Abstract
Background
Diabetic nephropathy (DN) is a prevalent microvascular diabetic complication all over the world.
Objective
This study was designed to measure oxidative stress, systemic inflammation and kidney function response to exercise training in patients with type 2 diabetic (T2DM) nephropathy.
Material and Methods
Eighty obese T2DM patients (50 males and 30 females), their body mass index (BMI) mean was 33.85±3.43 Kg/m2 and the mean of diabetes chronicity was 12.53±2.64 year participated in the present study and enrolled two groups; group I: received aerobic exercise training and group II: received no training intervention.
Results
The mean values of creatinine, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and malondialdehyde (MDA) were significantly decreased, while the mean values of interleukin-10 (IL-10), glutathione peroxidase (GPx) and glutathione (GSH) were significantly increased in group (A) after the aerobic exercise training, however the results of the control group were not significant. In addition, there were significant differences between both groups at the end of the study (P<0.05).
Conclusion
There is evidence that aerobic exercise training modulated oxidative stress and inflammatory cytokines and improved renal function among patients with diabetic nephropathy.
Keywords: Aerobic Exercise, Diabetic Nephropathy, Inflammatory Cytokines, Oxidative Stress
Introduction
Diabetic nephropathy (DN) considered as the most serious diabetic complication; while renal replacement is required for the majority of subjects with chronic renal disease among patients with T2DM1,2, where poor glycemic control3 is related to abnormal oxidative stress and systemic inflammation that induce progressive diabetic renal lesion4,5. Hyperglycemia induces oxidative stress and inflammation6. In addition, poor glycemic control induces abnormal level of oxidative stress markers7. In the other hand, oxidative stress induce dysfunction of β-cell that lead to insulin resistance development, diabetes and its associated microvascular complications8,9, so that patients with T2DM are under oxidative stress because of prolonged exposure to hyperglycemia10.
Researches proved that hyperglycemia that induced systemic inflammation and oxidative stress which induce DN11,12. Hyperglycemia in diabetic patients leads to mitochondrial dysfunction, advanced glycation end processes and other factors, and generate the reactive free radicals, then triggers the DNA fragmentation that lead to cell death13. However, Navarro et al. found an increase in the gene expression for pro-inflammatory cytokine in patients with DN14. Several studies reported that there was a significant elevation in inflammatory cytokines in T2DM with DN and there is an association between their levels and the incidence & the course of renal lesion among diabetics15–17.
Hyperglycemia also causes oxidative stress, decreases the regeneration of glutathione (GSH) from oxidized GSH and reduces the availability of nicotinamide adenine dinucleotide phosphate18,19. However, several reports stated that there was reduced level of GSH in diabetes associated with systemic inflammation20–22. In addition, in β-cell dysfunction may be related to abnormal GSH level induce long-term complications of diabetes23. Moreover, low GSH is related to DNA oxidative damage in T2DM24. Many studies reported decline in the level of SOD in diabetic tissue and blood25,26. While, study performed by Lucchesi and colleagues to observe the oxidative balance of diabetic rats reported diminished activity of SOD and other antioxidative enzymes in the liver tissue27. In the other hand, several studies reported an increased MDA level in patients with T2DM28,29. In addition, Baynes and Ramesh et al. reported that lipid peroxidation in diabetes induced many secondary chronic complications including atherosclerosis and neural disorders30,31.
Physical activity has several health benefits and plays an important role in treatment of chronic disorders. However, regular physical activity improves glucose control, blood lipid profile, insulin sensitivity and endothelial function that help to prevent diabetic complications32. Moreover, physical activity may reduce the risk and progression for diabetic nephropathy33.
This study was designed to measure oxidative stress, systemic inflammation and kidney function response to exercise training in patients with type 2 diabetic nephropathy.
Materials and Methods
Subjects
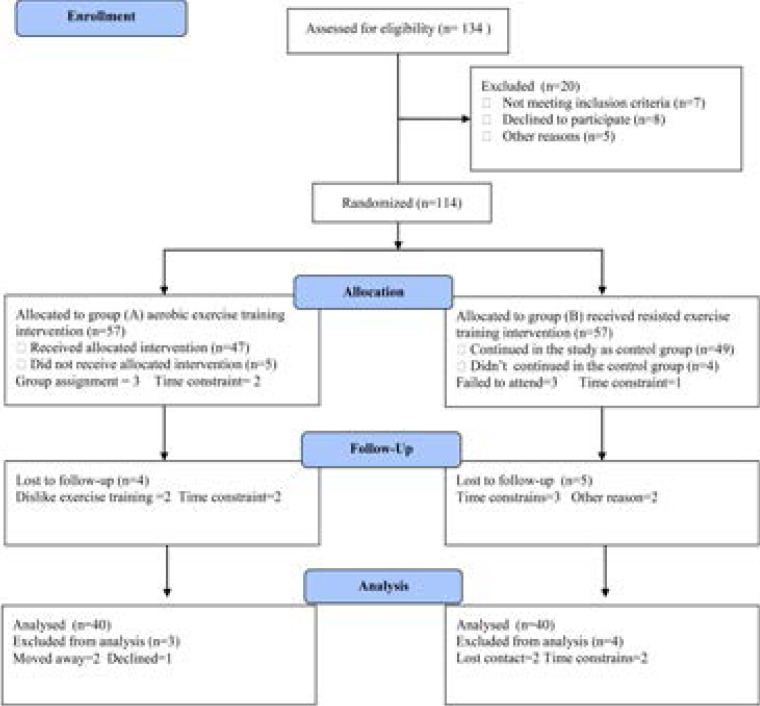
Eighty obese T2DM patients (50 males and 30 females), their body mass index (BMI) mean was 33.85±3.43 Kg/m2and the mean of diabetes chronicity was 12.53±2.64 year participated in the present study and enrolled two groups; group I: received aerobic exercise training and group II: received no training intervention. Exclusion criteria included smokers, kidney insufficiency, congestive heart failure, pregnant female patients, hepatitis and respiratory failure. Clinical evaluations and laboratory analysis were performed by independent assessors who were blinded to group assignment and not involved in the routine treatment of the patients. The CONSORT diagram outlining the details of the screening, run-in and randomization phases of the study and reasons for participant exclusion can be found in figure (1). Informed consent was obtained from all participants. This study was approved by the Scientific Research Ethical Committee, Faculty of Applied Medical Sciences at King University.
Figure 1.
Subjects screening and recruitment CONSORT diagram.
Measurements and procedures
A. Measurement of oxidative stress markers and anti-oxidant status
For all participants serum (from 10 ml blood in plain vial) and plasma (from 5 ml blood in EDTA vial) were separated from the sample within 30 min of collection and was stored in pyrogen free polypropylene cryo-tubes at (-80°C) until analysis. Assessment of lipid markers for peroxidation as malondialdehyde (MDA) was determined according to Buege and Aust34. However, Anti-oxidant status, glutathione (GSH) that was determined by the method of Beutler and colleagues35, in the other hand, glutathione peroxidase (GPx) was measured by the method of Nishikimi and colleagues36.
B. Measurement of inflammatory cytokines and serum creatinine
Blood samples were drained from the antecubital vein after a 12-hour fasting, the blood samples were centrifuged at + 4 °C (1000 = g for 10 min). Interleukin-6 (IL-6) and Interleukin-10 (IL-10) levels were analyzed by “Immulite 2000” immunassay analyzer (Siemens Healthcare Diagnostics, Deerfield, USA). However, tumor necrosis factor-alpha (TNF-α) was measured by ELISA kits (ELX 50) in addition to ELISA microplate reader (ELX 808; BioTek Instruments, USA). However, serum creatinine was measured with a kit obtained from Stanbio Laboratory (USA).
C. Aerobic exercise training program
Patients in group (A) were submitted to a 40 min aerobic session on a treadmill (the initial, 5-minute warm-up phase performed on the treadmill at a low load, each training session lasted 30 minutes and ended with 5-minute recovery and relaxation phase) either walking or running, based on heart rate, until the target heart rate was reached, according to American College of Sport Medicine guidelines. The program began with 10 min of stretching and was conducted using the maximal heart rate index (HRmax) estimated by: 220-age, with exercise intensity was 70–80% of HRmax34.
Statistical analysis
The mean values of the investigated parameters obtained before and after three months in both groups were compared using paired “t” test. Independent “t” test was used for the comparison between the two groups (P<0.05).
Results
Eighty obese patients with type 2 diabetes mellitus completed the screening evaluation. The baseline characteristics of the participants are shown in table (1). Most participants (60%) were men. Forty participants were assigned group (A) (n = 40; 24 males and 16 females) and group (B) (n =40, 26 males and 14 females). None of the baseline characteristics differed significantly between the two groups is listed in table (1).
Table (1).
Baseline characteristics of all participants
| Group (A) | Group (B) | Significance | |
| Age (year) | 48.34 ± 6.91 | 47.65 ± 7.28 | P > 0.05 |
| Gender (male/female) | 24/16 | 26/14 | P > 0.05 |
| BMI (kg/m2) | 34.15 ± 3.39 | 33.82 ± 3.47 | P > 0.05 |
| Duration of diabetes (year) | 13.12 ± 2.56 | 11.94 ± 2.72 | P > 0.05 |
| SBP (mmHg) | 148.53 ± 12.16 | 145.81 ± 13.44 | P > 0.05 |
| DBP (mmHg) | 92.62 ± 8.75 | 90.25 ± 7.28 | P > 0.05 |
| HBA1c (%) | 8.52 ± 2.43 | 8.37 ± 2.21 | P > 0.05 |
| Glucose (mmol/L) | 5.71 ±1.65 | 5.42 ± 1.48 | P > 0.05 |
| QUICKI | 0.149 ± 0.017 | 0.158 ± 0.016 | P > 0.05 |
| HOMA-IR | 5.13 ± 1.45 | 4.71 ± 1.32 | P > 0.05 |
BMI: Body Mass Index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HBA1c: glycosylated hemoglobin; QUICKI: The quantitative insulin-sensitivity check index; HOMA-IR: Homeostasis Modl Assessment-Insulin Resistance Index.
The mean values of creatinine, interleukin-6 (IL-6), tumor necrosis factor- alpha (TNF-α) and malondialdehyde (MDA) were significantly decreased, while the mean values of interleukin-10 (IL-10), glutathione peroxidase (GPx) and glutathione (GSH) were significantly increased in group (A) after the aerobic exercise training(table 2), however the results of the control group were not significant (table 3). In addition, there were significant differences between both groups at the end of the study (table 4).
Table 2.
Mean value and significance of creatinine, MDA, GSH, GPX, TNF-α, IL-6 and IL-10 in group (A) before and after treatment
| Mean + SD | t-value | Significance | ||
| Before | After | |||
| Creatinine (µmol/mol) | 86.41 ± 7.63 | 67.92 ± 5.15* | 9.24 | P <0.05 |
| MDA (nM/mL) | 0.32 ± 0.07 | 0.19 ± 0.06* | 5.12 | P <0.05 |
| GSH (nM/mL) | 3.54 ± 0.91 | 4.77 ± 1.23* | 6.48 | P <0.05 |
| GPX (UI/mL) | 2.73 ± 0.82 | 3.95 ± 1.15* | 6.26 | P <0.05 |
| TNF-α (pg/mL) | 5.36 ± 1.54 | 3.18 ± 1.32* | 6.17 | P <0.05 |
| IL-6 (pg/mL) | 2.85 ± 0.93 | 1.71 ± 0.67* | 5.32 | P <0.05 |
| IL-10 (pg/ml) | 6.24 ± 1.61 | 8.45 ± 1.73* | 7.12 | P <0.05 |
MDA: Malondialdehyde; GSH: Glutathione; GPx: Glutathione peroxidase; TNF-α: tumor necrosis factor – alpha; IL-6: Interleukin-6; IL-10: Interleukin-10
indicates a significant difference between the two groups, P < 0.05.
Table 3.
Mean value and significance of creatinine, MDA, GSH, GPX, TNF-α, IL-6 and IL-10 in group (B) before and at the end of the study
| Mean + SD | t-value | Significance | ||
| Before | After | |||
| Creatinine (µmol/mol) | 84.97 ± 6.85 | 87.26 ± 7.13 | 1.81 | P >0.05 |
| MDA (nM/mL) | 0.30 ± 0.08 | 0.33 ± 0.07 | 0.92 | P >0.05 |
| GSH (nM/mL) | 3.62 ± 0.87 | 3.56 ± 0.79 | 1.15 | P >0.05 |
| GPX (UI/mL) | 2.84 ± 0.93 | 2.67 ± 0.81 | 1.23 | P >0.05 |
| TNF-α (pg/mL) | 4.88 ± 1.37 | 5.25 ± 1.39 | 1.13 | P >0.05 |
| IL-6 (pg/mL) | 2.55 ± 0.72 | 2.86 ± 0.73 | 0.95 | P >0.05 |
| IL-10 (pg/ml) | 6.43 ± 1.54 | 6.12 ± 1.46 | 0.92 | P >0.05 |
MDA: Malondialdehyde; GSH: Glutathione; GPx: Glutathione peroxidase; TNF-α: tumor necrosis factor – alpha; IL-6: Interleukin-6; IL-10: Interleukin-10.
Table 4.
Mean value and significance of creatinine, MDA, GSH, GPX, TNF-α, IL-6 and IL-10 in group (A) and group (B) at the end of the study
| Mean + SD | t-value | Significance | ||
| Group (A) | Group (B) | |||
| Creatinine (µmol/mol) | 67.92 ± 5.15* | 87.26 ± 7.13 | 8.52 | P <0.05 |
| MDA (nM/mL) | 0.19 ± 0.06* | 0.33 ± 0.07 | 5.37 | P <0.05 |
| GSH (nM/mL) | 4.77 ± 1.23* | 3.56 ± 0.79 | 5.42 | P <0.05 |
| GPX (UI/mL) | 3.95 ± 1.15* | 2.67 ± 0.81 | 5.35 | P <0.05 |
| TNF-α (pg/mL) | 3.18 ± 1.32* | 5.25 ± 1.39 | 5.46 | P <0.05 |
| IL-6 (pg/mL) | 1.71 ± 0.67* | 2.86 ± 0.73 | 5.28 | P <0.05 |
| IL-10 (pg/ml) | 8.45 ± 1.73* | 6.12 ± 1.46 | 6.33 | P <0.05 |
MDA: Malondialdehyde; GSH: Glutathione; GPx: Glutathione peroxidase; TNF-α: tumor necrosis factor – alpha; IL-6: Interleukin-6; IL-10: Interleukin-10; (*) indicates a significant difference between the two groups, P < 0.05.
Discussion
Diabetic nephropathy (DN) is a worldwide prevalent medical problem affecting 20–40% of T2DM and characterized with high rate of morbidity and mortality as the DN is a principal etiology of renal failure35–37. Poor metabolic control, diabetes duration, race, heredity, life style, diet composition, aging, hypertension, systemic inflammation and oxidative stress are the common risk factors of DN38,39.
Our results demonstrate that aerobic exercise training reduced levels of TNF-α and IL-6, in addition to increased level of IL-10 that indicated reduced systemic inflammation. our results agreed with several studies have shown that aerobic exercise training promotes modulation of inflammatory cytokines40–42. Several large cohort studies have found a relationship between self-reported physical activity levels and systemic markers of inflammation: higher levels of physical activity are coupled to lower levels of circulating inflammatory markers in elderly individuals43–45. While, Nicklas et al. showed that regular aerobic exercise training was efficient in lowering IL-6 levels even without weight loss46. In addition, Santos and colleagues had twenty-two male, sedentary, healthy, elderly volunteers performed moderate aerobic exercise training for 60 min/day, 3 days/week for 24 week and concluded that 6 months of aerobic exercise training can improve sleep in the elderly via anti-inflammatory effect of aerobic training which modiies cytokine profiles (reduced IL-6 and TNF-α and increased IL-10)47. However, Kohut et al. reported that 10-months of aerobic, but not resistance exercise, significantly reduces serum inflammatory mediators in older adults48. Moreover, Bote et al. demonstrated that 8-months (2 sessions/week, 60-min/session) of aquatic-based exercise training tempered neutrophil activation (chemotaxis) and decreased systemic levels of IL-8 and noradrenalin compared to controls49. Similarly, Ploeger et al. reported that moderate aerobic exercise training has been recommended as an anti-inflammatory therapy50. The three possible mechanisms of exercise anti-inflammatory effects include reduction in visceral fat mass51; reduction in the circulating numbers of pro-inflammatory monocytes52 and an increase in the circulating numbers of regulatory T cells53. Moreover, Hong and colleagues show that cardiorespiratory fitness is associated with reduced low grade inflammation which may in part be mediated by enhancing the ability of immune cells to suppress inflammatory responses via adrenergic receptors54.
Concerning results of oxidative stress markers, results of our study agreed with other authors who reported that a six-months aerobic exercise was able to decrease lipid peroxidation, as well as to increase GSH and catalase activity in T2DM patients55,56. A similar study in obese individuals reported attenuation in exercise induced lipid peroxidation following 24 weeks of a moderate intensity resistance training57. In addition, Oliveira et al. compared the effects of 12 weeks training with three different types of exercise (aerobic training, strength training and combined training) on T2DM male and female human subjects, demonstrating that the aerobic exercise may help in minimizing oxidative stress and the development of the chronic complications of diabetes58. However, Vinetti et al. randomly assigned twenty male subjects with T2DM to an intervention group in a supervised exercise training (SET) consisted of a 12-month supervised aerobic, resistance and flexibility training. They concluded that SET was effective in improving cardiorespiratory fitness, cardiometabolic risk and oxidative stress status in T2DM59. While, Farinha et al. completed a 12-week treadmill exercise training, without modifications on dietary pattern in twenty-three women metabolic syndrome who had improved systemic oxidative stress and inflammatory biomarkers60. Similarly, Nojima et al. reported that 103 patients with type 2 diabetes mellitus were instructed to exercise at 50% of peak oxygen uptake for more than 30 minutes on at least 3 days per week over a 12 month period, their results proved that aerobic exercise training improved glycemic control and reduced oxidative stress in patients with type 2 diabetes mellitus61. Moreover, Gordon et al. reported that 3 months of Hatha yoga exercise and conventional exercise may have therapeutic preventative and protective effects on diabetes mellitus by decreasing oxidative stress and improving antioxidant status62. There are 2 mechanisms that underlie the anti-oxidative of aerobic exercise training. The first mechanism is that improvement in glycemic control associated with aerobic exercise training may result in a decrease in oxidative stress. Aerobic exercise training improves insulin sensitivity63 and glycemic control64. Hyperglycemia can induce oxidative stress via several mechanisms including glucose autoxidation, formation of advanced glycation end products, and activation of the polyol pathway65. Chugh et al. reported previously that 6 weeks of glycemic control with sulfonylurea resulted in an improvement of glycemic control and a reduction in serum malondialdehyde, a reliable measure of lipid peroxidation66. The other mechanism is that a decrease in oxidative stress caused by aerobic exercise training may lead to an improvement in glycemic control. Aerobic exercise may increase antioxidant activity and reduce oxidative stress. Elosua et al. reported that aerobic exercise training increased the activity of the endogenous antioxidants, glutathione peroxidase, and glutathione reductase and decreased oxidized low-density lipoprotein concentration67. There is evidence that oxidative stress is associated with insulin resistance, as Urakawa et al. demonstrated that plasma isoprostane levels were negatively correlated with glucose infusion rates in men68. These results therefore indicate that improved insulin sensitivity and glycemic control induced reduction in oxidative stress caused by aerobic exercise training.
Concerning renal function, results of the present study proved that aerobic exercise training improved creatinine in patients with DN, the possible cause for improving renal function following aerobic training may be due modulation of inflammatory cytokines and oxidative. Our results consistent with the studies of Chen et al., Shikano et al. and, Kafle et al. who confirmed the possible role of IL-6 and TNF-α and Gpx in diabetic renal damage progress69–71. While, Xu et al. conducted a cohort study on 176 patients with chronic kidney disease and 67 healthy controls and reported increased level of CRP, IL-6 and MDA in addition to decreased levels of SOD and GSHPX (glutathione peroxidase) along with inverse relationship between estimated glomerular filtration rate (eGFR) and MDA associated with positive relationship with SOD and GSH-PX among patients with chronic kidney disease (CKD)72. Moreover, Aslan et al. reported significant correlations between oxidative stress and microalbuminuria levels in patients with diabetic nephropathy73. However, Sreeram et al. reported that among 108 CKD patients, as the renal damage progressed the values of MDA & CRP increased while the values of GPx and SOD decreased74. The current study has important strengths and limitations. The major strength is the supervised nature of the study. However, all exercise sessions were supervised. Moreover, the study was randomized; hence, we can extrapolate adherence to the general population. In the other hand, the major limitations is only obese type 2 diabetic patients enrolled in the study, so the value of this study only related to obese patients with type 2 diabetic nephropathy, also small sample size in both groups may limit the possibility of generalization of the findings in the present study. Finally, within the limit of this study, aerobic exercise training is recommended for modulation of oxidative stress and inflammatory cytokines and improved renal function among patients with diabetic nephropathy. Further researches are needed to explore the impact of weight reduction on quality of life and other biochemical parameters among obese patients with type 2 diabetic nephropathy.
Conclusion
The current study provides evidence that aerobic exercise training modulated oxidative stress and inflammatory cytokines and improved renal function among patients with diabetic nephropathy.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G: 8-142-41). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
References
- 1.Bukhari SA, Shamshari WA, Ur-Rahman M, Zia-Ul-Haq M, Jaafar HZ. Computer aided screening of secreted frizzled related protein 4 (SFRP4): a potential control for diabetes mellitus. Molecules. 2014;19:10129–10136. doi: 10.3390/molecules190710129. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grace BS, Clayton P, McDonald SP. Increases in renal replacement therapy in Australia and New Zealand: understanding trends in diabetic nephropathy. Nephrology (Carlton) 2012;17:76–84. doi: 10.1111/j.1440-1797.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 3.Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes mediated vascular dysfunction: Unifying hypothesis of diabetes revisited. Vascular Pharmacology. 2012;57(5-6):139–139. doi: 10.1016/j.vph.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Kotur-Stevuljevic J, Simic-Ogrizovic S, Dopsaj V, et al. A hazardous link between malnutrition, inflammation and oxidative stress in renal patients. Clin Biochem. 2012;45(15):1202–1205. doi: 10.1016/j.clinbiochem.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Massy ZA, Stenvinkel P, Drueke TB. The role of oxidative stress in chronic kidney disease. Semin Dial. 2009;22(4):405–408. doi: 10.1111/j.1525-139X.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 6.Dandona P, Mohanty P, Chaudhuri A, Garg R, Aljada A. Insulin infusion in acute illness. The Journal of Clinical Investigation. 2005;115:2069–2072. doi: 10.1172/JCI26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Journal of the American Medical Association. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 8.Pan HZ, Zhang L, Guo MY, Sui H, Li H, Wu WH, Qu NQ, Liang MH, Chang D. The oxidative stress status in diabetes mellitus and diabetes nephropathy. Acta Diabetol. 2010;47:71–76. doi: 10.1007/s00592-009-0128-1. [DOI] [PubMed] [Google Scholar]
- 9.Shi YC, Pan TM. Red mold, diabetes, and oxidative stress: A review. Appl Microbiol Biotechnol. 2012;94:47–55. doi: 10.1007/s00253-012-3957-8. [DOI] [PubMed] [Google Scholar]
- 10.Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetes patients. J Med Assoc Thai. 2010;93:682–693. PubMed. [PubMed] [Google Scholar]
- 11.Bhattacharya S, Manna P, Gachhui R, Sil PC. D-saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kappaB and PKC signaling. Toxicol Appl Pharmacol. 2013;267:16–29. doi: 10.1016/j.taap.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 13.Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 14.Navarro JF, Milena FJ, Mora C, Leon C, Garcı´a J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562–570. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- 15.Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, et al. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52(5):605–608. doi: 10.1053/meta.2003.50096. [DOI] [PubMed] [Google Scholar]
- 16.Navarro JF(a), Mora C, Muros M, Garc´ıa J. Urinary tumour necrosis factor-alpha excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrology Dialysis Transplantation. 2006;21(12):3428–3434. doi: 10.1093/ndt/gfl469. [DOI] [PubMed] [Google Scholar]
- 17.El Mesallamy HO, Ahmed HH, Bassyouni AA, Ahmed AS. Clinical significance of inflammatory and fibrogenic cytokines in diabetic nephropathy. Clin Biochem. 2012 Jun;45(9):646–650. doi: 10.1016/j.clinbiochem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Vander Jagt DL, Hassebrook RK, Hunsaker LA, Brown WM, Royer RE. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: roles for glutathione in both enzymes and implications for diabetic complications. Chem Biol Interact. 2001;130e132:549–562. doi: 10.1016/s0009-2797(00)00298-2. [DOI] [PubMed] [Google Scholar]
- 19.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005;511:191–198. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Rahigude A, Bhutada P, Kaulaskar S, Aswar M, Otari K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience. 2012;226:62–72. doi: 10.1016/j.neuroscience.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese V, Cornelius C, Leso V, et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochimica et Biophysica Acta. 2012;1822(5):729–736. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Das J, Vasan V, Sil PC. Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicology and Applied Pharmacology. 2012;258(2):296–308. doi: 10.1016/j.taap.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Livingstone C, Davis J. Targeting therapeutics against glutathione depletion in diabetes and its complications. British Journal of Diabetes and Vascular Disease. 2007;7(6):258–265. [Google Scholar]
- 24.Dinc er Y, Akc ay T, Alademir Z, Ilkova H. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutation Research. 2002;505(1-2):75–81. doi: 10.1016/s0027-5107(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 25.Shukla K, Dikshit P, Tyagi MK, Shukla R, Gambhir JK. Ameliorative effect of With anticoagulants on dyslipidemia and oxidative stress in nicotinamide streptozotocin induced diabetes mellitus. Food and Chemical Toxicology. 2012;50(10):3595–3599. doi: 10.1016/j.fct.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Kim CH. Expression of extracellular superoxide dismutase protein indiabetes. Archives of Plastic Surgery. 2013;40(5):517–521. doi: 10.5999/aps.2013.40.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucchesi AN, Freitas NT, Cassettari LL, Marques SF, Spadella CT. Diabetes mellitus triggers oxidative stress in the liver of all oxan-treated rats: a mechanism for diabetic chronic liver disease. Acta Cirurgica Brasileira. 2013;28(7):502–508. doi: 10.1590/s0102-86502013000700005. [DOI] [PubMed] [Google Scholar]
- 28.Moussa SA. Oxidative stress in diabetes mellitus. Romanian Journal of Biophysics. 2008;18:225–236. [Google Scholar]
- 29.de M Bandeira S, da S Guedes G, da Fonseca LJS, Pires A S, Gelain DP, Moreira JC. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxidative Medicine and Cellular Longevity. 2012;2012 doi: 10.1155/2012/819310. Article ID 819310, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh B, Karuna R, Sreenivasa RS, et al. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine. 2012;2(11):895–900. doi: 10.1016/S2221-1691(12)60249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral LS, Silva FA, Correia VB, Andrade CE, Dutra BA, Oliveira MV, et al. Beneficial effects of previous exercise training on renal changes in streptozotocin-induced diabetic female rats. Exp Biol Med (Maywood) 2016;241:437–445. doi: 10.1177/1535370215609696. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerums G, MacIsaac RJ. Diabetic nephropathy: how does exercise affect kidney disease in T1DM? Nat Rev Endocrinol. 2015;11:324–325. doi: 10.1038/nrendo.2015.46. [DOI] [PubMed] [Google Scholar]
- 34.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 35.Whiting DR, Guariguta L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 37.Adeshara KA, Diwan AG, Tupe RS. Diabetes and Complications: Cellular Signaling Pathways, Current Understanding and Targeted Therapies. Curr Drug Targets. 2016;17:1309–1328. doi: 10.2174/1389450117666151209124007. [DOI] [PubMed] [Google Scholar]
- 38.Badal SS, Danesh FR. Diabetic Nephropathy: Emerging Biomarkers for Risk Assessment. Diabetes. 2015;64:3063–3065. doi: 10.2337/db15-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahimi Z, Mansouri Zaveleh O, Rahimi Z, Abbasi A. AT2R -1332 G: A polymorphism and diabetic nephropathy in type 2 diabetes mellitus patients. J Renal Inj Prev. 2013;2:97–101. doi: 10.12861/jrip.2013.31. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42:304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- 41.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–617. doi: 10.1016/j.numecd.2009.04.015. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Libardi CA, Souza GV, Cavaglieri CR, et al. Effect of resistance, endurance, and concurrent training on TNF-a, IL-6, CRP. Med Sci Sports Exerc. 2012;44:50–56. doi: 10.1249/MSS.0b013e318229d2e9. [DOI] [PubMed] [Google Scholar]
- 43.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am. J. Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 44.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu Z, Ye X, Wang J, Qi Q, Franco OH, Rennie KL, Pan A, Li H, Liu Y, Hu FB, Lin X. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older chinese people. Circulation. 2009;119:2969–2977. doi: 10.1161/CIRCULATIONAHA.108.833574. [DOI] [PubMed] [Google Scholar]
- 46.Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin- 6 in elderly people. J. Am. Geriatr. Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos R, Viana V, Boscolo R, Marques V, Santana M, Lira F, Tufik S, de Mello M. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. 2012;60:731–735. doi: 10.1016/j.cyto.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006;20(3):201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Bote ME, Garcia JJ, Hinchado MD, Ortega E. An exploratory study of the effect of regular aquatic exercise on the function of neutrophils from women with fibromyalgia: role of IL-8 and noradrenaline. Brain Behav. Brain Behav Immun. 2014 Jul;39:107–112. doi: 10.1016/j.bbi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc Immunol Rev. 2009;15:6–41. PubMed. [PubMed] [Google Scholar]
- 51.Mathur M, Pedersen B. Exercise as a mean to control low-grade inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timmerman K, Flynn M, Coen P, Markofski M, Pence B. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y. Effect of exercise training intensity on murine T regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22(5):643–652. doi: 10.1111/j.1600-0838.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 54.Hong S, Dimitrov S, Pruitt C, Shaikh F, Beg N. Benefit of physical fitness against inflammation in obesity: role of beta adrenergic receptors. Brain Behav Immun. 2014 Jul;39:113–120. doi: 10.1016/j.bbi.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazarevic G, Antic S, Cvetkovic T, Vlahovic P, Tasic I, Stefanovic V. A physical activity programme and its effects on insulin resistance and oxidative defense in obese male patients with type 2 diabetes mellitus. Diabetes and Metabolism. 2006;32(6):583–590. doi: 10.1016/S1262-3636(07)70312-9. [DOI] [PubMed] [Google Scholar]
- 56.Rector RS, Warner SO, Liu Y, et al. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. American Journal of Physiology. 2007;293(2):E500–E506. doi: 10.1152/ajpendo.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity. 2006;14(11):1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira VN, Bessa A, Jorge ML, et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Applied Physiology, Nutrition, and Metabolism. 2012;37(2):334–344. doi: 10.1139/h2012-004. [DOI] [PubMed] [Google Scholar]
- 59.Vinetti G, Mozzini C, Desenzani P, Boni E, Bulla L, Lorenzetti I, Romano C, Pasini A, Cominacini L, Assanelli D. Supervised exercise training reduces oxidative stress and cardiometabolic risk in adults with type 2 diabetes: a randomized controlled trial. Sci Rep. 2015;18(5):9238. doi: 10.1038/srep09238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farinha JB, Steckling FM, Stefanello ST, Cardoso MS, Nunes LS, Barcelos RP, Duarte T, Kretzmann NA, Mota CB, Bresciani G, Moresco RN, Duarte MM, Dos Santos DL, Soares FA. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med Open. 2015;1(1):3. doi: 10.1186/s40798-015-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, Yokoyama A, Inamizu T, Asahara T, Kohno N. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2008;57:170–176. doi: 10.1016/j.metabol.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 62.Gordon L, Morrison E, McGrowder D, Young R, Fraser Y, Zamora E, Alexander-Lindo R, Irving R. Effect exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med. 2008;8:21. doi: 10.1186/1472-6882-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanninen E, Uusitupa M, Siitonen O, Laitinen J, Lansimies E. Habitual physical activity, aerobic capacity and metabolic control in patients with newly diagnosed type 2 (non-insulin-dependent) diabetes mellitus: effect of 1-year diet and exercise intervention. Diabetologia. 1992;35:340–346. doi: 10.1007/BF00401201. [DOI] [PubMed] [Google Scholar]
- 64.Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36:434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- 65.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Chugh SN, Dhawan R, Kishore K, Sharma A, Chugh K. Glibenclamide vs gliclazide in reducing oxidative stress in patients of noninsulin dependent diabetes mellitus— a double blind randomized study. J Assoc Physicians India. 2001;49:803–807. [PubMed] [Google Scholar]
- 67.Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, et al. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–334. doi: 10.1016/s0021-9150(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 68.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 69.Chen S, Cohen MP, Lautenslager GT, Shearman CW, Ziyadeh FN. Glycated albumin stimulates TGF-beta 1 production and protein kinase C activity in glomerular endothelial cells. Kidney International. 2001;59:673–668. doi: 10.1046/j.1523-1755.2001.059002673.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 70.Shikano M, Sobajima H, Yoshikawa H, Toba T, Kushimoto H, Katsumata H, Tomita M, Kawashima S. Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Nephron. 2000 May;85(1):81–85. doi: 10.1159/000045634. [DOI] [PubMed] [Google Scholar]
- 71.Prasad Kafle Mukunda, Singh Shah Dibya, Shrestha Shailendra, Raj Sigdel Mahesh, Bahadur Raut Kanak. Prevalence of specific types of kidney disease in patients undergoing kidney bopsy: a single centre experience. Journal of Advances in Internal Medicine. 2014;03(01):5–10. [Google Scholar]
- 72.Xu Hong, Carrero Juan J. Insulin resistance in chronic kidney disease. Nephrology. 2017;22(Suppl. 4):31–34. doi: 10.1111/nep.13147. [DOI] [PubMed] [Google Scholar]
- 73.Aslan JE, David LL, McCarty OJ. Data detailing the platelet acetyl-lysine proteome. Data Brief. 2015 Oct 9;5:368–371. doi: 10.1016/j.dib.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramagopalan Sreeram, Leahy Thomas P, Stamp Elaine, Sammon Cormac. Approaches for the identification of chronic kidney disease in CPRD-HES-linked studies. J Comp Eff Res. 2020 May;9(7):441–446. doi: 10.2217/cer-2019-0190. [DOI] [PubMed] [Google Scholar]