Abstract
Introduction
We conducted a systematic review and meta‐analysis to review the relationship between midlife dyslipidemia and lifetime incident dementia.
Methods
The databases Medline, Embase, Scopus, Web of Science, and Cochrane were searched from inception to February 20, 2022. Longitudinal studies examining the relationship between midlife lipid levels on dementia, dementia subtypes, and/or cognitive impairment were pooled using inverse‐variance weighted random‐effects meta‐analysis.
Results
Seventeen studies (1.2 million participants) were included. Midlife hypercholesterolemia was associated with increased incidence of mild cognitive impairment (effect size [ES] = 2.01; 95% confidence interval [CI] 1.19 to 2.84; I2 = 0.0%) and all‐cause dementia (ES = 1.14; 95% CI: 1.07 to 1.21; I2 = 0.0%). Each 1 mmol/L increase in low‐density lipoprotein was associated with an 8% increase (ES = 1.08, 95% CI: 1.03 to 1.14; I2 = 0.3%) in incidence of all‐cause dementia.
Discussion
Midlife dyslipidemia is associated with an increased risk of cognitive impairment in later life.
Keywords: aging, cholesterol, cognitive, cognitive dysfunction, dementia, dyslipidemia, midlife
1. INTRODUCTION
Dementia is a neurodegenerative disease characterized by changes in cognition, behavior, and functioning. 1 Although the exact cognitive functions affected may depend on the subtype of dementia, impairment in various domains can occur including processing speed, attention, learning and memory, language, visuospatial and executive functions, and social cognition. 1 As of 2019, more than 55 million people were affected by the disease worldwide, with this projected to rise to 153 million people by 2050. 2 , 3 The burden of dementia is driven by its progressive and generally irreversible nature, placing long‐term stresses on both patients and carers.
Alzheimer's disease (AD) and vascular dementia (VaD) are the most common causes of dementia, frequently co‐occurring as a “mixed dementia,” and together account for ≈80% of global dementia cases. 4 AD is characterized by decline in two or more of the aforementioned cognitive domains, with resultant impact on the person's activities of daily living. 5 Mild cognitive impairment (MCI) is considered to be a prodrome or “at‐risk” stage for dementia. 6 It involves a decline in cognitive function beyond what is expected for normal aging, without the significant decline in daily functioning that would be evident in dementia. 6 More than50% of patients with MCI progress to dementia within 5 years. 7
Up to 40% of dementia risk has been attributed to modifiable risk factors. 8 Coupled with evidence that pathology leading to dementia begins 10 to 20 years before clinical symptoms emerge, 9 there is substantial interest in identifying early to midlife interventions that may prevent lifetime occurrence of dementia. One risk factor gaining interest is dyslipidemia, 10 , 11 which refers to an imbalance of lipid levels in the blood. 12 A proposed mechanism for the observed association between dyslipidemia and dementia is the conversion of systemic cholesterol into 27‐hydroxycholesterol, which may cross the blood‐brain barrier and promote deposition of amyloid beta and tau proteins characteristic of AD. 13 , 14 It is postulated that 27‐hydroxycholesterol increases beta‐site amyloid precursor protein cleaving enzyme 1, which is the rate‐limiting step (i.e., the slowest, most critical step that determines the overall reaction rate) in amyloid beta (Aβ) production. 14 A similar mechanism has been suggested to underlie an increased risk of MCI with early life exposure to elevated cholesterol. 15 Furthermore, hyperlipidemia may aggravate both carotid atherosclerosis contributing to VaD and oxidized low‐density lipoprotein (LDL)–mediated neuronal cell death implicated in dementia and MCI pathophysiology. 16 , 17
Despite a convincing theoretical basis, translation into clinical evidence has been equivocal. Studies examining dyslipidemia as part of a composite cardiovascular risk score have generally found a positive association with dementia. 18 , 19 , 20 , 21 However, results from studies isolating dyslipidemia as an independent risk factor have been inconsistent. 10 One contributing factor may be variation in study design, including the age at which lipid sampling takes place. 22 Cholesterol and dementia follow a non‐linear association with age, with evidence of a prodromal decrease in lipids prior to dementia onset. 23 Therefore, it is important to understand the risk associated with midlife and late‐life dyslipidemia separately. 23 We conducted a systematic review and meta‐analysis to update the evidence on the relationship between midlife dyslipidemia and lifetime incidence of dementia.
2. METHODS
This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guideline and was registered on the International Prospective Register of Systematic Reviews (PROSPERO: CRD42021278058). Two reviewers (JW and SS) independently performed the title and abstract screen, full‐text review of eligible studies, data extraction, and risk of bias assessment. Discrepancies were resolved by consensus or with involvement of a third reviewer (SB) if required.
2.1. Search strategy
A systematic literature search was conducted across Medline, Embase, Scopus, Web of Science, and Cochrane databases from inception to February 20, 2022, using the following keywords: “dementia” or, “cognitive impairment” or, “Alzheimer” and, “lipid” or, “cholesterol” or, “lipoprotein” or “triglyceride” and “incidence” or “prevalence” (see Appendix A for full search strategy). No language restriction was applied, and if required a medical translation service was used for non–English‐language texts requiring full‐text review. Reference lists of previous reviews and eligible studies were screened manually to identify additional relevant publications.
2.2. Eligibility criteria
We included cohort studies with ≥12 months of follow‐up that assessed the association between dyslipidemia and the development of dementia or MCI. Eligible participants were adults <65 years of age at baseline (i.e., at lipid sampling). Lipid subtypes included total cholesterol (TC), LDL, high‐density lipoprotein (HDL), and triglycerides (TGs). Eligible outcome measures were lifetime incidence of MCI, all‐cause dementia, and dementia subtypes. Diagnosis of the outcomes was accepted if studies used validated screening tools during neuropsychiatric assessment, including the Mini‐Mental State Examination (MMSE) for dementia; Peterson/Winblad and Albert diagnostic criteria for MCI; National Institute on Aging–Alzheimer's Association (NIA–AA), 24 International Working Group (IWG), or National Institute for Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria for AD 25 , 26 ; and National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria for VaD. Diagnostic codes identified in medical records were also accepted, 26 including the International Classification of Diseases (ICD) and Diagnostic and Statistical Manual of Mental Disorders (DSM) classifications.
2.3. Data collection and process
The following data items were extracted from studies: year of publication, population (including age, sex, and other baseline characteristics), setting, sample size, study design, inclusion and exclusion criteria, exposure (i.e., lipid subtypes measured), outcome measure(s), measure(s) of effect, effect size, and covariates. Missing or incomplete data were requested from the corresponding authors where applicable. 18 , 21 , 27 , 28 , 29 , 30
2.4. Assessment of midlife dyslipidemia
For the purposes of this review, “midlife” was defined as adults age <65 years. No lower age limit was applied to allow for flexibility of “midlife” definitions used by various studies and thus include as many studies as possible in our review. Although there is no universal definition for midlife, this upper threshold is used widely in clinical practice to delineate between middle‐aged versus older adults. 31 , 32 Dyslipidemia was defined as any lipid parameter (TC, LDL, HDL, TG) identified as outside of its normal range of values on a blood test. This normal range varied by country and institution of practice, and thus between studies. Data on specific thresholds for dyslipidemia and their associated outcomes were collected according to the original authors’ definition and reported as such in our analysis.
2.5. Data analysis
The quality of individual studies was assessed using the Newcastle‐Ottawa Scale, which has been validated for use in cohort studies. 33 Where studies analyzed the same patient cohorts, one article was selected for inclusion in the meta‐analysis based on sample size (i.e., total number of persons enrolled), quality assessment, and relevance. 18 , 19 , 34 , 35 , 36
When required, the effect size (ES) was calculated by pooling the hazard ratio, odds ratio, and relative risk as reported in individual studies, with these being treated as approximately equivalent measures given the relative rarity of dementia incidence (<15% per year). 37 , 38 , 39 The effect size of high versus low lipid levels, as defined by individual studies, were pooled using inverse‐variance weighted random‐effects meta‐analysis according to the method of DerSimonian and Laird. 40 For studies that reported both hazard and odds ratios, the hazard ratio was used for analysis. 34 Effect estimates from studies with continuous data were standardized to reflect a unit change in lipid levels by log transformation of the effect size and corresponding standard errors, then dividing by the reported lipid interval to yield change per 1 mmol/L of the respective lipid levels.
Sensitivity analyses were performed by excluding each study from the pooled analysis and by pooling studies that reported dementia incidence as a function of top‐versus‐bottom lipid threshold (e.g., ≥6.2 vs <5.18 mmol/L TC) or above‐versus‐below a certain lipid threshold (e.g., ≥6.5 vs <6.5 mmol/L TC). Subgroup analyses were performed for lipid and dementia subtypes, including MCI, where possible. These were conducted by extracting categories as originally reported by the authors, that is, analysis of “all‐cause dementia” included all papers reporting this as an outcome, which implicitly comprises AD, but did not include papers reporting AD as a standalone outcome. The I 2 statistic was used to assess heterogeneity of included studies, with values of <25%, 25 to 50%, and >50% corresponding to low, moderate, and high degrees of heterogeneity, respectively. 41 The effect of different lipid threshold values was investigated by meta‐regression that studied the effect of various high and low thresholds on overall pooled effect size. Publication bias was assessed by visual inspection of funnel plots and statistically using Egger's test.
All statistical analyses were conducted in STATA version 16 (Stata Corp, Texas, USA) using a two‐tailed alpha value of 0.05. Data are presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) unless stated otherwise.
3. Data availability
Anonymized data not published in this article will be made available by request from any qualified investigator.
4. RESULTS
4.1. Overview of included studies
Of 8183 studies identified by our search, 193 underwent full‐text review of which 176 were excluded (Appendix S2), and 17 were included in this review (Figure S1). Eleven studies examined the association between TC and all‐cause dementia. 18 , 19 , 21 , 27 , 28 , 29 , 34 , 35 , 42 , 43 , 44 Six examined the association between TC and AD specifically, 30 , 36 , 42 , 44 , 45 , 46 three investigated the relationship between LDL or HDL and dementia or MCI, 22 , 27 , 28 and two studied the link between TC and MCI. 20 , 30 The sample sizes of included studies ranged from 222 to 636,262 individuals with a mean age at baseline ranging between 42.4 and 56.7 years; follow‐up ranged from 7 to 36 years (median 21.2 years) (Tables 1 and 2). Most (n = 13) were prospective cohort studies, whereas the remainder (n = 4) were retrospective in design. Five were based in Finland, four in the United States, three in the United Kingdom, three in Sweden, and one each in Japan and Germany. Most studies (n = 14) had an overall low risk of bias (defined by Newcastle‐Ottawa Scale score 7 to 9) (Table 1). The studies by Rönnemaa et al., Rosengren et al., and Notkola et al. each scored 6 for reasons such as participants being exclusively male, limited adjustment for covariates (≤2 variables), and lack of description of participants lost to follow‐up (Table S1). 42 , 43 , 46
TABLE 1.
Characteristics of studies included in the systematic review
| Study (year) | n 1 | Year sampled | Follow‐up period, year | Country | Study design | Lipid measured | Outcome (effect measure) | Risk estimate | Diagnostic criteria | Mean (SD) age at baseline | Age range (baseline) | Female (%) | Covariates adjusted for in analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gong (2021) 27 | 273,262 | 2006‐2010 | 11.8a | United Kingdom | Prospective cohort study | TC, LDL, HDL | Incident dementia | HR | ICD‐10 codes via medical records, death registry | 56·3 (8 0) 2 | ·· | 100·0 | Age, BMI, CVA, diabetes, drugs b, c SBP, SES, smoking |
| Gong (2021) 27 | 228,964 | 2006‐2010 | 11·7a | United Kingdom | Prospective cohort study | TC, LDL, HDL | Incident dementia | HR | ICD10 codes via medical records, death registry | 56·7 (8·2) 2 | ·· | 0·0 | Age, BMI, CVA, diabetes, drugs b, c, SBP, SES, smoking |
| Iwagami (2021) 2 8 | 636,262 | 1992‐2009 |
7·4a (4·6, 10·4) |
United Kingdom | Retrospective cohort study | TC, LDL, HDL, TG | Incident dementia | HR | Medical codes via general practice records (CPRD GOLD) | 52·0 (7·2) | ·· | 49·9 | Age, BMI, country, CVA, CVD, diabetes, drugs b, c, d, ethnicity, EtOH, SES, sex, smoking, AF, COPD |
| Liang (2020) 19 | 744 | 1972‐1987 | 21·2* | Finland | Prospective cohort study | TC | Incident dementia | HR | DSM‐IV criteria via MMSE | 50·4 (6·0) | 40‐64 | 62·1 | Age, APOE genotype, BMI, BP, CVD, death, diabetes, education, glucose level, physical activity, sex, smoking |
| Sabia (2019) 29 | 7899 | 1985‐1988 | 24·7a | ||||||||||
| (19·1, 29·3) | United Kingdom | Prospective cohort study | TC | Incident dementia | HR | ICD10 codes via medical records | 50·4 (2·3) | 45‐55 | 32·4 | Age, CVD, ethnicity, education, marital status, SES, sex, | |||
| Svensson (2019) 22 | 1106 | 1990 | ·· | Japan | Prospective cohort study | TC, LDL, HDL, TG | Incident dementia, MCI | OR | DSM‐IV criteria via MMSE, WMS‐R, CDT, CDR scale | 54·3 (5·5) | ·· | 63·1 | Age, BMI, diabetes, drugs b, education, EtOH, HTN, sex, smoking |
| Vu (2019) 21 | 10,119 | 1967‐1973 | ·· | United States | Prospective cohort study | TC | Incident dementia | HR | ICD9 codes via Medicare claims | 35·3 (5·5) | 23‐47 | 32·4 | Age, death, education, ethnicity, No. of medical visits prior to diagnosis, sex |
| Toro (2014) 30 | 222 | 1993‐1995 | 14 a | Germany | Prospective cohort study | TC | Incident dementia, MCI | OR | DSM‐III via clinical assessment | 62·4 | 60‐64 | 46·8 | APOE genotype, BMI, diabetes, education, HTN, SES, sex |
| Exalto (2014) 34 | 9480 | 1964‐1973 | 36·1 (4·3)* | United States | Retrospective cohort study | TC | Incident dementia | HR | ICD9 codes via medical records | 46·1 (4·3) | 40‐55 | 55·1 | Age, BMI, education, depressed mood, diabetes, head trauma, lung function, obesity, SBP, sex, smoking |
| Rönnemaa (2011) 42 | 2268 | 1970 | 29* | Sweden | Prospective cohort study | TC | Incident dementia, AD, VD, FTLD | HR | DSM‐IV criteria via medical records | 49·6 (0·6) | ·· | 0·0 | Age, education |
| Solomon (2009) 45 | 9844 | 1964‐1973 | ·· | United States | Retrospective cohort study | TC | Incident AD | HR | ICD9 codes via medical records | 42·4 (1·7) | 40‐45 | 54·1 | Age, BMI, CVA (late‐life), diabetes, education, ethnicity, HTN, sex |
| Mielke (2010) 44 | 648 | 1968‐1969 | 32* | Sweden | Prospective cohort study | TC | Incident dementia, AD | HR | DSM‐III criteria via medical records | 46·8 2 | 38‐60 | 100·0 | Age, BMI, DBP, education |
| Whitmer (2005) 18 | 8845 | 1964‐1973 | 26·7* | United States | Retrospective cohort study | TC | Incident dementia | HR | ICD9 codes via medical records | 42·0 (1·4) | 40‐44 | 53·7 | Age, ethnicity, education, sex |
| Rosengren (2005) 43 | 7402 | 1970‐1973 | 23* | Sweden | Prospective cohort study | TC | Incident dementia | HR | ICD 8/9 codes via hospital records/death registry | 51·5 (2·3) | 47‐55 | 0·0 | Age |
| Kivipelto (2005) 35 | 1409 | 1972‐1987 | 21·0 (4·9)* | Finland | Prospective cohort study | TC | Incident AD | OR | DSM‐IV criteria via MMSE | 50·6 (6·0) | 40‐64 | 62·1 | Age, sex, education, BMI, SBP, follow‐up duration |
| Kivipelto (2001) 36 | 1400 | 1972‐1987 | 20·9 (4·9)* | Finland | Prospective cohort study | TC | Incident dementia | OR | DSM‐IV criteria via MMSE | 50·4 (6·0) | 40‐64 | 62·1 | Age, BMI, CVA, CVD, education, EtOH, smoking |
| Kivipelto (2001) 20 | 1352 | 1972‐1987 | 21·0 (4·9)* | Finland | Prospective cohort study | TC | Incident MCI | OR | MCADRC criteria | 50·4 (6·0) | 40‐64 | 62·1 | Age, BMI |
| Notkola (1998) 46 | 444 | 1959‐1974 | 30* | Finland | Prospective cohort study | TC | Incident AD | OR | DSM‐III‐R criteria | ·· | 40‐59 | 0·0 | Age, APOE genotype |
Number of participants from the original study who were eligible for analysis in the present review.
Due to data restrictions, values were based on the entire study sample rather than the eligible sub‐sample for our study.
*Follow‐up period in mean (SD) years; aFollow‐up period in median (IQR) years.
Drugs: b = lipid‐lowering, c = antihypertensive, d = hypoglycemic (antidiabetic).
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E ε4; AF, atrial fibrillation, BP, blood pressure; BMI, body‐mass index; CVD, cardiovascular disease; CVA, cerebrovascular accident/disease; COPD, chronic obstruction pulmonary disease; CDR, Clinical Dementia Rating; CPRD, Clinical Practice Research Datalink; CDT, clock drawing test; DSM, Diagnostic and Statistical Manual of Mental Disorders; DBP, diastolic blood pressure; EtOH, alcohol use; FTLD, frontotemporal lobe dementia; HDL, high‐density lipoprotein; HR, hazard ratio; HTN, hypertension; ICD, International Classification of Diseases; LDL, low‐density lipoprotein; OR, odds ratio; MCADRC, Mayo Clinic Alzheimer's Disease Research; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; RR, risk ratio; SES, socioeconomic status; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; VD, vascular dementia; WMS‐R, Wechsler Memory Scale‐Revised.
TABLE 2.
Baseline characteristics of participants in included studies
| Study (year) | TC, mmol/L (SD) | Dyslipidemia definition | Dyslipidemia, % | SBP | BMI | Diabetes, % | Smokers, % | Anti‐HTN drugs, % | Lipid‐lowering drugs, % | Education | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | % >140mm Hg | Mean (SD) | % >30 | mean (SD), years ¶ | % post‐secondary || | ||||||||
| Gong (2021) 27 | 5·9 (1·1) 1 | TC ≥ 6·2 mmol/L | 34·1 1 | 135·3 (19·2) 1 | ·· | 27·1 (5·2)‡, 1 | ·· | 3·8 1 | 40·7 1 | 14·1 1 | 10·8 1 | ·· | ·· |
| Gong (2021) 27 | 5·5 (1·1) 1 | TC ≥ 6·2 mmol/L | 23·8 1 | 140.9 (17·5) 1 | ·· | 27·8 (4·2)‡, 1 | ·· | 7·1 1 | 51·3 1 | 20·9 1 | 20·0 1 | ·· | ·· |
| Iwagami (2021)28 | 5·6 (1·1) | TC ≥ 6·2 mmol/L | 27·9 1 | ·· | ·· | 28·3 (5·7)‡ | ·· | 6·7 | 54·1 | 25·5 | 9·3 | ·· | ·· |
| Liang (2020) 19 | ·· | TC ≥ 6·2 mmol/L | 65·1 | ·· | 42·7 | ·· | 16.6 | 4·0 | 24·2 | ·· | ·· | 8·6 (3·4) | |
| Sabia (2019) 29 | 6·2 (1·2) | TC ≥ 6·2 mmol/L | 44·6 | 122·3 (15·1) | ·· | 25·5 (3·8)‡ | ·· | 1·4 | 19·7 | 5·24 | 0·7 | ·· | 39·1 |
| Svensson (2019) 22 | 5·0 | HDL < 1·3 mmol/L | ·· | ·· | 25·7 | 23·5 (2·6)‡ | ·· | 2·2 | 24·9 | ·· | 2·5 | ·· | 66·5 |
| Vu (2019) 21 | 5·1 (1·1) | TC ≥ 6·2 mmol/L | 11·5 | 131·8 (15·9) | ·· | 23·5 (2·6)‡ | ·· | 1·3 | 66·9 | ·· | ·· | 13·6 (2·6) | ·· |
| Toro (2014) 30 | 6·2 (1·2) | TC ≥ 6·9 mmol/L | ·· | ·· | ·· | 26.6 (3·5)‡ | ·· | ·· | ·· | ·· | ·· | 12·9 (2·8) | ·· |
| Exalto (2014) 34 | ·· | TC ≥ 6·5 mmol/L | 25·3 | ·· | 18·3 | – | 10.0 | 14·1 | 56·9 | ·· | ·· | ·· | ·· |
| Rönnemaa (2011) 42 | 6·9 (1·3) | TC > 7·0 mmol/L | ·· | 133·0 (18·0) | ·· | 25·1 (3·2)‡ | ·· | ·· | 51·0 | ·· | ·· | ·· | ·· |
| Solomon (2009) 45 | ·· | TC ≥ 6·2 mmol/L | 15·4 1 | 127·1 (14·7) 1 | ·· | ·· | ·· | ·· | 61·9 1 | ·· | ·· | ·· | ·· |
| Mielke (2010) 44 | 5·7 (1·1) | TC ≥ 6·2 mmol/L | 32·0 | ·· | 19·3 | 24·9 (3·9)‡ | ·· | 11·3 | ·· | ·· | ·· | ·· | 34·3|| |
| Whitmer (2005) 18 | ·· | TC ≥ 6·2 mmol/L | 32·2 | ·· | 19·4 | – | ·· | 11·4 | 59·8 | ·· | ·· | ·· | 50·9|| |
| Rosengren (2005) 43 | 6·4 (1·2) | TC ≥ 7·5mmol/L | ·· |
149 (22) |
·· | 25·5 (3·3)‡ | ·· | 2·0 | 50·2 | 5·4 | ·· | ·· | ·· |
| Kivipelto (2005) 35 | 6·7 (1·2) | TC ≥ 6·5 mmol/L | ·· | 144.3 (19.9) | ·· | 26·6 (3·7) | ·· | 6·7 | 43·0 | ·· | ·· | 8·7 (3·5) | ·· |
| Kivipelto (2001) 36 | 6·7 (1·2) | TC ≥ 6·5 mmol/L | 54·2 | 144·3 (19·9) | ·· | 26.6 (3.7) ‡ | ·· | 1·1 | 42·8 | 14·8 | ·· | 8·4 (3·5) | ·· |
| Kivipelto (2001) 20 | 6·7 (1·2) | TC ≥ 6·5 mmol/L | ·· | 143·9 (19·9) | ·· | 26·5 (3·7) | ·· | 1·1 | 42·7 | 14·4 | ·· | 8·7 (3·5) | ·· |
| Notkola (1998) 46 | 6·6 | TC ≥ 6·5 mmol/L | 58·6 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
Due to data restrictions, values were based on the entire study sample rather than the eligible sub‐sample for our study.
*Systolic blood pressure in mean (SD) mmHg; †Systolic blood pressure in % participants ≥140 mmHg.
‡ BMI in mean (SD) units; § BMI in % participants ≥30 units.
¶ Education in mean (SD) years; || Education in % participants who finished secondary school.
4.2. Association between midlife dyslipidemia and all‐cause dementia
High versus low level of TC was associated with an increase in incidence in all‐cause dementia (ES = 1.14; 95% confidence interval (CI): 1.07 to 1.21; I2 = 0·0%) (Figure 1). When TC was assessed as a continuous variable, each 1 mmol/L increase was associated with a 5% increase in incident dementia (ES = 1.05, 95% CI 1.02 to 1.08; I2 = 0.0) (Figure 1). Similarly, each 1 mmol/L rise in LDL corresponded to an 8% increase in incident in dementia (ES = 1.08, 95% CI: 1.03 to 1.14; I2 = 0.3%) (Figures 2). HDL levels were not associated with incidence of dementia, either as a continuous (ES 1.03 per 0.42 mmol/L increase in HDL; 95% CI: 0.98 to 1.08; n = 636,262 participants) or a categorical (ES = 0.44 for ≥1.84 vs <1.29 mmol/L HDL; 95% CI: 0.12 to 1.64; n = 651 participants) variable.
FIGURE 1.
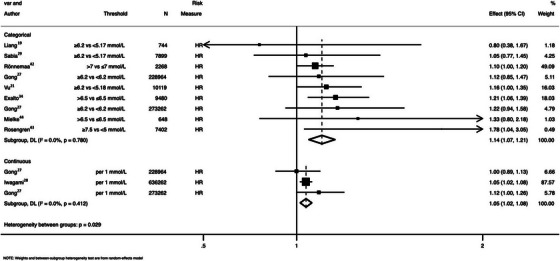
Pooled association between total cholesterol and all‐cause dementia. HR = hazard ratio
FIGURE 2.
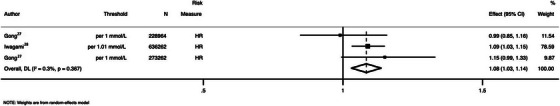
Pooled association between low‐density lipoprotein levels and all‐cause dementia. HR = hazard ratio
4.3. Association between midlife dyslipidemia and lifetime incidence of MCI
High versus low level of TC was associated with increased lifetime incidence of MCI (ES = 2.01; 95% CI: 1.19 to 2.84; I2 = 0.0%) (Figure 3). HDL was associated with a lower incidence of MCI (ES = 0.39; 95% CI: 0.22 to 0.70; n = 455 participants). 22
FIGURE 3.
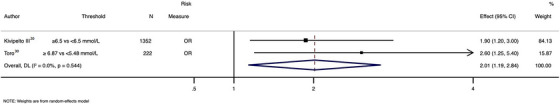
Pooled association between total cholesterol and mild cognitive impairment. OR = odds ratio
4.4. Association between midlife dyslipidemia and lifetime incidence of AD
There was a trend toward a greater incidence of AD with high versus low level of TC (ES = 1.41; 95% CI: 0.95 to 1.88; I2 = 57·5%) (Figure 4). Each 1 mmol/L increase in LDL was associated with an ≈17% increase in incidence of AD (ES = 1.17; 95% CI: 1.07 to 1.27; n = 636,262 participants). 28
FIGURE 4.
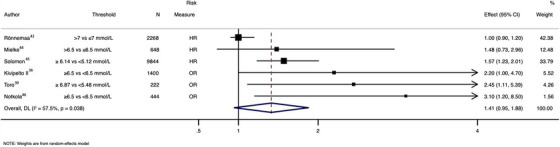
Pooled association between total cholesterol and Alzheimer's disease. HR = hazard ratio, OR = odds ratio
4.5. Sensitivity analysis
There was no clear evidence that different TC threshold values influenced the effect estimates of incident all‐cause dementia or AD (p ≥ 0.30). In sensitivity analysis examining the influence of individual studies on overall effect estimates, no significant change in the effect size of all‐cause dementia incidence was observed when individual studies were excluded (Figure S2), or when studies scoring 6 or lower on the Newcastle‐Ottawa Scale were excluded (Figure S3). 42 , 43 , 46 On the other hand, exclusion of the study by Rönnemaa and colleagues had a statistically significant influence on incidence of AD in individuals with high versus low TC (ES 1.62; 95% CI: 1.27 to 1.98) (Figure S4). 42 Furthermore, exclusion of studies with a Newcastle‐Ottawa Scale score of 6 or less had a marked effect on incidence of AD when comparing high and low levels of TC (ES 1.61; 95% CI: 1.25 to 1.96; I2 = 0·0%) (Figure S5). 42 , 46
No publication bias was observed in studies investigating the association between TC and incidence of dementia by visual inspection of the funnel plot (Figure S6) or statistically by Egger's test (slope P = 0.115; bias P = 0.074).
5. DISCUSSION
Findings from the current systematic review and meta‐analysis of 17 studies provide compelling evidence of an association between midlife dyslipidemia and lifetime incidence of dementia and MCI. Evidence was strongest for the relationship between TC in midlife and incidence of all‐cause dementia, which pooled results across eight studies encompassing over half a million participants. The findings suggest a 14% increased risk of incident dementia with elevated midlife TC compared to TC in the normal range. Likewise, results pooled from two large studies of over 1.1 million participants revealed a 5% increase in risk of incident dementia for every 1 mmol/L increase in TC. For MCI, results pooled across two studies demonstrated a two‐fold risk of incident MCI in those with high midlife TC; this risk was 61% less in those with high midlife HDL. Overall, the magnitude of dementia risk associated with midlife dyslipidemia in our review is comparable to that of excess alcohol consumption (relative risk [RR] 1.2), but less than other modifiable risk factors such as diabetes (RR 1.5), smoking (RR 1.6), hypertension (RR 1.6), and obesity (RR 1.6). 8
Our findings corroborate those of previous systematic reviews and meta‐analyses that found evidence of an association between midlife dyslipidemia and development of dementia. 10 , 39 Our review builds upon the work of Anstey et al. by incorporating more recently published studies, 10 including a large cohort study of more than 1.8 million individuals in the United Kingdom. 28 We further differentiate between lipid subtypes, as well as using lipid threshold values defined by individual studies rather than a singular cutoff of 6.5 mmol/L. We extended the findings of Anstey et al. 10 by exploring the relationship between midlife dyslipidemia and MCI and AD. The recent systematic review by Zhu et al. that included a sub‐analysis on midlife lipids and dementia found positive relationships between midlife TC and all‐cause dementia (ES = 1.16; 95% CI: 1.05 to 1.26) and AD (ES = 1.14; 95 CI: 1.02 to 1.27) that were comparable to our findings. 39 We add to this work by including major studies published after 2020, 27 , 28 analyzing midlife dyslipidemia on a per unit basis, and examining the relationship between midlife dyslipidemia and MCI. Overall, our study provides the most current and comprehensive synthesis of the literature pertaining to midlife dyslipidemia and later‐life incidence of MCI and dementia.
Results from our study have widespread public health implications. Targeting dyslipidemia as part of a comprehensive primary prevention strategy that includes modifiable risk factors has the potential to decrease the global burden of not only dementia but also MCI. 7 Dyslipidemia is an ideal risk factor to target due to its prevalence in the community (≈23% to 77%), 47 established treatment modalities, existing screening guidelines in the primary care setting, and benefits that extend to other diseases, most notably cardiovascular disease. 47 A systematic review and meta‐analysis of observational studies that investigated the effect of lipid‐lowering therapy with statins on cognitive impairment found that patients who used a statin had lower risk of all‐cause dementia and dementia subtypes, including AD and VaD. 37 Despite these compelling observational results, a Cochrane review of randomized controlled trials found that statins given to people at risk of vascular disease did not prevent cognitive decline or dementia.48 A potential explanation for this discrepancy is that the included studies focus on intervention during later life, when risk of cognitive decline may already be established. 48 It remains to be seen whether lipid‐lowering therapy given at midlife affords any protection from MCI and dementia later in life.
In our pooled analysis of six studies investigating the association between TC and AD, there was a trend toward greater incidence of AD in individuals with elevated midlife TC. It is worth noting that inclusion of only high‐quality studies (Newcastle‐Ottawa Scale score >6) in our sensitivity analysis resulted in a statistically significant change to the observed relationship between TC and AD. After excluding two studies of Newcastle‐Ottawa Scale score ≤6, 42 , 46 elevated midlife TC was associated with an ≈60% increased risk of incident AD. This may be explained partially by the study designs: Rönnemaa et al. applied stringent criteria to diagnose “pure Alzheimer's disease” that excluded participants with radiological evidence of VaD, which frequently co‐occurs with AD as a mixed dementia. In both studies, only male participants were enrolled, even though AD is known to disproportionately affect females. 42 , 46 In addition, both studies adjusted for only two covariates: age and apolipoprotein E (APOE) genotype by Notkola et al. and age and education by Rönnemaa et al. 42 , 46
Only one study examining midlife HDL, and cognitive impairment was identified, which showed a significant correlation between high HDL and lower incidence of MCI. 22 Because HDL metabolizes and effectively lowers LDL and TC, this effect aligns with the two studies that found a positive correlation between midlife TC and incidence of MCI. 20 , 30 Nonetheless, this review did not find a significant relationship between HDL and incident dementia, consistent with the findings of Zhu et al., although they did not specifically examine levels at midlife. 39
5.1. Strengths and limitations
The strengths of our review include a comprehensive search strategy that incorporated relevant lipid and dementia subtypes, a the over 1.2 million patients enrolled across six countries, long follow‐up duration, and overall low risk of bias of included studies. However, the findings of this review should be interpreted in context of some limitations. First, as mentioned, a limited number of studies were available for some exposure‐disease pairs, and thus results from those analyses should be seen as hypothesis generating. Second, some studies did not explicitly screen for dementia at enrollment, making it difficult to quantify baseline prevalence, although based on global prevalence rates this would be low (119 per 100,000 in those <65 years of age). 10 , 49 Likewise, the use of lipid‐lowering therapy at baseline was inconsistently reported between included studies, with only 12% of studies reporting data on the proportion of patients on lipid‐lowering therapy. Given that the proportion of patients on lipid lowering therapy in studies was relatively low (9.3% to 20%), exclusion of these studies in sensitivity analysis may not reflect a true effect of lipid‐lowering therapy. Third, different covariates were used across the studies. The most significant of these is arguably the APOE genotype, which has an established relationship with dementia. 50 In our review, only three studies adjusted for the APOE ε4 genotype 19 , 30 , 46 ; thus, the impact of dyslipidemia as a risk factor could not be separated entirely from genetic predisposition to dementia at baseline. In addition, there was heterogeneity in methodology between studies, including the use of different thresholds for dyslipidemia. Finally, owing to the limited nature of the data available from included studies, it was not possible to define lower TC and LDL thresholds from which each 1 mmol/L increase in lipid levels were calculated in the continuous analysis. It is possible that an increase in lipid levels, regardless of a lower threshold, may be associated with increasing dementia risk as highlighted by our results examining high versus low lipid levels. Until such thresholds have been defined in future studies, a cautionary approach to public health messaging may be prudent. Nonetheless, the inclusion of studies with higher lipid cutoffs is likely to provide a more conservative estimate of effect size. Furthermore, inclusion of studies with varying cutoff levels is likely to increase sensitivity, in contrast to prior work, which focused on a cutoff of >6.5 mmol/L. 10
Owing to the nature of population‐based studies, a single lipid measure was used as a marker for dyslipidemia in all but one included study. 46 Alteration in lipid levels in the period between lipid sampling and diagnosis of MCI or dementia, for example, due to lipid‐lowering therapies and/or lifestyle modifications, was not explored in these studies. Future research may aim to better understand the temporal behavior of lipids, especially as participants progress through stages of healthy cognition, aging, and cognitive impairment. More nuanced analysis may also help to delineate optimal timepoints for lipid sampling and intervention as well as to identify a target lipid level at which there is negligible added risk of cognitive impairment. Given the protective nature of high HDL against elevated TC and LDL, the association between HDL cholesterol and cognitive impairment in larger longitudinal studies may provide useful insights as to whether this lipid subtype is related to the onset of cognitive impairment.
6. CONCLUSION
In conclusion, this review identifies a compelling relationship between midlife dyslipidemia and increased risk of dementia and MCI. Efforts to reduce midlife cholesterol have the potential to decrease the total incidence and prevalence of dementia over time. Future research may build on our findings by examining the effect of midlife interventions on the temporal behavior of lipids and subsequent lifetime risk of cognitive decline. With reduced risk of disease incidence, it is expected that significant benefits will be realized in the way of health care cost savings, alleviated disease burden, and a shift toward preventative policy and care in cognitive health.
AUTHOR CONTRIBUTIONS
All authors provided critical input in analyzing, interpreting the data and revising the report. JW, SS, SB, NP, SN, and AM designed the study. AM coordinated the study. JW, SS, and SB designed and conducted the literature search. SB and MM did the statistical analysis. JW, SS, and SB wrote the report. All authors provided critical input in analyzing, interpreting the data and revising the report. JW and SS contributed equally and are recognized as co‐first authors of this article.
CONFLICT OF INTEREST
Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank the authors of included studies who provided us with supplementary data.
Wee J, Sukudom S, Bhat S, et al. The relationship between midlife dyslipidemia and lifetime incidence of dementia: A systematic review and meta‐analysis of cohort studies. Alzheimer's Dement. 2022;15:e12395. 10.1002/dad2.12395
Jason Wee and Sara Sukudom contributed as co‐first authors.
REFERENCES
- 1. Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21:591‐643. [Google Scholar]
- 2. Nichols E, Szoeke CE, Vollset SE, et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990‐2016: a systematic analysis for the Global Burden of Disease Study. 2016 Lancet Neurol. 2019;18(1):88‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Exchange GHD . GBD results tool. 2019;
- 4. Rizzi L, Rosset I, Roriz‐Cruz M. Global epidemiology of dementia: Alzheimer's and vascular types. Biomed Res Int. 2014;908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weller J, Budson A. Current understanding of Alzheimer's disease diagnosis and treatment. F1000Res. 2018;7(1): 1161‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. 2018;33(8):500‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262‐1270. [DOI] [PubMed] [Google Scholar]
- 8. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. [DOI] [PubMed] [Google Scholar]
- 10. Anstey KJ, Ashby‐Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late‐life dementia: review and meta‐analysis. J Alzheimers Dis. 2017;56(1):215‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Refolo LM, Pappolla MA, Malester B, et al. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7(4):321‐331. [DOI] [PubMed] [Google Scholar]
- 12. Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gamba P, Testa G, Gargiulo S, Staurenghi E, Poli G, Leonarduzzi G. Oxidized cholesterol as the driving force behind the development of Alzheimer's disease. Front Aging Neurosci. 2015;7:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marwarha G, Raza S, Prasanthi JR, Ghribi O. Gadd153 and NF‐κB crosstalk regulates 27‐hydroxycholesterol‐induced increase in BACE1 and β‐amyloid production in human neuroblastoma SH‐SY5Y cells. PloS One. 2013;8(8):e70773. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Zambón D, Quintana M, Mata P, et al. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am J Med. 2010;123(3):267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Draczynska‐Lusiak B, Doung A, Sun AY. Oxidized lipoproteins may play a role in neuronal cell death in Alzheimer disease. Mol Chem Neuropathol. 1998;33(2):139‐148. [DOI] [PubMed] [Google Scholar]
- 17. Stenberg D, Parthasarathy S, Carew T, Khoo J, Witztum J. Beyond cholesterol: modifications of low density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915‐24. [DOI] [PubMed] [Google Scholar]
- 18. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurol. 2005;64(2):277‐281. [DOI] [PubMed] [Google Scholar]
- 19. Liang Y, Ngandu T, Laatikainen T, et al. Cardiovascular health metrics from mid‐to late‐life and risk of dementia: A population‐based cohort study in Finland. PLoS Med. 2020;17(12):e1003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kivipelto M, Helkala E‐L, Hänninen T, et al. Midlife vascular risk factors and late‐life mild cognitive impairment: a population‐based study. Neurol. 2001;56(12):1683‐1689. [DOI] [PubMed] [Google Scholar]
- 21. Vu THT, Zhao L, Liu L, et al. Favorable cardiovascular health at young and middle ages and dementia in older age—the CHA study. J Am Heart Assoc. 2019;8(1):e009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Svensson T, Sawada N, Mimura M, Nozaki S, Shikimoto R, Tsugane S. The association between midlife serum high‐density lipoprotein and mild cognitive impairment and dementia after 19 years of follow‐up. Transl Psychiatry. 2019;9(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stewart R, White LR, Xue Q‐L, Launer LJ. Twenty‐six–year change in total cholesterol levels and incident dementia: the Honolulu‐Asia Aging Study. Arch Neurol. 2007;64(1):103‐107. [DOI] [PubMed] [Google Scholar]
- 24. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pimentel ÉML. Role of neuropsychological assessment in the differential diagnosis of Alzheimer's disease and vascular dementia. Dement Neuropsychol. 2009;3:214‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gong J, Harris K, Peters SA, Woodward M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med. 2021;19(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwagami M, Qizilbash N, Gregson J, et al. Blood cholesterol and risk of dementia in more than 1· 8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2021;2(8):e498‐e506. [DOI] [PubMed] [Google Scholar]
- 29. Sabia S, Fayosse A, Dumurgier J, et al. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow‐up of Whitehall II cohort study. BMJ. 2019;366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toro P, Degen C, Pierer M, Gustafson D, Schröder J, Schönknecht P. Cholesterol in mild cognitive impairment and Alzheimer's disease in a birth cohort over 14 years. Eur Arch Psychiatry Clin Neurosci. 2014;264(6):485‐492. [DOI] [PubMed] [Google Scholar]
- 31. Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatr Gerontol Int. 2006;6(3):149‐158. [Google Scholar]
- 32. Organization WH. Definition of an older or elderly person. 2010.
- 33. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Oxford; 2000. [Google Scholar]
- 34. Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement 2014;10(5):562‐570. [DOI] [PubMed] [Google Scholar]
- 35. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556‐1560. [DOI] [PubMed] [Google Scholar]
- 36. Kivipelto M, Helkala E‐L, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poly TN, Islam MM, Walther BA, et al. Association between use of statin and risk of dementia: a meta‐analysis of observational studies. Neuroepidemiology. 2020;54(3):214‐226. [DOI] [PubMed] [Google Scholar]
- 38. Symons M, Moore D. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893‐899. [DOI] [PubMed] [Google Scholar]
- 39. Zhu Y, Liu X, Zhu R, Zhao J, Wang Q. Lipid levels and the risk of dementia: A dose–response meta‐analysis of prospective cohort studies. Ann Clin Transl Neurol. 2022;9:296‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 41. Huedo‐Medina TB, Sánchez‐Meca J, Marin‐Martinez F, Botella J. Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193. [DOI] [PubMed] [Google Scholar]
- 42. Rönnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40‐year follow‐up of a population‐based cohort. Dement Geriatr Cogn Disord. 2011;31(6):460‐466. [DOI] [PubMed] [Google Scholar]
- 43. Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165(3):321‐326. [DOI] [PubMed] [Google Scholar]
- 44. Mielke M, Zandi P, Shao H, et al. The 32‐year relationship between cholesterol and dementia from midlife to late life. Neurol. 2010;75(21):1888‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Notkola I‐L, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E {FC12} e4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17(1):14‐20. [DOI] [PubMed] [Google Scholar]
- 47. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18(10):689‐700. [DOI] [PubMed] [Google Scholar]
- 48. McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2009;(2):CD003160‐CD003160. [DOI] [PubMed] [Google Scholar]
- 49. Hendriks S, Peetoom K, Verhey FR, de Vugt M, Koehler S. The prevalence of young onset dementia: a systematic review and meta‐analysis. ALZ; 2020: [Google Scholar]
- 50. Lumsden AL, Mulugeta A, Zhou A, Hyppönen E. Apolipoprotein E (APOE) genotype‐associated disease risks: a phenome‐wide, registry‐based, case‐control study utilising the UK Biobank. EBioMed. 2020;59:102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Anonymized data not published in this article will be made available by request from any qualified investigator.


