Abstract
Background
Bronchodilator responsiveness (BDR) in obstructive lung disease varies over time and may be associated with distinct clinical features.
Research Question
Is consistent BDR over time (always present) differentially associated with obstructive lung disease features relative to inconsistent (sometimes present) or never (never present) BDR in tobacco-exposed people with or without COPD?
Study Design and Methods
We retrospectively analyzed data from 2,269 tobacco-exposed participants in the Subpopulations and Intermediate Outcome Measures in COPD Study with or without COPD. We used various BDR definitions: change of ≥ 200 mL and ≥ 12% in FEV1 (FEV1-BDR), change in FVC (FVC-BDR), and change in in FEV1, FVC or both (ATS-BDR). Using generalized linear models adjusted for demographics, smoking history, FEV1 % predicted after bronchodilator administration, and number of visits that the participant completed, we assessed the association of BDR group: (1) consistent BDR, (2) inconsistent BDR, and (3) never BDR with asthma, CT scan features, blood eosinophil levels, and FEV1 decline in participants without COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 0) and the entire cohort (participants with or without COPD).
Results
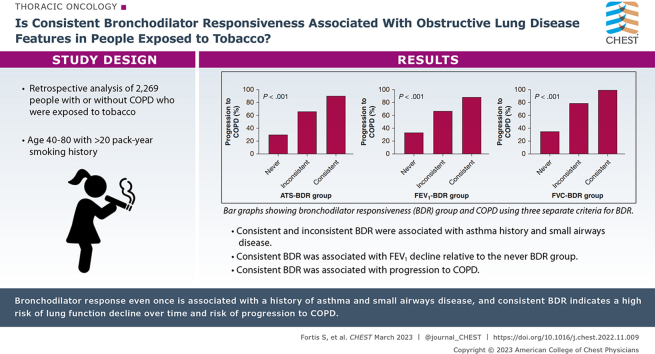
Both consistent and inconsistent ATS-BDR were associated with asthma history and greater small airways disease (%parametric response mapping functional small airways disease) relative to never ATS-BDR in participants with GOLD stage 0 disease and the entire cohort. We observed similar findings using FEV1-BDR and FVC-BDR definitions. Eosinophils did not vary consistently among BDR groups. Consistent BDR was associated with FEV1 decline over time relative to never BDR in the entire cohort. In participants with GOLD stage 0 disease, both the inconsistent ATS-BDR group (OR, 3.20; 95% CI, 2.21-4.66; P < .001) and consistent ATS-BDR group (OR, 9.48; 95% CI, 3.77-29.12; P < .001) were associated with progression to COPD relative to the never ATS-BDR group.
Interpretation
Demonstration of BDR, even once, describes an obstructive lung disease phenotype with a history of asthma and greater small airways disease. Consistent demonstration of BDR indicated a high risk of lung function decline over time in the entire cohort and was associated with higher risk of progression to COPD in patients with GOLD stage 0 disease.
Key Words: bronchodilator, bronchodilator response, bronchodilator responsiveness, bronchodilator reversibility, COPD
Abbreviations: ATS, American Thoracic Society; BDR, bronchodilator responsiveness; GOLD, Global Initiative for Chronic Obstructive Lung Disease; Pi10, square root of the airway wall area for a hypothetical airway with an internal perimeter of 10 mm; PRMfSAD, parametric response mapping functional small airways disease; SPIROMICS, Subpopulations and Intermediate Outcome Measures in COPD Study
Graphical Abstract
Take-home Points.
Study Question: Is consistent bronchodilator responsiveness (BDR) over time (always present) differentially associated with obstructive lung disease features relative to inconsistent (sometimes present) or never (never present) BDR in tobacco-exposed people with or without COPD?
Results: Although both consistent and inconsistent BDR were associated with asthma and small airways disease, consistent BDR was associated with a greater degree of small airways disease and FEV1 decline over time. Consistent BDR in individuals with normal spirometry findings is associated with higher risk for COPD progression.
Interpretation: Demonstration of BDR, even once, describes an obstructive lung disease phenotype with a history of asthma and greater small airways disease, but consistent demonstration of BDR indicates greater small airways disease and a higher risk of lung function decline over time. BDR in individuals with normal spirometry findings was associated with progression to COPD over time.
Assessment of spirometric bronchodilator responsiveness (BDR) is a commonly used pulmonary function test in patients with obstructive lung diseases. According to the American Thoracic Society (ATS)/European Respiratory Society 2005 guidelines, BDR is defined as an increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration.1 Traditionally, BDR has been considered a feature of asthma. According to Global Initiative for Asthma guidelines, BDR can confirm the diagnosis of asthma in patients with consistent clinical history and airflow limitation.2 The cut off for airflow limitation is not specified. BDR was used as the sole diagnostic criterion for asthma in several studies.3, 4, 5, 6 Nevertheless, other studies have shown that BDR is common among patients with COPD.7, 8, 9, 10, 11 The accuracy of BDR to distinguish between asthma and COPD is low.12, 13, 14, 15
The clinical value of BDR to identify phenotypes and to predict outcomes in COPD also is debatable.16 BDR is not necessarily consistent over time, and its variability between tests in the same individuals limits the usefulness of BDR to identify a stable clinical phenotype in COPD. However, lung function variability over time is a typical characteristic of asthma.2 Given that BDR variability over time may be associated differentially with different clinical features of obstructive lung disease, we hypothesized that in a population at high risk of COPD (people with history of heavy smoking), consistent BDR over time is associated differentially with obstructive lung disease features relative to inconsistent or absent BDR. To investigate our hypothesis, we analyzed data from the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). Using data from tobacco-exposed participants with or without COPD, we assessed the association of BDR category: (1) consistent BDR (BDR at every visit), (2) inconsistent BDR (BDR at some, but not all, visits), and (3) no BDR at any visit (BDR at none of the visits) with clinical asthma diagnosis, blood eosinophil counts, radiologic characteristics of airway inflammation, small airways disease and emphysema, and change in FEV1 after bronchodilator administration over time.
Study Design and Methods
We retrospectively analyzed data from the SPIROMICS, a prospective observational study conducted at multiple clinical centers in the United States (https://www.spiromics.org/spiromics/). The study protocol has been approved by the institutional review boards at each participating center (e-Appendix 1). All participants gave written informed consent. Details of the study protocol have been published previously.17
Study Participants
We used data of participants in the SPIROMICS with a ≥ 20 pack-year smoking exposure. The SPIROMICS enrolled participants with COPD (FEV1 to FVC ratio after bronchodilator administration of < 0.7) and without COPD (FEV1 to FVC ratio after bronchodilator administration of ≥ 0.7 with an FVC at or more than the lower limit of normal). Participants were individuals 40 to 80 years of age from the general population. Individuals with BMI of > 40 kg/m2, unstable cardiovascular disease, and lung disease other than asthma and COPD were excluded. Participants had up to five in-person visits over the course of up to 10 years. At the first visit, they answered questionnaires that included demographics, smoking exposure, medical history, and medication use; underwent CBC count testing; and underwent chest high-resolution CT scans. At each visit, participants underwent spirometry before and after bronchodilator administration performed according to ATS/European Respiratory Society guidelines18 and centralized quality assurance for acceptability and repeatability. Participants were instructed to withhold or refrain from vigorous exercise (0.5 h), smoking (1 h), eating a large meal (2 h), alcohol intake (4 h), caffeine intake (6 h), inhaled albuterol intake (6 h), inhaled ipratropium intake (8 h), and any other bronchodilator intake, but not inhaled glucocorticosteroids, for 24 h before spirometry. Spirometry after bronchodilator administration was performed between 15 and 30 min after four inhalations each of albuterol 90 μg/inhalation and ipratropium 18 μg/inhalation.
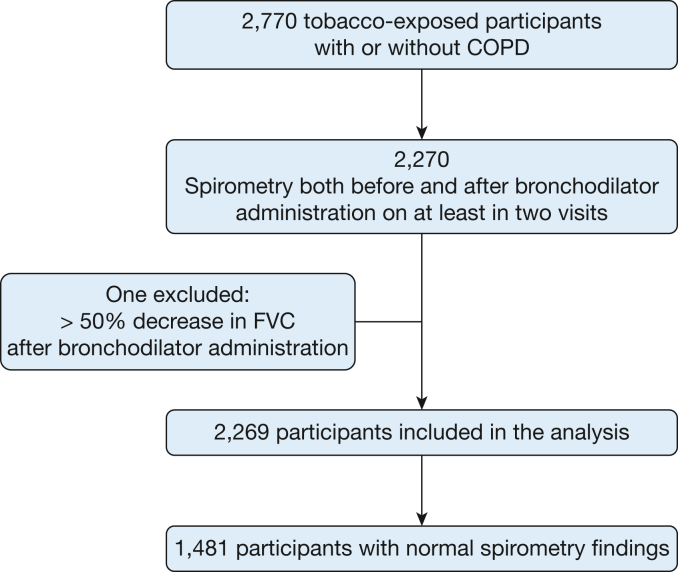
Of 2,770 tobacco-exposed participants with or without COPD, we included 2,270 individuals who underwent spirometry both before and after bronchodilator administration on at least two visits, but not necessarily at all five visits. After excluding one participant who showed a decrease in FVC of more than 50% after bronchodilator administration, 2,269 individuals were included in the analysis, with 1,481 of them showing normal spirometry findings (Fig 1). Of those participants, 1,481 showed normal spirometry findings (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 0).
Figure 1.
Flowchart showing participant disposition through the study.
Definitions
BDR was defined as an increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration (BDR in flow, volume, or both) according to the 2005 ATS/European Respiratory Society guidelines (ATS-BDR). Because the ATS-BDR definition is a composite of BDR in FEV1, FVC, or both and may have limited value to predict outcomes and to identify pathologic features,19,20 we also assessed additional BDR definitions (e-Fig 1). FEV1-BDR was defined as an increase in FEV1 of ≥ 12% and of ≥ 200 mL after bronchodilator administration. FVC-BDR was defined as an increase in FVC of ≥ 12% and ≥ 200 mL after bronchodilator administration. We did not examine the new (2021) ATS/European Respiratory Society BDR definition21 because it has not been adopted yet in clinical practice and no evidence is available to show that it is superior to the previous one (from 2005).22 History of asthma was self-reported (e-Table 1). Decline of FEV1 (in milliliters per year) was derived from the slope of a linear regression model that was fitted to values for FEV1 after bronchodilator administration as a function of the number of days since the first visit.
Imaging
At the first visit, participants underwent chest high-resolution CT scans at maximum inspiration (total lung capacity) and maximal expiration (residual volume). Quantitative image analysis was performed using VIDA software. Percent emphysema was defined as the percentage of voxels at maximum inspiration with attenuation of < –950 Hounsfield units, and gas trapping was quantified as the percentage of voxels at maximum expiration with attenuation values of < –856 Hounsfield units.23 Parametric response mapping was performed using the Imbio Lung Density Analysis software application (Imbio, LLC) to distinguish regions of emphysema from regions of nonemphysematous gas trapping, also called parametric response mapping functional small airways disease (PRMfSAD).24,25 The square root of the airway wall area for a hypothetical airway with an internal perimeter of 10 mm (Pi10) was used as a measure of airway wall thickness.26
Statistical Analysis
The main analysis included participants GOLD stage 0 disease. We categorized participants with GOLD stage 0 disease based on BDR variability into three groups: (1) those with consistent BDR when it is present at every visit; (2) those with inconsistent BDR when it is present at some, but not all, visits; and (3) those never with BDR when it is not present at any visit. We compared the characteristics of participants at the baseline (first) visit between groups using the analysis of variance or the Kruskal-Wallis test for continuous variables and the χ2 test or Fisher exact test for categorical variables.
Then, we examined the association of BDR groups (exposure) with a history of asthma and history of childhood asthma (outcome is a binary variable) using multivariate logistic regression models. Between BDR groups, we compared Pi10, % PRMfSAD, % emphysema, % gas trapping, blood eosinophil counts, and FEV1 decline using multivariate linear regression models. Least square means were used for pairwise comparisons with adjustment for multiple comparisons using Tukey’s method. We repeated the analysis using data from all participants (with and without COPD). In addition, we created multivariate logistic regression models to examine the association of BDR groups with progression to COPD at visit 5 (4 years from baseline) in participants with GOLD stage 0 disease.
In all multivariate analyses, we adjusted for age, sex, race, smoking status, pack-years smoked, FEV1 % predicted after bronchodilator administration at baseline, and number of visits that the participant completed because participants may have had a variable number of visits (two to five visits). All statistical analyses were conducted using R statistical software (R Foundation for Statistical Computing).
Results
Of 2,269 total participants without and without COPD, 813 never showed ATS-BDR, 991 showed inconsistent ATS-BDR, and 325 showed consistent ATS-BDR. The never and consistent ATS-BDR groups included more participants with only two visits relative to the inconsistent group (e-Table 2).We observed similar distributions using the FEV1-BDR and FVC-BDR definitions.
Among participants with GOLD stage 0 disease, those with consistent ATS-BDR were older, had more accumulated smoking exposure, were more likely to use inhaled bronchodilators and inhaled glucocorticosteroids, and showed lower FEV1 than the rest of the participants (Table 1). Changes in FEV1 and FVC after bronchodilators at baseline were greater in participants with consistent BDR. We found similar findings in the entire cohort (e-Table 3).
Table 1.
Baseline Characteristics of Participants With GOLD Stage 0 Diseasea Categorized by Bronchodilator Responsiveness (n = 1,481)
| Characteristics at Visit 1 | ATS-BDR |
P Valueb | ||
|---|---|---|---|---|
| Never | Inconsistent | Consistent | ||
| No. of patients | 725 | 629 | 127 | . . . |
| Age, y | 62.7 ± 9.5 | 64.0 ± 8.9 | 63.5 ± 8.6 | .026 |
| Female sex | 334 (46.1) | 301 (47.9) | 51 (40.2) | .28 |
| White race | 547 (75.4) | 486 (77.3) | 108 (85.0) | .06 |
| BMI, kg/m2 | 28.7 ± 5.1 | 28.6 ± 5.2 | 28.6 ± 5.4 | .94 |
| Pack-years of smoking | 45.3 ± 26.4 | 48.3 ± 23.0 | 52.3 ± 22.8 | .004 |
| Current individuals who smoke | 288 (40.1) | 261 (42.4) | 61 (48.4) | .20 |
| Asthma | 91 (12.6) | 136 (21.6) | 27 (21.3) | < .001 |
| Childhood asthma | 33 (4.6) | 69 (11.0) | 12 (9.4) | < .001 |
| Bronchodilator | 186 (25.9) | 262 (42.1) | 61 (48.4) | < .001 |
| Inhaled corticosteroids | 106 (14.8) | 162 (26.0) | 37 (29.6) | < .001 |
| Before bronchodilator administration | ||||
| FEV1, L | 2.59 ± 0.70 | 2.09 ± 0.66 | 1.83 ± 0.55 | < .001 |
| FEV1 % predicted | 89.1 ± 16.4 | 74.18 ± 17.4 | 61.2 ± 13.0 | < .001 |
| FVC, L | 3.65 ± 0.96 | 3.27 ± 0.96 | 3.19 ± 0.94 | < .001 |
| FVC % predicted | 95.8 ± 14.9 | 87.8 ± 16.9 | 80.9 ± 15.3 | < .001 |
| FEV1 to FVC ratio | 92.53 ± 9.62 | 83.85 ± 10.66 | 75.63 ± 8.97 | < .001 |
| After bronchodilator administration | ||||
| FEV1, L | 2.70 ± 0.73 | 2.32 ± 0.68 | 2.23 ± 0.62 | < .001 |
| FEV1 % predicted | 93.1 ± 16.6 | 82.3 ± 17.0 | 74.9 ± 13.3 | < .001 |
| FVC, L | 3.69 ± 0.96 | 3.48 ± 0.98 | 3.65 ± 0.99 | < .001 |
| FVC% predicted | 96.7 ± 14.7 | 93.4 ± 15.9 | 92.8 ± 14.9 | < .001 |
| FEV1/FVC | 95.86 ± 9.97 | 87.60 ± 10.06 | 80.44 ± 7.00 | < .001 |
| Change in FEV1, mL | 120 ± 110 | 230 ± 180 | 410 ± 190 | < .001 |
| Change in FEV1, % | 4.7 ± 4.2 | 12.4 ± 11.4 | 23.7 ± 12.8 | < .001 |
| Change in FVC, mL | 40 ± 150 | 210 ± 240 | 460 ± 270 | < .001 |
| Change in FVC, % | 1.1 ± 4.1 | 7.2 ± 8.6 | 15.7 ± 9.8 | < .001 |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. We categorized tobacco-exposed participants with normal spirometry findings based on ATS-BDR into three groups: consistent BDR when it is present at every visit; inconsistent BDR when it is present at some, but not all, visits; and never BDR when it is not present at any visit. ATS-BDR = increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration according to the American Thoracic Society/European Respiratory Society guidelines; BDR = bronchodilator responsiveness.
Normal spirometry findings.
Analysis of variance or Kruskal-Wallis test for continuous variables and χ2 or Fisher exact test for categorical variables.
History of Asthma
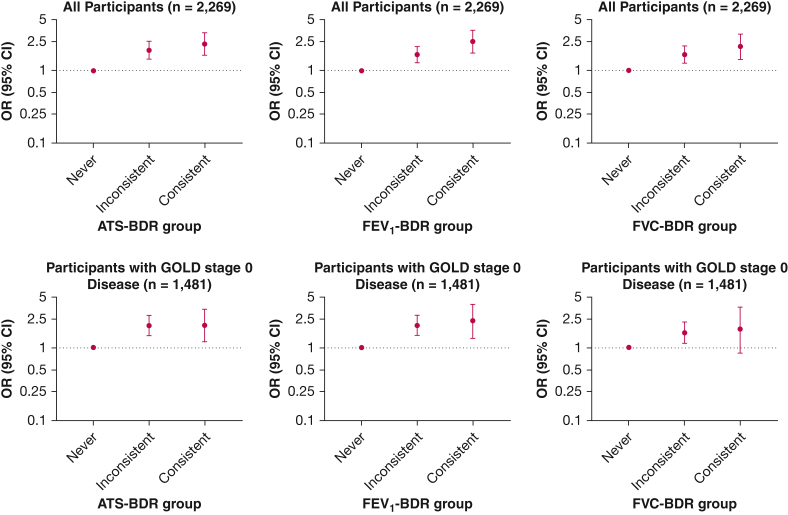
In adjusted analysis of the participants with GOLD stage 0 disease, using the ATS-BDR definition, we found associations with a history of asthma in both the consistent BDR group (OR, 2.03; 95% CI, 1.20-3.38; P = .007) and the inconsistent BDR group (OR, 2.00; 95% CI, 1.45-2.78; P < .001) and relative to the never BDR group (Fig 2). We observed a similar pattern using the FEV1-BDR and FVC-BDR definitions.
Figure 2.
Graphs showing the association of BDR group with asthma in participants with GOLD stage 0 disease (ie, normal spirometry findings; n = 1,481) and the entire cohort (n = 2,269). We categorized tobacco-exposed participants with or without COPD based on BDR variability into three groups: consistent BDR when it is present at every visit; inconsistent BDR when it is present at some, but not all, visits; and never BDR when it is not present at any visit. Multivariate logistic regression models used BDR group as the independent variable and asthma diagnosis as the dependent variable. All models included the following covariates: age, sex, race, smoking status, pack-years smoked, and FEV1 % predicted after bronchodilator administration at first visit, as well as number of visits. ATS-BDR = increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration; BDR = bronchodilator responsiveness; FEV1-BDR = increase in FEV1 of ≥ 12% and ≥ 200 mL after bronchodilator administration; FVC-BDR = increase in FVC of ≥ 12% and ≥ 200 mL after bronchodilator administration; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
In the analysis of the entire cohort, using the ATS-BDR definition, we found associations with a history of asthma in both the consistent BDR group (OR, 2.31; 95% CI, 1.62-3.31; P < .001) and the inconsistent BDR group (OR, 1.90; 95% CI, 1.44-2.52; P < .001) and relative to the never BDR group (Fig 2). We observed the same pattern using the FEV1-BDR and FVC-BDR definitions. We found similar results regarding the association of BDR variability with a history of childhood asthma (e-Fig 2).
Radiographic Findings
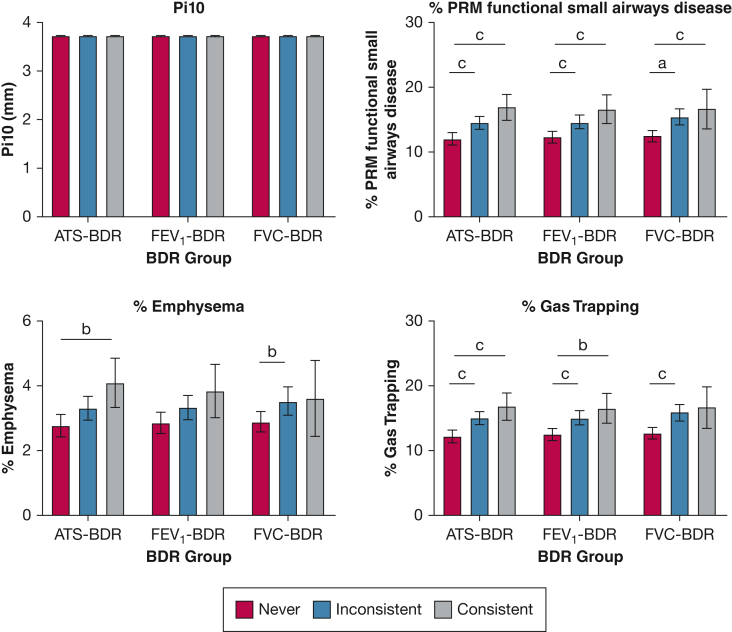
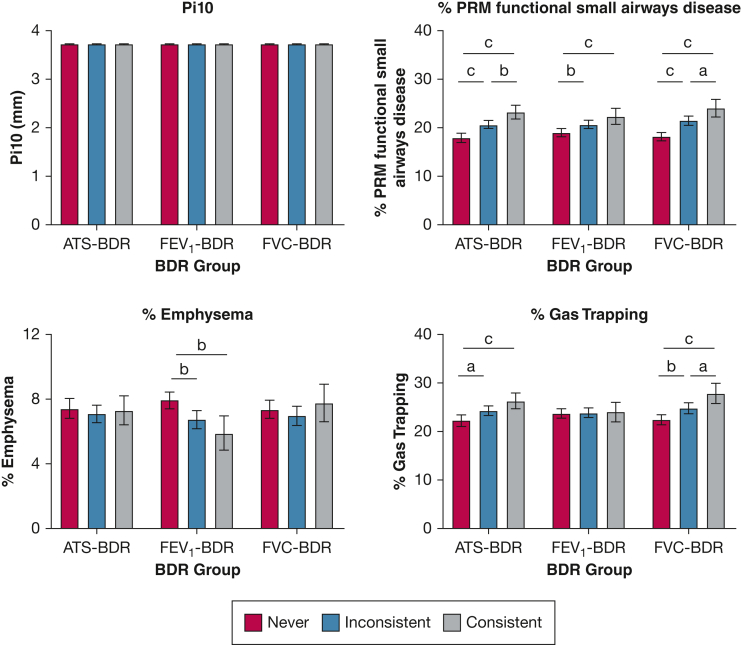
In the adjusted analysis, no difference was found in Pi10 across the BDR groups regardless of the BDR definition used both in the participants with GOLD stage 0 disease (Fig 3) and in the entire cohort (Fig 4).
Figure 3.
Bar graphs showing the association of BDR group with chest CT scan findings in participants with GOLD stage 0 disease (ie, normal spirometry findings; n = 1,481). We categorized tobacco-exposed participants with normal spirometry findings based on BDR variability into three groups: consistent BDR when it is present at every visit; inconsistent BDR when it is present at some, but not all, visits; and never BDR when it is not present at any visit. Multivariate linear regression models with BDR group as the independent variable and Pi10, % PRMfSAD, % emphysema, and % gas trapping as the dependent variables. All models included the following covariates: age, sex, race, smoking status and pack-years smoked, and FEV1 % predicted after bronchodilator administration at first visit, as well as number of visits. Based on these models, we calculated the least square mean (LSM). Pairwise comparisons using Tukey’s method correction for LSM were used. Values in the figures are presented as LSM with 95% CI. aP < .05. bP < .01. cP < .001. ATS-BDR = increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration; BDR = bronchodilator responsiveness; FEV1-BDR = increase in FEV1 of ≥ 12% and ≥ 200 mL after bronchodilator administration; FVC-BDR = increase in FVC of ≥ 12% and ≥ 200 mL after bronchodilator administration; Pi10 = square root of the airway wall area for a hypothetical airway with an internal perimeter of 10 mm; PRMfSAD = parametric response mapping functional small airways disease.
Figure 4.
Bar graphs showing the association of BDR group with chest CT scan findings in the entire cohort (n = 2,269). We categorized tobacco-exposed participants with or without COPD based on BDR variability into three groups: consistent BDR when it is present at every visit; inconsistent BDR when it is present at some, but not all, visits; and never BDR when it is not present at any visit. Multivariate linear regression models used BDR group as the independent variable and Pi10, % PRMfSAD, % emphysema, and % gas trapping as the dependent variables. All models included the following covariates: age, sex, race, smoking status and pack-years smoked, and FEV1 % predicted after bronchodilator administration at first visit, as well as number of visits. Based on these models, we calculated the least square mean (LSM). Pairwise comparisons using Tukey’s method correction for LSM were used. Values in the figures are presented as LSM with 95% CI. aP < .05. bP < .01. cP < .001. ATS-BDR = increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration; BDR = bronchodilator responsiveness; FEV1-BDR = increase in FEV1 of ≥ 12% and ≥ 200 mL after bronchodilator administration; FVC-BDR = increase in FVC of ≥ 12% and ≥ 200 mL after bronchodilator administration; Pi10 = square root of the airway wall area for a hypothetical airway with an internal perimeter of 10 mm; PRMfSAD = parametric response mapping functional small airways disease.
In adjusted analysis of the participants with GOLD stage 0 disease, % PRMfSAD was significantly greater in participants with consistent ATS-BDR (16.9%; 95% CI, 14.9%-18.9%) than % PRMfSAD in participants with never ATS-BDR (12.0%; 95% CI, 11.1%-13.0%; P < .001) (Fig 3). In the inconsistent compared with the never ATS-BDR group, % PRMfSAD was significantly greater (P < .001). We observed similar findings using the FEV1-BDR and FVC-BDR definitions. In the entire cohort, we observed a similar pattern (Fig 4).
In the analysis of participants with GOLD stage 0 disease, % emphysema did not vary between FEV1-BDR groups. Percent emphysema was greater in the consistent ATS-BDR group with an average of 4.1% (95% CI, 3.3%-4.8%) relative to the never ATS-BDR group with an average of 2.8% (95% CI, 2.4%-3.1%; P = .005) (Fig 3). Percent emphysema also was greater in the inconsistent FVC-BDR group (3.5%; 95% CI, 3.1%-4.0%) relative to the never FVC-BDR group (2.9%; 95% CI, 2.6%-3.2%; P = .045)
In the entire cohort analysis, % emphysema did not vary between BDR groups using the ATS-BDR and FVC-BDR definitions (Fig 4). When using the FEV1-BDR definition, % emphysema was greater in the never BDR group with an average of 8.0% (95% CI, 7.4%-8.5%) relative to the inconsistent BDR group (6.8%; 95% CI, 6.2%-7.3%; P = .003) and the consistent BDR group (5.9%; 95% CI, 4.9%-7.0%; P = .001).
In analysis of participants with GOLD stage 0 disease, % gas trapping was greater in participants with inconsistent BDR relative to participants with never BDR (Fig 3). Percent gas trapping also was greater in the consistent BDR group relative to the never BDR group when ATS-BDR and FEV1-BDR were applied. In the entire cohort, we observed a similar pattern (Fig 4) except that gas trapping did not vary between FEV1-BDR groups.
Eosinophil Counts
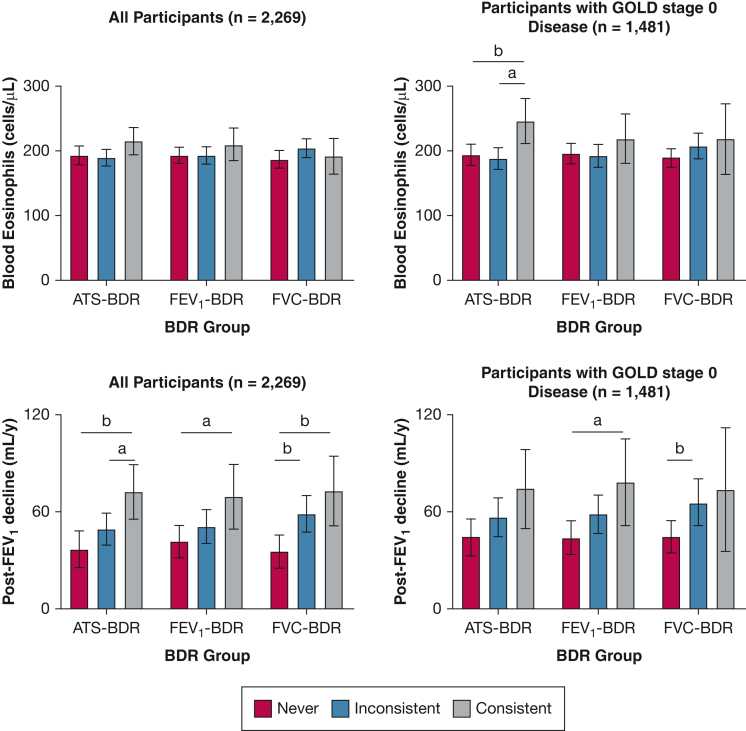
In adjusted analysis of participants with GOLD stage 0 disease, the absolute eosinophil counts were greater in the consistent ATS-BDR group relative to the inconsistent and never ATS-BDR groups (Fig 5). In the analysis of the entire cohort, no difference was found in blood eosinophil counts across BDR groups regardless of the BDR definition applied.
Figure 5.
Bar graphs showing the association of BDR group with blood eosinophil counts at baseline and decline in FEV1 after bronchodilator administration over time in participants with GOLD stage 0 disease (ie, normal spirometry findings; n = 1,481) and the entire cohort (n = 2,269). We categorized tobacco-exposed participants with or without COPD based on BDR variability into three groups: consistent BDR when it is present in every visit; inconsistent BDR when it is present at some, but not all, visits; and never BDR when it is not present at any visit. Multivariate linear regression models used BDR group as the independent variable and plasma eosinophil levels at baseline or decline in FEV1 % predicted after bronchodilator administration over time as the dependent variable. All models included the following covariates: age, sex, race, smoking status and pack-years smoked, and FEV1 % predicted after bronchodilator administration at first visit, as well as number of visits. Based on these models, we calculated the least square mean (LSM). Pairwise comparisons using Tukey method correction LSM were used. Values in the figures are presented as LSM with 95% CI. aP < .05. bP < .01. cP < .001. ATS-BDR = increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration; BDR = bronchodilator responsiveness; FEV1-BDR = increase in FEV1 of ≥ 12% and ≥ 200 mL after bronchodilator administration; FVC-BDR = increase in FVC of ≥ 12% and ≥ 200 mL after bronchodilator administration; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
FEV1 Decline After Bronchodilator Administration
In participants with GOLD stage 0 disease, the decline in FEV1 after bronchodilator administration was greater in participants with consistent FEV1-BDR (79 mL/y; 95% CI, 52-106 mL/y) than the decline in never FEV1-BDR (44 mL/y; 95% CI, 34-55 mL/y; P = .044) (Fig 5). The decline in FEV1 after bronchodilator administration was greater in the inconsistent FVC-BDR group (66 mL/y; 95% CI, 52-80 mL/y) than the decline in the never FVC-BDR group (45 mL/y; 95% CI, 35-55 mL/y; P = .034). In the entire cohort analysis, consistent BDR was associated with greater FEV1 decline relative to never BDR regardless of the BDR definition applied (Fig 5).
Progression to COPD
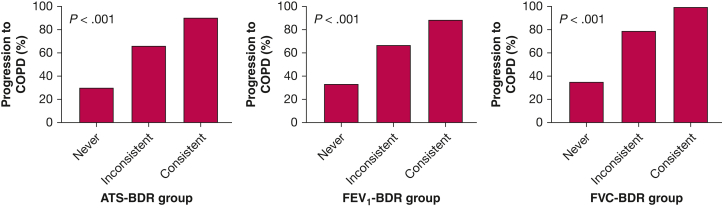
In 756 participants with GOLD stage 0 disease with available spirometric data at visit 5 (4 years from baseline), we found that 29.9% (100 of 334 participants) in the never ATS-BDR group, 66.7% (246 of 369 participants) in the inconsistent ATS-BDR group, and 90.6% (48 of 53 participants) in the consistent ATS-BDR group demonstrated COPD at visit 5 (Fig 6). In the adjusted analysis, both inconsistent ATS-BDR (OR, 3.20; 95% CI, 2.21-4.66; P < .001) and consistent ATS-BDR (OR, 9.48; 95% CI, 3.77-29.12; P < .001) were associated with progression to COPD at visit 5 relative to never ATS-BDR (e-Table 4). We observed the same pattern using the FEV1-BDR and FVC-BDR definitions.
Figure 6.
Bar graphs showing BDR group and COPD at visit 5 in participants with GOLD stage 0 disease (ie, normal spirometry findings; n = 756). We categorized tobacco-exposed participants with or without COPD based on BDR variability into three groups: consistent BDR when it is present at every visit; inconsistent BDR when it is present at some, but not all, visits; and never BDR when it is not present at any visit. ATS-BDR = increase in FEV1, FVC, or both of ≥ 12% and ≥ 200 mL after bronchodilator administration; BDR = bronchodilator responsiveness; FEV1-BDR = increase in FEV1 of ≥ 12% and ≥ 200 mL after bronchodilator administration; FVC-BDR = increase in FVC of ≥ 12% and ≥ 200 mL after bronchodilator administration; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Discussion
Among tobacco-exposed people with or without COPD, both inconsistent and consistent BDR was associated with a self-reported history of asthma. Consistent BDR also was associated with evidence of small airways disease on chest high-resolution CT imaging and greater lung function decline relative to never BDR regardless of the BDR definition applied. Among tobacco-exposed people with normal spirometry findings, both consistent and inconsistent BDR were associated with progression to COPD over time.
Earlier studies failed to show convincing clinical usefulness of BDR in COPD, likely because the BDR definition applied was not specific.10,27 The COPDGene study and the SPIROMICS showed that FEV1-BDR and FVC-BDR are associated differentially with clinical and radiographic features of obstructive lung disease.6,19,20 However, an important limitation of BDR to identify a phenotype is that it is not necessarily stable over time.16 To our knowledge, this is the first study in tobacco-exposed people with or without COPD examining the association of BDR over time with clinical and radiographic features.
Patients with asthma more often demonstrate BDR and typically BDR that is greater than patients with COPD, but BDR is common in both diseases.12 Global Initiative for Asthma guidelines often are misinterpreted, and BDR is considered equivalent to the diagnosis of asthma. It is no surprise that BDR was associated with clinical asthma diagnosis. Nonetheless, childhood asthma diagnosis is unlikely to be confounded based on the presence of BDR because it manifests in childhood with respiratory symptoms before spirometric evaluation.
We observed that BDR was not associated with airway wall thickness measured by Pi10, whereas a previous report by Kim and colleagues28 found an association in patients with COPD. This discrepancy can be explained by our inclusion of some participants without a spirometric diagnosis of COPD as well as differences in the protocols used to evaluate BDR. In the SPIROMICS, we aimed to elicit “maximal bronchodilatation” by administering both albuterol and ipratropium, as opposed to only albuterol.
Our findings complement previous reports in patients with COPD showing that the various BDR types are associated differentially with chest CT scan findings of obstructive lung disease.6,19,20 In the entire cohort that includes participants with a significant amount of emphysema, FEV1-BDR was associated inversely with emphysema. In a physiology study, Cerveri and colleagues29 showed that greater emphysema is associated with reduced BDR in FEV1 because the airway resistance and diameter mostly are determined by airway-parenchyma interdependence and airway smooth muscle does not play a significant role when the lungs are inflated close to total lung capacity at the beginning of exhalation (BDR in FEV1).30 This is the reason that in patients with significant emphysema, isolated BDR in FVC typically is present.20,30 However, in participants with GOLD stage 0 disease, the degree of emphysema is less and BDR maneuvers are not affected. In those individuals, we observed a positive association of BDR with emphysema likely because BDR was associated with small airways disease.
In both the entire cohort and those with GOLD stage 0 disease, more consistent BDR indicates greater small airways disease (PRMfSAD). We did not find an association of FEV1-BDR and traditional gas trapping in the entire cohort because traditional gas trapping cannot distinguish emphysema from true gas trapping because of small airways disease. More consistent FEV1-BDR is associated with less emphysema, but more true gas trapping resulting from small airways disease (PRMfSAD). Thus, the so-called sum of these two (that is, traditional gas trapping) does not vary between BDR groups.
Previous studies have shown that BDR is correlated weakly with sputum and blood eosinophil levels.31, 32, 33 Our findings failed to show a consistent pattern of BDR and blood eosinophils. This also may reflect that eosinophil levels vary over time and that one-time measurement may not be informative.34,35
We also found that BDR was associated with greater FEV1 decline over time. Other reports have shown that lung function decline over time in COPD is associated with methacholine reactivity and BDR,36,37 but these reports could have been confounded by less severe lung function at baseline.16,38,39 The higher the FEV1, the higher the chance of BDR.8 Nevertheless, a recent report showed an association of BDR with FEV1 decline even after adjusting for baseline lung function.20 Consistent BDR was associated with greater lung function decline relative to those with never BDR after taking into account the baseline lung function. Moreover, those with GOLD stage 0 disease and BDR, in particular those with consistent BDR, are at higher risk of progression to COPD. This important finding may be because individuals with consistent BDR show greater small airways disease and hence are at higher risk of lung function decline.25 Finding BDR may indicate small airways smooth muscle pathologic features playing a role in the inflammatory and remodeling process of the airway.40
Our observations suggest that the presence of BDR even at one visit (inconsistent BDR) describes an obstructive lung disease phenotype with a history of asthma and small airways disease, whereas consistent BDR provides additional characterization of this phenotyping by indicating a high risk of lung function decline over time. Tobacco-exposed people with or without COPD and consistent BDR showed a higher risk of lung function decline and greater severe small airways disease than individuals with never BDR independent of the FEV1 % predicted after bronchodilator administration.
Our study has several limitations. It included individuals with at least 20 pack-years cumulative smoking exposure, so that our results may not be generalizable in individuals with no or mild smoking exposure. Our main independent variable, BDR group, was based on spirometry in several visits, but most of the outcomes were based on baseline characteristics. Spirometry performed after administration of both albuterol and ipratropium, rather than only albuterol, reduces the chance of submaximal bronchodilation and potential BDR variation. In the adjusted analysis, we did not include medications (eg, long-acting bronchodilators) as covariates in the models because we could not confirm adherence to and durations of those treatments. Medications likely were confounded by indication based on the unadjusted analysis. Participants with consistent BDR showed worse lung function and more medication use. Finally, not all the participants underwent all five annual spirometry examinations. Most of those in the inconsistent BDR groups underwent four or five visits, whereas most of the participants in the never and consistent BDR groups underwent only two visits, thereby reducing the likelihood of demonstrating inconsistent BDR. Nonetheless, in the adjusted analysis, we adjusted for the number of visits that a participant completed. These limitations do not undermine the strengths of the study, which include sequential spirometry with stringent quality controls and a tightly defined chest CT scan protocol yielding a wealth of CT scan metrics that relate to lung structure.
Interpretation
In tobacco-exposed people with or without COPD, the presence of BDR even on one visit describes an obstructive lung disease phenotype with a greater likelihood of a history of asthma and more small airways disease. BDR in patients with GOLD stage 0 disease was associated with progression to COPD over time. Moreover, consistent BDR at every visit was associated with greater small airways disease and higher risk of lung function decline relative to those with no BDR.
Funding/Support
SPIROMICS was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health [Grants HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C, U01 HL137880, and U24 HL141762] and was supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan. S. F. has received grants from American Thoracic Society. S. P. B. is supported by the National Institutes of Health [Grants R01HL151421, R21EB027891, and UG3HL155806]. M. K. H. is supported by the National Institutes of Health. R. G. B. is support by the National Center for Advancing Translational Sciences, National Institutes of Health [Grant TL1TR001883-504 01]. M. B. D. has received research grants from the National Institutes of Health and the Department of Defense. S. P. P. is the primary investigator of the Wake Forest clinical site for the SPIROMICS COPD program, which is funded by the National Heart, Lung, and Blood Institute. N. P. has received grant funding from the National Institutes of Health. R. P. reports grants from the National Heart, Lung, and Blood Institute, the COPD Foundation, and the Department of Veterans Affairs outside the submitted work. R. E. K. has received grants from grants from the National Heart, Lung, and Blood Institute and the COPD Foundation. J. L. C. is supported by the Department of Veterans Affairs [Grant I01 CX002377], the Department of Defense, and the National Institutes of Health. R. P. B. is supported by the National Center for Advancing Translational Sciences, National Institutes of Health [Grant TL1TR001883-01]. N. N. H. reports grants from the National Institutes of Health and the COPD Foundation. J. A. K. has received research grants from the National Institutes of Health. D. C. has received grants from the National Institutes of Health and the COPD Foundation. C. B. C. reports grants from the National Heart, Lung, and Blood Institute, the National Institutes of Health, Foundation NIH, and the COPD Foundation, during the conduct of the study.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: S. F. has received grants from Fisher & Paykel and served as a consultant for Genentech. A. P. C. has consulted for GSK, AstraZeneca, and VIDA Diagnostics. S. P. B. has served as consultant or on advisory boards for Sanofi, Boehringer Ingelheim, Sunovion, GlaxoSmithKline, and IntegrityCE within the past three years. D. P. T. has consulted with AstraZeneca, Sunovion, Mylan, and Theravance. E. A. H. is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software. G. J. C. has received research grants from AstraZeneca, Boehringer Ingelheim, Novartis, Respironics, MedImmune, Actelion, Forest, Pearl, Ikaria, Aeris, PneumRX, and Pulmonx; is the founder of and has equity interest in HGE Health Care Solutions, Inc., and HGE Technologies; and has consulted for Amirall, Boehringer Ingelheim, and Holaira. M. K. H. has consulted for AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Pulmonx, Teva, Verona, Merck, Sanofi, DevPro, Aerogen, Polarian, Regeneron, and United Therapeutics; has given presentations for Cipla, Chiesi, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline; has received stock options from Meissa Vaccines; has received either in kind research support or funds paid to the institution from Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association; and has participated in data safety monitoring boards for Novartis and Medtronic with funds paid to the institution. M. A. reports grants from the Departments of Defense and Veterans Affairs, the Flight Attendant Medical Research Institute, and the California Tobacco-Related Disease Research Program during the conduct of the study; and has received research support from Guardant Health and Genentech. M. B. D. has received research grants from Boehringer-Ingelheim and Midmark and reports personal fees from Boehringer-Ingelheim, GlaxoSmithKline, AstraZeneca, Midmark, and Mylan-Theravance, outside the submitted work. V. K. has consulted for Boehringer Ingelheim, Gala Therapeutics, and AstraZeneca and received personal fees from ABIM. N. P. has received personal fees from CSL Behring and Pharmacosmos not related to the current work. S. I. R. was employed by and holds shares in AstraZeneca; has received grants from PCORI, the Greater Plains IDeA-CTR, and University of Nebraska; and has received consulting fees from the Alpha 1 Foundation, Bergenbio, GSK, Sanofi, Novoventures, and Verona outside the scope of this work. J. L. C. reports personal funds from AstraZeneca, Novartis, and CSL Behring, outside the submitted work. F. J. M. reports personal fees from Continuing Education, Forest Laboratories, Janssen, GlaxoSmithKline, Nycomed/Takeda, AstraZeneca, Boehringer Ingelheim, Bellerophon (formerly Ikaria), Genentech, Novartis, Pearl, Roche, Sunovion, Theravance, CME Incite, Annenberg Center for Health Sciences at Eisenhower, Integritas, InThought, National Association for Continuing Education, Paradigm Medical Communications, LLC, PeerVoice, UpToDate, Haymarket Communications, Western Society of Allergy and Immunology, Proterixbio (formerly Bioscale), Unity Biotechnology, ConCert Pharmaceuticals, Lucid, Methodist Hospital, Columbia University, Prime Healthcare Ltd., WebMD, PeerView Network, the California Society of Allergy and Immunology, Chiesi, and the Puerto Rico Thoracic Society, outside the submitted work. N. N. H. reports grants from Boehringer Ingelheim. J. A. K. has received research grants from NIH, the Patient Centered Outcomes Research Institute, ResMed, Inogen, and Sanofi, outside of the submitted work. P. G. W. reports personal fees from Theravance, GSK, NGM Pharmaceuticals, Amgen, Glenmark Pharmaceuticals, Regeneron, Sanofi, Clarus Ventures, 23andMe, and Astra Zeneca, all unrelated to this work. I. Z. B. has consulted with Astra Zeneca, Boehringer Ingelheim, Fisher and Paykel Healthcare, CSL Behring, Grifols, Verona Pharma, GE Healthcare, Mylan, Theravance, and GSK and has received research grants from AMGEN and GE Healthcare. C. B. C. reports personal fees from PulmonX, GlaxoSmithKline, NUVAIRA, and MGC Diagnostics, outside the submitted work. None declared (P. M. Q., B. A. D., W. H. A.).
Acknowledgments
Author contributions: S. F., P. M. Q., D. C., W. H. A., and C. B. C. were involved in the design of the analysis and drafting of the manuscript. S. F. had full access to the data and takes responsibility for the integrity of the data and accuracy of the analysis. All of authors were involved in editing the manuscript. All of the authors above approved this version of the manuscript for submission.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Role ofsponsors: Industry sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
∗ SPIROMICSInvestigators Collaborators: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Patricia Basta, PhD; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell, Jr, MD; Robert Paine III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN.
Other contributions: The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at www.spiromics.org. The authors thank the University of North Carolina at Chapel Hill BioSpecimen Processing Facility for sample processing, storage, and sample disbursements (http://bsp.web.unc.edu/).
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under “Supplementary Data.”
Contributor Information
Spyridon Fortis, Email: spyridon-fortis@uiowa.edu.
Subpopulations and Intermediate Outcome Measures in COPD Study Investigators:
Neil E. Alexis, Wayne H. Anderson, Mehrdad Arjomandi, Igor Barjaktarevic, R. Graham Barr, Patricia Basta, Lori A. Bateman, Surya P. Bhatt, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Brad Drummond, Christine M. Freeman, Craig Galban, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Yvonne Huang, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell, Jr., Robert Paine, III, Laura Paulin, Stephen P. Peters, Cheryl Pirozzi, Nirupama Putcha, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Sanjeev Raman, Stephen I. Rennard, Donald P. Tashkin, J. Michael Wells, Robert A. Wise, and Prescott G. Woodruff
Supplementary Data
References
- 1.Pellegrino R., Viegi G., Brusasco V., et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma Management and Prevention, Global Initiative for Asthma Management and Prevention website, 2021. Accessed September 2021. www.ginasthma.org
- 3.Aaron S.D., Vandemheen K.L., FitzGerald J.M., et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317(3):269–279. doi: 10.1001/jama.2016.19627. [DOI] [PubMed] [Google Scholar]
- 4.Aaron S.D., Vandemheen K.L., Boulet L.P., et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179(11):1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luks V.P., Vandemheen K.L., Aaron S.D. Confirmation of asthma in an era of overdiagnosis. Eur Respir J. 2010;36(2):255–260. doi: 10.1183/09031936.00165109. [DOI] [PubMed] [Google Scholar]
- 6.Barjaktarevic I.Z., Buhr R.G., Wang X., et al. Clinical significance of bronchodilator responsiveness evaluated by forced vital capacity in COPD: SPIROMICS cohort analysis. Int J Chron Obstruct Pulmon Dis. 2019;14:2927–2938. doi: 10.2147/COPD.S220164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han M.K., Wise R., Mumford J., et al. Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J. 2010;35(5):1048–1056. doi: 10.1183/09031936.00052509. [DOI] [PubMed] [Google Scholar]
- 8.Calverley P.M., Burge P.S., Spencer S., Anderson J.A., Jones P.W. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58(8):659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanania N.A., Sharafkhaneh A., Celli B., et al. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12(1):6. doi: 10.1186/1465-9921-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert P., Agusti A., Edwards L., et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 11.Tashkin D.P., Celli B., Decramer M., et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–750. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 12.Chhabra S.K. Acute bronchodilator response has limited value in differentiating bronchial asthma from COPD. J Asthma. 2005;42(5):367–372. doi: 10.1081/JAS-62992. [DOI] [PubMed] [Google Scholar]
- 13.Richter D.C., Joubert J.R., Nell H., Schuurmans M.M., Irusen E.M. Diagnostic value of post-bronchodilator pulmonary function testing to distinguish between stable, moderate to severe COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2008;3(4):693–699. doi: 10.2147/copd.s948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesten S., Rebuck A.S. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest. 1994;105(4):1042–1045. doi: 10.1378/chest.105.4.1042. [DOI] [PubMed] [Google Scholar]
- 15.Meslier N., Racineux J.L., Six P., Lockhart A. Diagnostic value of reversibility of chronic airway obstruction to separate asthma from chronic bronchitis: a statistical approach. Eur Respir J. 1989;2(6):497–505. [PubMed] [Google Scholar]
- 16.Calverley P.M., Albert P., Walker P.P. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med. 2013;1(7):564–573. doi: 10.1016/S2213-2600(13)70086-9. [DOI] [PubMed] [Google Scholar]
- 17.Couper D., LaVange L.M., Han M., et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M.R., Hankinson J., Brusasco V., et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Hansen J.E., Dilektasli A.G., Porszasz J., et al. A new bronchodilator response grading strategy identifies distinct patient populations. Ann Am Thorac Soc. 2019;16(12):1504–1517. doi: 10.1513/AnnalsATS.201901-030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortis S., Comellas A., Make B.J., et al. Combined FEV1 and FVC bronchodilator response, exacerbations, and mortality in COPD. Ann Am Thorac Soc. 2019;16(7):826–835. doi: 10.1513/AnnalsATS.201809-601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanojevic S., Kaminsky D.A., Miller M., et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt S.P., Fortis S., Bodduluri S. New guidelines for bronchodilator responsiveness in COPD: a test in search of a use. Am J Respir Crit Care Med. 2022;206(8):1042–1044. doi: 10.1164/rccm.202203-0458LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieren J.P., Newell J.D., Jr., Barr R.G., et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galban C.J., Han M.K., Boes J.L., et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S.P., Soler X., Wang X., et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(2):178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith B.M., Hoffman E.A., Rabinowitz D., et al. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(11):987–996. doi: 10.1136/thoraxjnl-2014-205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen E.F., Phanareth K., Laursen L.C., Kok-Jensen A., Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(4 pt 1):1267–1271. doi: 10.1164/ajrccm.159.4.9807121. [DOI] [PubMed] [Google Scholar]
- 28.Kim V., Desai P., Newell J.D., et al. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res. 2014;15:84. doi: 10.1186/s12931-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerveri I., Pellegrino R., Dore R., et al. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol (1985) 2000;88(6):1989–1995. doi: 10.1152/jappl.2000.88.6.1989. [DOI] [PubMed] [Google Scholar]
- 30.Newton M.F., O’Donnell D.E., Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042–1050. doi: 10.1378/chest.121.4.1042. [DOI] [PubMed] [Google Scholar]
- 31.Chou K.T., Su K.C., Hsiao Y.H., et al. Post-bronchodilator Reversibility of FEV1 and eosinophilic airway inflammation in COPD. Arch Bronconeumol. 2017;53(10):547–553. doi: 10.1016/j.arbres.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Papi A., Romagnoli M., Baraldo S., et al. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(5):1773–1777. doi: 10.1164/ajrccm.162.5.9910112. [DOI] [PubMed] [Google Scholar]
- 33.Proboszcz M., Mycroft K., Paplinska-Goryca M., et al. Relationship between blood and induced sputum eosinophils, bronchial hyperresponsiveness and reversibility of airway obstruction in mild-to-moderate chronic obstructive pulmonary disease. COPD. 2019;16(5-6):354–361. doi: 10.1080/15412555.2019.1675150. [DOI] [PubMed] [Google Scholar]
- 34.Van Rossem I., Vandevoorde J., Hanon S., Deridder S., Vanderhelst E. The stability of blood eosinophils in stable chronic obstructive pulmonary disease: a retrospective study in Belgian primary care. BMC Pulm Med. 2020;20(1):200. doi: 10.1186/s12890-020-01234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumann D.M., Tamm M., Kostikas K., Stolz D. Stability of the blood eosinophilic phenotype in stable and exacerbated COPD. Chest. 2019;156(3):456–465. doi: 10.1016/j.chest.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Tashkin D.P., Altose M.D., Connett J.E., Kanner R.E., Lee W.W., Wise R.A. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am J Respir Crit Care Med. 1996;153(6 pt 1):1802–1811. doi: 10.1164/ajrccm.153.6.8665038. [DOI] [PubMed] [Google Scholar]
- 37.Vestbo J., Edwards L.D., Scanlon P.D., et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 38.Anthonisen N.R., Lindgren P.G., Tashkin D.P., et al. Bronchodilator response in the lung health study over 11 yrs. Eur Respir J. 2005;26(1):45–51. doi: 10.1183/09031936.05.00102604. [DOI] [PubMed] [Google Scholar]
- 39.Scanlon P.D., Connett J.E., Waller L.A., et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 pt 1):381–390. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 40.Chung K.F. The role of airway smooth muscle in the pathogenesis of airway wall remodeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(4):347–354. doi: 10.1513/pats.200504-028SR. discussion 371-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.