Abstract
Rationale
We have previously shown that hospital strain is associated with intensive care unit (ICU) admission and that ICU admission, compared with ward admission, may benefit certain patients with acute respiratory failure (ARF).
Objectives
To understand how strain–process–outcomes relationships in patients with ARF may vary among hospitals and what hospital practice differences may account for such variation.
Methods
We examined high-acuity patients with ARF who did not require mechanical ventilation or vasopressors in the emergency department (ED) and were admitted to 27 U.S. hospitals from 2013 to 2018. Stratifying by hospital, we compared hospital strain–ICU admission relationships and hospital length of stay (LOS) and mortality among patients initially admitted to the ICU versus the ward using hospital strain as a previously validated instrumental variable. We also surveyed hospital practices and, in exploratory analyses, evaluated their associations with the above processes and outcomes.
Results
There was significant among-hospital variation in ICU admission rates, in hospital strain–ICU admission relationships, and in the association of ICU admission with hospital LOS and hospital mortality. Overall, ED patients with ARF (n = 45,339) experienced a 0.82-day shorter median hospital LOS if admitted initially to the ICU compared with the ward, but among the 27 hospitals (n = 224–3,324), this effect varied from 5.85 days shorter (95% confidence interval [CI], −8.84 to −2.86; P < 0.001) to 4.38 days longer (95% CI, 1.86–6.90; P = 0.001). Corresponding ranges for in-hospital mortality with ICU compared with ward admission revealed odds ratios from 0.08 (95% CI, 0.01–0.56; P < 0.007) to 8.89 (95% CI, 1.60–79.85; P = 0.016) among patients with ARF (pooled odds ratio, 0.75). In exploratory analyses, only a small number of measured hospital practices—the presence of a sepsis ED disposition guideline and maximum ED patient capacity—were potentially associated with hospital strain–ICU admission relationships.
Conclusions
Hospitals vary considerably in ICU admission rates, the sensitivity of those rates to hospital capacity strain, and the benefits of ICU admission for patients with ARF not requiring life support therapies in the ED. Future work is needed to more fully identify hospital-level factors contributing to these relationships.
Keywords: acute respiratory failure, intensive care unit, hospital strain, hospital variation, processes of care
Investigating variation in care delivery among hospitals facilitates the identification of practices that impact outcomes. Among other use cases, measuring among-hospital variability can enable the extrapolation of best practices from higher-performing to lower-performing hospitals through positive deviance studies (1). In critical care, prior work has confirmed significant among-hospital variation in intensive care units (ICUs) across many practices, including bed use (2), emergency department (ED) disposition decisions by diagnosis (3–7), end-of-life care (8, 9), and adherence to evidence-based practices (10, 11). In at least some circumstances, this variability can impact patient outcomes (12).
Motivated by the lack of consensus to guide optimal ED disposition for patients with acute respiratory failure (ARF) on the margins of critical illness—those who do not require life support such as mechanical ventilation or vasopressors in the ED (3–7)—we previously examined the relationship between ICU and ward admission decisions with regard to clinical outcomes (4, 13, 14). By leveraging a novel hospital strain index as a strong instrumental variable across 27 hospitals in two health systems (4), we found that ICU admission, compared with ward admission, was associated with reduced hospital length of stay (LOS), with deaths considered as long LOS, and reduced mortality in ARF (14).
However, what remains unknown is how these strain–process–outcome relationships vary among hospitals and, if they vary, whether measurable hospital-level factors contribute to such variability. In this study, we evaluated stratified analyses among participating hospitals and, in exploratory analyses, linked hospital-level variation to specific hospital policies and practices. Abstracts of this work were presented at the Society of Critical Care Medicine Critical Care Congress and the American Thoracic Society International Conference (15, 16).
Methods
Study Sites and Study Population
This study was performed using data from 27 hospitals across Penn Medicine and Kaiser Permanente Northern California. Although study hospitals were all in metropolitan areas of 1 million population or more (based on the U.S. Department of Agriculture Rural-Urban Continuum Codes) and would be classified as teaching hospitals on the basis of the presence of undergraduate or graduate medical education training programs or trainees, there was significant diversity in inpatient bed capacity (range, 50–776 beds; mean, 254 beds), and only two hospitals were primary university teaching hospitals (see Table E1 in the data supplement).
Details about the construction of the ARF study cohort have been reported previously (4, 14, 17). In summary, we studied “borderline” patients who met criteria for ARF in the ED on the basis of hypoxemia, hypercarbia, or respiratory support; had high acuity based on a Laboratory-based Acute Physiology Score version 2 ⩾100 (18, 19); and were admitted directly from the ED to a medical or medical-surgical ward, step-down unit, or ICU. We excluded patients who required mechanical ventilation or vasopressors in the ED (i.e., patients who would nearly always be admitted to the ICU) or who had a care limitation at the time of admission beyond a simple do-not-resuscitate/do-no-intubate order (because such patients may have ICU admission patterns, care delivery processes, and outcomes that differ substantially from those of other patients without such limitations). Patients requiring noninvasive respiratory support in the ED (e.g., high-flow nasal cannula and noninvasive ventilation such as bilevel positive airway pressure [BiPAP] and continuous positive airway pressure) were included.
Association of Hospital Strain with ICU Admission for ARF
We have previously reported the development and validation of a novel composite hospital strain index (4, 14, 20–23). Here, we performed a retrospective cohort study using multivariable logistic regression to assess the association of this hospital strain index and ICU (vs. ward) admission, stratified by hospital. Models were adjusted a priori for age, sex, race, ethnicity, insurance status, Laboratory-based Acute Physiology Score version 2 (a laboratory and vital sign-based acute severity of illness score), and Comorbidity Point Score version 2 (a 1-yr comorbidity score) (18, 19, 24).
Association of ICU Admission with ARF Outcomes
Building on prior work evaluating relationships between ICU admission and outcomes among 27 hospitals, here we used two-stage instrumental variable multivariable quantile regression and two-stage residual inclusion regression, stratified by hospital and adjusted for the same patient covariates as above, to assess hospital-specific associations between ICU admission and hospital LOS and hospital mortality, respectively (14, 25–29). In these analyses, we deployed the hospital-specific strain index as a within-hospital instrumental variable governing ICU versus ward admission. The exposure variable of ICU versus ward admission considered the initial site of admission directly from the ED; subsequent changes in level of care were not considered in these analyses. For the LOS analyses, death was ranked as equivalent to the 99th percentile of hospital LOS by clinical cohort, and hospice discharges were considered in-hospital deaths (28, 29). This approach seeks to surmount widely documented challenges in the handling of death in LOS analyses among critically ill study populations with high mortality (30), including potential among-hospital differences in the timing of withholding life-sustaining therapy in the face of capacity strain (31).
As reported previously, the validated hospital strain index is built on the hospital level (i.e., the coefficients used for weighted capacity strain metric contributions to the composite strain index are derived uniquely for individual hospitals) (4). Because it was previously deployed in pooled samples across all study hospitals, to further test robustness of the hospital strain index as an instrumental variable used on the hospital level, we measured whether the hospital-specific proportion of patients whose ICU versus ward admission decision was dictated by the degree of hospital strain—namely, hospital-level rates of compliance with the instrumental variable—was associated with outcome effect sizes. To do this, we first approximated the proportion of instrumental variable compliers by calculating the difference between the percentage of patients admitted to the ICU in the lowest two and highest two strain index deciles at each hospital. We then ranked hospitals by this instrumental variable complier proportion, plotted that ranking against a hospital’s ranking on the estimated effect size for both hospital LOS and mortality, and finally evaluated the resulting relationship using Pearson’s correlation coefficients. There were no observed correlations between the ranking of the difference between the percentage of patients admitted to the ICU in the lowest two and highest two strain index deciles by hospital—that is, the instrumental variable complier proportion ranking—and the ranking by effect estimate for hospital LOS (R = −0.17; P = 0.41; Figure E1) and hospital mortality (R = 0.12; P = 0.55; Figure E2).
Hospital Practices Survey
In parallel to the above quantitative analyses, we administered a survey at 26 of the study hospitals between June 2018 and June 2019 (one hospital closed during the study period and did not participate). We created the survey to measure the presence or absence of hospital practices hypothesized to be associated with ICU versus ward triage decisions and the potential benefit gained from ICU admission, which were informed by our previous research (32). Survey questions were divided into four sections: 1) general hospital practices for ED disposition and ICU admission decisions, 2) ICU organization, 3) ward organization, and 4) step-down unit organization (if applicable). The survey was iteratively reviewed by content experts on the investigative team specializing in pulmonary medicine, critical care, emergency medicine, and survey methodology who provided feedback on question content, form, and structure. The final survey instrument included 24 items that could be completed in approximately 20 minutes (Appendix E1). All study data were collected and managed using REDCap electronic data capture tools hosted at the University of Pennsylvania (33, 34).
The survey was administered using two approaches; at 21 hospitals, a trained researcher administered the survey in person and manually entered responses into the REDCap database, and at five hospitals, the survey was taken electronically via a unique REDCap link distributed via e-mail. Survey respondents included hospital administrators, attending physicians, medical directors, and nurse managers (Table E2). One respondent was identified at each hospital with a single ICU (n = 22); for hospitals with multiple ICUs (n = 4), multiple respondents were identified as needed, and we compared responses to confirm consistent practices across ICUs, or, if results were discordant, we used responses from the ICU with the dominant share of study patients with ARF.
Association of Hospital Practices with ARF Outcomes
We calculated descriptive statistics for hospital practices and identified by inspection hospital practices that displayed variation across study hospitals. In exploratory analyses, we used linear regression to assess the univariate association between individual hospital practices and four hospital-level outcomes for patients with ARF: mean predicted probability of ICU admission (calculated across hospital strain deciles), the range of predicted probability of ICU admission between the lowest and highest hospital strain deciles (e.g., the degree of association between hospital strain and ICU vs. ward admission), change in hospital LOS with ICU admission, and odds ratio (OR) for hospital mortality with ICU admission.
This and preceding related publications followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for cohort studies (35). The study protocol was approved with a waiver of informed consent by the institutional review boards of Kaiser Permanente Northern California (Oakland, CA) and the University of Pennsylvania (Philadelphia, PA).
Results
Patient Characteristics
Patient characteristics for this ARF cohort appear in Table E3 and have been reported previously (4, 14). In summary, among 45,339 patients with ARF, the mean age was 72.9 years, 15.0% were of Black race, and 70.8% were admitted directly to a ward or step-down unit, and the observed median hospital LOS was 3.9 (interquartile range, 2.2–5.8) days and observed hospital mortality was 20.2%.
Hospital ICU Staffing
Hospital staffing and practices are reported in Table 1. Of all hospitals’ ICUs, 92.3% were staffed by an intensivist as the primary physician, and the most common senior-most overnight clinician in the ICU was a nonintensivist attending physician (e.g., hospitalist) (73.1%), followed by a resident or advanced practice provider (15.4%) and intensivist attending physician (11.5%). A proportion of 57.7% of hospitals had ICU flex beds that could alternate between different levels of care or be used for overflow.
Table 1.
Hospital practices
| Hospital Practices | Statistics |
|---|---|
| Hospital triage practices | |
| General hospital protocols or criteria to guide the triage of patients from the ED to ICUs, SDUs, or wards; hospitals, n (%) | 19 (73.1) |
| Diagnosis-specific hospital protocols or criteria to guide the triage of patients from the ED to ICUs, SDUs, or wards; hospitals, n (%) | 16 (61.5) |
| ARF including BiPAP | 2 (7.7) |
| Sepsis | 10 (38.5) |
| ICUs | |
| ICUs that routinely admit medical patients, n (%) | |
| 1 ICU | 22 (84.6) |
| >1 ICU | 4 (15.4) |
| ICU beds that routinely admit medical patients, mean (SD) | 24 (12) |
| Hospitals with ICU flex beds that can alternate between different levels of care or that can be used for overflow; hospitals, n (%) | 15 (57.7) |
| ICUs staffed with an intensivist as the primary physician; hospitals, n (%) | 24 (92.3) |
| Most senior bedside clinician in ICU overnight; hospitals, n (%) | |
| Nonintensivist attending physician (e.g., hospitalist) | 19 (73.1) |
| Resident or advanced practice provider | 4 (15.4) |
| Intensivist attending physician | 3 (11.5) |
| Standard of two ICU patients per nurse; hospitals, n (%) | 25 (96.2) |
| ICU telemedicine or other remote monitoring; hospitals, n (%) | 4 (15.4) |
| Wards | |
| Mechanical ventilation permitted for some patients on wards not being imminently transferred to an ICU; hospitals, n (%) | 9 (34.6) |
| Permitted frequency for use of noninvasive ventilation (i.e., CPAP or BiPAP) on the ward; hospitals, n (%) | |
| Any time of day without a time limit | 11 (44.0) |
| Nighttime and during naps | 7 (28.0) |
| Any time of day with time limits | 5 (20.0) |
| Nighttime only | 2 (8.0) |
| Permitted use of high-flow nasal cannula on the ward; hospitals, n (%) | |
| Allowed without limits | 19 (73.1) |
| Allowed, but with limits on flow rate, oxygen concentration, or duration of use | 4 (15.4) |
| Not allowed or not available | 3 (11.5) |
| Emergency departments | |
| Maximum number of patients who can be under treatment in the ED at a given time (excluding patients in fast track, observation, or registered but not yet under treatment); mean patients, n (SD) | 34 (16) |
| Respiratory therapist(s) dedicated to the ED; hospitals, n (%) | 7 (26.9) |
| Standard maximum of four ED patients per nurse; hospitals, n (%) | 20 (83.3) |
| Primary responsibility for determining whether an ED patient will be admitted to the ICU during daytime hours; hospitals, n (%) | |
| ICU physician | 12 (46.2) |
| Collaborative between ICU, ED, and hospitalist physicians | 9 (35.6) |
| Hospitalist | 4 (15.4) |
| ED physician | 1 (3.9) |
| Primary responsibility for determining whether an ED patient will be admitted to the ICU during nighttime hours; hospitals, n (%) | |
| Hospitalist | 17 (65.4) |
| Collaborative between ICU, ED, and hospitalist physicians | 6 (23.1) |
| ICU physician | 2 (7.7) |
| ED physician | 1 (3.9) |
| Primary responsibility for managing patients boarding in the ED once a decision has been made to admit them to an ICU; hospital, n (%) | |
| Inpatient ICU team | 18 (72.0) |
| ED team | 4 (16.0) |
| Joint ED-ICU management | 3 (12.0) |
| Step-down units | |
| SDU available during study period,* hospitals, n (%) | 15 (57.7) |
| SDU can admit patients requiring noninvasive ventilation (i.e., BiPAP) or vasopressors/inotropes; hospitals, n (%) | 13 (100) |
| SDU places time limits on noninvasive ventilation (i.e., BiPAP) or vasopressors/inotropes before requiring ICU admission; hospitals, n (%) | 7 (53.9) |
| Standard of three SDU patients per nurse; hospitals, n (%) | 11 (84.6) |
Definition of abbreviations: ARF = acute respiratory failure; BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; ED = emergency department; ICU = intensive care unit; SD = standard deviation; SDU = step-down unit.
Notes: Data are reported as complete case responses; total study hospitals = 26; some hospitals did not supply answers to some questions.
Two hospitals’ SDUs are no longer open.
Triage Protocols and Practices
In the ED, the primary responsibility for determining whether a patient would be admitted to the ICU during daytime hours rested most commonly with the ICU physician (46.2%), followed by a collaboration between ICU, ED, and hospitalist physicians (35.6%); hospitalists alone (15.4%); or ED physicians alone (3.9%). At nighttime, it rested more predominantly with hospitalists alone (65.4%). ICU patients boarding in the ED were predominantly managed by the inpatient ICU team (72.0%) and less commonly by the ED team (16.0%), or they were jointly managed (12.0%).
For triage protocols, 73.1% of study hospitals had general hospital protocols or criteria to guide the triage of patients from the ED to ICUs, step-down units, or wards. A proportion of 61.5% of hospitals had diagnosis-specific triage protocols, but only 7.7% (n = 2) had a protocol for ARF, including patients requiring noninvasive ventilation (i.e., BiPAP), whereas 38.5% had one such protocol for sepsis, a common cause of ARF.
Ward and Step-Down ARF Practices
For ward practices, 34.6% of hospitals permitted mechanical ventilation for some patients not being imminently transferred to an ICU, and 44.0% and 73.1% permitted use of noninvasive ventilation and high-flow nasal cannulas, respectively, at any time of day without limits. Fifteen hospitals had step-down units, two of which closed during the study period. Among step-down units, 100% admitted patients requiring noninvasive ventilation (i.e., BiPAP) or vasopressors, and 53.9% placed time limits on the above therapies before requiring upgrade and ICU admission.
Association of Hospital Strain with ICU Admission for ARF
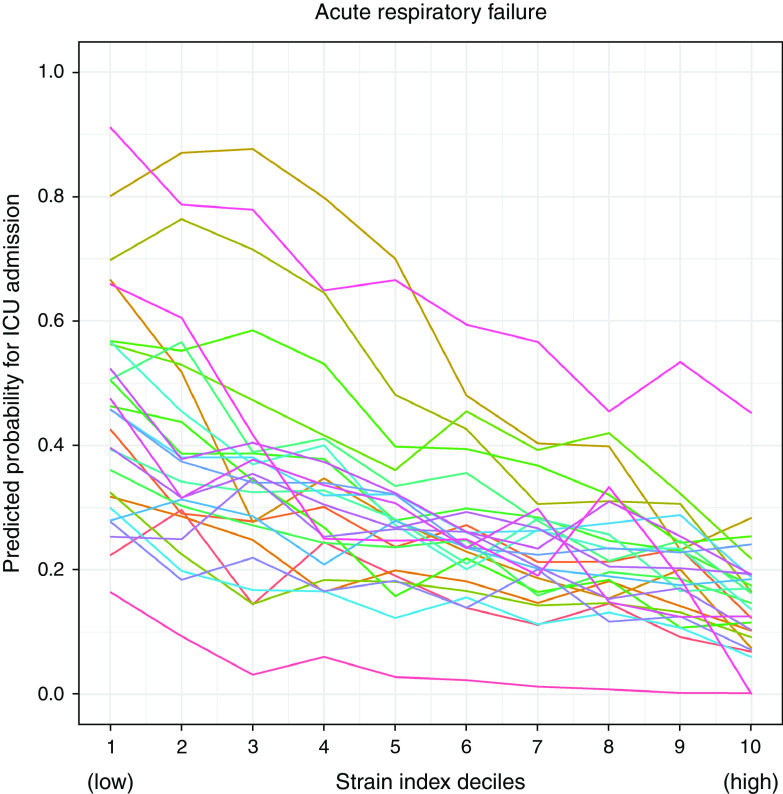
Hospitals varied in the proportion of patients with ARF they admitted to the ICU and in the strength of the association between hospital strain and ICU admission. The mean predicted probability of ICU admission across all deciles of strain by hospital ranged from 7.4% to 65.7% (Table E3). The difference in the predicted probabilities of ICU admission between the lowest and highest deciles of hospital strain (e.g., the degree of association between hospital strain and ICU vs. ward admission) ranged from 6.0% to 64.2% (Figure 1, Table E4).
Figure 1.
Hospital strain is associated with intensive care unit (ICU) admission and displays among-hospital variation. Hospitals varied in the proportion of patients with acute respiratory failure (ARF) they admitted to the ICU and in the strength of the association between hospital strain and ICU admission. The mean predicted probability of ICU admission for ARF across strain deciles by hospital ranged from 7.4% to 65.7%. The difference in the predicted probabilities of ICU admission for ARF between the lowest and highest deciles of hospital strain ranged from 6.0% to 64.2%. Each colored line represents an individual study hospital.
Association of ICU Admission with ARF Outcomes
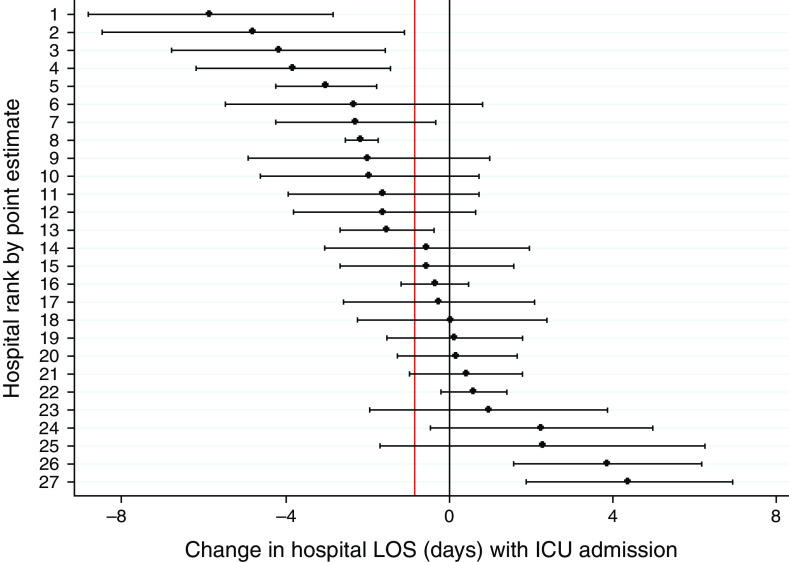
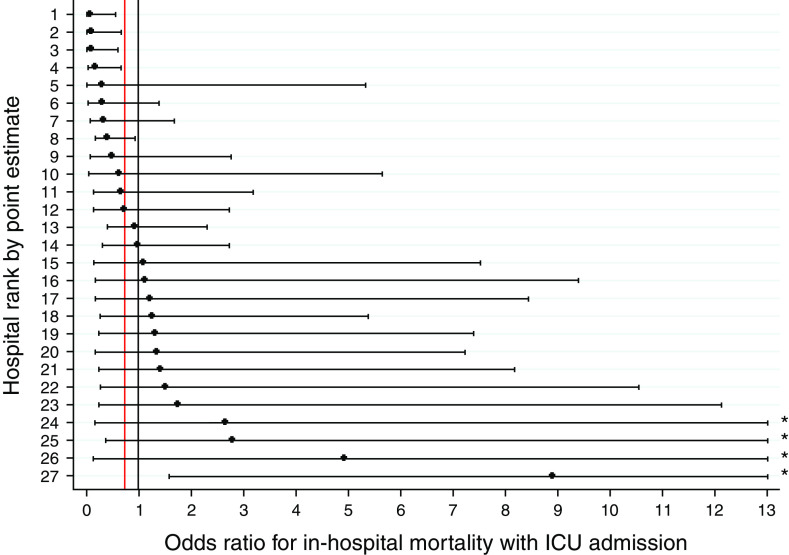
In previously published pooled analyses, ED patients with ARF (n = 45,339) experienced a 0.82-day shorter median hospital LOS if admitted initially to the ICU compared with the ward (14), but across the 27 study hospitals (n = 224–3,324), this effect varied from 5.85 days shorter (95% confidence interval [CI], −8.84 to −2.86; P < 0.001) to 4.38 days longer (95% CI, 1.86–6.90; P = 0.001) (Figure 2, Table E4). Corresponding ranges for in-hospital mortality with ICU compared with ward admission revealed ORs from 0.08 (95% CI, 0.01–0.56; P < 0.007) to 8.89 (95% CI, 1.60–79.85; P = 0.016) among patients with ARF (pooled OR, 0.75 [14]) (Figure 3, Table E5).
Figure 2.
Among-hospital variation in the association between ICU admission and hospital LOS in patients with ARF. Among patients with ARF (n = 45,339), initial ICU admission, compared with ward admission, conferred a 0.82-day shorter median hospital LOS in pooled analyses, but this effect varied among hospitals (n = 224–3,324) from 5.85 days shorter (95% CI, −8.84 to −2.86; P < 0.001) to 4.38 days longer (95% CI, 1.86–6.90; P = 0.001). The vertical black line displays no change in LOS, and the vertical red line displays the pooled point estimate of −0.82 days. Horizontal black bars represent 95% CIs. ARF = acute respiratory failure; CI = confidence interval; ICU = intensive care unit; LOS = length of stay.
Figure 3.
Among-hospital variation in the association between ICU admission and hospital mortality in patients with ARF. In pooled analyses, ED patients with ARF experienced an OR of 0.75 for in-hospital mortality if admitted initially to the ICU compared with the ward, but across the 27 study hospitals, this effect varied from an OR of 0.08 (95% CI, 0.01–0.56; P < 0.007) to 8.89 (95% CI, 1.60–79.85; P = 0.016). The vertical black line displays no change in mortality (OR = 1), and the vertical red line displays the pooled point estimate of OR 0.75. Horizontal black bars represent 95% CIs. *Upper bound extends beyond figure range; see Table E5 for complete CIs. ARF = acute respiratory failure; CI = confidence interval; ED = emergency department; ICU = intensive care unit; OR = odds ratio.
Association of Hospital Practices with ICU Admission and ICU Outcomes
Hospital practices that displayed among-hospital variation by inspection and were included in subsequent exploratory analyses are included in Table 1. Of note, the presence of an ARF-specific triage protocol was reported at only two study sites, and the availability of ICU telemedicine was perfectly colinear with study health system, and therefore neither was included in further analyses.
Among hospital practice variables, maximum ED patient capacity (β = −0.003; 95% CI, −0.006 to −0.00007; P = 0.046) and presence of a sepsis ED disposition guideline (β = −0.14; 95% CI, −0.24 to −0.04; P = 0.008) were correlated with the range of predicted probability of ICU admission for ARF between the lowest and highest hospital strain deciles (e.g., the degree of association between hospital strain and ICU vs. ward admission) (Table E6). This would equate to a 0.03% narrowing of the range of predicted probability of ICU admission with a one-patient increase in maximum ED capacity (a difference of 3 patients per 1,000 admitted to the ICU between the lowest and highest strain deciles) and a 14% narrowing of the range of predicted probability of ICU admission with the presence of a sepsis ED disposition guideline (a difference of 14 patients per 100 admitted to the ICU between the lowest and highest strain deciles). No other hospital practice–outcome pairings had statistically significant univariate associations, including for the outcomes of mean predicted probability of ICU admission, change in hospital LOS with ICU admission, and OR for hospital mortality with ICU admission.
Discussion
This study combines among-hospital patient-level and hospital-level analyses, including results of a survey of hospital practices, to examine among-hospital differences in critical care practices and outcomes for patients being admitted from the ED with ARF but not requiring life support therapies. Key findings of these analyses are that 1) there is among-hospital variation in ICU admission rates for patients with ARF not requiring life support therapies; 2) there is among-hospital variation in how sensitive those ICU admission decisions are to hospital capacity strain; 3) there is among-hospital variation in the association of ICU admission with hospital LOS and hospital mortality for patients with ARF not requiring life support therapies; and 4) in exploratory analyses, the presence of a sepsis ED disposition guideline and maximum ED patient capacity may be associated with reduced strain sensitivity of ICU admission decisions for patients with ARF.
The differences in the range of predicted probability of ICU admission for ARF between the lowest and highest hospital strain deciles (e.g., the degree of association between hospital strain and ICU vs. ward admission) were dramatic. The most strain-resistant study hospital showed just a 6.0% difference in ICU admission probabilities between the lowest and highest hospital strain deciles, evidence of little practice change in the face of changing hospital strain, whereas the most strain-sensitive hospital showed a 64.2% difference, evidence of significant changes in care delivery based on the degree of hospital strain at a given time.
Because knowing which patients should be admitted to the ICU and how hospitals should adapt to dynamic capacity strain remain incompletely understood, neither strain sensitivity nor strain resistance identified here is necessarily optimal or suboptimal (14, 36). The goal of ICU triage is to admit patients who are going to benefit specifically from ICU care compared with ward care and not to admit patients who are not going to benefit, because, for example, they are too well and would receive the same benefits outside of the ICU without the risk of ICU-related complications. ICU triage and whether it is optimal or suboptimal are the combination of overall rates of ICU admission, responses to changes in hospital strain and demand for ICU care, and how much ICU care benefits or harms specific patient populations. A strain-resistant hospital could be anchored at a relatively optimal ICU admission rate (i.e., admitting mostly and almost all patients who will benefit) or at a suboptimal ICU admission rate (i.e., admitting many patients who will not benefit or excluding many patients who would benefit). A highly strain-sensitive hospital could be optimally responsive to changes in supply and demand of critical care resources, preserving efficiency, or it could be allowing strain to induce too-frequent, too-large deviations from optimal care delivery practices.
The finding that there is among-hospital variation in the strain sensitivity of ICU admission decisions also suggests that there may be hospital-level differences that modify how hospitals respond to capacity strain. In our exploratory analysis of 24 expert-identified hospital practices spanning EDs, wards, ICUs, and step-down units, however, only the presence of an ED disposition guideline for sepsis (with a large magnitude), a common cause of ARF, and maximum ED patient capacity (with a relatively small magnitude) were associated with reduced strain sensitivity of ICU admission decisions for patients with ARF. Importantly, imprecision in the effect estimates in these exploratory analyses may hide important relationships, and hospital practices with null findings in the present analyses should not be discounted until further, dedicated, adequately powered, and nuanced studies are performed.
The fact that an ED disposition guideline appears to narrow the range of predicted probability of ICU admission for ARF between the lowest and highest hospital strain deciles, which has face validity based on a guideline intending to reduce practice variation, is again not obviously or necessarily beneficial or harmful. Such an impact would be beneficial if the narrowed range of ICU admission probability were more optimal for patients with ARF (e.g., admitting more patients who benefit and fewer patients who will not). Conversely, strain insensitivity induced by a triage guideline could be harmful if the newly narrowed range of ICU admission probability is miscalibrated (e.g., the hospital now underadmits patients with ARF who could benefit from the ICU) or if it overly reduces the hospital’s ability to optimally adapt to dynamic capacity strain. Of note, in contrast to sepsis protocols, the presence of general protocols and diagnosis-specific protocols (for any or all diagnoses) to guide ED disposition was not correlated with the strain sensitivity of ICU admission decisions for ARF, and ARF-specific triage protocols were rare (present at just two study hospitals) and therefore could not be meaningfully included in the reported analyses.
The finding of the association between maximum ED patient capacity and reduced strain sensitivity of ICU admission decisions, although of relatively small magnitude, also has face validity in that larger EDs may be better able to wait out delays in ICU bed availability. Understanding contextual factors that influence triage practices and their strain sensitivity requires more in-depth investigation.
Overall, in our exploratory analyses, only a small number of the hospital practices measured in our survey were associated with hospital strain–ICU admission relationships, and none were associated with hospital LOS and mortality outcomes related to ICU admission for ARF. This may suggest that hospital practices do not influence outcomes or more likely that alternative methods are required to identify aspects of the ED–ward–ICU hospital organizational structure and care delivery that are most impactful on triage and outcomes and/or most promising as targets of organizational interventions to improve triage practices and outcomes. This could be accomplished by positive deviance case studies that undertake in-depth qualitative examinations of the processes present at particularly high-performing hospitals with respect to ICU and ward net benefit.
In total, our findings expand the literature in a number of ways. The sensitivity of ICU admission decision to capacity strain and among-hospital variation in ICU admission rates are well documented (2, 4, 13, 37), but we now report that there is also variability between hospitals in how sensitive those ICU admission decisions are to dynamic changes in hospital capacity strain. Said another way, individual hospitals allocate ICU beds—a true or perceived scarce resource—differently both overall and in response to capacity strain. Similarly, among-hospital differences in outcomes in ARF of various etiologies is also well reported (38–42), but we now add the nuance that the benefit (or harm) specific to ICU admission for patients with ARF on the borderline of critical illness also varies significantly by hospital. In addition to hospital-level variation around the pooled estimate, which showed an overall net benefit of ICU admission to patients with ARF, there are also individual hospitals whose hospital-level point estimates reflect net harm (e.g., higher mortality and longer LOS associated) with ICU admission or, alternatively, net benefit for admission to the ward.
Limitations
The results of this study should be interpreted in the proper context and with the appropriate limitations. First, among-hospital and hospital-level analyses have considerably smaller samples than corresponding pooled analyses and thus considerably less precise estimates with wide CIs even when statistically significant; individual-hospital point estimates are likely less helpful than the overall pattern of among-hospital variation. Second, instrumental variable analyses present limitations in comparison to prospective randomization, which we have detailed previously when reporting pooled analyses, and these remain limitations in hospital-stratified analyses (4, 14). Namely, the possibility of residual confounding due to associations between the instrument and unmeasured confounders cannot be definitely proved to be absent as compared with true prospective randomization. Although we demonstrate here that there was no relationship between instrumental variable complier rates and instrumental variable analysis point estimates, if the instrumental variable compliers—namely, patients whose ED disposition decision differed as a result of hospital strain—were substantially different at different hospitals, the comparison of among-hospital instrumental variable results may reflect different types of patients, thus limiting their clinical applicability. In addition, hospitals at which the instrumental variable was weaker (i.e., fewer patients’ ED disposition decisions differed as a result of hospital strain), the estimates would be less reliable and would apply to fewer patients. Third, the hospital practices survey was limited to a nonexhaustive list of elements, was not powered to rule out hospital practices with important outcome associations, and allowed only minor answer clarification but not broader expanded answers. The survey also used a heterogeneous group of respondents, with single respondents potentially commenting on multiple hospital areas, which might introduce systemic differences in survey responses, although the largely quantitative nature of the questions reduces this risk. In sum, our analyses of these practices is therefore only exploratory and hypothesis generating in nature. More granular quantitative and qualitative work that allows the identification of other hospital practices that might shape outcomes is needed. Fourth, ARF is a syndrome with heterogeneity in both clinical picture and etiology, and this study was not designed to explore how different types of ARF are preferentially impacted by strain among or between hospitals. Finally, although our health systems and hospitals span multiple states and hospital types, they do not represent the full spectrum of hospital and patient diversity; similar studies at a range of hospital types and regions would be of further benefit.
Conclusions
Hospitals vary considerably in ICU admission rates, the sensitivity of those rates to hospital capacity strain, and the benefits of ICU admission for patients with ARF not requiring life support therapies in the ED. Future work is needed to more fully identify hospital-level factors contributing to these relationships.
Footnotes
Supported by National Institutes of Health grants R01HL136719 (S.D.H.), K24HL143289 (S.D.H.), and R35GM128672 (V.X.L.), The Permanente Medical Group, Inc. (G.J.E.), and National Institutes of Health grant K23HL161353 (G.L.A.). The funders had no role in the study design, execution, results interpretation, manuscript writing, or decision to submit for publication.
Author Contributions: Conception and design of study: G.L.A., V.X.L., E.D., D.S.S., M.K.D., B.B., G.J.E., S.D.H., and J.E.S. Data acquisition: G.L.A., V.X.L., M.C., E.D., B.B., F.X.B., and J.Z.W. Analysis and data interpretation: G.L.A., V.X.L., M.C., E.D., D.S.S., W.W., M.K.D., B.B., J.E.S., L.W.G., F.X.B., G.J.E., and S.D.H. Drafting and revision of the manuscript: G.L.A., V.X.L., M.C., E.D., D.S.S., W.W., M.K.D., B.B., J.E.S., L.W.G., F.X.B., J.Z.W., G.J.E., and S.D.H.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci . 2009;4:25. doi: 10.1186/1748-5908-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wunsch H, Angus DC, Harrison DA, Linde-Zwirble WT, Rowan KM. Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med . 2011;183:1666–1673. doi: 10.1164/rccm.201012-1961OC. [DOI] [PubMed] [Google Scholar]
- 3. Admon AJ, Seymour CW, Gershengorn HB, Wunsch H, Cooke CR. Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest . 2014;146:1452–1461. doi: 10.1378/chest.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anesi GL, Chowdhury M, Small DS, Delgado MK, Kohn R, Bayes B, et al. Association of a novel index of hospital capacity strain with admission to intensive care units. Ann Am Thorac Soc . 2020;17:1440–1447. doi: 10.1513/AnnalsATS.202003-228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation . 2013;127:923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA . 2015;314:1272–1279. doi: 10.1001/jama.2015.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Intensive care unit admission and survival among older patients with chronic obstructive pulmonary disease, heart failure, or myocardial infarction. Ann Am Thorac Soc . 2017;14:943–951. doi: 10.1513/AnnalsATS.201611-847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med . 2015;175:1019–1026. doi: 10.1001/jamainternmed.2015.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest . 2014;146:573–582. doi: 10.1378/chest.13-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weissman GE, Gabler NB, Brown SE, Halpern SD. Intensive care unit capacity strain and adherence to prophylaxis guidelines. J Crit Care . 2015;30:1303–1309. doi: 10.1016/j.jcrc.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dam TA, de Grooth HJ, Klausch T, Fleuren LM, de Bruin DP, Entjes R, et al. Some patients are more equal than others: variation in ventilator settings for coronavirus disease 2019 acute respiratory distress syndrome. Crit Care Explor . 2021;3:e0555. doi: 10.1097/CCE.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereira CCA, Martins M, Lima SML, de Andrade CLT, Soares FRG, Portela MC. Geographical variation in demand, utilization, and outcomes of hospital services for COVID-19 in Brazil: a descriptive serial cross-sectional study. PLoS One . 2021;16:e0257643. doi: 10.1371/journal.pone.0257643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anesi GL, Liu VX, Gabler NB, Delgado MK, Kohn R, Weissman GE, et al. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc . 2018;15:1328–1335. doi: 10.1513/AnnalsATS.201804-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anesi GL, Liu VX, Chowdhury M, Small DS, Wang W, Delgado MK, et al. Association of ICU admission and outcomes in sepsis and acute respiratory failure. Am J Respir Crit Care Med . 2022;205:520–528. doi: 10.1164/rccm.202106-1350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anesi GL, Liu VX, Chowdhury M, Small DS, Wang W, Delgado MK, Bayes B, Dress E, Escobar GJ, Halpern SD. Impact of intensive care unit admission on outcomes in sepsis and acute respiratory failure. Society of Critical Care Medicine (SCCM) Critical Care Congress (February 2021). Crit Care Med. 2021;49(1):230.
- 16.Anesi GL, Liu VX, Chowdhury M, Small DS, Wang W, Delgado MK, Bayes B, Dress E, Escobar GJ, Halpern SD. Among-Hospital Variation in the Impact of Intensive Care Unit Admission on Outcomes in Sepsis and Acute Respiratory Failure. American Thoracic Society International Conference (May 2021). Am J Respir Crit Care Med. 2021;203:A1677.
- 17. Anesi GL, Chelluri J, Qasim ZA, Chowdhury M, Kohn R, Weissman GE, et al. Association of an emergency department-embedded critical care unit with hospital outcomes and intensive care unit use. Ann Am Thorac Soc . 2020;17:1599–1609. doi: 10.1513/AnnalsATS.201912-912OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care . 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 19. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care . 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 20. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res . 2017;26:2333–2355. doi: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol . 2013;42:1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies NM, von Hinke Kessler Scholder S, Farbmacher H, Burgess S, Windmeijer F, Smith GD. The many weak instruments problem and Mendelian randomization. Stat Med . 2015;34:454–468. doi: 10.1002/sim.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Small DS, Rosenbaum PR. War and wages: the strength of instrumental variables and their sensitivity to unobserved biases. J Am Stat Assoc . 2008;103:924–933. [Google Scholar]
- 24. Liu V, Kipnis P, Gould MK, Escobar GJ. Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care . 2010;48:739–744. doi: 10.1097/MLR.0b013e3181e359f3. [DOI] [PubMed] [Google Scholar]
- 25. Chernozhukov V, Hansen C. Instrumental variable quantile regression: a robust inference approach. J Econom . 2008;142:379–398. [Google Scholar]
- 26. He X. Quantile curves without crossing. Am Stat . 1997;51:186–192. [Google Scholar]
- 27.Kwak DW.https://sites.google.com/site/dwkwak/dataset-and-code
- 28. Lin W, Halpern SD, Prasad Kerlin M, Small DSA. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res . 2017;26:292–311. doi: 10.1177/0962280214545121. [DOI] [PubMed] [Google Scholar]
- 29. Ranganathan P, Pramesh CS. Censoring in survival analysis: potential for bias. Perspect Clin Res . 2012;3:40. doi: 10.4103/2229-3485.92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harhay MO, Ratcliffe SJ, Small DS, Suttner LH, Crowther MJ, Halpern SD. Measuring and analyzing length of stay in critical care trials. Med Care . 2019;57:e53–e59. doi: 10.1097/MLR.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hua M, Halpern SD, Gabler NB, Wunsch H. Effect of ICU strain on timing of limitations in life-sustaining therapy and on death. Intensive Care Med . 2016;42:987–994. doi: 10.1007/s00134-016-4240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohn R, Madden V, Kahn JM, Asch DA, Barnato AE, Halpern SD, et al. Diffusion of evidence-based intensive care unit organizational practices. A state-wide analysis. Ann Am Thorac Soc . 2017;14:254–261. doi: 10.1513/AnnalsATS.201607-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap Consortium The REDCap consortium: building an international community of software platform partners. J Biomed Inform . 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform . 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med . 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 36. Anesi GL, Admon AJ, Halpern SD, Kerlin MP. Understanding irresponsible use of intensive care unit resources in the USA. Lancet Respir Med . 2019;7:605–612. doi: 10.1016/S2213-2600(19)30088-8. [DOI] [PubMed] [Google Scholar]
- 37. Trentini F, Marziano V, Guzzetta G, Tirani M, Cereda D, Poletti P, et al. Pressure on the health-care system and intensive care utilization during the COVID-19 outbreak in the Lombardy region of Italy: a retrospective observational study in 43,538 hospitalized patients. Am J Epidemiol . 2022;191:137–146. doi: 10.1093/aje/kwab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, et al. Association between the number of hospital admissions and in-hospital outcomes in patients with heart failure. Hypertens Res . 2020;43:1385–1391. doi: 10.1038/s41440-020-0505-2. [DOI] [PubMed] [Google Scholar]
- 39. LaBedz SL, Krishnan JA, Chung YC, Lindenauer PK, Spece LJ, Feemster LC, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis (Miami) . 2021;8:306–313. doi: 10.15326/jcopdf.2021.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cunningham LC, Fonarow GC, Yancy CW, Sheng S, Matsouaka RA, DeVore AD, et al. Regional variations in heart failure quality and outcomes: Get With the Guidelines–Heart Failure Registry. J Am Heart Assoc . 2021;10:e018696. doi: 10.1161/JAHA.120.018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Croft JB, Wheaton AG, Liu Y, Xu F, Lu H, Matthews KA, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease — United States, 2015. MMWR Morb Mortal Wkly Rep . 2018;67:205–211. doi: 10.15585/mmwr.mm6707a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumamaru H, Tsugawa Y, Horiguchi H, Kumamaru KK, Hashimoto H, Yasunaga H. Association between hospital case volume and mortality in non-elderly pneumonia patients stratified by severity: a retrospective cohort study. BMC Health Serv Res . 2014;14:302. doi: 10.1186/1472-6963-14-302. [DOI] [PMC free article] [PubMed] [Google Scholar]