Abstract
BACKGROUND:
Positive airway pressure (PAP) is the accepted standard treatment for obstructive sleep apnea. In the last decades, automatic PAP (APAP) adjustment modes have been increasingly used. Pressure auto adjustment offers better comfort to the patient and represents a valuable help for the clinician to provide optimal treatment. However, device performance differs among manufacturers. Furthermore, the success of the therapy relies greatly on unintentional air leak level for many reasons, hence the importance to investigate the performance of the most common devices. The aim of this study was to compare the performance of 3 APAP devices from the most common manufacturers in specific conditions (ie, obstructive sleep apnea, central sleep apnea, hypopnea), with and without unintentional air leak.
METHODS:
This was a bench test study. Performance tests were conducted on a breathing simulator using a Starling resistor, representing the upper airways, and an adjustable unintentional air leak valve. Three APAP devices (AirSense 10, DreamStation, and Prisma 20A) were tested in different scenarios.
RESULTS:
Without unintentional air leak, performance of the 3 devices was similar to existing literature. However, performance was altered with the addition of unintentional air leak in some scenario. The AirSense 10 was not able to respond correctly to obstructive apnea (intraclass correlation coefficient [ICC] 0.021, P = .61) and hypopnea (ICC 0.059, P = .26). Prisma 20A lowest performance was seen during simulated obstructive apnea (ICC 0.708, P < .001). DreamStation lowest performance was seen during simulated hypopnea events (ICC 0.755, P < .001).
CONCLUSIONS:
All 3 APAP devices reacted differently to the added unintentional air leak. Performance was altered with some devices, which could affect the therapy success in patients with sleep apnea syndrome. The variability of performance of some APAP devices with unintentional air leak should make clinicians evaluate their use in a home setting.
Keywords: CP, APAP, air leaks, obstructive sleep apnea, apnea syndrome
Introduction
Over the past decade, the prevalence of obstructive sleep apnea (OSA) has been increasing.1,2 It has been noticed that OSA is 2 times more prevalent in men than women.1 CPAP is the first-line treatment for most patients with OSA. It improves quality of sleep, daytime sleepiness, reduces apnea-hypopnea index (AHI), and cardiovascular morbidity and mortality.3-5 These health benefits are, however, directly correlated to adherence with a consensus of > 4 h use time per night.6 However, positive airway pressure (PAP) therapy is not tolerated by all patients. PAP devices can be uncomfortable or cumbersome and may lead to a poor adherence to the treatment, which is a well-recognized issue in OSA.7 Up to 50% of patients with OSA do not reach sufficient adherence.8,9 In the last 2 decades, automatic PAP (APAP) has been increasingly used to facilitate pressure level titration. In addition to providing better comfort to the patient, auto adjustment of the pressure improves therapy efficacy.10,11 Nevertheless, another key point to ensure adequate adherence to the treatment is to avoid unintentional air leaks, which are mainly due to leakage around the mask. Some studies have shown that APAP performance might be altered by unintentional air leak.11,12
Several bench studies have compared APAP performance among different manufacturers.13-17 Only one of them analyzed the addition of simulated unintentional air leak, in 2005; but devices have considerably changed and algorithm have been perfected throughout this time.18 The aim of this study was to investigate the impact of unintentional air leak on APAP performance in different simulated apnea events (ie, obstructive apnea, central apnea, and hypopnea) by simulating unintentional air leak.
QUICK LOOK.
Current Knowledge
Automatic positive airway pressure (APAP) devices facilitate pressure level titration. Autoadjustment of the pressure offers a valuable asset for the patient. Several studies already compared APAP performance amongst different manufacturers. However, only one of them analysed the impact of added unintentional airleaks.
What This Paper Contributes to Our Knowledge
In this bench evaluation model, added unintentional air leaks altered performance of some devices during obstructive sleep apnea and hypopnea simulated events. To ensure treatment efficacy, clinicians must pay utmost attention to unintentional air leak and find strategies to avoid them. Further research efforts would be necessary to deeper investigate APAP performance in these conditions.
Methods
Study Design
Performance of 3 common APAP devices was evaluated on a bench test. This allowed to compare APAP devices in reproducible and standardized conditions by avoiding bias that could have been encountered with a clinical study. The Active Servo Lung (ASL) 5000 simulator (IngMar Medical, Pittsburgh, Pennsylvania) was used in this study. A Starling resistor was added to the system to simulate obstructive apnea and hypopnea events. Its conjunct use with the ASL 5000 has been validated as efficient and reliable for apnea events simulation.19
ASL 5000 Breathing Simulator
The ASL 5000 is an active artificial lung that responds to set characteristics. The dedicated software (ASL 5000 3.6 version, IngMar Medical) was used to read and analyze the recorded scenarios.
Starling Resistor
The starling resistor has already been used in many studies to simulate upper airways.20,21 The pharynx is represented as a soft and malleable material where air flow can pass through. It is disposed between 2 solid and fixed ports, which evoke nasal cavity and trachea. Malleable material is placed into a hermetic tube, evoking tissues around pharynx. The pressure into the hermetic tube is controlled by a syringe. There was no manometer in the present study to monitor pressure level. With the use of a syringe, air flow can be reduced or abolished by increasing the pressure into the hermetic tube. This occurs when pressure in the hermetic tube is greater than pressure in the deformable conduct. Therefore, OSA and hypopnea events can be simulated.
Unintentional Air Leaks Valve
The unintentional air leak valve was developed by Haute Ecole d’Ingénierie de Genève.20,21 The device is connected to a computer and a software (Microsoft Visual Basic 6.0, Microsoft, Redmond, Washington) that controls the opening diameter of the valve between 0–10 mm. Maximal attainable air leak flow was 60 L/min for 25 cm H2O pressure. In this study, it was decided to set the maximal diameter 10 mm. The minimal and maximal air leak flows according to the pressure were measured and ranged from 24 L/min for 4 cm H2O pressure to 56.4 L/min for 20 cm H2O pressure. Detailed air leak flows can be found in Table 1.
Table 1.
Flows of the Unintentional Air Leak Valve According to Device Pressure Level
Bench Evaluation Configuration
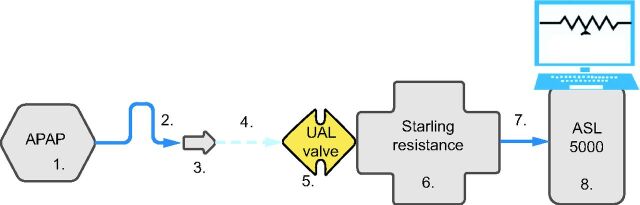
To ensure standardized and reproducible data, a similar configuration was used for OSA, central sleep apnea (CSA), and hypopnea. An exhalation port (Whisper Swivel II, Philips Respironics, Murrysville, Pennsylvania) was used in this evaluation to simulate the intentional leak of a vented mask. Detailed settings can be found in Figure 1.
Fig. 1.
Bench test setting. Configuration was set in the following order: 1. APAP device, 2. 180 cm SlimLine tubing, 3. Whisper Swivel II exhalation port, 4. connector, 5. Unintentional air leak (UAL) valve, 6. Starling resistance, 7. connector, and 8. ASL 5000 simulator. APAP = automatic positive airway pressure.
APAP Devices
Three APAP devices were tested in this study: AirSense 10 (ResMed, San Diego, California), DreamStation (Philips Respironics), and Prisma 20A (Löwenstein Medical, Bad Ems, Rheinland-Pfalz, Germany).
Protocol
Similar settings were used for the 3 APAPs as follows: range of pressure: 4–20 cm H2O, no ramp was added; mask type: face mask.
For all simulated events, scenario duration was 15 min. Respiratory mechanics were set as follows on the ASL 5000: compliance: 80 mL/cm H2O22-24; resistance: 5 cm H2O/L/s; inspiratory pressure: 7 cm H2O; frequency: 12 breaths/min; and inspiratory time 30% of breathing cycle. Tidal volume obtained with these parameters was 500 mL.
OSA and hypopnea were simulated through the Starling resistor. For CSA events, the simulation was made via the ASL 5000, where 25-s breathing pauses were set.
Every record started with 3 min of steady breathing (ie, no events). Then a 25-s event occurred every minute for the rest of the scenario. Therefore, 12 events appeared in each scenario. Each scenario was repeated twice, without unintentional air leak and with unintentional air leak, to ensure similar device response. Pressure data were recorded during the second scenario.
For hypopnea, the syringe of Starling resistor was used to increase pressure into the hermetic tube and reduce air flow. The injected air volume was adapted to ensure ≥ 50% air flow diminution as measured by the ASL 5000 simulator. For OSA, the injected air volume was adapted to ensure the collapse of the simulated upper airways. For CSA, respiratory pauses (ie, no breathing) were set on ASL 5000 program, and no manipulations were needed during the scenario.
Analysis
Pressure results were obtained via the ASL 5000 software and compared with data of the 3 devices. Pressure variations were analyzed with the ASL 5000 software (version 3.6). Pressure data were reported once every minute from the third minute to the 15th (ie, every 60 s from the 180–900 s) for each scenario.
Results are expressed as mean values. As values were obtained using a validated bench test, they were considered stable, and the SD was considered negligible.
To compare the effects of added unintentional air leak, the pressure bias was calculated between the scenario without and with added unintentional air leak for each device; the limits of agreement were presented as 95% CI for the difference between measurements (ie, 1.96 x SD) as proposed by Bland-Altman,25 and an intraclass correlation coefficient (ICC) analysis was performed.
Results
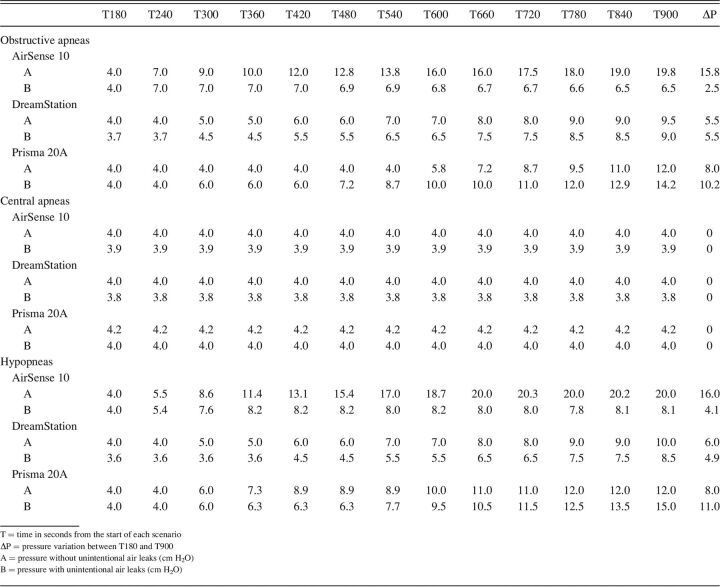
A total of 24 different scenarios were recorded. Detailed results can be found in Table 2.
Table 2.
Intra-device Pressure Comparison Per Events With and Without Unintentional Air Leaks
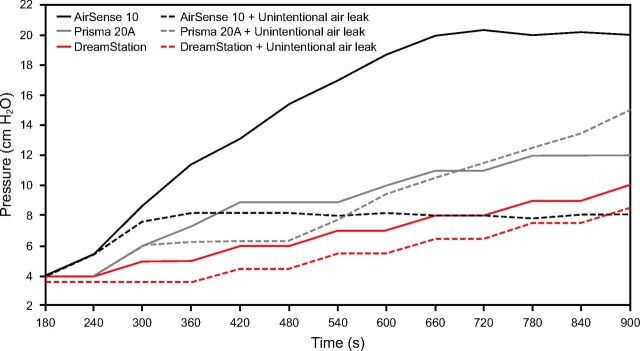
During simulated obstructive apnea events, DreamStation maintained a similar pressurization with unintentional air leak, with a variation of 0.5 cm H2O. Maximal pressurization reached for AirSense 10 was 6.5 cm H2O (ΔP = 2.5 cm H2O) with unintentional air leak compared to 19.8 cm H2O (ΔP = 15.8 cm H2O) without unintentional air leak. Pressurization of Prisma 20A was greater with unintentional air leak (ΔP = 10.2 cm H2O) than without (ΔP = 8.0 cm H2O). Without unintentional air leak, that APAP started to increase its pressurization only between the seventh and the eighth event (T540–T600). Graphic details of pressure variation during simulated obstructive apnea events are presented in Figure 2.
Fig. 2.
Pressure evolution without and with unintentional air leak during simulated obstructive apnea events. The 12 events occurred between 180–900 s, which corresponds to the 12-min data.
During simulated hypopnea events, pressure increased with and without unintentional air leak for DreamStation but was greater by 1.1 cm H2O without unintentional air leak. Similar performance as for OSA was observed for AirSense 10. ΔP was 16.0 cm H2O without unintentional air leak and 4.1 cm H2O with unintentional air leak. Prisma 20A pressurization was greater with unintentional air leak (ΔP = 11.0 cm H2O) than without (ΔP = 8.0 cm H2O). Graphic details of pressure variation during simulated hypopnea events are presented in Figure 3.
Fig. 3.
Pressure evolution without and with unintentional air leak during simulated hypopnea events. The 12 events occurred between 180–900 s, which corresponds to the 12-min data.
All 3 APAPs did not increase their pressurization (ΔP stayed at 0 cm H2O) when central apnea events were simulated.
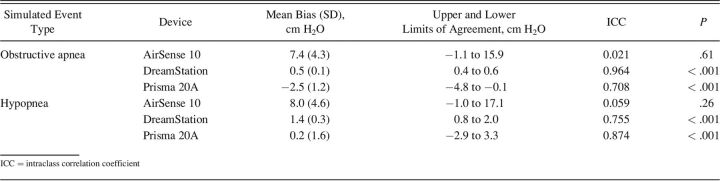
Overall, the DreamStation and the Prisma 20A demonstrated the smallest mean pressure bias and the best ICC in both simulated obstructive apnea and hypopnea events, ranging from −2.5 to 1.4 cm H2O and 0.708–0.964 (P < .001). The AirSense 10 demonstrated weak performance with a mean pressure bias of 7.4 (± 4.3) and 8.0 (± 4.6) and an ICC of 0.021 (P = .61) and 0.059 (P = .26) for simulated obstructive apnea and hypopnea events, respectively. Detailed results are presented in Table 3. Simulated central apnea events are not presented as none of the devices reacted to the events in both modalities (ie, with and without unintentional air leak).
Table 3.
Evaluation of the Pressure Bias and Correlation With and Without Unintentional Air Leaks
Discussion
The aim of this study was to investigate the performance of 3 APAP devices with and without unintentional air leak. Without unintentional air leak, results were similar to existing literature.11-13,17 However, performance was heterogeneous between the 3 devices when unintentional air leaks were simulated. The DreamStation was the device that demonstrated the most consistent results with and without unintentional air leak. Surprisingly, the AirSense 10 and Prisma 20A, which both rely on forced oscillation technique to differentiate central to obstructive events, demonstrated opposite behavior during simulated unintentional air leak. This difference might be explained by different algorithm performance or sensor sensitivity. Each manufacturer uses its own in-house algorithm, which details are not publicly revealed. Unfortunately, this limits interpretation and analysis of the results to very subjective assumptions. More information about algorithm could lead to a better understanding of results. The difference in algorithm performance was also observed without unintentional air leak between those 2 devices. During simulated OSA events without unintentional air leak, the Prisma 20A only started to pressurize between seventh and eighth event. After verifying the data, it was noticed that the first 6 events were classified as CSA by the algorithm. Our hypothesis is that, as flow limitation was total, the algorithm was in the beginning not able or not sensitive enough to differentiate CSA from OSA and only succeeded after some events. This confirms our thoughts that 2 devices both using forced oscillation technique can react differently to the same simulated events.
The AirSense 10 algorithm did not manage to identify OSA or hypopnea events when unintentional air leaks were simulated, and the pressure level remained similar throughout both scenarios. Altered APAP performance can affect treatment efficacy by not correcting apnea events. This can also lead to reduced patient adherence to treatment.5 On the other hand, the Prisma 20A delivered higher pressures with the presence of unintentional air leak. One hypothesis is that the device inaccurately estimates the level of unintentional air leak, resulting in an overcompensation. Even though it might prevent apnea events, higher pressure level could lead to discomfort and tolerance issue. Therefore, unintentional air leak generates risks of non-adherence to treatment in those 2 situations.26 However, some studies showed that adherence and amount of unintentional air leak did not differ between APAP versus CPAP modes, yet it was observed that APAP performance could be altered with unintentional air leak.11,12 This has been confirmed in our study. It is, therefore, necessary to find strategies to avoid unintentional air leak and ensure adequate devices functioning as well as a greater comfort.
An alternative is the use of an adequate interface to prevent unintentional air leak.26 It has been observed that interfaces are the major determinant of unintentional air leak. According to a study from 2018,27 the use of a nasal mask allowed to reduce unintentional air leak risks. This also helped to reduce residual AHI. Finally, nasal masks demonstrated to be easier to set and handle in comparison with a face mask. Clinicians should consider this aspect because a well-positioned mask leads to lower unintentional air leak. Nasal masks also offer many advantages such as a better stability, improved sleep, and a greater comfort during the night.27 However, clinicians must ensure that no leaks escape from the patient’s mouth for optimal treatment delivery.
Finally, it was observed that unintentional air leak impacts the whole therapy. Inadequate responses to sleep events inevitably lead to reduced therapeutic efficacy. Therefore, this study raises a central problem in the management of OSA that needs to be addressed as best as possible to ensure device performance and optimal adherence to treatment.
Conclusions
Our experimentation revealed heterogeneous responses of the devices to unintentional air leak, which altered some of the device performance. This could lead to a reduced therapeutic efficacy. Therefore, further research efforts would be necessary to deeper investigate APAP performance in these conditions. Thus, this would provide even more knowledge and possibilities to practitioners.
Footnotes
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3(4):310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177(9):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA 2020;323(14):1389-1400. [DOI] [PubMed] [Google Scholar]
- 4.Deacon NL, Jen R, Li Y, Malhotra A. Treatment of obstructive sleep apnea. Prospects for personalized combined modality therapy. Ann Am Thorac Soc 2016;13(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE Assessment. J Clin Sleep Med 2019;15(2):301-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masa JF, Corral-Penafiel J. Should use of 4 hours continuous positive airway pressure per night be considered acceptable compliance? Eur Respir J 2014;44(5):1119-1120. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 2011;15(6):343-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tingting X, Danming Y, Xin C. Non-surgical treatment of obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2018;275(2):335-346. [DOI] [PubMed] [Google Scholar]
- 9.Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real-world data. Sleep Med 2019;59:114-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman N. Treatment of obstructive sleep apnea: choosing the best positive airway pressure device. Sleep Med Clin 2020;15(2):205-218. [DOI] [PubMed] [Google Scholar]
- 11.Ryden A, Bando JM, Aysola RS. Auto-adjusting and advanced positive airway pressure therapeutic modalities. Semin Respir Crit Care Med 2014;35(5):593-603. [DOI] [PubMed] [Google Scholar]
- 12.Ip S, D’Ambrosio C, Patel K, Obadan N, Kitsios GD, Chung M, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev 2012;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isetta V, Navajas D, Montserrat JM, Farre R. Comparative assessment of several automatic CPAP devices’ responses: a bench test study. ERJ Open Res 2015;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhle KH, Karweina D, Domanski U, Nilius G. [Characteristics of auto-CPAP devices during the simulation of sleep-related breathing flow patterns.] Pneumologie 2009;63(7):390-398. [DOI] [PubMed] [Google Scholar]
- 15.Zhu K. Bench evaluation of the algorithms of ventilation treatment devices. Évaluation sur banc d'essai des algorithmes des machines ventilatoires. Thesis. Université Paris-Saclay; 2016; NNT:2016SACLS021(tel-01715011). [Google Scholar]
- 16.Zhu K, Aouf S, Roisman G, Escourrou P. Pressure-relief features of fixed and Auto-titrating continuous positive airway pressure may impair their efficacy: evaluation with a respiratory bench model. J Clin Sleep Med 2016;12(3):385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu K, Roisman G, Aouf S, Escourrou P. All APAPs are not equivalent for the treatment of sleep-disordered breathing: a bench evaluation of eleven commercially available devices. J Clin Sleep Med 2015;11(07):725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coller D, Stanley D, Parthasarathy S. Effect of air leak on the performance of auto-PAP devices: a bench study. Sleep Breath 2005;9(4):167-175. [DOI] [PubMed] [Google Scholar]
- 19.Barras A, Zaugg C. Validation de l’efficacité et de la fiabilité de l’ASL5000, combiné à une résistance de Starling, pour simuler un syndrome d’apnées du sommeil. Thesis. HES-SO University of Applied Sciences and Arts of Western Switzerland; 2020. doi: 10.22005/bcu.282114. [Google Scholar]
- 20.Schwartz AR, Smith PL. Cross-talk proposal: the human upper airway does behave like a Starling resistor during sleep. J Physiol 2013;591(9):2229-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le TB, Moghaddam MG, Woodson BT, Garcia GJM. Airflow limitation in a collapsible model of the human pharynx: physical mechanisms studied with fluid-structure interaction simulations and experiments. Physiol Rep 2019;7(10):e14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carteaux G, Lyazidi A, Cordoba-Izquierdo A, Vignaux L, Jolliet P, Thille AW, et al. Patient-ventilator asynchrony during noninvasive ventilation: a bench and clinical study. Chest 2012;142(2):367-376. [DOI] [PubMed] [Google Scholar]
- 23.Boussen S, Gainnier M, Michelet P. Evaluation of ventilators used during transport of critically ill patients: a bench study. Respir Care 2013;58(11):1911-1922. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Retory Y, Sagniez A, Hardy S, Cottin F, Roisman G, et al. New physiological bench test reproducing nocturnal breathing pattern of patients with sleep-disordered breathing. PLoS One 2019;14(12):e0225766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307-310. [PubMed] [Google Scholar]
- 26.Lebret M, Rotty MC, Argento C, Pepin JL, Tamisier R, Arbib F, et al. Comparison of auto- and fixed-continuous positive airway pressure on air leak in patients with obstructive sleep apnea: data from a randomized controlled trial. Can Respir J 2019;2019:6310956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowland S, Aiyappan V, Hennessy C, Catcheside P, Chai-Coezter CL, McEvoy RD, et al. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med 2018;14(01):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]