Key Points
Question
Does azithromycin administered orally during labor reduce neonatal sepsis and mortality?
Findings
This randomized trial in West Africa included 11 983 birthing parents and their infants. The incidence of the composite end point of neonatal sepsis or neonatal mortality was similar in the azithromycin (2.0%) and placebo (1.9%) groups. However, the incidence of noninvasive infections during the subsequent 4 weeks was reduced for newborns (skin infections) and parents (puerperal fever and mastitis) in the azithromycin group.
Meaning
These results do not support routine introduction of oral intrapartum azithromycin to reduce neonatal sepsis or mortality.
Abstract
Importance
Neonatal sepsis is a leading cause of neonatal mortality. New interventions are needed to decrease neonatal sepsis and mortality in regions with highest burden.
Objective
To evaluate the efficacy of intrapartum azithromycin to reduce neonatal sepsis or mortality, as well as neonatal and maternal infections.
Design, Setting, and Participants
This double-blind, placebo-controlled, randomized clinical trial enrolled and followed up birthing parents and their infants at 10 health facilities in The Gambia and Burkina Faso, West Africa, between October 2017 and May 2021.
Interventions
Participants were assigned at random to receive oral azithromycin (2 g) or placebo (ratio 1:1) during labor.
Main Outcomes and Measures
The primary outcome was a composite of neonatal sepsis or mortality, with the former defined based on microbiologic or clinical criteria. Secondary outcomes were neonatal infections (skin, umbilical, eye and ear infections), malaria, and fever; postpartum infections (puerperal sepsis, mastitis), fever, and malaria; and use of antibiotics during 4-week follow-up.
Results
The trial randomized 11 983 persons in labor (median age, 29.9 years). Overall, 225 newborns (1.9% of 11 783 live births) met the primary end point. The incidence of neonatal mortality or sepsis was similar in the azithromycin and placebo groups (2.0% [115/5889] vs 1.9% [110/5894]; risk difference [RD], 0.09 [95% CI, −0.39 to 0.57]), as was the incidence of neonatal mortality (0.8% vs 0.8%; RD, 0.04 [95% CI, −0.27 to 0.35]) and neonatal sepsis (1.3% vs 1.3%; RD, 0.02 [95% CI, −0.38 to 0.43]). Newborns in the azithromycin group compared with the placebo group had lower incidence of skin infections (0.8% vs 1.7%; RD, −0.90 [95% CI, −1.30 to −0.49]) and need for antibiotics (6.2% vs 7.8%; RD, −1.58 [95% CI, −2.49 to −0.67]). Postpartum parents in the azithromycin group had lower incidence of mastitis (0.3% vs 0.5%; RD, −0.24 [95% CI, −0.47 to −0.01]) and puerperal fever (0.1% vs 0.3%; RD, −0.19 [95% CI, −0.36 to −0.01]).
Conclusions and Relevance
Azithromycin administered orally during labor did not reduce neonatal sepsis or mortality. These results do not support routine introduction of oral intrapartum azithromycin for this purpose.
Trial Registration
ClinicalTrials.gov Identifier: NCT03199547
This randomized trial compares the efficacy of intrapartum azithromycin vs placebo to reduce neonatal sepsis or mortality, as well as neonatal and maternal infections, among birthing parents and their infants in West Africa.
Introduction
Each year, 4 million infants die during the first 28 days of life.1 Neonatal mortality accounts for 40% of deaths among children younger than 5 years worldwide. Neonatal mortality rates have not declined substantially during the past decade, in contrast to significant decreases in mortality among older children.1 Approximately one-third of neonatal deaths are attributable to sepsis.2 Sub-Saharan Africa has among the highest rates of neonatal mortality worldwide, and neonatal sepsis in that region is due to both gram-positive and gram-negative bacteria.3 New interventions are urgently needed.4
Azithromycin, a broad-spectrum macrolide,5 may have potential to reduce neonatal mortality. Early evaluations of mass drug administration campaigns to control trachoma in sub-Saharan Africa suggested that azithromycin reduced carriage of bacteria other than Chlamydia trachomatis and significantly reduced childhood mortality.6,7,8,9 A subsequent large-scale cluster-randomized trial in sub-Saharan Africa found that mass azithromycin administration reduced mortality in children younger than 5 years, with an stronger effect in infants younger than 6 months.10 A recent proof-of-concept trial found that oral azithromycin administered during labor reduced carriage of gram-positive bacteria during the subsequent 4 weeks,11 and a post hoc analysis found reduced mild to moderate disease in both birthing parents and newborns.12
This randomized clinical trial was designed to test the effectiveness of oral azithromycin administered during labor to reduce a composite end point of neonatal sepsis or neonatal mortality. Secondary objectives included evaluating the effect of the intervention on other infections in both newborns and birthing parents.
Methods
Overview
PregnAnZI-2 (Pre-delivery Administration of Azithromycin to Prevent Neonatal Sepsis & Death) was a double-blind, placebo-controlled, randomized clinical trial in which participants were assigned in a 1:1 ratio to receive either 2 g of oral azithromycin or placebo during labor.13 Pregnant persons were invited and provided written informed consent during antenatal care visits and were enrolled in the trial when they attended study health facilities during labor. The trial protocol is available in Supplement 1 and the full statistical analysis plan in Supplement 2. The trial was approved by The Gambia Government/MRCG (Medical Research Council Unit The Gambia) Joint Ethics Committee, the Comité d’Ethique pour la Recherche en Santé, the Ministry of Health of Burkina Faso, and the London School of Hygiene & Tropical Medicine Ethics Committee.
Study Settings
Participants were recruited in The Gambia and Burkina Faso between October 2017 and May 2021. Both countries have similar rates of neonatal mortality (26 and 30 deaths per 1000 live births in The Gambia and Burkina Faso, respectively). Approximately 15% of maternal deaths in these 2 countries are associated with puerperal infections.14,15
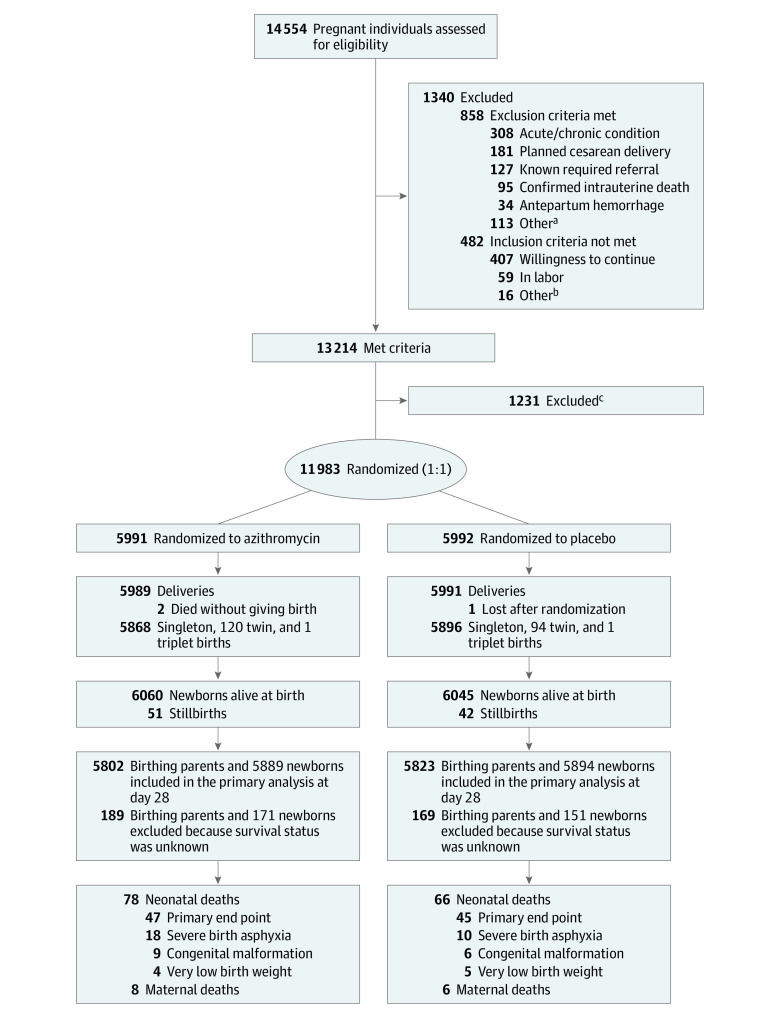
In The Gambia, participants were recruited from 2 government health facilities located in periurban areas close to the capital, Banjul. In Burkina Faso, participants were recruited in 8 peripheral health facilities in the rural districts of Nanoro and Yako (eFigure 1 in Supplement 3). In The Gambia, the first participant was recruited on October 22, 2017, and follow-up ended on May 31, 2021, while in Burkina Faso, recruitment began on January 8, 2018, and follow-up ended on April 10, 2021 (Figure 1).
Figure 1. Recruitment, Randomization, and Patient Flow in the Trial of Azithromycin to Prevent Neonatal Sepsis and Mortality.
aOther reasons included being HIV positive (n = 43), taking drugs that increased QT/QTc during the previous 2 weeks (n = 40), planning travel outside of the study area (n = 25), having severe congenital malformation (n = 4), and having a known allergy to macrolides (n = 1).
bOther reasons included age younger than 16 years (n = 10) and consent not found (n = 6).
cIndividuals excluded for the following reasons: late arrival to health facility, not enough time to screen/randomize and treat (n = 1149), study staff not available (n = 36), very early preterm or abortion (n = 22), unsure of fetal heart (n = 15), and partner declined participation (n = 9).
Study Procedures
A study nurse or a research clinician checked the eligibility of participants in labor who had previously signed consent during antenatal care visits. Inclusion criteria were active labor among individuals aged at least 16 years and confirmed willingness to be included in the trial. Exclusion criteria were (1) known HIV infection; (2) any chronic or acute condition; (3) planned cesarean delivery or known required referral; (4) known severe congenital malformation; (5) allergy to macrolides; and (6) use in the last 2 weeks of drugs known to prolong the QT interval, eg, chloroquine, quinine, piperaquine, or erythromycin.
Participants were assigned to azithromycin or placebo based on a list of 5-digit randomization numbers prepared by an independent statistician using block randomization (block size = 4). Packaging and labeling of the interventional medicinal products was done by an independent pharmaceutical company (Idifarma [now Ardena Pamplona]). Participants took oral azithromycin (2 g) or placebo under the supervision of a study nurse while in active labor.
Data Collection
Study data were collected at enrollment and during the birth hospitalization by research staff using an electronic case report form. Clinical data were subsequently collected before postdelivery discharge (generally between 6 and 24 hours after delivery). In The Gambia, participants were home-visited at day 28 until March 2020, after which (approximately 15% of the participants) visits were done via telephone to minimize risk of SARS-CoV-2 transmission. In Burkina Faso, participants attended study health facilities at day 28 post delivery. If participants did not attend, a home visit was done by a field worker within the following 4 days.
Hospitalizations and outpatient visits were recorded throughout the study period (details were reported elsewhere13).
Outcomes
The primary end point was a composite of sepsis or death during the first 28 days of life. As defined a priori, deaths due to severe birth asphyxia (1-minute Apgar score <4, including stillbirths), very low birth weight (<1.5 kg), and severe congenital malformations were excluded from the primary end point because we expected the azithromycin intervention not to have an effect on them.13
Neonatal sepsis was classified as early onset if it occurred within 3 days of birth and late onset if it occurred between 3 and 28 days.16 Early-onset sepsis was defined as hospitalization with at least 1 laboratory criterion and either respiratory distress or at least 2 other clinical criteria (eTable 1 in Supplement 3). Late-onset sepsis was defined as hospitalization with at least 1 laboratory criterion and respiratory distress (2 criteria required), or 1 feature of respiratory distress and another clinical criterion or at least 2 other clinical criteria (eTable 1 in Supplement 3). The study team was trained in identifying these clinical criteria.
Sepsis was culture confirmed if a microorganism was isolated from a normally sterile body site (or seen in the gram stain for the cerebrospinal fluid); contaminants were excluded.
Secondary End Points
For newborns, prespecified secondary end points included neonatal sepsis, neonatal mortality, culture-confirmed sepsis, fever (axillary temperature >38 oC), skin infections (details of definitions were reported elsewhere13), bacterially confirmed skin infections, conjunctivitis (details of definition elsewhere13) and bacterially confirmed if bacteria were isolated from an eye swab, umbilical infection/omphalitis, malaria (confirmed by either microscopy or rapid diagnostic test), prescribed antibiotics, and all-cause neonatal hospitalization (excluding injuries).
The cause of death was determined from the final serious adverse event diagnosis for deaths that occurred in a health facility and from the results of verbal autopsies (systematic oral interviews of family members to determine cause of death) for deaths that occurred at home.
For birthing parents, secondary outcomes included postpartum sepsis (maternal hospitalization with either sepsis diagnosis and 1 of the following criteria: uterine tenderness, foul-smelling lochia, abnormal vaginal discharge, or fever; at least 2 criteria in the absence of a sepsis diagnosis on admission); bacterially confirmed postpartum sepsis if bacteria were isolated from blood, cerebrospinal fluid, or a wound; mastitis (see definition reported elsewhere13) and bacterially confirmed if bacteria were isolated from a breast swab; malaria (see definition above) or puerperal fever (axillary temperature ≥38.0 °C); and prescribed antibiotics, any hospitalization, and mortality.
Adverse Events
Screening for adverse events was implemented with a combination of active (scheduled visits) and passive case detection (unscheduled visits or admissions to the study health facilities). Scheduled visits were done at hospital discharge after delivery, mostly by a research clinician, and at day 28, mostly by a research nurse. Unscheduled visits could occur any time during the follow-up period.13 Hypertrophic pyloric stenosis—the main safety consideration with the administration of azithromycin—was actively evaluated at each scheduled and unscheduled visit.
Sample Size
The study was initially designed to provide 80% power to detect a 40% reduction in neonatal mortality (as per the primary end point defined above).13 However, after an earlier cluster randomized trial10 of azithromycin in children aged 1 to 60 months reported lower-than-expected mortality, we decided to change the primary end point of the current study to a composite of either neonatal mortality or neonatal sepsis. The revised sample size calculation assumed the incidence of the composite end point would be 2.8% in the placebo group and used P = .05 for a 2-tailed test. The sample size of 12 000 participants was expected to provide 80% power to detect a 29% difference between groups in the composite outcome.13
Statistical Analysis
The analysis included birthing parents and live-born infants with known survival status at day 28 post delivery. Participants (parents and newborns) whose survival status could not be determined, or who had withdrawn from the trial, were excluded. No interim analysis was planned or conducted.
For the primary analysis, all randomized participants were assigned to their randomization group. Logistic regression was used to compare the primary composite outcome between trial groups. To improve statistical power, country was included in the model as a binary covariate, and birth weight and 1-minute Apgar score were included as continuous, linear covariates. Missing values for birth weight and 1-minute Apgar score were imputed using the mean value. Secondary outcomes were analyzed as binary outcomes at day 28, either with logistic regression (>40 events) or Fisher exact test (≤40 events), and as time-to-event outcomes using the Kaplan-Meier method. Risk differences (RDs) were computed from the logistic regression model by the g-computation approach that predicts the average risk under the counterfactual scenario where all participants receive azithromycin and compares this with the average risk under the scenario where all participants receive placebo.
All analyses were conducted with Stata version 17.0 (StataCorp) and R version 4.0.4 (The R Project). A P value threshold of .05 was used as the criterion for statistical significance.
Results
Baseline Characteristics
Between October 2017 and May 2021, 14 554 participants in labor were assessed for eligibility and 1340 (9.2%) were ineligible. Of the remaining 13 214 participants, 11 983 birthing parents (90.7%) and their newborns (11 764 singletons, 214 twin pairs, and 2 triplet sets) were recruited into the trial (Figure 1). Slightly more than half of participants were recruited in The Gambia (55.4%). Overall, 11 625 parents and 11 783 newborns were included in the day-28 analysis. A total of 93 stillbirths (0.8%) and 322 newborns (2.7%) who were lost to follow-up at day 28 were excluded from the neonatal analysis cohort; 358 birthing parents (3.0%) lost to follow-up were excluded from that analysis cohort. Fourteen birthing parents and 144 newborns died during the follow-up period (Figure 1).
Approximately two-thirds of the deliveries occurred during the dry season (November to May), 2.0% (233/11 621) were via cesarean delivery, and 1.8% (211/11 623) had a multiple pregnancy. The median time from administration of the trial intervention to delivery was 1.6 hours (IQR, 0.47-4.2). Overall, 7.1% (835/11 763) of newborns had a 1-minute Apgar score of 7 or less (mild to moderate or severe birth asphyxia) and 9.4% (1107/11 778) had low birth weight (<2.5 kg). Baseline characteristics of participants and their newborns by trial group are shown in Table 1. Twins and low-birth-weight deliveries were slightly more frequent in the intervention group.
Table 1. Baseline and Other Characteristics of the Study Participants.
| No. (%) | ||
|---|---|---|
| Azithromycin | Placebo | |
| All birthing parents | 5802 | 5823 |
| Country | ||
| The Gambia | 3210 (55.3) | 3232 (55.5) |
| Burkina Faso | 2592 (44.7) | 2591 (44.5) |
| Age, median (IQR), y | 27.0 (22.0-31.0) | 26.0 (22.0-31.0) |
| Delivery in dry season (Nov-May) | 3776 (65.1) | 3794 (65.2) |
| Cesarean delivery | 118/5800 (2.0) | 115/5821 (2.0) |
| Multiple pregnancy | ||
| Singleton | 5680/5800 (97.9) | 5732/5823 (98.4) |
| Twin | 119/5800 (2.1) | 90/5823 (1.5) |
| Triplet | 1/5800 (0.02) | 1/5823 (0.02) |
| Hours from membrane rupture to delivery, median (IQR) [No.] | 0.3 (0.1-1.5) [3453] | 0.3 (0.1-1.4) [3459] |
| Hours from treatment to delivery, median (IQR) [No.] | 1.6 (0.5-4.0) [5622] | 1.6 (0.5-4.3) [5638] |
| All newborns | 5889 | 5894 |
| Sex | ||
| Female | 2871 (48.8) | 2832 (48.0) |
| Male | 3018 (51.2) | 3062 (52.0) |
| Apgar scorea | ||
| 0-3 | 37/5875 (0.6) | 31/5888 (0.5) |
| 4-7 | 361/5875 (6.1) | 406/5888 (6.9) |
| 8-10 | 5477/5875 (93.2) | 5451/5888 (92.6) |
| Birth weight, kg | ||
| <1.5 | 10/5885 (0.2) | 15/5893 (0.3) |
| 1.5-2.4 | 550/5885 (9.3) | 532/5893 (9.0) |
| 2.5-3.9 | 5198/5885 (88.3) | 5235/5893 (88.8) |
| ≥4.0 | 127/5885 (2.2) | 111/5893 (1.9) |
| Birth weight, median (IQR), kg | 3.0 (2.7-3.3) | 3.0 (2.7-3.3) |
The Apgar score is a measure of intrapartum asphyxia (0-3: severe asphyxia, 4-7: mild to moderate asphyxia, and 8-10: no asphyxia).
Neonatal Sepsis and Mortality
In the primary analysis set, 225 newborns met the primary end point (133 with sepsis, 68 deaths, and 24 with both): 168 in The Gambia (114 with sepsis, 37 deaths, and 17 with both) and 57 in Burkina Faso (19 with sepsis, 31 deaths, and 7 with both). The incidence of the primary end point of neonatal sepsis or mortality was similar between trial groups (2.0% vs 1.9% in the azithromycin and placebo groups, respectively; RD, 0.09 [95% CI, −0.39 to 0.57]; odds ratio [OR], 1.06 [95% CI, 0.80 to 1.38]; P = .70). Likewise, the azithromycin and placebo groups had similar incidence of neonatal mortality (0.8% vs 0.8%; RD, 0.04 [95% CI, −0.27 to 0.35]; OR, 1.05 [95% CI, 0.70 to 1.60]; P = .80) and neonatal sepsis (1.3% vs 1.3%; RD, 0.02 [95% CI, −0.38 to 0.43]; OR, 1.02 [95% CI, 0.74 to 1.40]; P = .92) (Table 2). Time-to-event analyses of sepsis and mortality are shown in eFigure 2 in Supplement 3. Causes of death were similar between trial groups in both countries (eFigure 3 in Supplement 3).
Table 2. Composite Primary Outcome of Neonatal Sepsis or Mortality.
| No. (%) | Risk difference (95% CI)a | Odds ratio (95% CI)a | P valueb | ||
|---|---|---|---|---|---|
| Azithromycin | Placebo | ||||
| All newborns | |||||
| No. | 5889 | 5894 | |||
| Sepsis or mortalityc | 115 (2.0) | 110 (1.9) | 0.09 (−0.39 to 0.57) | 1.06 (0.80 to 1.38) | .70 |
| Sepsis | |||||
| Early (0-3 d) | 65 (1.1) | 59 (1.0) | 0.10 (−0.25 to 0.46) | 1.11 (0.77 to 1.61) | .57 |
| Late (4-28 d) | 14 (0.2) | 19 (0.3) | −0.08 (−0.28 to 0.11) | 0.74 (0.34 to 1.55) | .49 |
| Any sepsis | 79 (1.3) | 78 (1.3) | 0.02 (−0.38 to 0.43) | 1.02 (0.74 to 1.40) | .92 |
| Mortalityd | 47 (0.8) | 45 (0.8) | 0.04 (−0.27 to 0.35) | 1.05 (0.70 to 1.60) | .80 |
| The Gambia | |||||
| No. | 3256 | 3260 | |||
| Sepsis or mortalityc | 83 (2.5) | 85 (2.6) | −0.06 (−0.81 to 0.69) | 0.97 (0.71 to 1.34) | .87 |
| Sepsis | |||||
| Early (0-3 d) | 59 (1.8) | 50 (1.5) | 0.28 (−0.33 to 0.88) | 1.20 (0.81 to 1.77) | .37 |
| Late (4-28 d) | 11 (0.3) | 11 (0.3) | 0.00 (−0.29 to 0.29) | 1.00 (0.39 to 2.55) | >.99 |
| Any sepsis | 70 (2.1) | 61 (1.9) | 0.28 (−0.39 to 0.94) | 1.16 (0.81 to 1.66) | .41 |
| Mortalityd | 21 (0.6) | 33 (1.0) | −0.37 (−0.81 to 0.06) | 0.62 (0.36 to 1.09) | .10 |
| Burkina Faso | |||||
| No. | 2633 | 2634 | |||
| Sepsis or mortalityc | 32 (1.2) | 25 (0.9) | 0.29 (−0.26 to 0.84) | 1.33 (0.78 to 2.27) | .30 |
| Sepsis | |||||
| Early (0-3 d) | 6 (0.2) | 9 (0.3) | −0.11 (−0.42 to 0.19) | 0.67 (0.19 to 2.10) | .61 |
| Late (4-28 d) | 3 (0.1) | 8 (0.3) | −0.19 (−0.46 to 0.08) | 0.37 (0.06 to 1.56) | .23 |
| Any sepsis | 9 (0.3) | 17 (0.6) | −0.30 (−0.70 to 0.09) | 0.53 (0.21 to 1.26) | .17 |
| Mortalityd | 26 (1.0) | 12 (0.5) | 0.53 (0.06 to 1.00) | 2.18 (1.06 to 4.75) | .02 |
Risk differences and odds ratios adjusted for country, 1-minute Apgar score, and birth weight for outcomes with more than 40 events in both groups combined. Missing covariate values imputed using the mean value. For outcomes with 40 events or fewer, effects measures are unadjusted.
P value from logistic regression for outcomes with more than 40 events and from Fisher exact test for outcomes with 40 events or fewer.
A total of 24 deaths (11 in the azithromycin group and 13 in the placebo group) met the sepsis criteria, ie, contributed to both outcomes.
Mortality end point excluded deaths associated with very low birth weight (<1.5 kg), congenital malformations, and severe birth asphyxia.
In The Gambia, mortality was slightly lower in the azithromycin group than in the placebo group (0.6% vs 1.0%, P = .10) while in Burkina Faso, mortality was higher in the azithromycin group (1.0% vs 0.5%, P = .02). An opposite trend was observed for sepsis, which was higher in the azithromycin group in The Gambia and lower in the azithromycin group in Burkina Faso.
In other prespecified subgroup analyses, the incidence of the primary composite outcome was similar in both treatment groups (eTable 2 in Supplement 3).
Secondary Outcomes Among Newborns
There were 37 cases of culture-confirmed sepsis (13 in the azithromycin and 24 in the placebo groups; 0.2% vs 0.4%; RD, −0.19 [95% CI, −0.39 to 0.02]) (Table 3). Gram-negative bacteria represented 59.5% (22/37) of the confirmed sepsis cases (69.2% in the azithromycin group and 54.2% in the placebo group). Staphylococcus aureus (gram-positive) was the most commonly isolated bacterium (8/37, 21.6%), with most isolates detected in the placebo group (6 cases in the placebo group vs 2 in the azithromycin group).
Table 3. Etiologies of Blood Culture–Confirmed Sepsis.
| No. (%) | Risk difference (95% CI) | ||
|---|---|---|---|
| Azithromycin (n = 5889) | Placebo (n = 5894) | ||
| No. of children with a culture resulta | 163 (2.77) | 175 (2.97) | −0.20 (−0.80 to 0.40) |
| No growth | 123 (2.09) | 128 (2.17) | −0.08 (−0.60 to 0.44) |
| Contaminatedb | 27 (0.46) | 24 (0.41) | 0.05 (−0.19 to 0.29) |
| Any bacteria | 13 (0.22) | 24 (0.41) | −0.19 (−0.39 to 0.02) |
| Any gram-positive | 4 (0.07) | 11 (0.19) | −0.12 (−0.26 to 0.02) |
| Any gram-negative | 9 (0.15) | 13 (0.22) | −0.07 (−0.23 to 0.10) |
| Staphylococcus aureus | 2 (0.03) | 6 (0.10) | −0.07 (−0.17 to 0.04) |
| Burkholderia cepacia | 3 (0.05) | 4 (0.07) | −0.02 (−0.12 to 0.08) |
| Escherichia coli | 2 (0.03) | 3 (0.05) | −0.02 (−0.10 to 0.07) |
| Klebsiella spp | 0 | 2 (0.03) | −0.03 (−0.10 to 0.03) |
| Other bacteriac | 6 (0.10) | 9 (0.15) | −0.05 (−0.19 to 0.09) |
A total of 342 cultures done (2 children in each group with 2 culture results).
Bacteria considered to be contaminants were coagulase-negative Staphylococcus, Micrococci spp, Bacillus spp, diphtheroids, Viridians streptococci, Anthrobacter spp, and Rhodococcus spp.13
Other bacteria (number of isolates in the azithromycin and placebo groups, respectively) include Corynebacterium propinquum (1 and 1), Enterococcus spp (1 and 1), Pseudomonas (0 and 1), group A Streptococcus (0 and 1), Listeria spp (0 and 1), Acinetobacter baumannii (0 and 1), Citrobacter youngae (1 and 0), Enterobacter cloacae (0 and 1), Enterobacter kobei (1 and 0), Enterococcus faecium (0 and 1), Hafnia alvei (1 and 0), Salmonella spp (0 and 1), and Weeksella virosa (1 and 0).
The incidence of clinical skin infections was reduced by more than half in the azithromycin compared with the placebo group (0.8% vs 1.7%; RD, −0.90 [95% CI, −1.30 to −0.49]; P < .001). The incidence of bacterially confirmed skin infection (0.4% vs 0.8%; RD, −0.42 [95% CI, −0.70 to −0.15]; P = .003) and the need for antibiotic use (6.2% vs 7.8%; RD, −1.58 [95% CI, −2.49 to −0.67]; P < .001) were also reduced (Figure 2; eFigure 4 in Supplement 3). A post hoc analysis found that skin infections requiring hospitalization were also less frequent in the azithromycin group (0% vs 0.1%; RD, −0.12 [95% CI, −0.23 to −0.01]; P = .04). None of the other infections (ear and umbilical infections, conjunctivitis, or malaria) were significantly different between the study groups. Expressing the effects in terms of numbers needed to treat (NNT = 1/RD), 111 individuals would need to be treated with azithromycin to prevent 1 skin infection, 74 to prevent 1 case of any infection, and 63 individuals to prevent 1 case of antibiotic prescribing.
Figure 2. Secondary End Points by Study Group for Newborns and Birthing Parents.
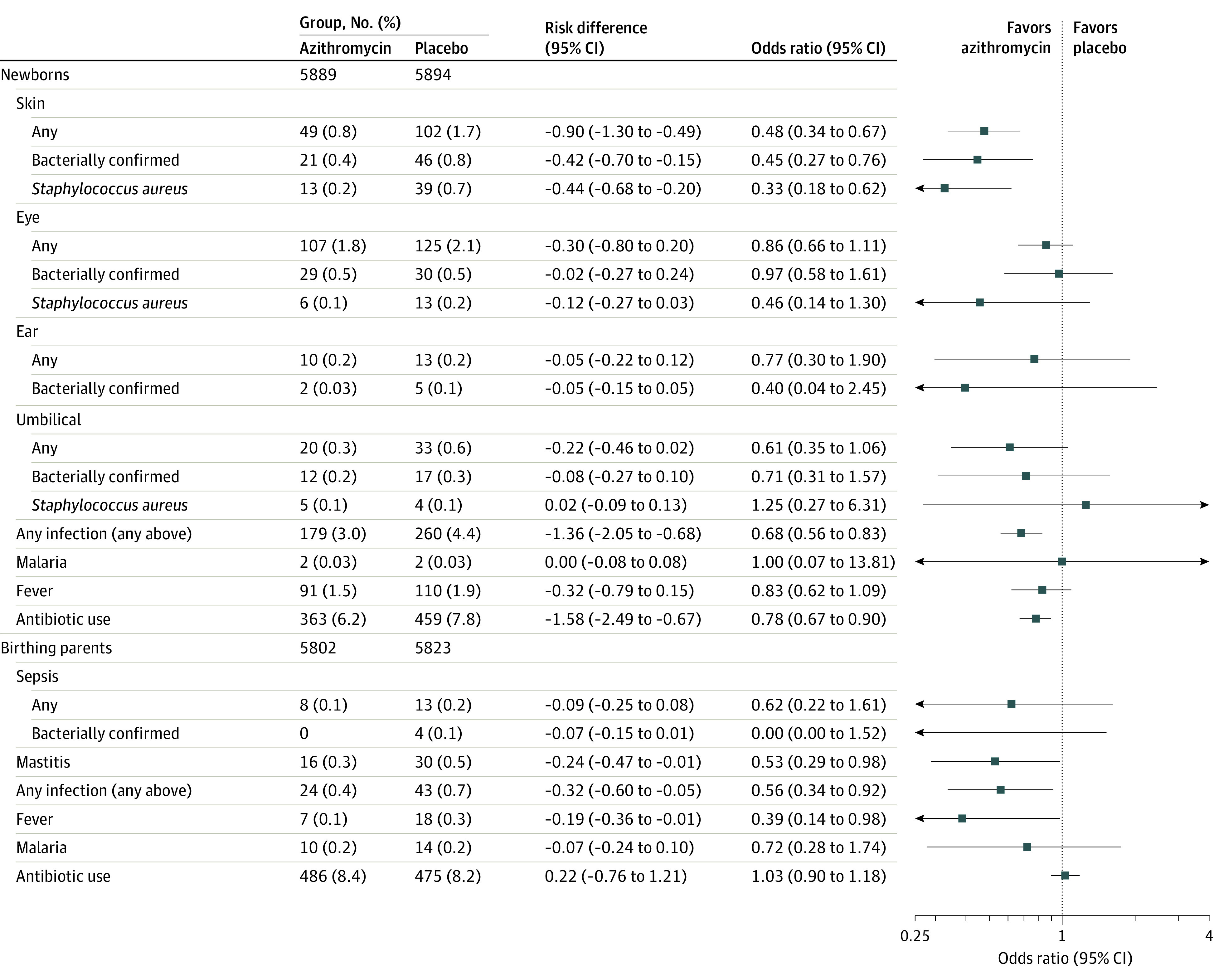
Secondary end points for newborns include skin infections, eye infections, ear infections, umbilical infections, malaria, fever, and antibiotic use, and for birthing parents includes puerperal sepsis, mastitis, puerperal fever, malaria, and antibiotic use; additional details are in the Methods section.
Secondary Outcomes Among Postpartum Parents
Mastitis and puerperal fever were significantly less common in the azithromycin group than in the placebo group (mastitis: 0.3% vs 0.5%, RD, −0.24 [95% CI, −0.47 to −0.01], P = .04; puerperal fever: 0.1% vs 0.3%, RD, −0.19 [95% CI, −0.36 to −0.01], P = .04), but there was no evidence of a difference between the study groups in the incidence of puerperal sepsis, malaria, or need for antibiotics (Figure 2; eFigure 5 in Supplement 3). In terms of number needed to treat, 313 individuals would need to be treated to prevent 1 case of infection (puerperal fever, puerperal sepsis, or mastitis) and 526 to prevent 1 case of fever.
Adverse Events
Four hundred sixty-nine newborns (4.0%) and 142 birthing parents (1.2%) were hospitalized during the follow-up period. The rate of mortality and hospitalization was similar between study groups for both parents and newborns (eTable 3 in Supplement 3). No cases of hypertrophic pyloric stenosis were detected. Nonserious adverse events for both birthing parents and newborns are described in eTable 4 in Supplement 3. In birthing parents, dizziness and reported fever were less common in the azithromycin group (dizziness: 4.4% vs 5.3%, RD, −0.86 [95% CI, −1.62 to −0.09], P = .03; reported fever: 0.8% vs 1.3%; RD, −0.41 [95% CI, −0.78 to −0.04], P = .03), while vomiting and edema were more common in the azithromycin group (vomiting: 10.1% vs 6.1%, RD, 3.99 [95% CI, 3.04 to 4.95], P < .001; edema: 4.2% vs 3.2%, RD, 1.08 [95% CI, 0.41 to 1.76], P = .002).
Discussion
This study found no effect of oral intrapartum azithromycin on neonatal sepsis or mortality. The intervention reduced skin infections in newborns and mastitis and puerperal fever in birthing parents. These results contrast a previous proof-of-concept trial that found intrapartum azithromycin reduced carriage of gram-positive bacteria in birthing parents and their newborns.
The lack of effect of intrapartum azithromycin on neonatal sepsis or mortality is consistent with a recent trial in Mali that found no effect of azithromycin given during the neonatal period on infant mortality at 6 and 12 months.17 It is not consistent with a trial that found oral administration of azithromycin to infants in Tanzania, Malawi, and Niger reduced mortality between 1 and 6 months of age by 25%.10 However, the current trial cannot be directly compared with these studies because the causes of mortality differ between newborns and older infants and children.18
Although azithromycin had no effect on neonatal sepsis or neonatal mortality, it significantly reduced infections in both mothers and newborns. Indeed, neonatal skin infections, including those requiring hospitalization, were reduced by more than half, confirming a previous ad hoc analysis of an earlier trial.12 This is probably due to the significant effect of intrapartum azithromycin reducing S aureus carriage.11 In Burkina Faso, azithromycin administered with seasonal malaria chemoprevention to children aged 3 to 59 months had a similar effect on nonsevere infections including skin infections.19 The observed reduction of the need for antibiotics in the azithromycin group is probably a consequence of the decrease in nonsevere infections in this group.
It is unclear why the effect of intrapartum azithromycin on S aureus carriage11 and noninvasive disease did not translate into a reduction in neonatal sepsis or neonatal mortality. A review of microbiologically confirmed sepsis cases in the West African region3 reported that 33% were attributable to S aureus, while in the current study, 25% of sepsis cases in the placebo group were attributable to S aureus, the most commonly isolated bacterium. However, fewer than 1 in 4 sepsis cases were microbiologically confirmed in the current study. It may be that sepsis and bacteriologically confirmed sepsis are caused by different pathogens, with gram-negative bacteria, viruses, and/or fungus being more prevalent causes of clinical sepsis. An alternative explanation is that a decrease in sepsis caused by some etiologies is balanced by an increase in other etiologies.
Regarding neonatal mortality, intrapartum azithromycin cannot prevent preterm births and intrapartum-related complications. These factors comprised about two-thirds of all neonatal deaths in the current study population.20 Intrapartum azithromycin is also unlikely to be effective against infections in the first few hours of life, and participants in the current trial had a short time interval (median, 1.6 hours) between azithromycin administration and delivery.
One potential advantage of administering antibiotics intrapartum is that this may benefit birthing parents as well as newborns. Both mastitis and puerperal fever were less frequent in the intervention group, confirming the ad hoc analysis of the previous trial.12 These results are consistent with a trial of azithromycin in cesarian deliveries conducted in the US that found that azithromycin reduced severe maternal infections by half.21 The results of the current study differ from the results of a trial of intrapartum azithromycin implemented in Cameroon that did not find a difference in maternal puerperal infections in a high-risk group of participants with prolonged labor or rupture of membranes at term.22 However, the Cameroon trial included only 255 to 257 participants per group and had limited statistical power to identify differences.22 Although the current study found that azithromycin reduced specific noninvasive infections in postpartum parents and newborns, the benefit of preventing these would need to be weighed against the potential harms of widespread antibiotic use.
In the analysis of adverse events, azithromycin was associated with an increase in vomiting and edema and a decrease in reported fever and dizziness. The effects of azithromycin on vomiting and fever were expected; vomiting has been previously described as an adverse effect of azithromycin,19 and the decrease in reported fever is likely a consequence of the decrease in nonsevere infections. The effects on edema and dizziness were unexpected, but the risk differences were small and could have been due to chance.
Limitations
Several limitations should be considered when interpreting this trial’s results. First, the proportion of neonatal sepsis that were microbiology-confirmed was low and there was a high rate of contamination. As a result, easy-to-grow bacteria, such as S aureus, may have been overrepresented among the culture-confirmed sepsis cases. Second, it is likely that cases of neonatal sepsis and other infections were missed, although this would have been likely random, therefore similar in both groups. Overall, it is likely that the incidence of infections was underestimated because active surveillance was limited to 1 scheduled visit at day 28 post delivery. In an earlier trial, where scheduled visits occurred daily during the first week and weekly thereafter, the incidence of neonatal and maternal infections was 4- to 5-fold higher than in the current study.12 The striking differences in the occurrence of early-onset sepsis between The Gambia and Burkina Faso suggests that some cases were missed in Burkina Faso, possibly because many participants did not live close to a study health facility. In The Gambia, late-onset sepsis may have been missed because C-reactive protein was measured qualitatively and could not meet the trial criterion of 40 mg/L. Third, the COVID-19 pandemic caused delays in sample collection, although this should not have had any influence on the trial end points.
Conclusions
Azithromycin administered orally during labor did not reduce neonatal sepsis or mortality. These results do not support the routine introduction of oral intrapartum azithromycin for this purpose.
Trial Protocol
Statistical Analysis Plan
eFigure 1. Map of the Trial Sites in (a) The Gambia and (b) Burkina Faso
eFigure 2. Kaplan-Meier Analysis By Trial Arm: Neonatal Mortality and Neonatal Sepsis
eFigure 3. Causes of Neonatal Death (Meeting the Primary Endpoint) in The Gambia and Burkina Faso
eFigure 4. Kaplan-Meier Analysis by Trial Arm: Neonatal Skin Infection, Umbilical Infection, Eye Infections and Ear Infections
eFigure 5. Kaplan-Meier Analysis by Trial Arm: Maternal Mastitis and Puerperal Sepsis
eTable 1. Neonatal Sepsis Criteria
eTable 2. Pre-specified Subgroup Analysis of the Neonatal Primary Endpoint by Trial Arm: Season, Mode of Delivery and Time From Treatment to Delivery
eTable 3. Safety Endpoints for Birthing Parents and Neonates by Trial Arm
eTable 4. Adverse Events for Birthing Parents and Neonates by Trial Arm
PregnAnZI-2 Working Group Members
Data Sharing Statement
References
- 1.Lawn JE, Blencowe H, Oza S, et al. ; Lancet Every Newborn Study Group . Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384(9938):189-205. doi: 10.1016/S0140-6736(14)60496-7 [DOI] [PubMed] [Google Scholar]
- 2.Seale AC, Mwaniki M, Newton CR, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. 2009;9(7):428-438. doi: 10.1016/S1473-3099(09)70172-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okomo U, Akpalu ENK, Le Doare K, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis. 2019;19(11):1219-1234. doi: 10.1016/S1473-3099(19)30414-1 [DOI] [PubMed] [Google Scholar]
- 4.Collaborators GBDU-M; GBD 2019 Under-5 Mortality Collaborators . Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet. 2021;398(10303):870-905. doi: 10.1016/S0140-6736(21)01207-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew RH, Gallis HA. Azithromycin: spectrum of activity, pharmacokinetics, and clinical applications. Pharmacotherapy. 1992;12(3):161-173. doi: 10.1002/j.1875-9114.1992.tb04504.x [DOI] [PubMed] [Google Scholar]
- 6.Porco TC, Gebre T, Ayele B, et al. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA. 2009;302(9):962-968. doi: 10.1001/jama.2009.1266 [DOI] [PubMed] [Google Scholar]
- 7.Leach AJ, Shelby-James TM, Mayo M, et al. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24(3):356-362. doi: 10.1093/clinids/24.3.356 [DOI] [PubMed] [Google Scholar]
- 8.Harding-Esch EM, Edwards T, Mkocha H, et al. ; PRET Partnership . Trachoma prevalence and associated risk factors in The Gambia and Tanzania: baseline results of a cluster randomised controlled trial. PLoS Negl Trop Dis. 2010;4(11):e861. doi: 10.1371/journal.pntd.0000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry AM, Jha HC, Lietman TM, et al. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis. 2002;35(4):395-402. doi: 10.1086/341414 [DOI] [PubMed] [Google Scholar]
- 10.Keenan JD, Bailey RL, West SK, et al. ; MORDOR Study Group . Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med. 2018;378(17):1583-1592. doi: 10.1056/NEJMoa1715474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roca A, Oluwalana C, Bojang A, et al. Oral azithromycin given during labour decreases bacterial carriage in the mothers and their offspring: a double-blind randomized trial. Clin Microbiol Infect. 2016;22(6):565.e1-565.e9. doi: 10.1016/j.cmi.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139(2):e20162281. doi: 10.1542/peds.2016-2281 [DOI] [PubMed] [Google Scholar]
- 13.Camara B, Bognini JD, Nakakana UN, et al. Pre-delivery administration of azithromycin to prevent neonatal sepsis and death: a phase III double-blind randomized clinical trial (PregnAnZI-2 trial). Int J Clin Trials. 2022;9(1). doi: 10.18203/2349-3259.ijct20220110 [DOI] [Google Scholar]
- 14.Burkina Faso CNdlS . Ministère de la Santé et de l’Hygiène Publique. Accessed September 21, 2022. https://www.sante.gov.bf/
- 15.Streatfield PK, Alam N, Compaoré Y, et al. Pregnancy-related mortality in Africa and Asia: evidence from INDEPTH Health and Demographic Surveillance System sites. Glob Health Action. 2014;7:25368. doi: 10.3402/gha.v7.25368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutland CL, Madhi SA, Zell ER, et al. ; PoPS Trial Team . Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet. 2009;374(9705):1909-1916. doi: 10.1016/S0140-6736(09)61339-8 [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg CE, Sié A, Bountogo M, et al. ; NAITRE Study Team . Neonatal azithromycin administration for prevention of infant mortality. NEJM Evid. 2022;1(4):a2100054. doi: 10.1056/EVIDoa2100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027-3035. doi: 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandramohan D, Dicko A, Zongo I, et al. Effect of adding azithromycin to seasonal malaria chemoprevention. N Engl J Med. 2019;380(23):2197-2206. doi: 10.1056/NEJMoa1811400 [DOI] [PubMed] [Google Scholar]
- 20.Healthy Newborn Network . Accessed September 21, 2022. https://www.healthynewbornnetwork.org/
- 21.Tita AT, Szychowski JM, Boggess K, et al. ; C/SOAP Trial Consortium . Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231-1241. doi: 10.1056/NEJMoa1602044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138(5):703-713. doi: 10.1097/AOG.0000000000004565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. Map of the Trial Sites in (a) The Gambia and (b) Burkina Faso
eFigure 2. Kaplan-Meier Analysis By Trial Arm: Neonatal Mortality and Neonatal Sepsis
eFigure 3. Causes of Neonatal Death (Meeting the Primary Endpoint) in The Gambia and Burkina Faso
eFigure 4. Kaplan-Meier Analysis by Trial Arm: Neonatal Skin Infection, Umbilical Infection, Eye Infections and Ear Infections
eFigure 5. Kaplan-Meier Analysis by Trial Arm: Maternal Mastitis and Puerperal Sepsis
eTable 1. Neonatal Sepsis Criteria
eTable 2. Pre-specified Subgroup Analysis of the Neonatal Primary Endpoint by Trial Arm: Season, Mode of Delivery and Time From Treatment to Delivery
eTable 3. Safety Endpoints for Birthing Parents and Neonates by Trial Arm
eTable 4. Adverse Events for Birthing Parents and Neonates by Trial Arm
PregnAnZI-2 Working Group Members
Data Sharing Statement