Abstract
Soluble mitogens and adhesion-dependent organization of the actin cytoskeleton are required for cells to enter S phase in fibroblasts. The induction of cyclin A is also required for S-phase entry, and we now report that distinct effects of mitogens and the actin cytoskeleton on the phosphorylation of CREB and pocket proteins regulate the extent and timing of cyclin A promoter activity, respectively. First, we show that CREB phosphorylation and binding to the cyclic AMP response element (CRE) determines the extent, but not the timing, of cyclin A promoter activity. Second, we show that pocket protein inactivation regulates the timing, but not the extent, of cyclin A promoter activity. CREB phosphorylation and CRE occupancy are regulated by soluble mitogens alone, while the phosphorylation of pocket proteins requires both mitogens and the organized actin cytoskeleton. Mechanistically, cytoskeletal integrity controls pocket protein phosphorylation by allowing for sustained ERK signaling and, thereby, the expression of cyclin D1. Our results lead to a model of cyclin A gene regulation in which mitogens play a permissive role by stimulating early G1-phase phosphorylation of CREB and a distinct regulatory role by cooperating with the organized actin cytoskeleton to regulate the duration of ERK signaling, the expression of cyclin D1, and the timing of pocket protein phosphorylation.
The proliferation of most cell types requires exposure to mitogenic growth factors, adhesion to the extracellular matrix and organization of the actin cytoskeleton. Growth factors, cell adhesion, and the organized actin cytoskeleton are all specifically required for cell cycle progression through the G1 phase (2). Organization of the actin cytoskeleton is a consequence of integrin-mediated adhesion, but cell adhesion and the organized cytoskeleton have distinguishable effects on G1-phase progression (14, 17). While many studies have focused on differences in signaling and cell cycle progression in adherent and nonadherent cells, the cell cycle consequence of disrupting the actin cytoskeleton in adherent cells has received much less attention.
Cell cycle progression is mediated by the cyclin-dependent kinases (cdk's) with the formation of active cyclin D-cdk4 (or -cdk6) and cyclin E-cdk2 complexes controlling progression through G1 phase (35). In fibroblasts, the activation of cyclin D1-cdk4 or -cdk6 is typically limited by the mid-G1-phase expression of cyclin D1, while the activation of cyclin E-cdk2 is controlled by the mid-to-late G1-phase downregulation of p21 family cdk inhibitors (typically p21cip1 and p27kip1). The G1-phase cyclin-cdk's are required for phosphorylation of the retinoblastoma protein (pRb) and p107 (referred to as pocket proteins); this phosphorylation results in the disruption of E2F-pocket protein complexes and the induction of pRb-regulated genes such as cyclin A (15, 40). We and others have shown that the expression of cyclin D1 and the downregulation of p21cip1 and p27kip1 are jointly dependent upon both growth factor stimulation and adhesion to the extracellular matrix (reviewed in reference 6). At least some of these effects reflect adhesion-dependent organization of the actin cytoskeleton because cytochalasin D blocks the expression of cyclin D1, pRb phosphorylation, and entry into S phase in human fibroblasts (5). The induction of cyclin A at the G1-S interface, and the consequent formation of catalytically active cyclin A-cdk2 complexes, signals the beginning of S phase (13, 30).
Several studies have shown that cell cycle-dependent expression of the cyclin A promoter is regulated by two contiguous cis elements. The upstream element is referred to as the cell cycle-dependent element (CDE), also called the cell cycle regulatory element, and the contiguous downstream element has been termed the cell cycle gene homology region (CHR) (47). The CDE site in the cyclin A promoter is required for transcriptional repression, preventing cyclin A gene expression in G0 and early G1 phase (18, 28, 29, 32, 47, 48). The CHR is thought to act as a corepressor cis element (48). Mutation of either the CDE/CHR sites leads to elevated cyclin A promoter activity throughout G1 phase (18, 32, 47). Despite significant efforts, the factors binding to the CDE and CHR remain unclear (see Discussion).
Several other studies indicate that cyclin A gene expression is regulated by E2F-pocket protein complexes. For example, overexpression of E2F1, cyclin D1, and human papillomavirus E7 rescues cyclin A expression and anchorage-independent growth (10, 31, 33, 42). Pocket proteins regulate cyclin A gene expression through the CDE/CHR site (4, 21, 31), but the mechanism of the pocket protein effect remains highly controversial. A few reports indicate that the CDE is a variant E2F site that interacts with E2F4/p107 complexes rather than E2F/pRb (32, 44). However, another group reported that E2Fs do not bind with high affinity to the CDE site in the cyclin A promoter (18), and a third group (21) reported that E2F1 and 3, but not E2F4, bind to the CDE and act as transcriptional activators at G1/S rather than repressors at G0 and early G1. Moreover, experiments with pRb-null and p107-null mouse embryo fibroblasts (MEFs) emphasize that pRb, and not p107, is important for cyclin A gene expression (28, 29). Thus, the exact mechanism by which pocket proteins control cyclin A gene expression through the CDE/CHR is poorly understood and may well involve both indirect and direct effects. p130, the third member of the pocket protein family, has not been implicated in the regulation of cyclin A; our use of the term pocket proteins in this study refers only to pRb and p107.
The cyclin A promoter also contains a cyclic AMP (cAMP)-responsive element (CRE), a CCAAT site, and putative sites for AP-1, p53, and Sp1 (11, 16, 18–20, 36). A comparison of the human and rodent cyclin A promoters shows strong sequence homology in a core region that contains the CRE, CCAAT, and CDE/CHR sites; identity is greatly reduced in sequences 5′ to this core (18). Additionally, mutation-deletion analysis indicates that the AP-1 and p53 sites upstream of the core sequence in the human cyclin A promoter are probably not functional (12, 16), and mutation or deletion of the Sp1 and CCAAT sites is without consistent effect (16, 18, 19, 25, 36, 43). In contrast, several reports show that the CRE site plays an important role in transcriptional activation of the cyclin A gene (3, 11, 25, 36, 38, 43). Most studies indicate that the CRE is constitutively occupied in G1-phase cells (3, 11, 36, 38, 43), and Zwicker and Müller (48) have proposed that the CDE/CHR acts in concert with upstream activating sequences, such as the CRE, to confer proper cell cycle regulation to the cyclin A promoter. Sylvester et al. (38) have provided initial evidence that the CRE and CDE sites cooperate to regulate cyclin A gene expression.
In this report, we sought to determine how soluble mitogens and the organized actin cytoskeleton regulate expression of the cyclin A gene. We show that mitogens and the actin cytoskeleton have distinct effects on CREB and pocket proteins and that these effects control the extent and timing of cyclin A promoter activity, respectively. In turn, we conclude that the pathways regulating the extent and timing of cyclin A promoter activity are distinguishable and regulated differently.
MATERIALS AND METHODS
Cell culture and cell cycle analysis.
NIH 3T3 fibroblasts, NRK fibroblasts, established MEFs, and early-passage cultures of human foreskin fibroblasts were maintained and G0 synchronized by serum starvation as described previously (7, 45). Quiescent cells were trypsinized, reseeded in Dulbecco modified Eagle medium (DMEM) in the absence or presence of 2 to 4 μM cytochalasin D (Calbiochem) and stimulated with the following mitogens: 5% fetal calf serum (FCS)–2 nM epidermal growth factor for 3T3 cells or 10% FCS for NRK cells, MEFs, and human fibroblasts. In some experiments, the effects of different actin-modifying drugs were compared by seeding cells in the presence of cytochalasin D, 0.5 μM latrunculin B (Calbiochem), 0.03 μM swinholide A (Calbiochem), or 0.03 μM jasplakinolide (Molecular Probes). NIH 3T3 cells expressing cyclin D1 under control of a tetracycline-repressible promoter were prepared and maintained as described previously (46). Cells were washed two to three times in phosphate-buffered saline, collected by scraping, and lysed in the appropriate buffer. Western blots were performed as described previously (7, 45) by using lysates of from 1 × 106 to 2 × 106 cells per sample.
EMSAs.
Nuclear extracts were prepared as described previously (1). Briefly, washed cell pellets (5 × 106 to 6 × 106 cells per 150-mm dish) were resuspended in 0.5 ml of cold hypotonic buffer (10 mM HEPES-KOH, pH 7.9; 10 mM KCl; 1.5 mM MgCl2; 0.5 mM dithiothreitol; 0.2 mM phenylmethylsulfonyl fluoride; 50 mM sodium fluoride; 10 μg of leupeptin/ml; 10 μg of aprotinin/ml) and allowed to swell on ice for 10 min. The cells were collected in an Eppendorf microcentrifuge, the supernatant was discarded, and the pellet was resuspended in 25 to 50 μl of cold lysis buffer (20 mM HEPES-KOH, pH 7.9; 25% glycerol; 420 mM NaCl; 1.5 mM MgCl2; 0.2 mM EDTA; 0.5 mM dithiothreitol; 0.2 mM phenylmethylsulfonyl fluoride; 50 mM sodium fluoride; 10 μg of leupeptin/ml; 10 μg of aprotinin/ml). After incubation on ice for 20 min, cellular debris was removed by full-speed centrifugation in an Eppendorf microcentrifuge (2 min, 4°C), and supernatants (nuclear extracts) were quick-frozen and stored as aliquots at −80°C. Proteins were quantified by the Bradford dye-binding procedure (Bio-Rad). Double-stranded oligonucleotides containing either wild-type (TGAATGACGTCAAGGCCGCGAG) or mutated (TGAATGAATTCAAGGCCGCGAG) cyclin A CRE was 5′ end labeled with [γ-32P]ATP (3,000 Ci/mmol) and T4 polynucleotide kinase (Life Technologies). The probe was purified by using a Microspin G-50 column (Amersham Pharmacia Biotech, Inc.). Specific activities were typically 5 × 108 to 10 × 108 cpm/μg. For electrophoretic mobility shift analyses (EMSAs; 3 to 6 μg of nuclear extract were incubated with 1 μg of poly(dI-dC)-poly(dI-dC) (Pharmacia) in binding buffer (10 mM Tris-HCl, pH 7.5; 50 mM NaCl; 0.5 mM dithiothreitol; 0.05% NP-40; 10% glycerol; 0.2 mM phenylmethylsulfonyl fluoride; 50 mM sodium fluoride; 10 μg of leupeptin/ml; 10 μg of aprotinin/ml) in a final volume of 20 μl. After incubation for 10 min at room temperature, DNA probe (6 × 104 cpm) was added to the mixture, followed by incubation for 10 min at room temperature. For competition experiments, a 100-fold molar excess of unlabeled competitor DNA was added to the reaction mixture prior to addition of the radioactive probe. For the supershift experiments, nuclear extracts in 20 μl of binding buffer were preincubated (30 min at room temperature) with 1 to 3 μl of antibodies against CREB (sc-271x), CREM (sc-440x), ATF-1 (sc-243x), c-jun (sc-44x), c-fos (sc-253x), and Sp1 (sc-420x) (all purchased from Santa Cruz Biotechnology, Inc.) or against phosphoCREB (06-519; Upstate Biotechnology, Inc.) prior to addition of the radioactive probe. Protein-DNA complexes were separated on a 5% polyacrylamide gel in 22.5 mM Tris–22.5 mM boric acid–62.5 mM EDTA (pH 8) at 160 V for 3 h at 4°C. The gels were dried and analyzed by autoradiography.
Expression vectors and luciferase constructs.
The cyclin A promoter-firefly luciferase (called luciferase) constructs were as described previously (16). p322/cycA-luc (1.3-kb promoter) contains bases 1 to 1,293 of the human cyclin A promoter upstream of the luciferase reporter. p367/cycA-luc (+CRE; bases 960 to 1,148) is a construct in which the sequences upstream of the CRE in the conserved core promoter have been deleted. p362/cycA-luc (−CRE; bases 988 to 1,148) is a construct in which the CRE site in the core promoter as well as all upstream sequences have been deleted. Refer to GenBank accession number x68303 for base numbering. The human papillomavirus type 18 E7 expression vector was the generous gift of Lou Laimins. The constitutively active MEK expression vector, pCMV-MEK(S218/222D), was the generous gift of Michael Weber.
Cell transfections and promoter-luciferase assays.
Transfections with pCMV-MEK(S218/222D) were performed in near-confluent 100-mm dishes by using 6 μg of plasmid and then analyzed for protein expression by Western blotting as described previously (7, 45). Unless noted otherwise, transient transfections of NIH 3T3 cells with promoter luciferase vectors were performed as described previously (7) by using 2 μg of cyclin A promoter-luciferase plasmid(s) and 0.1 μg of a Renilla luciferase expression plasmid (pRL-SV40 or pRL-CMV; Promega) to control for transfection efficiency. After an overnight recovery, the cells were synchronized in G0 by incubation in serum-free DMEM for 24 h. The G0-synchronized transfectants were trypsinized, reseeded at subconfluence in either 100-mm dishes (∼3 × 105 cells in 10 ml), 35-mm dishes (∼105 cells in 2 ml), or 24-well plates (∼104 cells in 0.5 ml) and then stimulated with mitogens in the absence or presence of actin-depolymerizing drugs. Cells were washed with phosphate-buffered saline, collected, lysed, and analyzed for luciferase and Renilla luciferase activity by using the Dual-Luciferase reporter assay system (Promega). Cyclin A promoter-driven luciferase activity was then normalized to a constant activity of Renilla luciferase. Analysis of protein concentration of lysates typically showed nearly identical recovery of cells within experiments.
RESULTS
Cytoskeletal integrity regulates cyclin A gene transcription.
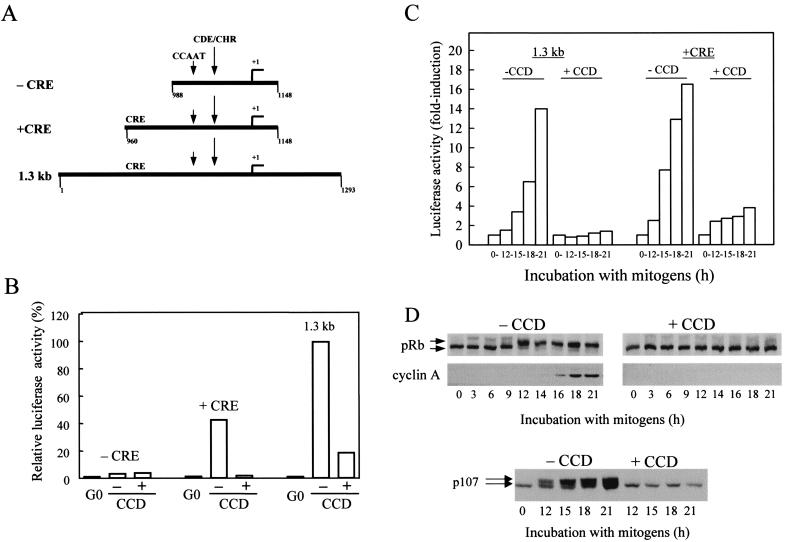
To explore the cooperative effects of the mitogens and the organized actin cytoskeleton on cyclin A promoter activity, we examined three cyclin A promoter-luciferase constructs: a 1.3-kb promoter, a deletion construct lacking 5′ upstream sequences that precede the highly conserved promoter core (+CRE), and an additional deletion construct that removes the CRE sequence from the conserved promoter core (−CRE) (Fig. 1A). These constructs were transiently transfected into NIH 3T3 cells, and their relative luciferase activities were measured at quiescence and after mitogen stimulation in the absence or presence of cytochalasin D. Cytochalasin D is commonly used to disrupt actin microfilaments and cytoskeletal structure.
FIG. 1.
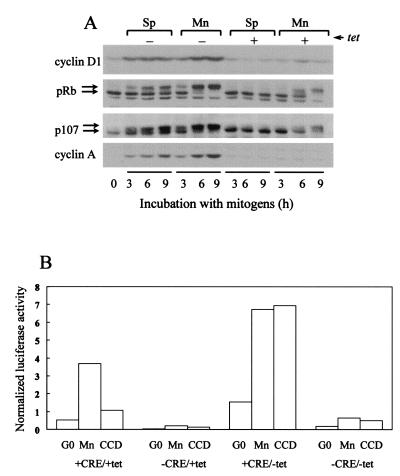
Cytoskeletal integrity regulates cyclin A gene transcription. (A) Diagram of the cyclin A promoter-luciferase constructs used in this study. (B and C) NIH 3T3 cells were transiently transfected with the cyclin A promoter-luciferase constructs and pRL-SV40 Renilla luciferase. Transfected cells were G0 synchronized and stimulated with mitogens (see Materials and Methods) in the absence or presence of cytochalasin D (CCD) for 18 h (B) or the various times indicated (C). Luciferase activity was normalized to a constant activity of Renilla luciferase. In panel B, normalized luciferase activity is plotted relative to the normalized activity obtained with the 1.3-kb cyclin A promoter from cells cultured in the absence of cytochalasin D (defined as 100%). In panel C, luciferase activity is plotted as the fold induction relative to G0. In panel D, NIH 3T3 cells were G0 synchronized by serum starvation and then stimulated with mitogens in the absence or presence of cytochalasin D for the times shown. Lysates were prepared and analyzed by Western blotting for the phosphorylation of pRb and p107 and the expression of cyclin A. The upper and lower arrows, respectively, in panel D show the positions of phosphorylated and unphosphorylated pRb and p107.
Relative to the activity seen with the 1.3-kb construct, deletion of 5′ upstream sequences of the conserved promoter core reduced the transcriptional activity of the cyclin A promoter (Fig. 1B; +CRE). However, activation of this core construct remained strongly dependent on mitogens and the organized actin cytoskeleton. Additional deletion of the CRE site completely blocked activation of the cyclin A promoter (Fig. 1B; −CRE). Others have already reported that mutation of the CRE greatly reduces transcriptional activation of a cyclin A promoter construct (11, 12, 36, 38). Thus, our results confirm the importance of the CRE site in the efficient induction of the cyclin A promoter. Additionally, we show that promoter activity is strongly inhibited when cytoskeletal integrity is lost.
We also compared the time-dependent activation of the 1.3-kb and core (+CRE) promoters. The constructs were transiently transfected into NIH 3T3 cells, the cells were synchronized into G0, and the levels of promoter activity were determined after stimulation with mitogens in the absence or presence of cytochalasin D (Fig. 1C). The results show that cyclin A promoter activity begins at ∼12 h and continues to at least 21 h. Both the 1.3-kb and the +CRE promoters showed similar fold inductions and time courses of promoter activity. Disruption of cytoskeletal integrity with cytochalasin D strongly inhibited activation of both the 1.3-kb and the core (+CRE) cyclin A promoters (Fig. 1C). Serum stimulation of transfected NRK cells and MEFs also induced the cyclin A promoter, and the induction was similarly dependent upon mitogens and an organized actin cytoskeleton (data not shown).
Parallel cultures of NIH 3T3 cells were used to assess the time course of pocket protein phosphorylation and expression of endogenous cyclin A protein in the presence and absence of an intact actin cytoskeleton. Phosphorylation of pRb and p107 was readily detectable by gel shift in mid-to-late G1 phase, and cyclin A expression was induced shortly thereafter (Fig. 1D). The phosphorylation of pRb and p107 and the expression of cyclin A were completely blocked by cytochalasin D (Fig. 1D). A comparison of the results in Fig. 1C and D shows that the cluster of transcription factor binding sites in the core (+CRE) promoter (CRE, CCAAT, CDE, and CHR motifs) retain the mitogen- and cytoskeletal-dependent regulation characteristic of the 1.3-kb promoter and the cyclin A protein itself.
Mitogen-dependent phosphorylation of CREB regulates the extent of cyclin A gene expression.
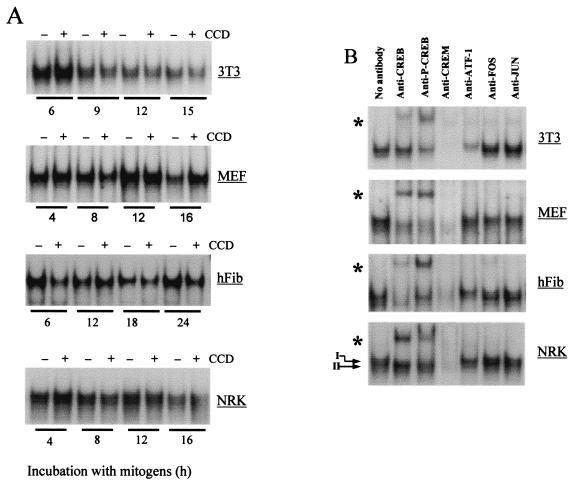
Nuclear lysates prepared from NIH 3T3 cells, MEFs, normal human fibroblasts, and NRK fibroblasts were used to address the role of cytoskeletal integrity in CRE occupancy and characterize the transcription factors that bind to the cyclin A promoter CRE site in fibroblasts. Consistent with results by others (3, 11, 36), Fig. 2A shows that CRE occupancy was similar through the G1 phase of serum-stimulated fibroblasts (although there was a slight but reproducible decrease in CRE occupancy in late G1 phase in 3T3 cells). Importantly, disruption of the actin cytoskeleton did not affect occupancy of the CRE in any of the four fibroblast cell lines we examined (Fig. 2A). Nuclear lysates from adherent cells were then preincubated with anti-CREB, anti-ATF-1, and anti-phosphoCREB (which recognizes Ser133 on phosphoCREB and the homologous serines in phosphoATF-1 and phosphoCREM). Readily apparent supershifts were obtained with anti-CREB and anti-phosphoCREB (Fig. 2B) in all of the fibroblast lines we tested. The same protein-CRE complexes were detected when cells were treated with cytochalasin D (data not shown). In human fibroblasts and NRK cells, the CRE complex was resolved into a doublet (e.g., refer to the arrows in Fig. 2B), and the slower-migrating band was supershifted with anti-CREB, while the faster one was supershifted with anti-ATF-1. Anti-fos, and anti-jun antibodies failed to supershift the CRE complexes (Fig. 2B). We especially note that the anti-CREM antibody (which cross-reacts with ATF-1 and CREB) resulted in a complete loss of the CRE complex from each of the four fibroblast lines. (The loss, rather than supershift, of the complex indicates that the CREM antibody is reacting with the DNA-binding domain of CREB family members.) Since Western blot analysis showed that CREM was not expressed in any of these fibroblasts (data not shown), the complete loss of the CRE complex in each of the four lines tested indicates that the transcription factors binding to the cyclin A CRE in fibroblasts are largely restricted to CREB and ATF-1. Immunoprecipitation-Western blot analysis of MEF, NIH 3T3, and NRK cell lysates showed that (i) ATF-1 and CREB formed both homodimers and heterodimers (to different degrees in the different cell lines) and (ii) phosphorylation was more apparent on CREB than on ATF-1 (data not shown).
FIG. 2.
Occupancy of the cyclin A CRE is independent of cytoskeletal integrity. NIH 3T3 cells, MEFs, early-passage human skin fibroblasts (hFib), and NRK fibroblasts were G0 synchronized and then stimulated with mitogens (see Materials and Methods) in the absence or presence of cytochalasin D (CCD) as described in Materials and Methods. (A) CRE EMSAs with nuclear extracts obtained after stimulation of cells with mitogens for the times shown. Unlabeled wild-type oligonucleotide (but not mutant oligonucleotide) effectively blocked formation of the complex, and a 32P-labeled mutant CRE oligonucleotide failed to assemble into a complex (data not shown). (B) Results obtained after preincubation of the nuclear lysates (prepared after 18 h of incubation of cells with mitogens in the absence of cytochalasin D) with anti-CREB, anti-phosphoCREB (p-CREB), anti-CREM, anti-ATF-1, anti-c-fos, and anti-c-jun. Controls demonstrated that these concentrations of antibodies were in excess (data not shown). The arrows show the doublet that was occasionally obtained during the EMSA. Asterisks indicate supershifted complexes.
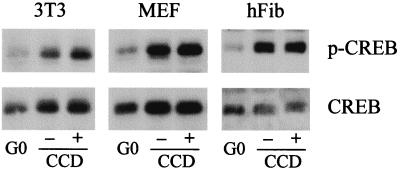
We then determined the relative effects of mitogens and the organized actin cytoskeleton on CREB phosphorylation. Western blot analysis with the phosphoCREB antibody showed that the activating phosphorylation of CREB is low in quiescent cells and stimulated by mitogens in each of the cell lines tested (Fig. 3). CREB phosphorylation was maximal 3 to 6 h after serum stimulation (data not shown), and disruption of the actin cytoskeleton had no effect on CREB phosphorylation (Fig. 3). Together, the results of Fig. 1 to 3 indicate that mitogen-stimulated CREB phosphorylation mediates the extent of promoter activity and that cytoskeletal integrity does not play a role in these events. However, since occupancy of the CRE (Fig. 2A) occurs well before induction of the cyclin A promoter (refer to Fig. 1C), it does not set the timing of promoter activity.
FIG. 3.
Mitogen-dependent phosphorylation of CREB. NIH 3T3 cells, MEFs, and normal human fibroblasts (hFib) were synchronized into G0 and stimulated with mitogens (see Materials and Methods) in the absence or presence of cytochalasin D (CCD). After 6 h, the cells were collected, lysed, and analyzed by Western blotting for the expression and phosphorylation of CREB by using specific antibodies.
Pocket protein phosphorylation by mitogens and the organized actin cytoskeleton regulates the timing of cyclin A gene expression.
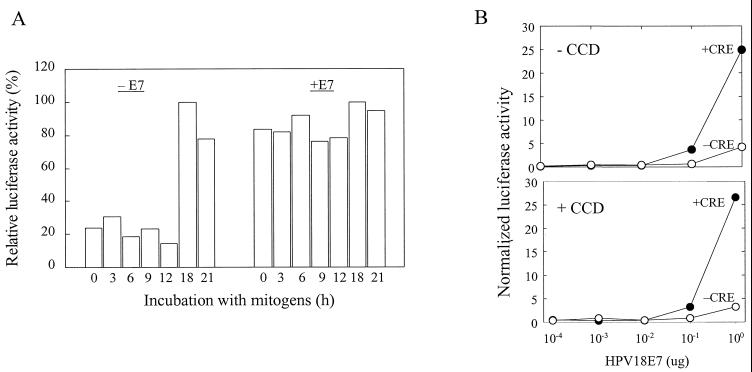
Our results showed a strong correlation between the kinetics of pRb and p107 phosphorylation and induction of the cyclin A promoter, and each of these events is completely dependent upon the organized actin cytoskeleton (see Fig. 1). To explore the kinetic link between pRb and p107 phosphorylation and cyclin A expression, we prematurely inactivated pocket protein function by transiently transfecting NIH 3T3 cells with a human papillomavirus-18 E7 expression vector (9). Overexpression of E7 deregulated the cyclin A promoter, allowing for promoter activity in serum-starved cells and throughout G1 phase (Fig. 4A). In contrast to results with control cells, cytochalasin D did not inhibit the ability of E7 to induce the cyclin A promoter (Fig. 4B), indicating that the organized actin cytoskeleton is not required after pocket protein inactivation. Moreover, E7 poorly induced luciferase activity if the cyclin A promoter lacked its CRE (Fig. 4B). These data agree well with our results showing that the CRE occupancy is necessary for efficient induction of cyclin A promoter activity and that this effect is independent of the organized actin cytoskeleton. Moreover, the finding that E7 allows for cyclin A expression in G0 and throughout G1 phase suggested that pocket proteins regulate the timing of cyclin A gene expression. The same conclusion was reached when we prematurely inactivated pocket proteins by conditional expression of cyclin D1 (see Fig. 7A).
FIG. 4.
E7 deregulates the timing of cyclin A expression. (A) NIH 3T3 cells were transiently cotransfected with (+E7) or without (−E7) 1 μg of E7 expression plasmid, 1 μg of the +CRE cyclin A luciferase reporter construct, or 0.1 μg of the pRL-CMV Renilla luciferase vector. The final amount of DNA transfected was brought to 2.1 μg for all samples by addition of empty vector as needed. The transfected cells were serum starved and stimulated with mitogens (see Materials and Methods) for the times shown. Luciferase activity was normalized to a constant activity of Renilla luciferase. In panel A, the luciferase activity is plotted relative to the amount of activity in the 18-h samples (defined as 100%) in both the absence and the presence of E7. In panel B, NIH 3T3 cells were transiently cotransfected with increasing amounts of the E7 expression plasmid, 1 μg of cyclin A luciferase reporter constructs, +CRE (●) or −CRE (○), and 0.1 μg of the pRL-SV40 Renilla luciferase vector. The final amount of DNA transfected was brought to 2.1 μg for all samples by the addition of empty vector as needed. The transfected cells were serum starved and stimulated with mitogens for 3 h in the absence or presence of cytochalasin D (CCD) as indicated. Luciferase activity was normalized to a constant activity of Renilla luciferase.
FIG. 7.
Deregulated cyclin D1 expression leads to deregulated cyclin A expression. (A) Serum-starved tet-cyclin D1 3T3 cells were trypsinized, reseeded in monolayer (Mn) or suspension (Sp) in the absence or presence of tetracycline, and stimulated with 10% FCS in DMEM for 0, 3, 6, and 9 h. Collected cells were lysed and analyzed by immunoblotting for the expression of cyclin D1, the phosphorylation of pRb and p107, and the expression of cyclin A. The upper and lower arrows show the phosphorylated and unphosphorylated forms of pRb and p107, respectively. (B) tet-cyclin D1 3T3 cells cultured with 2 μg of tetracycline/ml were transiently cotransfected with 1 μg of either +CRE or −CRE cyclin A promoter-luciferase vectors and 0.1 μg of pRL-SV40 Renilla luciferase vector. After serum starvation in the absence or presence of tetracycline (G0), the transfectants were trypsinized, suspended in 10% FCS-DMEM, and reseeded in tissue culture dishes in the absence (monolayer; Mn) or presence of cytochalasin D (CCD). Cells were incubated for 24 h with or without tetracycline prior to collection and lysis. Luciferase activity was normalized to a constant activity of Renilla luciferase.
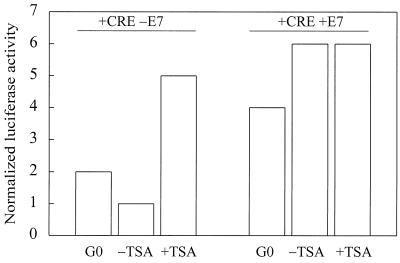
Although the transcription factors that bind to the CDE/CHR are still a subject of active investigation, several studies show that the CDE/CHR is required for transcriptional repression in the G0 and early G1 phases (18, 48). Transcriptional repression is typically regulated by the recruitment of histone deacetylases (HDACs) to repressor elements. For example, pRb recruits HDACs to E2F sites (8, 23). To examine the role of HDACs in cyclin A gene expression, NIH 3T3 cells were transiently transfected with the core cyclin A promoter luciferase construct (+CRE) and stimulated with mitogens in the presence or absence of the HDAC inhibitor, trichostatin A (TSA). Cyclin A promoter activity (which normally requires a >12-h mitogen stimulation; refer to Fig. 1) was stimulated 3 h after exposure to mitogens if the cells were treated with TSA (Fig. 5; −E7). The ability of TSA to accelerate the timing of cyclin A promoter activity agrees well with the results seen upon expression of E7 or cyclin D1 (refer to Fig. 4A and 7A). Moreover, if we inactivated pocket protein function with E7, TSA did not further stimulate cyclin A promoter activity (Fig. 5; compare –E7 and +E7). These results suggest that transcriptional repression of the cyclin A promoter reflects the effect of pocket protein-associated HDACs.
FIG. 5.
The HDAC inhibitor TSA accelerates cyclin A transcription. NIH 3T3 cells were transiently transfected with cyclin A promoter-luciferase constructs (+CRE; 1 μg), pRL-SV40 Renilla luciferase (0.1 μg), and either E7 (1 μg) or empty vector (1 μg). Serum-starved transfectants (G0) were stimulated with 10% FCS in DMEM in the absence or presence of 50 nM TSA for 3 h. Luciferase activity was normalized to a constant activity of Renilla luciferase.
In summary, the results presented in Fig. 1D show that pocket protein phosphorylation is completely dependent upon the organized actin cytoskeleton, whereas the results in Fig. 2 and 3 show that CRE occupancy and CREB phosphorylation is independent of the organized actin cytoskeleton. Thus, the organized actin cytoskeleton is required for induction of cyclin A gene expression because it is necessary for pocket protein phosphorylation. This conclusion is supported by our studies using ectopic expression of E7 (and cyclin D1 [see Fig. 7A]), which demonstrates that disruption of the actin cytoskeleton does not inhibit cyclin A promoter activity if pocket proteins have been inactivated. Finally, the direct relationship between the kinetics of pocket protein inactivation and cyclin A promoter activation indicates that the major effect of the organized actin cytoskeleton is on the timing, rather than the extent, of cyclin A promoter activity.
Mitogens and the organized actin cytoskeleton cooperate to regulate the duration of ERK signaling, the expression of cyclin D1, and the timing of pocket protein phosphorylation.
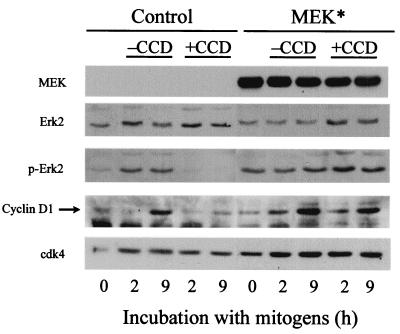
In addition to demonstrating the importance of cytoskeletal integrity in pocket protein-dependent cyclin A gene expression, we have also addressed the mechanism by which the organized actin cytoskeleton regulates pocket protein function. In nontransformed fibroblasts, the phosphorylation (inactivation) of pRb is initiated by cyclin D1-cdk4, and previous studies have indicated that a sustained ERK signal (for several hours) mediates the induction of cyclin D1 (24, 39). Since cyclin D1 expression is jointly dependent upon mitogens and an organized actin cytoskeleton (5), we sought to determine whether sustained ERK activity was dependent upon the organized actin cytoskeleton. Based on reactivity with an antibody specific for dually phosphorylated (active) ERK, we found that mitogens induced sustained ERK activity (∼9 h) and cyclin D1 only when the actin cytoskeleton was intact (Fig. 6; control). We (unpublished results) and others (39) find that sustained ERK activity for ∼6 h is required for the induction of cyclin D1 in mid-G1 phase, suggesting that the prolongation of ERK phosphorylation seen in response to the organized actin cytoskeleton causes the expression of cyclin D1. In support of this idea, we found that when NIH 3T3 cells were transiently transfected with a constitutively active MEK cDNA (to force the sustained phosphorylation of ERK), cyclin D1 was expressed in mid-G1 phase whether or not the actin cytoskeleton had been collapsed by exposure to cytochalasin D (Fig. 6; MEK*). Thus, mitogens and the organized actin cytoskeleton cooperate to allow for sustained ERK activity in G1 phase, and this cooperation is required for the expression of cyclin D1 in mid-G1 phase. Note that MEK* expression did not allow for pRb phosphorylation or cyclin A expression when the actin cytoskeleton was disrupted with cytochalasin D (data not shown). This result was expected given previous studies showing that strong ERK activation stimulates the expression of p21cip1 (7, 34, 41) and that both p21cip1 (7, 26) and p27kip1 (our unpublished results) levels are increased upon disruption of the actin cytoskeleton.
FIG. 6.
Forced activation of ERK leads to deregulated cyclin D1 expression. NIH 3T3 cells were transiently transfected with pCMV-MEK(S218/222D) (MEK*). The transfected cells were serum starved and stimulated with mitogens (see Materials and Methods) in the absence or presence of cytochalasin D (CCD) for the times shown. Collected cells were lysed, and equal amounts of cell protein were fractionated on a sodium dodecyl sulfate gel and analyzed by immunoblotting with anti-phosphoERK (p-ERK), anti-ERK (loading control), anti-cyclin D1, and anti-cdk4 (loading control).
To determine whether the organized actin cytoskeleton was required subsequent to the expression of cyclin D1, tet-cyclin D1 3T3 cells (see Materials and Methods) were serum starved and stimulated with mitogens in monolayer and suspension (Fig. 7A). In adherent cells cultured with tetracycline, the phosphorylation of pRb and p107 was detected at 6 to 9 h. Removal of tetracycline from the adherent cells allowed for the overexpression of cyclin D1 and an enhanced phosphorylation of pRb and p107 (e.g., compare the degree of phosho-pRb and phospho-p107 at 6 h; Mn ± tet). Importantly, tet-cyclin D1 3T3 cells cultured in suspension (which precludes cytoskeletal organization) completely failed to phosphorylate pRb and p107 when incubated with tetracycline, but suspended cells cultured without tetracycline expressed cyclin D1 and phosphorylated both pRb and p107 (Fig. 7A). Thus, cytoskeletal integrity was not required for pocket protein phosphorylation subsequent to cyclin D1 expression. Similarly, the phosphorylation of pocket proteins by ectopic expression of cyclin D1 allowed for cyclin A expression in suspended cells (Fig. 7A). Thus, the organized actin cytoskeleton is not necessary for the phosphorylation of pocket proteins or the expression of cyclin A once cyclin D1 has been induced. In turn, these results indicate that the actin cytoskeleton regulates cyclin A gene expression by sustaining ERK activity and thereby allowing for the expression of cyclin D1. Note, however, that the degree of pocket protein phosphorylation and cyclin A expression was somewhat reduced in suspended versus adherent cells, suggesting that there may be other, less-prominent effects of the organized actin cytoskeleton that contribute to pocket protein phosphorylation (e.g., effects on cyclin E-cdk2; see Discussion). We also emphasize that cyclin D1 was expressed prematurely (e.g., ∼3 h) upon removal of tetracycline and that premature cyclin D1 expression was associated with an accelerated phosphorylation of pRb and an accelerated expression of cyclin A (Fig. 7A, compare Mn ± tet). These results agree well with the E7 and TSA experiments (Fig. 4 and 5), again linking the timing of pocket protein inactivation to the timing of cyclin A promoter activity.
To assess the role of the CRE in cyclin D1-dependent activation of the cyclin A promoter, cyclin A promoter-luciferase constructs containing or lacking the CRE were transiently transfected into tet-cyclin D1 3T3 cells. Consistent with the results in Fig. 7A, the ectopic expression of cyclin D1 transactivated the cyclin A promoter even when cytoskeletal integrity was disrupted with cytochalasin D (Fig. 7B; compare +CRE/+tet and +CRE/−tet). But cyclin D1 overexpression did not overcome the need for the CRE (Fig. 7B; compare +CRE/–tet and –CRE/–tet). These results agree completely with those obtained upon expression of E7 (refer to Fig. 4B).
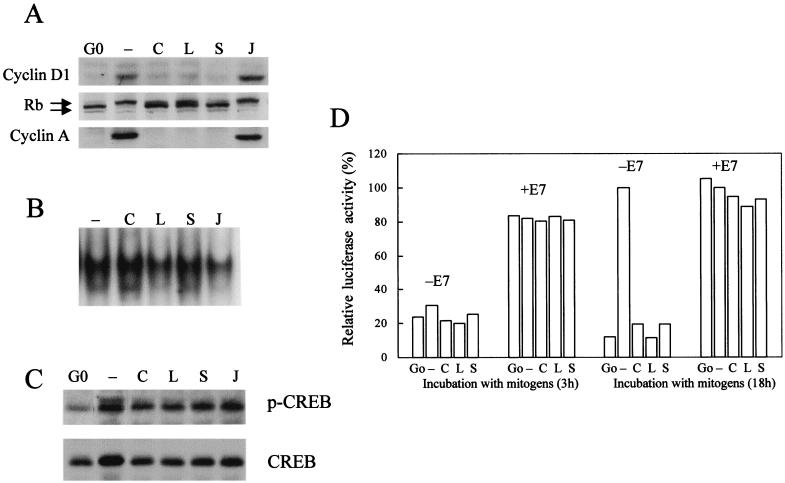
Finally, to confirm that the results we obtained with cytochalasin D reflect an overall inhibition of cytoskeletal integrity, we compared the effect of cytochalasin D to the effects of latrunculin B and swinholide A, two mechanistically distinct inhibitors of actin polymerization (37). We also examined the effect of jasplakinolide, which stabilizes F-actin (37). Similar to the results obtained in Fig. 1D and 6, time course analyses showed that cytochalasin D, latrunculin B, and swinholide A inhibited the mitogen-dependent induction of cyclin D1, the phosphorylation of pRb, and the induction of cyclin A (Fig. 8A), whereas jasplakinolide did not block these events. Moreover, in cells treated with cytochalasin D, occupancy of the CRE (Fig. 8B), CREB phosphorylation (Fig. 8C), and induction of the cyclin A promoter in response to E7 (Fig. 8D) were unaffected by latrunculin B or swinholide A. These data indicate that inhibition of actin polymerization, rather than any unique subcellular action of cytochalasin D, accounts for the effects we observed on cyclin A promoter activity. Additionally, they support our conclusion that the requirement for polymerized actin is restricted to pocket protein-dependent cyclin A gene expression.
FIG. 8.
Common effects of actin-depolymerizing agents on cyclin A gene expression. NIH 3T3 cells were serum starved (G0) and then stimulated with mitogens (see Materials and Methods) in the absence (–) or presence of cytochalasin D (C), latrunculin B (L), swinholide A (S), or jasplakinolide (J). (A) Lysates were prepared and analyzed by Western blotting for the expression of cyclin D1 (after 9 h of mitogen stimulation), the phosphorylation of pRb, and the expression of cyclin A (after 18 h of mitogen stimulation). The upper and lower arrows, respectively, show the positions of phosphorylated and unphosphorylated pRb. (B) CRE EMSAs were performed with nuclear extracts prepared after mitogen stimulation of cells for 18 h. (C) Cells were lysed and analyzed by Western blotting for the expression and phosphorylation of CREB by using specific antibodies. (D) Cells were transiently transfected with the +CRE cyclin A promoter-luciferase construct (1 μg), pRL-CMV Renilla luciferase (0.1 μg), and either E7 (1 μg) or empty vector (1 μg). Transfected cells were serum starved and stimulated with mitogens (see Materials and Methods) for 3 and 18 h. Luciferase activity was normalized to a constant activity of Renilla luciferase and plotted relative to the amount of activity in the 18-h samples (defined as 100%) in both the absence and the presence of E7.
DISCUSSION
The major goal of our studies was to understand the way that mitogens and the organized actin cytoskeleton cooperate in the regulation of the cyclin A gene. Induction of the cyclin A promoter at the G1/S interface is mediated by the CRE and the CDE/CHR sites, and our data support the importance of these sites. However, we also show that these sites have distinct roles in the induction of the cyclin A gene and are controlled by different signals. The CRE is important for efficient induction of the promoter, and this effect can be traced to a mitogen-dependent phosphorylation of CREB in early G1 phase. Disruption of cytoskeletal integrity does not block CREB phosphorylation or CRE occupancy. Thus, mitogens alone regulate the CRE. Moreover, since CREB phosphorylation and CRE occupancy occur well before promoter activity is detected, the CRE is not involved in setting the timing of cyclin A gene expression. Conversely, our results with E7 and cyclin D1 show that pocket protein function regulates the timing of cyclin A promoter activity. Pocket protein phosphorylation is both mitogen and actin cytoskeleton dependent. Thus, mitogens play two distinct roles in regulating the cyclin A promoter: a permissive role via the early G1 phosphorylation of CREB and a regulatory role in controlling pocket protein function via its cooperation with the organized actin cytoskeleton. In contrast, the actin cytoskeleton requirement for cyclin A gene expression is largely restricted to the regulation of pocket protein function. While others (37) have reported that different actin-depolymerizing drugs have distinct effects on serum response factor activation, our studies show that inhibition of actin polymerization with cytochalasin D, latrunculin B, or swinholide A leads to the same effect on cyclin D1 expression, pRb phosphorylation, and cyclin A gene expression. Similarly, none of these drugs affected CRE-dependent cyclin A gene expression.
Our studies also reveal a mechanism by which the organized actin cytoskeleton controls pocket protein function. In particular, we show that sustained ERK activity required for the induction of cyclin D1 is dependent on cytoskeletal structure. Since ectopic expression of cyclin D1 leads to pocket protein phosphorylation and cyclin A expression in suspended cells, the entire requirement for cytoskeletal integrity in cyclin A gene regulation seems largely due to its effect on sustained ERK signaling. However, it is worth noting that ectopic expression of cyclin D1 in tet-D1 3T3 cells also led to the activation of cyclin E-cdk2 (presumably by redistribution of the cdk inhibitors p21cip1 and p27kip1 [data not shown]). Activation of cyclin E-cdk2 is thought to be important for the phosphorylation of pRb (15); the effects of cytoskeletal integrity on these inhibitors may well contribute to pRb phosphorylation and cyclin A gene expression.
Pocket proteins are thought to regulate cyclin A promoter activity through the CDE/CHR site (18, 28, 29, 32, 33), but the proteins that bind to this site are very poorly defined. Some studies (32, 44) suggest that the CDE is occupied by E2F4/p107 complexes, but the binding of any E2F to the cyclin A promoter is highly controversial (see the introduction). Liu et al. (22) described a non-E2F protein, CDF-1, that interacts with both the CDE and the contiguous CHR motif. A recent study (21) suggests that CDF-1 and E2F1 (or E2F3) both bind to the CDE site in the cyclin A promoter but that E2F binding is dispensable for transcriptional repression. Instead, these authors propose that the binding of E2F activates the cyclin A promoter, and they reported very modest effects (∼2 h) of E2F binding on the timing of promoter activation. Others (27) have suggested that a distinct non-E2F factor, CHF, interacts specifically with the CHR. Neither CDF-1 nor CHF have been purified or cloned to date, precluding detailed analysis of this issue.
Recent work with p107-, p130-, and pRb-null MEFs strongly indicate that pRb, and not p107, is the critical pocket protein that regulates the cyclin A gene (28, 29). pRb regulates transcription by recruiting HDACs to promoters, and our results show that inhibition of HDAC activity stimulates cyclin A promoter activity. Moreover, we can link the HDAC effect to pocket protein function since inhibition of HDAC activity with TSA fails to stimulate cyclin A promoter activity in cells expressing E7. Thus, our studies support the importance of pRb-E2F complexes in the regulation of the cyclin A promoter. Moreover, we find that inactivation of pocket proteins allows for a very premature expression of cyclin A. The magnitude of this effect differs dramatically from that reported by Liu et al. (21). In our experiments, the timing of cyclin A promoter activity is almost completely under the control of pocket proteins. And regulation of pocket protein function is the major role of the organized actin cytoskeleton.
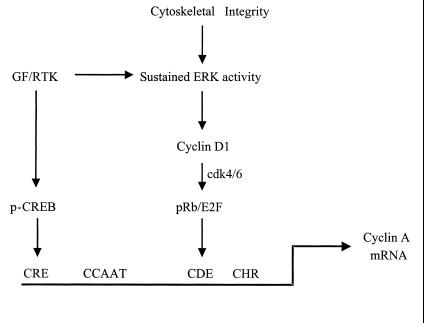
Together with other studies on the cyclin A promoter, our results lead us to the following model for regulation of the cyclin A gene (Fig. 9). Mitogens alone stimulate the early G1-phase phosphorylation of CREB, an effect that allows for occupancy of the cyclin A CRE. The organized actin cytoskeleton is required for sustained ERK activity in mitogen-treated cells. The sustained ERK signal then results in cyclin D1 expression which, in turn, initiates a series of actin cytoskeleton-independent effects including activation of cyclin D1-cdk4 and -cdk6 and phosphorylation of pRb. HDAC-pRb-E2F complexes control promoter activity at the CDE/CHR site, directly or indirectly. By these mechanisms, the cooperative effects of soluble mitogens and the actin cytoskeleton on CREB and pRb control the extent and timing of cyclin A promoter activity, respectively.
FIG. 9.
Cooperative effects of the cyclin A CRE and CDE sites. Activation of a receptor tyrosine kinase (RTK) by growth factor (GF) leads to the phosphorylation of CREB in early G1 phase, an effect that is permissive but not sufficient for induction of the cyclin A promoter. Receptor tyrosine kinases also cooperate with the organized actin cytoskeleton to sustain ERK activity and to regulate cyclin D1 expression, pocket protein phosphorylation, and occupancy of the CDE site. This effect is important for the timing of cyclin A promoter activity.
ACKNOWLEDGMENTS
M.E.B. and M.B. contributed equally to this study.
This work was supported by NIH grants GM48224 and CA72639 to R.K.A. M.E.B. was supported by a postdoctoral fellowship from the Department of the Army.
We thank Lou Laimins, Michael Weber, Joelle Sobczak, and Berthold Henglein for expression vectors.
REFERENCES
- 1.Andrews N C, Faller D V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assoian R K, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor-dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. doi: 10.1016/s0955-0674(97)80157-3. [DOI] [PubMed] [Google Scholar]
- 3.Barlat I, Henglein B, Plet A, Lamb N, Fernandez A, McKenzie F, Pouyssegur J, Vie A, Blanchard J M. TGF-β1 and cAMP attenuate cyclin A gene transcription via a cAMP responsive element through independent pathways. Oncogene. 1995;11:1309–1318. [PubMed] [Google Scholar]
- 4.Blanchard J M. Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem Pharmacol. 2000;60:1179–1184. doi: 10.1016/s0006-2952(00)00384-1. [DOI] [PubMed] [Google Scholar]
- 5.Bohmer R M, Scharf E, Assoian R K. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell. 1996;7:101–111. doi: 10.1091/mbc.7.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottazzi M E, Assoian R K. The extracellular matrix and mitogenic growth factors control G1-phase cyclins and cyclin-dependent kinase inhibitors. Trends Cell Biol. 1997;7:348–352. doi: 10.1016/S0962-8924(97)01114-8. [DOI] [PubMed] [Google Scholar]
- 7.Bottazzi M E, Zhu X, Bohmer R M, Assoian R K. Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J Cell Biol. 1999;146:1255–1264. doi: 10.1083/jcb.146.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 9.Chellappan S, Kraus V B, Kroger B, Munger K, Howley P M, Phelps W C, Nevins J R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeGregori J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. . (Erratum, 15:5846–5847.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desdouets C, Matesic G, Molina C A, Foulkes N S, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desdouets C, Ory C, Matesic G, Soussi T, Brechot C, Sobczak-Thepot J. ATF/CREB site-mediated transcriptional activation and p53-dependent repression of the cyclin A promoter. FEBS Lett. 1996;385:34–38. doi: 10.1016/0014-5793(96)00330-4. [DOI] [PubMed] [Google Scholar]
- 13.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 14.Hansen L K, Mooney D J, Vacanti J P, Ingber D E. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell. 1994;5:967–975. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbour J W, Luo R X, Dei Santi A, Postigo A A, Dean D C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 16.Henglein B, Chenivesse X, Wang J, Eick D, Brechot C. Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc Natl Acad Sci USA. 1994;91:5490–5494. doi: 10.1073/pnas.91.12.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, Chen C S, Ingber D E. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huet X, Rech J, Plet A, Vie A, Blanchard J M. Cyclin A expression is under negative transcriptional control during the cell cycle. Mol Cell Biol. 1996;16:3789–3798. doi: 10.1128/mcb.16.7.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer A, Carstens C P, Fahl W E. A novel CCAAT-binding protein necessary for adhesion-dependent cyclin A transcription at the G1/S boundary is sequestered by a retinoblastoma-like protein in G0. J Biol Chem. 1996;271:6579–6582. doi: 10.1074/jbc.271.12.6579. [DOI] [PubMed] [Google Scholar]
- 20.Kramer A, Carstens C P, Wasserman W W, Fahl W E. CBP/cycA, a CCAAT-binding protein necessary for adhesion-dependent cyclin A transcription, consists of NF-Y and a novel Mr 115,000 subunit. Cancer Res. 1997;57:5117–5121. [PubMed] [Google Scholar]
- 21.Liu N, Lucibello F C, Engeland K, Muller R. A new model of cell cycle-regulated transcription: repression of the cyclin A promoter by CDF-1 and anti-repression by E2F. Oncogene. 1998;16:2957–2963. doi: 10.1038/sj.onc.1201838. [DOI] [PubMed] [Google Scholar]
- 22.Liu N, Lucibello F C, Korner K, Wolfraim L A, Zwicker J, Muller R. CDF-1, a novel E2F-unrelated factor, interacts with cell cycle-regulated repressor elements in multiple promoters. Nucleic Acids Res. 1997;25:4915–4920. doi: 10.1093/nar/25.24.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo R X, Postigo A A, Dean D C. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 24.Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late-phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Okuyama S, Okamoto S, Nakajima T, Sekiya S, Oda K. Downregulation of the cyclin A promoter in differentiating human embryonal carcinoma cells is mediated by depletion of ATF-1 and ATF-2 in the complex at the ATF/CRE site. Exp Cell Res. 1995;216:422–430. doi: 10.1006/excr.1995.1053. [DOI] [PubMed] [Google Scholar]
- 26.Olson M F, Paterson H F, Marshall C J. Signals from Ras and Rho GTPases interact to regulate expression of p21.Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 27.Philips A, Chambeyron S, Lamb N, Vie A, Blanchard J M. CHF: a novel factor binding to cyclin A CHR corepressor element. Oncogene. 1999;18:6222–6232. doi: 10.1038/sj.onc.1203017. [DOI] [PubMed] [Google Scholar]
- 28.Philips A, Huet X, Plet A, Le Cam L, Vie A, Blanchard J M. The retinoblastoma protein is essential for cyclin A repression in quiescent cells. Oncogene. 1998;16:1373–1381. doi: 10.1038/sj.onc.1201655. [DOI] [PubMed] [Google Scholar]
- 29.Philips A, Huet X, Plet A, Rech J, Vie A, Blanchard J M. Anchorage-dependent expression of cyclin A in primary cells requires a negative DNA regulatory element and a functional Rb. Oncogene. 1999;18:1819–1825. doi: 10.1038/sj.onc.1202530. [DOI] [PubMed] [Google Scholar]
- 30.Resnitzky D, Hengst L, Reed S I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulze A, Mannhardt B, Zerfass-Thome K, Zwerschke W, Jansen-Durr P. Anchorage-independent transcription of the cyclin A gene induced by the E7 oncoprotein of human papillomavirus type 16. J Virol. 1998;72:2323–2334. doi: 10.1128/jvi.72.3.2323-2334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulze A, Zerfass K, Spitkovsky D, Middendorp S, Berges J, Helin K, Jansen-Durr P, Henglein B. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc Natl Acad Sci USA. 1995;92:11264–11268. doi: 10.1073/pnas.92.24.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulze A, Zerfass-Thome K, Berges J, Middendorp S, Jansen-Durr P, Henglein B. Anchorage-dependent transcription of the cyclin A gene. Mol Cell Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu M, Nomura Y, Suzuki H, Ichikawa E, Takeuchi A, Suzuki M, Nakamura T, Nakajima T, Oda K. Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp Cell Res. 1998;239:93–103. doi: 10.1006/excr.1997.3884. [DOI] [PubMed] [Google Scholar]
- 37.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 38.Sylvester A M, Chen D, Krasinski K, Andres V. Role of c-fos and E2F in the induction of cyclin A transcription and vascular smooth muscle cell proliferation. J Clin Investig. 1998;101:940–948. doi: 10.1172/JCI1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber J D, Raben D M, Phillips P J, Baldassare J J. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 41.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu G, Livingston D M, Krek W. Multiple members of the E2.F transcription factor family are the products of oncogenes. Proc Natl Acad Sci USA. 1995;92:1357–1361. doi: 10.1073/pnas.92.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshizumi M, Hsieh C M, Zhou F, Tsai J C, Patterson C, Perrella M A, Lee M E. The ATF site mediates downregulation of the cyclin A gene during contact inhibition in vascular endothelial cells. Mol Cell Biol. 1995;15:3266–3272. doi: 10.1128/mcb.15.6.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zerfass-Thome K, Schulze A, Zwerschke W, Vogt B, Helin K, Bartek J, Henglein B, Jansen-Durr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997;17:407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu X, Ohtsubo M, Bohmer R M, Roberts J M, Assoian R K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Scharf E, Assoian R K. Induction of anchorage-independent growth by transforming growth factor-beta linked to anchorage-independent expression of cyclin D1. J Biol Chem. 2000;275:6703–6706. doi: 10.1074/jbc.275.10.6703. [DOI] [PubMed] [Google Scholar]
- 47.Zwicker J, Lucibello F C, Wolfraim L A, Gross C, Truss M, Engeland K, Muller R. Cell cycle regulation of the cyclin A, cdc25C and cdc2 genes is based on a common mechanism of transcriptional repression. EMBO J. 1995;14:4514–4522. doi: 10.1002/j.1460-2075.1995.tb00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zwicker J, Muller R. Cell-cycle regulation of gene expression by transcriptional repression. Trends Genet. 1997;13:3–6. doi: 10.1016/s0168-9525(96)30112-1. [DOI] [PubMed] [Google Scholar]