Abstract
Rationale
Central sleep apnea (CSA) is associated with high mortality. Current knowledge stems from studies with limited sample size (fewer than 100 subjects) and in homogeneous populations such as heart failure (HF).
Objectives
To address this knowledge gap, we compared the mortality pattern and time to death between the CSA and obstructive sleep apnea (OSA) patients in the large Veterans Health Administration patient population using the big data analytic approach.
Methods
This is a retrospective study using national Veterans Health Administration electronic medical records from October 1, 1999, through September 30, 2020. We grouped the patients with underlying sleep disorders into CSA and OSA, using the International Classification of Diseases, Ninth and Tenth Revision codes. We applied Cox regression analysis to compare the mortality rate and hazard ratio (HR) among the two groups and adjusted HR by gender, race, body mass index (BMI), age, and Charlson Comorbidity Index. In CSA groups, a machine-learning algorithm was used to determine the most important predictor of time to death. Further subgroup analysis was also performed in patients that had comorbid HF.
Results
Evaluation of patients resulted in 2,961 grouped as CSA and 1,487,353 grouped as OSA. Patients with CSA were older (61.8 ± 15.6 yr) than those with OSA (56.7 ± 13.9 yr). A higher proportion of patients with CSA (25.1%) died during the study period compared with the OSA cohort (14.9%). The adjusted HR was 1.53 (95% confidence interval [CI], 1.43–4.65). Presence of HF history of cerebrovascular disease, hemiplegia, and having a BMI less than 18.5 were among the highest predictors of mortality in CSA. The subgroup analysis revealed that the presence of HF was associated with increased mortality both in CSA (HR, 7.4; 95% CI, 6.67–8.21) and OSA (HR, 4.3; 95% CI, 4.26–4.34) groups.
Conclusions
Clinically diagnosed CSA was associated with a shorter time to death from the index diagnostic date. Almost one-fifth of patients with CSA died within 5 years of diagnosis. The presence of HF, history of cerebrovascular disease and hemiplegia, male sex, and being underweight were among the highest predictors of mortality in CSA. CSA was associated with higher mortality than OSA, independent of associated comorbidity.
Keywords: central sleep apnea, obstructive sleep apnea, electronic medical records, machine learning
Central sleep apnea (CSA) is characterized by the repetitive reduction or cessation of airflow because of reduced ventilatory effort during sleep. It is less prevalent than obstructive sleep apnea (OSA), another breathing-related sleep disorder in which ventilatory effort is preserved (1–3). It is frequently associated with several other comorbid medical conditions, such as heart failure (HF) (4–7), and medications, such as opioids (8). Several studies have shown that CSA is associated with increased mortality (9–12). These studies are limited to a relatively smaller sample size (n = 36–88), a homogeneous population such as HF, and a sample bias, as patients were recruited from a single center. Thus, there is a knowledge gap in assessing the CSA-related mortality patterns involving a larger cohort of patients that is not homogeneous and involves various etiologies and comorbid conditions.
The Veteran Health Care system’s electronic medical record database was used to identify a cohort of patients to address this knowledge gap by analyzing its outpatient and inpatient administrative databases. The VHA (Veterans Health Administration) is the largest integrated healthcare system in the United States, providing care at 1,293 healthcare facilities and serving 9 million enrolled veterans each year (13). Given the breadth of available data, this dataset was uniquely positioned to answer this question at a longitudinal, national, system-based level. The primary objective of this study was to compare the average time to death from the diagnosis between patients with CSA and OSA in a real-world setting, using the big data analytic approach. These two mechanistically disparate sleep-disordered breathing syndromes have a similar final pathway of recurrent apneas and hypopneas, sleep fragmentation, and intermittent hypoxia.
Methods
Baylor College of Medicine’s institutional review board (H-35366) and Michael E. DeBakey Veteran Affairs medical center research and development committee approved this research.
Study Design and Setting
The study was performed using VHA electronic medical records from October 1, 1999, through September 30, 2020. The patients were followed until December 20, 2021. We included patients with any ICD-9 (International Classification of Disease, Ninth Edition) or ICD-10 (10th Edition) codes. Among eligible subjects, two cohorts were defined: 1) patients with CSA; and 2) patients with OSA.
The CSA or OSA are considered confirmed diagnoses on the basis of two separate outpatient encounters at least 30 days apart or one inpatient encounter. The CSA group was defined using the following ICD codes: ICD-9 codes: 327.21 and 327.27; ICD-10 codes: G47.31 and G47.37. The OSA group was defined using the following ICD codes: ICD-9 codes: 327.23, 327.20, and 327.2; ICD10 code: G47.33. The date of the patient’s first diagnosis was used as the index date. Cases with an overlapping diagnosis of both OSA and CSA were excluded to minimize the possibility of miscoding.
To assess if the ICD-9 and ICD-10 codes correctly defined the study cohorts, board-certified sleep medicine physicians (R.A. and A.S.) reviewed the clinical charts of a random sample of 1% of the CSA cohort. The charts were reviewed to find out if 1) CSA ICD-9 and ICD-10 codes were present in the chart; and 2) whether, on chart review, a diagnosis of CSA could be made with confidence. CSA diagnosis was defined by using the ICSD-3 (International Classification of Sleep Disorders, Third Edition) criteria (14). We found that 1) 100% of charts had the diagnostic code of CSA; and 2) CSA diagnosis was consistent with coded diagnosis in 60% of cases (0.6 positive predictive value); in the rest of the 40% of cases, CSA was miscoded for the diagnosis of OSA. Nearly 20% of cases in the miscoded group had less than 50% of total apnea and hypopnea events that were central in origin. Thus, it did not meet the diagnosis of CSA by the ICSD-3 definition; yet, the primary coding clinician felt that the overall clinical picture supported the CSA diagnosis. Polysomnography was available for review in 83.3% of charts, and in the rest, the coding accuracy was confirmed after reviewing clinicians’ documentation. Typically, in the missing cases, the polysomnography was conducted at an outside facility, and a report was not scanned into the electronic records. We did not perform a random sample analysis of the OSA cohort, as sensitivity and specificity data of our definition are available in the published literature (15).
Study Variables
The primary outcome of interest was all-cause mortality. We measured time-to-death by subtracting the death date and time from the index date at the time of diagnosis. Because the actual onset of disease in both OSA and CSA is generally unknown in both OSA and CSA, we chose the index diagnosis date for uniformity of calculation of time-to-death. All-cause mortality data were gathered from the corporate data warehouse (CDW) VHA Vital Status table (sensitivity 98.3% and specificity 99.8% relative to the National Death Index) (16, 17). Mortality data were recorded up to December 1, 2021.
We collected patient demographics such as age (categorized to <30, 30–40, 40–50, 50–65, 65–75, 75–85, and ⩾85 yr), body mass index (BMI) (categorized to <18.5, 18.5–25, 25–30, 30–35, and ⩾35), sex, race (White, Black, and others), ethnicity (Hispanic), and Charlson Comorbidity Index (CCI). The CCI calculated inpatient and outpatient problems 1 year before the index date (18).
Statistical Analysis
To characterize the two groups, continuous variables are presented as mean with standard deviation and categorical variables as number and percentage using SPSS, version 26 (SPSS, Inc.). We observed several missing values of BMI (n = 385 [13%] in CSA and 182,090 [12%] in OSA), and we performed a multivariate imputation by chained equations to impute missing values (R package, multivariate imputation by chained equations package 3.12.0). We used sex, race, age, and death status to impute BMI with five iterations.
We used Cox regression analyses to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for CSA and OSA groups by considering the OSA group as a reference. We adjusted the HRs by sex, race, body mass index, age, and CCI of two or more (R package, survival and epiDisplay). We used P < 0.05 as the level of significance.
Identifying important predictors of time to death in the CSA group
We took two steps to identify important variables that predict time to death. First, we excluded variables that were not statistically associated with mortality at P < 0.05. Then, we passed the remaining variables into the least absolute shrinkage and selection operator (LASSO) (19). LASSO is a machine-learning technique for selecting the most important variables to construct a model. LASSO forced the coefficients of not-so-significant variables to become zero; therefore, it reduced the number of variables. For internal validation, we used 10-fold crossvalidation with L1-penalization and Cox regression model (R package glmnet); see Figure E1.
CSA subgroup analysis
We performed a subgroup analysis on patients with HF, as it is one of the most widely studied groups on the impact of mortality. This subgroup of patients has been studied extensively. We reported the number of deaths, median and interquartile time to death, and HR using the absence of HF as a reference.
Additional subgroup analysis was done for patients with opiate prescriptions within 6 months of the index date. Sensitivity analysis after excluding comorbid conditions with high mortality (solid tumor [localized and metastatic], moderate-to-severe liver disease, and acquired immunodeficiency syndrome) was also performed.
Results
Patients
The baseline characteristics of patients in the two cohorts are shown in Table 1. Between 1999 and 2020, the total number of patients with CSA was 2,961 compared with 1,487,353 patients with OSA. The ratio of OSA to CSA was 502:1, confirming that, in U.S. veterans who sought sleep medicine services, the prevalence of CSA is substantially lower than OSA. The patients with CSA (61.8 ± 15.6 yr) were older than patients with OSA (56.7 ± 13.9 yr), and most patients in both groups were men (Table 1). The mean follow-up was 5.92 years (95% CI, 3.64–9.68). The minimum follow-up was 0.13 years, and the maximum follow-up was 22.23 years.
Table 1.
Characteristics and demographics of patients with central sleep apnea or obstructive sleep apnea
| CSA (n = 2,961) | OSA (n = 1,487,353) | |
|---|---|---|
| Age, M (SD) | 61.8 (15.6) | 56.7 (13.9) |
| <65 yr, n (%) | 1,477 (49.9) | 1,003,487 (67.5) |
| ⩾65 yr, n (%) | 1,484 (50.1) | 483,866 (32.5) |
| Body mass index, M (SD) | 31.2 (6.2) | 33.5 (6.2) |
| <18.5 | 22 (0.7) | 3,772 (0.3) |
| ⩾18.5 to <30 | 1,342 (45.3) | 453,195 (30.5) |
| ⩾30 | 1,597 (53.9) | 1,030,386 (69.3) |
| Race, n (%) | ||
| White | 2,325 (78.5) | 1,058,902 (71.2) |
| Black | 393 (13.3) | 298,392 (20.1) |
| Other | 243 (8.2) | 130,059 (8.7) |
| Sex (male), n (%) | 2,834 (95.7) | 1,389,393 (93.4) |
| Ethnicity (Hispanic), n (%) | 150 (5.1) | 117,779 (7.9) |
| Comorbidity | ||
| CCI, M (SD) | 1.8 (2.3) | 1.6 (2.1) |
| CCI ⩾2, n (%) | 1,137 (38.4) | 6,846 (37.1) |
| Myocardial infarction, n (%) | 138 (4.7) | 37,615 (2.5) |
| Heart failure, n (%) | 470 (15.9) | 99,919 (6.7) |
| Peripheral vascular disease, n (%) | 295 (10.0) | 75,627 (5.1) |
| Cerebrovascular disease, n (%) | 280 (9.5) | 73,510 (4.9) |
| Dementia, n (%) | 96 (3.2) | 12,385 (0.8) |
| Chronic obstructive pulmonary disease, n (%) | 618 (20.9) | 276,346 (18.6) |
| Connective tissue disease, n (%) | 45 (1.5) | 18,535 (1.2) |
| Peptic ulcer disease, n (%) | 25 (0.8) | 11,434 (0.8) |
| Mild liver disease, n (%) | 121 (4.1) | 37,880 (2.5) |
| Diabetes mellitus without complication, n (%) | 866 (29.2) | 425,423 (28.6) |
| Diabetes mellitus with end-organ damage, n (%) | 338 (11.4) | 137,269 (9.2) |
| Hemiplegia, n (%) | 46 (1.6) | 7,856 (0.5) |
| Moderate to severe chronic kidney disease, n (%) | 375 (12.7) | 94,393 (6.3) |
| Solid tumor (localized), n (%) | 241 (8.1) | 89,223 (6.0) |
| Moderate to severe liver disease, n (%) | 16 (0.5) | 3,795 (0.3) |
| Solid tumor (metastatic), n (%) | 28 (0.9) | 5,127 (0.3) |
| Acquired immunodeficiency syndrome, n (%) | 7 (0.2) | 4,657 (0.3) |
Definition of abbreviations: CCI = Charlson Comorbidity Index; CSA = central sleep apnea; M (SD) = mean (standard deviation); OSA = obstructive sleep apnea.
The CSA cohort (1.8 ± 2.3) had higher CCI than the OSA (1.6 ± 2.1) cohort. We observed that the CSA cohort has a higher percentage of patients with a CCI of two or higher (Table 1). Among the CCI component comorbid conditions, the CSA cohort had a higher proportion of HF (15.9%), peripheral vascular disease (10.0%), cerebrovascular disease (CVD) (9.5%), dementia (3.2%), mild liver disease (4.1%), hemiplegia (1.6%), myocardial infarction (4.7%), and moderate-to-severe chronic kidney disease (12.7%) (Table 1).
Hunter Cheyne Stokes breathing (HCSB) was present in 1.6% of patients with CSA and 0.05% of patients with OSA. Of 700 patients with CSA, 23.6% had an opiate prescription within 6 months of index diagnosis compared with 280,085 patients with OSA (18.8%).
Outcomes
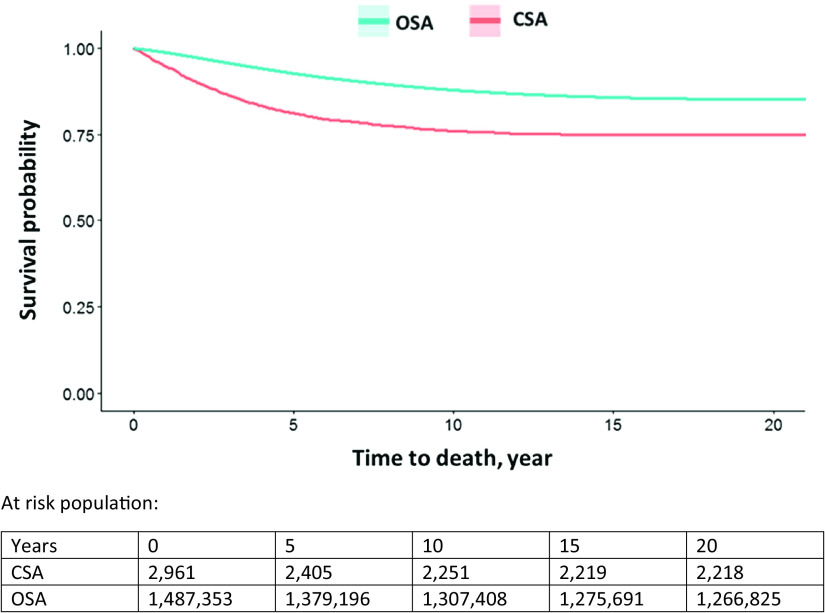
The median time to death in the CSA group (2.7 yr) was shorter than for the OSA group (5.1 yr) (Table 2). The unadjusted HR showed a significantly elevated rate of mortality in the CSA cohort (HR, 1.89; 95% CI, 1.76–2.03) compared with the OSA cohort; and, after adjusting HR by sex, race, BMI, age, and CCI, it remained significant (adjusted HR [aHR], 1.53; 95% CI, 1.43–4.65). We reported the Kaplan-Meier curve of the two groups in Figure 1. We observed a reduction in survival from the CSA group showing separation soon after diagnosis compared with the OSA group. About 18.7% of patients with CSA had died within approximately 5 years of diagnosis.
Table 2.
Comparing the hazard ratio of mortality in central sleep apnea and obstructive sleep apnea
| Death, n (%) | Time to death, years, M (IQR) | HR (95% CI) |
||
|---|---|---|---|---|
| Unadjusted | Adjusted* | |||
| CSA (n = 2,961) | 743 (25.1) | 2.7 (1.3–5.0) | 1.89 (1.76–2.03) | 1.53 (1.43–4.65) |
| OSA (n = 1,487,353) | 221,456 (14.9) | 5.1 (2.6–8.7) | Reference | Reference |
Definition of abbreviations: CI = confidence interval; CSA = central sleep apnea; HR = hazard ratio; IQR = interquartile range; M = median; OSA = obstructive sleep apnea.
Adjusted by sex, race, body mass index, age, and Charlson Comorbidity Index.
Figure 1.
Kaplan-Meier curves. Comparison of survival probability of CSA and OSA. CSA = central sleep apnea; OSA = obstructive sleep apnea.
After excluding comorbid conditions with high mortality (solid tumor [localized and metastatic], moderate-to-severe liver disease, and acquired immunodeficiency syndrome), the mortality hazard remained high in the CSA cohort when compared with the OSA cohort (HR, 1.79; 95% CI, 1.66–1.94). After adjusting for CCI, sex, race, BMI, and age, the hazard remained high (aHR, 1.38; 95% CI, 1.28–1.50).
Most Important Predictors of Time to Death in the CSA Group
Table E1 reports the most significant predictors of mortality in the CSA group. The presence of HF, history of CVD, hemiplegia, and BMI of less than 18.5 were among the highest predictors of mortality in CSA. The presence of male sex also suggested a higher risk of mortality. Younger age and obesity (BMI greater than 30) were associated with lower mortality.
Clinically Relevant Subgroup Analysis
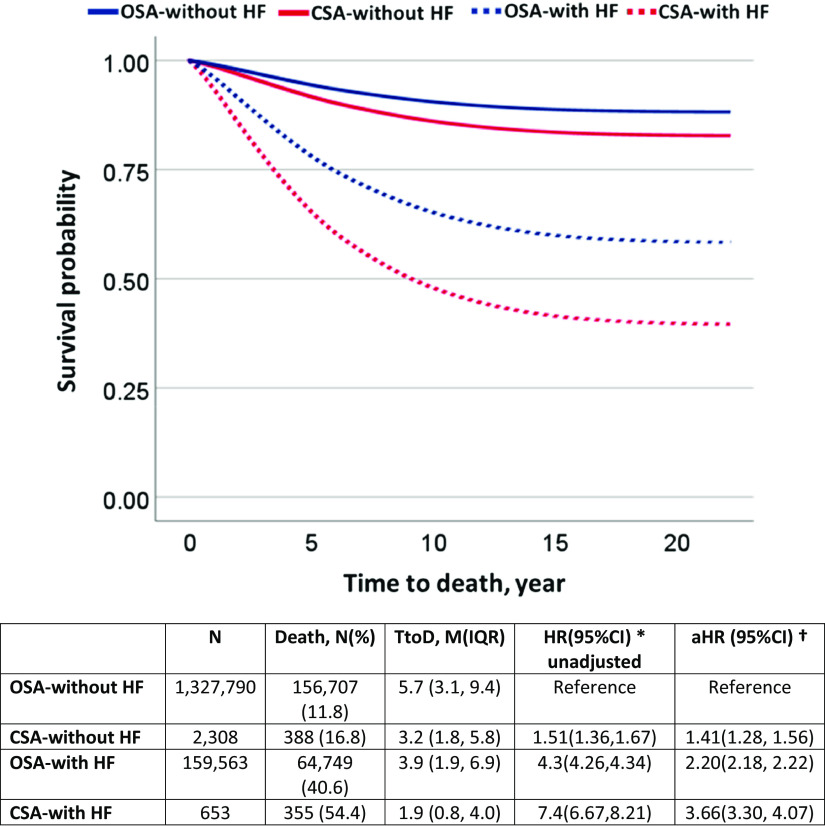
In this U.S. veteran-based study, 16% of patients had documented comorbid HF in the CSA cohort. As a reference, in a prior general population-based sleep heart health study, 7.4% (95% CI, 0.7–14.1) had comorbid HF among patients with CSA (2). The subgroup analysis revealed that the presence of HF is associated with increased mortality in both CSA (HR, 7.4; 95% CI, 6.67–8.21) and OSA (HR, 4.3; 95% CI, 4.26–4.34) groups. The Kaplan-Meir curve showed that the separation in morality started soon after index diagnosis (Figure 2). In general, the CSA group was associated with higher mortality in both HF and without HF groups compared with the respective OSA subgroup. After adjusting for CCI, sex, race, BMI, and age, we found the mortality hazard remained significant in both CSA with HF (aHR, 3.66; 95% CI, 3.30–4.07) and OSA (aHR, 2.20; 95% CI, 2.18–2.22) with HF subgroups.
Figure 2.
Kaplan-Meier curves. Comparison of survival probability on the basis of the presence and absence of HF. *HR (95%CI) = hazard ratio 95% percent confidence intervals and †adjusted by gender, race, body mass index, age, and Charlson comorbidity index. aHR = adjusted hazard ratio; CI = confidence interval; CSA = central sleep apnea; HF = heart failure; IQR = interquartile range; M = mean; OSA = obstructive sleep apnea; TtoD = time to death.
Patients with opiate prescriptions also demonstrated higher mortality hazard in the CSA group (HR, 1.91; 95% CI, 1.69–2.16; reference: patients with OSA who took opiates). After adjusting for CCI, sex, race, BMI, and age, we found the mortality hazard remained significant (aHR, 1.67; 95% CI, 1.48–1.89; reference: patients with OSA who took opiates).
Discussion
We constructed one of the largest retrospective, national, longitudinal cohorts of patients with CSA and OSA among the U.S. veteran population, spanning the last 2 decades. We observed that one-fifth of patients with CSA died within 5 years of diagnosis, with substantially higher mortality of patients with CSA than patients with OSA. The mortality hazard remained significant, even after adjusting for comorbidities. Further analysis showed that HF, history of CVD, hemiplegia, and male sex were among the highest predictors of mortality in CSA. The subgroup analysis showed that the presence of HF in both CSA and OSA groups is associated with significantly increased mortality compared with the patients without HF. After adjusting for comorbid conditions and demographics, we found CSA continued to be associated with higher mortality than OSA, independent of associated comorbidity.
Because CSA is associated with many conditions that lead to increased mortality, investigators have attempted to scrutinize CSA’s independent contribution to mortality. These studies analyzed mortality patterns in patients diagnosed with HF with reduced ejection fraction (HFrEF) by comparing the CSA cohort with the non-CSA cohort. The study data are inconsistent. Earlier reports suggested that in patients with HFrEF, nocturnal CSA with HCSB has no prognostic impact (12, 20); however, more recently, Javaheri and colleagues reported that the median survival in a CSA–HFrEF (n = 56) group was reduced (45 mo vs. 90 mo in HFrEF without CSA) (9). Our study investigated this question from a different perspective. The initial cohort comprised patients with CSA with varied comorbid conditions (including HF, opiate prescription, other causes of volume overload such as liver and kidney failure, and CVD). Our results affirmed that ICD-coded CSA is associated with higher mortality when compared with ICD-coded OSA. In our CSA cohort (n = 2,961), the time to death was 31 months (vs. 61 mo in the OSA group).
Our study’s Cox proportional hazard model investigating the survival probability demonstrated that the CSA cohort died at a higher rate soon after the index diagnosis. The curve demonstrated a steep decline until 5 years and flattened at around 10 years. These data should be interpreted in the context of the mean follow-up of 5.92 years (95% CI, 3.64–9.68). At around 5 years from diagnosis, approximately 19% of patients had died in the CSA cohort. These results suggest that, although OSA and CSA have a similar common final pathway of hypoxemia and apnea/hypopnea, their mortality patterns are distinct.
Furthermore, in our study, comorbid HF was present in 16% of patients in the CSA cohort but was associated with sevenfold higher mortality when compared with patients with OSA without HF. The remaining patients with CSA without HF also have 51% higher mortality when compared with the reference OSA group. Thus, the associated mortality in both OSA and CSA groups dramatically rises in the presence of comorbid HF. Reassuringly, the survival probability was similar to the HF–CSA group in a study by Roebuck and colleagues (n = 78, median follow-up 52 months) (Figure 2) (21). Even though this study included all patients with HF with and without sleep-disordered breathing, this study’s subgroup HF–CSA was similar to our definition. Thus, the current study expands on this smaller study on a much larger scale while offering a unique insight into the CSA–HF subgroup compared with those without HF.
There are several possible explanations for the shorter time to death in patients with CSA compared with OSA. As opposed to OSA, CSA is a heterogeneous syndrome in which different etiologies, such as HF, lead to a common final pathway of central apnea, hypopnea, and desaturation. Several comorbidities are associated with CSA; these include HF, CVD, advanced chronic kidney disease, and diabetes (14, 22–25). Although OSA is primarily related to a mechanical obstruction, the chemically driven alternation in central ventilatory control (26) may contribute to higher mortality. Second, there may be a lead time bias; patients with CSA may be diagnosed later than patients with OSA, as they frequently lack traditional OSA risk factors such as obesity. Nevertheless, in clinical practice, the diagnosis of CSA may forewarn a clinician regarding its conceivable link with mortality.
The CSA group had a higher proportion of comorbid conditions than the OSA group, suggesting this is a sicker cohort. However, when adjusted for these comorbid conditions and demographics, results showed that patients with CSA without HF continued to have an independent 41% higher mortality hazard. These data suggest that the deaths in this subgroup may not be fully explained by the comorbidities included in CCI. Whether CSA is an independent risk for increased mortality or simply a biomarker remains unknown. These findings should be explored further in prospective studies.
We also noted that in the CSA cohort, male sex was associated with an HR of more than two, suggesting an association of reduced survival in men. Like other studies involving U.S. veterans, this study had a predominance of men (96%). Furthermore, population-based studies have shown that the male sex itself is associated with a higher mortality risk than female sex (27). Thus, sex-related data should be interpreted within these constraints. Despite this limitation, these data may be worth exploring, as our data confirm those of earlier epidemiological studies that have shown that CSA is uncommon in women (28, 29). For example, even though approximately half of the sample comprised women in the sleep heart health study, in the CSA cohort, approximately 91% of patients were men (2). The presence of obesity (BMI greater than 30) was protective (aHR, 0.82), whereas BMI less than 18.5 was associated with higher odds of dying in the CSA cohort (aHR, 1.76) compared with the OSA cohort. This obesity paradox may reflect indication bias in sleep testing in that obese patients that are referred for testing are more likely to have OSA, whereas the sicker nonobese patients that are referred to testing are more likely to have CSA.
Limitations
This study has several limitations. Like many retrospective studies using large data sets, the current study was also at risk of several potential biases. The major bias was the reliability of the current cohort definition. On a random sample analysis, we noted that the CSA definition’s positive predictive value was 0.6. The rest of the patients had OSA and were miscoded. Because our study found that the mortality of the OSA cohort was lower than that for CSA, the presence of miscoded OSA likely diluted the mortality of the CSA group. In prior studies, the accuracy of ICD coding of primary diagnosis had been validated in sleep disorders such as OSA. These studies have reported that claims-based algorithms identify OSA in a definition similar to ours, with a positive predictive value of 74.8% and a negative predictive value of 18.3% (15). Thus, the OSA cohort may have a smaller subset of patients that may not have an OSA diagnosis. Only a small proportion of the CSA cohort had HCSB (1.6%). We suspect it is related to undercoding. When both CSA and HCSB are present, we suspect that the clinicians typically choose to document only the practically relevant CSA code. In addition, we could not assess granular data such as the severity of sleep-disordered breathing, treatment of underlying conditions, and the role of positive airway pressure therapy, including modalities such as adaptive servo-ventilation, left ventricular ejection fraction, atrial fibrillation, and other medications. Future studies using advanced tools such as natural language processing will help better understand these finer clinically relevant details.
Conclusions
We report that clinically diagnosed CSA was associated with a shorter time to death from the index diagnosis. The presence of CSA was associated with higher mortality, and almost one-fifth of patients with CSA died within 5 years of diagnosis. The subgroup analysis showed that the presence of HF in the CSA group is associated with significantly increased mortality compared with patients with CSA without HF.
Acknowledgments
Acknowledgment
The authors are grateful to the VA Informatics and Computing Infrastructure (VINCI).
Footnotes
Supported by the National Institute of Nursing Research (NINR) (R01NR018342 [S.N.]); National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) K25 funding (1K25HL152006-01 [J.R.]); ZOLL Respicardia (31002 [A.S.]); and the use of facilities and resources at the Center for Innovations in Quality, Effectiveness, and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, TX. The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs, the U.S. government, or Baylor College of Medicine.
Author Contributions: R.A.: conceptualization, methodology, investigation, writing/original draft preparation, and reviewing and editing. A.S.: conceptualization, methodology, writing/reviewing and editing, and funding acquisition. D.J.G. and S.N.: writing/reviewing and editing. J.R.: data curation, methodology, software, investigation, funding acquisition, and writing/reviewing and editing.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol . 2013;3:141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 2. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the sleep heart health study cohort. Sleep (Basel) . 2016;39:1353–1359. doi: 10.5665/sleep.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev . 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 4. Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation . 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 5. Sin DDD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med . 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 6. Bradley TD, Floras JSS. Sleep apnea and heart failure: part II: central sleep apnea. Circulation . 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 7. Sands SA, Owens RL. Congestive heart failure and central sleep apnea. Crit Care Clin . 2015;31:473–495. doi: 10.1016/j.ccc.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosen IM, Aurora RN, Kirsch DB, Carden KA, Malhotra RK, Ramar K, et al. American Academy of Sleep Medicine Board of Directors Chronic opioid therapy and sleep: an American Academy of Sleep Medicine position statement. J Clin Sleep Med . 2019;15:1671–1673. doi: 10.5664/jcsm.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol . 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 10. Lange RL, Hecht HH. The mechanism of Cheyne-Stokes respiration. J Clin Invest . 1962;41:42–52. doi: 10.1172/JCI104465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hagenah G, Zapf A, Schüttert JB. Cheyne-Stokes respiration and prognosis in modern-treated congestive heart failure. Lung . 2010;188:309–313. doi: 10.1007/s00408-009-9208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andreas S, Hagenah G, Moller C, Werner GS, Kreuzer H. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol . 1996;78:1260–1264. doi: 10.1016/s0002-9149(96)00608-x. [DOI] [PubMed] [Google Scholar]
- 13.Veterans Health Administration. 2021. https://www.va.gov/health/aboutvha.asp
- 14.Sateia MJ. International classification of sleep disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 15. Laratta CR, Tsai WH, Wick J, Pendharkar SR, Johannson KA, Ronksley PE. Validity of administrative data for identification of obstructive sleep apnea. J Sleep Res . 2017;26:132–138. doi: 10.1111/jsr.12465. [DOI] [PubMed] [Google Scholar]
- 16. Sohn M-W, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr . 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VIReC. VIReC factbook corporate data warehouse (CDW) vital sign 1.1 domain. Hines, IL: U.S. Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2018. [Google Scholar]
- 18. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol . 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Alelyani S, Liu H. In: Data classification: algorithms and applications. Aggarwal CC, editor. 2014. Feature selection for classification: a review; p. 37. [Google Scholar]
- 20. Luo Q, Zhang HL, Tao XC, Zhao ZH, Yang YJ, Liu ZH. Impact of untreated sleep apnea on prognosis of patients with congestive heart failure. Int J Cardiol . 2010;144:420–422. doi: 10.1016/j.ijcard.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 21. Roebuck T, Solin P, Kaye DM, Bergin P, Bailey M, Naughton MT. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. Eur Respir J . 2004;23:735–740. doi: 10.1183/09031936.04.00060404. [DOI] [PubMed] [Google Scholar]
- 22. Dharia SM, Unruh ML, Brown LK. Central sleep apnea in kidney disease. Semin Nephrol . 2015;35:335–346. doi: 10.1016/j.semnephrol.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 23. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep (Basel) . 2018;41:zsy058. doi: 10.1093/sleep/zsy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest . 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc . 2017;6:e004500. doi: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology . 2017;22:43–52. doi: 10.1111/resp.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu YT, Niubo AS, Daskalopoulou C, Moreno-Agostino D, Stefler D, Bobak M, et al. Sex differences in mortality: results from a population-based study of 12 longitudinal cohorts. CMAJ . 2021;193:E361–E370. doi: 10.1503/cmaj.200484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med . 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 29. Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol (1985) . 2000;89:192–199. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]