Abstract
Continuing insight into the molecular mechanisms of atopic disorders has enabled the development of biologics to precisely target these diseases. Food allergy (FA) and eosinophilic gastrointestinal disorders (EGIDs) are driven by similar inflammatory molecular mechanisms and exist along the same atopic disease spectrum. Therefore, many of the same biologics are being investigated to target key drivers of mechanisms shared across the disease states. The enormous potential of biologics for the treatment of FA and EGIDs is highlighted by significant increases in the number of ongoing clinical trials, over 30, evaluating their use in these disease states as well as the recent FDA approval of dupilumab for the treatment of eosinophilic esophagitis (EoE). Here we discuss past and current research into the use of biologics in FA and EGIDs and their potential role in improving treatment options in the future with the need to have biologics widely clinically available.
Keywords: Food allergy, eosinophilic gastrointestinal disorders, biologics, oral immunotherapy, eosinophilic esophagitis, eosinophilic gastritis, eosinophilic enteritis, eosinophilic colitis
Introduction
The prevalence of T helper 2 (TH2) cell-driven atopic conditions such as food allergy (FA)1 and eosinophilic gastrointestinal disorders (EGIDs)2 has increased over the last several decades. FA alone affects approximately 33 million people in just the US1, with approximately 1 in 20 of those with FA experiencing concomitant eosinophilic esophagitis (EoE), a 125-fold increase in prevalence over the general population3. This increased prevalence is likely due to substantial mechanistic overlap between not only the two conditions, but also other elements of the atopic march, which also include asthma and atopic dermatitis (AD). Indeed, many biologics that are under investigation in FA and EoE were first studied in asthma or AD, with their utility in the treatment of other atopic diseases being evaluated as early as 2003 (Figure 1).
FIG1.
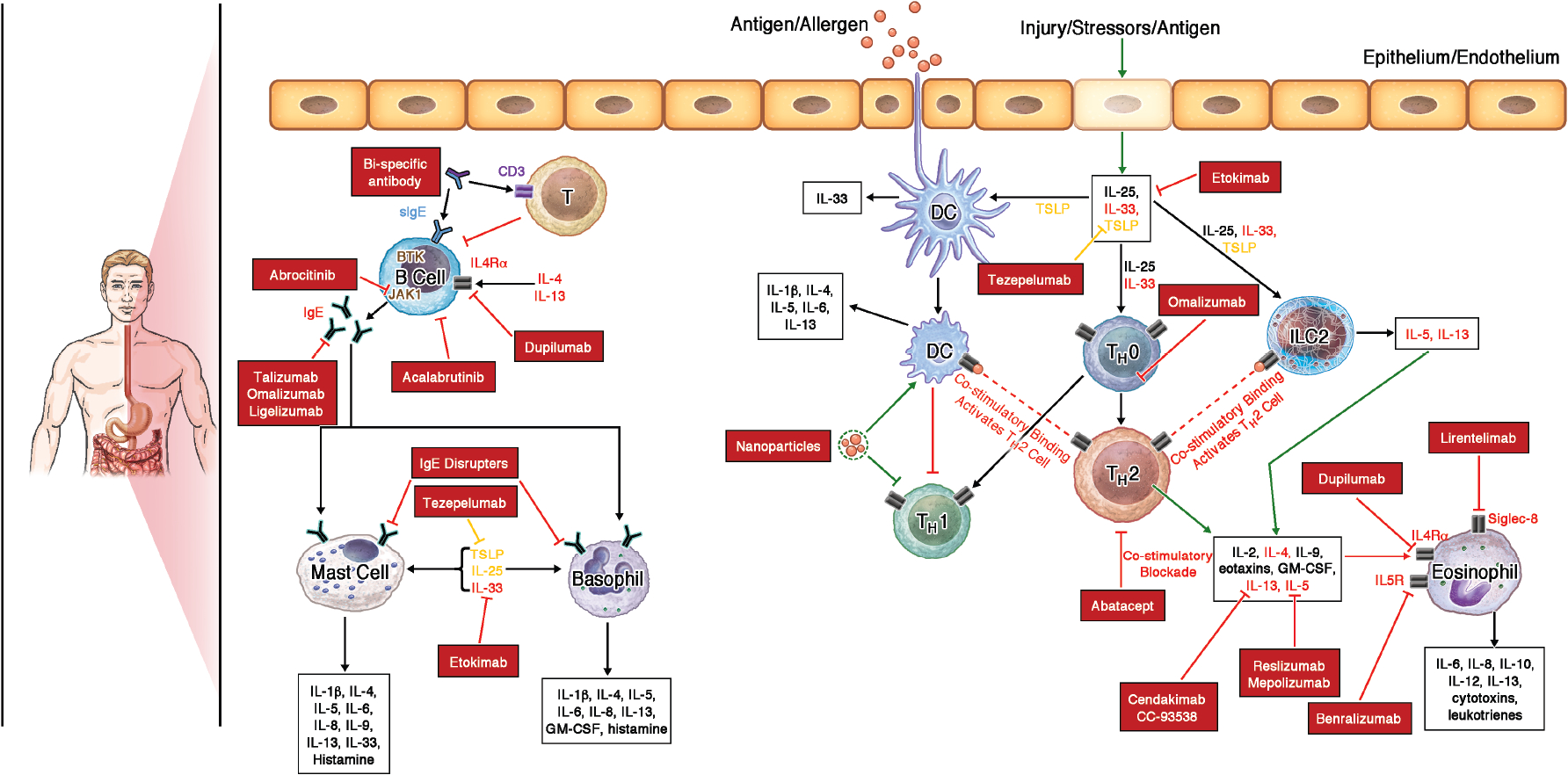
Biologics targeting key allergic pathways. Schematic of major allergic pathways that drive FA and EGIDs highlighting pathways that are targeted by biologics (red boxes) to treat these diseases.
With a better understanding of the mechanisms that drive atopic diseases, there has been an increased interest in using biologics that target the shared TH2, and to a lesser extent, non- TH2 molecular pathways (Figure 2). Atopic diseases can often co-exist; the earliest manifestation is AD which can arise in infancy. Skin barrier defects that are closely linked to the development of AD are also associated with the development of FA and EoE4, 5. These skin barrier defects induce the secretion of ‘alarmins’, signaling molecules such as IL-25, IL-33, and TSLP (Figure 2), which lead to downstream activation of type 2 allergic inflammation and pathological symptoms in atopic diseases, including AD and EoE (Figure 3). This is promising for the treatment of atopic diseases that share similar molecular mechanisms as agents selectively inhibiting such pathways could be used to simultaneously treat multiple related disease states. For example, the recent FDA approval of dupilumab for EoE in May 2022, a drug already approved for treatment of moderate-to-severe AD, nasal polyposis, and asthma. Similarly, Omalizumab has been approved for allergic asthma, chronic spontaneous urticaria, and is being investigated in FA. These examples have encouraged the investigation of biologics in other atopic diseases such as FA and non-esophageal EGIDs. Here, we discuss the role of biologics in FA and EGID in advancing treatment while highlighting key knowledge gaps that remain.
FIG2.
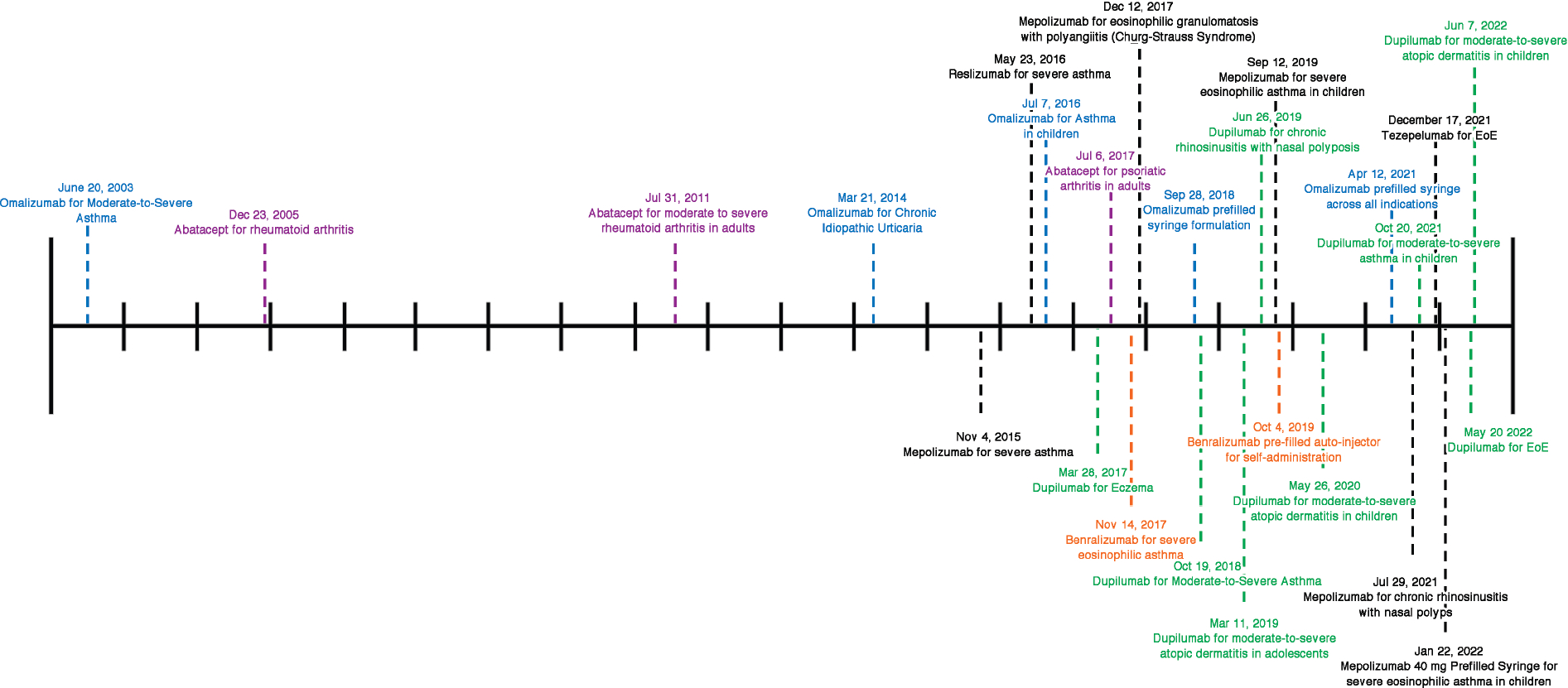
FDA approval of biologics that are used or are under investigation for the treatment of FA and/or EGIDs. Most biologics being investigated for use in FA and/or EGIDs have been approved for use in other atopic diseases. Recent years have seen an explosion of FDA approvals for the use of these biologics for several different diseases. Each tick in the timeline represents 1 year.
FIG3.
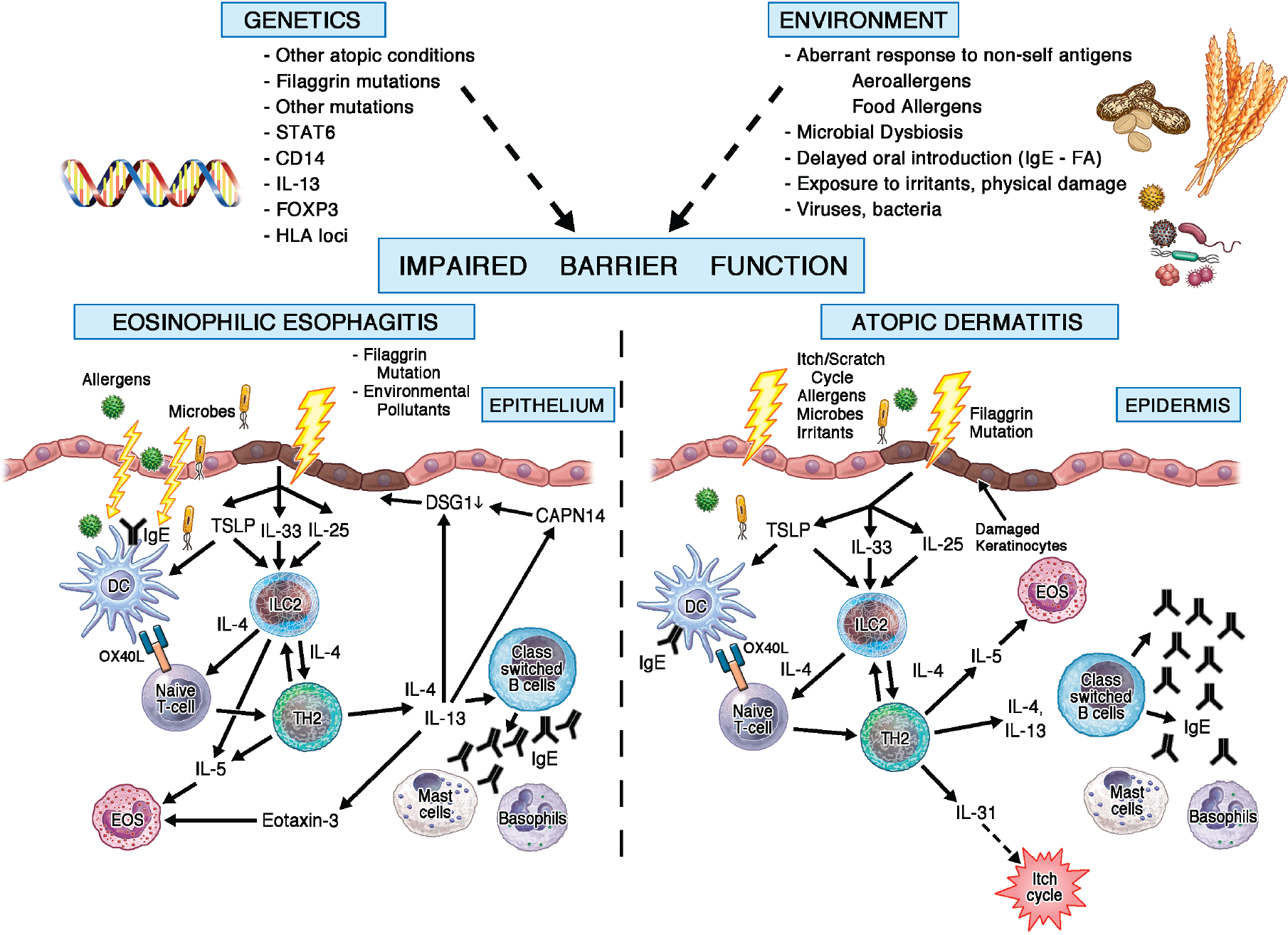
Impaired skin barrier in EoE and AD. Several genetic and environmental factors lead to the disruption of the epithelial skin barrier. This disrupted barrier initiates downstream signaling pathways that drive EoE and AD.
Role of Biologics in Food Allergy
Current knowledge of the mechanisms of FA stems primarily from animal models, however, studies monitoring the response to immunotherapy and biologics and studies on natural resolution in humans6 have helped clarify how tolerance is regulated. Respiratory, skin, and gut barrier disruption and dysfunction are key factors which allow food allergens to permeate through, promote the shift from tolerance to sensitization, and eventually activate antigen-specific B cells and downstream effector responses from eosinophils, basophils, and mast cells7 (Figure 3). Cytokines and alarmins produced by dysregulated epithelial cells and activated antigen presenting cells during sensitization, including IL-25, IL-33, and TSLP, induce naïve CD4+ T cell differentiation into TH2 cells and drive production of additional proinflammatory cytokines such as IL-4, IL-5, IL-9, and IL-13. Large amounts of these TH2 cytokines produced, in part, by innate lymphoid cells 8, disable the induction of tolerance by T cells and drive the recruitment and proliferation of effector cells7. The key drivers of the clinical symptoms associated with an allergic reaction include histamine, leukotrienes, cytokines, and prostaglandins. These inflammatory mediators are released upon degranulation of activated basophils and mast cells after exposure to an antigen (food allergen) resulting from crosslinking of antigen-specific IgE complexes on the cell surface9.
Although numerous studies have demonstrated the safety and efficacy of oral immunotherapy (OIT) for the treatment of FA10–15, adverse events (AEs) to OIT are common, especially during the initial build up phase16–18. This combined with the burden and stress of daily consumption of the allergenic food can make long-term adherence difficult17, 18. Furthermore, some patients may not tolerate OIT or have difficulty achieving desensitization goals, potentially due to differences in disease endotypes19, 20. Biologics have the potential to be used in a non-allergen-specific manner by targeting the underlying mechanisms driving the allergic state thus carrying the advantage of dampening allergic inflammation triggered by many allergens (food and environmental) when used alone or in combination with OIT (Table 1). To date, the most widely investigated strategy to minimize AEs associated with OIT has been the use of anti-IgE biologics21–26.
Table 1.
Ongoing clinical trials in FA.
| Intervention | Trial Number(s) | Phase | Population | Primary Endpoint | ||
|---|---|---|---|---|---|---|
| Biologic agents in food allergy | Anti-IgE | Omalizumab |
NCT04037176
NCT03964051 |
IV | 6–18 yo with allergy to 1 or more foods 18–70 yo with food allergy |
Change in challenge threshold after 18 weeks or 3 months of treatment in patients treated with Omalizumab versus placebo. |
| Omalizumab + peanut OIT | NCT01781637 | II | 7–25 yo with peanut allergy | Tolerance of 2000 mg 6 Weeks After Last Dose of Omalizumab/Placebo [Time Frame: 6 weeks after last dose of omalizumab/placebo] | ||
| Omalizumab +/− multi-allergen OIT | NCT03881696 | III | 1–56 yo with peanut allergy + 2 additional food allergies (milk, egg, wheat, cashew, hazelnut, walnut) | Number of participants to successfully consume a single dose of ≥ 600 mg of peanut protein. [Time Frame: During the DBPCFC at the end of Stage 1: 16 to 20 weeks after Stage 1 treatment initiation] | ||
| Omalizumab + multi-allergen OIT | NCT04045301 | IIb | 6–25 yo with multi-food allergy (≥3 foods) | Efficacy of omalizumab at decreasing time-to-maintenance (1500mg total protein) [Time Frame: 52 weeks) | ||
| Ligelizumab | NCT04984876 | III | 6–55 yo with peanut allergy | Proportion of participants to tolerate a single dose of ≥ 600 mg of peanut protein at week 12. [Time Frame: 12 weeks] | ||
| Anti-IL4Rα | Dupilumab |
NCT03793608
NCT04148352 |
II | 6–17 yo with peanut allergy 4–50 yo with cow’s milk allergy |
Proportion of patients treated with dupilumab monotherapy that pass DBPCFC with peanut protein. [Time Frame: week 24) Proportion of subjects treated with dupilumab plus milk protein OIT vs placebo plus milk protein OIT who tolerate at least 2040 mg (cumulative) cow’s milk protein during DBPCFC to milk at week 18 [Time Frame: Week 18] |
|
| Dupilumab + AR101 (peanut oral immunotherapy) | NCT03682770 | II | 6–17 yo with peanut allergy | Proportion of patients who successfully complete an exit food challenge with 2044 mg cumulative peanut protein. [Time Frame: Up to 40 weeks]. | ||
| Anti-IgE + Anti-IL4Rα | Omalizumab + dupilumab +/− multi-allergen OIT | NCT03679676 | II | 5–55 yo with peanut allergy + 1–2 other food allergies | The success rates of passing a FC to peanut and two other FAs [Time Frame: 44 weeks] | |
| Anti-JAK | Abrocitinib | NCT05069831 | I | 18–50 yo with food allergy | Change in skin prick and basophil activation tests. [Time Frame: baseline and after 4 months of treatment]. | |
| Anti-BTK | Acalabrutinab | NCT05038904 | II | 18+ yo with peanut or tree nut allergy | Change in the highest dose of peanut or tree nut that is tolerated during oral food challenge before and after taking acalabrutinib, as defined by the PRACTALL consensus grading system. [Time Frame: Baseline and Day 2]. | |
| co-stimulatory inhibitor | Abatacept | NCT04872218 | II | 14–50 yo with peanut allergy | Peanut specific/total IgE at week 24 [Time Frame: 24 weeks] | |
Anti-IgE Therapy
Talizumab
The first trial of biologics in FA evaluated the use of talizumab (TNX 901), an anti-IgE biologic, in 12–60-year-old patients with a history of peanut allergy (n=84). Treatment with talizumab was well-tolerated and significantly increased the reaction-eliciting dose of peanut protein from baseline to week 14 to 16 in those receiving 12 weeks of talizumab 450 mg therapy compared to placebo (mean increase, 710 mg vs 2627 mg; p<0.001), with increasing doses of talizumab associated with greater increases in tolerated dose (p<0.001)27. Although these findings supported the efficacy of talizumab, research has since shifted toward newer generations of anti-IgE biologics.
Omalizumab and Ligelizumab
Following in the footsteps of talizumab, the anti-IgE antibody omalizumab has emerged as the most widely investigated biologic in the setting of FA. Omalizumab is currently approved for the treatment of several atopic conditions, including moderate-to-severe persistent asthma in patients aged 6 years and older, chronic spontaneous urticaria in those aged 12 years or older, and nasal polyps in adult patients aged 18 years and older. The most common approach that has been studied for its incorporation in the treatment of FA is a short course of the agent prior to the initiation of OIT. In addition to improving the safety of OIT to single foods such as peanut and milk, pre- and concurrent treatment with omalizumab has shown promise in significantly reducing the time required to reach target maintenance doses of OIT23, 28.
Treatment with omalizumab prior to multi-allergen OIT to 2 or more foods has also been investigated in several trials29–33. Similar to findings of single-allergen therapy, a phase 1 study of 4–15-year-old multi food allergic children, pre-treatment with omalizumab facilitated a faster time to maintenance dose compared to multi-allergen OIT alone (median time 67 weeks vs 85 weeks, respectively)29, 11. Furthermore, in the phase 2 MAP-X clinical trial, in 4–15-year-old children allergic to 2–5 foods, adjunct omalizumab improved the safety of multi-allergen OIT, reduced the severity of AEs, and lowered median per-participant percentage of their OIT doses associated with any AE (27% vs 68% without omalizumab; p=0.0082)30. Gastrointestinal side effects, the most common type of AE during OIT, were diminished with the use of omalizumab (22% vs 54% without omalizumab; p=0.008), though not prevented. Omalizumab also increased the ability to pass a DBPCFC to at least 2 g food protein for 2 or more foods after 36 weeks (83% vs 33% without omalizumab, OR: 10, CI: 1.8–58.3, p=0.004). In line with the improved speed and safety of OIT escalation via adjunct omalizumab for multi-allergen OIT, long-term follow-up studies also demonstrate that omalizumab-facilitated multi-allergen OIT improves patient quality of life32, 33. Reported cases of EoE in some studies is a notable concern with OIT34; however, long-term follow-up is critical to capture the true incidence of EoE, which can occur after completion of study, during long-term maintenance.
Omalizumab has also been studied in a non-antigen-specific manner. In an observational efficacy study, 15 patients with severe allergic asthma and concomitant FA treated with omalizumab as monotherapy had an 8.6-fold increase in the reaction threshold dose across 16 foods, as well as a reduction in reactions related to accidental ingestions (47 in the 4 months before treatment down to 2; p <0.001)35. In an Italian cohort of 54 children with severe allergic asthma and FA, omalizumab monotherapy for approximately 5 months allowed 44% of participants to improve threshold sensitivity to culprit foods36. OUtMATCH (NCT03881696) is a prospective, phase 3 study investigating the safety and efficacy of omalizumab in multi-FAic children and adults (1–55 yo) in 3 stages. Stage 1 will assess monotherapy of omalizumab for 16 weeks compared to placebo37. Stage 2 will assess omalizumab facilitated multi-allergen OIT compared to omalizumab monotherapy. Stage 3 will assess the ability to switch to real-life food equivalents. The trial is currently ongoing and will provide much needed data on monotherapy and combination approaches, as well as long term outcomes.
Approval of self-administration of omalizumab at home for asthma, chronic idiopathic urticaria, and nasal polyposis, has improved access to omalizumab, and has proven to be cost effective38. These factors also make omalizumab an attractive treatment option for FA patients.
Despite the significant benefits to speed and safety of adjunct omalizumab, data on efficacy in promoting sustained unresponsiveness (SU) has been less promising. Similar to findings with OIT alone, sustained desensitization in omalizumab-facilitated multi-allergen OIT in the M-TAX study is more likely to occur with sustained multi-allergen OIT dosing in comparison to discontinuation of OIT dosing (85% with continuation of OIT dosing vs 55% with discontinuation, (OR: 4.5, CI: 1.1–19.3, p= 0.03) in 4–55-year-old patients31. Ligelizumab, another anti-IgE agent with higher binding affinities for free IgE compared to omalizumab, is currently under investigation as monotherapy in a phase 3, 52-week study for peanut allergic patients 6–55 years of age (NCT04984876)39 and may hold promise for food allergy children and adults.
Anti IL-4 and IL-13
Dupilumab
Dupilumab is an anti-IL-4 receptor alpha (IL-4Rα) antibody that is FDA approved for moderate to severe asthma in patients 6 years or older, moderate to severe AD in patients 6 months or older, EoE in patients 12 years or older, and chronic rhinosinusitis with nasal polyps in adults. IL-4Rα is the receptor for the pro-atopy cytokines IL-4 and IL-13, thus making it an ideal target in various allergic diseases including FA. Dupilumab has potential benefits over anti-IgE therapy through a wider inhibitory impact on the allergic inflammation pathway.
There are several clinical trials recently underway assessing its efficacy with or without concomitant peanut and milk OIT as well as in multi-allergen OIT. A clinical trial using dupilumab as monotherapy for peanut allergic patients aged 6–17 years old was recently completed (NCT03793608) with results currently pending. Dupilumab’s potential as an adjunct treatment with OIT is currently being examined in 2 phase 2 clinical trials, a study in peanut allergic children aged 6–17 (NCT03682770) and the MAGIC study in milk-allergic individuals aged 4–50 years old (NCT04148352). In a novel phase 2 trial, the COMBINE study (NCT03679676), children and adults aged 4–55 years old are randomized to the sequential use of omalizumab and/or dupilumab to target both the IgE and IL-4/13 pathways during multi-allergen OIT to understand safety and efficacy of combination therapy. Given dupilumab’s recent approval for use in EoE, it is of great interest to see whether GI side effects are mitigated during OIT.
Optimal dosing for biologics
While the use of omalizumab in FA has largely followed the dosing guidelines used in allergic asthma based on patient weight and total IgE23, 28, 30, the optimal dosing is still under investigation. Previously a retrospective analysis of 181 patients undergoing omalizumab as adjunct therapy with OIT found that the ideal dose of omalizumab was best predicted by weight-based dosing, without adjusting for IgE40. Ongoing studies such as the BOOM trial (NCT04045301) aim to understand if weight-based dosing is more effective in FA compared to weight and IgE-based dosing40. Alternatively, fixed dosing of omalizumab was investigated in the MIMIX phase 2 clinical trial. Participants with multi-FAs were given a fixed dose of omalizumab (150 mg, 3 doses, every 4 weeks; similar to chronic idiopathic urticaria dosing) and a multi-allergen OIT with maintenance dose of either 300 or 1200 mg. In the ITT population, 70% of participants showed increase by 25% in sIgG4/sIgE ratios after just 18 weeks of therapy. Further analysis showed that standard omalizumab (asthma-based dosing) facilitated success at higher maintenance doses, but did not have a significant impact at lower OIT doses41. Although these studies have largely focused on omalizumab, similar issues exist for other biologics, highlighting the need for further studies on the optimal dosing of biologics for individual diseases.
Eosinophilic Gastrointestinal Disorders during Food Allergy Therapy
Asymptomatic gastrointestinal eosinophilia often coexists with FA, with published rates of 24–43%42, 43 and is comparable histologically to EoE44. FA may develop in individuals with EoE during periods of food elimination45 and, conversely, EoE and asymptomatic gastrointestinal eosinophilia may develop following successful oral challenge, introduction of a previously avoided IgE-mediated food allergen, and OIT46, 47. A pilot study investigating 20 adults undergoing peanut OIT in the POISED cohort found OIT-induced gastrointestinal eosinophilia is usually transient and asymptomatic with one adult developing EoE48. Most cases of treatment-associated disease fortunately resolve with dose modification or cessation of OIT49, though a small subset may have EGIDs that persist after stopping OIT, suggesting there may be a non-OIT food trigger50. The impact of biologics on OIT-induced gastrointestinal eosinophilia is of particular interest and currently under investigation using the minimally invasive esophageal string test (NCT04943744, NCT04148352).
Role Of Biologics In Eosinophilic Gastrointestinal Disorders
In healthy individuals, eosinophils are found throughout the gastrointestinal (GI) tract with the notable exception of the esophagus51. Eosinophil counts do not normally rise above 5 eosinophils per high-power field (eos/hpf) in the esophagus, 30 eos/hpf in the stomach52, and 26 eos/hpf in the duodenum42. Elevation of eosinophils in the GI tract with the presence of clinical symptoms suggests the presence of EGIDs53 including EoE, eosinophilic gastritis (EoG), and eosinophilic enteritis (EoN) which affect 5254, 5.155, and 2854 people per 100,000, respectively.
EoE is defined clinically and pathologically as the presence of gastrointestinal symptoms (nausea, vomiting, food impaction, dysphagia) with 15 or greater mucosal eosinophils per high power field in biopsies taken at any level of the esophagus, in patients with no other identified cause of esophageal eosinophilia such as gastroesophageal reflux disease, parasitic and fungal infections, Crohn’s disease, celiac disease, and connective tissue disease, among others. Although not required for diagnosis, additional histologic features include eosinophil density, basal zone hyperplasia, eosinophilic abscesses, eosinophil surface layering, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis. These features comprise the EoE histologic scoring system (EoEHSS)56 that is both reliable57, 58 and treatment-responsive59–64. In addition to proton pump inhibitors (PPIs), dietary elimination of ‘identified’ offending foods, endoscopic dilation, and topical corticosteroids (TCS) have been a mainstay of treatment for EoE65. However, TCS induces Candida in esophagitis in 5–30% of patients66, can induce adrenal insufficiency after prolonged use in 5–43% of patients67, 68 and can lose efficacy with long-term use69, resulting in problems with adherence, cost, and recurrence of disease which may lead to consideration of biologics68, 70–75.
Several biologics are used or being investigated in clinical trials for the treatment of EoE which act on the cytokines and cell receptors that govern the production and homing of eosinophils (Table 2). IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are cytokines that are vital in eosinophil development76, 77. IL-5 is the most specific cytokine for eosinophils and acts at multiple functional levels to impact eosinophil production, activation, and survival78. Other cell surface structures are relatively specific for eosinophils, such as CC-chemokine receptor 3 (CCR3), which mediates eosinophil chemotaxis in response to eotaxins, and sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8), whose engagement induces the apoptosis of activated eosinophils79, 80. Epidermal growth factor (EGF) module containing mucin-like hormone-like receptor 1 (EMR1) is a surface receptor that is unique to the eosinophil and represents a potential therapeutic target in eosinophilic diseases81. Furthermore, epithelial cell-derived alarmins IL-25 (also known as IL-17E), IL-33, and thymic stromal lymphopoietin (TSLP) promote eosinophilopoiesis by increasing IL-5 production by group 2 innate lymphoid cells (ILC2)82. Extravasation of eosinophils out of the circulation and into tissue sites is dependent on the function of integrins and their counter-ligands on activated endothelium and eosinophil-selective chemoattractants such as the eotaxins83. Notably, eotaxin-3 is markedly induced by IL-13, providing a synergistic mechanism by which TH2 and ILC2 cells, co-producing IL-5 and IL-13, regulate tissue eosinophilia84.
Table 2.
Ongoing clinical trials in EGID.
| Intervention | Trial Number(s) | Phase | Population | Primary Endpoint | ||
|---|---|---|---|---|---|---|
| Biologic agents in eosinophilic gastrointestinal disorders | S1P receptor modulator | Etrasimod* | NCT04682639 | II | 18–65 yo with eosinophilic esophagitis | Percent Change From Baseline in Esophageal Peak Eosinophil Count (PEC) [Time Frame: Baseline to Week 16] |
| Anti-IL5 | Mepolizumab | NCT03656380 | II | 16–75 yo with EoE | Mean Change in Dysphagia from Baseline to 3 months Post-treatment [Time Frame: Baseline, Month 3 Post-Treatment] | |
| Anti-IL5Rα | Benralizumab |
NCT04543409
NCT05251909 |
III | 12–65 yo with eosinophilic esophagitis 12–130 yo with Eosinophilic Gastritis and/or Gastroenteritis |
Proportion of patients with a histologic response at Week 24, defined as a peak esophageal intraepithelial eosinophil count ≤ 6 eos/hpf. [Time Frame: Week 24] Proportion of patients achieving a histological response in the stomach and/or in the duodenum [Time Frame: Week 24] |
|
| Anti-IL-13 | CC-93538 |
NCT05175352
NCT04753697 |
I II |
18–75 yo with eosinophilic esophagitis 12–75 yo with eosinophilic esophagitis |
Pharmacokinetics [Time Frame: Up to 18 Weeks] Change in dysphagia days clinical response [Time Frame: At week 24] |
|
| CC-93538 |
NCT04991935
NCT05214768 |
III | 12–75 with eosinophilic esophagitis 12–75 yo with eosinophilic gastroenteritis |
Incidence of Adverse Events (AEs) [Time Frame: For a minimum of 28 months] Changes in mean number of peak eosinophils (eos) per high-power field (hpf) in gastrointestinal (GI) biopsies from baseline to Week 16 [Time Frame: At Week 16] |
||
| Anti-IL4Rα | Dupilumab |
NCT03678545
NCT04394351 NCT05247866 |
II III IV |
12–70 yo with Eosinophilic Gastritis 1–11 yo with eosinophilic esophagitis 6–25 yo with eosinophilic esophagitis |
Relative change of peak eosinophil counts in the stomach [Time Frame: 12 weeks] Proportion of patients achieving peak esophageal intraepithelial eosinophil count ≤6 eos/hpf (400×) [Time Frame: Week 16] Esophageal Eosinophilia (number of eosinophils in the esophagus) [Time Frame: up to week 48] |
|
| Anti-Siglec-8 | Lirentelimab |
NCT03664960
NCT04322708 NCT05152563 NCT04322604 NCT04620811 NCT04856891 |
II II/III III |
18–80 yo with Eosinophilic Gastritis and/or Eosinophilic Duodenitis 12–80 yo with eosinophilic esophagitis 18–80 yo with Eosinophilic Gastritis and/or Eosinophilic Duodenitis 18–80 yo with Eosinophilic Duodenitis |
The safety and tolerability of AK002 by evaluating adverse events assessed using the CTCAE version 4.03 [Time Frame: Day 785 (End of Study)] The proportion of patients who achieve esophageal intraepithelial eosinophil count of ≤6 eosinophils/hpf [Time Frame: At Week 24] Proportion of Responders as determined by gastric or duodenal tissue eosinophil counts. [Time Frame: At Week 24] The safety and tolerability of lirentelimab by evaluating adverse events assessed using the CTCAE version 5.0 [Time Frame: Day 561 (End of Study)] Proportion of Responders, where a responder is a patient achieving a mean peak duodenal eosinophil count ≤15 cells/3 duodenal hpf. [Time Frame: At Week 24] |
|
| Anti-IL15 | CALY-002* | NCT04593251 | I | 18–50 yo with either eosinophilic esophagitis or celiac disease | Incidence of treatment-emergent adverse event [Time Frame: through study completion, an average of 3 months post last dose] | |
In contrast to EoE, our understanding of the pathophysiology of non-EoE EGIDs is hampered by several important factors, including small sample size due to low disease prevalence, non-specific symptoms (such as early satiety, abdominal pain, nausea, vomiting, and diarrhea)85 that may lead to diagnostic delay, and a lack of validated patient-reported outcome measures. A poor response to elimination diets and topical steroid preparations often necessitates systemic steroid use, underlining the need for effective new treatments for these diseases. In fact, many of the biologics studied in EoE have also been trialed in EoG and/or EoN52, 86–90.
Anti-IL-4 & 13
Dupilumab
Antibodies targeting IL-13 in adults were shown to significantly reduce eosinophilic inflammation60, 91, with a reduction in histologic and endoscopic features after treatment compared to placebo60. Dupilumab with dual IL-4 and IL-13 blockade led to promising results in a phase 2 study of 47 adult subjects with EoE and 12 weeks of treatment with dupilumab reduced peak esophageal intraepithelial eosinophil counts by 107.1% (CI: 73.0–141.2 p= <.0001), EoEHSS grade 68.3% (CI: 50.3–86.2 p= <.0001), and endoscopic reference scores by 1.6 points (CI: −2.5 to −0.7; p= .0006)61. A phase 3 study has confirmed histologic improvements with dupilumab in addition to demonstrating improvement in symptoms, including dysphagia92–95(NCT03633617). Given these encouraging results as well as emerging real-world data96, dupilumab was the first to receive FDA approval in May 2022 for the treatment of EoE. This has paved the way for a trial examining dupilumab for the treatment of EoG (NCT03678545).
Anti-IL-5
Reslizumab
Despite FA and EGID being driven by TH2 signaling, they have very different clinical presentations, which could be due to FA being driven by responses from IL-5− TH2 cells whereas EGID are driven by IL-5+ TH2 cells97. Given the presumed role of eosinophils in EGID, a number of biologic agents that reduce eosinophils to varying degrees via the IL-5 pathway have been tested. In a cohort of 226 patients with moderate or severe EoE, neutralization of IL-5 with 1, 2, or 3 mg of reslizumab for 12 weeks reduced peak esophageal eosinophil counts by 59% ,67%, or 64%, respectively, compared to 24% in the placebo group by week 15; however there was no difference in physician EoE global assessment between treatment groups98.
Mepolizumab
Similarly, IL-5 neutralization with mepolizumab subcutaneous injections for 12 weeks in a cohort of 59 EoE patients by Assa’ad et al reduced peak and mean eosinophils to less than 20 eos/hpf in 31.6% and 89.5% of subjects, respectively. However, no difference in EoE symptoms was observed after mepolizumab treatment99. Case studies of two severe adult asthmatics treated with mepolizumab, one with comorbid EoG and the other with EoN, exhibited improvement in their concurrent GI disease, but further study is required87, 88. An ongoing phase 2 clinical trial aims to enroll 16–75 year old patients (N=66) with EoE to assess the efficacy of 2 different doses of mepolizumab (NCT03656380).
Benralizumab
Another possible explanation for the lack of efficacy of reslizumab and mepolizumab is that they reduce, but do not deplete, eosinophils100, 101. A limited set of patients with hypereosinophilic syndrome involving gastrointestinal eosinophilia (N=7) received the anti-IL5RA antibody, benralizumab, in a phase 2 clinical trial89. All 7 patients had reduced gastrointestinal eosinophils, although 4 experienced disease flares while on therapy102. A trial of benralizumab for the treatment of PDGRFA-negative hypereosinophilic syndrome included 6 patients with concurrent gastric eosinophilia consistent with EoG, of whom 4 also had duodenal involvement. Peripheral blood and GI tissue eosinophils were depleted in each of these patients with a variable reduction in initial symptoms that returned in some with treatment reduction or diet liberalization89. Furthermore, an ongoing phase 3 clinical trial (NCT04543409) aims to recruit 12–65-year-old EoE patients (N=211) to test the efficacy of benralizumab recently released topline results where although treatment with benralizumab showed improvement in histologic disease remission, it failed to improve symptoms of dysphagia compared to placebo103.
In summary, biologics targeting eosinophils reduces eosinophilic inflammation in gastrointestinal tissues, but the persistence of symptoms suggests that eosinophils are only a part of the pathophysiology of EGIDs.
Anti-IgE
Omalizumab
In contrast to FA, omalizumab has not shown great success in EoE. In a cohort of 30 adults with EoE, omalizumab, given every 2–4 weeks for 16 weeks did not significantly reduce eosinophilic inflammation104, consistent with non-IgE-mediated food reactions in most EoE patients. An early trial with omalizumab did not demonstrate improvement in tissue eosinophilia but did show a 42% reduction in absolute eosinophil count 86.
Anti-SIGLEC-8
Lirentelimab
The ENIGMA phase 2 clinical trial of lirentelimab, an eosinophil-depleting anti-Siglec-8 antibody, given monthly for 4 months at a low dose or high dose schedule demonstrated a dramatic reduction in gastrointestinal eosinophil counts and improvement in clinical symptoms in patients with EoG and eosinophilic duodenitis (EoD)52. The KRYPTOS phase 2/3 clinical trial of lirentelimab, in 276 subjects with EoE reported that 87.9% of high dose for 5 months and 92.5% of low dose treated subjects achieved the histologic endpoint of 6 or fewer eos/hpf compared to 10.9% of those receiving placebo. However, no associated symptomatic improvement was seen between groups, thus failing to meet the symptomatic co-primary endpoint105. These results add to a growing body of evidence suggesting that the pathogenesis of EGID involves mechanisms that are, at least partially, independent of eosinophilia, therefore biologics targeting eosinophils only may not alleviate the disease process.
Non TH2-specific Inflammation Targets
Biologics used to treat inflammatory bowel disease have also been explored as a treatment for EoE in mainly small single-institution studies. Infliximab, an inhibitor of TNF-α, failed to reduce eosinophilic infiltration or resolve symptoms in three adults with severe corticosteroid-dependent EoE after 4–8 weeks of treatment106. Early studies on the α4β7 integrin inhibitor, vedolizumab, suggest that it may reduce dysphagia and esophageal eosinophilia in refractory EoG/EoN and in patients with concomitant Crohn’s disease and symptomatic EoE, however more research is needed90, 107, 108.
Upcoming novel biologics
Additional targets for biologics include the upstream alarmins: IL-25, IL-33, and TSLP (Figure 2). IL-33 was studied in a phase 2a study; 73% of peanut allergic adults tolerated 275 mg of peanut protein 15 days after a single infusion of the anti-IL-33 monoclonal antibody, etokimab, compared to 0% who received placebo109. A different anti-IL-33 biologic, itepekimab, has shown efficacy by improvement in lung function and asthma control in a Phase 3 asthma study with and without combination therapy with dupilumab110. The anti-TSLP biologic, tezepelumab, has also shown promise in asthma (NAVIGATOR study)111 and might have benefit in FA and EGID112, 113.
Other clinical trials are also underway evaluating the co-stimulatory inhibitor abatacept (NCT04872218) in the treatment of peanut allergy. Looking beyond FA and EGIDs, several exciting Phase 1 and 2 clinical trials are studying novel biologics in AD, including ADX-914, a fully human anti-IL-7R antibody (NCT0550902); PF-07242813, a CD1a inhibitor (NCT04668066); and fezakinumab, an IL-22 inhibitor (NCT01941537). If these early investigations demonstrate safety and efficacy in AD, it is feasible that future studies will expand to other atopic diseases, including EoE and FA. Additional targets such as Th1 adjuvants added to allergens to induce a Th1-skewed response are being explored for FA and may be a potential target for allergic disease114. Other novel biologics being investigated include disruptive IgE inhibitors115 and passive blockade with monoclonal antibodies to sIgE epitopes116.
Conclusion
There is substantial mechanistic overlap between FA, asthma, AD, and EGIDs. Indeed, many biologics that are under investigation in FA and EoE were first studied in asthma or AD. Examples include omalizumab, which was first approved for asthma in 2003, and now being studied in food allergy, and dupilumab, which was first approved for atopic dermatitis in 2017 and only recently approved for EoE as well. Leveraging insights into mechanisms of action of these biologics in other atopic conditions will enable more precise identification of how biologics can play a role in FA and EGID and facilitate a more personalized targeted approach to therapy. More research is still needed to achieve this goal however, and some of the gaps in knowledge are noted in Table 3.
Table 3.
Gaps in knowledge for the use of biologics in FA and EGID.
| Remaining questions | Steps moving forward |
|---|---|
| What is the optimal dose and timing of the biologic? | Inclusion of different arms with different doses or timecourses for the biologic. |
| How effective are these biologics in comparison with each other? | Design clinical trials aimed at comparing the efficacy of different biologics either individually or in combination. |
| How can we track the effectiveness of a biologic? | Identification of biomarkers for clinical response to the biologic through application of omics technology at various timepoints throughout treatment: pre-treatment, during treatment, and post treatment. |
| How do we identify which biologic is best suited for a food allergic individual? | Identification of baseline biomarkers suggestive of optimal response to specific biologics. |
| Would using these biologics in combination with each other increase their effectiveness? | Incentivize research investigating combination therapies. |
| How durable are the benefits of these biologics? | Long-term follow-up from completed studies can assess how long these benefits last post-study completion and screen for the potential of long-term adverse events. |
With the rapid advancement in knowledge in this area and further clinical trials underway, we also need to pay close attention to developing strategies to mitigate the impact of the high cost of biologics and reducing barriers to access for diverse patient populations. Home self-administration of many of these biologics may both improve access and reduce cost, but ensuring adherence with therapy and utilizing digital means of remote monitoring will be the key to continued success. With the plethora of biologics that will soon be available for use in FA and EGIDs, there is a great need for developing treatment algorithms that incorporate clinical presentation as well as biomarkers. Additionally, clinical practice guidelines developed by experts in the field through consensus building should differentiate between ill- and well-founded off-label practices to allow for the use of biologics pending regulatory approval. Although data integrity should not be compromised, biologics that are already approved in adults and have a track record of safety in children for other indications could be utilized for well-founded off-label practices through special access programs until full approval is available. Long-term studies are also critically important and will help identify durability and safety of these approaches. These strategies would allow physicians to better care for their pediatric and adult patients and bring severe disease under tighter control.
Acknowledgments
This study was supported by National Institutes of Health (NIH) U54 AI117804 for the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). CEGIR is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), and is co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NCATS, and the Intramural Research Program of the NIH. CEGIR is also supported by patient advocacy groups, including the American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Disease (CURED), and Eosinophilic Family Coalition (EFC). As a member of the RDCRN, CEGIR is also supported by its Data Management and Coordinating Center (DMCC) (U2CTR002818). Funding support for the DMCC is provided by NCATS and the National Institute of Neurological Disorders and Stroke (NINDS). Additional funding also includes 5UM1 AI130839-04 NIAID and 5U19 AI1104209-07 NIAID.
Abbreviations
- AD
Atopic dermatitis
- AEs
Adverse events
- CCR3
CC-chemokine receptor 3
- EGF
Epidermal growth factor
- EGIDs
Eosinophilic gastrointestinal disorders
- EMR1
EGF module containing mucin-like hormone-like receptor 1
- EoD
Eosinophilic duodenitis
- EoG
Eosinophilic gastritis
- EoEHSS
EoE histologic scoring system
- EoN
Eosinophilic enteritis
- eos/hpf
Eosinophils per high-power field
- FA
Food allergy
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IL-4Rα
IL-4 receptor alpha
- ILC2
Group 2 innate lymphoid cells
- OIT
Oral immunotherapy
- Siglec-8
Sialic acid-binding immunoglobulin-like lectin 8
- SU
Sustained unresponsiveness
- TCS
Topical corticosteroids
- TH2 cells
T helper 2 cells
- TSLP
thymic stromal lymphopoietin
- EoE
Eosinophilic esophagitis
Footnotes
Conflicts of interest
SBS reports grants from NIH, Regeneron, DBV Technologies, Aimmune, Novartis, CoFAR, and FARE. She is an Advisory member at Genentech and DBV Technologies. SA reports participation as an advisory board member, and/or consultant, and/or speaker for Novartis, and Ulrich outside the submitted work. MB has received consulting fees from Sanofi and GLG consulting. ADe reports grants from Fondation du Souffle; Conseil Régional Hauts-de-France; consulting fees or honoraria from Novartis, ALK, GSK, Sanofi, Regeneron, AstraZeneca, Boehringer Ingelheim, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science; participation in data safety monitoring board for BOOM study, outside the submitted work. GTF is a- Chief Medical Officer at EnteroTrack, and reports research grants from NIH, Holocalara, and Arena]. AL reports consultant fees from COUR Pharmaceuticals. KCN reports gr ants from National Institute of Allergy and Infectious Diseases (NIAID), National Heart, Lung, and Blood Institute (NHLBI), National Institute of Environmental Health Sciences (NIEHS), and Food Allergy Research & Education (FARE); stock options from IgGenix, Seed Health, ClostraBio, and ImmuneID; is Director of the World Allergy Organization Center of Excellence for Stanford, Advisor at Cour Pharma, Consultant for Excellergy, Red tree ventures, Eli Lilly, and Phylaxis, Co-founder of Before Brands, Alladapt, Latitude, and IgGenix; and National Scientific Committee member at Immune Tolerance Network (ITN), and National Institutes of Health (NIH) clinical research centers, outside the submitted work; patents include, “Mixed allergen composition and methods for using the same,” “Granulocyte-based methods for detecting and monitoring immune system disorders,” and “Methods and Assays for Detecting and Quantifying Pure Subpopulations of White Blood Cells in Immune System Disorders.”. MER is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Nextstone One, Bristol Myers Squibb, Astra Zeneca, Ellodi Pharma, GlaxoSmith Kline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoint and has an equity interest in the first seven listed, and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate. M.E.R. is an inventor of patents owned by Cincinnati Children’s Hospital. JMS has grant support from Regeneron/Sanofi, NIH. Consultant agreements with Regeneron/Sanofi, Allakos, and Celgene, Royalties from Uptodate. PB reports personal fees from Novartis, Pfizer, Sanofi-Genzyme, Bausch Health, Astra Zenaca, ALK and Aralez, as well as grants from DBV technologies, Regeneron, Novartis and Sanofi outside the submitted work. MHC has received research funding from AstraZeneca, Ception, GSK, Meritage Pharma Inc., Receptos/Celgene/BMS, Regeneron Pharmaceuticals and Shire, a Takeda company, and is a consultant for Allakos, Arena Pharmaceuticals, AstraZeneca, Calypso Biotech, EsoCap Biotech, GlaxoSmithKline, Receptos/Celgene/BMS, Regeneron Pharmaceuticals, Robarts Clinical Trials Inc./Alimentiv, Inc. and Shire, a Takeda company. AD has received fees for lectures and honoraria for attending advisory boards from Novartis, GSK, Sanofi, Regeneron, AstraZeneca, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science, ALK, Stallergènes-Greer outside the submitted work; participates in data safety monitoring board for BOOM study, outside the submitted work.. JW receives research support from National Institute of Allergy and Infectious Diseases, Aimmune, DBV Technologies, and Regeneron, and consultancy fees from ALK Abello and Jubilant HollisterStier. BLW reports research support from the National Institute of Allergy and Infectious Disease. RAW receives research support from NIH, Aimmune, DBV, Genentech, Novartis, Regeneron, FARE, and Siolta, and royalties from Up To Date. TZ reports industry consulting, research grants and/or honoraria from AImmune, Ajanta Pharma, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, Bio Cryst, Celldex, FAES, HAL, Henkel, Kryolan, Leti, Lofarma, L’Oreal, Meda, Medi Wound, Menarini, Merck, MMV Medicines for Malaria Venture, MSD, Novartis, PCM Scientific, Pfizer, Sanofi, Sanoflore, Stallergenes, Takeda, Teva, and UCB. He is a committee member of WHO-Initiative “Allergic Rhinitis and its Impact on Asthma” (ARIA), Member of the Board for German Society for Allergy and Clinical Immunology (DGAKI), Chairman of the Board for European Centre for Allergy Research Foundation (ECARF), President of Global Allergy and Asthma European Network (GA2LEN), and member of Committee on Allergy Diagnosis and Molecular Allergology for World Allergy Organisation (WAO). RSC receives grant support from the Consortium for Food Allergy Research (CoFAR), National Institute of Allergy and Infectious Disease (NIAID), Food Allergy Research & Education (FARE), Aimmune, DBV Technologies, Astellas, Novartis, Regeneron, and Astra Zeneca, and is an advisory board member for Alladapt Immunotherapeutics, Novartis, Sanofi, Allergenis, Intrommune Therapeutics, and Genentech. All other authors indicate no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open 2019; 2:e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018; 154:319–32 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract 2017; 5:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook-Mills JM, Emmerson LN. Epithelial barrier regulation, antigen sampling, and food allergy. J Allergy Clin Immunol 2022; 150:493–502. [DOI] [PubMed] [Google Scholar]
- 5.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018; 141:1711–25 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andorf S, Bunning B, Tupa D, Cao S, Long AJ, Borres MP, et al. Trends in egg specific immunoglobulin levels during natural tolerance and oral immunotherapy. Allergy 2020; 75:1454–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anvari S, Miller J, Yeh CY, Davis CM. IgE-Mediated Food Allergy. Clin Rev Allergy Immunol 2019; 57:244–60. [DOI] [PubMed] [Google Scholar]
- 8.Sahiner UM, Layhadi JA, Golebski K, István Komlósi Z, Peng Y, Sekerel B, et al. Innate lymphoid cells: The missing part of a puzzle in food allergy. Allergy 2021; 76:2002–16. [DOI] [PubMed] [Google Scholar]
- 9.Sampath V, Sindher SB, Alvarez Pinzon AM, Nadeau KC. Can food allergy be cured? What are the future prospects? Allergy 2020; 75:1316–26. [DOI] [PubMed] [Google Scholar]
- 10.Investigators PGoC Vickery BP, Vereda A Casale TB, Beyer K, du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med 2018; 379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 11.Begin P, Winterroth LC, Dominguez T, Wilson SP, Bacal L, Mehrotra A, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol 2014; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.J OBH, Beyer K, Abbas A, Fernandez-Rivas M, Turner PJ, Blumchen K, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health 2020; 4:728–39. [DOI] [PubMed] [Google Scholar]
- 13.Bird JA, Spergel JM, Jones SM, Rachid R, Assa’ad AH, Wang J, et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J Allergy Clin Immunol Pract 2018; 6:476–85 e3. [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet 2022; 399:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol 2017; 139:173–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brozek JL, Firmino RT, Bognanni A, Arasi S, Ansotegui I, Assa’ad AH, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guideline update - XIV - Recommendations on CMA immunotherapy. World Allergy Organ J 2022; 15:100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet 2019; 393:2222–32. [DOI] [PubMed] [Google Scholar]
- 18.Chinthrajah RS, Purington N, Andorf S, Long A, O’Laughlin KL, Lyu SC, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019; 394:1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoos AM, Chawes BL, Rasmussen MA, Bloch J, Bonnelykke K, Bisgaard H. Atopic endotype in childhood. J Allergy Clin Immunol 2016; 137:844–51 e4. [DOI] [PubMed] [Google Scholar]
- 20.Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens in children. Korean J Pediatr 2013; 56:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee CSK, Albuhairi S, Noh E, El-Khoury K, Rezaei S, Abdel-Gadir A, et al. Long-Term Outcome of Peanut Oral Immunotherapy Facilitated Initially by Omalizumab. J Allergy Clin Immunol Pract 2019; 7:451–61 e7. [DOI] [PubMed] [Google Scholar]
- 22.Brandstrom J, Vetander M, Sundqvist AC, Lilja G, Johansson SGO, Melen E, et al. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin Exp Allergy 2019; 49:1328–41. [DOI] [PubMed] [Google Scholar]
- 23.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 2017; 139:873–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol 2013; 132:1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martorell-Calatayud C, Michavila-Gomez A, Martorell-Aragones A, Molini-Menchon N, Cerda-Mir JC, Felix-Toledo R, et al. Anti-IgE-assisted desensitization to egg and cow’s milk in patients refractory to conventional oral immunotherapy. Pediatr Allergy Immunol 2016; 27:544–6. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Soejima K, Taniuchi S, Hatano Y, Yamanouchi S, Ishikawa H, et al. Oral immunotherapy combined with omalizumab for high-risk cow’s milk allergy: a randomized controlled trial. Sci Rep 2017; 7:17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung DY, Sampson HA, Yunginger JW, Burks AW Jr., Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003; 348:986–93. [DOI] [PubMed] [Google Scholar]
- 28.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2016; 137:1103–10 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol 2014; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol 2018; 3:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andorf S, Purington N, Kumar D, Long A, O’Laughlin KL, Sicherer S, et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. EClinicalMedicine 2019; 7:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otani IM, Begin P, Kearney C, Dominguez TL, Mehrotra A, Bacal LR, et al. Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol 2014; 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crespo JB, Domingo MV, Arauzo NH, Castillo MJ, Delavalle MB, Foix MPS, et al. Real life study of the use of omalizumab for pediatric patients with multiple food allergies. Allergol Immunopathol (Madr) 2021; 49:15–22. [DOI] [PubMed] [Google Scholar]
- 34.Burk CM, Dellon ES, Steele PH, Virkud YV, Kulis M, Burks AW, et al. Eosinophilic esophagitis during peanut oral immunotherapy with omalizumab. J Allergy Clin Immunol Pract 2017; 5:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiocchi A, Artesani MC, Riccardi C, Mennini M, Pecora V, Fierro V, et al. Impact of Omalizumab on Food Allergy in Patients Treated for Asthma: A Real-Life Study. J Allergy Clin Immunol Pract 2019; 7:1901–9 e5. [DOI] [PubMed] [Google Scholar]
- 36.Abstracts from the European Academy of Allergy and Clinical Immunology Digital Congress, 06–08 June 2020. Allergy 2020; 75 Suppl 109:5–610. [DOI] [PubMed] [Google Scholar]
- 37.Robert A, Wood RSC, Amanda K. Rudman Spergel, Denise C Babineau, Scott H. Sicherer, Edwin H. Kim, Wayne G. Shreffler, Jones Stacie M., Donald Y.M Leung, Brian P. Vickery, Andrew Bird J, Jonathan M, Spergel, Michael Kulis, Iqbal Ahmar, Kaufman Derrick, Umetsu Dale T., Monica Ligueros-Saylan Alkaz Uddin, Fogel Robert B., Lussier Stephanie, Mudd Kim, Poyser Julian, Martin MacPhee Maria Veri, Davidson Wendy, Hamrah Sanaz, Long Andrew, Togias Alkis, on behalf of theOUtMATCH study team. Protocol design and synopsis: Omalizumab as Monotherapy and as Adjunct Therapy to Multiallergen OIT in Children and Adults with Food Allergy (OUtMATCH). Journal of Allergy and Clinical Immunology: Global 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaker M, Briggs A, Dbouk A, Dutille E, Oppenheimer J, Greenhawt M. Estimation of Health and Economic Benefits of Clinic Versus Home Administration of Omalizumab and Mepolizumab. J Allergy Clin Immunol Pract 2020; 8:565–72. [DOI] [PubMed] [Google Scholar]
- 39.Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbaren N, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun 2020; 11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azzano P, Paquin M, Langlois A, Morin C, Parizeault G, Lacombe-Barrios J, et al. Determinants of omalizumab dose-related efficacy in oral immunotherapy: Evidence from a cohort of 181 patients. J Allergy Clin Immunol 2021; 147:233–43. [DOI] [PubMed] [Google Scholar]
- 41.Sindher SB, Kumar D, Cao S, Purington N, Long A, Sampath V, et al. Phase 2, randomized multi oral immunotherapy with omalizumab ‘real life’ study. Allergy 2022. [DOI] [PubMed] [Google Scholar]
- 42.Wright BL, Fernandez-Becker NQ, Kambham N, Purington N, Tupa D, Zhang W, et al. Baseline Gastrointestinal Eosinophilia Is Common in Oral Immunotherapy Subjects With IgE-Mediated Peanut Allergy. Front Immunol 2018; 9:2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbosa AC, Castro FM, Meireles PR, Arruda LK, Cardoso SR, Kalil J, et al. Eosinophilic Esophagitis: Latent Disease in Patients with Anaphylactic Reaction to Cow’s Milk. J Allergy Clin Immunol Pract 2018; 6:451–6 e1. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura H, Tanaka F, Nadatani Y, Otani K, Hosomi S, Kamata N, et al. Eosinophilic esophagitis and asymptomatic esophageal eosinophilia display similar immunohistological profiles. J Clin Biochem Nutr 2021; 68:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho HE, Chehade M. Development of IgE-mediated immediate hypersensitivity to a previously tolerated food following its avoidance for eosinophilic gastrointestinal diseases. J Allergy Clin Immunol Pract 2018; 6:649–50. [DOI] [PubMed] [Google Scholar]
- 46.Hill DA, Shuker M, Cianferoni A, Wong T, Ruchelli E, Spergel JM, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. J Allergy Clin Immunol Pract 2015; 3:123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014; 113:624–9. [DOI] [PubMed] [Google Scholar]
- 48.Wright BL, Fernandez-Becker NQ, Kambham N, Purington N, Cao S, Tupa D, et al. Gastrointestinal Eosinophil Responses in a Longitudinal, Randomized Trial of Peanut Oral Immunotherapy. Clin Gastroenterol Hepatol 2021; 19:1151–9 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldberg MR, Nachshon L, Levy MB, Elizur A, Katz Y. Risk Factors and Treatment Outcomes for Oral Immunotherapy-Induced Gastrointestinal Symptoms and Eosinophilic Responses (OITIGER). J Allergy Clin Immunol Pract 2020; 8:125–31. [DOI] [PubMed] [Google Scholar]
- 50.Hamant L, Freeman C, Garg S, Wright BL, Schroeder S. Eosinophilic esophagitis may persist after discontinuation of oral immunotherapy. Ann Allergy Asthma Immunol 2021; 126:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kita H Eosinophils: multifunctional and distinctive properties. Int Arch Allergy Immunol 2013; 161 Suppl 2:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med 2020; 383:1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright BL, Schwartz JT, Ruffner MA, Furuta GT, Gonsalves N, Dellon ES, et al. Eosinophilic gastrointestinal diseases make a name for themselves: A new consensus statement with updated nomenclature. J Allergy Clin Immunol 2022; 150:291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr 2011; 52:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansoor E, Saleh MA, Cooper GS. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin Gastroenterol Hepatol 2017; 15:1733–41. [DOI] [PubMed] [Google Scholar]
- 56.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017; 30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warners MJ, Ambarus CA, Bredenoord AJ, Verheij J, Lauwers GY, Walsh JC, et al. Reliability of histologic assessment in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2018; 47:940–50. [DOI] [PubMed] [Google Scholar]
- 58.Vieira MC, Gugelmin ES, Percicote AP, Ribeiro MG, de Miranda RA, Vieira GG, et al. Intra- and interobserver agreement of histopathological findings in pediatric patients with eosinophilic esophagitis. J Pediatr (Rio J) 2022; 98:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins MH, Dellon ES, Katzka DA, Hirano I, Williams J, Lan L. Budesonide Oral Suspension Significantly Improves Eosinophilic Esophagitis Histology Scoring System Results: Analyses From a 12-Week, Phase 2, Randomized, Placebo-controlled Trial. Am J Surg Pathol 2019; 43:1501–9. [DOI] [PubMed] [Google Scholar]
- 60.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology 2019; 156:592–603 e10. [DOI] [PubMed] [Google Scholar]
- 61.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis. Gastroenterology 2020; 158:111–22 e10. [DOI] [PubMed] [Google Scholar]
- 62.Dellon ES, Collins MH, Rothenberg ME, Assouline-Dayan Y, Evans L, Gupta S, et al. Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol 2021; 19:473–83 e17. [DOI] [PubMed] [Google Scholar]
- 63.Ma C, Jairath V, Feagan BG, Guizzetti L, Zou G, McFarlane SC, et al. Responsiveness of a Histologic Scoring System Compared With Peak Eosinophil Count in Eosinophilic Esophagitis. Am J Gastroenterol 2022; 117:264–71. [DOI] [PubMed] [Google Scholar]
- 64.Cruz J, Irvine MA, Avinashi V, Chan ES, Vallance BA, Soller L, et al. Application of the Eosinophilic Esophagitis Histology Scoring System Grade Scores in Patients at British Columbia Children’s Hospital. Fetal Pediatr Pathol 2022:1–15. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira CT, Vieira MC, Furuta GT, Barros F, Chehade M. Eosinophilic esophagitis-Where are we today? J Pediatr (Rio J) 2019; 95:275–81. [DOI] [PubMed] [Google Scholar]
- 66.von Arnim U, Malfertheiner P. Eosinophilic esophagitis--treatment of eosinophilic esophagitis with drugs: corticosteroids. Dig Dis 2014; 32:126–9. [DOI] [PubMed] [Google Scholar]
- 67.Harel S, Hursh BE, Chan ES, Avinashi V, Panagiotopoulos C. Adrenal Suppression in Children Treated With Oral Viscous Budesonide for Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2015; 61:190–3. [DOI] [PubMed] [Google Scholar]
- 68.Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal Insufficiency after Chronic Swallowed Glucocorticoid Therapy for Eosinophilic Esophagitis. J Pediatr 2016; 170:240–5. [DOI] [PubMed] [Google Scholar]
- 69.Eluri S, Runge TM, Hansen J, Kochar B, Reed CC, Robey BS, et al. Diminishing Effectiveness of Long-Term Maintenance Topical Steroid Therapy in PPI Non-Responsive Eosinophilic Esophagitis. Clin Transl Gastroenterol 2017; 8:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bose P, Kumar S, Nebesio TD, Li C, Hon EC, Atkins D, et al. Adrenal Insufficiency in Children With Eosinophilic Esophagitis Treated With Topical Corticosteroids. J Pediatr Gastroenterol Nutr 2020; 70:324–9. [DOI] [PubMed] [Google Scholar]
- 71.Hsu S, Wood C, Pan Z, Rahat H, Zeitler P, Fleischer D, et al. Adrenal Insufficiency in Pediatric Eosinophilic Esophagitis Patients Treated with Swallowed Topical Steroids. Pediatr Allergy Immunol Pulmonol 2017; 30:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucendo AJ, Miehlke S, Schlag C, Vieth M, von Arnim U, Molina-Infante J, et al. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology 2019; 157:74–86 e15. [DOI] [PubMed] [Google Scholar]
- 73.Straumann A, Lucendo AJ, Miehlke S, Vieth M, Schlag C, Biedermann L, et al. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients With Eosinophilic Esophagitis. Gastroenterology 2020; 159:1672–85 e5. [DOI] [PubMed] [Google Scholar]
- 74.Kochis SR, Cooke DW, Dantzer J, Wood R, Keet C. Low detection of adrenal suppression secondary to swallowed steroids for eosinophilic esophagitis in a quality improvement project. J Allergy Clin Immunol Pract 2020; 8:3647–9 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen ET, Huang KZ, Chen HX, Landes LE, McConnell KA, Almond MA, et al. Longitudinal Growth Outcomes Following First-line Treatment for Pediatric Patients With Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2019; 68:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weller PF. Cytokine regulation of eosinophil function. Clin Immunol Immunopathol 1992; 62:S55–9. [DOI] [PubMed] [Google Scholar]
- 77.Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019; 50:796–811. [DOI] [PubMed] [Google Scholar]
- 78.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006; 24:147–74. [DOI] [PubMed] [Google Scholar]
- 79.Lamkhioued B, Abdelilah SG, Hamid Q, Mansour N, Delespesse G, Renzi PM. The CCR3 receptor is involved in eosinophil differentiation and is up-regulated by Th2 cytokines in CD34+ progenitor cells. J Immunol 2003; 170:537–47. [DOI] [PubMed] [Google Scholar]
- 80.Youngblood BA, Brock EC, Leung J, Falahati R, Bochner BS, Rasmussen HS, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Legrand F, Tomasevic N, Simakova O, Lee CC, Wang Z, Raffeld M, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol 2014; 133:1439–47, 47 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitchell PD, O’Byrne PM. Epithelial-Derived Cytokines in Asthma. Chest 2017; 151:1338–44. [DOI] [PubMed] [Google Scholar]
- 83.Klion AD, Ackerman SJ, Bochner BS. Contributions of Eosinophils to Human Health and Disease. Annu Rev Pathol 2020; 15:179–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doran E, Cai F, Holweg CTJ, Wong K, Brumm J, Arron JR. Interleukin-13 in Asthma and Other Eosinophilic Disorders. Front Med (Lausanne) 2017; 4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Genta RM, Dellon ES, Turner KO. Non-oesophageal eosinophilic gastrointestinal diseases are undersuspected clinically and underdiagnosed pathologically. Aliment Pharmacol Ther 2022; 56:240–50. [DOI] [PubMed] [Google Scholar]
- 86.Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol 2007; 120:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han D, Lee JK. Severe asthma with eosinophilic gastroenteritis effectively managed by mepolizumab and omalizumab. Ann Allergy Asthma Immunol 2018; 121:742–3. [DOI] [PubMed] [Google Scholar]
- 88.Caruso C, Colantuono S, Pugliese D, Di Mario C, Tolusso B, Gremese E, et al. Severe eosinophilic asthma and aspirin-exacerbated respiratory disease associated to eosinophilic gastroenteritis treated with mepolizumab: a case report. Allergy Asthma Clin Immunol 2020; 16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuang FL, Legrand F, Makiya M, Ware J, Wetzler L, Brown T, et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N Engl J Med 2019; 380:1336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim HP, Reed CC, Herfarth HH, Dellon ES. Vedolizumab Treatment May Reduce Steroid Burden and Improve Histology in Patients With Eosinophilic Gastroenteritis. Clin Gastroenterol Hepatol 2018; 16:1992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2015; 135:500–7. [DOI] [PubMed] [Google Scholar]
- 92.Dellon ES, Rothenberg ME, Collins MH, Hirano I, Chehade M, Bredenoord AJ, et al. A Phase 3, Randomized, 3-Part Study to Investigate the Efficacy and Safety of Dupilumab in Adult and Adolescent Patients with Eosinophilic Esophagitis: results from Part A. Am J Gastroenterol 2020; 115 (Suppl 1):LB3. [Google Scholar]
- 93.Dellon ES, Rothenberg ME, Collins MH, Hirano I, Chehade M, Bredenoord AJ, et al. LIBERTY EoE TREET: Results from Parts A and C of the Phase 3, Randomized, 3-Part LIBERTY EoE TREET Study to Investigate the Efficacy and Safety of Dupilumab in Adult and Adolescent Patients with Eosinophilic Esophagitis up to 52-Weeks. Am J Gastroenterol 2021; 116 (suppl):Oral presentation 52. [Google Scholar]
- 94.Rothenberg ME, Dellon ES, Bredenoord AJ, Collins MH, Hirano I, Chehade M, et al. Dupilumab Improves Clinical and Histologic Aspects of Disease in Adult and Adolescent Patients With Eosinophilic Esophagitis at Week 24: Results from Part B of the 3-Part LIBERTY EoE TREET Study. J Allergy Clin Immunol 2022; 149 (Suppl):AB312 (#L02). [Google Scholar]
- 95.Dellon ES, Rothenberg ME, Bredenoord AJ, Collins MH, Hirano I, Chehade M, et al. Clinical and Histologic Improvements with Weekly Dupilumab Treatment in Adult and Adolescent Patients With Eosinophilic Esophagitis at Week 24: Weekly and Every 2 Week Results from Part B of the 3-Part LIBERTY EoE TREET Study. Gastroenterology in press, 2022:Oral late-breaking abstract. [Google Scholar]
- 96.Spergel BL, Ruffner MA, Godwin BC, Liacouras CA, Cianferoni A, Gober L, et al. Improvement in eosinophilic esophagitis when using dupilumab for other indications or compassionate use. Ann Allergy Asthma Immunol 2022. [DOI] [PubMed] [Google Scholar]
- 97.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. J Allergy Clin Immunol 2009; 124:1326–32 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2012; 129:456–63, 63 e1–3. [DOI] [PubMed] [Google Scholar]
- 99.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011; 141:1593–604. [DOI] [PubMed] [Google Scholar]
- 100.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184:1125–32. [DOI] [PubMed] [Google Scholar]
- 101.Moran AM, Ramakrishnan S, Borg CA, Connolly CM, Couillard S, Mwasuku CM, et al. Blood Eosinophil Depletion with Mepolizumab, Benralizumab, and Prednisolone in Eosinophilic Asthma. Am J Respir Crit Care Med 2020; 202:1314–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuang FL, De Melo MS, Makiya M, Kumar S, Brown T, Wetzler L, et al. Benralizumab Completely Depletes Gastrointestinal Tissue Eosinophils and Improves Symptoms in Eosinophilic Gastrointestinal Disease. J Allergy Clin Immunol Pract 2022; 10:1598–605 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Update on the MESSINA Phase III trial for Fasenra in eosinophilic esophagitis. 2022.] Available from https://www.astrazeneca.com/media-centre/press-releases/2022/update-on-messina-phase-iii-trial.html.
- 104.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014; 147:602–9. [DOI] [PubMed] [Google Scholar]
- 105.Allakos. Allakos announces topline phase 3 data from the ENIGMA 2 study and phase 2/3 data from the KRYPTOS study in patients with eosinophilic gastrointestinal diseases [press release]. 2021. (Accessed April 25, 2022, at https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-enigma-2-study-and-phase.).
- 106.Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol 2008; 122:425–7. [DOI] [PubMed] [Google Scholar]
- 107.Nhu QM, Chiao H, Moawad FJ, Bao F, Konijeti GG. The Anti-alpha4beta7 Integrin Therapeutic Antibody for Inflammatory Bowel Disease, Vedolizumab, Ameliorates Eosinophilic Esophagitis: a Novel Clinical Observation. Am J Gastroenterol 2018; 113:1261–3. [DOI] [PubMed] [Google Scholar]
- 108.Taft TH, Mutlu EA. The Potential Role of Vedolizumab in Concomitant Eosinophilic Esophagitis and Crohn’s Disease. Clin Gastroenterol Hepatol 2018; 16:1840–1. [DOI] [PubMed] [Google Scholar]
- 109.Chinthrajah S, Cao S, Liu C, Lyu SC, Sindher SB, Long A, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wechsler ME, Ruddy MK, Pavord ID, Israel E, Rabe KF, Ford LB, et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N Engl J Med 2021; 385:1656–68. [DOI] [PubMed] [Google Scholar]
- 111.Menzies-Gow A, Colice G, Griffiths JM, Almqvist G, Ponnarambil S, Kaur P, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res 2020; 21:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med 2021; 384:1800–9. [DOI] [PubMed] [Google Scholar]
- 113.Corren J, Ambrose CS, Sałapa K, Roseti SL, Griffiths JM, Parnes JR, et al. Efficacy of Tezepelumab in Patients with Severe, Uncontrolled Asthma and Perennial Allergy. J Allergy Clin Immunol Pract 2021; 9:4334–42.e6. [DOI] [PubMed] [Google Scholar]
- 114.Kulis M, Gorentla B, Burks AW, Zhong XP. Type B CpG oligodeoxynucleotides induce Th1 responses to peanut antigens: modulation of sensitization and utility in a truncated immunotherapy regimen in mice. Mol Nutr Food Res 2013; 57:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pennington LF, Gasser P, Brigger D, Guntern P, Eggel A, Jardetzky TS. Structure-guided design of ultrapotent disruptive IgE inhibitors to rapidly terminate acute allergic reactions. J Allergy Clin Immunol 2021; 148:1049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atanasio A, Franklin MC, Kamat V, Hernandez AR, Badithe A, Ben LH, et al. Targeting immunodominant Bet v 1 epitopes with monoclonal antibodies prevents the birch allergic response. J Allergy Clin Immunol 2022; 149:200–11. [DOI] [PubMed] [Google Scholar]


