Abstract
Rationale
Sleep apnea is the manifestation of key endotypic traits, including greater pharyngeal collapsibility, reduced dilator muscle compensation, and elevated chemoreflex loop gain.
Objectives
We investigated how endotypic traits vary with obesity, age, sex, and race/ethnicity to influence sleep apnea disease severity (apnea–hypopnea index [AHI]).
Methods
Endotypic traits were estimated from polysomnography in a diverse community-based cohort study (Multi-Ethnic Study of Atherosclerosis, N = 1,971; age range, 54–93 yr). Regression models assessed associations between each exposure (continuous variables per 2 standard deviations [SDs]) and endotypic traits (per SD) or AHI (events/h), independent of other exposures. Generalizability was assessed in two independent cohorts.
Results
Greater AHI was associated with obesity (+19 events/h per 11 kg/m2 [2 SD]), male sex (+13 events/h vs. female), older age (+7 events/h per 20 yr), and Chinese ancestry (+5 events/h vs. White, obesity adjusted). Obesity-related increase in AHI was best explained by elevated collapsibility (+0.40 SD) and greater loop gain (+0.38 SD; percentage mediated, 26% [95% confidence interval (CI), 20–32%]). Male-related increase in AHI was explained by elevated collapsibility (+0.86 SD) and reduced compensation (−0.40 SD; percentage mediated, 57% [95% CI, 50–66%]). Age-related AHI increase was explained by elevated collapsibility (+0.37 SD) and greater loop gain (+0.15 SD; percentage mediated, 48% [95% CI, 34–63%]). Increased AHI with Chinese ancestry was explained by collapsibility (+0.57 SD; percentage mediated, 87% [95% CI, 57–100]). Black race was associated with reduced collapsibility (−0.30 SD) and elevated loop gain (+0.29 SD). Similar patterns were observed in the other cohorts.
Conclusions
Different subgroups exhibit different underlying pathophysiological pathways to sleep apnea, highlighting the variability in mechanisms that could be targeted for intervention.
Keywords: pathophysiology, epidemiology, precision medicine, endotype
Sleep apnea is a highly prevalent condition (1) characterized by a heterogeneous etiology. Patients differ not only by pharyngeal collapsibility but also in key nonanatomical traits, including poor dilator muscle compensation and exaggerated ventilatory chemoreflex sensitivity (higher loop gain) (2–13). Multiple observational and interventional studies support the notion that reduced muscle compensation (5, 8, 10, 12–14) and elevated loop gain (2, 9, 12, 15, 16) contribute to sleep apnea independent of pharyngeal collapsibility. Currently, it is unclear whether increased risk of sleep apnea with obesity, older age, or male versus female sex involves deficits in ventilatory control or dilator muscle compensation independent of established differences in collapsibility. It is also unclear whether pathophysiological mechanisms vary with race/ethnicity. Improved understanding of the upstream determinants of obstructive sleep apnea (OSA) endotypes is needed to develop precision medicine approaches to therapy for patients with sleep apnea (2–7).
To shed light on the pathways between obesity, age, sex, and race/ethnicity exposures and sleep apnea pathophysiology, we estimated sleep apnea endotypic traits from routine polysomnography (17, 18) in a large, ethnically diverse community sample (MESA [Multi-Ethnic Study of Atherosclerosis]) of middle-aged and older adults (19). We tested primary specific hypotheses, including that obesity raises loop gain (20, 21), women have greater compensation versus men (22), and older participants have elevated loop gain and reduced compensation (23). We also sought to discover new associations between traits and race/ethnicity and to confirm previous findings that collapsibility is compromised in men versus women, in older versus younger adults, with obesity, or with Chinese ancestry (24, 25). Emphasis was placed on three primary traits (collapsibility, compensation, and loop gain) shown to be adversely associated with sleep apnea severity (apnea–hypopnea index [AHI]). We subsequently explored potential mechanisms of obesity-, age-, and sex-related deficits in traits. We also sought to replicate the main findings in two additional cohorts (CFS [Cleveland Family Study] and MrOS [Osteoporotic Fractures in Men Study]).
Methods
Participants and Study Design
MESA is a prospective observational cohort study examining risk factors for cardiovascular disease in four self-identified mutually exclusive racial/ethnic groups: White, Black/African American, Hispanic, and Chinese (19). Participants were initially free of clinical cardiovascular disease at Examination 1 (2000–2002). A subgroup (n = 2,060) participated in the MESA Sleep Ancillary Study (MESA Examination 5, 2010–2013), which included in-home nocturnal polysomnography (19). Details are described in the data supplement. Institutional review board approval was obtained at each site, and written informed consent was also obtained. A prespecified statistical analysis plan was approved by the MESA Steering Committee.
Polysomnography
Full polysomnography included electroencephalography and nasal pressure cannula (direct current coupled; 32 Hz) plus other standard signals (19). Sleep, arousals, and respiratory events were based on standard criteria (hypopneas, 30% reduction in flow with ⩾3% desaturation or arousal).
Pathophysiological Traits
Sleep apnea pathophysiological (endotypic) traits were estimated using established automated methods (17, 18) deployed recently to predict therapeutic responses (2, 3, 6, 7) and found to have high within- and across-night repeatability (intraclass correlations, ∼0.8 for collapsibility and loop gain, ∼0.7 for compensation; see Supplemental Methods—Reliability in the data supplement) (26, 27). Briefly, each trait is defined by spontaneous fluctuations in ventilation (from nasal pressure, mean normalized) and ventilatory drive (intended ventilation estimated using a chemoreflex model via least-squares regression; data supplement).
Collapsibility was based on the median ventilation at normal/eupneic ventilatory drive (passive); lower values of passive indicate greater collapsibility (8, 18, 28). Compensation, the increase in ventilation with rising ventilatory drive, was determined by calculating active (ventilation when ventilatory drive is at the arousal threshold). active minus passive reflects dilator muscle compensation. Lower values represent a greater neuromuscular contribution to sleep apnea (10, 11, 18). Loop gain (LG1; ventilatory control sensitivity) is the ventilatory drive response to reduced ventilation. Higher values represent a greater ventilatory control contribution to sleep apnea (17) because of either chemoreflex (increased hypoxic/hypercapnic ventilatory response) or pulmonary (e.g., lower lung volume) mechanisms.
Median values during non–rapid eye movement (non-REM) sleep were used for each participant. Trait values were included for any individual if there were at least three windows of data available for analysis of a given trait. (Otherwise, no minimum AHI was set for primary analysis.) Analyses were executed using custom software (Phenotyping Using Polysomnography, MATLAB; MathWorks).
Arousal threshold was also calculated but was not considered a mechanistic driver of increased AHI. Although a low arousal threshold may contribute to OSA severity in some circumstances (e.g., higher AHI in REM; lower AHI in N3 sleep [29, 30]), cross-sectional analyses (12, 30) find increased arousal threshold with greater OSA severity (12, 31), opposite to the proposed causal mechanistic direction. This reversed association is considered the consequence of a strong adaptive effect of OSA on an increased arousal threshold (32).
Exposures
Primary exposures were age, sex, body mass index (BMI), and race/ethnicity (self-report, using categories White, Black/African American, Hispanic, and Chinese).
Exploratory exposure variables
Exploratory analysis of the mechanisms underlying obesity associations used measures from core MESA examinations (data supplement), including total body fat (bioelectrical impedance) and waist-to-height ratio (abdominal obesity), forced vital capacity (FVC; to explore potential obesity-related reduction in lung volumes), and obesity-related biomarkers (fasting glucose and insulin [glucose–insulin homeostasis], leptin [adipose tissue biomarker], and C-reactive protein concentrations [inflammation]). Exploratory analysis of the mechanisms underlying sex differences examined total testosterone and estradiol concentrations. Mechanisms underlying age differences examined the cardiac dysfunction biomarker N-terminal prohormone B-type natriuretic peptide and renal dysfunction (glomerular filtration rate).
Statistical Analysis
Primary models
Regression models were used to describe the associations between six primary exposures (age, sex, BMI, and race/ethnicity subgroups) and four outcomes (AHI and three endotypic traits). For each trait (and AHI), a multivariable regression model assessed the association with each exposure, adjusting for other exposures. Analyses were also adjusted for sleeping position (percentage in nonsupine sleep).
Endotypic traits and AHI were transformed for normality (see Table E1 in the data supplement) to achieve Gaussian residuals across multiple linear models. Effects on collapsibility (passive) were modeled using a sigmoidal link function (modified logistic regression with slope = 1 at 50% eupnea) to handle known floor and ceiling effects (5). Likewise, effects on pharyngeal muscle compensation were estimated by modeling active (same link function), adjusting for collapsibility (both passive and passive2 terms); thus, coefficients reflect the influence on active with passive effects regressed out (5). The linear model for loop gain also adjusted for collapsibility (both passive and passive2 terms) (Table E2). Model coefficients for endotypic traits were standardized (per standard deviation [SD]) to facilitate coefficient comparisons. Continuous exposures were standardized per 2 SD for comparisons with binary exposures (BMI per 11.1 kg/m2; age per 20 yr [exact 2 SD = 18.3 yr]) (33).
A separate multivariable model was developed to quantify the independent contributions of primary endotypic traits (collapsibility, compensation, loop gain per 2 SD) to AHI (data supplement). Variance inflation factors were examined to assess multicollinearity (<3 for exposures and traits in the above models).
Mediation analysis (Baron and Kenny method [34, 35]) described the extent to which different endotypic traits (alone or in combination) explained the observed associations between each exposure and AHI (primary multivariable model analysis); 95% confidence intervals (CIs) were calculated using bootstrapping (5,000 iterations). Exploratory analyses incorporated further adjustment for selected potential confounders.
Multiplicity
Significance was accepted at a P threshold of 0.0021 (24 comparisons, four independent variables [AHI, collapsibility, compensation, and loop gain] × 6 exposure variables; Bonferroni method).
Replication Analyses
To assess the generalizability of the main findings, we repeated the primary model analyses in two independent cohorts: CFS (n = 537 men and women) and MrOS (n = 2,608; males only) (36–38) (data supplement). The race/ethnicity analyses were limited to differences between Black and White participants because of the limited sample size of other groups. Meta-analysis (39, 40) provided pooled estimates across multiple cohorts.
Results
Of the 2,060 MESA Sleep participants, 16 had unscorable studies, 24 had insufficient nasal pressure signal, and 49 had fewer than three windows with respiratory events eligible for endotypic trait analysis; thus, traits were obtained in n = 1,971 participants. Baseline characteristics of these participants are described in Table 1. Trait distributions are illustrated in Figure E1.
Table 1.
Participant characteristics in Multi-Ethnic Study of Atherosclerosis
| Characteristics | Values | No. of Participants* |
|---|---|---|
| Demographics | ||
| Age, yr | 68.5 ± 9.1 | 1,968 |
| Sex, M/F, % | 916/1,052, 47% | 1,968 |
| Race/ethnicity, Black/Hispanic/Chinese/White | 548/464/241/715 | 1,968 |
| Obesity and related characteristics | ||
| Body mass index, kg/m2 | 28.6 ± 5.5 | 1,964 |
| Waist circumference/height, % | 60.2 ± 9.0 | 1,963 |
| Total body fat, % | 31.1 ± 9.6 | 1,859 |
| Forced vital capacity, % predicted | 97.0 ± 17.5 | 1,448 |
| Fasting glucose, ng/ml | 950 [880–1,050] | 1,944 |
| Fasting insulin, ng/ml | 510 [330–760] | 1,759 |
| C-reactive protein, high sensitivity, ng/ml | 1.8 [0.8–4.1] | 1,957 |
| Leptin, ng/ml | 14.3 [6.3–29.8] | 765 |
| Sex hormones | ||
| Testosterone, men, pg/ml | 4,114 [3273–5,012] | 1,729 |
| Testosterone, women, pg/ml | 250 [160–360] | |
| Estradiol, men pg/ml | 31 [24–38] | 1,729 |
| Estradiol, women, pg/ml | 21 [13–53] | |
| Polysomnography | ||
| OSA severity, n, normal/mild/moderate/severe | 191/592/591/586 | 1,960 |
| AHI, total, events/h | 18.7 [10.0–33.4] | 1,960 |
| AHI non-REM, events/h | 15.1 [6.7–31.1] | 1,962 |
| AHI REM, events/h | 30.7 [15.2–50.9] | 1,943 |
| Central events, % respiratory events | 0 [0–2] | 1,769 |
| Apneas, % respiratory events | 8 [2–20] | 1,769 |
| Arousal index, events/h | 19.8 [14.0–28.1] | 1,962 |
| Total sleep time, min | 360.8 ± 81.3 | 1,968 |
| Non-REM 1, % total sleep time | 12.1 [8.4–18.0] | 1,965 |
| Non-REM 2, % total sleep time | 58.0 [51.3–64.3] | 1,965 |
| Non-REM 3, % total sleep time | 8.2 [2.1–15.7] | 1,965 |
| REM, % total sleep time | 18.3 [13.8–22.4] | 1,965 |
| Supine, % total sleep time | 34.8 [12.1–63.9] | 1,968 |
Definition of abbreviations: AHI = apnea–hypopnea index; OSA = obstructive sleep apnea; REM = rapid eye movement.
Continuous variables are represented by the mean ± standard deviation or median [interquartile range] as appropriate.
Sample size shows the number of participants available from among the 1,971 participants with endotypic traits. OSA severity was defined as normal (AHI, <5 events/h), mild (5 ⩽ AHI < 15 events/h), moderate (15 ⩽ AHI < 30 events/h), and severe (AHI ⩾30 events/h).
Endotypes and Sleep Apnea Severity
In mutually adjusted analyses, increased collapsibility, elevated loop gain, and reduced compensation were each associated with higher AHI (Table 2; see also Figure E2), suggesting that independent mechanistic pathways contribute to OSA severity.
Table 2.
Associations between apnea–hypopnea index and endotypes in Multi-Ethnic Study of Atherosclerosis
| Endotypic Traits | β ± SEM | Interpretation |
|---|---|---|
| Collapsibility | 15.3 ± 0.5* | Greater collapsibility → higher AHI |
| Compensation | −6.4 ± 0.8* | Reduced compensation → higher AHI |
| Loop gain | 9.7 ± 0.7* | Higher loop gain → higher AHI |
Definition of abbreviations: AHI = apnea–hypopnea index; SEM = standard error of the mean.
Data shown are standardized β (events/h per 2 standard deviations [SDs]) relating primary endotypic traits (collapsibility, compensation, loop gain; mean centered) to AHI, suggesting that greater collapsibility (per ventilation under passive conditions [passive]), reduced compensation (per ventilation under active conditions [active], adjusted for collapsibility), and elevated loop gain (per LG1) each contribute to obstructive sleep apnea (multivariable model R2 = 0.45). Each trait association shown is adjusted for other endotypic traits. Note that AHI data were square root transformed for analysis; results were back transformed for interpretability. passive and active were also transformed (see Methods). Coefficients for traits (and interaction term) were standardized by 2 SD to facilitate comparisons. Model: AHI0.5 = 4.69 − 2.10 passive − 0.74 active + 0.94 LG1 − 0.72 passive × active (exposures are mean centered and 2-SD standardized). β for collapsibility (passive) was similar in bivariate analysis.
P < 0.00001.
Associations of Demographics and Obesity with Sleep Apnea Severity and Endotypic Traits
The results of multivariable adjusted regression models for each exposure are detailed in Table 3 and Figures 1 and 2.
Table 3.
Estimated effects of exposures on endotypic traits and apnea–hypopnea index in Multi-Ethnic Study of Atherosclerosis
| Exposures | Apnea–Hypopnea Index (events/h) |
Collapsibility (SD) |
Compensation (SD) |
Loop Gain (SD) |
|---|---|---|---|---|
| BMI (2 SD) |
19.5 ± 1.1
(<0.00001) |
0.40 ± 0.07
(<0.00001) * |
0.17 ± 0.12 (0.18) |
0.38 ± 0.05
(<0.00001) * |
| Age (20 yr) | 7.2 ± 1.0 (<0.00001) |
0.37 ± 0.07
(<0.00001) * |
0.72 ± 0.13 (<0.00001) † |
0.15 ± 0.05
(0.0017) * |
| Male vs. Female | 13.2 ± 0.9 (<0.00001) |
0.86 ± 0.06
(<0.00001) * |
−0.40 ± 0.13
(0.0019) * |
0.05 ± 0.04 (0.24) |
| Black vs. White |
−5.2 ± 1.0
(<0.00001) |
−0.30 ± 0.08
(0.0004) |
0.33 ± 0.13 (0.042) |
0.29 ± 0.05
(<0.00001) |
| Hispanic vs. White | −0.6 ± 1.0 (0.5) |
−0.08 ± 0.08 (0.3) | −0.03 ± 0.14 (0.9) | −0.01 ± 0.06 (0.8) |
| Chinese vs. White |
5.0 ± 1.4
(0.0003) |
0.57 ± 0.10 (<0.00001) * | −0.07 ± 0.16 (0.7) | 0.01 ± 0.07 (0.9) |
Definition of abbreviations: BMI = body mass index; SD = standard deviation.
Data shown are β ± standard error of the mean (SEM) (P value) with apnea–hypopnea index (AHI) represented by events per hour and traits by SD; continuous exposures are standardized using 2 SD to facilitate comparison with binary exposures (sex, race/ethnicity). A 2-SD change for BMI was +11.1 kg/m2 and for age was 18.3 years (data were standardized using 20 yr for simplicity). Each column describes a single multivariable model. β-Estimates include adjustment for age, sex, BMI, race, and body position. The loop gain model includes adjustment for collapsibility (passive and passive2) as a confounder. Note that AHI data were square root transformed for analysis; results were back transformed for interpretability. SEM for back-transformed AHI data (AHI0.5) was based on the lower confidence bound, such that 1.96 × SEM indicates the lower 95% limit. Bold type indicates P < 0.05. P < 0.0021 was considered significant after adjusting for multiple comparisons (Bonferroni method).
Finding is in support of a specific hypothesis.
Finding is not in support of a specific hypothesis.
Figure 1.
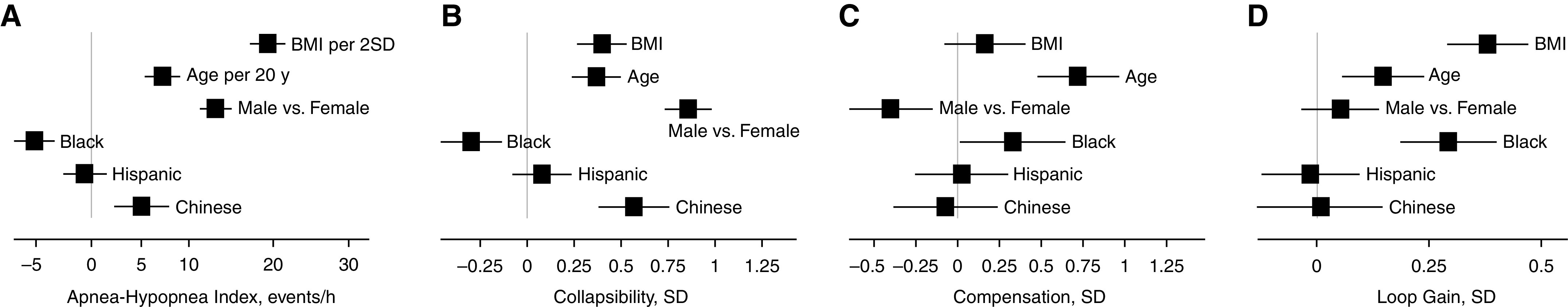
Effects of demographics and obesity on apnea–hypopnea index (AHI) (A) and primary endotypic traits (B–D) in the MESA study (Multi-Ethnic Study of Atherosclerosis). Squares and horizontal lines indicate the strength of each association (β with 95% confidence interval). Reference group for race/ethnicity is White. Note that the nonlinear scaling for AHI is due to the square root transformation; results were back transformed for interpretability. BMI = body mass index; SD = standard deviation.
Figure 2.
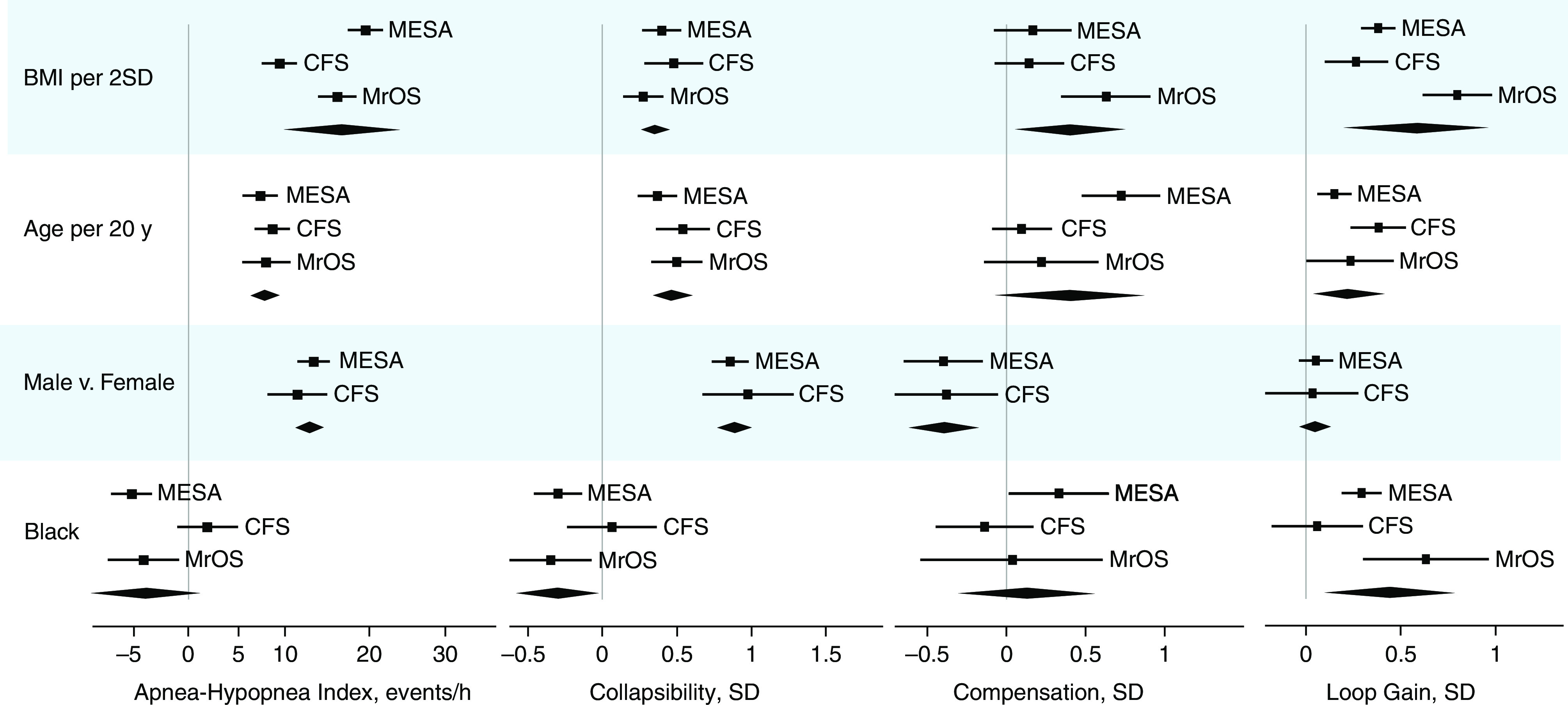
Replication of effects of obesity and demographics on apnea–hypopnea index and primary endotypic traits in the CFS (Cleveland Family Study) and MrOS (Osteoporotic Fractures in Men Study) studies. Data from the MESA study (Multi-Ethnic Study of Atherosclerosis) are repeated for comparison. Squares and horizontal lines indicate the strength of each association (β with 95% confidence interval [CI]). Meta-analysis results for the three cohorts are also shown (diamond describes β and 95% CI). Sample sizes were as follows: MESA, n = 1,971; CFS, n = 537; MrOS, n = 2,608. SD standardization was based on data from MESA to facilitate comparison across cohorts. Results for Hispanic and Chinese participants could not be reassessed in the additional cohorts. BMI = body mass index; SD = standard deviation.
Sleep apnea severity
Greater AHI was associated with increased BMI (β = +19 events/h per 11.1 kg/m2), male sex (+13 events/h), older age (+7 events/h per 20 yr), and Chinese ancestry (+5 events/h vs. White race); reduced AHI was observed with Black race (−5 events/h vs. White race, obesity adjusted) (Table 3, Figure 1A).
Collapsibility
As hypothesized, greater pharyngeal collapsibility was associated with older age, male sex, obesity (BMI), and Chinese ancestry (vs. White race) (Table 3, Figure 1B). The male-related deficit (β = 0.86 SD) was estimated to be approximately twofold that of a 20-year increase in age or 11 BMI points and was ∼50% greater than the estimate for Chinese ancestry (vs. White race). Collapsibility was lower in Black than in White participants.
Compensation
As hypothesized, reduced pharyngeal compensation was associated with male sex (Table 3, Figure 1C). Greater compensation in Black participants was observed but did not meet the significance threshold (β = 0.33 SD; P = 0.042). Our results did not support the hypothesized reduction in compensation with increasing age.
Loop gain
As hypothesized, increased loop gain (ventilatory control sensitivity) was associated with increased BMI (β = 0.38 SD) and older age (β = 0.15 SD). We also observed increased loop gain in Black versus White participants (Table 3, Figure 1D).
Endotypic Mechanisms that May Mediate Population Differences in Sleep Apnea
Mediation analyses described the degree to which endotypic traits explained the greater AHI with obesity, older age, male sex, Chinese ancestry, and the lower AHI in Black participants (Table 4). Greater collapsibility was a significant mediator of increased AHI observed with age (39%), male sex (52%), and Chinese ancestry (87%), but it was only a modest mediator of increased AHI with obesity (17%). Loop gain modestly mediated greater AHI with obesity (17%) and age (21%). The lower AHI in association with Black race was mediated by less severe collapsibility (41%; 52% if also considering greater compensation).
Table 4.
Extent to which traits explain differences in apnea–hypopnea index with obesity and demographics in Multi-Ethnic Study of Atherosclerosis
| Percentage Mediated by Endotypic Traits [95% CI] |
||||||
|---|---|---|---|---|---|---|
| Factor | Association with AHI (β ± SEM) | Collapsibility | Loop Gain | Collapsibility + Compensation | Collapsibility + Loop Gain | All Traits |
| BMI (2 SD) | 19.1 ± 1.1 | 17 [12, 22] * | 17 [13, 22] * | 17 [12, 22] * | 26 [20, 32] * | 26 [20, 32] * |
| Age (20 yr) | 8.3 ± 1.3 | 39 [26, 53] † | 21 [12, 32] * | 38 [24, 52] † | 48 [34, 63] † | 47 [33, 62] † |
| Male vs. Female | 13.4 ± 1.0 | 52 [45, 60] † | 11 [7, 16] | 57 [50, 66] † | 54 [46, 61] † | 59 [51, 67] † |
| Black vs. White | −5.0 ± 1.0 | 41 [24, 64] † | <0 | 52 [34, 77] † | 21 [−3, 40]* | 32 [10, 52] † |
| Chinese vs. White | 5.4 ± 1.5 | 87 [57, 100] ‡ | 16 [−1, 36]* | 94 [63, 100] ‡ | 88 [59, 100] ‡ | 95 [65, 100] ‡ |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CI = confidence interval; SEM = standard error of the mean.
Data describe the percentage reduction in association strength (β) with the addition of individual endotypic traits to the primary model between AHI and obesity/demographics (95% confidence intervals were calculated using bootstrapping with 5,000 iterations). Bold denotes a significant mediation effect. Exposures with an observed association with AHI (per Table 2) were examined. For example, the association between Chinese (vs. White) and AHI (5.4 events/h) was diminished by 87% after collapsibility was included; greater collapsibility was therefore considered a potential explanation for increased AHI in Chinese versus White participants. Note that AHI data were square root transformed for analysis; results were back transformed for interpretability.
Denotes 15–30% mediation (“modest”).
Denotes 30–60% mediation (“substantial”).
Denotes >60% mediation (“strong”).
Exploratory Pathway Analyses
Obesity-related pathways
Mechanistic exploration of whether obesity might augment loop gain via chemoreflex (“controller gain”; fat tissue mass, obesity biomarkers) rather than pulmonary pathways (“plant gain”; abdominal obesity, lung volume) revealed the following. 1) Compared with associations with BMI (β = 0.38 SD), loop gain was more strongly associated with total body fat (β = 0.51 SD) but less strongly associated with abdominal obesity (0.27 SD; Table E3); similar findings were found for AHI. 2) Leptin, but not other biomarkers (glucose, insulin, C-reactive protein, FVC; Table E4), was associated with loop gain and modestly accounted for the association between BMI and loop gain.
Age-related pathways
The association between loop gain and older age was substantially diminished with adjustment for either cardiac or renal dysfunction (Table E5). The age-related increase in collapsibility was modestly diminished with adjustment for cardiac dysfunction.
Sex-related pathways
Adjustment for testosterone diminished the male-related deficits in compensation and AHI but not collapsibility (Table E6); testosterone was also associated with reduced compensation within men (Table E6). No association with estradiol was observed.
Replication Analyses
The main analyses performed in MESA (Table 3, Figure 1) were repeated in CFS and MrOS (datasets described in Table E7); meta-analysis summarized the pooled results across all three cohorts (Figure 2, Table E8). Findings that were supported by the additional cohorts per meta-analysis were as follows. 1) Increased AHI with obesity, older age, and male versus female sex. 2) Increased collapsibility with obesity, age, and male sex and reduced collapsibility with Black versus White race; notably, associations between obesity and collapsibility remained modest compared with stronger associations between obesity and AHI. 3) Compensation was reduced in men versus women. 4) Loop gain was increased with obesity, age, and Black versus White. Cross-cohort analyses identified reduced AHI in Black versus White participants in the MESA and MrOS cohorts, but not in the younger CFS cohort. Meta-analysis did not confirm the increase in compensation with age or in Black versus White participants (seen in MESA).
Discussion
This study assessed the heterogeneity of pathophysiologic traits causing OSA in large, diverse community samples to provide insight into how traits vary with obesity, age, sex, and race/ethnicity and how variation in these traits influences sleep apnea disease severity (AHI). Major findings that were consistent across samples were as follows. Greater AHI with obesity was accompanied by hypersensitive ventilatory control (higher loop gain) and unexpectedly modest effects on collapsibility. Greater AHI with older age was accompanied by increased collapsibility and increased loop gain. Greater AHI in men versus women was accompanied by reduced muscle compensation plus a pronounced deficit in collapsibility. Lower AHI in Black versus White participants in MESA was accompanied by less severe collapsibility despite higher loop gain; these findings were also observed in another cohort of older participants but not in a younger sample. Greater collapsibility was the sole identified physiological determinant of heightened disease severity in Chinese (vs. White) participants in MESA. Overall, our study provides novel evidence that obesity, age, sex, and race/ethnicity factors are associated with OSA severity via different pathophysiological pathways.
Physiological Insights
Obesity
Obesity is the strongest population-level OSA risk factor (∼11- to ∼21-fold vs. nonobese) (1) and is understood to raise disease risk via increased pharyngeal collapsibility (25), putatively via greater tongue fat and reduced lung volume (41, 42). This study demonstrates that obesity was associated with both increased collapsibility and increased loop gain, supporting influences of obesity beyond established deleterious effects on upper airway anatomy (8, 43). The modest association between collapsibility and obesity observed is consistent with prior studies conducted during non-REM sleep (just 1–2 cm H2O greater collapsibility with obesity) (25). Effects of obesity beyond anatomical traits causing OSA have been postulated (44) but have not clearly been demonstrated. We previously identified a ventilatory control deficit in the form of elevated loop gain (steady-state method) in overweight/obese participants versus control participants in a smaller sample (13). Here, in a large community sample and replicated across cohorts, we confirmed the hypothesis that obesity is associated with elevated loop gain. Notably, obesity-related increases in AHI were explained by loop gain to an extent similar to collapsibility. Our study did not find evidence that higher BMI is associated with ineffective muscle compensatory responses to obstruction, consistent with prior work showing maintained or enhanced compensation in many obese participants (13).
Analysis of obesity-related pathways also revealed several insights. 1) Compared with BMI, total body fat was a stronger determinant of both AHI and loop gain but not a stronger determinant of collapsibility. These associations suggest that total body fat may better capture the effects of adiposity on ventilatory control than BMI or waist-to-hip ratio. 2) Adjusting for lung volume (per FVC, used as a surrogate for the preferred variable functional residual capacity; see Supplemental Methods in the data supplement) had no impact on the link between obesity and loop gain. Thus, the increased loop gain with obesity appears less likely to involve mechanical deficits (reduced lung gas volume) and more likely to involve fat tissue–related signaling pathways that ultimately augment chemosensitivity (21) (Tables E3 and E4).
Age
Older versus younger adults are at greater risk of sleep apnea (approximately two- to eightfold) (1), but the mechanisms underlying this increased risk are not understood. As expected, we found that increasing age is associated with greater collapsibility (8, 45). However, we did not find evidence supporting an age-related decrement in compensation (23), consistent with some smaller studies (8, 45). We also identified that older age is associated with higher loop gain, which builds on the known heightened risk of central sleep apnea (46). However, the higher loop gain with age was largely explained by age-related decrements in cardiac and renal function. The increase in loop gain with age contrasts with smaller physiology studies suggesting lower loop gain in older sleep apnea patients (45), possibly reflecting differences in methodology or selection biases in the small experimental studies.
Sex
Men have a two- to fivefold greater odds of sleep apnea than women (1) for reasons that are not well understood. Consistent with our previous unadjusted analysis (47), here we showed that male sex is associated with triple the magnitude of a 20-year increase in age and double the effect of obesity (11 BMI points), a stronger association than previously appreciated from smaller physiology studies (25, 48). Yet, as suggested previously (8), the increased AHI in men versus women is only modestly explained by elevated collapsibility (Table 4).
We showed, for the first time, to our knowledge, that men exhibit a deficit in pharyngeal compensation compared with women, a finding that was seen consistently across cohorts (MESA and CFS). Although smaller studies have not demonstrated such differences (48, 49), this finding is consistent with 1) the observation that women have lower AHI than men in non-REM sleep despite similar AHI in REM (during hypotonic conditions) (47) and 2) reduced baseline tone and responsiveness of the genioglossus in men during wakefulness (50). Exploratory analysis suggests that the lower compensation in men may be explained by differences in testosterone (Table E6), consistent with observations that testosterone administration can raise non-REM AHI (51). Analysis adjusted for collapsibility did not show greater loop gain in men than in women (in contrast to prior unadjusted findings [47]); thus, loop gain is unlikely to be an independent contributor to greater OSA risk in men.
Black race
Prior reports on sleep apnea risk in Black versus White adults have been conflicting. Several studies report similar obesity-adjusted prevalence (19), whereas one study reported higher prevalence of severe sleep apnea in Black versus White participants (52). In fully adjusted analysis in MESA, we demonstrated a reduction in AHI in Black participants (−5 events/h vs. White participants; also seen in BMI unadjusted analyses) (Table E9), explained by a protective effect of reduced collapsibility despite an increase in loop gain. Similar findings were observed in MrOS, but not in CFS, a younger family-based sample. Overall, the present study findings suggest that sleep apnea in older Black participants may be characterized more by a ventilatory control phenotype than by deficits in pharyngeal function. Indeed, in a recent study, Black versus White patients responded preferentially to supplemental oxygen therapy for sleep apnea (2).
Chinese ancestry
Prior studies reported that participants of Chinese background consistently exhibit increased OSA risk in obesity-adjusted analyses (19). Here, Chinese ancestry was associated with greater collapsibility, with no evidence of deficits in pharyngeal compensation or ventilatory control. Indeed, the AHI increase (+5 events/h) is almost entirely explained by the observed increase in collapsibility (Table 4). Etiology may involve differences in craniofacial structure (24). The notion that nonanatomical pathways may be less pertinent to the Chinese population is consistent with lower loop gain seen in Chinese versus White patients in the clinical setting (53).
Hispanic ethnicity
Hispanic ethnicity (vs. White race) was not independently associated with differences in AHI or endotypic traits.
Strengths and Limitations
This study is the largest investigation of sleep apnea endotypes to date. Study strengths included the analyses of standardized polysomnography in well-characterized and diverse samples, independent cohort replication analyses, and use of rigorous and comprehensive statistical analysis. However, we recognize several limitations. 1) Traits were indirectly measured using noninvasive polysomnographic estimates that can be applied to large community samples rather than gold standard invasive measurements, which could potentially affect the capacity to detect associations with demographics and obesity. Although these methods appear reliable enough to describe how different demographic and obesity-related risk factors promote OSA (see Supplemental Methods—Reliability in the data supplement), we note that the reliability of the compensation measure is lower than that for loop gain and collapsibility (intraclass correlation coefficient, ∼0.7 compared with ∼0.8). We note that associations between traits (compensation in particular) and exposures may benefit from further analysis using additional tools as they are developed in the future. 2) The analytic approaches were validated in populations exhibiting at least mild sleep apnea. However, the fundamental principles (model fit to ventilation) are relevant if events are present. In secondary analyses, we showed consistency of findings when analyses were limited to participants with AHI >5 events/h (Table E10). 3) Race/ethnicity associations were adjusted for BMI, which may capture obesity differently across racial/ethnic groups, although endotype findings were robust to sensitivity analyses (Table E9). Our study does not distinguish between genetic differences and environmental/social risk factors. 4) Care must be taken when inferring causality using observational data. Untreated recurrent obstruction may raise loop gain; thus, associations between loop gain and AHI must be interpreted carefully. We sought to address this by adjusting for pharyngeal collapsibility in each analysis of loop gain. Notably, loop gain was not universally elevated in all circumstances in which increased AHI was identified (e.g., in men vs. women, Chinese vs. White participants). 5) Endotypes were based on data from non-REM sleep; therefore, our associations may not apply to mechanisms in REM sleep (see the data supplement). 6) Although overall consistency of associations was observed across three independent cohorts, some differences were seen, particularly for associations with Black race for CFS compared with the two older cohorts. 7) Arousal threshold data were not emphasized to focus on factors causing increased AHI; however, a lower arousal threshold in women versus men was observed (Table 5), which may have implications for OSA presentation and responses to therapy. 8) The loop gain measure used captures hypersensitive rather than unstable ventilatory control; an alternative measure is described in Table 5. 9) An alternative surrogate measure of collapsibility (ventilation at minimal ventilatory drive) may better reflect pharyngeal collapsibility at hypotonic conditions (54); collapsibility findings were similar using minimal ventilatory drive in place of passive (Table E11).
Table 5.
Association between exposures and additional endotypic traits in Multi-Ethnic Study of Atherosclerosis
| Factors | Ventilatory Instability (SD) | Arousal Threshold (SD) |
|---|---|---|
| BMI (2 SD) | −0.07 ± 0.05 (0.11) |
0.04 ± 0.04 (0.25) |
| Age (20 yr) |
0.32 ± 0.05
(<0.00001) |
0.04 ± 0.04 (0.3) |
| Male vs. Female |
0.29 ± 0.04
(<0.00001) |
0.14 ± 0.03 (0.00004) |
| Black vs. White |
0.17 ± 0.06
(0.002) |
0.04 ± 0.04 (0.4) |
| Hispanic vs. White | 0.03 ± 0.06 (0.6) | −0.04 ± 0.04 (0.3) |
| Chinese vs. White | 0.05 ± 0.07 (0.5) | 0.07 ± 0.06 (0.21) |
Definition of abbreviations: BMI = body mass index; SD = standard deviation; SEM = standard error of the mean.
Data shown are β ± SEM (P value) with units for traits expressed in SD. See Table 3 footnote for further details. Bold type indicates P < 0.05. Results were adjusted for collapsibility (passive and passive2). A lower arousal threshold in women was observed, indicating a significantly greater frequency of arousals per respiratory event in women (18% [10–26]; estimate [95% confidence interval]). Male sex was associated with ventilatory instability and was confirmed by analysis demonstrating that men (vs. women) also exhibited greater central apnea index (+0.5 [0.3–0.7] events/h) and a greater non-rapid eye movement (REM)/REM ratio of events (15% [12–18%]; [AHInon-REM − AHIREM]/[AHInon-REM + AHIREM]).
Clinical Implications
Evidence is accumulating that treatments for sleep apnea are likely to succeed or fail depending on the underlying endotype of sleep apnea. For example, residual events with anatomical interventions (e.g., continuous positive airway pressure, oral appliances, surgery, hypoglossal nerve stimulation) occur in patients with a higher loop gain (3, 6, 7). Our data suggest that obese, older, male, and Black patients might be most susceptible to residual sleep apnea with therapies that solely address anatomic disease mechanisms. Notably, higher loop gain in obese patients may contribute to the failure of non–continuous positive airway pressure anatomical treatments; targeting loop gain and collapsibility together might be of value in obese patients. Because of greater collapsibility, participants of Chinese ancestry may require stronger anatomical interventions, and nonanatomical interventions may be less efficacious. Moreover, the elevated loop gain and less severe collapsibility in Black participants implies greater potential for loop gain–lowering interventions (2) but reduced efficacy of mechanical interventions (3, 6, 7), providing an opportunity for novel therapy. Our study highlights the pitfalls of interpreting the results of trials testing therapies in predominantly White, male, older populations without due consideration of population diversity. The greater upper airway compensation in women may have implications for the efficacy of interventions aimed at increasing dilator muscle activity (3, 14) or therapies that rely on a functional upper airway apparatus (2). Finally, the lower arousal threshold also observed in women could feasibly reduce the efficacy and tolerability of pharmacological muscle stimulation and device therapies for OSA (3, 55).
Endotypic heterogeneity also has potential implications for sleep apnea detection in the community because sleep apnea is underdiagnosed, particularly in Black adults (56). Existing screening approaches that rely on surrogates of collapsibility (e.g., snoring) may have reduced capacity to detect sleep apnea with a major ventilatory control etiology.
Conclusions
The present community-based study shows that endotypic traits for sleep apnea vary with age, sex, race/ethnicity, and obesity and provides the largest collection of data that describes differences in predominant OSA etiologic pathways across population subgroups and with obesity. Consequently, subgroups of the population may be expected to respond differently to treatments that target different disease mechanisms and may feasibly exhibit different susceptibility to the adverse outcomes of sleep apnea, findings that may help inform endotype-focused precision medicine interventions (2, 3, 6).
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supported by the American Heart Association (15SDG25890059; principal investigator, S.A.S.) and the National Institutes of Health, National Heart, Lung, and Blood Institute (R35HL135818, principal investigator, S.R.; R01HL146697, principal investigator, S.A.S.). The parent Multi-Ethnic Study of Atherosclerosis Sleep Ancillary study was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL098433), as well as by National Heart, Lung, and Blood Institute–funded contracts (HHSN268201500003I, N01HC95159, N01HC95160, N01HC95161, N01HC95162, N01HC95163, N01HC95164, N01HC95165, N01HC95166, N01HC95167, N01HC95168, N01HC95169) and by cooperative agreements funded by the National Center for Advancing Translational Sciences (UL1TR000040, UL1TR001079, UL1TR001420). The parent Osteoporotic Fractures in Men Study ancillary study, “Outcomes of Sleep Disorders in Older Men,” was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL071194, R01HL070848, R01HL070847, R01HL070842, R01HL070841, R01HL070837, R01HL070838, R01HL070839). The parent Cleveland Family Study was funded by the National Institutes of Health (R01HL046380, M01RR00080-39, T32HL07567). The National Sleep Research Resource was also supported by the National Institutes of Health (R24HL114473, 75N92019R002).
Author Contributions: Conception: S.A.S., S.R.P., S.R. Study design: S.A.S. Endotyping design and development: S.A.S., R.M.A., D.M., D.V., P.I.T., L.K.G. Endotype analysis: S.A.S., R.M.A. Sleep data analysis/management: S.R., M.R., S.A.S. Statistical analysis: S.A.S., R.M.A., T.S., L.T.-M., S.R. Manuscript drafting: S.A.S., R.M.A., S.R.P., S.R. Funding: S.A.S., S.R. All authors interpreted data, edited the manuscript for important intellectual content, and approved the final draft. S.A.S. is the guarantor responsible for overall content.
Data Sharing: MESA (Multi-Ethnic Study of Atherosclerosis) data access is managed by the MESA Coordinating Center and requires completion of a Data Distribution Agreement, review and approval of a detailed proposal by MESA publications and steering committees (see mesa-nhlbi.org), and subsequent investigator approval. For requests for statistical analysis code, please contact the corresponding author.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol . 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sands SA, Edwards BA, Terrill PI, Butler JP, Owens RL, Taranto-Montemurro L, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J . 2018;52:1800674. doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Op de Beeck S, Wellman A, Dieltjens M, Strohl KP, Willemen M, Van de Heyning PH, et al. STAR Trial Investigators Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea. Am J Respir Crit Care Med . 2021;203:746–755. doi: 10.1164/rccm.202006-2176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vena D, Azarbarzin A, Marques M, Op de Beeck S, Vanderveken OM, Edwards BA, et al. Predicting sleep apnea responses to oral appliance therapy using polysomnographic airflow. Sleep . 2020;43:zsaa004. doi: 10.1093/sleep/zsaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taranto-Montemurro L, Messineo L, Azarbarzin A, Vena D, Hess LB, Calianese NA, et al. Effects of the combination of atomoxetine and oxybutynin on OSA endotypic traits. Chest . 2020;157:1626–1636. doi: 10.1016/j.chest.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bamagoos AA, Cistulli PA, Sutherland K, Madronio M, Eckert DJ, Hess L, et al. Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc . 2019;16:1422–1431. doi: 10.1513/AnnalsATS.201903-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joosten SA, Leong P, Landry SA, Sands SA, Terrill PI, Mann D, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnea. Sleep (Basel) . 2017;40:zsx094. doi: 10.1093/sleep/zsx094. [DOI] [PubMed] [Google Scholar]
- 8. Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med . 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 9. Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol . 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol (1985) . 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 11. Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) . 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med . 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med . 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taranto-Montemurro L, Messineo L, Sands SA, Azarbarzin A, Marques M, Edwards BA, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med . 2019;199:1267–1276. doi: 10.1164/rccm.201808-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edwards BA, Sands SA, Owens RL, Eckert DJ, Landry S, White DP, et al. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep (Basel) . 2016;39:1973–1983. doi: 10.5665/sleep.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards BA, Sands SA, Owens RL, White DP, Genta PR, Butler JP, et al. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol . 2014;592:4523–4535. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J . 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med . 2018;197:1187–1197. doi: 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep (Basel) . 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bassi M, Giusti H, Leite CM, Anselmo-Franci JA, do Carmo JM, da Silva AA, et al. Central leptin replacement enhances chemorespiratory responses in leptin-deficient mice independent of changes in body weight. Pflugers Arch . 2012;464:145–153. doi: 10.1007/s00424-012-1111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, et al. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med . 1999;159:1477–1484. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]
- 22. Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath . 2008;12:259–264. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 23. Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med . 2006;119:72.e9–72.e14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee RW, Vasudavan S, Hui DS, Prvan T, Petocz P, Darendeliler MA, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep . 2010;33:1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol (1985) . 2008;104:1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tolbert T, Parekh A, Mullins A, Osorio R, Ayappa I, Rapoport DM. Variability of physiologic traits determined by phenotyping using polysomnography on consecutive nights [abstract] Am J Respir Crit Care Med . 2022;205:A4816. [Google Scholar]
- 27. Alex RM, Sofer T, Azarbarzin A, Vena D, Gell LK, Wellman A, et al. Within-night repeatability and long-term consistency of sleep apnea endotypes: the Multi-Ethnic Study of Atherosclerosis and Osteoporotic Fractures in Men Study. Sleep (Basel) . 2022;45:zsac129. doi: 10.1093/sleep/zsac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) . 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax . 2010;65:107–112. doi: 10.1136/thx.2008.112953. [DOI] [PubMed] [Google Scholar]
- 30. Messineo L, Eckert DJ, Taranto-Montemurro L, Vena D, Azarbarzin A, Hess LB, et al. Ventilatory drive withdrawal rather than reduced genioglossus compensation as a mechanism of obstructive sleep apnea in REM sleep. Am J Respir Crit Care Med . 2022;205:219–232. doi: 10.1164/rccm.202101-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med . 2014;190:1293–1300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep . 2009;32:1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med . 2008;27:2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- 34. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis J Stat Softw 2014. 59 1 38 26917999 [Google Scholar]
- 35. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol . 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 36. Redline S, Tishler PV, Tosteson TD, Williamson J, Kump K, Browner I, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med . 1995;151:682–687. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 37. Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Osteoporotic Fractures in Men Study Group Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc . 2011;59:2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang GQ, Cui L, Mueller R, Tao S, Kim M, Rueschman M, et al. The National Sleep Research Resource: towards a sleep data commons. J Am Med Inform Assoc . 2018;25:1351–1358. doi: 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health . 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deeks JJ, Altman DG, Bradburn MJ.Statistical methods for examining heterogeneity and combining results from several studies in meta-analysisEgger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context 2nd edLondon: BMJ Books; 2001. pp. 285–312. [Google Scholar]
- 41. Wang SH, Keenan BT, Wiemken A, Zang Y, Staley B, Sarwer DB, et al. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat. Am J Respir Crit Care Med . 2020;201:718–727. doi: 10.1164/rccm.201903-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abdeyrim A, Zhang Y, Li N, Zhao M, Wang Y, Yao X, et al. Impact of obstructive sleep apnea on lung volumes and mechanical properties of the respiratory system in overweight and obese individuals. BMC Pulm Med . 2015;15:76. doi: 10.1186/s12890-015-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirkness JP. Obesity-related ventilatory phenotypes of sleep-disordered breathing. Am J Respir Crit Care Med . 2014;190:853–854. doi: 10.1164/rccm.201409-1674ED. [DOI] [PubMed] [Google Scholar]
- 44. Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology . 2009;110:908–921. doi: 10.1097/ALN.0b013e31819c74be. [DOI] [PubMed] [Google Scholar]
- 45. Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep (Basel) . 2014;37:1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med . 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 47. Won CHJ, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, et al. Sex differences in obstructive sleep apnea phenotypes, the Multi-Ethnic Study of Atherosclerosis. Sleep (Basel) . 2020;43:zsz274. doi: 10.1093/sleep/zsz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med . 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 49. Jordan AS, Catcheside PG, O’Donoghue FJ, Saunders NA, McEvoy RD. Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol (1985) . 2002;92:410–417. doi: 10.1152/japplphysiol.00461.2001. [DOI] [PubMed] [Google Scholar]
- 50. Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med . 1995;152:725–731. doi: 10.1164/ajrccm.152.2.7633734. [DOI] [PubMed] [Google Scholar]
- 51. Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, et al. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin Endocrinol (Oxf) . 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 52. Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med . 1995;152:1946–1949. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 53. O’Driscoll DM, Landry SA, Pham J, Young A, Sands SA, Hamilton GS, et al. The physiological phenotype of obstructive sleep apnea differs between Caucasian and Chinese patients. Sleep (Basel) . 2019;42:zsz186. doi: 10.1093/sleep/zsz186. [DOI] [PubMed] [Google Scholar]
- 54. Vena D, Taranto-Montemurro L, Azarbarzin A, Op de Beeck S, Marques M, Vanderveken OM, et al. Clinical polysomnographic methods for estimating pharyngeal collapsibility in obstructive sleep apnea. Sleep (Basel) . 2022;45:zsac050. doi: 10.1093/sleep/zsac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zinchuk AV, Chu JH, Liang J, Celik Y, Op de Beeck S, Redeker NS, et al. Physiological traits and adherence to sleep apnea therapy in individuals with coronary artery disease. Am J Respir Crit Care Med . 2021;204:703–712. doi: 10.1164/rccm.202101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnson DA, Guo N, Rueschman M, Wang R, Wilson JG, Redline S. Prevalence and correlates of obstructive sleep apnea among African Americans: the Jackson Heart Sleep Study. Sleep (Basel) . 2018;41:zsy154. doi: 10.1093/sleep/zsy154. [DOI] [PMC free article] [PubMed] [Google Scholar]


