Abstract
Background:
This retrospective database analysis describes clinical characteristics and treatment patterns of U.S. women with a diagnosis for uterine fibroids (UF), both with and without heavy menstrual bleeding (HMB).
Materials and Methods:
Two cohorts aged 18–50 years with an incident UF diagnosis, comprising women with and without claims for HMB (UF-HMB and UF-only), were identified from the IQVIA PharMetrics® Plus database (January 1, 2010–December 31, 2019). The index date was the first UF claim following diagnosis; treatment patterns were documented for postindex years 1 and 2 and the full duration of postindex follow-up. Also identified were claims for symptoms or signs potentially associated with UF. Outcomes were the proportion of patients treated with pharmacologic therapies of interest and gynecologic procedures. Logistic regression was used to identify factors associated with postdiagnosis hysterectomy and hormonal therapy.
Results:
A total of 66,313 (71.8%) women were included in the UF-HMB cohort (mean age [standard deviation]) 42.6 [5.4] years), and 26,068 (28.2%) in the UF-only cohort (41.8 [6.3]). Median follow-up was ∼4 years. Pain was the most common symptom (42.7% in patients with UF-HMB and 36.6% with UF-only); also common were abnormal bleeding (15.6%, 11.5%) and fatigue (22.2%, 15.5%). Within 1 year of UF diagnosis, 28.8% and 49.2% of women with UF-HMB and UF-only, respectively, had no claims for relevant pharmacologic or surgical treatment. In logistic regression, multiple factors were associated with a higher likelihood of receiving hysterectomy or hormonal therapy.
Conclusions:
Patients with UF-HMB were more likely to receive UF treatment, either surgical or pharmacologic, than women with UF-only. Apart from HMB, pain was the most commonly documented symptom of UF.
Keywords: uterine fibroids, leiomyoma, heavy menstrual bleeding, menorrhagia
Introduction
Uterine fibroids (UF) are the most common benign pelvic tumors among women in the United States.1,2 The prevalence of UF increases with age until menopause, with >60% of cases occurring in women aged 30–44 years.3,4 Although UF can be asymptomatic, ∼25% of women are symptomatic,2 with heavy menstrual bleeding (HMB) being the most frequently reported symptom.5–8 Although HMB commonly drives the need for treatment in patients with UF, it is not the only factor; other symptoms, such as pain and fatigue, can severely impair multiple aspects of women's health-related quality of life and lead patients to seek medical attention.3,8,9
No clear consensus currently exists regarding appropriate treatment protocols for patients with symptomatic UF,10 although it is acknowledged that shared decision-making should be used in treatment selection, and that quality of life should be a central consideration.11 Recently published guidelines from the American College of Obstetricians and Gynecologists (ACOG) regarding the management of symptomatic UF suggest that treatment decisions should be guided by individual patient symptoms and long- and short-term treatment goals.12 However, very few drugs are indicated for UF,13–16 and only limited evidence is available for many commonly used pharmacologic options.17 Medical treatments used for UF or its symptoms include nonsteroidal anti-inflammatory drugs, tranexamic acid, hormonal therapy, aromatase inhibitors, gonadotropin-releasing hormone (GnRH) analogs, and progesterone modulators.7 These typically provide only short-term symptom control, and the long-term use of some of these drugs can lead to adverse effects such as reduced bone density or dyslipidemia.12
Inpatient and outpatient surgical options include hysterectomy, myomectomy, uterine artery embolization, and endometrial ablation. These treatments are effective but may lead to complications and may not preserve fertility or the uterus.18,19 Based on a 2020 retrospective database analysis, hysterectomy was the most common surgery in the United States for symptomatic UF management between 2010 and 2015, accounting for 68%–76% of initial UF-related surgeries.18
The 2012 multinational Uterine Bleeding and Pain Women's Research Study surveyed reproductive-aged women with and without UF (n = 1,533 and n = 20,213, respectively) and found that women with UF consistently experienced a significantly higher frequency and severity of pain symptoms than women without UF.8 Although most women with symptomatic UF choose nonsurgical first-line management,10 current evidence indicates that medical therapy use is limited in the year following UF diagnosis.18,20 There is a need to better understand the real-world clinical characteristics and treatment patterns of women with UF who are symptomatic due to HMB or for other reasons, such as pain.
The objective of this retrospective database analysis was to describe the clinical characteristics and treatment patterns (hormonal therapy, pain management, and surgery) of U.S. women with medical claims for UF who did and did not have medical claims for HMB (UF-HMB and UF-only, respectively). The deidentified records of these women were followed for a minimum of 2 years after their index diagnosis of UF. Patients were identified at incident diagnosis and followed for a minimum of 2 years after their index claim.
Materials and Methods
Study design and data source
Women with UF-HMB and UF-only, aged 18–50 years, with an initial UF diagnosis between January 1, 2010 and December 31, 2019 were identified from the IQVIA PharMetrics® Plus database. This database includes longitudinal, medical, and pharmacy claims that have been processed for payment by national and subnational health plans and self-insured employer groups covering >190 million enrollees, primarily commercial patients.21
Study population
Two mutually exclusive cohorts were created using selection criteria applied to identify patients with a minimum of 2 years of continuous follow-up subsequent to initial diagnosis. In the first cohort (UF-HMB), patients were required to have ≥2 UF claims ≥30 days apart, (International Classification of Diseases ICD-10 D25.X and ICD-9 218.X) and ≥2 claims for HMB at any time that were ≥30 days apart (ICD-10, N92.0-N92.4; N93.8-N93.9; ICD-9 626.2, 626.5–626.6, 626.8–626.9, 627.0). These claims comprised an initial claim and a confirmatory claim (required in case the initial UF or HMB claims were an exploratory diagnosis). In the second cohort (UF-only), patients were required to have ≥2 UF claims that were ≥30 days apart, and no claims for HMB at any time.
The index date was defined as the date of the first UF diagnosis claim, and patients were required to have a minimum of 1 year of continuous preindex date enrollment. Patients could not have any UF diagnosis claims in the year before the index date (i.e., women were newly diagnosed), and were excluded if they did not have ≥2 years of continuous enrollment postindex date (making the index date range between January 2011 and December 2017). Patients were allowed to have one 30-day gap in enrollment coverage each year.
Also identified were claims for other symptoms or signs potentially associated with UF that were submitted either before or on the same day as the first UF diagnosis. Patients with symptomatic UF were required to have at least one additional symptom related to pain (abdominal/pelvic), abnormal bleeding, infertility, anemia and/or fatigue. Symptom data were evaluated for all UF-HMB patients (who were all considered symptomatic, due to HMB) and for UF-only patients who had documented symptoms. In Supplementary Table S1, the ICD-9 and ICD-10 codes were used to identify these claims.
Outcomes and statistical analyses
Descriptive statistics were used to summarize study population characteristics. Treatment patterns over the first and second years following the index date, and for the full duration of postindex follow-up, were assessed as the proportion of patients treated with gynecologic procedures and/or prescribed pharmacologic therapies of interest that were reimbursed by insurance. Pharmacologic therapies of interest were hormonal treatments (oral and nonoral contraceptives), including intrauterine devices (IUDs, except ParaGard®/copper IUD), estrogen, progestin, aromatase inhibitors, elagolix, danazol, leuprolide, or any luteinizing hormone-releasing hormone agonists.
Also evaluated were the use of tranexamic acid and pain medicines, including narcotic (prescribed for ≥30 days) and prescription non-narcotic analgesics. Not available for analysis were over-the-counter products not captured in medical claims and prescriptions not reimbursed by the payer. Gynecologic procedures of interest were hysterectomy, operative laparoscopy, myomectomy, oophorectomy, ablation of the endometrium and/or fibroids, excision, and salpingectomy. Finally, data were collected for pharmacologic treatments of interest (hormonal or analgesic) received by patients in the year preceding the index date.
Patients in both cohorts who underwent hysterectomy within 1 year postindex date were further stratified by age. Logistic regression models were constructed to determine factors associated with specific treatments (hysterectomy and hormonal therapy) in patients with UF-HMB and UF-only. To isolate these findings to patients who received hysterectomy due to UF, the regression analysis excluded patients with a claim for endometriosis (ICD-9 617.X or ICD-10 N80.X). This exclusion was applied because of the potential for confounding due to concomitant comorbidity. The variables included in the logistic regression were factors that could contribute to treatment decision-making and that could be captured in claims data. These were age, abnormal bleeding, anemia, fatigue, infertility, pain, prior- and post-UF diagnosis use of medications, including hormonal treatment, non-narcotic, or narcotic analgesic treatment, and inpatient or outpatient diagnosis site. Data were analyzed using SAS/STAT(r) software, version 15.1 (2016 SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Patient selection based on study criteria is summarized in Table 1; a total of 1,513,396 women had a UF claim. After inclusion and exclusion criteria were applied, 92,399 met the criteria for further analysis; of these, 66,313 (71.8%) were assigned to the UF-HMB cohort and 26,068 (28.2%) to the UF-only cohort. In addition, a total of 50,409 (UF-HMB) and 22,384 (UF-only) women were evaluated in regression analysis.
Table 1.
Study Criteria and Exclusions Applied for Cohort Identification, Patients with UF-HMB and UF-only
| Characteristic | UF-HMB | UF-only |
|---|---|---|
| All patients with ≥1 UF diagnostic claim in the database | 1,513,396 | |
| Female, aged 18–50 years | 1,084,061 | |
| ≥2 dataset claims for HMB ever (≥30 days apart) (with HMB) or no claims for HMB ever (without HMB)a | 422,558 | 385,158 |
| ≥2 diagnoses for UF ever in the dataset | 237,060 | 117,431 |
| 12-month history without a UF diagnosis before index date | 109,825 | 47,129 |
| ≥2 years continuous follow-up after the index date and included in the study (allowing for one 30-day gap in coverage each year) | 66,313 | 26,068 |
| Regression analysis only: no claims for endometriosis | 50,409 | 22,384 |
Bold values are final cohort numbers used in the overall analysis during the follow-up period.
Patients with UF plus one claim for HMB were excluded.
HMB, heavy menstrual bleeding; UF, uterine fibroids.
Table 2 summarizes patient characteristics related to insurance type and patient age. The large majority of patients, both UF-HMB and UF-only (95%–96%), had commercial health insurance coverage, and most were aged 35–50 years (91% UF-HMB and 85% UF-only). Among UF-HMB patients, mean age (standard deviation [SD]) was 42.6 (5.4) years, and median time from index date to last claim was 1499 days (4.1 years). Among UF-only patients, mean age (SD) was 41.8 (6.3) years, and median time from index date to last claim was 1407 days (3.9 years).
Table 2.
Health Insurance and Age Groups of Patients with UF-HMB and UF-only
| Group | UF-HMB (n = 66,313) | UF-only (n = 26,068) |
|---|---|---|
| Health insurance type, n (%) | ||
| Commerciala | 63,050 (95.1) | 25,031 (96.0) |
| Government-sponsored planb | 2882 (4.3) | 871 (3.3) |
| Unknown | 381 (0.6) | 166 (0.6) |
| Age group, years, n (%) | ||
| 18̶24 | 280 (0.4) | 181 (0.7) |
| 25̶34 | 5517 (8.3) | 3,777 (14.5) |
| 35̶44 | 31,955 (48.2) | 11,185 (42.9) |
| 45̶50 | 28,561 (43.1) | 10,925 (41.9) |
| Mean patient age (SD) | 42.6 (5.4) | 41.8 (6.3) |
| Median time from index date to last claim, years | 4.1 | 3.9 |
Employer-sponsored or individual coverage plans.
Medicare or Medicaid.
SD, standard deviation.
Symptomatology
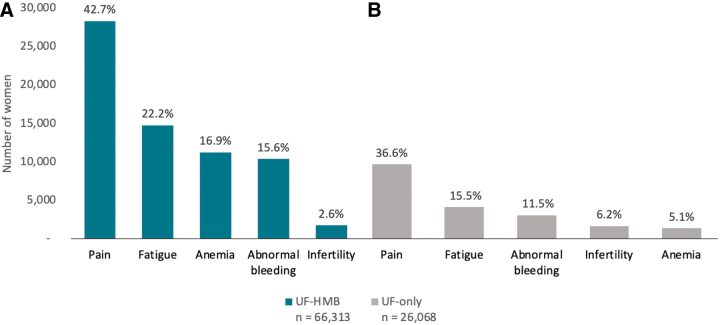
Figure 1 shows other symptoms and signs of UF that were diagnosed either before or on the same day as patients' first UF diagnosis. With the exception of infertility, all symptoms and signs were diagnosed at a somewhat higher rate in women with UF-HMB than in symptomatic women with UF-only. Pain was the most common symptom for all women (42.7% [UF-HMB] and 36.6% [UF-only]); abnormal bleeding (15.6%, 11.5%) and fatigue (22.2%, 15.5%) were also common in both cohorts.
FIG. 1.
Symptom-specific claims filed before or on same day as UF diagnosis, patients with (A) UF-HMB or (B) UF-only. HMB, heavy menstrual bleeding; UF, uterine fibroids.
Treatment patterns
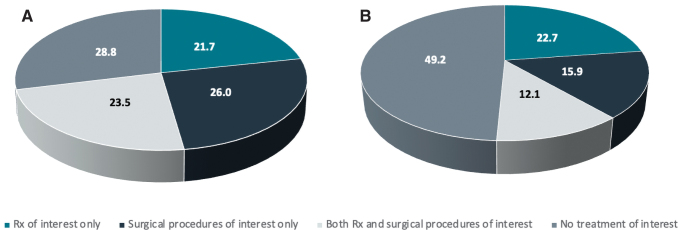
As shown in Figure 2, within 1 year of the index date, 28.8% of UF-HMB patients and 49.2% of UF-only patients received no surgical or pharmacologic treatment of interest. Table 3 shows treatment status by prescription and study year. Within 1 year of the index date, 54.7% (UF-HMB) and 65.1% (UF-only) of patients, respectively, had not received any pharmacologic therapy of interest. However, this decreased over time: for patients with UF-HMB, this rate was 46.3% at year 2 and 35.7% at any time during the median 4.1 years of follow-up. For patients with UF-only, this rate was 57.8% at year 2 and 49.2% at any time during the median 3.9 years of follow-up.
FIG. 2.
Overall treatment status within 1 year of index date, patients with (A) UF-HMB or (B) UF-only. Rx, pharmacologic therapy.
Table 3.
Treatment Status by Prescription (by Study Year) or Gynecologic Procedure (Year 1 Only), Patients with UF-HMB and UF-only
| Treatment, n (%) | UF-HMB (n = 66,313) | UF-only (n = 26,068) |
|---|---|---|
| Use of any pharmacologic treatment of interest | ||
| Year 1 | 30,017 (45.3) | 9,085 (34.9) |
| Year 2 | 35,579 (53.7) | 10,993 (42.2) |
| Anytime | 42,617 (64.3) | 13,254 (50.8) |
| Use of any hormonala treatment | ||
| Year 1 | 24,526 (37.0) | 7,606 (29.2) |
| Year 2 | 28,876 (43.5) | 9,063 (34.8) |
| Anytime | 34,713 (52.3) | 10,771 (41.3) |
| Any gynecologic procedure of interest (year 1) | 32,819 (49.5) | 7,311 (28.0) |
| Hysterectomy | 20,499 (30.9) | 4,326 (16.6) |
| Other procedures | 16,029 (24.2) | 3,562 (13.7) |
Hormonal treatment includes aromatase inhibitors, leuprolide, other luteinizing hormone-releasing hormone agonists, elagolix, estrogen, progestin, nonoral hormonal contraceptives (except for ParaGard® copper intrauterine device J73000, NDC 51285020401, 51285020402), and oral hormonal contraceptives.
Similarly, a large proportion of patients with UF received no hormonal therapy, and this trend declined modestly over time. In women with UF-HMB, the percentage receiving no hormonal therapy was 63.0% at year 1, 56.5% at year 2, and 47.7% at any time during follow-up. In women with UF-only, the percentage receiving no hormonal therapy was 70.8% at year 1, 65.2% at year 2, and 58.7% at any time during follow-up.
Within 1 year of the index date, 49.5% (UF-HMB) and 28.0% (UF-only) of patients underwent a gynecologic procedure of interest, most frequently hysterectomy (30.9% and 16.6%, respectively; Table 3). Table 4 shows the number of patients who underwent hysterectomy, both by age and cumulatively by study year. In both cohorts, hysterectomies were most common in older women (45–50 years; UF-HMB, 35.7%, UF-only, 21.9%). Patients with UF-HMB aged 35–44 years also had a high rate of hysterectomy (30.2%). Regardless of age, patients with UF-HMB were more likely to have a hysterectomy than those with UF-only (30.9% and 16.6%), and the percentage of women with UF-HMB having a hysterectomy increased over time: 38.7% at 2 years and 50.0% at any time during follow-up. The proportion of women with UF-only claims receiving hysterectomy also increased over time (19.7% at 2 years and 23.9% at any time during follow-up).
Table 4.
Hysterectomies After the Index Date in Patients with UF, patients with UF-HMB and UF-only, by Age Group (Year 1 Only) and by Study Year
| Count (%) of patients with a hysterectomy | ||
|---|---|---|
| Age group (year 1 data) | UF-HMB (n = 66,313) | UF-only (n = 26,068) |
| 18 – 24 years, count (%) | 7 (2.5) n = 280 | 2 (1.1) n = 181 |
| 25 – 34 years, count (%) | 651 (11.8) n = 5517 | 150 (4.0) n = 3777 |
| 35 – 44 years, count (%) | 9636 (30.2) n = 31,955 | 1,783 (15.9) n = 11,185 |
| 45 – 50 years, count (%) | 10,205 (35.7) n = 28,561 | 2,391 (21.9) n = 10,925 |
| Total hysterectomies, by study year (cumulative incidence) | ||
| Year 1, % | 30.9 n = 20,499 | 16.6 n = 4,326 |
| Year 2, % | 38.7 n = 25,670 | 19.7 n = 5,147 |
| Anytime, % | 50.0 n = 31,155 | 23.9 n = 6,234 |
Factors associated with hysterectomy and hormonal treatment
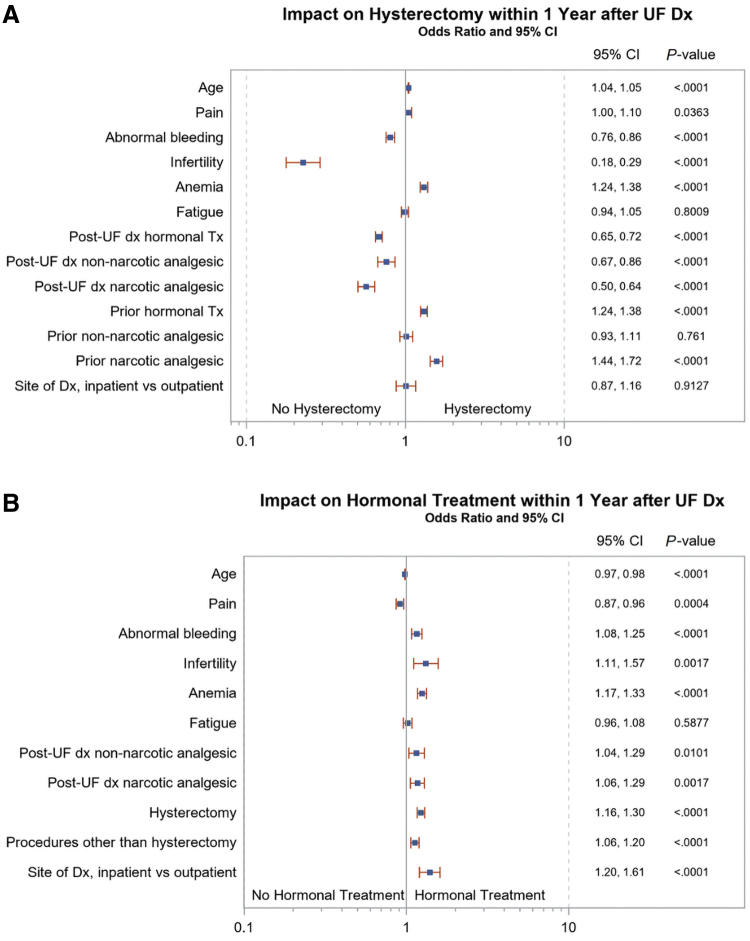
Based on logistic regression (Fig. 3), in patients with UF-HMB, multiple factors were significantly associated with hysterectomy within 1 year of UF diagnosis. Factors with the most substantial odds ratios (OR) for a higher rate of hysterectomy were prior (pre-UF diagnosis) use of narcotic analgesics (OR, 95% confidence interval [CI]: 1.44–1.72) or hormonal therapy (1.24–1.38), and existing anemia (1.24–1.38). Factors with the most substantial OR for a lower likelihood of receiving hysterectomy were infertility (OR, 0.18–0.29), and the post-UF diagnosis use of narcotic analgesics (0.50–0.64), hormonal therapy (0.65–0.72), and non-narcotic analgesics (0.67–0.86).
FIG. 3.
Impact of symptoms and prior treatment on (A) hysterectomy and (B) hormonal treatment within 1 Year of UF diagnosis in patients with UF-HMB.
CI, confidence interval; dx, diagnosis; Tx, treatment.
In the UF-HMB cohort, factors associated with the highest OR of receiving hormonal treatment within 1 year of UF diagnosis were infertility (OR, 1.11–1.57) and the site of diagnosis (inpatient vs. outpatient) (1.20–1.61). Pain and higher age were modestly associated with a lower likelihood of receiving hormonal treatment (ORs 0.87–0.96 and 0.97–0.98, respectively).
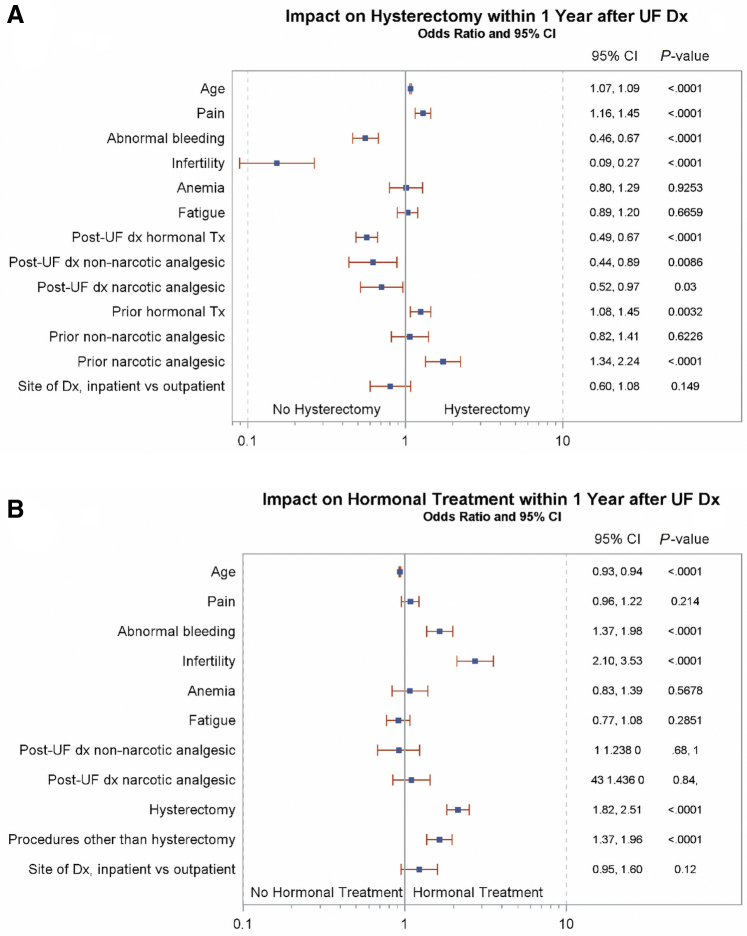
Multiple factors were also significantly associated with hysterectomy in patients with UF-only (Fig. 4). Factors with the most substantial OR for a higher rate of hysterectomy were prior narcotic analgesic use (95% CI, 1.34–2.24) and pain (95% CI, 1.16–1.45), and prior hormonal therapy (1.08–1.45). Factors with the most substantial OR for a lower likelihood of receiving hysterectomy were infertility (0.09–0.27), abnormal bleeding (0.46–0.67), postdiagnosis use of hormonal therapy (0.49–0.67), and the post-UF diagnosis use of non-narcotic (0.44–0.89) and narcotic analgesics (0.52–0.97). In the UF-only cohort, factors associated with the highest OR of receiving hormonal treatment within 1 year of UF diagnosis were infertility (2.10–3.53), abnormal bleeding (1.37–1.98), hysterectomy (1.82–2.51), and surgical procedures other than hysterectomy (1.37–1.96). Patient age was modestly associated with a lower likelihood of receiving hormonal treatment (OR 0.93–0.94).
FIG. 4.
Impact of symptoms and prior treatment on (A) hysterectomy and (B) hormonal treatment within 1 Year of UF diagnosis in patients with UF-only.
Discussion
In this retrospective analysis of women aged ≤50 years with a diagnosis of UF, both with and without claims for HMB, a relatively large proportion of patients received surgical and/or pharmacologic UF treatment in the year following diagnosis (71.2% of patients with UF-HMB and 50.8% with UF-only). Over the 1-year postindex period, hormonal or analgesic drug therapy was administered to 45.3% of women with UF-HMB and 34.9% of those with UF-only, and the proportion of both cohorts prescribed drug treatment increased over time. Nonetheless, after ∼4 years of median follow-up, nearly two-thirds (64.3%) of UF-HMB and one-half (50.8%) of UF-only patients had a claim for any hormonal or analgesic pharmacologic therapy.
These findings are consistent with two recent retrospective cohort studies of insured patients with UF with and without claims for HMB. Wang et al, evaluated commercially insured patients (2007–2018) with claims for UF-only, HMB-only, UF-HMB, and controls. The authors found that 68% of women with UF-HMB received treatment (medical or surgical) in the first year following diagnosis, while 38% of those with UF-only received treatment.20 Likewise, Bonine et al, conducted a large database analysis of patients with symptomatic UF with Commercial (n = 225,737) or Medicaid (n = 19,062) insurance.
In this study, 31.7% (Commercial) and 53.0% (Medicaid) of patients received pharmacologic treatment for UF in the 12 months following diagnosis, while 31.6% of patients with symptomatic UF (defined as at least one diagnosis of a related symptom, including anemia due to blood loss, vaginal bleeding, or other menstrual bleeding disorders, pain associated with female genital organs, and/or urinary symptoms) did not receive any treatment within 12 months of diagnosis.18
The current data also confirm existing research indicating that many women in the United States undergo gynecologic surgical procedures (primarily, hysterectomy) in the year following UF diagnosis, without having any prior prescription claims for UF pharmacologic therapy.18,20,22 In the first postindex year, 49.5% and 28.0% of UF-HMB and UF-only patients received a surgical procedure, including 30.9% and 16.6% who had a hysterectomy. In the same time frame, 26.0% and 15.9% of UF-HMB and UF-only patients, respectively, underwent a surgical procedure without any prior claims for pharmacologic therapies of interest. Similar findings were observed in Wang et al, which found that 37% (UF-HMB) and 14% (UF-only) received only surgical/procedural treatment in the 12 months following UF diagnosis.
In addition, in the Wang study, women with claims for HMB were also more likely to have surgical procedures than those without claims for HMB, and hysterectomy was the most common procedure received at any time point.20 Bonine et al, also found that hysterectomy was the most common surgery in the United States for symptomatic UF management, and accounted for 68%–76% of initial UF-related surgeries.18 Although hysterectomy effectively eliminates the possibility of UF recurrence, this intervention is not an option for premenopausal women who desire fertility or want to keep their uterus.5,12
Furthermore, this analysis also confirms prior research showing that pain is a common symptom driving the need for treatment among women with UF.8,23 In this study, pain was the most frequently reported symptom, 36.6% with UF-only and 42.7% with UF-HMB. This matches prior research showing a high incidence of pain in patients with UF. In the 2012 multinational Uterine Bleeding and Pain Women's Research Study survey, more than 50% of women with UF reported at least one pain symptom, and many reported multiple pain symptoms that included chronic pelvic pain, pain due to bladder pressure, and pain experienced multiple times during the monthly cycle (e.g., during ovulation or before, during, and/or after menstruation).8 Similarly, in a 2017 U.S. survey of women with UF and no history of hysterectomy (n = 955), more than 60% reported pain symptoms.23
In the current study, regression analysis showed that the presence of pain had a small but significant association with subsequent hysterectomy, while the use of postdiagnosis hormonal treatment was associated with a reduced likelihood of hysterectomy. Prior narcotic analgesic use was the variable most strongly associated with an increased likelihood of receiving a hysterectomy. These findings suggest that patients with UF who are experiencing pain at diagnosis (expressed either directly via medical claims, or indirectly via prior prescriptions for narcotic medications) are more likely to receive a hysterectomy.
These results also suggest that our understanding of how women are affected by UF-related pain, and why physicians choose specific responses to patient-reported pain, is not fully understood. It is recognized that the symptoms of UF, especially pain and HMB, can severely impair multiple aspects of women's health-related quality of life, including productivity, sexuality, physical and emotional well-being, work performance, and relationships.8,24 As such, appropriate interventions are needed to alleviate UF symptoms and signs, as well as to improve quality of life.24 The recently updated (2021) U.S. ACOG guidelines acknowledge that insufficient comparative evidence exists to offer recommendations for first-line medical therapy in patients with UF, and state that individualized medical management, based on patient-specific symptoms and their severity, rather than a prespecified clinical sequencing algorithm, should be used to guide treatment.
ACOG also highlights the importance of conducting research that centers UF as a clinical issue for women that requires safe and effective approved nonsurgical treatment options.12 With that said, the regular off-label use of pharmacologic therapies and high rates of hysterectomy in patients with symptomatic UF suggest a need for the individualized management and use of existing drugs and for the development of new, safe, and effective noninvasive treatments.11,18,25 Historically, pharmacologic treatment options for UF have primarily addressed bleeding, and not pain, symptoms.12 The recently approved therapies containing GnRH antagonists (elagolix and relugolix) reduce HMB, and relugolix combination therapy has also been demonstrated to address pain13,14 and/or bulk symptoms.15,16
Study strengths and limitations
A key strength of the current analysis is the generalizability of its findings to commercially insured women with UF aged 50 years and younger in the United States. According to an epidemiologic analysis of 1999–2005 National Health and Nutrition Examination Survey data, 95.5% of women with UF are diagnosed before 50 years of age.4 This study's primary findings are further supported by two recent retrospective database analyses, both of which were conducted using different source databases than the current study (Wang et al used Truven MarketScan® Commercial Claims and Encounters and Bonine et al used the IBM Watson Health MarketScan® Commercial Claims and Encounters and Medicaid Multi-State databases).18,20 Furthermore, by using regression analysis to model patient data, this study expands on existing research to show the relationship between prediagnosis treatment patterns, pain, and other signs and symptoms of UF, and subsequent hysterectomy and hormonal therapy use.
In terms of limitations, this study was retrospective and descriptive in nature and was conducted using medical and pharmacy claims data. Considered broadly, claims data are collected for insurance payments, not research, and may be subject to coding errors, data gaps, and data limitations. For this analysis, no claims data were available for over-the-counter or other drugs that required out-of-pocket payment (i.e., for pain or birth control), or for prescriptions not reimbursed by the payer; both of these factors could lead to an underestimate of drug use. Likewise, any free drug samples provided to patients, or prescriptions that were left unfilled, would not be captured in this analysis. Any patient who was lacking an insurance claim for UF diagnosis was not included in this analysis.
The retrospective analysis of claims data also limits the ability to make conclusions regarding causality or to draw inferences regarding the medical decision-making processes behind treatment choices. For example, this study does not fully explain patient-specific factors leading to treatment decisions: young patients wishing to preserve fertility might prefer medical treatment, but might also avoid medical therapies contraindicated in pregnancy; on the contrary, older women who have completed their families might prefer surgical treatment. Furthermore, no standardization exists among physicians or other health care providers regarding the most appropriate approach to coding patient encounters (i.e., whether to code for diagnosis only, diagnosis with symptomatology, and/or diagnosis plus separate claims for specific symptoms). It is also important to note that although ∼75% of study patients reported symptoms commonly associated with UF, some patients with UF-only had no reported symptoms.
For these women, it is not possible to know whether a specific complaint led to these UF diagnoses or if they were incidental. It is possible that a larger number of study patients were symptomatic than reported; as not all symptoms reported by patients will be documented in electronic health records and captured in claims data. It is also not possible to confirm that the symptom claims evaluated in this analysis were related to UF, as patients may have experienced these symptoms for other reasons. Likewise, patients may have been administered study drugs for reasons other than UF (since National Drug Code claims are not associated with a diagnosis); if this occurred, it could have led to an overestimation of the drug treatment rate. It was also not feasible to evaluate any adverse events that patients might have experienced with drug or other treatments.
In this study, age was the only patient demographic characteristic that was independently evaluated; this was due to limitations of the claims dataset; the impact of additional patient characteristics will be investigated in further research. In addition, premenopausal women aged >50 years were not included in the analysis, which limits the ability to generalize study results to an older population. Finally, despite efforts to limit the patient population to women with recently diagnosed UF with and without claims for HMB, patients could have been diagnosed with UF or incurred claims for HMB in the >1 year before 2010. Similarly, patients who reported symptoms that were not accompanied by an applicable diagnostic claim for UF would have been excluded from this study.
Implications for practice and/or policy
There is a need to better understand why women with symptomatic UF may fail to receive pharmacologic treatment and why hysterectomy is frequently the initial intervention in this patient population. The current study results suggest a need for more in-depth research, such as a comprehensive electronic health record analysis, to obtain a more accurate and detailed profile of patient utilization and treatment patterns, and to better understand the clinical rationale and patient characteristics that inform treatment decision-making. This study also supports previous findings that pain is a common symptom in patients with UF, and highlights the need for clinicians to consider the impact of pain and other UF signs and symptoms when designing treatment plans.
Conclusions
In this analysis, in the year following incident diagnosis, U.S. patients aged ≤50 years with UF and a history of HMB were more likely to receive UF treatment, either surgical or pharmacologic, than women without claims for HMB. Apart from HMB, pain was the most commonly documented symptom of UF. These findings highlight the need for additional treatment options, including pharmacologic therapy, for women with symptomatic UF.
Supplementary Material
Acknowledgments
Medical writing support was provided by Caitlin Rothermel MA, MPH of MedLitera as overseen by Simcoe Consultants, Inc., and funded by Myovant Sciences GmbH, in collaboration with Pfizer, Inc.; K.E. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The conclusions set forth herein are based on independent research and publicly available material.
Disclaimer
The views expressed are the views and opinions of the authors and do not reflect or represent the views of Charles River Associates or any organizations with which the authors are affiliated.
Author Disclosure Statement
L.M., C.L., and R.D. are Myovant Sciences, Inc. stockholders, and C.L. and R.D. are employees of Myovant Sciences Inc.; K.E. is a Principal with Charles River Associates, which received consulting fees from Myovant Sciences, Inc., to conduct this research.
Funding Information
This study was funded by Myovant Sciences, Inc. and conducted by Charles River Associates.
Supplementary Material
References
- 1. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100–107; doi: 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 2. Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: A systematic review. BJOG 2017;124(10):1501–1512; doi: 10.1111/1471-0528.14640 [DOI] [PubMed] [Google Scholar]
- 3. Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: Burden and unmet medical need. Semin Reprod Med 2017;35(6):473–480; doi: 10.1055/s-0037-1607264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cacheris WP, Hunsche EG. PIH15 - Prevalence of diagnosed uterine fibroids and endometriosis in the US: Data from a nationally representative population-based survey. Value Health 2018;21:S216; doi: 10.1016/j.jval.2018.09.1280 [DOI] [Google Scholar]
- 5. De La Cruz MS, Buchanan EM. Uterine fibroids: Diagnosis and treatment. Am Fam Physician 2017;95(2):100–107. https://www.ncbi.nlm.nih.gov/pubmed/28084714. [PubMed] [Google Scholar]
- 6. Khan AT, Shehmar M, Gupta JK. Uterine fibroids: Current perspectives. Int J Womens Health 2014;6:95–114; doi: 10.2147/IJWH.S51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis TD, Malik M, Britten J, et al. A comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res Int 2018;2018:2414609; doi: 10.1155/2018/2414609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmermann A, Bernuit D, Gerlinger C, et al. Prevalence, symptoms and management of uterine fibroids: An international internet-based survey of 21,746 women. BMC Womens Health 2012;12:6; doi: 10.1186/1472-6874-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: From menarche to menopause. Clin Obstet Gynecol 2016;59(1):2–24; doi: 10.1097/GRF.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Havryliuk Y, Setton R, Carlow JJ, et al. Symptomatic fibroid management: Systematic review of the literature. JSLS 2017;21(3); doi: 10.4293/JSLS.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mas A, Tarazona M, Dasi Carrasco J, et al. Updated approaches for management of uterine fibroids. Int J Womens Health 2017;9:607–617; doi: 10.2147/IJWH.S138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American College of Obstetricians & Gynecologists. Management of symptomatic uterine leiomyomas: ACOG Practice Bulletin, Number 228. Obstet Gynecol 2021;137(6):e100–e115; doi: 10.1097/AOG.0000000000004401. [DOI] [PubMed] [Google Scholar]
- 13. Al-Hendy A, Lukes AS, Poindexter AN, 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med 2021;384(7):630–642; doi: 10.1056/NEJMoa2008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MYFEMBREE® (relugolix, estradiol, and norethindrone acetate) tablets, for oral use [prescribing information]. Brisbane, CA: Myovant Sciences Inc.; 2021. [Google Scholar]
- 15. Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med 2020;382(4):328–340; doi: 10.1056/NEJMoa1904351. [DOI] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration (FDA). FDA approves new option to treat heavy menstrual bleeding associated with fibroids in women, 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-option-treat-heavy-menstrual-bleeding-associated-fibroids-women [Last accessed: June 15, 2021].
- 17. Duckitt K, Collins S. Menorrhagia. BMJ Clin Evid 2012;2012(0805). [PMC free article] [PubMed] [Google Scholar]
- 18. Bonine NG, Banks E, Harrington A, et al. Contemporary treatment utilization among women diagnosed with symptomatic uterine fibroids in the United States. BMC Womens Health 2020;20(1):174; doi: 10.1186/s12905-020-01005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043; doi: 10.1038/nrdp.2016.43. [DOI] [PubMed] [Google Scholar]
- 20. Wang A, Wang S, Owens C, et al. Health care costs and treatment patterns associated with uterine fibroids and heavy menstrual bleeding: A claims analysis. J Womens Health (Larchmt) 2021; 10.1089/jwh.2020.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marth MP, De AP, Roach A. Support Your Clinical and Economic Value Proposition with Real World Data - Part 2 in a Series. IQVIA website. Published March 24, 2021. Available from: https://www.iqvia.com/locations/united-states/blogs/2021/03/support-clinical-economic-value-prop-with-rwd-part-2 [Last accessed: July 15, 2022].
- 22. Bonafede MM, Pohlman SK, Miller JD, et al. Women with newly diagnosed uterine fibroids: Treatment patterns and cost comparison for select treatment options. Popul Health Manag 2018;21(S1):S13–S20; doi: 10.1089/pop.2017.0151. [DOI] [PubMed] [Google Scholar]
- 23. Soliman AM, Margolis MK, Castelli-Haley J, et al. Impact of uterine fibroid symptoms on health-related quality of life of US women: Evidence from a cross-sectional survey. Curr Med Res Opin 2017;33(11):1971–1978; doi: 10.1080/03007995.2017.1372107. [DOI] [PubMed] [Google Scholar]
- 24. Fortin C, Flyckt R, Falcone T. Alternatives to hysterectomy: The burden of fibroids and the quality of life. Best Pract Res Clin Obstet Gynaecol 2018;46:31–42; doi: 10.1016/j.bpobgyn.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 25. Vilos GA, Allaire C, Laberge PY, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can 2015;37(2):157–178; doi: 10.1016/S1701-2163(15)30338-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.