?) clinical signs in animals with considerable socio‐economic impacts and a possible risk for transmission to humans
- Risk‐based surveillance
- Use of information about the probability of occurrence and the magnitude of the biological and/or economic consequence of health hazards to plan, design and/or interpret the results obtained from surveillance systems. Risk‐based surveillance can include one or several of the following four approaches:
- Risk‐based prioritisation.
- Risk‐based requirement.
- Risk‐based sampling.
- Risk‐based analysis.
Annex A – Description of how to use the RiBESS tool for the calculation of the sample size for probability‐based surveillance components
The RiBESS+ tool is a free web application, developed in the R4EU platform environment, implementing statistical methods for estimating important parameters for the design of surveillance activities (see Figure A.1).
Figure A.1.
Start screen of the RiBESS+ tool
The tool, originally developed to support countries in substantiating freedom from a given disease (EFSA, 2012), demonstrated its potential to be applied also for other purposes like, e.g. early detection, where the concept of ‘early’ is translated in a prevalence threshold. The tool allows to design the surveillance activity so that, with a measurable level of confidence, at least one exam (laboratory testing, clinical inspection, etc.) will return alerting feedback (presence of a pathogen, presence of a given symptom, etc.) in case the prevalence in the population raises above the chosen threshold.
It is important to note that:
in case all exams are negative, it will be possible to conclude (with a measurable level of confidence) that the prevalence is below the chosen threshold;
in case at least one exam is positive, it is NOT possible to conclude that the prevalence of the disease in the target population is above the chosen threshold.
This statistical consideration finds its roots in the statistical hypothesis testing methodology. In designing a surveillance activity with the RiBESS+ tool, the user implicitly is testing the hypothesis that the disease is absent (i.e. the prevalence in the population is below the chosen threshold). When all exams are negative, the Null Hypothesis cannot be rejected (the disease is absent). When one exam is positive (or more), the Null Hypothesis is simply rejected and the Alternative Hypothesis is true, i.e. the disease is present, but without ANY information about the prevalence in the target population.
This is to say that the tool cannot be used to estimate the prevalence in a given population, but it can be of use for:
estimating the sample size (design phase) to achieve a certain surveillance sensitivity (i.e. a certain probability that at least on exam will return a ‘positive’ result, in case the prevalence in the target population raises above the chosen threshold);
estimating the sensitivity achieved (after implementation of the sampling and testing activity) based on the samples collected and tested (or planned to be collected and tested).
Here follow two simple and not exhaustive examples of the applications listed above.
Example 1: Estimation of the sample size
A typical simple scenario, for purely illustrative purposes, is represented by a single farm, in which we want to detect the presence of a given disease.
In this case, the input parameters are:
The level of confidence to be achieved, 15 i.e. the probability that the round of tests in the farm will detect the disease: This parameter, in a surveillance context, is the sensitivity that it is wanted to achieve.
The population size, i.e. the number of animals in the farm.
The test sensitivity, i.e. the probability that the test used (e.g. a laboratory test, but also a clinical examination, based on the case definition) returns a positive signal, given that the animal is truly infected.
The design prevalence, i.e. the prevalence threshold set by the designer. Ideally, in case the goal is to detect the disease as soon as it is present, the design prevalence should be zero. For mathematical reasons, however, this value cannot be used as the equation will return a sample size equal to infinite. More pragmatically, this value should be somewhat ‘synonymous’ of absence or, as an alternative, a level of prevalence that is somewhat ‘acceptable’ (e.g. because up to a certain number of animals infected the disease is still controllable). In a surveillance context, this parameter could be called ‘detection prevalence’.
Let us quantify these parameters for the example:
| PARAMETER | ESTIMATION / MEASUREMENT |
|---|---|
| Sensitivity to be achieved at farm level | 95% |
| Population size | 2,500 |
| Test sensitivity | 0.9 |
| Design prevalence | 0.01 (25 infected animals) |
The RiBESS+ tool returns a sample size of 313 animals that need to be randomly selected, sampled and tested. In other terms, by sampling and testing 313 animals, randomly selected, if they all test negative, it is possible to conclude (with a 95% confidence) that the disease is absent. From another perspective, it is possible to state that in case in the farm there will be more than 25 infected animals, at least on test out of the 313 will return a positive result with a 95% probability.
Note: Random sampling is an important assumption in this methodology. The tool, however, allows the design of surveillance activities based on a convenient sampling approach. To do so, the target population should be divided in homogenous ‘probability groups’ (i.e. groups in which each animal has the same probability of being infected). In this case, the surveillance could ‘conveniently’ target only one probability‐group, i.e. the one in which a simple random sample is possible. In the RiBESS+ tool, the probability‐groups can be captured by means of the Risk Factors tab (see Figure A.2).
Figure A.2.
Risk Factors of the RiBESS+ tool. Example with a single risk factor with three levels
The tool also returns another value (332), calculated based on a Binomial distribution, therefore assuming an infinite population. The first value (313) is taking into account the fact that the population is finite, so using a Hypergeometric distribution (sampling without replacement). This simple feature allows in reality to deal with the more complex, but quite common in certain circumstances, situation where the size of the population is not known. In those cases, to be on the safe side and avoiding the risk of overestimating the sensitivity of the surveillance, it is suggested to use the Binomial result, assuming that the population is infinite and the sampling activity has no effect on the probability of selecting an infected animal.
It is important to highlight that the approach and the RiBESS tool are completely scalable, and the same approach can be used at a higher level, e.g. to estimate the number of farms to be visited, simply changing the parameter in the tool, as follows.
| PARAMETER | ESTIMATION/MEASUREMENT |
|---|---|
| Sensitivity to be achieved at (e.g.) regional level | 95% |
| Population size | 110 (farms) |
| Test sensitivity | 0.95*** |
| Design prevalence | 0.01 (1 infected farm) |
In this case, the RiBESS+ tool returns a value of 123 farms to be visited. In case two of them have infected animals, at least one of the 123 visits will report the presence of the disease.
Note: The test sensitivity value used in this higher level (0.95***) is the ‘Sensitivity to be achieved at farm level’, obtained at individual farm level in the previous step by sampling and testing 313 animals.
Example 2: Estimation of the sensitivity achieved
Let us bring forward the scenario explored in the previous section: during the surveillance period, it was not possible to achieve the goal of sampling 123 farms and test 313 animals each. What would be the sensitivity achieved?
By selecting the proper parameter to be estimated in the RiBESS+ tool, it is possible to make this estimation inserting the actual numbers of the required parameters (see Figure A.3).
Figure A.3.
Selection of a new parameter to be estimated (Surveillance Sensitivity achieved)
For purely illustrative purpose, we consider the following situation:
Farm level
| PARAMETER | ESTIMATION/MEASUREMENT |
|---|---|
| Sample size (tested) | 100 |
| Population size | 2,500 |
| Test sensitivity | 0.9 |
| Design prevalence | 0.01 (25 infected animals) |
For each farm, only 100 animals were tested (instead of 313 as planned). In this case, the probability that at least 1 of the 100 samples tested positive in case more than 25 animals were positive is 62%. This is the actual ‘test’ sensitivity at farm level that was used during the surveillance.
Regional level
| PARAMETER | ESTIMATION/MEASUREMENT |
|---|---|
| Sample size (tested) | 90 |
| Population size | 110 |
| Test sensitivity | 0.62*** (from the previous step) |
| Design prevalence | 0.01 (1 infected farm) |
Overall, visiting 90 farms out of 110 and testing in each 100 animals (for a total of 9,000 laboratory tests) achieves an overall sensitivity of 51%… which is very close to the toss of a coin. This does not mean that the surveillance activity will not be able to detect the disease (the probability is not 0% and there are, actually, a few people winning the national lottery), but it gives an indication, indeed, of the performance, based on which the country can evaluate, e.g. if it more proficient to sample more animals in each farm and visit less farms or the reverse.
Final remarks
The examples given cover only some situations in the real world and in the context of a One Health approach, in which more than one surveillance component are run at the same time to detect the same pathogen (e.g. domestic animals, vectors, environment, etc.). The RiBESS tool gives the possibility to estimate the sensitivity achieved for each of them which, combined, can give a more realistic idea of the probability of detecting the pathogen.
In some contexts, e.g. wildlife, some of the parameters may be uncertain. The RiBESS+ tool allows to input the required parameters including the uncertainty around the estimate, by means of probabilistic distributions.
The RiBESS+ tool must be seen by the countries as an instrument for the self‐assessment of the performance of the surveillance activities that they are implementing (or they plan to implement), with the goal of (i) optimising the efforts and (ii) highlighting weak areas of knowledge that may contribute to the optimisation of the activities (e.g. estimation of the target population or of the test sensitivity), and finally (iii) leading to dedicated research studies.
Extra reading on this topic
The Manual of the RiBESS+ is available in the Start page of the tool.
The RiBESS+ tool has been extensively applied in the context of the Plan Pest Surveillance activities. Many and more complex examples are given in the Technical Report (EFSA, 2020) which, by analogy, may be useful for the countries to design (or evaluate) their surveillance activities.
Annex B – Overview of information provided by the European Commission regarding co‐funded surveillance projects focusing on animals or on the environment (including water)
EboSursy: a project under WOAH umbrella in Africa strengthening surveillance and early detection of pathogens in wildlife and human‐animal interaction incl. bush meat.
‘SAFE ‐ Safety across Asia For the global Environment’ project with key stakeholders in China, Viet Nam, Laos, and Thailand, to advance on the EU/UN/Asia cooperation to contribute to the prevention of wildlife‐related pandemics by targeting risks at facilities and locations with a high risk of disease agents' transmission from wild animals to humans.
EASIN European Alien Species Information Network. MS report invasive alien species (only the ones listed) and list their management measures.
JRC application for the general public to report invasive alien species.
the Insignia work uses honeybees to survey pollutants.
H2020 projects:
Projects looking at vector‐borne diseases, with an international dimension:
PREPARE4VBD: https://cordis.europa.eu/project/id/101000365
DELTA‐FLU (https://delta-flu.fli.de/de/home)
Project looking at data for epidemiology and prioritisation of animal diseases, but focusing on (non‐EU regulated) pig diseases that are not (yet?) relevant for human health:
ICRAD ERA‐NET (https://www.icrad.eu/) launched several projects:
TechPEPCon;
FluNuance;
Preventer;
MuseCov;
PIGIE;
ConVErgence
Projects launched by Biodiversa
Co‐funded Veterinary programmes:
Details on the type of expenditures covered can be found in the relevant 2021–2022 Guidelines attached herein and also available online: https://ec.europa.eu/food/system/files/2021-06/cff_animal_vet-progs_guidance_progs_erad_wd-2021-10502.pdf
The programmes of previous years (2020 and earlier), may be found here https://ec.europa.eu/food/horizontal-topics/funding-procurement-grants/food-chain-funding/funding-animal-health-measures-1_en
The 2021–2022 programmes are not published yet, but should be expected any time soon on the HaDEA website: https://hadea.ec.europa.eu/index_en
Water‐related projects:
Advanced urban water management to efficiently ensure bathing water quality, Reference: LIFE17 ENV/ES/000396 | Acronym: LIFE iBATHWATER.
The LIFE iBATHWATER project aims to demonstrate how better technology and interoperability can reduce pollution levels in water bodies located near urban centres. Reduce the load of contaminants, including pathogens and organic matter, discharged in urban wastewater to counter the contamination of nearby water bodies;
Reactive barriers for water renaturalization during managed aquifer recharge in the Baix Camp region, Reference: LIFE20 ENV/ES/000284 | Acronym: LIFE REMAR.
The goal of the LIFE REMAR project is to demonstrate the viability of recharging an aquifer with treated wastewater using Managed Aquifer Recharge (MAR) technology. Monitoring of contaminants of emerging concern (CECs), pathogens, nitrogen and phosphorous, microplastics and antibiotic resistance genes (ARGs); The technology provides water of sufficient quality to meet standards for water reuse (EU Regulation 2020/741 for water reuse); Reduction of pollutants from the secondary WWTP effluent, especially emerging contaminants and pathogens (2.5 kg/yr), nitrogen (2,240 kg/yr), phosphorous (36 kg/yr), suspended solids (559 kg/yr) and ARGs;
Integrating circular economy and biodiversity in sustainable wastewater treatments based on constructed wetlands, Reference: LIFE19 ENV/ES/000197 | Acronym: LIFE RENATURWAT.
Improve the quality of WWTP effluents, reducing their concentration of nutrients, emerging pollutants, priority substances and pathogens. Demonstration that the proposed treatment significantly improves the environmental quality of WWTP effluent. Specifically, the following removal efficiencies, based on pilot plant studies, are expected: total phosphorus (>50%), ammonium (>60%), organic matter (>25%), emerging contaminants (20–50%), pathogens (>80%).
Innovative cost‐effective multibarrier treatments for reusing water for agricultural irrigation, Reference: LIFE19 ENV/ES/000278 | Acronym: LIFE PHOENIX.
New treatment will be applied in highly water‐stressed areas in central and southern Spain, and central Portugal. It will increase removal of organic matter, solids and pathogens using high‐efficiency settling, filtration, flotation and biological technologies.
Ensure water quality by online monitoring (e.g. toxins and pathogens) and offline analysis (e.g. eco‐toxicity and ARBs); Validation of indicator microorganisms and performance targets: (4) E. coli for pathogenic bacteria (5.0 log10 reduction), (5) coliphages for pathogenic viruses (6.0 log10 reduction), and (6) Clostridium perfringens spores for protozoa (5.0 log10 reduction);
INNOVATIVE COMBINATION OF WWT TECHNOLOGIES FOR WATER REUSE: ANAEROBIC‐AEROBIC, MICROALGAE AND AOP PROCESSES, Reference: LIFE18 ENV/ES/000170 | Acronym: LIFE AMIA.
LIFE AMIA aims to reuse wastewater in agricultural irrigation and aquifer recharge to protect the aquatic environment against pollution caused by pathogens and micropollutants not removed by conventional wastewater treatment plants (WWTPs).
Low energy treatment technology for leachate valorisation, Reference: LIFE15 ENV/ES/000530 | Acronym: LIFE LEACHLESS.
The LIFE LEACHLESS project aims to demonstrate the technical and economic feasibility of an innovative and cost‐efficient technology for leachate treatment, based on solar evaporation/condensation and forward osmosis. Expected results: A high‐quality final effluent obtained that is 100% free of pathogens and xenobiotic compounds and that can be reused or discharged into watercourses;
Validation of adsorbent materials and advanced oxidation techniques to remove emerging pollutants in treated wastewater, Reference: LIFE16 ENV/ES/000169 | Acronym: LIFE CLEAN UP.
LIFE CLEAN UP aims to validate an innovative, efficient and environmentally friendly system to remove EPs and other pathogens from wastewater. On its way out of the adsorption system, the project will test the treating of the water with an advanced oxidation process (AOP) involving light pulses, photocatalysis and photosensitisers to degrade pollutants and pathogens that were not previously retained.
Adding sustainability to the fruit and vegetable processing industry through solar‐powered algal wastewater treatment, Reference: LIFE16 ENV/ES/000180 | Acronym: LIFE ALGAECAN.
The LIFE ALGAECAN project will demonstrate the feasibility of applying solar‐powered algal treatment to the effluents generated by fruit and vegetable processing. Production of a high‐quality final effluent that is 100% free of pathogens and xenobiotic compounds;
Early detection and advanced management systems to reduce forest decline caused by invasive and pathogenic agents, Reference: LIFE14 ENV/ES/000179 | Acronym: LIFE HEALTHY FOREST.
The main aim of the LIFE HEALTHY FOREST project was to design, apply and monitor advanced methodologies for achieving more sustainable forestry management at an EU level. These methods would especially target forest decline caused by invasive and pathogenic agents, taking into account both environmental and socio‐economic impacts. Implement an early detection system in large‐scale demonstration plots to give a comprehensive overview of the status of forest health for informing EU policy. This system encompasses field monitoring and sampling, morphological, molecular and physiological techniques, and remote sensing. It will have a positive effect on biodiversity by intervening against invasive and pathogenic agents that are directly related to forest decline and mortality. A guide book would be produced to explain the system. The LIFE HEALTHY FOREST project developed of protocols for the early detection of decline agents. The protocols not only permit the detection of native agents but also have been designed to detect quarantined organisms introduced to Spain and elsewhere in Europe that represent a serious threat for European forests. They were validated on small‐scale plots and tested at larger plots. Furthermore, tests for the virulence of the pathogens were concluded both in field and in greenhouse conditions.
Synergic TPAD and O3 process in WWTPs for Resoruce Efficient waste management, Reference: LIFE14 ENV/ES/000150 | Acronym: LIFE STO3RE.
Demo that Dry anaerobic digestion technology offers improvement in effectiveness, cost‐effectiveness and sustainability over other methods for sludge treatment in small to medium sized WWTPs. expected reduction of 50–99% in pathogen content in sludge.
LIFE15 NAT/ES/000757 ‐ LIFE Tritó Montseny.
There is one action (D3) for the surveillance of infectious illnesses transmitted by amphibians that could have a negative impact on the wild population of the targeted species (Calotriton arnoldi), in order to develop an early response if necessary.
Suggested citation: EFSA (European Food Safety Authority) , Berezowski J, De Balogh K, Dórea FC, Ruegg S, Broglia A, Zancanaro G and Gervelmeyer A, 2023. Scientific Report on the coordinated surveillance system under the One Health approach for cross‐border pathogens that threaten the Union – options for sustainable surveillance strategies for priority pathogens. EFSA Journal 2023;21(3):7882, 39 pp. 10.2903/j.efsa.2023.7882
Requestor European Commission
Question number FSA‐Q‐2022‐00294
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements EFSA wishes to thank the following for the support provided to this scientific output: Marzia GNOCCHI, Celine GOSSNER, Marieta BRAKS, Joaquin VICENTE BAÑOS, Ezio FERROGLIO and Dolores GAVIER‐WIDEN.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 31 January 2023
Notes
Note: the RiBESS tool refers to this parameter as ‘Target confidence of freedom’ as Freedom From Disease was the original context in which it was developed. In a surveillance context, this is the sensitivity of the surveillance activity.
References
- Aenishaenslin C, Häsler B, Ravel A, Parmley EJ, Mediouni S, Bennani H, Stärk KDC and Buckeridge DL, 2021. Evaluating the integration of One Health in surveillance systems for antimicrobial use and resistance: a conceptual framework. Frontiers in Veterinary Science, 8, 611931. 10.3389/fvets.2021.611931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier M, Uea‐Anuwong T, Binot A, Hendrikx P and Goutard FL, 2020. Characteristics of One Health surveillance systems: a systematic literature review. Preventive Veterinary Medicine, 181, 104560. 10.1016/j.prevetmed.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Braks M, Medlock JM, Hubalek Z, Hjertqvist M, Perrin Y, Lancelot R, Ducheyne E, Hendricks G, Stroo A, Heyman P and Sprong H, 2014. Vector‐borne disease intelligence: strategies to deal with disease burden and threats. Frontiers in Public Health, 2, 280 pp. 10.3389/fpubh.2014.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks M, Schaffner F, Medlock JM, Berriatua E, Balenghien T, Mihalca AG, Hendrickx G, Marsboom C, Van Bortel W, Smallegange RC, Sprong H, Gossner CM, Czwienczek E, Dhollander S, Briët O and Wint W, 2022. VectorNet: Putting vectors on the map. Frontiers in Public Health, 10, 809763. 10.3389/fpubh.2022.809763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin S, McGillycuddy C, Carvelli A, Dorea F, Mantero J, Muellner P, Muellner U, Weaver J and Dhingra M, 2022. Characterizing the risk landscape (riskscape) for global one health intelligence. 7th World One Health Congress, Singapore, 7–11 November 2022. Available online: https://drive.google.com/file/d/1wu3GyK1unKNj45Cs0zwWC7E_93Ht7Kze/view
- Cameron AR, Meyer A, Faverjon C and Mackenzie C, 2020. Quantification of the sensitivity of early detection surveillance. Transboundary and Emerging Diseases, 67(6), 2532–2543. 10.1111/tbed.13598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati M, 2023. Surveillance plan proposal for early detection of zoonotic pathogens in Equidae (horses, donkeys). EFSA supporting publication 2023:EN‐7854, 52 pp. 10.2903/sp.efsa.2023.EN-7854 [DOI]
- ECDC (European Centre for Disease Prevention and Control) , 2014. Guidelines for the surveillance of native mosquitoes in Europe. Stockholm: ECDC; 2014. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/surveillance-of%20native-mosquitoes%20-guidelines.pdf
- ECDC (European Centre for Disease Prevention and Control) , 2021. Organisation of vector surveillance and control in Europe. Stockholm: ECDC; 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Organisation-vector-surveillance-control-Europe_0.pdf
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority) , 2018. Field sampling methods for mosquitoes, sandflies, biting midges and ticks – VectorNet project 2014–2018. Stockholm and Parma: ECDC and EFSA; 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Vector-sampling-field-protocol-2018.pdf
- EcoHealth Alliance , 2022. Disease X – The next Pandemic. Available online: https://www.ecohealthalliance.org/2018/03/disease-x
- EFSA (European Food Safety Authority) ; Scientific and technical assistance on Echinococcus multilocularis infection in animals. EFSA Journal 2012;10(11):2973, 22 pp. 10.2903/j.efsa.2012.2973 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Lázaro E, Parnell S, Vicent Civera A, Schans J, Schenk M, Cortiñas Abrahantes J, Zancanaro G and Vos S, 2020. General guidelines for statistically sound and risk‐based surveys of plant pests. EFSA supporting publication 2020:EN‐1919, 65 pp. 10.2903/sp.efsa.2020.EN-1919 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Berezowski J, De Balogh K, Dórea F, Rüegg S, Suk J, Broglia A, Gervelmeyer A and Kohnle L, 2023. Prioritisation of zoonotic diseases for coordinated surveillance systems under the One Health approach for cross‐border pathogens that threaten the Union. EFSA Journal 2023;21(3):7853, 54 pp. 10.2903/j.efsa.2023.7853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENETWILD‐consortium , Zanet Stefania, Vada Rachele, Gavier‐Widen Dolores, Gonçalves Catarina, Vicente Joaquín, Ferroglio Ezio, 2022a. Literature review on worldwide surveillance systems targeting transboundary zoonotic and emerging diseases within the holistic One‐Health perspective. EFSA supporting publication 2022:EN‐7767, 61 pp. 10.2903/sp.efsa.2022.EN-7767 [DOI] [Google Scholar]
- ENETWILD‐consortium , Alves PC, Gavier‐Widen D, Ferroglio E, Queirós J, Rafael M, Santos N, Silva T, Gonçalves C, Vada R, Zanet S, Smith G, Gethöffer F, Keuling O, Staubach C, Sauter‐Louis C, Blanco JA, Podgorski T, Larska M, Richomme C, Knauf S, Rijks JM, Pasetto C, Benatti F, Poncina M, Gómez A, Dups‐Bergmann J, Neimanis A and Vicente J, 2022b. Literature review on the main existing structures and systematic/academic initiatives for surveillance in the EU for zoonoses in the environment and the methods for surveillance of pathogens in the environment. EFSA supporting publication 2022:EN‐7792, 115 pp. 10.2903/sp.efsa.2022.EN-7792 [DOI] [Google Scholar]
- ENETWILD‐consortium , Ferroglio E, Gavier‐Widen D, Gonçalves C, Vada R, Zanet S, Smith G, Gethöffer F, Keuling O, Staubach C, Sauter‐Louis C, Blanco JA, Podgorski T, Larska M, Richomme C, Knauf S, Jolianne M, Rijks JM, Gómez A, Alves PC, Queirós J, Rafael M, Santos N, Silva T, Dups‐Bergmann J, Neimanis A and Vicente J, 2022c. Describing and mapping of the main existing structures and systematic initiatives and academic activities for surveillance in the EU for zoonoses (transboundary, emerging and re‐emerging) in domestic animals and wildlife. EFSA supporting publication 2022:EN‐7795, 116 pp. 10.2903/sp.efsa.2022.EN-7795 [DOI] [Google Scholar]
- ENETWILD‐consortium , Klaas M, Sebastian M, Ferroglio E, Goncalves C, Vada R, Zanet S, Gavier‐Widén D and Vicente J., 2022d. A review of endangered wildlife hosts in Europe for selected pathogens to be targeted by One Health surveillance. EFSA Supporting publication 2022:EN‐7766, 12 pp. 10.2903/sp.efsa.2022.EN-7766 [DOI] [Google Scholar]
- ENETWILD‐consortium , Gavier‐Widen D, Ferroglio E, Smith G, Gonçalves C, Vada R, Zanet S, Gethöffer F, Keuling O, Staubach C, Sauter‐Louis C, Blanco JA, Fernández de Mera IG, Podgorski T, Larska M, Richomme C, Knauf S, Rijks JM, Gómez A, Alves PV, Santos N, Queirós J, Dups‐Bergmann J, Neimanis A and Vicente J, 2023. Formulating recommendations/technical specifications for sustainable surveillance of zoonotic pathogens where wildlife is implicated. EFSA supporting publication 2023:EN‐7812, 111 pp. 10.2903/sp.efsa.2023.EN-7812 [DOI] [Google Scholar]
- Filter M, Buschhardt T, Dórea F, de Abechuco EL, Günther T, Sundermann EM, Gethmann J, Dups‐Bergmann J, Lagesen K and Ellis‐Iversen J, 2021. One Health Surveillance Codex: promoting the adoption of One Health solutions within and across European countries. One Health, 12, 100233. 10.1016/j.onehlt.2021.100233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitziger M, Esposito R, Canali M, Aragrande M, Häsler B and Rüegg SR, 2018. Knowledge integration in One Health policy formulation, implementation and evaluation. Bulletin of the World Health Organization, 96, 211–218. 10.2471/BLT.17.202705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoinville LJ, Alban L, Drewe JA, Gibbens JC, Gustafson L, Häsler B, Salman M and Stärk KDC, 2013. Proposed terms and concepts for describing and evaluating animal‐health surveillance systems. Preventive Veterinary Medicine, 112, 1–12. 10.1016/j.prevetmed.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Nigsch A, 2023. Surveillance plan proposal for early detection of zoonotic pathogens in pigs and poultry. EFSA supporting publication 2023:EN‐7855, 32 pp. 10.2903/sp.efsa.2023.EN-7855 [DOI] [Google Scholar]
- One Health High‐Level Expert Panel (OHHLEP) , Adisasmito WB, Almuhairi S, Behravesh CB, Bilivogui P, Bukachi SA, Casas N, Cediel Becerra N, Charron DF, Chaudhary A, Ciacci Zanella JR, Cunningham AA, Dar O, Debnath N, Dungu B, Farag E, Gao GF, Hayman DTS, Khaitsa M, Koopmans MGP, Machalaba C, Mackenzie JS, Markotter W, Mettenleiter TC, Morand S, Smolenskiy V and Zhou L, 2022. One Health: A new definition for a sustainable and healthy future. PLoS Pathog, 18(6), e1010537. 10.1371/journal.ppat.1010537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach G, Cunha Silva L and Buzzell‐Hatay A, 2023. Surveillance plan proposal for early detection of zoonotic pathogens in ruminants. EFSA supporting publication 2023:EN‐7887 (in press). [Google Scholar]
- Shiroyama H, Yarime M, Matsuo M, Schroeder H, Scholz R and Ulrich AE, 2012. Governance for sustainability: knowledge integration and multi‐actor dimensions in risk management. Sustainability Science, 7, 45–55. 10.1007/s11625-011-0155-z [DOI] [Google Scholar]
- Springborn MR, Weill JA, Lips KR, Ibáñez R and Ghosh A, 2022. Amphibian collapses increased malaria incidence in Central America. Environmental Research Letters, 17(10), 104012. 10.1088/1748-9326/ac8e1d [DOI] [Google Scholar]
- Taylor LH, Latham SM and Woolhouse ME, 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 356(1411), 983–989. 10.1098/rstb.2001.0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrestrial animal code of the World Organisation for Animal Health (WOAH, 2022). Available at: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/
- World Health Organisation (WHO) , 2022. WHO to identify pathogens that could cause future outbreaks and pandemics. Available online: https://www.who.int/news/item/21-11-2022-who-to-identify-pathogens-that-could-cause-future-outbreaks-and-pandemics
- World Health Organisation (WHO), World Organisation for Animal Health (WOAH) and Food and Agricultural Organisation (FAO) , 2019. Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. Available online: https://www.fao.org/3/ca2942en/CA2942EN.pdf
- World Health Organisation (WHO), World Organisation for Animal Health (WOAH) and Food and Agricultural Organisation (FAO) , 2020. Joint Risk Assessment Operational Tool (JRA OT): An Operational Tool of the Tripartite Zoonoses Guide – Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. Geneva. Available online: https://www.fao.org/documents/card/en/c/cb1520en/
- World Health Organisation (WHO), World Organisation for Animal Health (WOAH) and Food and Agricultural Organisation (FAO) , 2022a. Surveillance and Information Sharing Operational Tool ‐ An operational tool of the Tripartite Zoonoses Guide. Geneva, Rome and Paris. Available online: https://www.fao.org/3/cc0716en/cc0716en.pdf
- World Health Organisation (WHO), World Organisation for Animal Health (WOAH) and Food and Agricultural Organisation (FAO) , 2022b. Multisectoral coordination mechanisms operational tool: an operational tool of the tripartite zoonoses guide. Available online: https://www.who.int/publications/i/item/9789240053236


