Abstract
An idiopathic macular hole is an anatomic defect of the neurosensory retina that develops in the fovea. In this report, we present three macular hole cases that are refractory standard macular hole surgery and treated with AM transplantation (AMT). We reached anatomical success in all three cases without any complications or adverse effects. AMT is effective in achieving satisfactory hole closure for cases that are refractory standard surgery.
Keywords: Amnoitic membrane, macular hole, retina pigment epithelial cell
Introduction
Idiopathic macular hole is a full thickness defect involving the neurosensory retina at the fovea and treated surgically (1,2). Kelly and Wendel first described the surgical technique as pars plana vitrectomy, posterior cortical vitreous ± internal limiting membrane (ILM) removing, intraocular gas tamponade, and face down positioning in 1991 (1). Nowadays, surgical success rates reach over 90% with modified surgical techniques including triamsinolon asetonide using during surgery, ILM peeling and using inverted ILM flap (3,4). Other modifications in the technique are larger ILM peelings, using autologus gluconated blood clumps, transforming growth factor-beta-2, tissue glue, gelatin plug, or human amniotic membrane (AM) (4-6). These modifications can be used in larger, Stages 3–4 or recurrent holes (3,4-6).
AM consists of a single layer of an epithelium, a thick basement membrane and the avascular mesenchymal tissue (7,8). AM cells have pluripotent differentiation potentials, anti-inflammatory, anti-bacterial, anti-viral effects, and a low immunogenicity that makes it a suitable source for transplantation (8,9). AM simplifies epithelial cell migration, strenghtens basal epithelial cell adhesion, promotes epitheal cell differentiation, prevents epithelial apoptosis, and produces growth factors to stimulate epithelization (9,10).
Here, we report three macular hole cases that refractory standard surgery and treated with AM transplantation (AMT). All surgeries were made by one of the authors (YT). Written informed consent for publication was obtained from patients for publication of this case report and any accompanying images.
Case Report
Case 1 – A 65-year-old man complained of decreased vision in his right eye for 1 year. He underwent retinal detachment surgery (pars plana vitrectomy, ILM peeling, laser photocoagulation, and silicon oil injection) 1 year ago. His best-corrected visual acuity (BCVA) was counting fingers from 50 cm in his right eye and 20/20 in the left. Anterior segment examination revealed an emulsified silicon oil in the anterior chamber in his right eye, he was bilateral pseudophakic. Funduscopy revealed an image of a full thickness macular hole that was confirmed by spectral domain optical coherence tomography (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany) (Fig.1a).
Figure 1.
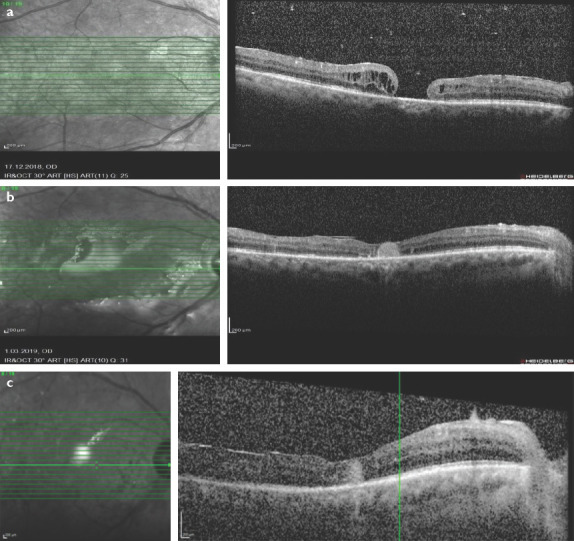
(a) Pre-operative optical coherence tomography (OCT) image revealed a full thickness macular hole. (b) 10 days after amniotic membrane transplantation surgery macular hole was closed in the OCT scan. (c) 1.5 months after surgery after silicon intake hole was still closed and neurosensory retina was overfilling the AM in the OCT scan.
The patient underwent 25 G 3-port vitrectomy, silicon oil removal, anterior chamber lavage, AMT, and fluid-air-decaline-air-silicon exchange. Visualization during vitrectomy was achieved with a non-contact wide-angle system (Eibos system, Moller-Wedel international). AM was taken from human AM patch. The AM inserted through the trocar into the vitreous cavity by vitreoretinal forceps. It is manipulated under decaline transplanted through macular hole into the subretinal space and extended to cover all area of the hole. The patient was ordered to prone position for 3 days after surgery. Ten days after surgery his BCVA was 5/100 in his right eye, hole was closed, and neurosensory retina was overfilling the AM (Fig. 1b). 1.5 months after surgery his BCVA was 8/100 in his right eye and hole was still closed (Fig. 1c).
Case 2 – A 65-year-old woman complained of decreased vision in her right eye for 6 months. Her BCVA was 3/20 in her right eye and 20/20 in the left. Anterior segment examination revealed a nuclear cataract on her both eyes. Funduscopy revealed an image of a full thickness macular hole with epiretinal membrane that was confirmed by SD-OCT (Heidelberg Engineering, Heidelberg, Germany) (Fig. 2a).
Figure 2.
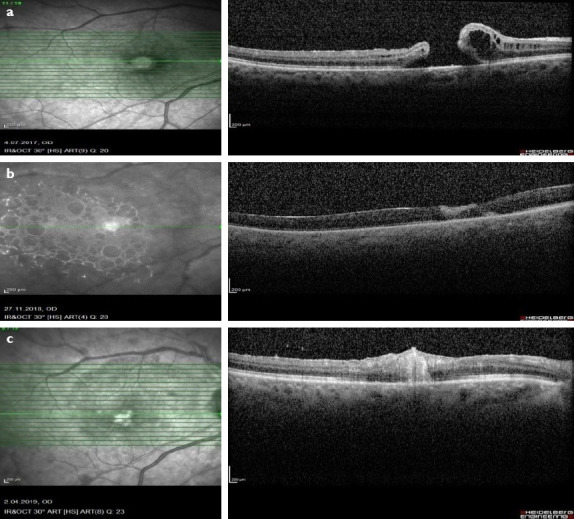
(a) Pre-operative optical coherence tomography (OCT) image revealed a full thickness macular. (b) OCT scan showed that hole was closed and AM was in place 20 days after surgery. (c) 3 months after surgery after silicon intake OCT scan revealed macular hole was closed.
The patient underwent phacoemulsification, 25 G 3-port vitrectomy, epiretinal membrane peeling, fluid-air-12% sulfur hexafluoride (SF6) exchange. Visualization during vitrectomy was achieved with a noncontact wide-angle system (Eibos system, Moller-Wedel international). Eleven months after surgery, the hole did not close. Then, she underwent 25 G 3-port vitrectomy, ILM peeling, and fluid-decaline-air-SF6 exchange. Eighteen months after surgery, macular hole was still present. Then, she had 25 G 3-port vitrectomy, AMT (with same technique), and fluid-air-silicon exchange. The patient was ordered to prone position for 3 days after surgery. Twenty days after surgery, her BCVA was 1/20 on her right eye, macular hole was closed, and neurosensory retina was overfilling the AM (Fig. 2b). Three months after surgery, her BCVA was 1/20 in her right eye and hole was still closed (Fig. 2c).
Case 3 – A 72-year-old man complained of decreased vision in his left eye for 1 year. His BCVA was 20/20 in his right eye and 2/20 in the left. Anterior segment examination revealed no further abnormalities, beyond his bilateral pseudophakic status. Funduscopy and SD-OCT revealed an image of a cystoid macular edema. After two injections of intravitreal triamsinolone acetonide, he developed full thickness macular hole that was confirmed by SD-OCT (Heidelberg Engineering, Heidelberg, Germany). His BCVA was 1/20 in his left eye.
He underwent 3-port vitrectomy, ILM peeling, and fluid-air-12% SF6 exchange. Visualization during vitrectomy was achieved with a noncontact wide-angle system (Eibos system, Moller-Wedel international). Ten days after surgery, his BCVA was 1/10 on his left eye, hole was still open and he had cystoid macular edema. He had 1 times intravitreal triamsinolon acetonid, 2 times intravitreal dexamethasone implant injection. 1.5 years after the first operation, his BCVA was counting fingers from 1 m and hole was open (Fig. 3a). Then, he underwent three-port vitrectomy, anterior vitrectomy for adherent vitreous band on the nasal side of the iris, subretinal fluid aspiration from the hole region, and fluid-air-20% SF6 exchange. Three months after surgery hole did not close, his BCVA was same. Then, he had three-port vitrectomy, AMT (with same technique), and fluid-air-silicon exchange. The patient was ordered to prone position for 3 days after surgery. Twenty days after surgery, his BCVA was counting fingers from 20 centimeters, hole was closed, and neurosensory retina was overfilling the AM (Fig. 3b). Six months after surgery, his BCVA was same and hole was still closed (Fig. 3c).
Figure 3.
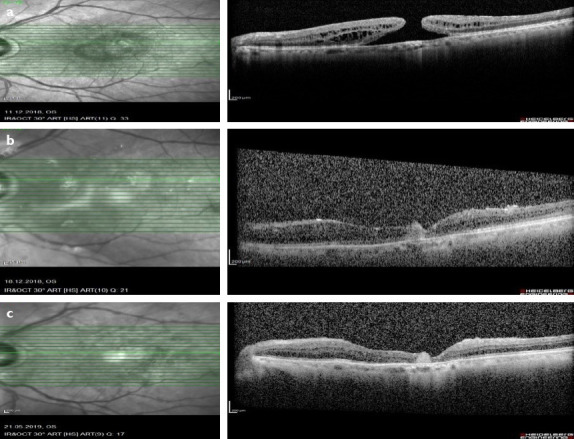
(a) Full thickness macular hole was seen in preoperatively optical coherence tomography (OCT) image. (b) OCT scan showed that hole was closed and AM was in place 20 days after surgery. (c) 6 months after surgery hole was still closed and neurosensory retina was overfilling the AM in the OCT scan.
Discussion
AM is widely used for ocular surface pathologies in ophthalmology for about 80 years. AM has many good features such as anti-fibrotic, anti-inflammatory, antimicrobial properties; pluripotent differentiation potentials, fascilitating epithelization, and promoting epithelial cell differentiation (9-11).
Rosenfeld was the first who had the idea of using AM in retinal pathologies. He implantated AM in the subretinal space in a rabbit model without any inflammation or side effects in 1999 (12). Then, Capeans cultured human RPE cells over human AM. They observed attachment and growth of RPE cells over the AM and cells maintained epithelial phenotype (13). In 2004, Stanzel et al. showed that AM could be used as a basement membrane containing matrix to promote growth and differentiation of rabbit RPE cells in culture (14).
In 2012, Kiilgaard et al. transplanted AM to the subretinal space in pigs and showed that AM was covered with a monolayer of pigmented cells that had a contact with the host RPE, showed epithelial phenotype, produced growth factors for continuing retinal homeostasis (15). In 2017 Zhu et al. used biocompatible AM plug for choroidal hole repairing in a case and they reported that the hole was closed, the plugged AM organized and vascularized after 3 months (16).
Rizzo et al. used human AM plug in their prospective study. They enrolled eight patients with recurrent macular hole and six patients with retinal detachment. In the both of the groups, macular hole closure demonstrated 1 week after surgery in all cases and BCVA was improved. They reported that the repaired retina organized in normal layers like original retina and had tight adhesion with the bellowing AM. They did not notice any side effects or complications (5).
In this report, we present three persistent macular hole cases. All cases had undergone PPV with ILM peeling firstly. Case 1 had previous retinal detachment surgery. We put the human AM plug in the subretinal space. We used decline during surgery and silicone oil tamponade after surgery maintaining the position of AM plug. Rizzo et al. used 20% SF6 in macular hole group, silicion oil tamponade in the retinal detachment group. In all cases, we showed macular hole closure by OCT. Similar to Rizzo et al.’s study, our patients OCT scans demonstrated that repaired retina organized in normal layers like original retina and had tight adhesion with the bellowing AM. We reached anathomical success in all cases but only one case gain vision increase. In Rizzo’s study, both of the groups showed increase in BCVA. We did not report any side effects or complications similarly. The disadvantages of the technique are its difficulty and long learning time. AMT is not a first step surgery for retinal diseases such as macular hole, retinal breaks, retinal detachments, or choroidal holes but a suitable technique for refractory or recurrent cases.
In recurrent macular holes, ILM plug taken from the periphery of the posterior pole, lens capsule fragments or retinal free flap, transforming growth factor beta 2 or autologus platelet concentrate application are recommended procedures in the literature (17-23). All these techniques increased closure rates from 58% to 96%.
Conclusion
We obtained anatomical success in all three cases without any complication or adverse effects. Further, prospective studies with large study populations and longer follow-up are needed to determine the efficiency of this surgical technique.
Footnotes
Disclosures
Informed consent: Written informed consent was obtained from the patient for the publication of the case report and the accompanying images.
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Surgical and Medical Practices – Y.T.; Concept – F.C.K., Y.T., M.A.Y.; Design – F.C.K.; Data Collection or Processing – F.C.K.; Analysis or Interpretation – F.C.K., Y.T., M.A.Y.; Literature Search – F.C.K.; Writing: F.C.K., Y.T., M.A.Y.
References
- 1.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–9. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RN, Gass JD. Idiopathic macular holes:Observations, stages of formation, and implications for surgical intervention. Ophthalmology. 1988;95:917–24. doi: 10.1016/s0161-6420(88)33075-7. [DOI] [PubMed] [Google Scholar]
- 3.Lai MM, Williams GA. Anatomical and visual outcomes of idiopathic macular hole surgery with internal limiting membrane removal using low-concentration indocyanine green. Retina. 2007;27:477–82. doi: 10.1097/01.iae.0000247166.11120.21. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti M, Benjamin P, Chakrabarti K, Chakrabarti A. Closing macular holes with ''macular plug''without gas tamponade and postoperative posturing. Retina. 2017;37:451–9. doi: 10.1097/IAE.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo S, Caporossi T, Tartaro R, Finoccihio L, Franco F, Barca F, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39:95–103. doi: 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 6.Peyman GA, Daun M, Greve MD, Yang D, Wafapoor H, Rifai A. Surgical closure of macular hole using an absorbable macular plug. Int Ophthalmol. 1997;21:87–91. doi: 10.1023/a:1005866002930. [DOI] [PubMed] [Google Scholar]
- 7.Bourne GL. The microscopic anatomy of the human amnion and chorion. Am J Obstet Gynecol. 1970;79:1070–3. doi: 10.1016/0002-9378(60)90512-3. [DOI] [PubMed] [Google Scholar]
- 8.Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–28. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 9.Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane:From structure and functions to clinical applications. Cell Tissue Res. 2012;349:447–58. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi N, Inatomi T, Sotozono C, Fullwood N, Quantock A, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 11.Rotth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23:522–5. [Google Scholar]
- 12.Rosenfeld PJ, Merritt J, Hernandez E, Mollor D, Rosa RH, Tsong SC. Subretinal implantation of human amniotic membrane:A rabbit model for the replacement of Bruch's membrane during submacular surgery. Invest Ophthalmol Vis Sci. 1999;40:206. [Google Scholar]
- 13.Capeans C, Pineiro A, Pardo M, Seuiro-Lopez C, Blanco MJ, Dominguez A. Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand. 2003;81:271–7. doi: 10.1034/j.1600-0420.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 14.Stanzel BV, Espana EM, Grueterich M, Kawakita T, Parel JM, Tseng SC. Amniotic membrane maintains the phenotype of rabbit retinal pigment epithelialcells in culture. Exp Eye Res. 2005;80:103–12. doi: 10.1016/j.exer.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Kiilgaard JF, Scherfig E, Prause JU, La Cour M. Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cell Int. 2012;2012:716968. doi: 10.1155/2012/716968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu D, Jin X, Zhou J. Transplantation of amniotic membrane for choroidal hole to treat suprachoroidal silicone oil migration. Acta Ophthalmol. 2017;95:522–3. doi: 10.1111/aos.13516. [DOI] [PubMed] [Google Scholar]
- 17.Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157:861–9. doi: 10.1016/j.ajo.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134:229–30. doi: 10.1001/jamaophthalmol.2015.5237. [DOI] [PubMed] [Google Scholar]
- 19.Thomas AS, Mahmoud TH. Subretinal transplantation of an autologous retinal free flap for chronic retinal detachment with proliferative vitreoretinopathy with and without macular hole. Retina. 2017;38:121–4. doi: 10.1097/IAE.0000000000002026. [DOI] [PubMed] [Google Scholar]
- 20.Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36:163–70. doi: 10.1097/IAE.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 21.Peng J, Chen C, Jin H. Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina. 2018;38:2177–83. doi: 10.1097/IAE.0000000000001830. [DOI] [PubMed] [Google Scholar]
- 22.Ie D, Glaser BM, Thompson JT, Sjaarda RN, Gordon LW. Retreatment of full thickness macular holes persisting after prior vitrectomy. A pilot study. Ophthalmology. 1993;100:1787–93. doi: 10.1016/s0161-6420(93)31397-7. [DOI] [PubMed] [Google Scholar]
- 23.Korobelnik JF, Hannouche D, Belayachi N, Branger M, Guez JE. Autologous platelet concentrate as an adjunct in macular hole healing:A pilot study. Ophthalmology. 1996;103:590–4. doi: 10.1016/s0161-6420(96)30648-9. [DOI] [PubMed] [Google Scholar]


