Abstract
BACKGROUND:
In the midst of the COVID-19 pandemic, noninvasive respiratory support (NRS) therapies such as high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV) were central to respiratory care. The extent to which these treatments increase the generation and dispersion of infectious respiratory aerosols is not fully understood. The objective of this study was to characterize SARS-CoV-2 aerosol dispersion from subjects with COVID-19 undergoing NRS therapy.
METHODS:
Several different aerosol sampling devices were used to collect air samples in the vicinity of 31 subjects with COVID-19, most of whom were receiving NRS therapy, primarily HFNC. Aerosols were collected onto filters and analyzed for the presence of SARS-CoV-2 RNA. Additional measurements were collected in an aerosol chamber with healthy adult subjects using respiratory therapy devices under controlled and reproducible conditions.
RESULTS:
Fifty aerosol samples were collected from subjects receiving HFNC or NIV therapy, whereas 6 samples were collected from subjects not receiving NRS. Only 4 of the 56 aerosol samples were positive for SARS-CoV-2 RNA, and all positive samples were collected using a high air flow scavenger mask collection device placed in close proximity to the subject. The chamber measurements with healthy subjects did not show any significant increase in aerosol dispersion caused by the respiratory therapy devices compared to baseline.
CONCLUSIONS:
Our findings demonstrate very limited detection of SARS-CoV-2–containing aerosols in the vicinity of subjects with COVID-19 receiving NRS therapies in the clinical setting. These results, combined with controlled chamber measurements showing that HFNC and NIV device usage was not associated with increased aerosol dispersion, suggest that NRS therapies do not result in increased dispersal of aerosols in the clinical setting.
Keywords: COVID-19, noninvasive ventilation, critical care, transmission, communicable diseases, aerosol
Introduction
COVID-19, caused by SARS-CoV-2, was declared a pandemic by the World Health Organization in March 2020. Transmission of SARS-CoV-2 occurs predominantly via the respiratory route. Infected individuals produce respiratory particles that contain infectious virus, and susceptible individuals can become infected by inhaling virus-containing particles.1 Multiple studies have shown that SARS-CoV-2 RNA and infectious virus are detectable in air samples collected from the hospital rooms of subjects with COVID-19.2-7
In the midst of the ongoing pandemic, noninvasive respiratory support (NRS) therapies such as high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV) are essential to respiratory care. However, the extent to which HFNC or NIV increases the generation and dispersion of infectious respiratory aerosols by patients with COVID-19 is not fully understood. Quantifying aerosol dispersion from these treatments is of clinical interest in order to assess exposure risk and guide appropriate use of personal protective equipment and building infrastructure. In some centers, respiratory adjuncts such as HFNC and NIV were abandoned altogether in favor of early endotracheal intubation to control the spread of infectious aerosols, possibly with negative consequences.8
Prior studies characterizing aerosol dispersion during use of NRS therapies include simulations with tracer particles and manikins using a variety of noninvasive devices as well as a more limited set of studies collecting samples in clinical settings and directly measuring the presence of pathogens.9 These studies showed differing amounts of aerosol dispersal depending on the type and fit of the mask interface and the particular tracer being used.10-13 Studies measuring aerosol dispersion near healthy human participants using HFNC or bi-level positive airway pressure devices did not detect an increase in aerosol dispersion.14-15 Likewise, studies conducted on individuals with pneumonia, upper respiratory tract symptoms, or chronic lung disease found limited evidence of increased aerosol dispersion during HFNC or NIV use, with the exception of some larger particle sizes.16-17 A few recent studies characterizing environmental contamination specifically in the context of subjects with COVID-19 receiving NRS therapies also found no evidence for increased dispersion of SARS-CoV-2–containing aerosols.18-20 Only one of these studies quantified levels of the pathogen of interest (in this case SARS-CoV-2) in both the nasopharynx of the subject and in collected aerosol samples.18
In the current study, we used 2 measurement strategies to characterize aerosol dispersion in the context of NRS therapies: The collection of air samples in clinical settings soon after subjects tested positive for SARS-CoV-2 RNA, and measurements of aerosol dispersion by healthy subjects using NRS devices in an aerosol chamber under controlled conditions. The objective of this study was to characterize SARS-CoV-2 aerosol dispersion from subjects with COVID-19 undergoing NRS therapy and to contextualize these measurements for clinical care providers.
QUICK LOOK.
Current Knowledge
Noninvasive respiratory support (NRS) therapies such as high-flow nasal cannula and noninvasive ventilation have been frequently applied to treat COVID-induced hypoxemic respiratory failure. However, these therapies raised substantial concern among caregivers regarding aerosol dispersion from these treatments and increased risk of transmission of disease. Quantification of this risk is of great clinical interest in order to assess exposure risk and guide appropriate use of personal protective equipment and building infrastructure.
What This Paper Contributes to Our Knowledge
Air samples were collected in the hospital rooms of subjects receiving respiratory therapy for COVID-19. Samples were assayed for the presence of SARS-CoV-2 RNA. Very high air flow collection in close proximity to the subjects was required to detect SARS-CoV-2 in aerosol samples, indicative of low virus-containing aerosol concentrations under the conditions tested. Additionally, aerosol dispersion by healthy subjects using NRS devices in an aerosol chamber was also measured. No increase in aerosol dispersion compared to baseline was observed for these healthy subjects.
Methods
The funding sources for this work played no role in the preparation, review, or approval of this manuscript.
Study Participants
Clinical sampling.
Hospital rooms were selected for sampling based on the presence of a subject with a proximate positive COVID-19 test who was undergoing treatment with HFNC, NIV, or standard oxygen. The protocol was approved by the Tufts Medical Center Institutional Review Board (STUDY00000551), which waived the need for written informed consent but required that all subject and caregiver participants be given an information sheet explaining the study and informing them that they had an option to decline. Sampling was conducted near 37 subjects between April 2020–March 2021. No identifying information was retained after the initial sample collection, and a participant number was used to associate clinical metadata with sampling results. The subjects were able to withdraw from study participation at any point after initiation of aerosol collection.
Chamber measurements.
Five healthy subjects participated in the aerosol chamber study. All participants attested to being nonsmokers, not having current lung ailments, and not having COVID-19 symptoms or recent contact with COVID-19–positive individuals. The study protocol was approved by the institutional review board at MIT (the Committee on the Use of Humans as Experimental Subjects) under protocol 2010000251. Informed consent was obtained from the subjects.
Measurement Equipment and Sampling Procedures
Clinical sampling.
Subjects provided a clinical saliva sample in a glass sample jar to enable determination of active viral shedding at the time of aerosol collection. Subjects with artificial airways underwent tracheal suctioning.
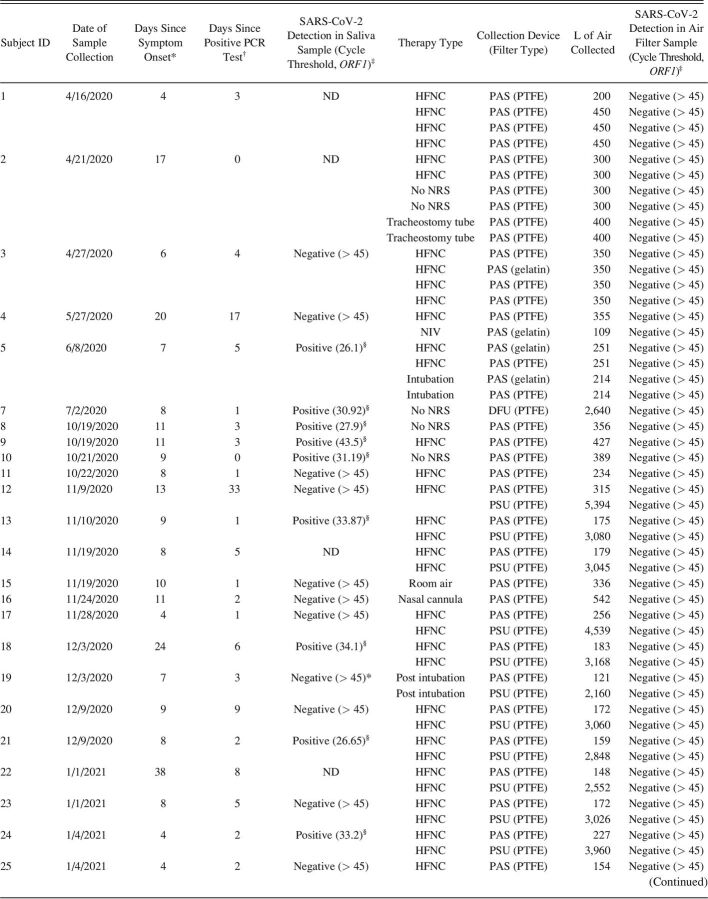
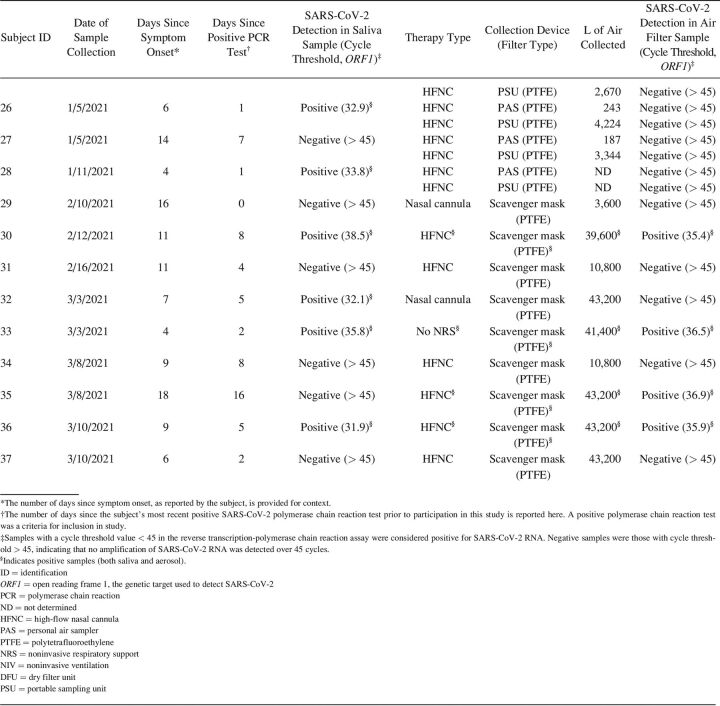
Small AirChek TOUCH personal air sampling (PAS) pumps (5 L/min flow; SKC, Eighty Four, Pennsylvania) were used to collect aerosol samples onto 37-mm diameter polytetrafluoroethylene (PTFE, 0.3 µm pore size; SKC) or gelatin filters (SKC). The filters were housed in 3-piece plastic cassettes and attached to the pump via a 3-foot length of Tygon tubing. PAS pumps were placed approximately 30–45 cm in front of the subject at chest level on a standard hospital tray. In some cases, a second PAS pump was placed approximately 2 m from the subject’s bed with the intent to assess the spatial extent of aerosol dispersion. Higher air flow sampling onto 47-mm PTFE filters (3 µm pore size) was conducted using 2 devices: a modified dry filter unit collector (83 L/min) and a portable sampling unit (PSU, 90 L/min). Due to the loud operating volume of the dry filter unit and PSU, these devices were placed approximately 2–3 m from the subject’s bed. Sampling was conducted for between 20–90 min. The number of liters of air collected for each sample, which is based on the sampling time and device flow, is provided in Table 2.
Table 2.
Detailed Information for All Collected Aerosol Samples
In order to contain and direct sampling, we devised a scavenger mask setup that allowed sampling to be conducted directly in front of the subject’s mouth. The scavenger mask consisted of a tent mask intended for oxygen delivery attached to a Concha Tubing Adapter and an in-line PTFE filter and subsequently connected to full wall suction (see Figure E7 in the online supplement, see related supplementary materials at http://www.rcjournal.com). The flow provided by this setup was approximately 30 L/min, and sampling was allowed to proceed overnight. The number of liters of air collected for each sample, which is based on the sampling time and device flow, is provided in Table 2.
After collection, filter cassettes were stored on ice for processing within 24 h. Alternatively, samples were placed in a −80°C freezer (stability of viral samples was demonstrated for at least a week) and transported on ice to the New England Regional Biosafety Laboratory for extraction of SARS-CoV-2 RNA under biosafety level 3 conditions.
Chamber measurements.
Measurements were collected inside a high-efficiency particulate air−filtered chamber. The subjects were seated at a table with their face approximately 6 inches away from the inlets of 2 real-time aerosol sensors (see Figure E1 in the online supplement, see related supplementary materials at http://www.rcjournal.com). A plastic shielding box was placed over the sensors and subject’s head to help concentrate any generated respiratory aerosols near the sensor inlets. The aerosol sensors included (1) an Optical Particle Sizer 3330 (TSI, Shoreview, Minnesota) that counts particles in 16 size bins ranging from 0.37−10.0 µm in 1-s time intervals and (2) a Wideband Integrated Bioaerosol Sensor, (Droplet Measurement Technologies, Longmont, Colorado) that measures size (0.5 µm−30.0 µm), asphericity, and ultraviolet-induced fluorescence (an indicator of the presence of biological material) of each particle passing through the instrument.
Prior to data collection for each subject, a small burst of deionized water mist was sprayed inside the plastic shielding box to ensure the aerosol sensors were properly responding. Two NRS devices were tested for each participant: an Airvo high-flow system (Fisher & Paykel Healthcare, Auckland, New Zealand) set at 37°C and 60 L/min flow (maximum for the device) via medium standard nasal prongs placed firmly in the nostrils, with straps adjusted to comfort. NIV was delivered using a V60 bi-level positive airway pressure device (Phillips Respironics, Murrysville, Pennsylvania) set at inspiratory pressure of 12 cm H2O and expiratory pressure of 5 cm H2O. NIV was delivered via a standard full face mask with standard ventilator circuitry with a viral/bacterial filter affixed to the exhalation port.
For each device, a series of background measurements (eg, with the participant wearing the cannula or mask but the respiratory equipment turned off) and “on” measurements (eg, the same conditions with the equipment running) were collected for 4 min each. During the first 2 min, the participant sat still while trying to keep their mouth closed; during the final 2 min, they moved their head around near the sensor inlets, talked, and adjusted the cannula or face mask to disrupt the fitting and create air leaks. Additional details regarding the experimental setup are provided in the online supplement (see related supplementary materials at http://www.rcjournal.com).
Clinical Sample Processing and Detection of SARS-CoV-2
Viral material was eluted from PTFE and gelatin filters by submerging the filters in a lysis buffer derived from the Omega Mag-Max Viral RNA/DNA kit (Omega Bio-tek, Norcross, Georgia). The gelatin filters dissolved in the buffer, and the resultant viscous solution was diluted prior to RNA extraction. Saliva samples were diluted 1:1 in lysis buffer and treated with proteinase K prior to RNA extraction. See online supplement for more details (see related supplementary materials at http://www.rcjournal.com).
Viral RNA was extracted using the Omega Mag-Max viral RNA/DNA kit. Extracted RNA was screened for the presence of SARS-CoV-2 sequences by semi-quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR). See the online supplement for additional details (see related supplementary materials at http://www.rcjournal.com). Any sample with a cycle threshold value < 45 was considered positive. Negative samples were those for which amplification was not detected within 45 cycles (cycle threshold > 45). Variant identification was not performed on the samples. The limit of detection of the RT-PCR assay was determined to be 2 genomic copies based on analysis of a dilution curve of the template standard. Given the limit of detection of the RT-PCR assay along with a conservative estimate of extraction efficiency (50%) and the specific processing procedures, the end-to-end sensitivity of the assay was estimated to be ∼450 SARS-CoV-2 genomic copies per filter.
Statistical Analysis
Clinical samples.
Because of the small number of samples collected and the high prevalence of samples negative for SARS-CoV-2 RNA, a statistical analysis of the results was not performed.
Chamber measurements.
To evaluate whether HFNC or NIV significantly changed aerosol concentrations in the chamber study, 4 separate models were fitted: one for each of the HFNC and NIV devices, as observed by the 2 different sampling devices. In each case, the total aerosol concentrations, sampled at a 1-s cadence, were modeled using a linear mixed-effects model. Details of the model are provided in the online supplement (see related supplementary materials at http://www.rcjournal.com). The primary parameters of interest were the marginal effects each device had on the background aerosol concentration.
Results
Clinical Aerosol Sample Collection
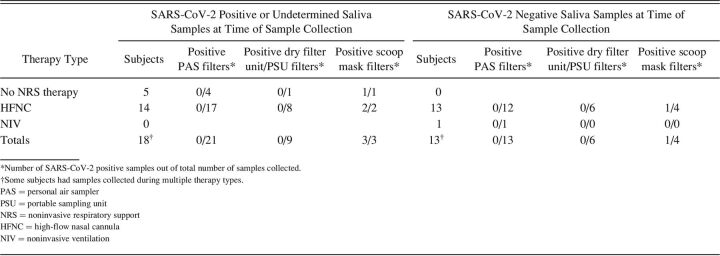
A total of 56 aerosol samples were collected from 31 subjects in the clinical setting. Five of the subjects were receiving no NRS; one subject was receiving NIV, and 27 subjects were receiving HFNC (some subjects had samples collected during multiple therapy types) (Table 1). A limited number of additional samples (10 samples) was collected from subjects receiving other interventions such as nasal cannula or intubation. Because of the very small sample sizes in these cases and the fact that these interventions do not represent NRS therapies, these results are not discussed here. However, details are provided for all collected samples in Table 2.
Table 1.
Summary of Aerosol Samples Collected During the Study
All of the subjects included in the clinical portion of this study had tested positive for SARS-CoV-2 RNA prior to participation in the study (average 5.3 d, representing the most recent PCR test result). In order to confirm viral shedding at the time of aerosol sample collection, saliva samples were collected from most subjects. Of the 31 subjects, only 14 produced saliva samples that were positive for SARS-CoV-2 RNA (Table 1). Saliva samples were not collected for 4 of the subjects, and SARS-CoV-2 RNA was undetectable in saliva samples from the remaining 13 subjects (Table 1). On average, subjects with a positive saliva sample at the time of aerosol collection had received their initial positive PCR test result more recently than subjects with a negative saliva sample (2.9 d vs 8.4 d, P = .036 by a 2-tailed t test). Duration since symptom onset was also tracked for all subjects (Table 2) to provide additional context. Subjects were generally later in their disease course: average 10.4 d between symptom onset and aerosol sample collection, with no difference between subjects with positive versus negative saliva samples (P = .80 by a 2-tailed t test).
Initial sampling efforts in the clinical setting used low air flow PAS pumps. Twenty-one aerosol samples were collected from subjects with positive saliva samples or from subjects for whom a saliva sample was not collected. None of these aerosol samples were positive for SARS-CoV-2 RNA (Tables 1 and 2). Given the volume of air collected through the PAS pump filters (100–450 L depending on the duration of sampling) (Table 2) and the sensitivity of the RT-PCR assay (see Methods section), the limit of detection for PAS pump sampling was estimated to be 2 viral particles/L air.
To increase sampling sensitivity, higher-flow dry filter unit and PSU samplers were used to sample larger volumes of air (2,500–6,000 L depending on the duration of sampling, which corresponds to a sampling limit of detection of approximately 0.1 viral particles/L (Table 2), although sampling was conducted at a greater distance from the subject. One dry filter unit aerosol sample was collected from a subject with a positive saliva sample who was not receiving NRS therapy, whereas 8 PSU aerosol samples were collected from subjects undergoing HFNC treatment. None of these samples were positive for SARS-CoV-2 RNA (Tables 1 and 2).
In order to obtain high-flow collection of aerosols in the very close proximity of the subjects, a scavenger mask setup was developed using the house suction line (see Figure E7 in the online supplement, see related supplementary materials at http://www.rcjournal.com). Three scavenger mask aerosol samples were collected from 3 subjects with positive saliva samples. All 3 samples were positive for SARS-CoV-2 RNA: 2 for subjects undergoing HFNC and one for a subject not undergoing any NRS therapy (Tables 1 and 2). The scavenger mask samples represent aerosols collected from > 10,000 L of air (depending on the duration of sampling (Table 2), with a corresponding sampling limit of detection of approximately 0.01 viral particles/L.
Twenty-three aerosol samples were collected from subjects with negative saliva samples (Tables 1 and 2). As expected, all of these aerosol samples were negative for SARS-CoV-2 RNA, with the exception of one scavenger mask sample. In the case of this sample, it is possible that the saliva result for this particular subject (#35, Table 2) was a false negative, the mask sample was a false positive, or that the very high volume of air collected for the aerosol sample provided much higher levels of sensitivity compared to the saliva sample.
Aerosol Chamber Measurements
Measurements were also collected from 5 healthy subjects in an aerosol chamber to further characterize aerosol dispersion during usage of either HFNC or NIV devices. The intention of these chamber studies was to collect aerosol measurements during NRS device usage under more controlled and reproducible conditions compared to the clinical setting. In the event that aerosol dispersion was detected, these studies could also help inform optimal placement of sampling devices relative to the subject in the clinical setting.
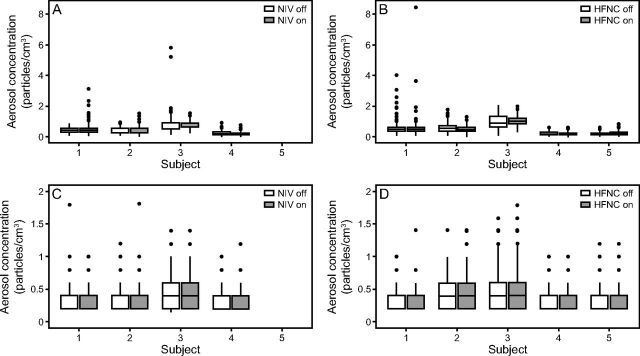
Figure 1 shows the distribution of total measured aerosol concentration for each subject using the HFNC and NIV devices. The distribution of aerosol concentrations with the equipment off (background) is shown as well as the distribution with the equipment on. Whereas there was variation in the background aerosol concentrations between subjects, the distributions of concentrations were very similar regardless of whether the device being tested was turned on or off. The only consistent increase in aerosol concentration from either respiratory device was observed during the warm-up period of the HFNC. Aerosols dispersed during this period had properties consistent with water droplets (ie, spherical and nonfluorescent) (see related supplementary materials at http://www.rcjournal.com). Once the HFNC device had reached its operating temperature, no increase in aerosol concentration over baseline was observed.
Fig. 1.
Box-and-whisker plots of aerosol concentrations measured by the Optical Particle Sizer 3330 (A and B) and Wideband Integrated Bioaerosol Sensor (C and D) for each participant with the high-flow nasal cannula (HFNC) (B and D) and noninvasive ventilation (NIV) (A and C) off versus on. Participant #5 was not able to complete the NIV measurements due to time constraints, which is why that data point is missing. Note that these distributions do not include measurements during the warm-up period for the HFNC. The center line of each box indicates the median, the edges of the boxes indicate the 25th and 75th percentiles, and whiskers indicate the largest or smallest values within 1.5 times the interquartile range from quartiles 3 and 1, respectively.
To evaluate statistical differences in aerosol concentrations for device on versus off states, linear mixed-effect models were used to estimate the marginal means for the device on and off conditions with associated 95% CI, which are shown in Figure E6 (see related supplementary materials at http://www.rcjournal.com). In all cases, t tests yielded insignificant differences in the mean concentration in the device states. These results suggest that the HFNC and NIV devices had no effect on the total aerosol concentrations, at least in healthy adults. Estimates, standard errors, and P values for tests of contrasts between the HFNC or NIV device on and device off states are provided in Table E1 (see related supplementary materials at http://www.rcjournal.com).
Discussion
Our study pairs the collection of aerosol samples in the clinical setting with controlled chamber measurements to characterize exposure risk during NRS treatment of subjects with COVID-19. Importantly, collected aerosol samples were analyzed for the presence of SARS-CoV-2 RNA along with saliva samples to assess potential infection risk of samples, something that to our knowledge only one prior study has done.18 Overall, our results suggest that NRS therapy is not associated with increased aerosol dispersion in healthy adults compared to baseline and that exposure to infectious aerosols near patients with COVID-19 receiving NRS therapy is expected to be very low (ie, below 0.1 viral particles/L air). Based on these results, our study suggests that NRS therapies should continue to be considered for the management of patients with COVID-19.
Of the 31 subjects involved in the clinical measurement portion of the study, approximately half no longer had positive saliva samples for SARS-CoV-2 RNA at the time of aerosol sample collection. Prior studies have shown that SARS-CoV-2 viral loads peak in the respiratory tract soon after symptom onset (ie, within 3–5 d).21 On average, the subjects in our study were 5.3 d past their most recent PCR test result and 10.4 d post-symptom onset. It is possible that the apparent low levels of SARS-CoV-2-containing aerosols measured in our study may be due to a mismatch in the timelines of peak viral shedding and onset of severe respiratory symptoms requiring hospitalization and respiratory support. Subjects in this study were also receiving a range of different treatments, which may have affected viral shedding (eTable 2, see related supplementary materials at http://www.rcjournal.com).
Of the 33 aerosol samples collected from subjects with positive or undetermined saliva samples, only 3 contained detectable levels of SARS-CoV-2 RNA, all of which were collected using the scavenger mask setup, suggesting that very high-volume sampling in the immediate vicinity of the subject was required for detection. These results also suggest that the engineering controls generally in place in hospital rooms, such as high air exchange rates and air filtration, are effective at reducing aerosol concentrations. The rooms used for sample collection in this study exhibited at least 4–6 air changes per h (eTable 2, see related supplementary materials at http://www.rcjournal.com).
The clinical measurements were supplemented with controlled measurements of healthy subjects using HFNC and NIV devices in a high-efficiency particulate air-filtered room. Aerosol concentrations near 5 healthy subjects using HFNC or NIV respiratory therapy devices did not increase above baseline once the HFNC device had warmed up (as measured by 2 real-time particle sensors; see Fig. 1 and Figure E6 in the online supplement). Positive controls such as spraying water or asking subjects to sing, cough, or yell loudly, on the other hand, led to spikes in detectable aerosol. Whereas the chamber testing of NRS did not reveal any increase in aerosol concentrations, it is important to consider that the sensitivity of these real-time measurements in the aerosol chamber may be insufficient to completely rule out a clinically relevant level of respiratory aerosol dispersion. For example, an increased aerosol concentration of 100 particles/L would be below the minimal measurable level of our study (250 particles/L) (eTable 1, see related supplementary materials at http://www.rcjournal.com) but would correspond to a caregiver inhaling an additional 5,000 respiratory particles over a period of 10 min (assuming a minute ventilation of 5 L/min). The infectious dosage of SARS-CoV-2 is unknown but is estimated to be in the range of hundreds to thousands of viral particles.22
In contrast, detection of SARS-CoV-2 RNA in the clinical sampling study provides much higher sensitivity and specificity and thus higher confidence regarding respiratory aerosol concentrations. Depending on the type of sampling device and the volume of air collected (Table 2), the limit of detection of the aerosol sampling approaches was between 0.01 viral particles/L (for the scavenger mask) and 2.00 viral particles/L (for the PAS pump). Given that very high-volume air sampling was required to detect SARS-CoV-2, respiratory aerosol concentrations during NRS therapies are likely near the lower end of this concentration range.
Our results corroborate those of previous studies that found no increase in aerosol concentration during HFNC and NIV use in healthy adults14,15,17 and also corroborate a more recent study that specifically found no evidence of increased aerosol dispersion during NRS treatment of subjects with COVID-19 in the United Kingdom.18 The current study provides additional context, pairing clinical sampling with aerosol chamber measurements and using state-of-the-art real-time particle sensors to characterize aerosol properties. Our stepwise sampling approach of conducting progressively higher-flow sampling in increasing proximity to the subject is a strength of the study and provides additional context regarding the spatial extent of contamination throughout the room, suggesting that aerosol concentrations are very low at distances as small as 30–45 cm from the subject.
Limitations of the current study include the small overall sample size and the lack of subjects with COVID-19 on NRS. Initially, our study was designed to encompass a variety of procedures including NIV, nebulizers, and bag-mask ventilation. In the wake of the pandemic, many of these clinical behaviors were modified with the intent to reduce aerosol dispersion. This modification resulted in fewer patients at our center being offered NIV following failure of HFNC, instead being transitioned to invasive mechanical ventilation. It is also important to point out that samples were being collected while other mitigation procedures were in place, such as use of rooms with at least 4–6 air exchanges per h and of viral/bacterial filters on NIV during our aerosol chamber measurements. Dispersion of particles may have differed in the absence of those mitigations or in rooms with different air recirculation rates. Furthermore, we must acknowledge that it is unclear how the results of the current study extend to patients infected with other respiratory diseases or novel SARS-CoV-2 variants. All samples were collected between April 2020–March 2021, before SARS-CoV-2 variants like Delta and Omicron were predominant in Massachusetts.
Conclusions
The findings presented here suggest that NRS therapies should continue to be considered as treatment options for managing hypoxemic respiratory failure induced by COVID-19 and that dispersion of infectious aerosol during these therapies is limited. Our study suggests that hospital care personnel are not at heightened risk when attending patients with COVID-19 on NRS therapy due to a combination of later stage of disease at therapy initiation (and therefore lower rates of viral shedding), the presence of environmental controls in patient rooms, the integrity of NIV with a viral-bacterial exhalation filter and HFNC relative to aerosol dispersion (little to no increase), and the use of concomitant therapies that may further limit viral shedding. Our study also underscores the need to develop more rigorous clinical data regarding aerosol dispersion during respiratory care for this and future respiratory failure–associated pandemics in order to optimize their control and management in the hospital environment. Our results should not be construed as a justification to relax personal protective equipment guidelines because we did not examine the effects of such changes on viral transmission.
Acknowledgments
The authors would like to thank Trina Vian and Lynn Kerr of MIT Lincoln Laboratory for helping design the aerosol measurements and equipment setup. We also thank Leslie Lussier and Beth Gill of Tufts Medical Center for providing training on the respiratory equipment as well as the respiratory therapists and nurses at Tufts Medical Center for their cooperation and support as we carried out sampling.
Footnotes
See the Related Editorial on Page 169
The authors have disclosed no conflicts of interest.
This study was supported by Greater Boston Consortium on Pathogen Readiness, National Institutes of Health/National Institute of Allergy and Infectious Diseases Centers of Excellence for Influenza Research and Response (HHSN272201400008C).
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1.Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med 2021;174(1):69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lednicky JA, Lauzard M, Fan ZH, Jutla A, Tilly TB, Gangwar M, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis 2020;100:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santarpia JL, Rivera DN, Herrera VL, Morwitzer MJ, Creager HM, Santarpia GW, et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep 2020;10(1):12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, et al. ; Singapore 2019 Novel Coronavirus Outbreak Research Team. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun 2020;11(1):2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020;582(7813):557-560. [DOI] [PubMed] [Google Scholar]
- 6.Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis 2020;26(7):1583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habibi N, Uddin S, Behbehani M, Abdul Razzack N, Zakir F, Shajan A. SARS-CoV-2 in hospital air as revealed by comprehensive respiratory viral panel sequencing. Infect Prev Pract 2022;4(1):100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care 2020;10(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Alolaiwat A, Fink JB, Dhand R. Aerosol-generating procedures and virus transmission. Respir Care 2022;67(8):1022-1042. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J 2019;53(4):1802339. [DOI] [PubMed] [Google Scholar]
- 11.McGrath JA, O’Toole C, Bennett G, Joyce M, Byrne MA, MacLoughlin R. Investigation of fugitive aerosols released into the environment during high-flow therapy. Pharmaceutics 2019;11(6):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avari H, Hiebert RJ, Ryzynski AA, Levy A, Nardi J, Kanji-Jaffer H, et al. Quantitative assessment of viral dispersion associated with respiratory support devices in a simulated critical care environment. Am J Respir Crit Care Med 2021;203(9):1112-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotoda M, Hishiyama S, Mitsui K, Tanikawa T, Morikawa S, Takamino A, et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect 2020;104(4):534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Boehman A, Capelcelatro J, Cohn AM, Cooke JM, Costa DK, et al. Variation in aerosol production across oxygen delivery devices in spontaneously breathing human subjects. MedRxiv 2020. doi: 10.1101/2020.04.15.20066688. [Google Scholar]
- 15.Gaeckle NT, Lee J, Park Y, Kreykes G, Evans MD, Hogan CJ., Jr. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med 2020;202(8):1115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung CCH, Joynt GM, Gomersall CD, Wong WT, Lee A, Ling L, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect 2019;101(1):84-87. [DOI] [PubMed] [Google Scholar]
- 17.Simonds AK, Hanak A, Chatwin M, Morrell M, Hall A, Parker KH, et al. Evaluation of droplet dispersion during noninvasive ventilation, oxygen therapy, nebulizer treatment, and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess 2010;14(46):131-172. [DOI] [PubMed] [Google Scholar]
- 18.Winslow RL, Zhou J, Windle EF, Nur I, Lall R, Ji C, et al. SARS-CoV-2 environmental contamination from hospitalized patients with COVID-19 receiving aerosol-generating procedures. Thorax 2022;77(3):259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebreil AL, Greux V, Glenet M, Huguenin A, N’Guyen Y, Berri F, et al. Surfaces and air contamination by severe acute respiratory syndrome coronavirus 2 using high-flow nasal oxygenation or assisted mechanical ventilation in intensive care unit rooms of patients with coronavirus disease 2019. J Infect Dis 2022;225(3):385-391. [DOI] [PubMed] [Google Scholar]
- 20.Roca O, Pacheco A, Rodon J, Anton A, Vergara-Alert J, Armadans L, et al. Nasal high-flow oxygen therapy in COVID-19 patients does not cause environmental surface contamination. J Hosp Infect 2021;116:103-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021;2(1):e13-e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popa A, Genger JW, Nicholson MD, Penz T, Schmid D, Aberle SW, et al. Genomic epidemiology of superspreading events in Austria reveals mutational dynamics and transmission properties of SARS-CoV-2. Sci Transl Med 2020;12(573). [DOI] [PMC free article] [PubMed] [Google Scholar]