As intensivists, decisions about whom not to admit to our care are often as challenging as decisions regarding patients already in the intensive care unit (ICU). For marginal patients—those with high acuity but no clear-cut ICU indications—admission to the general care ward typically means losing the reassurance and rapid ability to intervene provided by frequent vital signs, other advanced monitoring, and close geographic proximity to critical care nurses, physicians, and respiratory therapists. Not surprisingly, triage decisions are consequential; mortality is higher among critically ill patients initially triaged to the general care ward compared with those directly admitted to the ICU or among those with delays in accessing the ICU once ill (1, 2). Deteriorating ward patients may also have collateral effects on neighboring patients who share clinicians and clinical resources (3). On the other hand, overtriage of patients at low likelihood of benefit might expose patients to unnecessary risk and exacerbate limitations in ICU clinicians and beds (4, 5). Accurate, objective triage guidelines are essential, and central to this goal are studies that identify who benefits from ICU admission and why.
In an excellent series of papers, Anesi and colleagues have sought to provide strong observational evidence in support of these goals. The authors began with foundational work in 2020 when they identified hospital capacity strain as a determinant of ICU triage (6). Hospitals under strain—measured using markers of occupancy, acuity, and activity—admit fewer marginal patients to the ICU than they do when they are less strained. The authors leveraged this variability as a tool to study the potential benefits of ICU admission among patients not on mechanical ventilation or vasopressors (7). Specifically, they conducted an instrumental variable analysis in which capacity strain functioned akin to a coin flip in a randomized controlled trial by helping determine whether a marginal patient with sepsis or respiratory failure would go to the ICU or to the general care ward. Ultimately, they found that admission to the ICU among such patients was associated with shorter lengths of stay and improved survival in respiratory failure. The opposite was true among patients in the study with sepsis.
In this issue of AnnalsATS, Anesi and colleagues (pp. 406–413) ask two related questions about whether hospitals vary in their response to strain and in the benefits associated with ICU versus ward triage for respiratory failure (8). They begin with an established cohort of patients with acute respiratory failure presenting to emergency departments in each of 27 hospitals. They excluded people requiring mechanical ventilation or vasopressors and others with treatment limitation decisions that may have precluded ICU care. They then characterized strain temporally within each hospital and used regression to identify the relationship between strain and ICU versus ward triage. Models were adjusted for demographic characteristics, comorbidities, and severity of illness using laboratory and vital sign measurements.
Hospitals in the authors’ study varied widely in the degree to which their triage decisions were influenced by strain; whereas some hospitals’ practices were fairly static, others’ predicted probability of ICU admission differed by up to 60% between times of high and low strain. That a potentially consequential decision varies so much depending on whether the hospital is strained is by itself a noteworthy finding. Workforce limitations, resource shortages, and high-strain events (e.g., periodic pandemic surges, natural disasters) are unlikely to abate in the near term and seem to cause tangible changes in how we care for patients with respiratory failure.
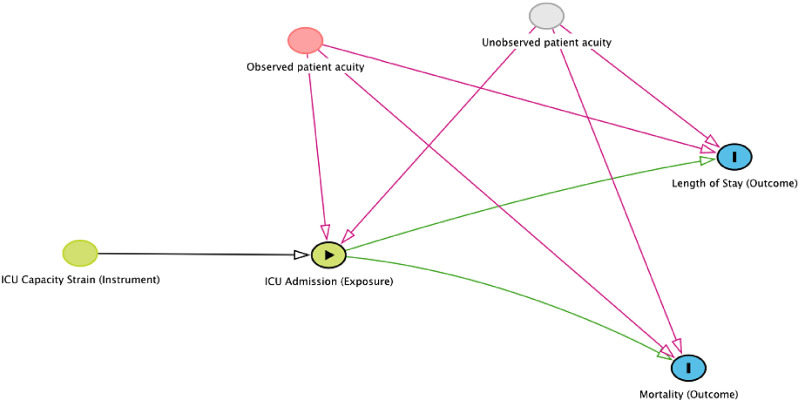
Next, the authors test whether the effects of ICU versus ward admission for marginal patients vary across hospitals. As in their earlier work, they use instrumental variable methods (Figure 1), with ICU capacity strain as an instrument (9). Within hospitals, they assume (with justifications where possible) that 1) capacity strain is associated with ICU admission (there is a causal “arrow” between strain and ICU admission on the directed acyclic graph), 2) capacity strain is associated with outcomes only via ICU admission (the only paths between strain and outcomes go through ICU admission), and 3) there are no common causes of hospital strain and ICU admission (there are no other variables with arrows between both capacity strain and ICU admission). This method allows them to measure unbiased (e.g., unconfounded) benefits or harms associated with ICU admission among marginal patients (i.e., those without organ failure and whose emergency department triage destination is determined by whether the hospital is strained). In doing so, they find substantial hospital-level differences in both length of stay and mortality attributed to ICU versus ward triage. Put another way, an otherwise identical patient with moderate illness severity might be harmed by ICU admission at one hospital and saved by ICU over ward admission at another.
Figure 1.
Directed acyclic graph describing the authors’ instrumental variable analysis (created using DAGitty version 3.0; dagitty.net). ICU = intensive care unit.
This finding suggests that the optimal location for an otherwise similar patient with moderate illness severity—for whom triage decisions are likely variable—is a hospital-specific characteristic, probably driven by features local to the ICU and ward environments. So, universal ICU triage guidelines that are valid across hospitals might not be plausible. Instead, future work might focus on identifying and implementing the features of wards or ICUs that make them more effective. The authors evaluated a broad range of protocols, staffing models, and capabilities across degrees of care at study hospitals. None of the surveyed practices, however, influenced the relative benefits of ICU versus ward admission. Beyond a deeper dive into the characteristics evaluated by the authors, several areas are ripe for further work leveraging modern advances in data availability, analytical techniques, and computational power.
First, we ought to continue leveraging electronic health record data to understand why the ICU does or does not improve outcomes for certain patients. What elements do high-functioning wards and ICUs share in common? Similarly, which complications do or do not occur that might explain the observed variation in benefit? In answering these questions, we will be better positioned to improve care across degrees of care while reducing reliance on overburdened ICUs for patients who can safely receive care elsewhere.
Second, locally developed clinical prediction models that apply rich electronic health record data may provide better identification of patients’ potential for benefit from ICU transfer compared with more general guidelines. Modern machine learning techniques have the ability to incorporate patient diagnoses, vital signs, laboratory results, imaging data, and other characteristics into their predictions; clinical prediction models on the basis of these techniques can take advantage of this added nuance to identify patients who are most likely to deteriorate without higher degrees of care (10, 11). Triage rules applying such models warrant further evaluation.
Finally, we might reimagine ICU triage as matching specific patient needs to staff and resource availability in real time. It goes without saying that a patient hospitalized with respiratory failure requiring frequent nebulized medications or chest physiotherapy might do better in a location in which respiratory therapists and trained nurses are more available to care for them. At a given time, this might be in a specific ward, or it might be in an ICU. Real-time analytical tools that take into account the characteristics and needs of patients undergoing triage, the acuity and needs of other patients in the potential destination areas, and the specific resources currently available in those areas may be more effective than static triage guidelines. Such models might be able to deliver more effective care that responds to dynamic changes in staffing and workload.
This paper by Anesi and colleagues (8) and their related work represent important steps toward understanding relationships between ICU triage and subsequent outcomes for patients with respiratory failure and other critical illness syndromes. It should prompt further work on understanding how and why specific degrees of care matter for patients with respiratory failure, and efforts to increase access to the beneficial components of that care to all patients.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants K08HL155407 (A.J.A.) and K01HL155404 (S.A.). This work does not represent the views of the U.S. government or the U.S. Department of Veterans Affairs.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Harris S, Singer M, Rowan K, Sanderson C. Delay to admission to critical care and mortality among deteriorating ward patients in UK hospitals: a multicentre, prospective, observational cohort study. Lancet . 2015;385:S40. doi: 10.1016/S0140-6736(15)60355-5. [DOI] [PubMed] [Google Scholar]
- 2. Sankey CB, McAvay G, Siner JM, Barsky CL, Chaudhry SI. “Deterioration to door time”: an exploratory analysis of delays in escalation of care for hospitalized patients. J Gen Intern Med . 2016;31:895–900. doi: 10.1007/s11606-016-3654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, Edelson DP, Howell MD, Churpek MM. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA . 2016;316:2674–2675. doi: 10.1001/jama.2016.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halpern NA, Pastores SM, Oropello JM, Kvetan V. Critical care medicine in the United States: addressing the intensivist shortage and image of the specialty. Crit Care Med . 2013;41:2754–2761. doi: 10.1097/CCM.0b013e318298a6fb. [DOI] [PubMed] [Google Scholar]
- 5. Guidet B, Leblanc G, Simon T, Woimant M, Quenot JP, Ganansia O, et al. ICE-CUB 2 Study Network Effect of systematic intensive care unit triage on long-term mortality among critically ill elderly patients in France: a randomized clinical trial. JAMA . 2017;318:1450–1459. doi: 10.1001/jama.2017.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anesi GL, Chowdhury M, Small DS, Delgado MK, Kohn R, Bayes B, et al. Association of a novel index of hospital capacity strain with admission to intensive care units. Ann Am Thorac Soc . 2020;17:1440–1447. doi: 10.1513/AnnalsATS.202003-228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anesi GL, Liu VX, Chowdhury M, Small DS, Wang W, Delgado MK, et al. Association of ICU admission and outcomes in sepsis and acute respiratory failure. Am J Respir Crit Care Med . 2022;205:520–528. doi: 10.1164/rccm.202106-1350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anesi GL, Dress E, Chowdhury M, Wang W, Small DS, Delgado MK, et al. Among-hospital variation in ICU admission practices and associated outcomes for patients with acute respiratory failure. Ann Am Thorac Soc . 2023;20:406–413. doi: 10.1513/AnnalsATS.202205-429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maciejewski ML, Brookhart MA. Using instrumental variables to address bias from unobserved confounders. JAMA . 2019;321:2124–2125. doi: 10.1001/jama.2019.5646. [DOI] [PubMed] [Google Scholar]
- 10. Peelen RV, Eddahchouri Y, Koeneman M, van de Belt TH, van Goor H, Bredie SJ. Algorithms for prediction of clinical deterioration on the general wards: a scoping review. J Hosp Med . 2021;16:612–619. doi: 10.12788/jhm.3630. [DOI] [PubMed] [Google Scholar]
- 11. Cummings BC, Ansari S, Motyka JR, Wang G, Medlin RP, Jr, Kronick SL, et al. Predicting intensive care transfers and other unforeseen events: analytic model validation study and comparison to existing methods. JMIR Med Inform . 2021;9:e25066. doi: 10.2196/25066. [DOI] [PMC free article] [PubMed] [Google Scholar]