Abstract
Circadian sleep wake disorders are common. As they represent conflict between the timing of the patient’s endogenous rhythms and desired timing of sleep, the presenting complaints may include both difficulty of sleep initiation or maintenance, as well as undesired or unplanned daytime or early evening sleepiness. Therefore, circadian disorders may be misdiagnosed as either a primary insomnia or hypersomnia disorder, depending on which complaint is more troublesome for the patient. Objective information about sleep and wake patterns over long periods of time is crucial for accurate diagnosis. Actigraphy provides long-term information about the rest/activity pattern about an individual. However, caution should be applied in interpretation of the results, as the information provided only includes information of movements, and activity is only an indirect circadian phase marker. Timing of light and melatonin therapy is critical for successful treatment of circadian rhythm disorders. Therefore, results of actigraphy are useful and should be used in conjunction with additional measurements, including 24 hour sleep-wake history, sleep log, and melatonin measurements.
Keywords: Circadian rhythm disorders, actigraphy
Circadian rhythms are among the two key processes that regulate the timing and continuity of sleep and wakefulness. Directed by the suprachiasmatic nucleus, the circadian system influences a wide variety of functions, including metabolism, hormone release, sleep-wake propensity, and activity.
1. CIRCADIAN RHYTHM DISORDERS
Circadian rhythm sleep-wake disorders (CRSWDs) are characterized by a misalignment between the desired timing of sleep and the ability to fall asleep and remain asleep. While circadian rhythm disorders usually manifest as extremely early or late sleep times, they may also frequently present as insomnia, and/or excessive daytime sleepiness (EDS). When a patient is attempting to sleep at a conventional clock time instead of aligning sleep according to his or her underlying circadian rhythm of sleep-wake propensity, they would experience difficulty initiating or maintaining sleep. Conversely, when that patient tries to stay awake and remain alert during the endogenous sleep phase of their circadian cycle, he or she will experience excessive daytime sleepiness, as commonly seen with night shift workers or patients with delayed sleep-wake phase disorder. Regardless of type, circadian rhythm sleep disorders can cause a major impairment of work, school, and social activities, lead to disruptions of social and family life, and increase the risk of developing many other medical complaints and disorders.
While prevalence in the population remains unknown (1), some estimates hold that up to 3% of the adult population suffers from a circadian rhythm sleep-wake disorder (2), with a higher prevalence (7–16%) in adolescents and young adults (1).
Despite their high prevalence, CRSWDs are commonly misdiagnosed as a primary insomnia disorder or, in some situations, a hypersomnia disorder, due to insufficient history taking. In these cases, hypnotic treatments for insomnia or wake promoting treatments for hypersomnia symptoms are often unsuccessful and, expensive, and sometimes harmful or lead to delay in accurate diagnosis and effective treatment when there is an underlying CRSWDed. The diagnosis of CRSWDs circadian rhythm disorders is based on a careful sleep history. While these disorders are presumed to their name implies that they result from an abnormality of the circadian timing system, current diagnostic criteria do not require assessment of circadian rhythms in the diagnosis of a CRSWD. However, measurable and/or objective evidence is needed to better assess the sleep-wake pattern to support an accurate diagnosis and tailoring of the timing of administration of appropriate light therapy and melatonin or other medication or behavioral treatment options.
Currently, multiple instruments including sleep logs and sleep diaries are used in the diagnostic process, as well as standardized questionnaires about chronotype (Morningness-Eveningness Questionnaires, Munich Chronotype Questionnaire) (3,4). Plasma melatonin and salivary dim light melatonin onset (DLMO) measurements and core body temperature are used in research applications and aid diagnostic precision of CRSWDs (5,6) but have remained of limited utility in usual clinical practice settings due to their labor intensiveness, expense, and special resources and settings that are required for accurate measurement, so these are typically not employed for routine clinic use, although home sampling of DLMO may be considered (see further discussion in Section 3 below). Urinary 6-sulfatoxymelatonin may be useful in very specific clinical situations such as non-24-hour sleep wake disorder (7). Polysomnography (PSG), while a gold standard for assessment of sleep duration and quality, does not provide information about circadian phase and is not routinely used with CRSWD patients unless a comorbid sleep disorder is suspected. Actigraphy is an objective measure that is useful in clinical practice for assessment of multi-day sleep/wake patterns to aid diagnosis of CRSWDs.
2. ACTIGRAPHY
Actigraphy is helpful for the diagnosis and management of insomnia, circadian rhythm disorders, and excessive daytime sleepiness (EDS) as it provides objective data regarding longitudinal sleep-wake patterns in the patient’s usual home and work environment. Actigraphy, in addition to a sleep log, is particularly helpful for the evaluation and treatment of CRSWDs (1,8). An actigraph is a small, light-weight wrist (or ankle) -worn computerized accelerometer with the ability to measure rest-activity patterns continuously over long periods of time (several days, or as long as weeks to months) (10,11). After at least 7 days of data collection (to include both weekdays and a weekend), data are downloaded to a computer where off-line analysis is performed.
Minimal actigraph technical requirements include: a triaxial accelerometer, the ability to record data in 30 seconds or 1-minute epochs, battery power to record data, the ability to store at least 7 days of data, and support for both device and software. Additional recommended features include: a water proof case, an ambient light sensor, an event marker, a nonvolatile memory, a battery that can store data for a minimum of two weeks, and a small, comfortable profile (9). Although actigraphs provide objective data about rest-activity patterns, supplemental information from a sleep log or diary recording the patient’s habitual, bed and wake times and any awakenings and their durations, a light sensor, as well as a sensor to light spectrum, and an event marker can improve the usefulness and accuracy of the activity data. Data collected over longer periods can be useful in the diagnosis of non-24 or irregular sleep wake rhythm disorder (11). Most activity monitors come with automated software packages that score the data as sleep or wake, and the estimates of sleep and wake are typically improved by manual overscoring referencing the additional data provided by sleep logs and light detection patterns to correctly interpret the actigraphy data. Scoring algorithms differ between manufacturers and can produce different sleep-wake scoring on the same data file. Some are optimized for specific patient populations (adult vs. pediatric).
The evaluation of the sensitivity, specificity, and agreement of actigraphy compared to PSG is related to the device itself, the software (algorithm) used to analyze the data, and often settings within the software that can be adjusted by the user. Actigraphy tends to overestimate sleep and underestimate wake time and the accuracy declines as sleep quality declines. (11, 12, 13, 14).
Various actigraphy devices and software algorithms have been compared in validation studies to polysomnography with high levels of sensitivity and specificity for rest/activity patterns reported in various patient populations, in terms of rest/activity patterns (11,15,16). However, it is important to remember that the diagnosis of CRSWDs circadian rhythm disorders does not routinely include polysomnography, which classically in the US captures a single night of sleep and thus does not provide information about longer term sleep-wake patterns that are necessary to observe for accurate diagnosis of CRSWDs. (28)
3. CIRCADIAN RHYTHM DISORDER ASSESSMENT -DLMO
To accurately determine the timing of the circadian system in a patient with a suspected CRSWD, an at-home or clinic assessment of their dim light salivary melatonin onset may be useful (20, 21). Melatonin is a hormone produced by the pineal gland, and production of melatonin is suppressed by light. Melatonin levels are typically undetectable during the daytime hours and begin to rise in the evening, 1–2 hours before the individual’s usual bedtime. The levels reach a peak in the middle of the usual sleep episode, and then decline throughout the latter part of the night, reaching undetectable levels again the next day. Due to suppression by light, melatonin levels must be measured in dim light conditions (ideally less than 20 lux). DMLO measurement are, traditionally performed in a laboratory over several days, but can also be performed by the patient in their home (21). For CRSWD patients, a sleep-log should be used to determine usual bedtime. The DLMO assessment process should begin 5–7 hours before usual bedtime (average time from the past week), with the patient maintained in a dim light environment throughout the study, including for one hour before the first sample is taken and ensuing hourly saliva samples which are collected every hour from the start until at least 1 hour after usual bedtime. Each sample is frozen after collection, and the next day the samples are transferred to an assay facility where a commercial ELISA or RIA kit is used to determine the melatonin level in each sample. Linear interpolation between the resulting assay values is used to determine the time at which melatonin values rise above a set threshold (typically 3 pg/mL; 22), the dim light salivary melatonin onset (DLSMO). The timing of the DLSMO in the patient can then be compared with their usual sleep onset time, as well as with normative data, to guide diagnosis and treatment.
4. TIMING AND EFFECT OF LIGHT.
The period (cycle length) of the circadian system is close to, (although not exactly), 24 hours. In healthy sighted humans the endogenous period/cycle length averages 24.2 hours, ranging from about 23.5 to 24.5 hours (23, 24). The endogenous circadian clock must be regularly reset to remain synchronized to the 24-hour clock time in the environment, a process called entrainment. Light is the most powerful environmental influence on the human circadian timing system, and it is through regular light-dark exposure that the circadian timing system of humans is synchronized on a daily basis and is reset when traveling across time zones. Many features of the light to which we are exposed determine the entrainment or resetting process, including the spectral composition, intensity, duration, timing, and overall pattern (including timing and duration of darkness) (25). One feature of the circadian response to light is that it is phase dependent. That means that the same light stimulus, when applied at different phases (times of day) can produce different responses. Light in the late afternoon or early evening (i.e. 4 to 7 p.m.) produces phase delay shifts (shifts to a later hour), whereas light exposure in the late biological night or early morning biological day produces phase advance shifts (shifts earlier), as determined by relationship of light exposure to the body core temperature nadir point in the early morning hours. Light exposure in the middle of the biological day typically produces only minimal changes in phase. These responses can be summarized in a phase response curve (26, 27). From our understanding that most humans have a circadian period longer than 24 hours, most people need their overall daily light exposure to produce a slight phase advance (on average 0.2 hours, or 12 minutes) in order to remain entrained. To achieve entrainment most people need to get more morning light exposure than evening light exposure. Being able to correctly identify the timing of the circadian phase is critical for effective light therapy for CRSWDs. Mistiming the light by even a few hours can result in either no effect on phase or a phase shift in the wrong direction, which can exacerbate misentrainment and resultant symptoms of insomnia or hypersomnia.
5. CASES
Case 1
A 27 year old non-sighted man presented to clinic with a complaint of inability to control a shifting sleep schedule. The patient lost his sight 7 years earlier due to retinitis pigmentosa, but was otherwise in good health. Following vision loss, his bedtime continually shifted 15–30 minutes later each night with a corresponding drift in his awakening time. After 3–4 weeks he would reset to an earlier bedtime and the shifting would begin again. Four months of actigraphy data are shown below in the form of a double raster plot, which compresses the activity data over long periods of time. Activity, shown by the dark bars, and sleep time, shown by the white space, shift gradually later until they reset after approximately two weeks (Fig1). This pattern is consistent with non 24h sleep wake phase disorder.
Figure 1:

Actigraphy data of Case 1
Actigraphy is more accurate than sleep logs, particularly for long periods of time. While melatonin is more sensitive than actigraphy, in this case multiple samples would be required because of the shifting circadian phase. History and actigraphy confirmed diagnosis of Non-24 hour sleep wake disorder.
Case 2:
Figure 2: Melatonin levels (pg\mL) of case 2 by time
Figure 2:

Activity pattern of case 2
A 44-year-old woman reported lifelong difficulty awakening and performing any cognitive tasks in the morning and preferred performing work-related tasks at night, and it was most common for her to go to her office late in the evening and work overnight until 5–6 am.
The top graph shows the daily activity pattern over 7 days (Fig 2). A cumulative graph of the activity pattern for this patient is shown below (Fig 3). As this patient’s activity pattern was fairly consistent over the course of the recording, this combined graph allows easier visualization of the activity pattern, with minimal activity between 7am and 2:30 pm. Since DLMO usually precedes typical bedtime by 1–2 hours, based on this activity pattern, one might expect that the patient’s DLMO is close to 4–5 am. However, the following melatonin profile was obtained:
Figure 3:
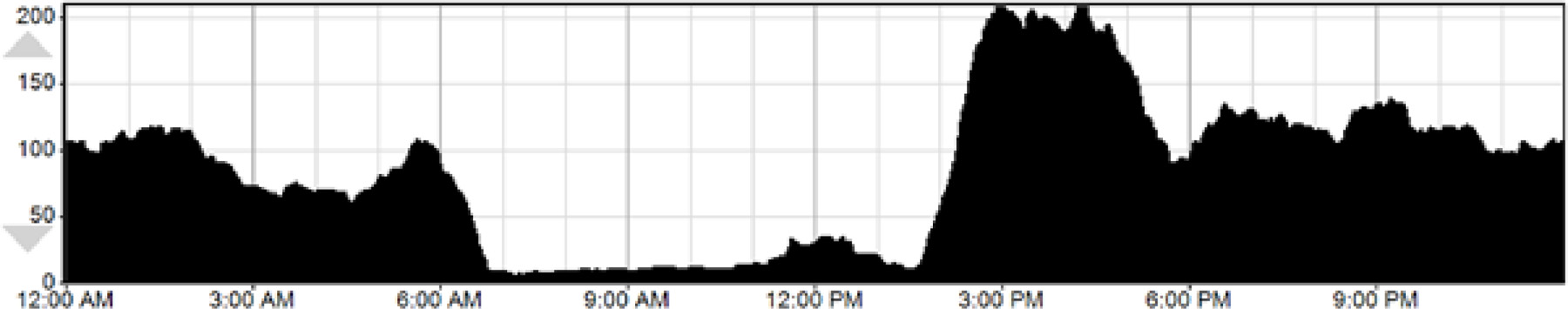
Daily activity patterns of Case 2
As seen, DLSMO was earlier at 12:30 am (Fig 4). For treatment, melatonin 0.5 mg was used to advance this patient’s sleep phase, taken at 11:30 pm, and maintaining light levels low after that. Based on the patient’s sleep log and actigraphy data, light therapy given at 8 am, the patient’s desired rise time, might fall on the part of the phase response curve that would produce phase delay. However, the melatonin measurements provide more precise information, and thus, light therapy at rise time may be beneficial to help advance this patient’s sleep phase.
Figure 4:

DLSMO of Case 2
Case 3:
An 82 year old man was reported by his family to have a five year history of short term memory loss, paralleled by erratic sleep habits. Before his memory loss began, he had a regular sleep schedule with a usual 10 pm bedtime and 6 am awakening time. However, for the last 2–3 years, he would become agitated in the evenings, and he would often be unable to initiate sleep until 1 am, and would awaken by 4 or 5 am, frequently noted to doze off and on through the daytime hours and he would also take 2 or 3 naps each lasting 1–2 hours in duration throughout the afternoon and early evening. His actigraphy is shown below.
Actigraphy shows a highly chaotic sleep wake schedule, without an average consistent bed or rise time, several short bouts of inactivity and probable sleep, with intervening brief periods of movement and wakefulness (Fig 5).
Figure 5:

Actigraphy of Case 3
These actigraphy findings support the clinical history and diagnosis of an irregular sleep wake rhythm disorder. This disorder is most common in elderly individuals with a neurodegenerative disorder, and likely arise from degeneration of the suprachiasmatic nucleus, the brain’s master clock which coordinates circadian rhythmicity and a consolidated sleep wake schedule. Less frequently, this disorder may also be seen in children or adolescents with an underlying neurodevelopmental disorder.
Summary and conclusions:
Actigraphy provides an objective, relatively inexpensive, and helpful information about long term patterns of activity and rest, which can be helpful in evaluating patients with circadian rhythm sleep wake disorders. Actigraphy provides data on sleep wake patterns, and is not a specific circadian phase marker, so any information provided by actigraphy should be reviewed in the context of each individual patient’s clinical scenario, sleep log, and if possible, results of dim light melatonin onset profile for optimal guidance of therapy.
Funding source:
None
Appendix
Section 1. Diagnostic Criteria for Delayed Sleep-Wake Phase Disordera
A. There is a significant delay in the phase of the major sleep episode in relation to the desired or required sleep time and wake-up time, as evidenced by a chronic or recurrent complaint by the patient or a caregiver of the inability to fall asleep and difficulty awakening at a desired or required clock time.
B. The symptoms are present for at least 3 months.
C. When patients are allowed to choose their ad libitum schedule, they will exhibit improved sleep quality and duration for age and maintain a delayed phase of the 24-hour sleep-wake pattern.
D. Sleep log and, whenever possible, actigraphy monitoring for at least 7 days (preferably 14 days) demonstrate a delay in the timing of the habitual sleep period. Both work/school days and free days must be included within this monitoring.
E. The sleep disturbance is not better explained by another current sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder.
Section 2. Diagnostic Criteria for Advanced Sleep-Wake Phase Disordera
A. There is an advance (early timing) in the phase of the major sleep episode in relation to the desired or required sleep time and wake-up time, as evidenced by a chronic or recurrent complaint of difficulty staying awake until the required or desired conventional bedtime, together with an inability to remain asleep until the required or desired time for awakening.
B. Symptoms are present for at least 3 months.
C. When patients are allowed to sleep in accordance with their internal biological clock, sleep quality and duration are improved with a consistent but advanced timing of the major sleep episode.
D. Sleep log and, whenever possible, actigraphy monitoring for at least 7 days (preferably 14 days) demonstrate a stable advance in the timing of the habitual sleep period. Both work/school days and free days must be included within this monitoring.
E. The sleep disturbance is not better explained by another current sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder.
Section 3. Diagnostic Criteria for Irregular Sleep-Wake Rhythm Disordera
A. The patient or caregiver reports a chronic or recurrent pattern of irregular sleep and wake episodes throughout the 24-hour period, characterized by symptoms of insomnia during the scheduled sleep period (usually at night), excessive sleepiness (napping) during the day, or both.
B. Symptoms are present for at least 3 months.
C. Sleep log and, whenever possible, actigraphy monitoring for at least 7 days (preferably 14 days) demonstrate no major sleep period and multiple irregular sleep bouts with at least three brief sleep periods during a 24-hour period.
D. The sleep disturbance is not better explained by another current sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder.
Section 4. Diagnostic Criteria for Non-24-Hour Sleep-Wake Rhythm Disorder
A. There is a history of insomnia, excessive daytime sleepiness, or both, which alternate with asymptomatic episodes, due to misalignment between the 24-hour light-dark cycle and the non-entrained endogenous circadian rhythm of sleep-wake propensity.
B. Symptoms persist over the course of at least three months.
C. Daily sleep logs and actigraphy for at least 14 days, preferably longer for blind persons, demonstrate a pattern of sleep and wake times that typically delay each day, with a circadian period that is usually longer than 24 hours.
D. The sleep disturbance is not better explained by another current sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder.
Footnotes
Conflicts of interest: None
Modified from the American Academy of Sleep Medicine.40 2014 American Academy of Sleep Medicine.
References
- 1.American Academy of Sleep Medicine. International classification of sleep disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 2.Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res 1993; 2:51–55. [DOI] [PubMed] [Google Scholar]
- 3.Horne JA, Östberg O A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms”. Int J Chronobiol 1976;4(2):97–110. [PubMed] [Google Scholar]
- 4.The search for circadian clock components in humans: new perspectives for association studies. Allebrandt KV, Roenneberg T. Braz J Med Biol Res 2008. Aug;41(8):716–21 [DOI] [PubMed] [Google Scholar]
- 5.Pavlova M Circadian Rhythm Sleep-Wake Disorders. Contin Lifelong Learn Neurol 2017;23(4, SleepNeurology):1051–1063. [DOI] [PubMed] [Google Scholar]
- 6.Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, Kayumov L. Dim light melatonin onset (DLMO): A toll for the analysis of the circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry 2007. Jan 30;31(1) [DOI] [PubMed] [Google Scholar]
- 7.Flynn-Evans EE, Tabandeh H, Skene DJ, Lockley SW. Circadian Rhythm Disorders and Melatonin Production in 127 Blind Women with and without Light Perception. J Biol Rhythms 2014;29(3):215–224. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. Use of Actigraphy for the Evaluation of Sleep Disorders J Clin Sleep Med 2018. Jul 15;14(7):1231–1237). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Society of behavioral sleep medicine guide to actigraphy monitoring: clinical and research application. Behavioral Sleep Medicine 2015. 13: S1–S3 [DOI] [PubMed] [Google Scholar]
- 10.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med 2001;2 :389–96. [DOI] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003; 26:342–92 [DOI] [PubMed] [Google Scholar]
- 12.Lichstein KL1, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, Lester KW, Aguillard RN. Actigraphy validation with insomnia. Sleep 2006. Feb;29(2):232–9.) [PubMed] [Google Scholar]
- 13.Hauri PJ, Wisbey J. Actigraphy and insomnia: a closer look. Part 2. Sleep 1994. Aug;17(5):408–410 [PubMed] [Google Scholar]
- 14.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev 2002. Apr;6(2):113–24 [DOI] [PubMed] [Google Scholar]
- 15.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A Jr, Coleman J, Lee-Chiong T, Pancer J, Swick TJ; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007. Apr;30(4):519–29 [DOI] [PubMed] [Google Scholar]
- 16.Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013. Nov 1;36(11):1747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak CP1, Tryon WW, Nagaraja H, Dzwonczyk R How accurately does wrist actigraphy identify state of sleep and wakefulness? Sleep 2001. Dec 15;24(8):957–65 [DOI] [PubMed] [Google Scholar]
- 18.Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res 2001. Oct;31(3):264–72 [DOI] [PubMed] [Google Scholar]
- 19.Kantermann T, Sung H, Burgess HJ. Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J Biol Rhythms 2015. Oct;30(5):449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullman RE, Roepke SE, Duffy JF. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO). Sleep Medicine 2012; 13:703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess HJ, Park M, Wyatt JK, Fogg LF. Home dim light melatonin onsets with measures of compliance in delayed sleep phase disorder. J Sleep Res 2016. Jun;25(3):314–7. [DOI] [PubMed] [Google Scholar]
- 22.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med 2008. Feb 15;4(1):66–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999; 284:2177–2181. [DOI] [PubMed] [Google Scholar]
- 24.Duffy JF, Cain SW, Chang A-M, Phillips AJK, Munch MY, Gronfier C, Wyatt JK, Dijk D-J, Wright Jr, KP, Czeisler, CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proceedings of the National Academy of Sciences USA 2011; 108 Suppl 3:15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy JF, Czeisler CA. Effect of light on human circadian physiology. [Invited review, Peer-reviewed] Sleep Medicine Clinics 2009; 4:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol 2003. Jun 15;549(Pt 3):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol 2012. Jul 1;590(13):3035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Loube DL, Owens J, Pancer JP, Wise M. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 2005. Apr;28(4):499–521. Review. [DOI] [PubMed] [Google Scholar]


