Abstract
Pneumonia imposes a significant clinical burden on people with immunocompromising conditions. Millions of individuals live with compromised immunity because of cytotoxic cancer treatments, biological therapies, organ transplants, inherited and acquired immunodeficiencies, and other immune disorders. Despite broad awareness among clinicians that these patients are at increased risk for developing infectious pneumonia, immunocompromised people are often excluded from pneumonia clinical guidelines and treatment trials. The absence of a widely accepted definition for immunocompromised host pneumonia is a significant knowledge gap that hampers consistent clinical care and research for infectious pneumonia in these vulnerable populations. To address this gap, the American Thoracic Society convened a workshop whose participants had expertise in pulmonary disease, infectious diseases, immunology, genetics, and laboratory medicine, with the goal of defining the entity of immunocompromised host pneumonia and its diagnostic criteria.
Keywords: pneumonia, immunocompromised host, immunosuppression, diagnosis
Contents
Overview
Introduction
Workshop Format and Methods
Defining ICHP
-
ICH Populations
Cancer and HCT Recipients
HIV in the Era of Antiretroviral Therapy
Chronic Immunosuppression and Novel Biologics
Solid Organ Transplantation
Inborn Errors of Immunity
Summary
Consensus Definition
Defining Diagnostic Criteria of ICHP
-
Current Diagnostic Approaches
Clinical Diagnosis of Pneumonia: Lessons Learned from HAP/VAP and CAP Guidelines
Culture-dependent Pathogen Identification in ICHP
Culture-independent Pathogen Identification in ICHP
Metagenomics and Future Approaches to ICHP
Consensus Statement
Conclusions
Overview
Pneumonia imposes a significant clinical burden on people with immunocompromising conditions because of cytotoxic treatments, biological therapies, organ transplants, inherited and acquired immunodeficiencies, and other immune disorders. The absence of a widely accepted definition for immunocompromised host pneumonia (ICHP) is a significant knowledge gap that hampers consistent clinical care and research for infectious pneumonia in these vulnerable populations. To address this gap, the ATS (American Thoracic Society) convened a workshop whose participants had expertise in pulmonary disease, infectious diseases, immunology, genetics, and laboratory medicine, with the goal of defining the entity of ICHP and its diagnostic criteria. Our conclusions include the following:
-
•
ICHP is defined as infectious pneumonia that occurs in an individual with a quantitative or functional host immune defense disorder.
-
•
The diagnostic criteria of ICHP include clinical suspicion of a lung infection, with or without compatible clinical signs and symptoms, and with radiographic evidence of a new or worsening pulmonary infiltrate.
Introduction
Pneumonia is an important cause of morbidity and mortality for all populations, but it disproportionately impacts individuals with impaired immunity to diverse etiologies. In addition to patients undergoing bone marrow-suppressive therapies to treat malignancies, millions of individuals take immunosuppressants and targeted biologics to control autoimmune and inflammatory diseases (1). Nearly all solid organ transplant (SOT) recipients require lifelong immunosuppression to prevent allograft rejection (2). In 2020, more than 37 million people worldwide were living with human immunodeficiency virus (HIV), with 1.5 million new HIV infections diagnosed annually (3). Inherited immunodeficiencies, although less common than treatment-associated immune impairments, also place patients at risk for infections. Immunocompromised hosts (ICHs) can develop pneumonia because of unusual pathogens that are difficult to treat, as well as common pathogens such as Streptococcus pneumoniae or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that can lead to more severe outcomes (4–7).
Despite general recognition of the burden of pneumonia in ICHs, the entity ICHP remains poorly defined. Community-acquired pneumonia (CAP) treatment guidelines explicitly exclude immunocompromised patients because of the heterogeneity of the underlying immunocompromising conditions, challenges in diagnosis because of atypical presentations, as well as the wide array of opportunistic pathogens that confound empiric treatment approaches (8). Only recently has a treatment approach for CAP in ICH been considered, acknowledging that no consensus exists around who should be considered immunocompromised (9). Often ICHs are considered in broad terms without acknowledgment of heterogeneity and degree of immune impairment. To date, no formally endorsed definition of ICHP exists to guide clinical care or to inform the design of interventional trials. The lack of a uniform definition is a significant limitation to consistent clinical care and standardized identification of patients for targeted interventional trials.
To address these challenges, an ATS workshop was convened with the goal of defining ICHP and developing diagnostic criteria for this entity.
Workshop Format and Methods
Workshop participants were invited on the basis of expertise in lung infections and immunocompromised populations, representing fields of pulmonary medicine, laboratory medicine, infectious diseases, immunology, genetics, and research methodology. All participants were vetted for conflicts of interest as per ATS policies. To focus workshop discussions, two rounds of a Delphi survey created by the co-chairs (S.E.E., G.-S.C., and K.C.) were administered electronically to all participants several weeks before the live workshop (see data supplement). These allowed the co-chairs to characterize which topics elicited consensus versus disagreement. Round one consisted of 34 questions pertaining to the definition of ICHP, including patient populations and risk categories, diagnostic criteria involving microbiology and radiology, and clinical trial considerations. Participants responded to each Delphi statement on a Likert scale (strongly agree/agree/neutral/disagree/strongly disagree), with consensus defined as at least 70% agreement of participant responses. A second set of Delphi statements was generated on the basis of responses to the first set, allowing for the refinement of areas of consensus. Areas of strong consensus were retained as initial elements of the definitional statements, and areas of less clear agreement were included in the live workshop agenda for discussion by the entire panel.
The live workshop was conducted May 6–7, 2021, via a two-part videoconference. The first day consisted of brief presentations considering specific ICH populations to conceptually frame the ICHP definition. The second day focused on methods of pathogen detection to derive a diagnostic algorithm for ICHP. After the live workshop, two follow-up videoconferences were held to achieve consensus on proposed definitional statements. These statements do not constitute an evidence-based guideline but rather an expert consensus. The summary of the workshop presentations and consensus statements are reported here.
Defining ICHP
To derive a definition of ICHP, we considered the spectrum of immunocompromised host populations. Discussion centered around the shared characteristics of the patient populations that are conventionally considered immunocompromised, which patients should be considered in this rubric, and under what clinical scenarios should ICHP be suspected. Three key questions framed the discussion: What is the defect in the host immune defense? For what pathogens is the immune-compromised population at increased risk? Is there a quantifiable biomarker that correlates with the degree of risk?
ICH Populations
Cancer and Hematopoietic Cell Transplant (HCT) Recipients
In studies of vaccine efficacy and acute respiratory failure (9–11), patients with cancer are categorized together broadly, regardless of malignancy type or disease stage. However, whereas the morbidity and mortality of CAP are elevated for cancer patients in general, the incidence varies among types of malignancies and treatment phase: patients with lung cancer have a 21-fold increase in pneumonia compared with a 1.7-fold increase in those with breast cancer compared with the general population (12). Patients with myelodysplastic syndromes have a greater risk of developing pneumonia after induction chemotherapy than those with acute leukemias, largely because of profound and persistent cytopenias (13).
The most readily quantifiable immune defect in clinical practice for patients with cancer and receiving HCT is neutropenia, which occurs during different phases of cytotoxic chemotherapy for different cancers. Neutropenia is also intrinsic to marrow-infiltrating disease processes such as hematologic malignancies. The depth and duration of neutropenia confer heterogeneous degrees of vulnerability to infections in these populations (14). The period of neutropenia for most patients with solid tumors receiving chemotherapy is brief, often less than 7 days. Patients with acute leukemias and pre-engraftment HCT recipients frequently have more than 10 days of neutropenia, placing them at substantial risk for ICHP. Profound neutropenia may occur together with or independent of lymphopenia, monocytopenia, and hypogammaglobulinemia, which further increase risk. These immune defects depend on the mechanism of action for the cancer treatments administered. Lymphopenia is also prevalent in patients undergoing cytotoxic cancer treatments. Although the use of absolute lymphocyte count to stratify infection risk is not well defined, it may be used to guide the need for Pneumocystis pneumonia (15) prophylaxis or indicate the likelihood of viral pneumonia (16–19).
Novel cancer therapies, particularly those that exploit intrinsic immune activity against cancer cells, have introduced new iatrogenic immune impairments. In patients who receive chimeric antigen receptor T-cell therapy, the initial neutropenia of conditioning is brief, whereas off-tumor hypogammaglobulinemia can be chronic, increasing the risk for recurrent sinopulmonary infections (20). Patients with lymphoid malignancies (as well as autoimmune diseases and other conditions) receiving anti-CD20 therapies, such as rituximab, have notably poor B-cell responses to vaccination even more than 12 months after receipt of therapy (21). Other mechanisms of immune compromise in patients with cancer include exposure to corticosteroids and other immunosuppressive therapy for the treatment of graft-versus-host disease or checkpoint inhibitor pneumonitis. Patients with cancer who recover their neutrophil counts after cytotoxic therapy are not considered at high risk for infections (14); there may still be persistent leukocyte-mediated defects from cumulative myelosuppressive therapy, and the risk of pneumonia often remains elevated because of other conditions, such as epithelial barrier disruption (i.e., mucositis), poor nutrition, and structural lung disease (22).
HIV in the Era of Antiretroviral Therapy (ART)
In 1997, the advent of ART transformed HIV into a chronic disease and dramatically reduced mortality from opportunistic pulmonary infections such as Pneumocystis pneumonia (23, 24). People with HIV (PWH) (particularly those not on effective ART) remain vulnerable to a variety of opportunistic lung infections depending on their CD4+ T-cell count (25).
The CD4 cell count is the most readily quantifiable immune defect in PWH, with the risk for many opportunistic infections increasing sharply once the CD4 cell count drops below 200 cells/microliter. The risk for bacterial pneumonia also increases with a decreasing CD4 cell count. However, even patients on ART with well-controlled HIV disease and a normal CD4 count have significant impairments to the immune system (26) and an increased risk of nonopportunistic bacterial pneumonia by organisms such as Streptococcus pneumoniae, Hemophilus influenza, Staphylococcus aureus, and Klebsiella pneumoniae (27). PWH with CD4 counts above 500 cells/microliter have a fivefold increased risk of S. pneumoniae pneumonia and invasive pneumococcal disease compared with the general population (28, 29). In addition, the HIV epidemic contributed to a significant resurgence in global rates of Mycobacterium tuberculosis infection, and PWH remain at high risk for tuberculosis even with appropriate ART and preserved CD4 cell counts (30). Thus, although the CD4 cell count is a recognized marker of immunocompromise, a variety of other immune defects, including quantitative and qualitative B-cell abnormalities that result in impaired pathogen-specific antibody production, abnormalities in neutrophil function or numbers, abnormalities in alveolar macrophage function, and alterations in mucociliary function and soluble defense molecules in respiratory secretions, also confer risk for lung infections in PWH (31).
Chronic Immunosuppression and Novel Biologics
Chronic use of corticosteroids is perhaps the most common cause of iatrogenic immune suppression. Corticosteroids remain the first-line therapy for a variety of autoimmune diseases, inflammatory conditions, cancer, SOT rejection, and graft-versus-host disease. Mechanisms of immune suppression include the downregulation of proinflammatory cytokines and chemokines and the dampening of the function of multiple immune cell types, notably macrophages and T cells (32). This renders patients at increased risk for a number of infections, including Pneumocystis pneumonia, invasive aspergillosis, as well as typical causes of CAP (33–35), although this risk is dose-dependent. Increased risk generally occurs when exposed to at least 20 mg equivalent of prednisone daily for more than 2–4 weeks (36); lower doses (i.e., 10 mg/d) taken for prolonged periods can also confer an increased risk (5).
Persons taking novel biologics for autoimmune and inflammatory diseases are susceptible to pneumonia in a predictable manner because of the impairment in both the myeloid and T- and B-cell arms of the immune response (37). Blockade of TNFα (tumor necrosis factor-α), which regulates macrophage and T-cell responses to mycobacteria and granuloma formation, increases susceptibility to tuberculosis (TB) (38, 39) and less commonly, other granulomatous infections, including endemic fungal infections (40). The use of TNFα blockers, including etanercept and infliximab, is increasing (41); consequently, TB cases reported with their use may be increasing in endemic areas, even with latent TB infection screening (42, 43). IL-6 (interleukin 6) inhibitors and targeted small molecule inhibitors like JAK kinase inhibitors have been associated with higher TB risk (44–48). B-cell depletion agents and T-cell costimulatory therapy (abatacept) carry lower risk. Other cytokine blockers (anti–IL-12/IL-23, anti–IL-23, and anti–IL-17) also carry TB warnings, but an elevated TB risk has not been demonstrated to date (49, 50). However, an increased incidence of TB may be anticipated as experience with novel agents accumulates over time. Additional risk accrues with the use of biologics in the setting of other risk factors, including diabetes, cigarette smoking, advanced age, and concomitant use of other immunosuppressants (51).
SOT
With the increasing number of SOT procedures performed around the world, the transplant population at risk for pneumonia continues to expand. The risk of pneumonia is dynamic and depends on the time elapsed from transplant and net state of immunosuppression, which depends on the nature of the induction and maintenance immunosuppressive regimen, underlying comorbidities, and epidemiologic exposure (52). As SOT recipients are typically on multiple immunosuppressive agents concurrently, they are at heightened risk for pneumonia because of opportunistic pathogens, as well as those typically identified in immunocompetent hosts. SOT recipients exhibit disproportionate defects in cellular immunity, predisposing them to viral, fungal, and mycobacterial pulmonary infections. Lung transplant recipients are at the highest risk for pneumonia among SOT recipients, with high attributable mortality, especially in the first year after transplant (53). Certain respiratory pathogens, namely community-acquired respiratory viruses, are associated with an increased risk of lung allograft dysfunction and chronic rejection (54).
Inborn Errors of Immunity
Primary immunodeficiencies (PIDs) comprise a heterogeneous group of diseases caused by inborn errors of immunity (IEI) or specific genetic mutations that confer susceptibility to infections, autoimmune diseases, allergies, bone marrow failure, and malignancy (55). Fungal pneumonia presenting in otherwise apparently healthy individuals has led to the discovery of single-gene IEI and insights into mechanisms of host defense (56). Germline variants of genes involved in the oxidative burst in cytoskeleton/actin polymerization, in cytokine signaling, in C-type lectin receptor signaling, or other transcription factors underlie the basis of many PID syndromes, such as chronic granulomatous disease (e.g., CYBB/gp91phox [X-linked] and NCF1/p47phox [autosomal recessive, AR]), Wiskott-Aldrich syndrome (WAS/WASP [X-linked]), hyper-IgE syndrome (e.g., IL6ST/gp130 [autosomal recessive (AR) or autosomal dominant (AD)], IL6R/IL-6R [AR], STAT3/STAT3 [AD]), to name a few (57). These loss- or gain-of-function gene variants lead to impaired immune cell function and/or lung epithelial cell function, predisposing individuals to severe viral, bacterial, disseminated mycobacterial, and fungal infections. In addition, acquired anticytokine autoantibodies are an important and expanding set of PID mimics that are directed against such targets as IFNγ (interferon γ) (mycobacterial susceptibility), IFNα (severe coronavirus disease [COVID-19] susceptibility), and GM-CSF (granulocyte-macrophage colony-stimulating factor) (cryptococcal and Nocardia susceptibility) (58, 59).
Summary
There was broad consensus that an ICH is an individual with a quantitative or functional immune disorder, and therefore the ICHP definition should be anchored on the host with an identifiable immune defect. The panel acknowledged that the clinical characterization of the host response and its functional impairment is limited to imprecise indices (e.g., leukocyte count and exposure to immunosuppressive medications). A quantifiable immune defect may be feasible for some conditions, such as HIV, in which immune status is at least partially reflected by the CD4 cell count. However, extrapolation of these markers to other disease conditions is not necessarily warranted, accurate, or predictive. Some individuals may lack a quantifiable immune defect by currently available laboratory assays and yet have functional immunocompromise, such as those on chronic corticosteroids or those with chronic granulomatous disease. Other individuals, such as recipients of SOT and HCT, are exposed to various immune-suppressive and immune-modulating agents that cause varying and overlapping effects on the innate, cellular, and humoral arms of the immune system that change over time (60). A method to measure the net state of immunosuppression, as proposed for SOT (61), should be investigated and validated for other ICH populations. Available indirect markers of the immune state can include serum drug concentrations, circulating numbers of immune cells (CD3, CD4, CD8, and natural killer), soluble markers (Ig, C3, MLB, and sCD30), and markers of T-cell function. In the absence of unique biomarkers, the risk of pneumonia in ICH is best gauged using a multifactorial approach that considers various immune and clinical factors in a dynamic fashion (62). Considering all of these elements, the panel endorsed the following statements.
Consensus Definition
ICHP is an infectious pneumonia that occurs in an individual with a quantitative or functional host immune defense disorder.
-
•
Clarifying Statement 1: The host immune defense disorder is a systemic process that results in the impairment of pathogen detection, killing, and/or clearance. Thus, the definition of ICHP is on the basis of the host rather than the identification of a pathogen. Host immune defense disorders encompass innate, cellular, and humoral immune mechanisms (Table 1).
-
•
Clarifying Statement 2: The host immune defense disorder is present at the time of infection. Transient impairment of immune function, as may occur in the setting of severe sepsis, is not sufficient for the patient to be considered inherently immunocompromised. Patients who have a history of immunocompromising conditions that have resolved should not be considered ICHs. For example, patients with cancer without ongoing leukopenia, lymphopenia, immunoglobulin defects, or recent use of immunosuppressive medications are no longer immunocompromised. This includes patients with solid malignancies who have only received local therapy, such as surgical resection or organ-specific radiation therapy.
-
•
Clarifying Statement 3: Systemic conditions with known metabolic effects on immune function should be considered comorbidities but not immunocompromise-defining conditions. These conditions include diabetes and chronic liver dysfunction. Persons of advanced age, although at increased risk for pneumonia, would not be considered ICH under the proposed definition, as increased susceptibility to pneumonia is not synonymous with immunocompromise.
-
•
Clarifying Statement 4: Mechanical and structural lung diseases that increase the risk for pneumonia are not included in this definition of ICHP. These include conditions leading to recurrent aspiration, large airway obstruction, nonsystemic causes of bronchiectasis, chronic obstructive pulmonary disease, or other chronic lung diseases. Although these conditions add additional risk for pneumonia, they are not systemic immune defects.
-
•
Clarifying Statement 5: The host immune defense disorder increases the individual’s risk for more frequent or severe disease caused by common community pathogens. This statement acknowledges that CAP in ICHs with typical organisms, such as S. pneumoniae, can present with more frequent, severe, prolonged, or recurrent manifestations compared with immunocompetent persons.
-
•
Clarifying Statement 6: The host immune defense disorder increases the risk of pneumonia because of uncommon or opportunistic pathogens. ICHs are susceptible to a broader range of lung infections than the general population.
-
•
Clarifying Statement 7: An ICH with pneumonia warrants consideration of additional diagnostic and/or alternative therapeutic strategies beyond those recommended in pneumonia guidelines targeting the general patient population. Opportunistic and multiorganism infections can occur with a host immune defense disorder; hence, pursuing the identification of a specific etiology is important while considering empiric coverage for patients who are ICHs on the basis of the underlying immune impairment.
Table 1.
Types of systemic host defense defects and suggested evaluation
| Defect | Type of Defect | Potential Evaluation |
|---|---|---|
| Innate immunity | Deficiency or dysfunction of innate immune cells: macrophages, monocytes, dendritic cells, neutrophils, NK cells, complement, pulmonary epithelium | CBC with differential, HIV testing, and peripheral blood flow cytometry |
| Consider genetic screening for primary immunodeficiencies and anticytokine autoantibodies (76) | ||
| Cellular immunity | T-cell deficiency or dysfunction | CBC with differential, peripheral blood smear, HIV testing, bone marrow biopsy if testing suggestive of malignancy or lineage failure, peripheral blood flow cytometry, immunoglobulin concentrations |
| Immunosuppressive drug concentrations (if applicable) | ||
| Consider genetic screening for primary immunodeficiencies and anticytokine autoantibodies | ||
| Humoral immunity | B-cell deficiency or dysfunction | CBC with differential, HIV testing, peripheral blood flow cytometry, immunoglobulin concentrations with antibody concentrations after vaccination |
| Immunosuppressive drug concentrations (if applicable) | ||
| Antibody deficiency | Consider genetic screening for primary immunodeficiencies | |
| Immunoglobulin panel |
Definition of abbreviations: CBC = complete blood count; HIV = human immunodeficiency virus; NK = natural killer.
Defining Diagnostic Criteria of ICHP
The definition of ICHP is predicated on a clinical diagnosis of pneumonia and host factors rather than on infectious etiology. However, pathogen identification is central to the appropriate management of ICHP. Just as we acknowledge that the definition of ICHP is limited by a lack of universally reportable markers of host immune dysfunction, diagnostic criteria for ICHP are complicated by limitations in diagnostic tools. The second part of the workshop focused on diagnostic criteria for ICHP. A discussion of recent guidelines, the clinical diagnosis, and currently available diagnostic technologies for pathogen identification is followed by a proposed diagnostic algorithm and clarifying statements.
Current Diagnostic Approaches
Clinical Diagnosis of Pneumonia: Lessons Learned from Hospital-acquired Pneumonia (HAP)/Ventilator-associated Pneumonia (VAP) and CAP Guidelines
The panel that wrote the 2016 HAP/VAP (63, 64) and 2019 CAP (8) guidelines previously encountered challenges that informed our current approach to formulating recommendations for the diagnosis of ICHP. All three clinical entities share similarities: an extensive subject matter, a heterogeneous patient population, and a relative paucity of high-quality evidence. The HAP/VAP and CAP guideline panelists believed that the previously accepted clinical definition of pneumonia, which requires compatible clinical signs and symptoms with radiographic evidence of a new or worsening pulmonary infiltrate, although clearly not 100% sensitive or specific, was clinically useful at the bedside and generally accepted. In other words, any diagnostic criteria or definitions that rely on test results that are not available at the time of initial clinical decision-making will have limited use for clinical care. We, therefore, used this framework as the basis to define the clinical diagnosis of pneumonia in ICH patients, as detailed below, recognizing that this population may lack typical clinical signs such as fever or leukocytosis.
Culture-dependent Pathogen Identification in ICHP
Microbial growth-based methods are specific for the detection of most clinically encountered pathogens, although sensitivity varies depending on the clinical scenario. Identification of a bacterial pathogen with an antimicrobial susceptibility pattern can be established when there is microbial growth. Nonetheless, several significant problems with culture-based approaches remain. Reporting is slow, necessitating the frequent use of empiric antimicrobials in ICHP. Mold infections present a particular challenge in patients who are ICHs when diagnostic confirmation is necessary to optimize therapy and avoid toxicities of empiric antifungals. Unfortunately, mold growth rates are slow, and our ability to recover molds, such as Aspergillus spp., Scedosporium spp., and various mucormycetes, is less than 10% (65–67). Demand for bronchoalveolar lavage (BAL) specimens is increasing, in part because of the introduction of new diagnostic assays (Table 2), which imposes an additional burden, risk for the patient, and additional time to acquire data.
Table 2.
Available diagnostic tests from bronchoalveolar lavage specimens for the evaluation of immunocompromised host pneumonia
| Laboratory | Test(s) | Volume Required (ml) |
|---|---|---|
| Microbiology | Bacterial PCR, culture, and sensitivities Legionella culture and PCR AFB smear and mycobacterial culture and NAAT Fungal PCR, smear, and culture Pneumocystis stains and PCR Aspergillus galactomannan assay β–D-glucan assay |
10 |
| Virology | Respiratory viral PCR CMV rapid culture and/or PCR HHV-6 PCR VZV and HSV PCR |
5 |
| Cytopathology | When malignancy is suspected | 3–5 |
| Hematology | Cell count and differential CD4/CD8 ratio Flow cytometry if hematologic malignancy is suspected |
3–5 |
| Pathology | Pathogen review Staining for microorganisms Tests for noninfectious etiologies (i.e., PAS stain for PAP) |
3–5 |
Definition of abbreviations: AFB = acid-fast bacilli; CMV = cytomegalovirus; HHV = human herpes virus; HSV = herpes simplex virus; NAAT = nucleic acid amplification test; PAP = pulmonary alveolar proteinosis; PAS = periodic acid-Schiff; PCR = polymerase chain reaction; VZV = varicella zoster virus.
Appropriate test selection will be dictated by the clinical scenario and clinical suspicion for opportunistic microorganisms and noninfectious diagnoses.
Culture-independent Pathogen Identification in ICHP
The past decade has seen rapid growth in culture-independent diagnostics for respiratory pathogens. These include the ubiquitous reverse transcription–quantitative polymerase chain reaction (qPCR) testing for SARS-CoV-2, broad multiplex panels for upper and lower respiratory tract specimens, and plasma cell-free DNA for lower respiratory tract specimens. Although not technically molecular tests, enzyme immunoassay antigen tests for Legionella pneumophilia and endemic fungi are culture-independent assays that can be performed on urine or BAL samples. These tests offer faster turnaround times and greater sensitivity than respiratory cultures. Their use has demonstrated the broad array of pathogens that cause ICHP, including Pneumocystis, as well as the lack of respiratory virus seasonality (68) and long-term shedding of viral nucleic acids often seen in ICHs (69). Before the COVID-19 pandemic, new lower respiratory tract multiplex panels offered broad testing of over 24 targets with less than a 90-minute turnaround together with quantitation of the detected pathogens (70); COVID-19 has further accelerated the availability of high-throughput molecular diagnostics. These panels, unsurprisingly, often detect more pathogens than typical culture-based approaches (71). However, these tools lack coverage for fungi, cytomegalovirus, and several important bacterial pathogens in ICH, such as Nocardia, Actinomyces, and Stenotrophomonas (72).
Metagenomics and Future Approaches to ICHP
The existing clinical definition of pneumonia is syndromic and lacks specificity. A promising diagnostic strategy for identifying pathogens in ICHP is the incorporation of metagenomic and metatranscriptomic techniques, in which DNA or RNA from respiratory specimens is extracted, sequenced (with or without PCR amplification), classified, and interpreted. This approach has the advantage of being taxonomically agnostic: capable of identifying pathogens independent of their phylogeny (bacterial, fungal, viral, and protozoal). Rapid detection of resistance genes could inform antimicrobial agent selection faster than culture-based resistance testing. Recent advances in nanopore sequencing have made real-time, on-demand sequencing possible, and early proof-of-principle studies have demonstrated its potential to identify respiratory pathogens within hours of sampling (73, 74). Furthermore, real-time metatranscriptomics could provide clinicians with a rich, high-dimensional characterization of the host response and its impairments. However, the clinical implementation may be challenging because of detection characteristics, bioinformatics requirements, and reimbursement issues (75). Moreover, the increased sensitivity of sequencing-based techniques has blurred the distinctions between pathogens, colonizers, commensals, and contaminants, as the detection of typical pathogens in respiratory specimens does not necessarily imply acute infection.
Culture-based approaches to microbial identification are often too slow and insensitive to inform timely targeted antimicrobial therapy, necessitating the use of empiric treatment regimens. PCR-based methods are faster, but these require a predefined target for identification; even a broad multiplex panel cannot encompass the wide breadth of pathogens in ICHP. Metagenomics may represent the next frontier for understanding pathogen–host interactions, but the clinical application requires further research. With these limitations in mind, the panel developed a clinically practical diagnostic algorithm for ICHP, which accounts for the diversity of clinical presentations and a broad differential of pathogens because of underlying mechanisms of impaired host response and currently available diagnostic modalities.
Consensus Statement
The diagnosis of ICHP requires clinical suspicion of a lung infection, with or without compatible clinical signs and symptoms, and with radiographic evidence of a new or worsening pulmonary infiltrate.
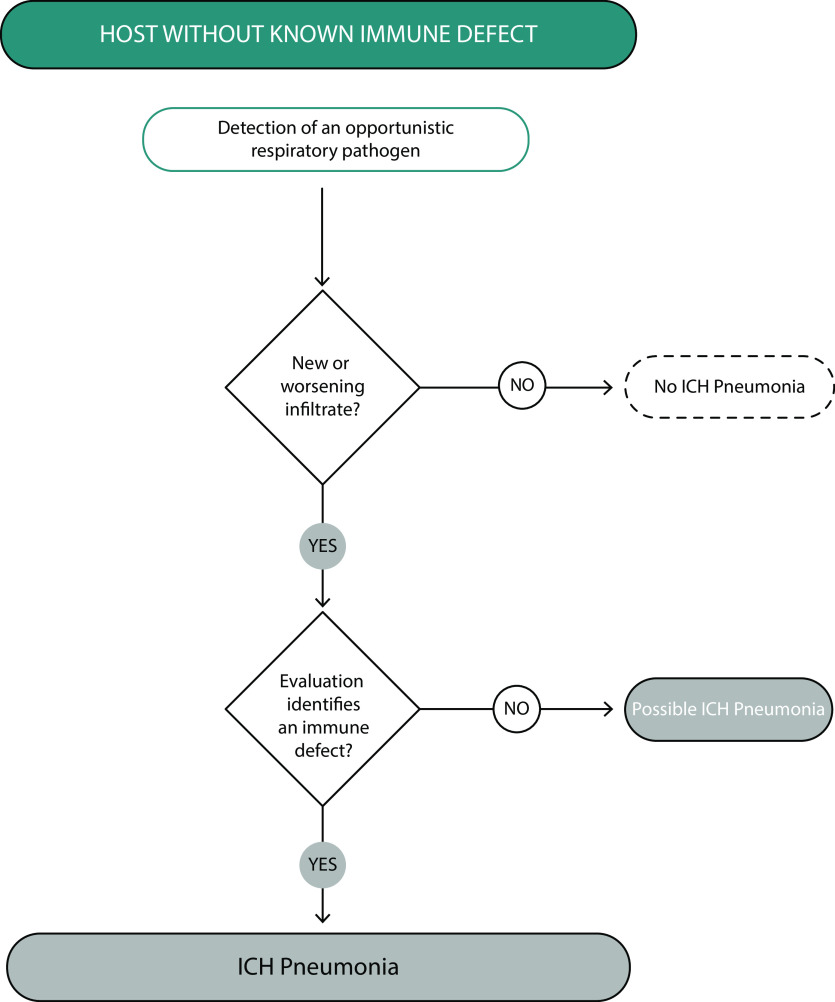
Because of the numerous potential host defects and pathogens that can present as ICHP, the panel determined that the formal diagnostic criteria for ICHP would be best presented as an algorithm. As presented in Figure 1, in a host with a know immune defect, the diagnosis of ICHP is further determined by compatible clinical presentation, possible pathogen identification, and the exclusion of competing causes of the clinical, microbiologic, and radiographic manifestations. Figure 2 presents an alternate diagnostic algorithm for ICHP addressing patients without previously known immune defects who present with evidence of pneumonia caused by an unusual or opportunistic pathogen, raising the possibility of ICHP.
Figure 1.
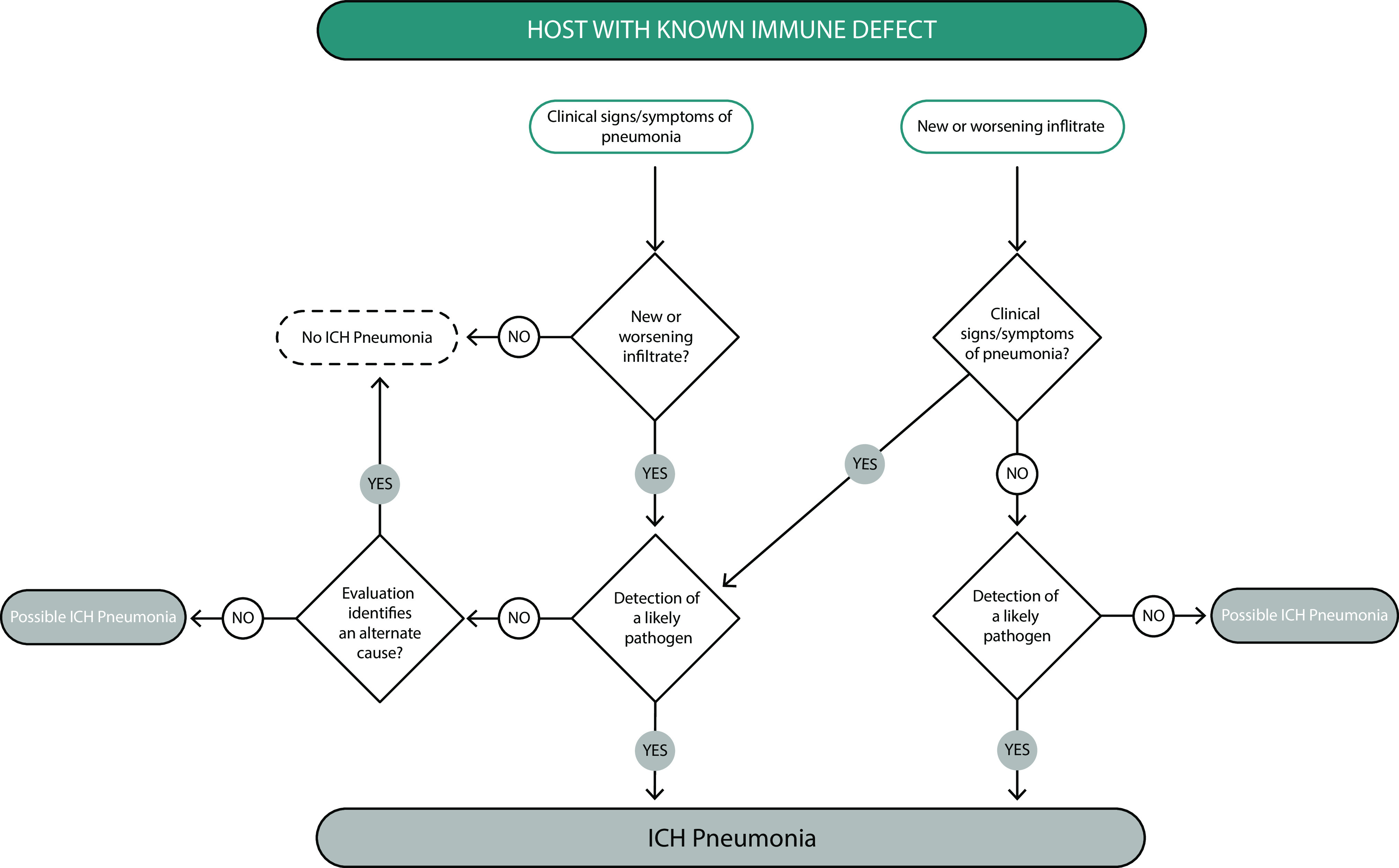
Diagnostic criteria and algorithm for pneumonia in a host with a known immune defect. Clinical signs and symptoms include fever, cough, sputum production, as well as hypoxemia, which should prompt evaluation with chest imaging. In the immunocompromised host, lung infiltrates may not be evident by chest X-ray and may require a computed tomography scan for detection. It should be noted that immunocompromised hosts may have concurrent and multiple infectious and noninfectious etiologies of lung infiltrates (e.g., viral pneumonia, invasive aspergillosis, and congestive heart failure). ICH = immunocompromised hosts.
Figure 2.
Diagnostic criteria and algorithm for pneumonia in a host without a known immune defect. In this scenario, an opportunistic pathogen is detected in a patient with radiographic infiltrates. Underlying immunocompromise may also be suspected with an unusual radiographic presentation that suggests an opportunistic infection (e.g., cystic lesions as a presentation of Pneumocystis pneumonia). ICH = immunocompromised hosts.
-
•
Clarifying statement 1: Clinical presentations of pneumonia are often atypical in ICHs; therefore, signs and symptoms of pneumonia are not required. However, there should be radiographic evidence of a lower respiratory tract infection for a diagnosis of ICHP. Classic signs and symptoms compatible with lung infection include new onset cough, fever, leukocytosis, hypoxemia, shortness of breath, pleuritic chest pain, and bronchial breath sounds. These signs and symptoms are not always present in ICHs, even when radiographic opacities suggestive of pneumonia are present. For example, invasive pulmonary aspergillosis in an HCT recipient on corticosteroids for graft-versus-host disease may present asymptomatically, with lung disease detected incidentally on chest computed tomography. Conversely, there may be high clinical suspicion for pneumonia in an ICH individual with compatible symptoms but a paucity of findings on routine chest X-rays. This should prompt additional evaluation with a chest computed tomography scan. Given multiple nonpulmonary causes of infection and inflammation in the ICH, findings consistent with an infection on imaging must be present for ICHP diagnosis.
-
•
Clarifying Statement 2: Although radiographic abnormalities are required for a diagnosis of infectious pneumonia, not all radiographic findings represent infection. ICH populations are also at risk for a variety of noninfectious pulmonary conditions, which need to be distinguished from infectious pneumonia. For example, the differential diagnosis of lung nodules in a patient with rheumatoid arthritis on etanercept includes rheumatoid nodules and malignancy as well as TB and endemic fungal disease.
-
•
Clarifying Statement 3: Diagnosis of ICHP does not require detection of a causative microorganism, particularly if no compatible alternative noninfectious diagnosis is present. Timely identification of an etiology in ICHP is not always feasible but should not delay empiric therapy if prompt treatment is dictated by the clinical situation. Although there was broad consensus that the diagnosis of ICHP does not require the detection of a causative microorganism, concerted efforts should be made to identify a specific pathogen through available culture-dependent and -independent methods given the broad differential in ICHP and implications for treatment. This will often involve bronchoscopy with BAL and/or transbronchial biopsies to evaluate for typical and opportunistic pathogens, as well as alternative noninfectious diagnoses (Table 2).
-
•
Clarifying statement 4: Identification of an opportunistic organism in the lungs should prompt evaluation for an underlying host immune defect (Figure 2 and Table 3). Detection of an unusual pathogen in an apparently normal host may be indicative of an underlying immune defect. Examples include the case of invasive aspergillosis without apparent risk factors, which may prompt investigation into PID because of inborn errors of immunity. Pneumocystis pneumonia in an individual without iatrogenic immunosuppression should prompt investigation of HIV infection or PID. If an immune defect is found in the setting of such pneumonia, then ICHP is diagnosed.
Table 3.
Opportunistic pathogens in the lung that should prompt consideration of a host immune defect (if not already known to be an immunocompromised host)
| Organism or Clinical Scenario | Type of Defect | Typical Host/Comments | |
|---|---|---|---|
| Bacteria | Recurrent pneumonia or recurrent infections with encapsulated organisms (ex: Streptococcus pneumoniae, Haemophilus influenza, and Neisseria meningitidis) (77–80) | Humoral immunity (B-cell–mediated/antibody deficiency) | Splenectomy/asplenia, CVID, CLL, multiple myeloma, hypogammaglobulinemia, SOT, complement deficiency, IRAK4/MyD88 deficiencies, NEMO deficiency, STAT3 deficiency, IL6ST deficiency |
| Innate immunity (complement deficiency, signaling defect) | Recurrent pneumonia is defined by two or more episodes within a 1-yr period and should be considered an AIDS-defining condition. HIV testing should be pursued if the status is unknown | ||
| Pneumococcal pneumonia and invasive pneumococcal disease (e.g., bacteremia and meningitis) | Humoral immunity | IRAK4/MyD88 deficiencies, NEMO deficiency, SOT | |
| Consider HIV testing with pneumococcal pneumonia and IPD | |||
| Mycobacterium tuberculosis (active TB disease and latent TB infection) (81, 82) | Cellular immunity | Absolute and functional leukopenia, as well as cytokine signaling defects, SOT | |
| CDC recommends HIV screening for all patients with TB disease and LTBI | |||
| Disseminated NTM (83) | Cellular immunity | Hairy cell leukemia, acute leukemia, T-cell lymphoma, advanced HIV infection with CD4 generally < 100 cells/µl, GATA2 deficiency, cytokine signaling defects, anti-IFNγ autoantibodies | |
| Pulmonary NTM | Innate immunity (pulmonary epithelial function) | Bronchiectasis, including cystic fibrosis and other forms of ciliary dyskinesia | |
| Nocardia (84) | Innate immunity (macrophage and neutrophil dysfunction) | Chronic granulomatous disease, MDS, AML, neutropenia, pulmonary alveolar proteinosis, anti–GM-CSF autoantibodies, high-dose systemic corticosteroid use, SOT | |
| Cellular immunity | |||
| Legionella (85) | Cellular immunity | HIV, SOT, hairy cell leukemia, and systemic corticosteroid use | |
| Viruses | Herpes simplex virus (86) | Cellular immunity | CLL, SOT, IEI/PID |
| CMV (87) | Cellular immunity | SOT and HCT, as well as advanced HIV disease (CD4 < 100 cells/µl) | |
| Respiratory infections because of CMV are rare in immunocompetent hosts | |||
| Human herpesvirus-6 (88, 89) | Cellular immunity | HCT, associated with idiopathic pneumonia syndrome | |
| VZV (90) | Cellular immunity | SOT. Although rare, cutaneous, oropharyngeal, or esophageal VZV can disseminate to the lungs | |
| Community-acquired respiratory viruses (10, 91–96) | Cellular immunity | HCT, SOT, hematologic malignancies, corticosteroids, lung transplant, inborn errors of type I IFN immunity or autoantibodies to them | |
| Humoral immunity | The risk of mortality with lower tract disease is greatest with influenza, parainfluenza, respiratory syncytial virus, human metapneumovirus, adenovirus, and SARS-CoV-2 | ||
| Antibody responses to vaccinations may be weak | |||
| Fungi (97, 98) | Invasive pulmonary aspergillosis (99) | Innate immune defects (neutropenia and phagocyte defects) | Neutropenia, chronic granulomatous disease, SOT, STAT3 deficiency, IL6ST deficiency, cytokine signaling defects, high dose systemic corticosteroid use |
| Mucor (Rhizopus spp., Mucor spp., Lichtheimia spp., Cunninghamella, Rhizomucor, and Apophysomyces spp.) | Innate immunity (neutrophil and macrophage dysfunction) | MDS, AML, neutropenia, diabetes mellitus, SOT | |
| Cryptococcus neoformans | Cellular immunity | Hairy cell leukemia, acute leukemia, T-cell lymphoma, SOT, STAT3 deficiency, HIV disease with CD4 < 200 | |
| Pneumocystis jirovecii (100, 101) | Cellular immunity | Hairy cell leukemia, acute leukemia, T-cell lymphoma, severe combined immunodeficiency, cytokine signaling defects, SOT, and other causes of lymphopenia, including HIV disease with CD4 < 200 | |
| Humoral Immunity | |||
| Histoplasmosis | Cellular immunity | SOT, cytokine signaling defects, STAT3 deficiency, STAT1 gain-of-function mutations, and HIV disease with CD4 < 100–200 | |
| Coccidioidomycosis | Cellular immunity | SOT, cytokine signaling defects, STAT3 deficiency, STAT1 gain-of-function mutations, and HIV disease (typically with CD4 < 250) | |
| Parasites (102) | Strongyloides stercoralis | Cellular immunity | HTLV-1 infection, HIV, SOT, hypogammaglobulinemia, and corticosteroid use |
| Toxoplasma gondii | Cellular immunity | Hairy cell leukemia, SOT, acute leukemia, T-cell lymphoma, and HIV disease with CD4 < 100 |
Definition of abbreviations: AIDS = acquired immunodeficiency syndrome; AML = acute myelogenous leukemia; CDC = Centers for Disease Control and Prevention; CLL = chronic lymphoblastic leukemia; CMV = cytomegalovirus; CVID = common variable immunodeficiency; GATA2 = GATA-binding protein 2; GM-CSF = granulocyte-macrophage colony-stimulating factor; HCT = hematopoietic cell transplant; HIV = human immunodeficiency virus; HTLV-1 = human T-lymphotropic virus-1; IEI = inborn errors of immunity; IFN = interferon; IL6ST = interleukin 6 cytokine family signal transducer; IPD = invasive pneumococcal disease; IRAK4 = interleukin-1 receptor-associated kinase 4; LTBI = latent tuberculosis infection; MDS = myelodysplastic syndrome; MyD88 = myeloid differentiation primary response 88; NEMO = nuclear factor-kappa B essential modulator; NTM = nontuberculous mycobacteria; PID = primary immunodeficiency; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SOT = solid organ transplant; STAT = signal transducer and activator of transcription; TB = tuberculosis; VZV = varicella zoster virus.
Conclusions
The use of the proposed definition of ICHP and diagnostic algorithms is twofold: first, to provide a conceptual framework for clinicians to recognize and diagnose pneumonia in ICHs; second, to provide consistent criteria for the inclusion of ICH in clinical studies and interventional trials. By consensus, we anchor the definition of ICHP on the presence of a host immune defense disorder that precedes infectious pneumonia. The definitions of ICH and ICHP will evolve with a further understanding of immunity. We have highlighted the need to identify additional quantifiable biomarkers and composite indices of immune impairment that more accurately reflect an individual’s state of immunocompromise. Immunophenotyping of ICH populations and correlation with disease presentations and pathogen burden would allow for ICHP risk stratification that could be translated to the clinic. The development of more sensitive techniques in pathogen detection, such as metagenomics, alongside clinical immunophenotyping, will improve diagnostic and therapeutic approaches and aid the refinement of our understanding of ICHP. ICHs are a vulnerable and underserved population whose care will benefit from clearer definitions and further investigations.
Acknowledgments
Acknowledgment
The authors would like to thank Larry Mose and Marty Levkova for their assistance with the graphic design of the figures and Darrah Thomas for assistance in preparing the manuscript. The authors would also like to thank Kimberly Lawrence of the ATS for her guidance in managing the online workshop during the COVID-19 pandemic.
This official workshop report was developed by an ad hoc subcommittee of the ATS Assembly on Pulmonary Infections and Tuberculosis.
Members of the subcommittee are as follows:
Guang-Shing Cheng, M.D. (Co-Chair)1,2
Kristina Crothers, M.D. (Co-Chair)2,3
Scott E. Evans, M.D. (Co-Chair)4
Stefano Aliberti, M.D.5*
Anne Bergeron, M.D., Ph.D.6*
Michael Boeckh, M.D., Ph.D.1,2*
Jason W. Chien, M.D., M.S.7*
Catia Cilloniz, M.D.8,9*
Keira Cohen, M.D.10*
Nathan Dean, M.D.11,12*
Charles S. Dela Cruz, M.D., Ph.D.13,14*
Robert P. Dickson, M.D.15‡
Alexander L. Greninger, M.D., Ph.D., M.S., M.Phil.16‡
Chadi A. Hage, M.D.17‡
Tobias M. Hohl, M.D., Ph.D.18*
Steven M. Holland, M.D.19*
Barbara E. Jones, M.D.12,20*
Joseph Keane, M.D.21‡
Mark Metersky, M.D.22‡
Rachel Miller, M.D.23*
Anne Puel, Ph.D.24,25,26‡
Julio Ramirez, M.D.27*
Marcos I. Restrepo, M.D.28,29*
Ajay Sheshadri, M.D., M.S.C.I.4*
Bashar Staitieh, M.D.30‡
Jeffrey Tarrand, M.D.31‡
Kevin L. Winthrop, M.D.32*
Richard G. Wunderink, M.D.33*
1Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington; 2Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington; 3Veterans Administration Puget Sound Health Care System, Seattle, Washington; 4Department of Pulmonary Medicine, University of Texas MD Anderson Cancer Center, Houston, Texas; 5University of Milan, Milan, Italy; 6Pneumology Department, University of Geneva, Geneva, Switzerland; 7Janssen Pharmaceuticals, Brisbane, California; 8University of Barcelona, Barcelona, Spain; 9Faculty of Health Sciences, Continental University, Huancayo, Peru; 10Johns Hopkins University, Baltimore, Maryland; 11Intermountain Medical Center, Murray, Utah; 12Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah, Salt Lake City, Utah; 13Section of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, Yale University, New Haven, Connecticut; 14Veterans Affairs Connecticut Healthcare System, New Haven, Connecticut; 15University of Michigan Medical School, Ann Arbor, Michigan; 16Division of Virology, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington; 17Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh, Lung Transplant-UPMC, Pittsburgh, Pennsylvania; 18Department of Medicine, Human Oncology and Pathogenesis Program, and Immunology Program, Memorial Sloan Kettering Cancer Center, New York, New York; 19National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; 20Veterans Administration, Salt Lake City, Utah; 21Clinical Medicine, St. James’s Hospital, Trinity College, Dublin, Ireland; 22University of Connecticut School of Medicine, Farmington, Connecticut; 23Division of Infectious Diseases, Department of Internal Medicine, Duke University, Durham, North Carolina; 24Laboratory of Human Genetics of Infectious Diseases Paris, INSERM, Paris, France; 25University of Paris Cité, Imagine Institute, Paris, France; 26Rockefeller University, New York, New York; 27Division of Infectious Diseases, University of Louisville, Louisville, Kentucky; 28South Texas Veterans Health Care System, San Antonio, Texas; 29Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Texas Health San Antonio, San Antonio, Texas; 30Division of Pulmonary, Allergy, Critical Care & Sleep Medicine, Department of Medicine, Emory University, Atlanta, Georgia; 31Department of Laboratory Medicine, University of Texas MD Anderson Cancer Center, Houston, Texas; 32Oregon Health Sciences University, Portland, Oregon; and 33Pulmonary and Critical Care Division, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
*Participant.
‡Speaker.
Footnotes
This official Workshop Report of the American Thoracic Society was approved October 2022
The Department of Veterans Affairs did not have a role in the conduct of the study, in the collection, management, analysis, interpretation of data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
This document has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: G.-S.C. served on advisory board for Fred Hutch Protocol; served as board member for The Firland Foundation; served as consultant for Janssen; received research from NIH; received travel support from Joan Clark Funds for Fred Hutch Pulmonary Faculty. A.B. served as consultant for Zambon; served as speaker for Pfizer and Shire; received research support from Johnson & Johnson, SOS Oxygen, and Zambon. M.B. served as consultant for Allovir, EvrysBio, GlaxoSmithKline, Helocyte, Janssen, Kyorin, Merck, ReViral, Symbio, and Vir Bio; financial stake in Helocyte and Evrys Bio; received research support from Ansun Biopharma, Astellas, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Regeneron, Ridgeback, and Vir Bio; received royalties from UptoDate. J.W.C. is employed by Gilead Sciences and Johnson & Johnson; has financial stake in Johnson & Johnson. K. Cohen is employed by Johnson and Johnson; served on advisory committee for Paratek; served as consultant for AN2, HillRom, Insmed, Merck, Microbion, and Paratek; received research support from Burroughs Welcome Fund, COPD Foundation, Cystic Fibrosis Foundation, HillRom, Insmed, NIH/NHLBI, and Spero Therapeutics. N.D. served as consultant for Biofire and Merck; served on data safety and monitoring board for Clinipace and Contrafect. A.L.G. served as expert witness for Olympus; financial stake (partner) in LabCorp; received research support from Abbott, Bill & Melinda Gates Foundation, Cepheid, Gilead, Hologic, Janssen, Merck, Novavax, and Pfizer. M.M. served on advisory committee for Insmed, International Biophysics, and Savara; served as consultant for Shionogi and Zambon; served as speaker for Prime Education; received research support from Insmed. J.R. received research support from Pfizer. A.S. served as consultant for Enanta Pharmaceuticals and PsiOxus Therapeutics; received honoraria from Med Learning Group; received research support from NIH and Gateway for Cancer Research. B.S. received research support from the National Institute of Alcohol Abuse and Alcoholism, 1K08AA024512-01A1. K.L.W. served as consultant for Bristol Myers Squibb, Insmed, Lilly, Pfizer, and UCB; served on data safety and monitoring board for Abbvie and Roche; received research support for Bristol Myers Squibb. R.G.W. served on advisory committee for bioMerieux and Merck; served as consultant for La Jolla and Shionogi; served on data safety and monitoring board for Pfizer and Vir; served as speaker for bioMerieux and WebMD. S.E.E. has financial stake in and receives royalties from Pulmotect, Inc; holds US and international patents related to stimulating lung defenses. K. Crothers, S.A., C.C., C.S.D.C., R.P.D., C.A.H., T.M.H., S.M.H., B.J., J.K., R.M., A.P., M.I.R., J.T. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA . 2016;316:2547–2548. doi: 10.1001/jama.2016.16477. [DOI] [PubMed] [Google Scholar]
- 2. Pilch NA, Bowman LJ, Taber DJ. Immunosuppression trends in solid organ transplantation: the future of individualization, monitoring, and management. Pharmacotherapy . 2021;41:119–131. doi: 10.1002/phar.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. 2022. https://www.unaids.org/en/resources/fact-sheet
- 4. Yoke LH, Lee JM, Krantz EM, Morris J, Marquis S, Bhattacharyya P, et al. Clinical and virologic characteristics and outcomes of coronavirus disease 2019 at a cancer center. Open Forum Infect Dis . 2021;8:ofab193. doi: 10.1093/ofid/ofab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ward D, Gørtz S, Thomson Ernst M, Andersen NN, Kjær SK, Hallas J, et al. The effect of immunosuppressants on the prognosis of SARS-CoV-2 infection. Eur Respir J . 2022;59:2100769. doi: 10.1183/13993003.00769-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sousa D, Justo I, Domínguez A, Manzur A, Izquierdo C, Ruiz L, et al. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin Microbiol Infect . 2013;19:187–192. doi: 10.1111/j.1469-0691.2012.03765.x. [DOI] [PubMed] [Google Scholar]
- 7. Di Pasquale MF, Sotgiu G, Gramegna A, Radovanovic D, Terraneo S, Reyes LF, et al. GLIMP Investigators Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis . 2019;68:1482–1493. doi: 10.1093/cid/ciy723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramirez JA, Musher DM, Evans SE, Dela Cruz C, Crothers KA, Hage CA, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest . 2020;158:1896–1911. doi: 10.1016/j.chest.2020.05.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes K, Middleton DB, Nowalk MP, Balasubramani GK, Martin ET, Gaglani M, et al. HAIVEN Study Investigators Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in immunocompromised adults. Clin Infect Dis . 2021;73:e4353–e4360. doi: 10.1093/cid/ciaa1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azoulay E, Russell L, Van de Louw A, Metaxa V, Bauer P, Povoa P, et al. Nine-i Investigators Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med . 2020;46:298–314. doi: 10.1007/s00134-019-05906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmedt N, Heuer OD, Häckl D, Sato R, Theilacker C. Burden of community-acquired pneumonia, predisposing factors and health-care related costs in patients with cancer. BMC Health Serv Res . 2019;19:30. doi: 10.1186/s12913-018-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia JB, Lei X, Wierda W, Cortes JE, Dickey BF, Evans SE, et al. Pneumonia during remission induction chemotherapy in patients with acute leukemia. Ann Am Thorac Soc . 2013;10:432–440. doi: 10.1513/AnnalsATS.201304-097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. 2020. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1457
- 15. Williams KM, Ahn KW, Chen M, Aljurf MD, Agwu AL, Chen AR, et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant . 2016;51:573–580. doi: 10.1038/bmt.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah DP, Ghantoji SS, Ariza-Heredia EJ, Shah JN, El Taoum KK, Shah PK, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood . 2014;123:3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chemaly RF, Hanmod SS, Rathod DB, Ghantoji SS, Jiang Y, Doshi A, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood . 2012;119:2738–2745. doi: 10.1182/blood-2011-08-371112. [DOI] [PubMed] [Google Scholar]
- 18. Martino R, Porras RP, Rabella N, Williams JV, Rámila E, Margall N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant . 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seo S, Gooley TA, Kuypers JM, Stednick Z, Jerome KR, Englund JA, et al. Human metapneumovirus infections following hematopoietic cell transplantation: factors associated with disease progression. Clin Infect Dis . 2016;63:178–185. doi: 10.1093/cid/ciw284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kampouri E, Walti CS, Gauthier J, Hill JA. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev Hematol . 2022;15:305–320. doi: 10.1080/17474086.2022.2063833. [DOI] [PubMed] [Google Scholar]
- 21. Ito Y, Honda A, Kurokawa M. COVID-19 mRNA vaccine in patients with lymphoid malignancy or anti-CD20 antibody therapy: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk . 2022;22:e691–e707. doi: 10.1016/j.clml.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong JL, Evans SE. Bacterial pneumonia in patients with cancer: novel risk factors and management. Clin Chest Med . 2017;38:263–277. doi: 10.1016/j.ccm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest . 1998;113:1225–1229. doi: 10.1378/chest.113.5.1225. [DOI] [PubMed] [Google Scholar]
- 24. Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS . 2006;20:1095–1107. doi: 10.1097/01.aids.0000226949.64600.f9. [DOI] [PubMed] [Google Scholar]
- 25. Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev . 2020;100:603–632. doi: 10.1152/physrev.00039.2018. [DOI] [PubMed] [Google Scholar]
- 26. Staitieh BS, Egea EE, Guidot DM. Pulmonary innate immune dysfunction in human immunodeficiency virus. Am J Respir Cell Mol Biol . 2017;56:563–567. doi: 10.1165/rcmb.2016-0213TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zifodya JS, Crothers K. Treating bacterial pneumonia in people living with HIV. Expert Rev Respir Med . 2019;13:771–786. doi: 10.1080/17476348.2019.1634546. [DOI] [PubMed] [Google Scholar]
- 28. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garcia Garrido HM, Mak AMR, Wit FWNM, Wong GWM, Knol MJ, Vollaard A, et al. Incidence and risk factors for invasive pneumococcal disease and community-acquired pneumonia in human immunodeficiency virus-infected individuals in a high-income setting. Clin Infect Dis . 2020;71:41–50. doi: 10.1093/cid/ciz728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auld SC, Staitieh BS. HIV and the tuberculosis “set point”: how HIV impairs alveolar macrophage responses to tuberculosis and sets the stage for progressive disease. Retrovirology . 2020;17:32. doi: 10.1186/s12977-020-00540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charles TP, Shellito JE. Human immunodeficiency virus infection and host defense in the lungs. Semin Respir Crit Care Med . 2016;37:147–156. doi: 10.1055/s-0036-1572553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol . 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ledoux MP, Guffroy B, Nivoix Y, Simand C, Herbrecht R. Invasive pulmonary aspergillosis. Semin Respir Crit Care Med . 2020;41:80–98. doi: 10.1055/s-0039-3401990. [DOI] [PubMed] [Google Scholar]
- 34. Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic PA, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect . 2020;27:790.e1–790.e5. doi: 10.1016/j.cmi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration . 2017;94:299–311. doi: 10.1159/000479089. [DOI] [PubMed] [Google Scholar]
- 36. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am . 2016;42:157–76. doi: 10.1016/j.rdc.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keane J, Bresnihan B. Tuberculosis reactivation during immunosuppressive therapy in rheumatic diseases: diagnostic and therapeutic strategies. Curr Opin Rheumatol . 2008;20:443–449. doi: 10.1097/BOR.0b013e3283025ec2. [DOI] [PubMed] [Google Scholar]
- 38. Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis . 2003;3:148–155. doi: 10.1016/s1473-3099(03)00545-0. [DOI] [PubMed] [Google Scholar]
- 39. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med . 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 40. Baddley JW, Cantini F, Goletti D, Gómez-Reino JJ, Mylonakis E, San-Juan R, et al. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-α agents) Clin Microbiol Infect . 2018;24:S10–S20. doi: 10.1016/j.cmi.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 41. Hanly JG, Lethbridge L. Use of disease-modifying antirheumatic drugs, biologics, and corticosteroids in older patients with rheumatoid arthritis over 20 years. J Rheumatol . 2021;48:977–984. doi: 10.3899/jrheum.200310. [DOI] [PubMed] [Google Scholar]
- 42. Minozzi S, Bonovas S, Lytras T, Pecoraro V, Gonzalez-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf . 2016;15:11–34. doi: 10.1080/14740338.2016.1240783. [DOI] [PubMed] [Google Scholar]
- 43. Cantini F, Nannini C, Niccoli L, Iannone F, Delogu G, Garlaschi G, et al. SAFEBIO (Italian multidisciplinary task force for screening of tuberculosis before and during biologic therapy) Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev . 2015;14:503–509. doi: 10.1016/j.autrev.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 44. Winthrop KL, Mariette X, Silva JT, Benamu E, Calabrese LH, Dumusc A, et al. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors) Clin Microbiol Infect . 2018;24:S21–S40. doi: 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 45. Winthrop KL, Harigai M, Genovese MC, Lindsey S, Takeuchi T, Fleischmann R, et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis . 2020;79:1290–1297. doi: 10.1136/annrheumdis-2019-216852. [DOI] [PubMed] [Google Scholar]
- 46. Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, Tanaka Y, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis . 2016;75:1133–1138. doi: 10.1136/annrheumdis-2015-207319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol . 2014;41:15–23. doi: 10.3899/jrheum.130466. [DOI] [PubMed] [Google Scholar]
- 48. Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther . 2011;13:R141. doi: 10.1186/ar3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evangelatos G, Koulouri V, Iliopoulos A, Fragoulis GE. Tuberculosis and targeted synthetic or biologic DMARDs, beyond tumor necrosis factor inhibitors. Ther Adv Musculoskelet Dis . 2020;12:1759720X20930116. doi: 10.1177/1759720X20930116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nogueira M, Warren RB, Torres T. Risk of tuberculosis reactivation with interleukin (IL)-17 and IL-23 inhibitors in psoriasis—time for a paradigm change. J Eur Acad Dermatol Venereol . 2021;35:824–834. doi: 10.1111/jdv.16866. [DOI] [PubMed] [Google Scholar]
- 51. Harigai M, Koike R, Miyasaka N, Pneumocystis Pneumonia under Anti-Tumor Necrosis Factor Therapy (PAT) Study Group Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med . 2007;357:1874–1876. doi: 10.1056/NEJMc070728. [DOI] [PubMed] [Google Scholar]
- 52. Roberts MB, Fishman JA. Immunosuppressive agents and infectious risk in transplantation: managing the “net state of immunosuppression”. Clin Infect Dis . 2021;73:e1302–e1317. doi: 10.1093/cid/ciaa1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dulek DE, Mueller NJ, AST Infectious Diseases Community of Practice Pneumonia in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant . 2019;33:e13545. doi: 10.1111/ctr.13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fisher CE, Preiksaitis CM, Lease ED, Edelman J, Kirby KA, Leisenring WM, et al. Symptomatic respiratory virus infection and chronic lung allograft dysfunction. Clin Infect Dis . 2016;62:313–319. doi: 10.1093/cid/civ871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies expert committee. J Clin Immunol . 2022;42:1473–1507. doi: 10.1007/s10875-022-01289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li J, Vinh DC, Casanova JL, Puel A. Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol . 2017;40:46–57. doi: 10.1016/j.mib.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. The ever-increasing array of novel inborn errors of immunity: an interim update by the IUIS committee. J Clin Immunol . 2021;41:666–679. doi: 10.1007/s10875-021-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng A, Holland SM. Anticytokine autoantibodies: autoimmunity trespassing on antimicrobial immunity. J Allergy Clin Immunol . 2022;149:24–28. doi: 10.1016/j.jaci.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Puel A, Bastard P, Bustamante J, Casanova JL. Human autoantibodies underlying infectious diseases. J Exp Med . 2022;219:e20211387. doi: 10.1084/jem.20211387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Kessel DA, Hoffman TW, Kwakkel-van Erp JM, Oudijk ED, Zanen P, Rijkers GT, et al. Long-term follow-up of humoral immune status in adult lung transplant recipients. Transplantation . 2017;101:2477–2483. doi: 10.1097/TP.0000000000001685. [DOI] [PubMed] [Google Scholar]
- 61. Ling X, Xiong J, Liang W, Schroder PM, Wu L, Ju W, et al. Can immune cell function assay identify patients at risk of infection or rejection? A meta-analysis. Transplantation . 2012;93:737–743. doi: 10.1097/TP.0b013e3182466248. [DOI] [PubMed] [Google Scholar]
- 62. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med . 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 63. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63:575–582. doi: 10.1093/cid/ciw504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tarrand JJ, Lichterfeld M, Warraich I, Luna M, Han XY, May GS, et al. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol . 2003;119:854–858. doi: 10.1309/EXBV-YAUP-ENBM-285Y. [DOI] [PubMed] [Google Scholar]
- 66. Tarrand JJ, Han XY, Kontoyiannis DP, May GS. Aspergillus hyphae in infected tissue: evidence of physiologic adaptation and effect on culture recovery. J Clin Microbiol . 2005;43:382–386. doi: 10.1128/JCM.43.1.382-386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kontoyiannis DP, Chamilos G, Hassan SA, Lewis RE, Albert ND, Tarrand JJ. Increased culture recovery of zygomycetes under physiologic temperature conditions. Am J Clin Pathol . 2007;127:208–212. doi: 10.1309/7KU5XWURYM0151YN. [DOI] [PubMed] [Google Scholar]
- 68. Milano F, Campbell AP, Guthrie KA, Kuypers J, Englund JA, Corey L, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood . 2010;115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. de Lima CR, Mirandolli TB, Carneiro LC, Tusset C, Romer CM, Andreolla HF, et al. Prolonged respiratory viral shedding in transplant patients. Transpl Infect Dis . 2014;16:165–169. doi: 10.1111/tid.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Buchan BW, Armand-Lefevre L, Anderson N. Molecular diagnosis of pneumonia (including multiplex panels) Clin Chem . 2021;68:59–68. doi: 10.1093/clinchem/hvab143. [DOI] [PubMed] [Google Scholar]
- 71. Buchan BW, Windham S, Balada-Llasat JM, Leber A, Harrington A, Relich R, et al. Practical comparison of the BioFire FilmArray pneumonia panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol . 2020;58:e00135-20. doi: 10.1128/JCM.00135-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Murphy CN, Fowler R, Balada-Llasat JM, Carroll A, Stone H, Akerele O, et al. Multicenter evaluation of the BioFire FilmArray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol . 2020;58:e00128-20. doi: 10.1128/JCM.00128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pendleton KM, Erb-Downward JR, Bao Y, Branton WR, Falkowski NR, Newton DW, et al. Rapid pathogen identification in bacterial pneumonia using real-time metagenomics. Am J Respir Crit Care Med . 2017;196:1610–1612. doi: 10.1164/rccm.201703-0537LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Charalampous T, Kay GL, Richardson H, Aydin A, Baldan R, Jeanes C, et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat Biotechnol . 2019;37:783–792. doi: 10.1038/s41587-019-0156-5. [DOI] [PubMed] [Google Scholar]
- 75. Greninger AL. The challenge of diagnostic metagenomics. Expert Rev Mol Diagn . 2018;18:605–615. doi: 10.1080/14737159.2018.1487292. [DOI] [PubMed] [Google Scholar]
- 76. Zhang H, He F, Li P, Hardwidge PR, Li N, Peng Y. The role of innate immunity in pulmonary infections. BioMed Res Int . 2021;2021:6646071. doi: 10.1155/2021/6646071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bonanni P, Grazzini M, Niccolai G, Paolini D, Varone O, Bartoloni A, et al. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum Vaccin Immunother . 2017;13:359–368. doi: 10.1080/21645515.2017.1264797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nonas S. Pulmonary manifestations of primary immunodeficiency disorders. Immunol Allergy Clin North Am . 2015;35:753–766. doi: 10.1016/j.iac.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 79. Cinetto F, Scarpa R, Rattazzi M, Agostini C. The broad spectrum of lung diseases in primary antibody deficiencies. Eur Respir Rev . 2018;27:180019. doi: 10.1183/16000617.0019-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Skattum L, van Deuren M, van der Poll T, Truedsson L. Complement deficiency states and associated infections. Mol Immunol . 2011;48:1643–1655. doi: 10.1016/j.molimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 81. Lombardi A, Villa S, Castelli V, Bandera A, Gori A. T-cell exhaustion in Mycobacterium tuberculosis and nontuberculous mycobacteria infection: pathophysiology and therapeutic perspectives. Microorganisms . 2021;9:2460. doi: 10.3390/microorganisms9122460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lewinsohn DM, Lewinsohn DA. New concepts in tuberculosis host defense. Clin Chest Med . 2019;40:703–719. doi: 10.1016/j.ccm.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 83. Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis . 2015;15:968–980. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 84. Lynch JP, III, Reid G, Clark NM. Nocardia spp.: a rare cause of pneumonia globally. Semin Respir Crit Care Med . 2020;41:538–554. doi: 10.1055/s-0040-1708816. [DOI] [PubMed] [Google Scholar]
- 85. Schlossberg D, Bonoan J. Legionella and immunosuppression. Semin Respir Infect . 1998;13:128–131. [PubMed] [Google Scholar]
- 86. Jellinge ME, Hansen F, Coia JE, Song Z. Herpes simplex virus type 1 pneumonia—a review. J Intensive Care Med . 2021;36:1398–1402. doi: 10.1177/0885066620965941. [DOI] [PubMed] [Google Scholar]
- 87. Fonseca Brito L, Brune W, Stahl FR. Cytomegalovirus (CMV) pneumonitis: cell tropism, inflammation, and immunity. Int J Mol Sci . 2019;20:3865. doi: 10.3390/ijms20163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hill JA, Vande Vusse LK, Xie H, Chung EL, Yeung CCS, Seo S, et al. Human herpesvirus 6b and lower respiratory tract disease after hematopoietic cell transplantation. J Clin Oncol . 2019;37:2670–2681. doi: 10.1200/JCO.19.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Seo S, Renaud C, Kuypers JM, Chiu CY, Huang ML, Samayoa E, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood . 2015;125:3789–3797. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert Rev Vaccines . 2017;16:391–400. doi: 10.1080/14760584.2017.1294069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Piñana JL, Pérez A, Montoro J, Hernani R, Lorenzo I, Giménez E, et al. The effect of timing on community acquired respiratory virus infection mortality during the first year after allogeneic hematopoietic stem cell transplantation: a prospective epidemiological survey. Bone Marrow Transplant . 2020;55:431–440. doi: 10.1038/s41409-019-0698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pochon C, Voigt S. Respiratory virus infections in hematopoietic cell transplant recipients. Front Microbiol . 2019;9:3294. doi: 10.3389/fmicb.2018.03294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis . 2014;59:S344–S351. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. COVID-STORM Clinicians COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science . 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Munting A, Manuel O. Viral infections in lung transplantation. J Thorac Dis . 2021;13:6673–6694. doi: 10.21037/jtd-2021-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA . 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology . 2012;17:913–926. doi: 10.1111/j.1440-1843.2012.02150.x. [DOI] [PubMed] [Google Scholar]
- 98. Pergam SA. Fungal pneumonia in patients with hematologic malignancies and hematopoietic cell transplantation. Clin Chest Med . 2017;38:279–294. doi: 10.1016/j.ccm.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 99. Thompson GR, III, Young JH. Aspergillus infections. N Engl J Med . 2021;385:1496–1509. doi: 10.1056/NEJMra2027424. [DOI] [PubMed] [Google Scholar]
- 100. Charpentier E, Ménard S, Marques C, Berry A, Iriart X. Immune response in Pneumocystis infections according to the host immune system status. J Fungi (Basel) . 2021;7:625. doi: 10.3390/jof7080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fishman JA. Pneumocystis jiroveci. Semin Respir Crit Care Med . 2020;41:141–157. doi: 10.1055/s-0039-3399559. [DOI] [PubMed] [Google Scholar]
- 102. Kunst H, Mack D, Kon OM, Banerjee AK, Chiodini P, Grant A. Parasitic infections of the lung: a guide for the respiratory physician. Thorax . 2011;66:528–536. doi: 10.1136/thx.2009.132217. [DOI] [PubMed] [Google Scholar]