Abstract
Objective:
To examine whether demographic and cancer-related characteristics and factors such as fertility discussion with a medical provider and fertility preservation use are associated with attempting pregnancy after adolescent and young adult cancer.
Design:
Cross-sectional online survey
Subjects:
Women with lymphoma, breast cancer, thyroid cancer, or gynecologic cancer diagnosed at ages 15–39 years during 2004–2016 were identified from the North Carolina Cancer Registry and the Kaiser Permanente Northern and Southern California healthcare systems and responded to an online survey addressing survivorship concerns, including fertility and reproductive outcomes.
Exposures:
Demographic characteristics, cancer characteristics, fertility discussion with a medical provider or fertility specialist between cancer diagnosis and starting cancer treatment, use of fertility preservation strategies (freezing embryos or oocytes) after cancer diagnosis
Main outcome measures:
Pregnancy attempt after cancer diagnosis, defined by either a pregnancy or 12 months of trying to become pregnant without pregnancy
Results:
Among 801 participants who had not reached their desired family size at diagnosis, 77% had a fertility discussion with any medical provider between cancer diagnosis and starting treatment and 8% used fertility preservation after cancer diagnosis. At survey (median=7 years post-diagnosis; IQR=4–10), 32% had attempted pregnancy. Neither fertility discussion with any medical provider nor fertility counseling with a fertility specialist was significantly associated with pregnancy attempts. However, use of fertility preservation was significantly associated with attempting pregnancy (PR=1.74; 95% CI: 1.312.32). Other characteristics positively associated with pregnancy attempts included younger age at diagnosis, longer time since diagnosis, having a partner (at diagnosis or at survey), and having a history of infertility prior to cancer diagnosis.
Conclusion:
Use of fertility preservation strategies was uncommon in our cohort but was associated with attempting pregnancy after cancer. Ensuring access to fertility preservation methods may help adolescent and young adult cancer survivors to plan and initiate future fertility.
Keywords: Cancer survivors, adolescents and young adults, pregnancy attempts
Introduction
In the United States, estimated new cancer diagnoses among adolescents and young adults (AYAs, ages 15–39 years) exceeded 88,000 in 2021, with over half of these occurring among AYA women.(1, 2) Five-year survival for this age group has increased in recent decades and currently stands at 85%, meaning most AYAs with cancer will go on to become long-term survivors.(1) For AYA women, a cancer diagnosis may coincide with critical life decisions regarding future pregnancy and desired family size. These decisions, complex for any young adult, may be further complicated by exposure to cancer therapies and the psychological impacts of a cancer diagnosis and the possibility of recurrence.
The American Society of Clinical Oncology (ASCO) recommends that cancer patients of reproductive age receive counseling from healthcare providers to discuss the potential impacts of cancer treatments on future fertility and options for fertility preservation (e.g., freezing of oocytes or embryos).(3–5) The American Society for Reproductive Medicine (ASRM) has published similar recommendations for younger patients receiving gonadotoxic cancer treatment.(6–8) As these recommendations have gained widespread recognition, a growing number of studies have reported on rates of receipt of fertility counseling and use of fertility preservation strategies among young women with cancer.(9–15) Likewise, several population-based studies have reported rates of live births among cancer survivors and how these differ from those of the general population.(16, 17) However, little research has focused on how often AYAs attempt pregnancy after cancer, or whether fertility counseling or fertility preservation use impact the likelihood of a future pregnancy attempt, as this information is seldom available in studies with sufficient sample size or follow up to investigate these associations. Fertility-related care may allow survivors to be better informed about their fertility status and therefore influence whether they attempt pregnancy after cancer. An understanding of the factors associated with pregnancy attempts in this population may help healthcare providers to better support AYA cancer survivors and their reproductive goals.
In this study, we investigated post-diagnosis pregnancy attempts among AYA cancer survivors who had not yet reached their desired family size at the time of cancer diagnosis. The goal of our analysis was to identify factors associated with attempting pregnancy after cancer to increase the evidence base for understanding reproductive patterns in the AYA oncology population.
Materials and methods
Study design and participants
These analyses used data from the AYA Horizon Study, a cohort study focused on reproductive outcomes for young women diagnosed with cancer. The parent study and ancillary online survey have been described in detail previously.(18) Women with an incident diagnosis of breast cancer, thyroid cancer, melanoma, lymphoma (Hodgkin lymphoma or non-Hodgkin lymphoma) or gynecologic cancer (ovarian, cervical, or uterine cancers) at ages 15–39 years during 2004–2016 were identified using data from the North Carolina (NC) Central Cancer Registry and the Kaiser Permanente Northern California and Southern California (KPNC and KPSC) Cancer Registries. The AYA Site recodes ICD-O-3/WHO definition was used to define cancer types.(19) Both in situ and invasive breast cancers were included; only invasive cancer diagnoses were included for other cancer types. This research was approved by Institutional Review Boards at the University of North Carolina, KPNC, and KPSC.
Between September 2018 and November 2019, eligible women who were alive and 18 years or older were mailed letters inviting them to participate in an online survey about a broad range of survivorship topics. The 130-item survey was developed by a collaboration of oncologists, epidemiologists, psychiatrists, and other experts and has been previously published in full.(20) Of the 13,132 eligible individuals who were mailed letters inviting survey participation, a total of 1,679 completed the online survey (participation rate=12.8%). Detailed information on the characteristics of respondents and the overall invited sample have been described elsewhere.(18) In brief, survey respondents were more likely than the overall invited sample to be White (vs. Black or Asian) and non-Hispanic (vs Hispanic) and less likely to have gynecologic cancer (vs other cancer types) and to have received surgery alone (vs any chemotherapy). For the current analysis, we excluded survey respondents who reported a history of hysterectomy, tubal ligation, or bilateral oophorectomy prior to cancer diagnosis (N=246) or were missing information on these characteristics (N=5);those missing information on post-diagnosis pregnancy attempts (N=13); and those with melanoma (N=151), who would be unlikely to be exposed to gonadotoxic therapies. Finally, we excluded those who indicated that they had already reached their desired family size at the time of their cancer diagnosis (N=460) and those with missing responses on this item (N=3). The analyses presented here thus include a total of 801 women who either had not reached their desired family size at diagnosis (N=666) or were unsure (N=135).
Outcome
The primary outcome of interest was pregnancy attempts after cancer diagnosis. In the online survey, women were asked whether they experienced infertility (12 months of trying to become pregnant without pregnancy) after cancer diagnosis, and to list all pregnancies and pregnancy outcomes (currently pregnant, live birth, miscarriage, termination or other) and their dates. Women were considered to have a post-diagnosis pregnancy attempt if they reported either infertility or any pregnancy (regardless of pregnancy outcome) beginning after their cancer diagnosis.
Covariates
Information on cancer type, stage, and age at diagnosis came from the NC Central Cancer Registry or the KPNC/KPSC Cancer Registries. Demographic and cancer treatment characteristics were self-reported in the online survey or obtained from the cancer registries. Number of live births and infertility prior to cancer diagnosis were obtained from survey responses. Participants were asked whether they discussed their future fertility with any medical provider between cancer diagnosis and the start of cancer treatment. In a separate question, they were asked whether they saw a fertility specialist during this time window. These were treated as separate exposures in analyses to distinguish the impact of care focused specifically on fertility from possibly brief discussions about fertility that may have occurred with a non-fertility specialist during the course of cancer care. Fertility-specific care could provide survivors with more accurate information on their fertility status, and therefore could be more likely to influence future pregnancy attempts. Participants also reported whether they froze embryos and/or oocytes for fertility preservation.
Statistical analysis
Modified Poisson regression models with robust error variance were used to estimate prevalence ratios (PR) and 95% confidence intervals (CI) for pregnancy attempts. Multivariable models were adjusted for variables selected a priori as those we expected to be most strongly associated with pregnancy attempts (age at diagnosis, time from diagnosis to survey, partnered status at diagnosis, and number of live births before cancer diagnosis), as well as any other variables significantly associated with pregnancy attempts in unadjusted models. All participants reported that they had not gone through menopause prior to their cancer diagnosis. However, 125 women (N=41 gynecologic cancer, 76 breast cancer, 8 lymphoma) reported an age at menopause that was within 2 years of their age at diagnosis. We performed sensitivity analyses excluding these participants. All analyses were performed with SAS, version 9.4 (Cary, NC, USA).
Results
Participant characteristics are shown in Table 1. The median ages at cancer diagnosis and survey were 32 years (IQR: 27, 36) and 39 years (IQR: 34, 43), respectively. Overall, 60% were at least 6 years out from diagnosis at the time of survey. The majority were white (76%), non-Hispanic (833%), and had health insurance at the time of diagnosis (97%). More than half of participants (59%) had 0 live births before cancer diagnosis, and 15% reported prior infertility. The most common cancer diagnoses were breast (39%) and thyroid (30%),and about half had localized stage disease (49%). Initial cancer treatments included any chemotherapy (51%), radiation without chemotherapy (27%), and surgery only (21%).
Table 1.
Characteristics of Female AYA Cancer Survivors identified in North Carolina and California (n=801)
| Na | % | |
|---|---|---|
|
| ||
| Demographic characteristics | ||
| Study site | ||
| North Carolina Cancer Registry | 426 | 53% |
| Kaiser Permanente Northern California | 179 | 22% |
| Kaiser Permanente Southern California | 196 | 24% |
| Age at survey, median (IQR) | 39 | (34, 43) |
| Age at diagnosis in years | ||
| 15–24 | 121 | 15% |
| 25–29 | 167 | 21% |
| 30–34 | 237 | 30% |
| 35–39 | 276 | 34% |
| Median (IQR) | 32 | (27, 36) |
| Years Since Diagnosis | ||
| <6 | 320 | 40% |
| 6–<10 | 257 | 32% |
| 10+ | 224 | 28% |
| Median (IQR) | 7 | (4, 10) |
| Race | ||
| White | 577 | 76% |
| Black | 41 | 5% |
| Asian | 51 | 7% |
| Other | 88 | 12% |
| Ethnicity | ||
| Non-Hispanic | 655 | 83% |
| Hispanic | 136 | 17% |
| Education Level | ||
| < Some College | 259 | 33% |
| College | 299 | 38% |
| Post Grad | 233 | 29% |
| Partnered at Diagnosis | ||
| Not Partnered | 299 | 37% |
| Partnered | 501 | 63% |
| Partnered at Survey | ||
| Not Partnered | 233 | 29% |
| Partnered | 557 | 71% |
| Health insurance at diagnosis | ||
| No | 26 | 3% |
| Yes | 775 | 97% |
| Reproductive characteristics | ||
| Live births before diagnosis | ||
| None | 476 | 59% |
| One | 189 | 24% |
| More than one | 136 | 17% |
| Infertility Prior to Diagnosis | ||
| No Prior Infertility | 682 | 85% |
| Prior Infertility | 118 | 15% |
| Cancer characteristics | ||
| Cancer Type | ||
| Thyroid cancer | 243 | 30% |
| Gynecologic (cervical, endometrial, ovarian) | 117 | 15% |
| Breast cancer | 313 | 39% |
| Lymphoma | 128 | 16% |
| Cancer Stage | ||
| In situ (Breast only) | 26 | 3% |
| Localized | 367 | 49% |
| Regional | 314 | 42% |
| Distant | 41 | 5% |
| Unstaged | 6 | 1% |
| Initial treatment | ||
| Surgery Only | 161 | 21% |
| Radiation (No Chemo) | 203 | 27% |
| Chemotherapy | 383 | 51% |
| No recorded treatment | 11 | 1% |
Ns may not sum to the total due to missing responses
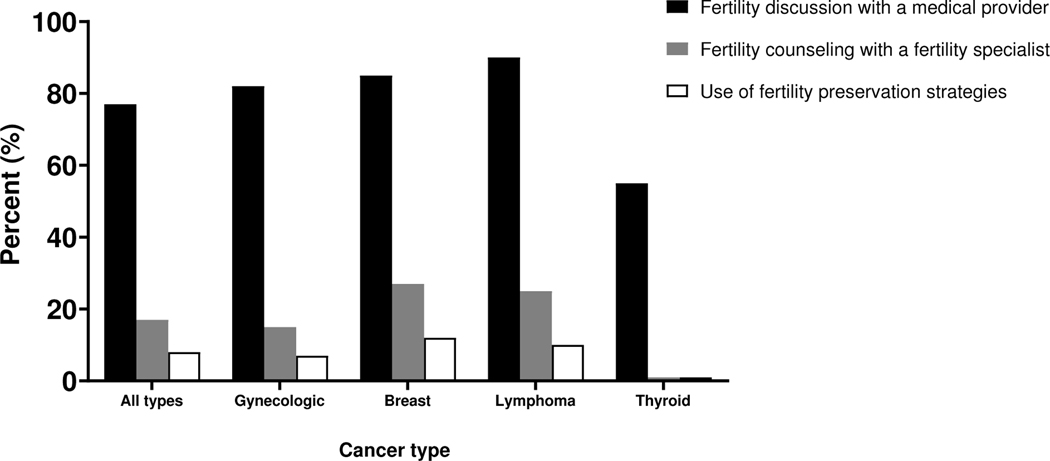
Overall, 77% of women discussed future fertility with a medical provider between cancer diagnosis and starting cancer treatment (Figure 1). Across cancer types, this proportion ranged from 55% for thyroid cancer to 90% for lymphoma. In total, 17% reported fertility counseling from a fertility specialist, and 8% completed fertility preservation with either embryo or oocyte cryopreservation. Receipt of fertility counseling with a fertility specialist ranged from 1% for thyroid cancer to 27% for breast cancer, while use of fertility preservation strategies ranged from 1% for thyroid cancer to 12% for breast cancer.
Figure 1.
Fertility discussion, fertility counseling, and use of fertility preservation strategies, overall and by cancer type
A total of 259 women (32%) in the overall sample reported at least one pregnancy attempt after cancer diagnosis, with proportions ranging from 21% among women with gynecologic cancers to 49% among women with lymphoma (Table 2). While only 21% of women <6 years from diagnosis at survey had attempted pregnancy, 37% and 42% of women 6-<10 years and 10+ years from diagnosis, respectively, had done so. Outcomes of the first post-diagnosis pregnancy attempt included live birth (53%), miscarriage (22%), infertility only (14%), pregnancy termination (7%), current pregnancy (3%), and stillbirth or ectopic pregnancy (0.4%). Among the 30 women who used fertility preservation strategies and had at least one post-diagnosis pregnancy attempt, 28 (93%) reported at least one post-diagnosis pregnancy, and 9 (30%) reported at least one post-diagnosis pregnancy conceived with fertility treatments.
Table 2.
Associations between participant characteristics and post-diagnosis pregnancy attempts
| No pregnancy attempt (N=542) | Pregnancy attempt (N=259) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Na | % | Na | % | PR (95% CI) | PR (95% CI)b | |
|
| ||||||
| Demographic characteristics | ||||||
| Study site | ||||||
| North Carolina Cancer Registry | 290 | 54% | 136 | 53% | 1 | 1 |
| Kaiser Permanente Northern California | 116 | 21% | 63 | 24% | 1.10 (0.87, 1.40) | 1.07 (0.85, 1.33) |
| Kaiser Permanente Southern California | 136 | 25% | 60 | 23% | 0.96 (0.75, 1.23) | 0.99 (0.79, 1.25) |
| Age at diagnosis in years | ||||||
| 15–24 | 76 | 14% | 45 | 17% | 1 | 1 |
| 25–29 | 88 | 16% | 79 | 31% | 1.27 (0.96, 1.69) | 1.28 (0.99, 1.67) |
| 30–34 | 159 | 29% | 78 | 30% | 0.89 (0.66, 1.19) | 0.88 (0.64, 1.20) |
| 35–39 | 219 | 40% | 57 | 22% | 0.56 (0.40, 0.77) | 0.59 (0.41, 0.86) |
| Years Since Diagnosis | ||||||
| <6 | 252 | 46% | 68 | 26% | 1 | 1 |
| 6–<10 | 161 | 30% | 96 | 37% | 1.76 (1.35, 2.29) | 1.56 (1.22, 2.00) |
| 10+ | 129 | 24% | 95 | 37% | 2.00 (1.54, 2.59) | 1.73 (1.35, 2.21) |
| Race | ||||||
| White | 383 | 75% | 194 | 79% | 1 | 1 |
| Black | 29 | 6% | 12 | 5% | 0.70 (0.42, 1.16) | 0.96 (0.62, 1.49) |
| Asian | 39 | 8% | 12 | 5% | 0.87 (0.53, 1.42) | 1.44 (0.89, 2.33) |
| Other | 60 | 12% | 28 | 11% | 0.95 (0.68, 1.31) | 1.04 (0.77, 1.39) |
| Ethnicity | ||||||
| Non-Hispanic | 451 | 84% | 204 | 80% | 1 | 1 |
| Hispanic | 85 | 16% | 51 | 20% | 1.20 (0.94, 1.54) | 1.14 (0.92, 1.43) |
| Education Level | ||||||
| < Some College | 171 | 32% | 88 | 35% | 1 | 1 |
| College | 206 | 38% | 93 | 36% | 0.92 (0.72, 1.16) | 1.00 (0.80, 1.25) |
| Post Grad | 159 | 30% | 74 | 29% | 0.93 (0.73, 1.20) | 0.98 (0.78, 1.24) |
| Partnered at Diagnosis | ||||||
| Not Partnered | 235 | 43% | 64 | 25% | 1 | 1 |
| Partnered | 306 | 57% | 195 | 75% | 1.82 (1.43, 2.32) | 1.62 (1.23, 2.12) |
| Partnered at Survey | ||||||
| Not Partnered | 200 | 37% | 33 | 13% | 1 | 1 |
| Partnered | 335 | 63% | 222 | 87% | 2.81 (2.02, 3.92) | 2.14 (1.48, 3.10) |
| Health insurance at diagnosis | ||||||
| No | 14 | 3% | 12 | 5% | 1 | 1 |
| Yes | 528 | 97% | 247 | 95% | 0.69 (0.45, 1.06) | 0.80 (0.55, 1.15) |
| Reproductive characteristics | ||||||
| Live births before diagnosis | ||||||
| None | 324 | 60% | 152 | 59% | 1 | 1 |
| One | 121 | 22% | 68 | 26% | 0.90 (0.67, 1.21) | 0.73 (0.55, 0.98) |
| More than one | 97 | 18% | 39 | 15% | 1.13 (0.89, 1.42) | 0.97 (0.77, 1.22) |
| Infertility Prior to Diagnosis | ||||||
| No Prior Infertility | 471 | 87% | 211 | 82% | 1 | 1 |
| Prior Infertility | 71 | 13% | 47 | 18% | 1.29 (1.00, 1.65) | 1.42 (1.10, 1.83) |
| Cancer characteristics | ||||||
| Cancer Type | ||||||
| Thyroid cancer | 143 | 26% | 100 | 39% | 1 | 1 |
| Gynecologic (cervical, endometrial, ovarian) | 92 | 17% | 25 | 10% | 0.52 (0.36, 0.76) | 0.46 (0.32, 0.67) |
| Breast cancer | 242 | 45% | 71 | 27% | 0.55 (0.43, 0.71) | 0.60 (0.46, 0.77) |
| Lymphoma | 65 | 12% | 63 | 24% | 1.20 (0.95, 1.51) | 1.16 (0.94, 1.43) |
| Cancer Stage c | ||||||
| In situ (breast only) | 22 | 4% | 4 | 2% | 0.48 (0.19, 1.20) | NC |
| Localized | 250 | 49% | 117 | 49% | 1 | 1 |
| Regional | 218 | 42% | 96 | 40% | 0.96 (0.77, 1.20) | 0.90 (0.73, 1.11) |
| Distant | 20 | 4% | 21 | 9% | 1.61 (1.15, 2.24) | 1.38 (1.01, 1.88) |
| Unstaged | 3 | 1% | 3 | 1% | 1.57 (0.69, 3.54) | NC |
| Initial treatment c | ||||||
| Surgery Only | 116 | 23% | 45 | 19% | 1 | 1 |
| Radiation (no chemo) | 127 | 25% | 76 | 31% | 1.34 (0.99, 1.82) | 1.35 (0.99, 1.81) |
| Chemotherapy | 266 | 52% | 117 | 48% | 1.09 (0.82, 1.46) | 1.19 (0.90, 1.56) |
| No recorded treatment | 6 | 1% | 5 | 2% | 1.63 (0.81, 3.25) | NC |
NC, not calculated due to small sample
Ns may not sum to the total due to missing responses
Models include the listed variable of interest as well as the following covariates: age at diagnosis, time since diagnosis, partnered at diagnosis, partnered at survey, prior infertility, number of livebirths before cancer diagnosis, and cancer type
Models include the listed variable of interest as well as the following covariates: age at diagnosis, time since diagnosis, partnered at diagnosis, partnered at survey, prior infertility, number of livebirths before cancer diagnosis; not adjusted for cancer type due to nonoverlapping distributions of listed variable of interest and cancer type
Associations between demographic, cancer, and reproductive characteristics and pregnancy attempts are shown in Table 2. In multivariable-adjusted models, women who were aged 35–39 (PR=0.59; 95% CI: 0.41, 0.86) years at diagnosis were less likely to attempt pregnancy than those aged 15–24 years. Women were more likely to have attempted pregnancy if they were further from diagnosis at the survey (6-<10 years vs <6 years: PR=1.56, 95% CI: 1.22, 2.00; 10+ years vs <6 years: PR=1.73, 95% CI: 1.35, 2.21). Having a partner at diagnosis (PR=1.62; 95% CI: 1.23, 2.12) or survey (PR=2.14; 95% CI: 1.48, 3.10) was also predictive of pregnancy attempts, as was prior infertility (PR=1.42; 95% CI: 1.10, 1.83). Across cancer characteristics, cancer type was significantly associated with pregnancy attempts, with survivors of gynecologic cancer (PR=0.46; 95% CI: 0.32, 0.67) and breast cancer (PR=0.60; 95% CI: 0.46, 0.77) less likely to attempt pregnancy than thyroid cancer survivors. Women with distant stage disease appeared to be more likely to have a pregnancy attempt compared to localized stage (PR=1.38; 95% CI: 1.01, 1.88), though there were few women in this group. Those with regional stage did not differ significantly from localized stage in likelihood of pregnancy attempts. Study site, race, ethnicity, education, health insurance at diagnosis, number of live births before diagnosis, and initial treatment were not strongly associated with pregnancy attempts.
Overall, neither fertility discussions with any medical provider nor fertility counseling with a fertility specialist between cancer diagnosis and starting treatment was significantly associated with attempting pregnancy (Table 3). However, women who used fertility preservation strategies were more likely to attempt pregnancy (PR=1.74; 95% CI: 1.31, 2.32).
Table 3.
Associations between fertility discussion, fertility counseling, and fertility preservation use and pregnancy attempts
| No pregnancy attempt (N=542) | Pregnancy attempt (N=259) | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Na | % | Na | % | PR (95% CI) | PR (95% CI)b | |
|
| ||||||
| Fertility discussion with a medical provider | ||||||
| No | 130 | 24% | 58 | 22% | 1 | 1 |
| Yes | 411 | 76% | 201 | 78% | 1.06 (0.84, 1.36) | 1.02 (0.81, 1.28) |
| Fertility counseling by a fertility specialist | ||||||
| No | 456 | 84% | 208 | 80% | 1 | 1 |
| Yes | 86 | 16% | 51 | 20% | 1.19 (0.93, 1.52) | 1.25 (0.98, 1.61) |
| Use of fertility preservation strategies | ||||||
| No | 511 | 94% | 229 | 88% | 1 | 1 |
| Yes | 31 | 6% | 30 | 12% | 1.59 (1.20, 2.10) | 1.74 (1.31, 2.32) |
Ns may not sum to the total due to missing responses
Models include the listed variable of interest as well as the following covariates: age at diagnosis, time since diagnosis, partnered at diagnosis, partnered at survey, prior infertility, number of livebirths before cancer diagnosis, and cancer type
Prevalence ratio estimates in sensitivity analyses excluding women with an age at menopause within 2 years of age at diagnosis were similar to those in primary analyses (data not shown).
Discussion
In this cohort of women with a history of AYA cancer who had not yet reached their desired family size at diagnosis, more than three-quarters of women reported that they had discussed fertility with any medical provider before starting their cancer treatment. However, receipt of fertility counseling (with any medical provider or with a fertility specialist) was not significantly associated with attempting pregnancy after cancer. In contrast, the use of fertility preservation strategies (embryo or oocyte freezing) was a significant predictor of post-cancer pregnancy attempts. Other characteristics that were positively associated with attempting pregnancy among survivors in our cohort included younger age at diagnosis, longer time since diagnosis, having a partner at diagnosis, and having a history of infertility.
It is encouraging that over 75% of our overall sample, and 80–90% of women with breast and gynecologic cancers and lymphoma, reported discussing future fertility with a medical provider after their cancer diagnosis and before initiating cancer treatment. Yet across cancer types, fewer than one-third reported conversations with a fertility specialist, and very few reported use of embryo or oocyte cryopreservation for preserving fertility. These results are similar to other reports documenting low rates of fertility preservation use after an AYA cancer diagnosis.(11, 14, 15) For example, among 176 women diagnosed with AYA cancer who participated in the AYA HOPE study, only 6.8% made arrangements for fertility preservation.(15) Our findings and those of others may reflect inconsistent access to fertility specialists and/or the high cost of fertility preservation methods for those without insurance coverage for these services. While the decision to preserve fertility is undoubtedly a complex and personal one, our findings highlight the importance of ensuring access to fertility specialists and embryo/oocyte cryopreservation methods for women who may wish to have children after cancer treatment.
Few studies have investigated the likelihood of pregnancy attempts among young women with a cancer history. Among 251 women with a cancer diagnosis (any type) at ages <45 years in the Fertility Information Research Study (FIRST), 21% had attempted pregnancy at a median of 2.4 years post-diagnosis.(11) Women in our cohort were, on average, older and further out from their diagnosis at the time of survey than those in the FIRST cohort. We also restricted our analyses to women who indicated that they had not yet reached their desired family size at diagnosis, and still just 36% had attempted pregnancy by a median of 7 years post-diagnosis. Even among those women who were 10+ years from diagnosis, fewer than half reported a pregnancy attempt after their cancer. It is possible that some women changed their mind in the intervening years, or still planned to attempt pregnancy at the time of our survey. Nevertheless, our results suggest a need for research to identify potential barriers to pregnancy among AYA cancer survivors and for further efforts to support women in achieving their post-cancer reproductive goals.
AYA cancer survivors in our cohort were more likely to report a pregnancy attempt if they were partnered and at least 6 years out from diagnosis at the time of survey. These findings align with the need for AYAs to reach both personal and cancer-related milestones before attempting pregnancy. Reasons for the observed association with prior infertility are less immediately clear, but this finding suggests that women with a history of unsuccessful pre-cancer pregnancy attempts may be more motivated to make additional attempts after their cancer diagnosis. In contrast, women who were older than 30 years at diagnosis and those with breast or gynecologic cancers (who tend to be older at diagnosis, on average, than those with other AYA cancers) were less likely to report a pregnancy attempt. For women at the upper end of the AYA age range, cancer diagnosis and treatment may cause them to postpone pregnancy until they are beyond their childbearing years. For those with gynecologic cancers, removal of reproductive organs during cancer treatment may also help explain the lower likelihood of pregnancy attempts. Women with more advanced cancer stage and those who received radiation or chemotherapy were not less likely to attempt pregnancy in our analyses. For clinicians, these findings highlight the importance of not assuming that female AYAs with more severe disease or more intense treatment will be less likely to attempt pregnancy in the future and therefore less likely to need fertility-related care.
In our overall cohort, receipt of fertility counseling, either with any medical provider or specifically with a fertility specialist, was not significantly associated with attempting pregnancy. However, fertility preservation, through embryo or oocyte cryopreservation, was a significant predictor of pregnancy attempts, suggesting that women who are able to access these methods and choose to use them may be more motivated and better informed about attempting pregnancy after cancer. These findings contrast with those from the FIRST cohort, in which, contrary to expectation, those who did not use fertility preservation were approximately twice as likely to attempt pregnancy as those who did (RR=2.38; 95% CI: 1.31, 4.32).(11) Further investigation may be warranted to better understand these discrepant findings and the potential impact of fertility preservation use on post-cancer pregnancy attempts.
Our analysis has some limitations. Information on fertility counseling and fertility preservation use was obtained through participant self-report and was not verified through medical records. Given the relatively long period between cancer diagnosis and survey for many women in our cohort, it is possible that some participants were unable to accurately recall whether they accessed these services. We also lacked information on fertility discussions or fertility specialist visits that occurred after cancer treatment and could therefore not assess their impact on later pregnancy attempts. Our outcome of interest, post-diagnosis pregnancy attempts, may also have been misclassified due to inaccurate recall. Because we considered all reported pregnancies to reflect pregnancy attempts, women with only unintended pregnancies after their cancer may also have been misclassified. We also did not capture attempts that did not result in pregnancy and did not meet the 1-year threshold for infertility. The overall proportion of women with a pregnancy attempt in our cohort would likely be somewhat higher if we had included those who attempted to conceive for less than 12 months but did not do so. We did not have detailed information on cancer therapies and therefore could not distinguish between women who were exposed to potentially gonadotoxic therapies and those who were not. For women with gynecologic cancer specifically, we could not distinguish fertility-sparing surgical procedures from those that would preclude future pregnancy. Small numbers of women in certain subgroups (e.g. distant stage, uninsured at diagnosis) limited our ability to draw conclusions about associations between these characteristics and pregnancy attempts. The participation rate in the parent study was low overall (~13%), and women who chose to participate may differ in important ways from those who did not. However, with over 800 survey participants, our analysis is one of the largest studies to date to address patient-reported reproductive issues and outcomes among AYA women with cancer.
Conclusions
Reproductive issues are a priority survivorship concern for AYA women with cancer. In our cohort, use of fertility preservation strategies, but not fertility counseling, was a significant predictor of attempting pregnancy after AYA cancer. Ensuring access to fertility preservation methods may help AYA survivors to plan and initiate future fertility. Our findings on pregnancy attempts may inform the interpretation of population-based studies of birth rates after AYA cancer.
Attestation Statement:
Data regarding any of the subjects in the study has not been previously published unless specified.
Data will be made available to the editors of the journal for review or query upon request.
Capsule: Among women with cancer diagnosed during adolescence or young adulthood (ages 15–39 years), use of fertility preservation strategies was uncommon but was associated with attempting pregnancy after cancer.
Funding Statement:
This research was supported in part by the National Cancer Institute (R01 CA204258) and a developmental award from University of North Carolina (UNC) Lineberger Comprehensive Cancer Center.
Footnotes
Disclosure Statement: The authors declare that they have not competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cancer Institute. Cancer stat facts: Cancer among adolescents and young adults (AYAs) (ages 15–39). Available from: https://seer.cancer.gov/statfacts/html/aya.html. Accessed Apr. 13, 2022.
- 2.American Cancer Society. Cancer Facts & Figures 2020. Special section: Cancer in adolescents and young adults. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancerfacts-and-statistics/annual-cancer-facts-and-figures/2020/special-section-cancer-in-adolescents-and-young-adults-2020.pdf. Accessed Apr. 13, 2022.
- 3.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917–31. [DOI] [PubMed] [Google Scholar]
- 4.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- 6.Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril 2018;110:380–6. [DOI] [PubMed] [Google Scholar]
- 7.Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril 2013;100:1224–31. [DOI] [PubMed] [Google Scholar]
- 8.Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–33. [DOI] [PubMed] [Google Scholar]
- 9.Young K, Shliakhtsitsava K, Natarajan L, Myers E, Dietz AC, Gorman JR, Martínez ME, Whitcomb BW, Su HI. Fertility counseling before cancer treatment and subsequent reproductive concerns among female adolescent and young adult cancer survivors. Cancer 2019;125:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skaczkowski G, White V, Thompson K, Bibby H, Coory M, Orme LM, Conyers R, Phillips MB, Osborn M, Harrup R, Anazodo A. Factors influencing the provision of fertility counseling and impact on quality of life in adolescents and young adults with cancer. J Psychosoc Oncol 2018;36:484–502. [DOI] [PubMed] [Google Scholar]
- 11.Dominick SA, Whitcomb BW, Gorman JR, Mersereau JE, Chung K, Su HI. Factors associated with pregnancy attempts among female young adult cancer survivors. J Cancer Surviv 2014;8:571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins Bressler L, Mersereau JE, Anderson C, Rodriguez JL, Hodgson ME, Weinberg CR, Sandler DP, Nichols HB. Fertility-related experiences after breast cancer diagnosis in the Sister and Two Sister Studies. Cancer 2019;125:2675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bann CM, Treiman K, Squiers L, Tzeng J, Nutt S, Arvey S, McGoldrick D, Rechis R. Cancer Survivors’ Use of Fertility Preservation. J Womens Health (Larchmt) 2015;24:1030–7. [DOI] [PubMed] [Google Scholar]
- 14.Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012;118:1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shnorhavorian M, Harlan LC, Smith AW, Keegan TH, Lynch CF, Prasad PK, Cress RD, Wu XC, Hamilton AS, Parsons HM, Keel G, Charlesworth SE, Schwartz SM. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: A population-based study. Cancer 2015;121:3499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols HB, Anderson C, Ruddy KJ, Black KZ, Luke B, Engel SM, Mersereau JE. Childbirth after adolescent and young adult cancer: a population-based study. J Cancer Surviv 2018;12:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg D, Meeks HD, Johnstone E, Berga SL, Smith KR, Hotaling J, Letourneau JM. Cancer treatment is associated with a measurable decrease in live births in a large, population-based study. F S Rep 2021;2:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols HB, Baggett CD, Engel SM, Getahun D, Anderson C, Cannizzaro NT, Green L, Gupta P, Laurent CA, Lin PC, Meernik C, Moy LM, Wantman E, Xu L, Kwan ML, Mersereau JE, Chao CR, Kushi LH. The Adolescent and Young Adult (AYA) Horizon Study: An AYA Cancer Survivorship Cohort. Cancer Epidemiol Biomarkers Prev 2021;30:857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results Program. AYA Site Recode/WHO 2008. Definition. Available from: https://seer.cancer.gov/ayarecode/aya-who2008.html. Accessed Apr. 13, 2022. [Google Scholar]
- 20.Meernik C, Kirchhoff AC, Anderson C, Edwards TP, Deal AM, Baggett CD, Kushi LH, Chao CR, Nichols HB. Material and psychological financial hardship related to employment disruption among female adolescent and young adult cancer survivors. Cancer 2020;127:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]