Abstract
Background:
Preconception diabetes is strongly associated with adverse birth outcomes. Less is known about the effects of elevated glycemia at levels below clinical cutoffs for diabetes. In this study, we estimated associations between preconception diabetes, prediabetes, and hemoglobin A1c (HbA1c) on the risk of preterm birth, and evaluated whether associations were modified by access to or utilization of health care services.
Materials and Methods:
We used data from Add Health, a US prospective cohort study with five study waves to date. At Wave IV (ages 24–32), glucose and HbA1c were measured. At Wave V (ages 32–42), women with a live birth reported whether the baby was born preterm. The analytic sample size was 1989.
Results:
The prevalence of preterm birth was 13%. Before pregnancy, 6.9% of women had diabetes, 23.7% had prediabetes, and 69.4% were normoglycemic. Compared to the normoglycemic group, women with diabetes had 2.1 (confidence interval [95% CI]: 1.5–2.9) times the risk of preterm birth, while women with prediabetes had 1.3 (95% CI: 1.0, 1.7) times the risk of preterm birth. There was a nonlinear relationship between HbA1c and preterm birth such that risk of preterm birth emerged after HbA1c = 5.7%, a standard cutoff for prediabetes. The excess risks of preterm birth associated with elevated HbA1c were four to five times larger among women who reported unstable health care coverage and among women who used the emergency room as usual source of care.
Conclusion:
Our findings replicate prior research showing strong associations between preconception diabetes and preterm birth, adding that prediabetes is also associated with higher risk. Policies and interventions to enhance access and utilization of health care among women before pregnancy should be examined.
Keywords: preconception, health care access, preterm birth, diabetes, glycemia
Introduction
Among US women of reproductive age (i.e., ages 18 to 44 years), the prevalence of diabetes is 4.5% and half of these cases are poorly controlled.1 Approximately 1%–2% of women enter pregnancy with diabetes.2,3 An additional estimated 20% of women in this age range have prediabetes, a metabolic state characterized by higher than normal blood glucose levels, but do not meet criteria for a diagnosis of diabetes mellitus.2 The proportion of women entering pregnancy with prediabetes is not well documented.
Preconception diabetes is associated with adverse pregnancy outcomes.3–5 For example, a 2017 meta-analysis of 55 studies reported that preconception diabetes is associated with 3.5 (3.1 and 4.0) times the odds of preterm birth.6 Notably, only a few studies have evaluated risks associated with preconception prediabetes. Of those available, two found that glucose levels in this range were associated with slightly higher birth weight, but not with gestation length.7,8 Another study reported that glucose levels in this range were associated with small increased risks of preterm birth.9
The American College of Obstetricians and Gynecologists (ACOG) and American Diabetes Association (ADA) recommend that women with preconception diabetes achieve optimal glycemic control before conception to prevent adverse outcomes.3,4,10 However, adherence to this guidance is complicated by limited access and utilization of health care services for some groups.11–13 More specifically, 12% of reproductive-aged women are uninsured.14 Furthermore, the proportion of preventive care visits among nonpregnant women that include recommended screening for diabetes is low (15%), ranging from 5.2% at obstetrician/gynecologist visits to 21.8% at primary care physician visits.15 Thus, prediabetes and diabetes can easily go undetected. Indeed, one-third of individuals with diabetes and >80% of individuals with prediabetes are undiagnosed in this population group.2,11
In the analysis presented below, we examined associations between both preconception diabetes status and hemoglobin A1c (HbA1c) with preterm birth. We hypothesized that both preconception diabetes and prediabetes would be associated with increased risk of preterm birth. Second, we assessed whether associations were modified by access and utilization of health care and hypothesized that the associations would be larger among women with less engagement in health care before pregnancy.
Materials and Methods
Study sample
The National Longitudinal Study of Adolescent to Adult Health (Add Health) is a prospective cohort study. Participants were recruited while in the 7th–12th grade from a sample of schools across the United States. The sampling frame included schools with at least 30 students enrolled and was stratified by region, urbanicity, school size, school type, and ethnicity.16 The initial sample included 20,745 adolescents (Wave I, 1994–1995) and follow-up is ongoing, with five assessment waves completed to date.16 Detailed information about the Add Health study design is accessible at https://addhealth.cpc.unc.edu/data. We obtained Add Health data through a restricted contract with the University of North Carolina at Chapel Hill, Carolina Population Center. Secondary analyses of these data were deemed exempt from the Institutional Review Board review by the UC San Diego Human Research Protections Program.
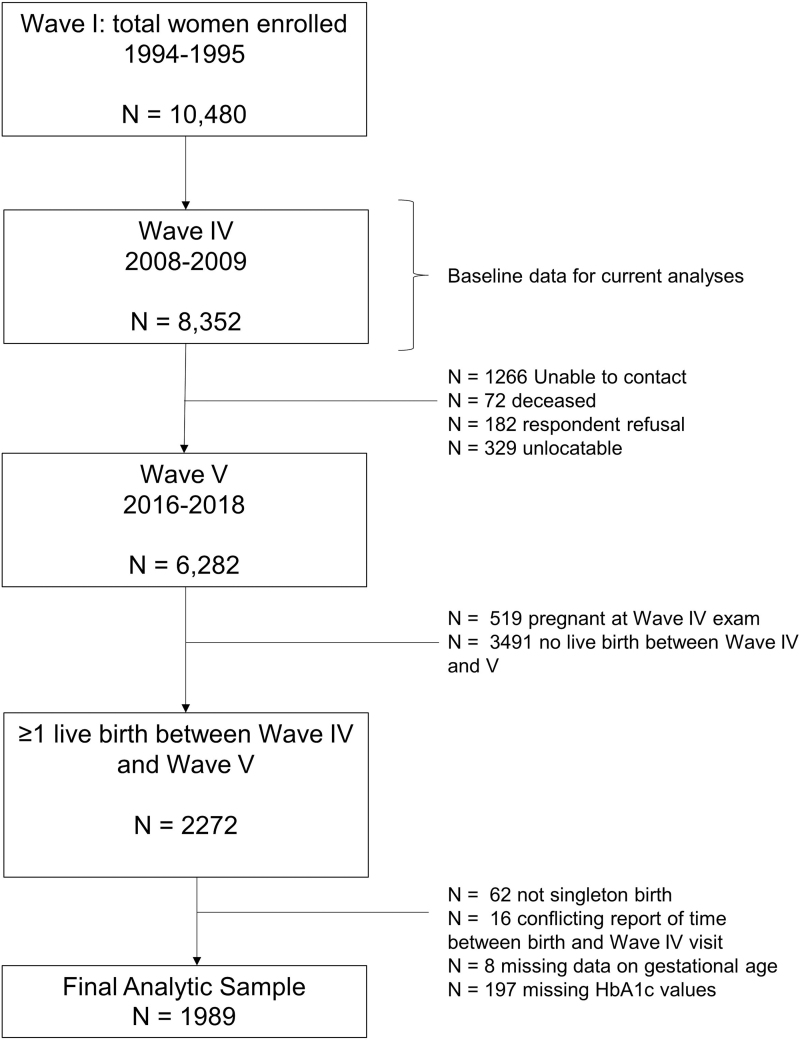
For this analysis, we used data collected at the Wave IV (2008–2009) and V (2016–2018) assessments, when participants were between ages 24–32 and 32–42 years, respectively. We included women who reported at least one pregnancy between the waves and were not pregnant at Wave IV. We excluded 62 participants with non-singleton births, 197 with missing laboratory values, and 24 with missing data for gestational age, resulting in a final analytic sample of 1989 women (Fig. 1). A comparison of sociodemographic characteristics between women assessed at the Wave I examination and those included in our analysis is provided in Supplementary Table S1.
FIG. 1.
Derivation of analytic sample.
Preconception diabetes status
At Wave IV, capillary whole blood was collected using finger stick from voluntary fasting (n = 273) and nonfasting participants (n = 1944). Samples were assayed for HbA1c (%) and glucose (mg/dL) using standard protocols.17 Reliability was tested in a random subsample of participants who provided two blood samples, 1 week apart, and showed an intraclass correlation coefficient of 0.97 for HbA1c (%) and 0.67 for fasting glucose.17 Self-reported history of diabetes was measured by the questionnaire item: “Has a doctor, nurse, or other health care provider ever told you that you have or had high blood sugar or diabetes when you were not pregnant?” In addition, prescription medication containers were reviewed for therapeutic classification.17
Using the Wave IV data, we created a three-level preconception diabetes status variable (diabetes, prediabetes, and normoglycemia). Criteria for diabetes included self-reported history of diabetes, antidiabetes medication use, HbA1c ≥6.5%, nonfasting glucose ≥200 mg/dL, or fasting glucose ≥126 mg/dL.18 Criteria for prediabetes included HbA1c 5.7%–6.4% or fasting glucose 100–125 mg/day. We classified women who did not meet criteria for diabetes or prediabetes as normoglycemic.2,18 In secondary analyses, we created a four-level preconception diabetes variable that further stratified the diabetes group into those with optimal versus suboptimal glycemic control using the ADA-recommended cutoff for preconception glycemia (optimal: HbA1c <6.5%).10
In sensitivity analyses, we recreated the three-level preconception diabetes using different criteria to evaluate potential misclassification bias. Specifically, we first excluded self-reported data from the classification, and then excluded glucose laboratory values. We found good agreement across the different classification schemes (Supplementary Table S2).
Preterm birth
The outcome variable was preterm birth. At the Wave V visit, women who gave birth since Wave IV responded to the questionnaire item, “A preterm delivery is one that occurs before 37 weeks in pregnancy (more than 3 weeks early). Was this baby born preterm?” If someone had multiple births between Wave IV and Wave V, we analyzed outcome data from the birth most proximal to the Wave IV visit.
Health care access and utilization
We created four binary indices of health care access and utilization based on self-reported data from Wave IV. They were (1) stable health care coverage (coded “yes” if participant answered “12 months” to the questionnaire item, “Over the past 12 months, how many months did you have health insurance?”), (2) unmet health care needs (coded “yes” if participant responded “yes” to the questionnaire item, “Has there been a time in the past 12 months when you thought you should get medical care, but you did not?”), (3) emergency room as usual source of care (coded “yes” if participant responded “emergency room department or other nonprimary care site” to the questionnaire item, “Where do you usually go when you are sick or need health care?”), and (4) last routine check-up (coded as “≤12 months” or “>12 months” based on participant response to the questionnaire item, “How long ago did you last have a routine check-up?”).
In addition to these binary variables, we created a 4-level insurance status variable based on responses to the questionnaire item, “Which of the following best describes your current health insurance situation?” The categories were as follows: uninsured, Medicaid, private insurance, and other. The “other” category included people who were covered through the Indian Health Service or active-duty military.
Covariates
We identified potential confounding variables by literature review. These were maternal age at birth, months between glucose measurement and birth, gravidity, US region, maternal race, maternal education status, Hispanic ethnicity, past year health care coverage, systolic blood pressure, waist circumference, and smoking status.
We calculated maternal age (in years) at birth from maternal birth date and offspring birth date and calculated the time (in months) between the Wave IV visit when diabetes status was assessed and the offspring birth date. Preconception systolic blood pressure (mmHg) and preconception waist circumference (cm) were measured by research personnel.19 We used all four of these variables as continuous variables in analysis.
The remaining covariates were self-reported by the participant and used as categorical variables in analysis. At Wave I, participants indicated their race using the following response options: White, Black or African American, American Indian or Native American, Asian or Pacific Islander, and other. They also responded to the questionnaire item, “Are you of Hispanic or Latino origin?” (yes/no).
At Wave IV, participants responded to the questionnaire item, “What is the highest grade or year of regular school you completed.” This questionnaire item had 13 response options ranging from “8th grade or less” to “completed postbaccalaureate professional education.” For analysis, we collapsed maternal education status into three categories: less than high school, high school to some college, and college or more.
Gravidity was reported with the questionnaire item, “How many times have you been pregnant?” For analysis, we created a three-level gravidity variable: 0, 1, or 2+ prior pregnancies. We also created a three-level smoking status variable (current, former, and never) based on participant response to “During the past 30 days, on how many days did you smoke cigarettes?” and “Have you ever smoked cigarettes regularly—that is, at least once cigarette every day for 30 days?” Finally, US region was coded based on the participant's residential address at Wave IV (Northeast, Midwest, South, and West).
Statistical analysis
Restricted use, de-identified Add Health participant data were obtained from the University of North Carolina at Chapel Hill, Carolina Population Center. All analyses were carried out in SAS 9.4 (Cary, NC).
We first described demographic, socioeconomic, and health care access and utilization variables in the full sample and stratified by preconception diabetes status. Then, we used multivariable Poisson regression models (link = log)20 to estimate risk ratios and linear regression models (link = identity)21 to estimate risk differences (RDs) for the association between preconception diabetes status and preterm birth. In a separate set of models, we used the four-level diabetes status variable to distinguish between participants with optimal versus suboptimal glycemic control. Models adjusted for all covariates listed above.
Next, we analyzed HbA1c as an independent variable. To assess a potential nonlinear relationship between HbA1c and preterm birth, we modeled HbA1c as a continuous variable using restricted cubic splines with five knots placed at the 5th, 35th, 65th, 75th, and 98th percentiles of HbA1c.22 Knot locations were selected based on standard recommendations for a four-knot model,23 with an added knot at the 75th percentile, which in this sample was HbA1c = 5.7, a clinically meaningful cut point used to indicate prediabetes.24
Finally, we assessed effect modification by health care access and utilization on the additive scale. To do so, we first described the percentage of participants with elevated HbA1c (HbA1c ≥5.7) and preterm birth by level of each health care variable. Then we estimated RDs stratified by health care access and utilization to evaluate a change in magnitude of the association across groups.
Results
The average age of women at the Wave IV assessment was 28.6 years (range 24–34 years) and average age at delivery was 32 years (range 26–40) (Table 1). At Wave IV, 6.9% of women had diabetes, 23.7% had prediabetes, and 69.4% were normoglycemic. Among women with diabetes, 61.6% were aware of their status, 27.5% were currently taking antidiabetic medicine, and 54.4% had met ADA guidelines for optimal glycemic control preconception (Table 2).
Table 1.
Demographic, Socioeconomic, and Health Care Access and Utilization Characteristics at the Wave IV Assessment Stratified by Preconception Diabetes Category, Add Health 2008–2009 (N = 1989)
| Characteristics | Full sample | Normoglycemia (N = 1380, 69.4%) | Prediabetes (N = 471, 23.7%) | Diabetes (N = 138, 6.9%) |
|---|---|---|---|---|
| Demographic | ||||
| Maternal age, years | 28.5 ± 1.7 | 28.5 ± 1.7 | 28.6 ± 1.6 | 28.8 ± 1.7 |
| US region, % | ||||
| Northeast | 303 (15.2) | 233 (16.9) | 51 (10.8) | 19 (12.8) |
| Midwest | 475 (23.9) | 342 (24.8) | 104 (22.1) | 29 (21.0) |
| South | 723 (36.4) | 476 (34.5) | 190 (40.3) | 57 (41.3) |
| West | 488 (24.5) | 329 (23.8) | 126 (26.8) | 33 (23.9) |
| Race, % | ||||
| White | 1311 (66.0) | 992 (71.9) | 245 (52.0) | 74 (53.6) |
| Black or African American | 365 (18.4) | 174 (12.6) | 141 (29.9) | 50 (36.2) |
| American Indian or Alaska Native | 43 (2.2) | 32 (2.3) | 8 (1.7) | 3 (2.2) |
| Asian or Pacific Islander | 127 (6.4) | 85 (6.2) | 36 (7.6) | 6 (4.3) |
| Other | 140 (7.1) | 96 (7.0) | 39 (8.3) | 5 (3.6) |
| Hispanic or Latina, % | 278 (14.0) | 180 (13.0) | 82 (17.4) | 16 (11.6) |
| Socioeconomic | ||||
| Household annual income, $ | ||||
| <25,000 | 261 (13.8) | 163 (11.8) | 67 (14.2) | 31 (22.5) |
| 25,000–50,000 | 459 (24.3) | 310 (22.5) | 117 (24.8) | 32 (23.2) |
| 50,000–100,000 | 819 (43.3) | 579 (42.0) | 191 (40.6) | 49 (35.5) |
| 100,000–150,000 | 240 (12.7) | 184 (13.3) | 43 (9.1) | 13 (9.4) |
| >150,000 | 114 (6.0) | 82 (5.9) | 27 (5.7) | 5 (3.6) |
| Highest education completed, % | ||||
| Less than high school | 66 (3.4) | 40 (2.9) | 20 (4.2) | 6 (4.3) |
| High school to some college | 961 (49.7) | 610 (44.2) | 261 (55.4) | 90 (65.2) |
| College or more | 114 (46.8) | 686 (49.7) | 181 (38.4) | 38 (27.5) |
| Health care access and utilization | ||||
| Insurance status, % | ||||
| Private insurance | 1550 (78.2) | 1105 (80.1) | 349 (74.1) | 96 (69.6) |
| Medicaid | 147 (7.4) | 83 (6.0) | 47 (10.0) | 17 (12.3) |
| Uninsured | 273 (13.8) | 177 (12.8) | 72 (15.3) | 24 (17.4) |
| Other | 12 (0.6) | 9 (0.7) | n/a | n/a |
| Unstable coverage past 12 months | 457 (23.0) | 292 (21.2) | 129 (27.4) | 36 (26.1) |
| Last routine checkup | ||||
| ≤12 months ago | 1464 (73.6) | 1003 (68.5) | 352 (74.7) | 109 (79.0) |
| >12 months ago | 524 (26.4) | 376 (27.2) | 119 (25.3) | 29 (21.0) |
| Emergency room as usual source of care, % | 168 (8.5) | 96 (7.0) | 54 (11.5) | 18 (13.0) |
| Unmet need for medical care, % | 429 (21.6) | 272 (19.7) | 108 (22.9) | 49 (35.5) |
Mean ± SD reported for continuous variables, and Column N (%) reported for categorical. Missing data: insurance status (n = 7), education (n = 57), income (n = 96), Hispanic ethnicity (n = 4), race (n = 3), last routine checkup (n = 1).
n/a, not listed due to cell size <3.
Table 2.
Summary Statistics for Preconception Diabetes and Blood Glucose Measures Collected at Wave IV, Add Health (N = 1989)
| Characteristic | Mean ± SD (range) or N (%) |
|---|---|
| Full sample | |
| Diabetes classificationa | |
| Normoglycemia, % | 1380 (69.4) |
| Prediabetes, % | 471 (23.7) |
| Diabetes, % | 138 (6.9) |
| HbA1c, mean % | 5.5 ± 0.7 (4.5–15.1) |
| HbA1c categoryb | |
| <5.7% | 1490 (74.9) |
| 5.7%–6.4% | 436 (21.9) |
| ≥6.5% | 63 (3.2) |
| Participants with diabetes (N = 138) | |
| Self-reported history of diabetes, % | 85 (61.6) |
| Current antidiabetic medication use, % | 38 (27.5) |
| Current insulin use, % | 5 (6.0) |
| Optimal glycemic control (i.e., HbA1c <6.5%), % | 75 (54.4) |
| Fasting participants (≤8 hours) (N = 271) | |
| HbA1c, mean % | 5.7 ± 0.8 (4.7–11.6) |
| Blood glucose, mg/dL | 100.3 ± 22.0 (59.0–287.0) |
| Nonfasting participants (N = 1937) | |
| HbA1c, mean % | 5.5 ± 0.7 (4.5–15.1) |
| Blood glucose, mg/dL | 104.4 ± 28.0 (40.0–597.0) |
Criteria for diabetes included self-reported history of diabetes, antidiabetes medication use, HbA1c ≥6.5%, nonfasting glucose ≥200 mg/dL, or fasting glucose ≥126 mg/dL.18 Criteria for prediabetes included HbA1c 5.7%–6.4% or fasting glucose 100–125 mg/day.
Categories determined by HbA1c alone.
HbA1c, hemoglobin A1c.
Among women with preconception diabetes and prediabetes, the prevalence of preterm birth was 25.4% and 15.1%, respectively. Compared to the normoglycemic group (prevalence: 11%), women with diabetes had 2.1 (confidence interval [95% CI]: 1.5–2.9) times the risk of preterm birth, while women with prediabetes had 1.3 (95% CI: 1.0–1.67) times the risk of preterm birth. Furthermore, 37% of women with suboptimal control of diabetes had a preterm birth compared to 16% of those with optimal control (Table 3).
Table 3.
Risk of Preterm Birth Associated with Preconception Diabetes Status (N = 1989)
| Exposure variable | N | % PTB | Preterm birth |
|
|---|---|---|---|---|
| aRR (95%CI) | aRD (95%CI) | |||
| Diabetes classification | ||||
| Normoglycemia | 1380 | 11.0% | Ref. | Ref. |
| Prediabetes | 471 | 15.1% | 1.30 (0.99 to 1.70) | 3.34 (−0.42 to 7.09) |
| Diabetes | 138 | 25.4% | 2.07 (1.46 to 2.94) | 12.89 (5.25 to 20.52) |
| Optimal controla | 75 | 16.0% | 1.54 (0.89 to 2.67) | 5.76 (−3.16 to 14.67) |
| Suboptimal control | 63 | 36.5% | 2.67 (1.73 to 4.13) | 21.87 (9.49 to 34.25) |
Models adjust for age at pregnancy, months between pregnancy and glucose measurement, gravidity, US region, race, maternal education status, Hispanic ethnicity, past year health care coverage, systolic blood pressure, waist circumference, and smoking status.
Optimal glycemic control: HbA1c <6.5%
aRD, adjusted risk difference, interpreted as cases per 100 persons; aRR, adjusted risk ratio; CI, confidence interval.
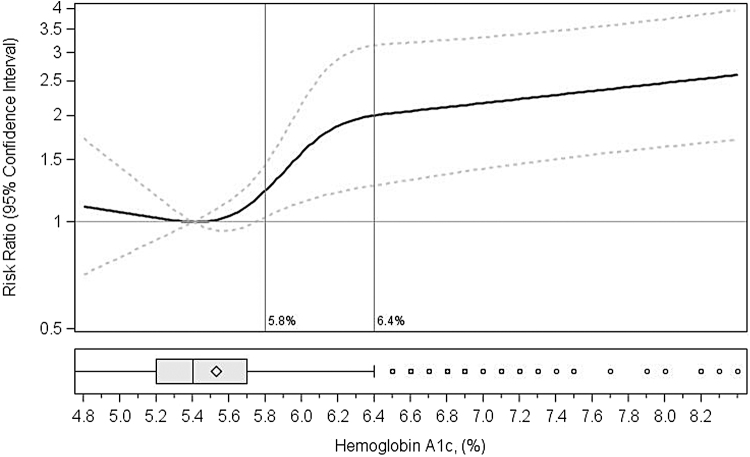
When evaluating HbA1c as a continuous variable, we found no significant increase in risk of preterm birth in the normoglycemic range. The risk of preterm birth began to increase with HbA1c values above 5.7% (Fig. 2). Using 5.4% (sample median) as the reference, we estimated risk ratios of 1.3 (95% CI: 1.0–1.7) for HbA1c of 5.6% and 2.1 (95% CI: 1.3–3.3) for HbA1c of 6.5%. In the full sample, and compared to women with HbA1c <5.7, having an HbA1c level ≥5.7% was associated with 6.2 per 100 (95% CI: 2.5–9.9) additional cases of preterm birth.
FIG. 2.
Nonlinear association between preconception HbA1c (%) and preterm birth (N = 1989). F2 Footnote: results from restricted cubic spline with four knots (5th, 35th, 65th, 75th, and 98th percentiles). Models adjusted for age at pregnancy, US region, gravidity, race, Hispanic ethnicity, education, past year health care coverage, smoking status, systolic blood pressure, and waist circumference. Reference is median HbA1c (5.4%). X-axis trimmed at 1st (HbA1c = 4.8) and 99th (HbA1c = 8.4) percentiles. Vertical reference lines at 5.8% and 6.5% used to indicate standard cutoffs for prediabetes and diabetes, respectively. HbA1c, hemoglobin A1c.
The prevalence of preterm birth was higher among women without versus with stable health care coverage in the last year (14.7% vs. 12.5%), among those with unmet versus met health care needs (18.0% vs. 11.6%), among those indicating the emergency room as a usual source of care versus not (17.3% vs. 12.6%), and among those who were covered by Medicaid (19.7%) versus those who were privately insured (12.3%) or uninsured (13.6%) (Table 4).
Table 4.
Associations Between Preconception Hyperglycemia and Preterm Birth Stratified by Health Care Access and Utilization (N = 1989)
| Characteristic | Sample size | % HbA1c ≥5.7% | % preterm birth | aRD (95% CI) |
|---|---|---|---|---|
| Full sample | 1989 | 25.1% | 13.0% | 6.17 (2.47 to 9.86) |
| Stable health care coverage | ||||
| Yes | 1532 | 23.8% | 12.5% | 3.71 (−0.49 to 7.91) |
| No | 457 | 29.5% | 14.7% | 13.11 (6.23 to 19.99) |
| Unmet health care needs | ||||
| Yes | 429 | 29.4% | 18.0% | 6.97 (0.20 to 14.15) |
| No | 1560 | 23.9% | 11.6% | 5.70 (1.57 to 9.84) |
| Emergency room as usual source | ||||
| Yes | 168 | 37.5% | 17.3% | 17.65 (7.14 to 28.17) |
| No | 1819 | 23.9% | 12.6% | 4.76 (0.87 to 8.66) |
| Insurance provider | ||||
| Uninsured | 273 | 28.9% | 13.6% | 13.6 (4.69 to 22.49) |
| Medicaid | 147 | 36.7% | 19.7% | 1.13 (−10.13 to 12.39) |
| Private | 1550 | 23.4% | 12.3% | 5.16 (0.93 to 9.39) |
| Last routine checkup | ||||
| ≤12 months | 1464 | 26.1% | 13.5% | 5.20 (1.05 to 9.35) |
| >12 months | 524 | 22.3% | 11.5% | 9.04 (1.90 to 16.18) |
Models adjust for age at pregnancy, months between pregnancy and glucose measurement, gravidity, US region, race, maternal education status, Hispanic ethnicity, systolic blood pressure, waist circumference, and smoking status.
RD, risk difference (interpreted as cases per 100 persons).
The RD for preterm birth comparing women with HbA1c level ≥5.7% to women with HbA1c <5.7 was larger among those with unstable health care coverage (RD = 13.1, 95% CI: 6.2–20.0), among those who used the emergency room as usual source of care (RD = 17.7, 95% CI: 7.1–28.2), among those who were uninsured (RD = 13.6, 95% CI: 4.7–22.5), and among those who had not had a routine checkup in the year prior (RD = 9.0, 95% CI: 1.9–16.2) (Table 4).
Discussion
In this cohort of US women who gave birth between 2008 and 2018, both preconception diabetes and prediabetes were associated with increased risk of preterm birth. Notably, the association between higher HbA1c and risk of preterm birth emerged at HbA1c ≥5.7%, a clinical cutoff for prediabetes. The associations between preconception elevated HbA1c and preterm birth were larger among women without stable health care coverage.
Our findings relating preconception diabetes and risk of preterm birth are largely consistent with a previously published meta-analysis reporting that pre-existing diabetes was associated with 3.5 times the odds of preterm birth.6 Similarly, our findings provide support for the ADA guidelines recommending optimal glycemic control before conception.10 In this sample, women with preconception diabetes, who had HbA1c <6.5%, the ADA recommended target, had substantially lower risk of preterm birth compared to those with HbA1c ≥6.5%. Unfortunately, only 54% of women had optimal glycemic control, a percentage consistent with the National Health and Nutrition Examination Survey estimate of poor glycemic control (51.5%) among women ages 20–44.1 Furthermore, nearly 40% of women with diabetes were unaware of their diagnosis.
Our study extends existing literature by evaluating effects of glucose impairment in the prediabetic range, as well as continuous measures of preconception HbA1c. We estimated an ∼30% increased risk in preterm birth among women with prediabetes. A study conducted in Norway found similarly increased odds of preterm birth (OR 1.4, 95% CI: 1.4–2.1) among participants with preconception glucose levels between 5.4 and 11.5 mmol/L (values in prediabetes and diabetes range) compared to those with glucose levels between 1.0 and 4.4 mmol/L.8 Another study from Guangdong Province, China, found that preconception prediabetes was associated with ∼10% increased risk of preterm birth.9
Of note, when evaluating preconception glucose as a continuous variable, the Guangdong Province study reported null associations with preterm birth.9 Two other studies that analyzed preconception glucose levels as a continuous variable (one conducted in the Cardiovascular Risk in Young Finns Study25 and one in the Bogalusa Heart Study26) also reported null associations of higher glucose levels with preterm birth. When we examined the effects of HbA1c (a proxy for average plasma glucose concentration in the prior 2–3 months) continuously, we similarly found no effect of increasing HbA1c within the normoglycemic range. Instead, we identified HbA1c = 5.7% as a potential threshold at which an increased risk of preterm birth emerges.
The mechanisms underlying associations between preconception diabetes, prediabetes, and preterm birth are unclear, although hyperglycemia at conception and in early pregnancy is associated with increased chronic inflammation and oxidative stress. These stressors may adversely affect placental growth during the first trimester and placental function throughout pregnancy, as evidenced by the higher rate of preeclampsia noted in pregnancies complicated by diabetes.4,27–29 The nonlinear effect between HbA1c and preterm birth in our study suggests that there is a specific point at which glucose dysregulation becomes harmful to developmental processes. These findings must be replicated by future research to inform understanding of the specific physiologic mechanism. In addition, studies with glucose measured both preconception and during pregnancy will be critical to disentangling pathways and identifying periods of risk because the association between preconception diabetes and preterm birth may be explained by higher incidence of gestational diabetes in this group.
Our findings, in aggregate with the existing literature, suggest that screening for hyperglycemia before pregnancy is important to identifying women who may experience greater risks of adverse birth outcomes. HbA1c is simple to collect in clinical settings, is valid irrespective of fasting status, and may be a useful tool for screening. Our data show that preconception HbA1c is associated with preterm birth with a similar magnitude as diabetes status (which requires fasting glucose measurements). Future studies may consider the benefits, harms, and added health care costs of universal HbA1c screening among women of reproductive age. We add that the potential benefits of universal HbA1c screening are highlighted by the statistic that almost half of US pregnancies are unintended.30 If a provider waits until an individual is pregnant or is seeking preconception care to screen for hyperglycemia, they will miss a large portion of the population that may have benefited from earlier screening.
Moreover, our finding that women with diabetes meeting optimal glycemic control before pregnancy have considerably better outcomes emphasizes the importance of preconception care.31,32 A simulation model based on US estimates found that universal preconception glycemic care could prevent 8397 annual preterm deliveries among women with diagnosed diabetes and 2267 preterm deliveries among women with undiagnosed diabetes, and reduce costs attributed to preterm birth by >500 million dollars.31
In discussing strategies to improve screening and preconception care, we acknowledge that engagement in health care among women ages 18–44 is low. Before the implementation of the Affordable Care Act (ACA) (corresponding to the period under study), 25% of women reported being uninsured at some point in the prior year.12 After ACA implementation, this number decreased to 12%.14 In our study, we reported substantially greater burden of preconception hyperglycemia among women with limited health care access and utilization. This subgroup of women also experienced excess relative burden of preterm birth associated with preconception HbA1c levels. This excess relative burden may be due to delayed detection of diabetes, longer duration of uncontrolled diabetes, and fewer resources to control glycemia.
This study has several limitations. Preterm birth was self-reported and did not distinguish spontaneous versus indicated preterm births. The prevalence of preterm birth in the full sample (13%) was higher than anticipated, indicating some women may have misreported the outcome. However, we would not expect this misclassification to be differential by diabetes status. For the exposure, we classified women who reported being told by a doctor that they had “high blood sugar or diabetes” as having diabetes. This may have resulted in misclassification if women with prediabetes also responded positively to this question. Furthermore, we were not able to distinguish between Type I and Type II diabetes, measured glucose and HbA1c only once, and did not consider other complications of pregnancy such as preeclampsia and gestational hypertension, which could explain some of the increased preterm birth risk associated with hyperglycemia.
Finally, we included women who had a pregnancy between Waves IV and Wave V. These women were more educated and older during pregnancy, and a greater proportion were White than in the baseline cohort. Due to small sample sizes and resultant unstable estimates, we did not use Add Health sampling weights. However, a previous study conducted within Add Health reported the weighted prevalence of diabetes, prediabetes, and glycemic control among all women participants at Wave IV.11 The unweighted estimates presented in our analytic sample are similar to those previously published.11
Despite these limitations, this research is strengthened by the measurement of glycemia before pregnancy. We were not reliant on retrospectively reported exposures, and we were able to examine risk of adverse birth outcomes across the full range of HbA1c.
Conclusions
We reported increased risk of preterm birth associated with blood glucose levels below clinical cutoffs for diabetes and showed the disproportionate burden of elevated preconception glucose levels among women with limited access to and utilization of health care. Additional monitoring could help identify at-risk women earlier, providing increased opportunities for preconception intervention and substantial benefit to population health.
Supplementary Material
Acknowledgments
This work was completed as part of Dr. Delker's PhD thesis, which is available at ProQuest Dissertations & Theses Global. The citation is Delker, E.M. (2020). Preconception cardiometabolic health and risk of adverse birth outcomes among US women (Order No. 28154976). Available from ProQuest Dissertations & Theses Global: The Sciences and Engineering Collection. (2477874497). Retrieved from http://libproxy.sdsu.edu/login?url=https://www-proquest-com.libproxy.sdsu.edu/dissertations-theses/preconception-cardiometabolic-health-risk-adverse/docview/2477874497/se-2.
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining Data Files from Add Health should contact Add Health, The University of North Carolina at Chapel Hill, Carolina Population Center, Carolina Square, Suite 210, 123 W. Franklin Street, Chapel Hill, NC 27516 (addhealth_contracts@unc.edu). No direct support was received from grant P01 HD31921 for this analysis.
Authors' Contributions
E.D.: conceptualization, methodology, formal analysis, and writing—original draft. G.A.R.: conceptualization, supervision, and writing—review and editing. G.B.: conceptualization, methodology, formal analysis, and writing—review and editing. D.Y.L.: conceptualization and writing—review and editing. K.F.: methodology and writing—review and editing. L.C.G.: writing—review and editing. E.O.: methodology and writing—review and editing. S.G.: writing—review and editing. M.A.: resources, supervision, writing—review and editing, and funding acquisition.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
E.D. was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Grant number: T32HL079891, the UCSD Integrated Cardiovascular Epidemiology Fellowship. This is a training grant; the funder was not involved in the study design, data collection, analysis and interpretation of data, writing the report, and decision to submit the article for publication.
Supplementary Material
References
- 1. Azeez O, Kulkarni A, Kuklina EV, et al. Hypertension and diabetes in non-pregnant women of reproductive age in the United States. Prev Chronic Dis 2019;16; doi: 10.5888/pcd16.190105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services: Atlanta, GA; 2020. [Google Scholar]
- 3. Alexopoulos AS, Blair R, Peters AL. Management of preexisting diabetes in pregnancy: A Review. JAMA 2019;321(18):1811–1819; doi: 10.1001/jama.2019.4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 201: Pregestational Diabetes Mellitus. Obstet Gynecol 2018;132(6):e228–e248; doi: 10.1097/AOG.0000000000002960 [DOI] [PubMed] [Google Scholar]
- 5. Berry DC, Boggess K, Johnson QB. Management of pregnant women with type 2 diabetes mellitus and the consequences of fetal programming in their offspring. Curr Diab Rep 2016;16(5):36; doi: 10.1007/s11892-016-0733-7 [DOI] [PubMed] [Google Scholar]
- 6. Yu L, Zeng XL, Cheng ML, et al. Quantitative assessment of the effect of pre-gestational diabetes and risk of adverse maternal, perinatal and neonatal outcomes. Oncotarget 2017;8(37):61048–61056; doi: 10.18632/oncotarget.17824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romundstad PR, Davey Smith G, Nilsen TIL, et al. Associations of prepregnancy cardiovascular risk factors with the offspring's birth weight. Am J Epidemiol 2007;166(12):1359–1364; doi: 10.1093/aje/kwm272 [DOI] [PubMed] [Google Scholar]
- 8. Magnussen EB, Vatten LJ, Myklestad K, et al. Cardiovascular risk factors prior to conception and the length of pregnancy: Population-based cohort study. Am J Obstet Gynecol 2011;204(6):526.e1–8; doi: 10.1016/j.ajog.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 9. Tang J, Zhu X, Li M, et al. The impact of maternal prepregnancy impaired fasting glucose on preterm birth and large for gestational age: A large population-based cohort study. Am J Obstet Gynecol 2020;222(3):265.e1–265.e19; doi: 10.1016/j.ajog.2019.09.037 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of medical care in diabetes-2019. Diabetes Care 2019;42(Suppl 1):S165–S172; doi: 10.2337/dc19-S014 [DOI] [PubMed] [Google Scholar]
- 11. Britton LE, Hussey JM, Crandell JL, et al. Racial/ethnic disparities in diabetes diagnosis and glycemic control among women of reproductive age. J Womens Health (Larchmt) 2002 2018;27(10):1271–1277; doi: 10.1089/jwh.2017.6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kozhimannil KB, Abraham JM, Virnig BA. National trends in health insurance coverage of pregnant and reproductive-age women, 2000 to 2009. Womens Health Issues 2012;22(2):e135–e141; doi: 10.1016/j.whi.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 13. Easter SR, Rosenthal EW, Morton-Eggleston E, et al. Disparities in care for publicly insured women with pregestational diabetes. Obstet Gynecol 2017;130(5):946–952; doi: 10.1097/AOG.0000000000002252 [DOI] [PubMed] [Google Scholar]
- 14. Gains in Insurance Coverage for Reproductive-Age Women at a Crossroads. Guttmacher Institute; 2018. Available from: https://www.guttmacher.org/article/2018/12/gains-insurance-coverage-reproductive-age-women-crossroads [Last accessed: August 6, 2020].
- 15. Stormo AR, Saraiya M, Hing E, et al. Women's clinical preventive services in the United States: Who is doing what? JAMA Intern Med 2014;174(9):1512–1514; doi: 10.1001/jamainternmed.2014.3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris KM, Halpern CT, Whitsel EA, et al. Cohort Profile: The National Longitudinal Study of Adolescent to Adult Health (Add Health). Int J Epidemiol 2019;48(5):1415–1415k; doi: 10.1093/ije/dyz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitsel EA, Tabor JW, Nguyen QC, et al. Add Health Wave IV Documentation: Measures of glucose homeostasis. UNC Chapel Hill: Carolina Population Center; 2021b. Available from: https://addhealth.cpc.unc.edu/wp-content/uploads/docs/user_guides/Glucose_HbA1c.pdf [Last accessed: January 25, 2023].
- 18. Standards of Medical Care in Diabetes—2017 abridged for primary care providers. Clin Diabetes Publ Am Diabetes Assoc 2017;35(1):5–26; doi: 10.2337/cd16-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Entzel P, Whitsel E, Richardson A, et al. Add Health Wave IV Documentation: Cardiovascular and anthropometric measures; 2009. Available from: https://addhealth.cpc.unc.edu/wp-content/uploads/docs/user_guides/Wave_IV_Cardiovascular_and_anthropometric_documentation.pdf [Last accessed: January 25, 2023].
- 20. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159(7):702–206; doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 21. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162(3):199–200; doi: 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 22. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29(9):1037–1057; doi: 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 23. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer-Verlag; 2001; doi: 10.1007/978-1-4757-3462-1 [DOI] [Google Scholar]
- 24. Marshall C, Adams S, Dyer W, et al. Opportunities to reduce diabetes risk in women of reproductive age: Assessment and treatment of prediabetes within a large integrated delivery system. Womens Health Issues 2017;27(6):666–672; doi: 10.1016/j.whi.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 25. Harville EW, Viikari JSA, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiol Camb Mass 2011;22(5):724–730; doi: 10.1097/EDE.0b013e318225c960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harville EW, Myers L, Shu T, et al. Pre-pregnancy cardiovascular risk factors and racial disparities in birth outcomes: The Bogalusa Heart Study. BMC Pregnancy Childbirth 2018;18:339; doi: 10.1186/s12884-018-1959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han CS, Herrin MA, Pitruzzello MC, et al. Glucose and metformin modulate human first trimester trophoblast function:A model and potential therapy for diabetes-associated uteroplacental insufficiency. Am J Reprod Immunol N Y N 1989 2015;73(4):362–371; doi: 10.1111/aji.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desoye G. The human placenta in diabetes and obesity: Friend or foe? The 2017 Norbert Freinkel Award Lecture. Diabetes Care 2018;41(7):1362–1369; doi: 10.2337/dci17-0045 [DOI] [PubMed] [Google Scholar]
- 29. Shub A, Lappas M. Pregestational diabetes in pregnancy: Complications, management, surveillance, and mechanisms of disease—A review. Prenat Diagn 2020;40(9):1092–1098; doi: 10.1002/pd.5718 [DOI] [PubMed] [Google Scholar]
- 30. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016;374(9):843–852; doi: 10.1056/NEJMsa1506575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson C, Grosse SD, Li R, et al. Preventable health and cost burden of adverse birth outcomes associated with pregestational diabetes in the United States. Am J Obstet Gynecol 2015;212(1):74.e1–9; doi: 10.1016/j.ajog.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott KA, Britton L, McLemore MR. The ethics of perinatal care for Black women: Dismantling the structural racism in “Mother Blame” narratives. J Perinat Neonatal Nurs 2019;33(2):108–115; doi: 10.1097/JPN.0000000000000394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.