Abstract
Agricultural crops infected with vector-borne pathogens can suffer severe negative consequences, but the extent to which phytopathogens affect the fitness of their vector hosts remains unclear. Evolutionary theory predicts that selection on vector-borne pathogens will favour low virulence or mutualistic phenotypes in the vector, traits facilitating effective transmission between plant hosts. Here, we use a multivariate meta-analytic approach on 115 effect sizes across 34 unique plant–vector–pathogen systems to quantify the overall effect of phytopathogens on vector host fitness. In support of theoretical models, we report that phytopathogens overall have a neutral fitness effect on vector hosts. However, the range of fitness outcomes is diverse and span the parasitism–mutualism continuum. We found no evidence that various transmission strategies, or direct effects and indirect (plant-mediated) effects, of phytopathogens have divergent fitness outcomes for the vector. Our finding emphasizes diversity in tripartite interactions and the necessity for pathosystem-specific approaches to vector control.
Keywords: vector-borne, phytopathogen, multi-trophic interactions, symbiosis continuum, vector–pathogen interactions, parasitism–mutualism continuum
1. Introduction
Many viral and bacterial pathogens that cause plant disease epidemics rely on herbivorous insect vectors for transmission [1,2]. Vector-borne pathogens should be selected to enhance their transmission to plant hosts, via direct effects in the vector host or indirectly by manipulating the host plant [3]. However, high virulence to the vector can negatively impact transmission as phytopathogens rely on the mobility of the vector for transmission and dispersal to non-motile plant hosts [4–6]. Consequently, evolutionary theory predicts that vector-borne agents will be relatively less virulent to the vector or even have beneficial phenotypes in the vector host [7–10]. Despite predictions, a wide range of fitness effects have been reported across vector species. Squash vein yellowing virus (SqVYV) improves the longevity and fecundity of its whitefly vector [11], whereas Watermelon bud necrosis virus (WBNV) reduces these fitness-associated parameters in its vector Thrips palmi [12]. Contradictory results have also been reported within the same taxa: Candidatus Liberibacter asiaticus positively affects the citrus psyllid's fecundity [13,14], but negatively affects survival and longevity [14,15], underscoring the complexity of vector–pathogen interactions. The extent to which phytopathogens affect the fitness of vector hosts remains unquantified (figure 1a), despite the importance for the ecology and evolution of these pathosystems (a plant–pathogen–vector association) [3].
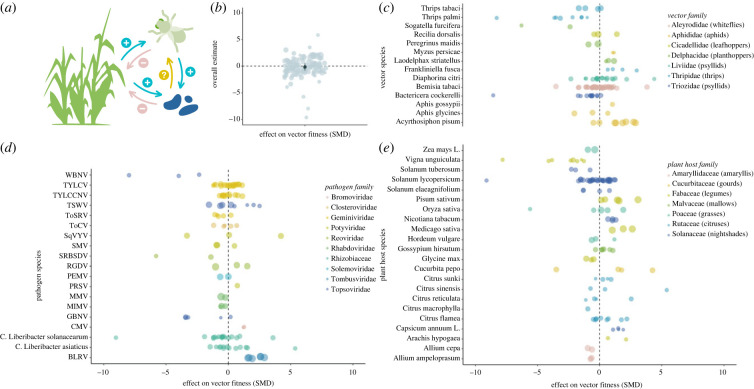
Figure 1.
Crops infected with vector-borne phytopathogens suffer negative consequences, but the extent to which phytopathogens affect the fitness of their vector hosts is unclear (a). Vector–phytopathogen interactions are diverse and range from parasitic to mutualistic (SMD = −0.1354, 95% CI: −0.8088–0.5381) (b). Mean effect and 95% confidence intervals are plotted in black. Effect sizes reported according to vector species (c), pathogen species (d) and plant host species (e). Individual effect sizes are jittered and coloured by family. Point size represents study sample size.
The majority of attention has focused on the nature of pathogen tropism, retention and replication in the vector (referred to as transmission mode) [3]. Non-circulative pathogens are restricted to the vector's stylet or foregut for short periods. Circulative viruses, and all bacteria, enter the haemocoel and render the vector infectious for longer periods [16], with some also propagating within the vector [17]. Consequently, it has been suggested that non-circulative, non-persistent pathogens will predominantly have indirect effects on vector fitness, for example by affecting the volatile profile of infected plants [18] or altering plant defence against herbivory [19]. By contrast, circulative, propagative viruses and bacteria are more likely to affect vector fitness directly, for example by hijacking the vector's cellular machinery, in addition to plant-mediated indirect effects. The outcome of vector–pathogen interactions may be affected by additional factors such as pathogen and vector taxonomy [20,21], differences in study methods [22], and whether vertical transmission occurs in the vector (see electronic supplementary material, table S1 for hypotheses).
Previous vote-counting studies highlight a positive effect of phytopathogens on their vectors [3,23,24]; these have focused either exclusively on viruses or included effects on vector behaviour. Here, we use a meta-analytic approach to test the hypothesis that vector-borne bacterial and viral phytopathogens benefit their insect vectors. We include only fitness-associated metrics to assess the scope for mutualism in these interactions. Further analysis tests for ecological drivers of variation in vector–pathogen interactions. Our synthesis of 34 pathosystems suggests the mean effect of infection on vector fitness is neutral, but the range of outcomes is considerable and spans the parasitism–mutualism continuum. As fitness costs affect the abundance and population dynamics of insect vectors, an improved understanding of vector–pathogen interactions will be critical for preventing plant disease epidemics.
2. Methods
(a) . Literature review
We searched Web of Science for relevant studies and retrieved 888 papers published up to July 2021 (electronic supplementary material, information and figure S3). Studies on plant pathosystems were included when each experiment had a treatment and control group with independent samples; an infection challenge, where greater than or equal to 70% of individuals were infected; a standardized way to quantify fitness; and greater than or equal to five observations. Studies that evaluated the effects of temperature, co-infections, or starvation, and studies testing the effect of infection on host preference and feeding behaviours, were excluded. Overall, 26 studies were included covering 34 unique pathosystems.
(b) . Calculation of effect sizes
Of the 115 effect sizes extracted, 85 were in count form and 30 were dichotomous. To combine the datasets, dichotomous data were first expressed as odds ratios and accompanying s.e. as described in [25], then converted into standardized mean differences (SMDs) [26]. Where count data were provided, the SMD was calculated as the difference in mean outcome between the infected and control groups, divided by the standard deviation of the outcome among participants. Where mean outcomes were not reported, data were obtained from plots by extracting data points using webplotdigitiser [27]. Missing s.e. and sample sizes were extrapolated using methods described in [28].
(c) . Meta-analytical model
Most studies reported multiple effect sizes. To account for any dependencies within studies, multi-level models were fitted using restricted maximum-likelihood estimation using the metafor package (v3.0-2) [29] in R v. 4.0.5 [30,31]. Nesting the effects reported within the study ID allowed for differentiation of the effect sizes due to sampling variation within and between studies [32]. Pathogen species, host species and vector species were also included as random effects, allowing multiple representations of the same species to be accounted for [33]. Taxonomic subgroup analysis was performed by calculating mean effect sizes for each pathogen genus and vector genus. The contribution of ecological and methodological predictors to the overall effect was then assessed using univariate models (electronic supplementary material, table S1). Omnibus tests were used to assess differences in mean effect size between groups, and likelihood ratio tests using maximum-likelihood estimation were used to assess the significance of each predictor.
(d) . Assessing bias
Publication bias was visually assessed using funnel plots (electronic supplementary material, figure S4). The weighted Rosenberg method [34] was used to calculate a fail-safe number, which estimates the number of studies averaging a null result that would have to be added to the dataset to reduce the significance level to a target alpha level (e.g. 0.05). The fail-safe number was 581, which is not greater than the threshold considered for a robust analysis (nfs > 5 N + 10 where N = no. effect sizes). These results suggest that the dataset could be affected by bias towards positive results. However, as noted by others [23], negative results would be biologically interesting in this case and less likely to remain unpublished.
3. Results
All 34 pathosystems included domesticated crop hosts and insect vectors, 29% of the vector-borne agents were bacteria and 71% were viruses (table 1).
Table 1.
Summary of pathosystems included.
| plant host | vector | pathogen | fitness effectsa | effectb | references |
|---|---|---|---|---|---|
| Viral pathosystems | |||||
| Allium ampeloprasum (wild leek) | Thrips tabaci (onion thrips) | tomato spotted wilt virus (TSWV) | adult longevity, fecundity | both | [35] |
| Allium cepa (onion) | Thrips tabaci | tomato spotted wilt virus (TSWV) | adult longevity, survival | both | [35] |
| Arachis hypogaea (groundnut) | Frankliniella fusca (tobacco thrips) | tomato spotted wilt virus (TSWV) | fecundity | likely both | [36] |
| Capsicum annuum L. (red pepper) | Frankliniella fusca (tobacco thrips) | cucumber mosaic virus (CMV) | fecundity | indirect | [37] |
| Capsicum annuum L. | Frankliniella fusca | tomato spotted wilt virus (TSWV) | fecundity | likely both | [37] |
| Capsicum annuum L. | Myzus persicae (green peach aphid) | cucumber mosaic virus (CMV) | fecundity | indirect | [37] |
| Capsicum annuum L. | Myzus persicae | tomato spotted wilt virus (TSWV) | fecundity | likely both | [37] |
| Cucurbita pepo (pumpkin) | Aphis gossypii (melon aphid) | papaya ring spot virus (PRSV) | fecundity | indirect | [38] |
| Cucurbita pepo | Bemisia tabaci (whitefly) | squash vein yellowing virus (SqVYV) | adult longevity, fecundity | indirect | [11] |
| Glycine max (soya bean) | Aphis glycines (soya bean aphid) | soya bean mosaic virus (SMV) | fecundity | indirect | [39,40] |
| Gossypium hirsutum (cotton) | Bemisia tabaci (whitefly) | tomato yellow leaf curl China virus (TYLCCNV) | fecundity | direct | [41] |
| Gossypium hirsutum | Bemisia tabaci | tomato yellow leaf curl virus (TYLCV) | adult longevity, fecundity | likely both | [42] |
| Hordeum vulgare (barley) | Laodelphax striatellus (small brown planthopper) | maize Iranian mosaic virus (MIMV) | adult longevity, fecundity | direct | [43] |
| Medicago sativa (alfalfa) | Acyrthosiphon pisum (pea aphid) | bean leafroll virus (BLRV) | survival | likely both | [44] |
| Nicotiana tabacum (tobacco) | Bemisia tabaci (whitefly) | tomato yellow leaf curl China virus (TYLCCNV) | adult longevity, fecundity | likely both | [41] |
| Oryza sativa (rice) | Recilia dorsalis (zigzag leafhopper) | rice gall dwarf virus (RGDV) | adult longevity, fecundity, survival | direct | [45] |
| Oryza sativa (rice) | Sogatella furcifera (white-backed planthopper) | southern rice black-streaked dwarf virus (SRBSDV) | fecundity, fertility | direct | [46] |
| Pisum sativum (pea plant) | Acyrthosiphon pisum (pea aphid) | bean leafroll virus | survival | both | [44] |
| Pisum sativum | Acyrthosiphon pisum | pea enation mosaic virus (PEMV) | fecundity, survival | likely both | [47] |
| Solanum lycopersicum (tomato) | Bemisia tabaci (whitefly) | tomato chlorosis virus (ToCV) | adult longevity, fecundity, fertility, survival | indirect | [48] |
| Solanum lycopersicum | Bemisia tabaci | tomato severe rugose virus (ToSRV) | adult longevity, fecundity, fertility, survival | indirect | [48] |
| Solanum lycopersicum | Bemisia tabaci | tomato yellow leaf curl China virus (TYLCCNV) | adult longevity, fecundity, survival | likely both | [49] |
| Solanum lycopersicum | Bemisia tabaci | tomato yellow leaf curl virus (TYLCV) | adult longevity, fecundity, survival | likely both | [42,49] |
| Vigna unguiculata (cowpea) | Thrips palmi (melon thrips) | groundnut bud necrosis virus (GBNV) | adult longevity, fecundity | direct, likely both | [50] |
| Vigna unguiculata | Thrips palmi | WBNV | adult longevity, fecundity | likely both | [12] |
| Zea mays L. (corn) | Peregrinus maidis (corn planthopper) | maize mosaic virus (MMV) | adult longevity, fecundity | both | [51] |
| Bacterial pathosystems | |||||
| Citrus flamea (shatangju) | Diaphorina citri (Asian citrus psyllid) | Candidatus Liberibacter asiaticus | adult longevity, fecundity, survival | both | [14] |
| Citrus macrophylla (alemow) | Diaphorina citri | Candidatus Liberibacter asiaticus | adult longevity | likely both | [15] |
| Citrus reticulata (tangerine) | Diaphorina citri | Candidatus Liberibacter solanacearum | adult longevity, fecundity, fertility, survival | likely both | [52] |
| Citrus sinensis (sweet orange) | Diaphorina citri | Candidatus Liberibacter asiaticus | fecundity, fertility, survival | both | [13] |
| Citrus sunki (sour mandarin) | Diaphorina citri | Candidatus Liberibacter solanacearum | adult longevity, fecundity, fertility, survival | likely both | [52] |
| Solanum elaeagnifolium (silverleaf nightshade) | Bactericera cockerelli (potato psyllid) | Candidatus Liberibacter solanacearum | adult longevity, fecundity, survival | direct | [53] |
| Solanum lycopersicum (tomato) | Bactericera cockerelli | Candidatus Liberibacter solanacearum | fecundity, fertility | direct | [54,55] |
| Solanum tuberosum (potato) | Bactericera cockerelli | Candidatus Liberibacter solanacearum | adult longevity, fecundity | direct | [53] |
aMeasure/proxy of vector fitness.
bWhether the study tested direct effects of phytopathogen association with vector (Direct); indirect effects driven by altered plant host state (Indirect); a combination of direct and indirect effects when both vector and plant were confirmed infected (Both); or a likely combination of the two in cases where the plant was infected but vector may have gained infection (but not confirmed) from feeding (Likely both). See electronic supplementary material, figure S2 for detail.
(a) . Neutral effect of infection on vector host
The mean effect of phytopathogen infection on vector hosts was neutral (figure 1b), but highly variable (full model: SMD = −0.1354, s.e. = 0.3436, 95% CI: −0.8088, 0.5381, p = 0.6936) with interactions spanning from parasitic to mutualistic. Heterogeneity across studies was high (Q(d.f. = 114) = 1090.6284, p < 0.0001).
(b) . Subgroup analysis according to phylogeny
Mean effects were calculated for taxonomic subgroups at the family level and most showed neutral effects overall. However, associations in the vector family Triozidae (jumping plant lice) were associated with a negative impact on vector fitness, (SMD = −1.2066, s.e. = 0.5329, 95% CI = −2.2511, −0.1621, p = 0.0236) and in the pathogen family Tombusviridae (single-stranded RNA viruses) a positive effect was observed (SMD = 2.0543, s.e. = 0.2404, 95% CI = 1.5831, 2.5255, p = <0.0001).
(c) . Biological predictors
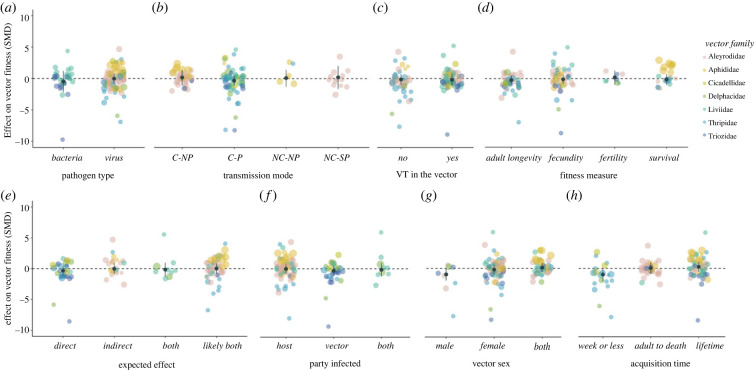
We tested multiple ecological factors to assess if they accounted for variation in the effect of infection on vector fitness (figure 2). Fitness effects did not vary between bacterial and viral pathogens (QM(d.f. = 1) = 0.2041, p = 0.6514), and transmission mode did not significantly affect the fitness of the vector host (QM(d.f. = 3) = 0.7933, p = 0.8511). Estimates for vector fitness were not impacted by pathogens that are also transmitted vertically in the vector (horizontal/mixed-mode transmission, QM(d.f. = 1) = 0.0090, p = 0.9244). We did not detect significant differences between the measures used to estimate fitness (e.g. longevity, fecundity, fertility and survival (QM(d.f. = 3) = 1.0689, p = 0.7846).
Figure 2.
Variation in the effect of infection on vector fitness was not significantly affected by pathogen type (a), transmission mode (circulative non-propagative; circulative-propagative; non-circulative, non-persistent; non-circulative, semi-persistent) (b), vertical transmission in vector (c), fitness measure (d), direct or indirect effects (e), vector sex (f), party infected (g) or acquisition time (h). Individual effect sizes are jittered and coloured by vector family. Point size represents study sample size. Mean effect sizes and 95% confidence intervals are plotted in black.
Pathosystems were classified by whether the infections were expected to directly affect fitness as they infect the vector host (Direct effects); indirectly affect fitness by altering plant host defences to herbivory and/or facilitating predation (Indirect effects); a certain combination of direct and indirect effects (Both) or a likely combination of the two (Likely both). For classification criteria see electronic supplementary material, figure S2. We were unable to detect significant differences in the fitness outcomes conferred by infections across these categories (QM(d.f. = 3) = 0.6531, p = 0.8842).
Overall, sex was not a significant driver of variation in vector–phytopathogen outcomes (QM(d.f. = 2) = 5.5433, p = 0.0626). However, infected males were generally more negatively affected by infection (SMD = −0.9706, 95% CI = −1.9570, 0.0159) than females (SMD = −0.2159, 95% CI = −0.9220, 0.4902) or mixed groups (SMD = 0.1208, 95% CI = −0.6425, 0.8842).
(d) . Methodological predictors
We found limited evidence that methodological factors influenced the effect of infection on vector host fitness. The method of pathogen inoculation to the vector host did not significantly affect fitness outcomes (QM(d.f. = 2) = 0.2068, p = 0.9018), and for studies that inoculated the plant host no effect of inoculation method was detected (QM(d.f. = 3) = 2.2931, p = 0.5138). The parties experimentally infected during the study also did not have a significant impact on the infection outcome (QM(d.f. = 2) = 0.6016, p = 0.7402). However, vector fitness was more negatively affected in studies where both plant and vector hosts, or only the vector host was infected (SMD = −0.1619, 95% CI = −1.276, 0.9522; SMD = −0.3198, 95% CI = −1.1064, 0.4668), whereas vectors showed neutral fitness effects in studies where only the plant host was infected (SMD = −0.0189, 95% CI = −0.7388, 0.7011).
Finally, the amount of time allotted for the vector to acquire the pathogen (acquisition time) did not significantly affect the outcome of infection (QM(d.f. = 2) = 3.7372, p = 0.1543). That said, vectors that had shorter acquisition periods of a week or less generally had reduced fitness (SMD = −1.0049, 95% CI = −2.1278, 0.118) but those with longer acquisition periods (adult to death/lifetime) showed neutral fitness effects (SMD = 0.0542, 95% CI = −0.8832, 0.9917; SMD = 0.2564, 95% CI = −0.5433, 1.0561).
4. Discussion
Evolutionary theory suggests that vector-borne pathogens will be selected for low virulence or even beneficial phenotypes in the vector host, as mobility of this host is crucial for transmission [5,6,56]. Across 34 pathosystems, we show the average effect of infection on vector fitness is neutral, in line with theoretical models [7–10]. However, our analysis shows vector–pathogen interactions span the symbiosis continuum, like many other host–microbe interactions [57], with infections ranging from beneficial to highly detrimental for vectors.
Phylogeny is frequently an important predictor of host–microbe interactions [20,21]. Our analysis revealed some differences in infection outcomes among pathogen and vector families; however, the number of species represented was limited. Sex-specific differences also drive variation in host–parasite interactions [58], and in our study male vectors appeared to suffer more from associations. The negative trend for males may be influenced by differences in immunocompetence [59]; alternatively, less harm may be favoured in females who are generally the dominant transmitters [60].
For the systems studied herein, circulative pathogens had similar fitness effects on vectors to those that do not circulate within the host. Similarly, we found no evidence that direct effects of infection differ significantly from indirect effects. These findings, contrary to our initial hypothesis, come with two caveats: the number of studies remains relatively small, and vectors typically acquire pathogens by feeding on infected plants, making it challenging to disentangle the direct and indirect effects of the pathogen on the insect vector. Some studies limited the contribution of indirect effects by infecting vectors in vitro [14,41,43,45,46,50,53–55] or transferring them regularly onto unexposed plants [50]. For most other studies, whether effects were direct, indirect, likely both, or certainly both were inferred based on pathogen transmission mode and the experimentally infected party (electronic supplementary material, figure S2). More studies are needed that distinguish the direct and indirect effects of plant pathogens on vectors, and establish whether mutualistic phenotypes evolve more readily within a given effect class.
Methodological differences frequently drive variation in study outcomes. For example, plant hosts inoculated mechanically versus vector inoculated will not induce processes such as herbivore effector-triggered immunity or other changes to plant chemical composition [61–63]. However, inoculation method explained little of the variation in vector fitness, suggesting methods may be relatively comparable. Notably, negative fitness outcomes were slightly more prevalent if the vector was the only party infected in the experiment. This suggests that beneficial phenotypes in the vector may depend upon the interaction of both direct and plant-mediated (indirect) effects of a pathogen. To disentangle the contribution of direct and indirect effects, studies must infect each party individually and in combination.
Domesticated crop hosts that are closely related, and grown in dense, low diversity, cultures are overrepresented here. These characteristics likely shape the interaction between vector and phytopathogen. Non-domesticated pathosystems differ greatly in these characteristics [64], and would be a valuable point of comparison for the evolution of tripartite interactions. Such systems are underrepresented in the literature. Given the controlled conditions of experiments, it is also likely that context-dependent cost/benefits for vector hosts are unaccounted for here.
Our study formally quantifies the effect of agriculturally important phytopathogens on insect vectors. We report an overall neutral fitness effect, but one that is underpinned by considerable diversity in both parasitic and mutualistic phenotypes. This finding highlights the value of vector–phytopathogen systems for exploring the evolution of tripartite symbiotic interactions and emphasizes the necessity for pathosystem-specific approaches to vector control.
Acknowledgements
We thank Kim Hoang and Jingdi Li for sharing code, those authors who provided access to their data, and three anonymous reviewers for their comments.
Data accessibility
All data, code and supplementary files associated with the manuscript are publicly available at: https://doi.org/10.6084/m9.figshare.c.6215267.v2 [65].
Authors' contributions
M.F.M.S.: data curation, formal analysis, investigation, methodology, visualization, writing—original draft; K.C.K.: conceptualization, funding acquisition, supervision and writing—review and editing; G.C.D.: conceptualization, supervision, validation, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
K.C.K. is funded by a European Research Council Starter Grant (COEVOPRO 802242) and Natural Environment Research Council Standard Grant (NE/X000540/1). M.F.M.S. is funded by a Jardine Scholarship.
References
- 1.Whitfield AE, Falk BW, Rotenberg D. 2015. Insect vector-mediated transmission of plant viruses. Virology 479–480, 278-289. ( 10.1016/j.virol.2015.03.026) [DOI] [PubMed] [Google Scholar]
- 2.Perilla-Henao L, Casteel C. 2016. Vector-borne bacterial plant pathogens: interactions with hemipteran insects and plants. Front Plant Sci. 7, 1163. ( 10.3389/fpls.2016.01163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eigenbrode SD, Bosque-Pérez NA, Davis TS. 2018. Insect-borne plant pathogens and their vectors: ecology, evolution, and complex interactions. Annu. Rev. Entomol. 63, 169-191. ( 10.1146/annurev-ento-020117-043119) [DOI] [PubMed] [Google Scholar]
- 4.Cunniffe NJ, Taylor NP, Hamelin FM, Jeger MJ. 2021. Epidemiological and ecological consequences of virus manipulation of host and vector in plant virus transmission. PLoS Comput. Biol. 17, e1009759. ( 10.1371/journal.pcbi.1009759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlovskis Z, Canale MC, Thole V, Pecher P, Lopes JR, Hogenhout SA. 2015. Insect-borne plant pathogenic bacteria: getting a ride goes beyond physical contact. Curr. Opin. Insect Sci. 9, 16-23. ( 10.1016/j.cois.2015.04.007) [DOI] [PubMed] [Google Scholar]
- 6.Allen LJS, Bokil VA, Cunniffe NJ, Hamelin FM, Hilker FM, Jeger MJ. 2019. Modelling vector transmission and epidemiology of co-infecting plant viruses. Viruses 11, E1153. ( 10.3390/v11121153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliot SL, Adler FR, Sabelis MW. 2003. How virulent should a parasite be to its vector? Ecology 84, 2568-2574. ( 10.1890/02-8013) [DOI] [Google Scholar]
- 8.Jeger MJ, Holt J, Van Den Bosch F, Madden LV. 2004. Epidemiology of insect-transmitted plant viruses: modelling disease dynamics and control interventions. Physiol. Entomol. 29, 291-304. ( 10.1111/j.0307-6962.2004.00394.x) [DOI] [Google Scholar]
- 9.Holt J, Jeger MJ, Thresh JM, Otim-Nape GW. 1997. A model of plant virus disease dynamics incorporating vector population processes: its application to the control of African cassava mosaic disease in Uganda. J. Appl. Ecol. 34, 793-806. ( 10.2307/2404924) [DOI] [Google Scholar]
- 10.Madden L, Jeger M, Bosch F. 2000. A theoretical assessment of the effects of vector-virus transmission mechanism on plant virus disease epidemics. Phytopathology 90, 576-594. ( 10.1094/PHYTO.2000.90.6.576) [DOI] [PubMed] [Google Scholar]
- 11.Shrestha D, McAuslane HJ, Adkins ST, Smith HA, Dufault N, Colee J, Webb SE. 2017. Host-mediated effects of semipersistently transmitted Squash vein yellowing virus on sweetpotato whitefly (Hemiptera: Aleyrodidae) behavior and fitness. J. Econ. Entomol. 110, 1433-1441. ( 10.1093/jee/tox161) [DOI] [PubMed] [Google Scholar]
- 12.Ghosh A, Basavaraj YB, Jangra S, Das A. 2019. Exposure to watermelon bud necrosis virus and groundnut bud necrosis virus alters the life history traits of their vector, Thrips palmi (Thysanoptera: Thripidae). Arch. Virol. 164, 2799-2804. ( 10.1007/s00705-019-04381-z) [DOI] [PubMed] [Google Scholar]
- 13.Pelz-Stelinski KS, Killiny N. 2016. Better together: association with ‘Candidatus Liberibacter asiaticus' increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Ann. Entomol. Soc. Am. 109, 371-376. ( 10.1093/aesa/saw007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren SL, Li YH, Zhou YT, Xu WM, Cuthbertson AGS, Guo YJ, Qiu BL. 2016. Effects of Candidatus Liberibacter asiaticus on the fitness of the vector Diaphorina citri. J. Appl. Microbiol. 121, 1718-1726. ( 10.1111/jam.13302) [DOI] [PubMed] [Google Scholar]
- 15.Nehela Y, Killiny N. 2018. Infection with phytopathogenic bacterium inhibits melatonin biosynthesis, decreases longevity of its vector, and suppresses the free radical-defense. J. Pineal Res. 65, e12511. ( 10.1111/jpi.12511) [DOI] [PubMed] [Google Scholar]
- 16.Ng JCK, Perry KL. 2004. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 5, 505-511. ( 10.1111/j.1364-3703.2004.00240.x) [DOI] [PubMed] [Google Scholar]
- 17.Nault LR. 1997. Arthropod transmission of plant viruses: a new synthesis. Ann. Entomol. Soc. Am. 90, 521-541. ( 10.1093/aesa/90.5.521) [DOI] [Google Scholar]
- 18.Eigenbrode SD, Ding H, Shiel P, Berger PH. 2002. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. B 269, 455-460. ( 10.1098/rspb.2001.1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luan JB, Yao DM, Zhang T, Walling LL, Yang M, Wang YJ, Liu SS. 2013. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 16, 390-398. ( 10.1111/ele.12055) [DOI] [PubMed] [Google Scholar]
- 20.Longdon B, Hadfield JD, Webster CL, Obbard DJ, Jiggins FM. 2011. Host phylogeny determines viral persistence and replication in novel hosts. PLoS Pathog. 7, e1002260. ( 10.1371/journal.ppat.1002260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelstädter J, Hurst G. 2006. The dynamics of parasite incidence across host species. Evol. Ecol. 20, 603-616. ( 10.1007/s10682-006-9120-1) [DOI] [Google Scholar]
- 22.Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. 2005. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70-79. ( 10.1111/j.1461-0248.2004.00699.x) [DOI] [Google Scholar]
- 23.Mauck K, Bosque-Pérez NA, Eigenbrode SD, De Moraes CM, Mescher MC. 2012. Transmission mechanisms shape pathogen effects on host–vector interactions: evidence from plant viruses. Funct. Ecol. 26, 1162-1175. ( 10.1111/j.1365-2435.2012.02026.x) [DOI] [Google Scholar]
- 24.Ray S, Casteel CL. 2022. Effector-mediated plant–virus–vector interactions. Plant Cell. 34, 1514-1531. ( 10.1093/plcell/koac058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks JJ, Higgins JP, Altman DJ. 2022. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane handbook for systematic reviews of interventions, version 6.3 (updated February 2022). London, UK: Cochrane. See https://training.cochrane.org/handbook/current/chapter-10 (accessed 5 September 2022). [Google Scholar]
- 26.Higgins JP, Li T, Deeks JJ. 2022. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane handbook for systematic reviews of interventions, version 6.3 (updated February 2022). London, UK: Cochrane. See https://training.cochrane.org/handbook/current/chapter-06 (accessed 5 September 2022). [Google Scholar]
- 27.Rohatgi A. 2021. WebPlotDigitizer: extract data from plots, images, and maps, version 4.5. See https://automeris.io/WebPlotDigitizer/ (accessed 5 September 2022).
- 28.Rosenberg MS, Rothstein HR, Gurevitch J. 2013. Effect sizes: conventional choices and calculations. In Handbook of meta-analysis in ecology and evolution (eds Koricheva J, Gurevitch J, Mengersen K), pp. 61-71. Princeton, NJ: Princeton University Press. [Google Scholar]
- 29.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1-48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 30.R Core Team. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.r-project.org/ (accessed 5 September 2022). [Google Scholar]
- 31.RStudio Team. 2022. RStudio: integrated development for R. Boston, MA: RStudio, PBC. See https://www.rstudio.com/ (accessed 5 September 2022). [Google Scholar]
- 32.Mengersen K, Gurevitch J, Schmid CH. 2013. 18 Meta-analysis of primary data, pp. 300-312. Princeton, NJ: Princeton University Press. See https://www.degruyter.com/document/doi/10.1515/9781400846184-020/html (accessed 5 September 2022). [Google Scholar]
- 33.Fernández-Castilla B, Maes M, Declercq L, Jamshidi L, Beretvas SN, Onghena P, Van den Noortgate W. 2019. A demonstration and evaluation of the use of cross-classified random-effects models for meta-analysis. Behav. Res. Methods 51, 1286-1304. ( 10.3758/s13428-018-1063-2) [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg MS. 2005. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59, 464-468. [PubMed] [Google Scholar]
- 35.Inoue T, Sakurai T. 2006. Infection of tomato spotted wilt virus (TSWV) shortens the life span of thelytokous Thrips tabaci (Thysanoptera: Thripidae). Appl. Entomol. Zool. 41, 239-246. ( 10.1303/aez.2006.239) [DOI] [Google Scholar]
- 36.Shrestha A, Srinivasan R, Riley DG, Culbreath AK. 2012. Direct and indirect effects of a thrips-transmitted Tospovirus on the preference and fitness of its vector, Frankliniella fusca. Entomol. Exp. Appl. 145, 260-271. ( 10.1111/eea.12011) [DOI] [Google Scholar]
- 37.Gautam S, Mugerwa H, Sundaraj S, Gadhave KR, Murphy JF, Dutta B, Srinivasan R. 2020. Specific and spillover effects on vectors following infection of two RNA viruses in pepper plants. Insects 11, E602. ( 10.3390/insects11090602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadhave KR, Dutta B, Coolong T, Srinivasan R. 2019. A non-persistent aphid-transmitted Potyvirus differentially alters the vector and non-vector biology through host plant quality manipulation. Sci. Rep. 9, 2503. ( 10.1038/s41598-019-39256-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Liu X, Liu X, Michaud JP, Zhi H, Li K, Li X, Li Z. 2018. Host plant infection by soybean mosaic virus reduces the fitness of its vector, Aphis glycines (Hemiptera: Aphididae). J. Econ. Entomol. 111, 2017-2023. ( 10.1093/jee/toy165) [DOI] [PubMed] [Google Scholar]
- 40.Cassone BJ, Michel AP, Stewart LR, Bansal R, Mian MAR, Redinbaugh MG. 2014. Reduction in fecundity and shifts in cellular processes by a native virus on an invasive insect. Genome Biol. Evol. 6, 873-885. ( 10.1093/gbe/evu057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Li M, Li JM, Huang CJ, Zhou XP, Xu FC, Liu SS. 2010. Viral infection of tobacco plants improves performance of Bemisia tabaci but more so for an invasive than for an indigenous biotype of the whitefly. J. Zhejiang Univ. Sci. B 11, 30-40. ( 10.1631/jzus.B0900213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Liu J, Liu SS. 2011. Tomato yellow leaf curl virus infection of tomato does not affect the performance of the Q and ZHJ2 biotypes of the viral vector Bemisia tabaci. Insect Sci. 18, 40-49. ( 10.1111/j.1744-7917.2010.01354.x) [DOI] [Google Scholar]
- 43.Moeini P, Afsharifar A, Izadpanah K, Sadeghi SE, Eigenbrode SD. 2020. Maize Iranian mosaic virus (family Rhabdoviridae) improves biological traits of its vector Laodelphax striatellus. Arch. Virol. 165, 169-178. ( 10.1007/s00705-019-04450-3) [DOI] [PubMed] [Google Scholar]
- 44.Davis TS, Wu Y, Eigenbrode SD. 2017. The effects of bean leafroll virus on life history traits and host selection behavior of specialized pea aphid (Acyrthosiphon pisum, Hemiptera: Aphididae) genotypes. Environ. Entomol. 46, 68-74. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Lu C, Li M, Wu W, Zhou G, Wei T. 2016. Adverse effects of rice gall dwarf virus upon its insect vector Recilia dorsalis (Hemiptera: Cicadellidae). Plant Dis. 100, 784-790. ( 10.1094/PDIS-06-15-0713-RE) [DOI] [PubMed] [Google Scholar]
- 46.Xu H, He X, Zheng X, Yang Y, Tian J, Lu Z. 2014. Southern rice black-streaked dwarf virus (SRBSDV) directly affects the feeding and reproduction behavior of its vector, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Virol. J. 11, 55. ( 10.1186/1743-422X-11-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodge S, Powell G. 2010. Conditional facilitation of an aphid vector, Acyrthosiphon pisum, by the plant pathogen, pea enation mosaic virus. J. Insect Sci. Online. 10, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maluta N, Fereres A, Lopes JRS. 2019. Plant-mediated indirect effects of two viruses with different transmission modes on Bemisia tabaci feeding behavior and fitness. J. Pest Sci. 92, 405-416. ( 10.1007/s10340-018-1039-0) [DOI] [Google Scholar]
- 49.Liu J, Zhao H, Jiang K, Zhou XP, Liu SS. 2009. Differential indirect effects of two plant viruses on an invasive and an indigenous whitefly vector: implications for competitive displacement. Ann. Appl. Biol. 155, 439-448. ( 10.1111/j.1744-7348.2009.00366.x) [DOI] [Google Scholar]
- 50.Daimei G, Raina HS, Devi PP, Saurav GK, Renukadevi P, Malathi VG, Senthilraja C, Mandal B, Rajagopal R. 2017. Influence of groundnut bud necrosis virus on the life history traits and feeding preference of its vector, Thrips palmi. Phytopathology 107, 1440-1445. ( 10.1094/PHYTO-08-16-0296-R) [DOI] [PubMed] [Google Scholar]
- 51.Higashi CHV, Bressan A. 2013. Influence of a propagative plant virus on the fitness and wing dimorphism of infected and exposed insect vectors. Oecologia 172, 847-856. ( 10.1007/s00442-012-2540-4) [DOI] [PubMed] [Google Scholar]
- 52.Wu F, Qureshi JA, Huang J, Fox EGP, Deng X, Wan F, Liang G, Cen Y. 2018. Host plant-mediated interactions between ‘Candidatus Liberibacter asiaticus’ and its vector Diaphorina citri Kuwayama (Hemiptera: Liviidae). J. Econ. Entomol. 111, 2038-2045. [DOI] [PubMed] [Google Scholar]
- 53.Thinakaran J, Yang XB, Munyaneza JE, Rush CM, Henne DC. 2015. Comparative biology and life tables of ‘Candidatus Liberibacter solanacearum’-infected and -free Bactericera cockerelli (Hemiptera: Triozidae) on potato and silverleaf nightshade. Ann. Entomol. Soc. Am. 108, 459-467. ( 10.1093/aesa/sav030) [DOI] [Google Scholar]
- 54.Albuquerque Tomilhero Frias A, Ibanez F, Mendoza A, de Carvalho Nunes WM, Tamborindeguy C. 2020. Effects of ‘Candidatus Liberibacter solanacearum’ (haplotype B) on Bactericera cockerelli fitness and vitellogenesis. Insect Sci. 27, 58-68. ( 10.1111/1744-7917.12599) [DOI] [PubMed] [Google Scholar]
- 55.Nachappa P, Levy J, Pierson E, Tamborindeguy C. 2014. Correlation between ‘Candidatus Liberibacter solanacearum’ infection levels and fecundity in its psyllid vector. J. Invertebr. Pathol. 115, 55-61. ( 10.1016/j.jip.2013.10.008) [DOI] [PubMed] [Google Scholar]
- 56.Gutiérrez S, Michalakis Y, Van Munster M, Blanc S. 2013. Plant feeding by insect vectors can affect life cycle, population genetics and evolution of plant viruses. Funct. Ecol. 27, 610-622. ( 10.1111/1365-2435.12070) [DOI] [Google Scholar]
- 57.Drew GC, Stevens EJ, King KC. 2021. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 19, 623-638. ( 10.1038/s41579-021-00550-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26, 1009-1024. ( 10.1016/S0020-7519(96)80001-4) [DOI] [PubMed] [Google Scholar]
- 59.Retschnig G, Williams GR, Mehmann MM, Yañez O, de Miranda JR, Neumann P. 2014. Sex-specific differences in pathogen susceptibility in honey bees (Apis mellifera). PLoS ONE 9, e85261. ( 10.1371/journal.pone.0085261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kellen WR, Chapman HC, Clark TB, Lindegreen JE. 1965. Host–parasite relationships of some Thelohania from mosquitoes (Nosematidae: Microsporidia). J. Invertebr. Pathol. 7, 161-166. ( 10.1016/0022-2011(65)90030-3) [DOI] [PubMed] [Google Scholar]
- 61.Cui H, Tsuda K, Parker JE. 2015. Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol. 66, 487-511. ( 10.1146/annurev-arplant-050213-040012) [DOI] [PubMed] [Google Scholar]
- 62.Naalden D, van Kleeff PJM, Dangol S, Mastop M, Corkill R, Hogenhout SA, Kant MR, Schuurink RC. 2021. Spotlight on the roles of whitefly effectors in insect–plant interactions. Front. Plant Sci. 12, 661141. ( 10.3389/fpls.2021.661141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogenhout SA, Bos JIB. 2011. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 14, 422-428. ( 10.1016/j.pbi.2011.05.003) [DOI] [PubMed] [Google Scholar]
- 64.Stukenbrock EH, McDonald BA. 2008. The origins of plant pathogens in agro-ecosystems. Annu. Rev. Phytopathol. 46, 75-100. ( 10.1146/annurev.phyto.010708.154114) [DOI] [PubMed] [Google Scholar]
- 65.Santiago MFM, King KC, Drew GC. 2023. Interactions between insect vectors and plant pathogens–span the parasitism-mutualism continuum. Figshare. ( 10.6084/m9.figshare.c.6215267) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Santiago MFM, King KC, Drew GC. 2023. Interactions between insect vectors and plant pathogens–span the parasitism-mutualism continuum. Figshare. ( 10.6084/m9.figshare.c.6215267) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data, code and supplementary files associated with the manuscript are publicly available at: https://doi.org/10.6084/m9.figshare.c.6215267.v2 [65].