Abstract
The ARF and p53 tumor suppressors mediate Myc-induced apoptosis and suppress lymphoma development in Eμ-myc transgenic mice. Here we report that the proapoptotic Bcl-2 family member Bax also mediates apoptosis triggered by Myc and inhibits Myc-induced lymphomagenesis. Bax-deficient primary pre-B cells are resistant to the apoptotic effects of Myc, and Bax loss accelerates lymphoma development in Eμ-myc transgenics in a dose-dependent fashion. Eighty percent of lymphomas arising in wild-type Eμ-myc transgenics have alterations in the ARF-Mdm2-p53 tumor suppressor pathway characterized by deletions in ARF, mutations or deletions of p53, and overexpression of Mdm2. The absence of Bax did not alter the frequency of biallelic deletion of ARF in lymphomas arising in Eμ-myc transgenic mice or the rate of tumorigenesis in ARF-null mice. Furthermore, Mdm2 was overexpressed at the same frequency in lymphomas irrespective of Bax status, suggesting that Bax resides in a pathway separate from ARF and Mdm2. Strikingly, lymphomas from Bax-null Eμ-myc transgenics lacked p53 alterations, whereas 27% of the tumors in Bax+/− Eμ-myc transgenic mice contained p53 mutations or deletions. Thus, the loss of Bax eliminates the selection of p53 mutations and deletions, but not ARF deletions or Mdm2 overexpression, during Myc-induced tumorigenesis, formally demonstrating that Myc-induced apoptotic signals through ARF/Mdm2 and p53 must bifurcate: p53 signals through Bax, whereas this is not necessarily the case for ARF and Mdm2.
The oncoprotein c-Myc, paradoxically, is an inducer of both cell proliferation and cell death, and the levels of Myc and/or the conditions in which it is expressed dictate cell fate (2, 7, 40). Most cancer cells that overexpress Myc, by translocation, amplification, or other means, harness the full growth potential of this oncogene by inactivating the apoptotic effectors of Myc, including the tumor suppressors ARF and p53 (5, 50). ARF is a nucleolar protein that binds to and sequesters Mdm2 (55, 59). Mdm2 is a p53 transcription target (3, 61) that inhibits p53's transactivation functions (37) and ubiquitinates p53 (12), leading to p53 degradation (48). Myc activation induces the sustained expression of both ARF and p53, and this triggers apoptosis; as a consequence, primary ARF- and p53-null hematopoietic and fibroblast cells are impaired in their apoptotic response to Myc (5, 63). Furthermore, deletion of ARF, mutation or deletion of p53, and Mdm2 overexpression occur in 24, 28, and 48%, respectively, of the lymphomas that arise in Eμ-myc transgenic mice (80% overall [5]), and ARF- or p53-null Eμ-myc transgenic mice have a markedly accelerated course of lymphoma (5, 17, 50).
Loss of the antiapoptotic protein Bcl-XL or Bcl-2 compromises hematopoietic cell survival, whereas loss of ARF or p53 has no effect upon hematopoietic cell development (6, 38, 39, 41, 57). Bax is a proapoptotic Bcl-2 family member whose deletion has modest effects on lymphocyte numbers (22). However, the combined loss of Bax and Bak, another proapoptotic Bcl-2 family member, results in profound defects in both development and lymphocyte homeostasis (24). Bax normally resides in the cytosol of healthy cells, yet it relocalizes and inserts into the outer mitochondrial membrane after stimulation with a variety of apoptotic stimuli (reviewed in reference 9). In turn, this leads to mitochondrial dysfunction with alterations in the permeability transition pore, the release of cytochrome c, and the activation of Apaf-1 and caspases, which cleave intracellular targets required for cell survival (9). The balance of proapoptotic and antiapoptotic Bcl-2 family members regulates the susceptibility of cells to apoptosis (reviewed in reference 23). For example, an excess of Bax can overwhelm the cell and trigger an apoptotic response, whereas the antiapoptotic Bcl-2 family members Bcl-2 and Bcl-XL inhibit the deleterious effects of Bax (23).
Bcl-2 and Bcl-XL are overexpressed in many human malignancies (reviewed in reference 46), and Bcl-XL expression is activated by retroviral insertions in some murine T-cell leukemias and lymphomas (41). Bcl-2 and Myc have been shown to cooperate in transformation (8, 56), and Eμ-myc/Eμ-bcl-2 double transgenic mice develop an aggressive and rapid lymphoma composed of primitive lymphoid cells (54). Although the cooperation between Bcl-2 and Myc and the regulation of Bax by Bcl-2 are well documented, the precise role that Bax plays in Myc functions is less clear.
Bax-deficient mice manifest a modest lymphoid hyperplasia but are not prone to spontaneous tumor development (20, 22). However, mutations that inactivate Bax are found in a subset of human colon adenocarcinomas (45) and some human hematopoietic cancer cell lines (4, 30, 31), and Bax loss cooperates with simian immunodeficiency virus (SV40) large T antigen in transgenic mouse models of cancer (52, 62). Recently, bax has been suggested to be a direct transcriptional target of c-Myc in human tumor cell lines (32). However, it is unclear how Bax influences Myc-induced hematopoietic cell apoptosis and tumorigenesis and whether Bax expression influences the ARF-Mdm2-p53 tumor suppressor pathway. Here we report that, although Myc activation fails to regulate Bax levels in primary murine pre-B cells, Bax-deficient cells are markedly resistant to Myc-induced apoptosis. More importantly, Bax loss accelerates Myc-induced tumorigenesis in Eμ-myc transgenic mice, and the lymphomas arising in Bax-null transgenics selectively lack mutations or deletions of p53. However, Bax-null Eμ-myc transgenics still display the same frequency of alterations of ARF and Mdm2, indicating that Bax is not necessarily a target of ARF or Mdm2 even though both can function with p53 in this tumor suppressor pathway. The results support a model whereby Bax functions as a critical downstream effector of the p53 apoptotic pathway, and thus ARF and Mdm2 must have other mediators important for tumorigenesis.
MATERIALS AND METHODS
Transgenic and knockout mice.
The inbred C57BL/6 Eμ-myc transgenic mouse strain was kindly provided by Alan Harris (Walter & Eliza Hall Institute, Melbourne) and Charles Sidman (University of Cincinnati). Bax-null mice have been previously described and were C57BL/6 × 129/svj (22). The ARF-null (C57BL/6 × 129/svj) and p53-null (C57BL/6 × 129/svev) mice were generously provided by Charles Sherr and Gerard Grosveld, respectively. Eμ-myc transgenics were mated to Bax+/− mice and the F1 littermates were then mated to each other to obtain Bax+/+, Bax+/−, and Bax−/− Eμ-myc transgenics.
Primary B cells.
Primary pre-B cell cultures were generated from the bone marrow of 6- to 8-week-old wild-type, Bax-, ARF-, p53-, and ARF/p53-double null mice as previously described (5). Briefly, culture of bone marrow in interleukin-7 (IL-7)-containing medium after 12 to 14 days established >98% pure population of pre-B cells as determined by phenotype analysis using B-cell-specific antibodies and fluorescence-activated cell sorting (FACS). The pre-B cells expressed CD19, B220, and CD24 and were negative for surface immunoglobulin M (IgM) and CD43 irrespective of genotype. IgM+/CD19+ B cells were sorted from spleens from age- and gender-matched mice: one wild-type and two precancerous Eμ-myc transgenics. All antibodies used for phenotypic analyses were from PharMingen (San Diego, Calif.) or Southern Biotechnology (Birmingham, Ala.).
Virus infection.
Virus was produced and used to infect primary pre-B cells as previously described (5). Briefly, MSCV-Myc-ER-IRES-GFP or control MSCV-IRES-GFP virus was cotransfected with helper virus into 293T cells, and live virus was then collected at intervals, pooled, and filtered (49). Viral stocks, MSCV-Myc-ER-IRES-GFP virus or the MSCV-IRES-GFP control virus, were used to infect primary pre-B cells in the presence of 8 μg of Polybrene/ml. Green fluorescent protein (GFP)-positive infected cells were isolated 3 to 4 days postinfection by sterile sorting with a Cytomation MoFlo cell sorter (Fort Collins, Colo.). GFP-positive cells were expanded in IL-7-containing medium and analyzed for levels of Myc-ER (previously referred to as Myc-ERTM [5, 6]) protein and sensitivity to Myc-induced apoptosis. Myc-ER is a fusion protein of c-Myc linked to a modified estrogen receptor hormone binding domain (25) and is designed to hold Myc-ER in heat shock complexes in the cytosol. Upon addition of 1 μM 4-hydroxytamoxifen (4-HT), which binds to the ER portion of Myc-ER, Myc-ER then translocates to the nucleus and activates transcription (25). As reported elsewhere (5), addition of 4-HT to uninfected or MSCV-IRES-GFP control virus-infected cells had no effect on pre-B cell growth or viability.
Viability and apoptosis assays.
Cell viability was determined at specific intervals by trypan blue dye exclusion after the removal of IL-7 or the addition of 1 μM 4-HT (Sigma, St. Louis, Mo.) to the culture medium to activate Myc-ER. For the IL-7 deprivation experiments, wild-type and Bax−/− pre-B cells were washed twice with phosphate-buffered saline and resuspended in medium lacking IL-7 but still containing 10% fetal calf serum. Apoptosis was measured by propidium iodide staining of DNA and quantitation of fragmented (sub-G1) DNA.
Western blotting.
Whole-cell protein extracts from primary pre-B cells or pre-B- or B-cell lymphomas from Eμ-myc transgenic mice were isolated as previously described (5, 63). Briefly, ice-cold lysis buffer (50 mM HEPES, pH 7.5; 150 mM NaCl; 1 mM EDTA; 2.5 mM EGTA; 0.1% Tween 20; 1 mM phenylmethylsulfonyl fluoride; 0.4 U of aprotinin/ml; 1 mM NaF; 10 mM β-glycerophosphate; 0.1 mM sodium orthovanadate; 10 μg of leupeptin/ml) was added to cells pellets or small (3- to 5-mm2) tumor chunks. Samples were then subjected twice to sonication for 8 s and centrifuged (4°C, 7 min, 14,000 rpm) to sediment the undissolved cellular material, and then the protein in the supernatant was quantified by using a Bio-Rad Protein Assay (Hercules, Calif.). Equal amounts of protein (200 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10%), transferred to nitrocellulose membranes (Protran; Schleicher & Schuell, Dassel, Germany), and blotted with antibodies specific for the p19ARF (44), p53 (Ab-7), and poly(ADP-ribose) polymerase (PARP; Ab-2) (both from, Calbiochem, La Jolla, Calif.), Mdm2 (C-18; Santa Cruz, Inc., Santa Cruz, Calif.), c-Myc (06–340; Upstate Biotechnology, New York, N.Y.), and Bax (13686E; PharMingen, San Diego, Calif.). Bound immunocomplexes were detected by enhanced chemiluminescence (Amersham, Piscataway, N.J.) or Supersignal (Pierce, Rockford, Ill.).
Southern blotting.
Genomic DNA was isolated from lymphomas arising in Bax+/− and Bax−/− Eμ-myc transgenic mice and digested with AflII or BamHI. Equal amounts of DNA were electrophoretically separated in agarose gels, transferred to nitrocellulose membranes, and then probed with cDNAs coding for ARF (exon 1β) (AflII digested) and p53 (exons 2 to 10) and the joining region of immunoglobulin heavy chain (JH) (both BamHI digested). Genomic DNA isolated from the spleen of a wild-type littermate was used as a control.
Northern blotting.
Total RNA was isolated by using TRIzol Reagent according to the manufacturer's directions (Life Technologies, Grand Island, N.Y.) at intervals (0, 1, 3, or 6 h) from primary pre-B cells after addition of 1 μM 4-HT after a 30-min pretreatment with 10 μg of cycloheximide or vehicle control (100% ethyl alcohol)/ml. Northern blotting with 20 μg of total RNA per lane was performed using conventional techniques and probed with the coding portion of murine bax cDNA (kindly provided by John Reed, The Burnham Institute).
RESULTS
Bax loss impairs Myc-induced apoptosis.
Bax-null mice have modest increases in B- and T-lymphocyte numbers, presumably due to decreased apoptosis (22). To determine whether Bax-deficient B lymphocytes were intrinsically more resistant to apoptosis than wild-type lymphocytes, we harvested bone marrow cells and expanded pre-B cells in IL-7-containing medium and measured proliferation rates and viability. The Bax-null pre-B cells grew at a rate similar to wild-type pre-B cells (data not shown) and showed no difference in viability (Fig. 1A, open symbols). Moreover, deprivation of IL-7 induced apoptosis in Bax-null pre-B cells with kinetics virtually identical to that of wild-type pre-B cells (Fig. 1A, closed symbols). Similar results were obtained when wild-type and Bax−/− thymocytes were deprived of cytokines (22). Therefore, Bax deficiency does not confer a growth or survival advantage to lymphocytes during ex vivo culture.
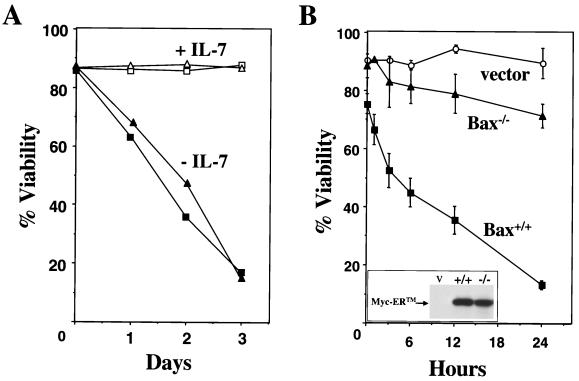
FIG. 1.
Bax does not influence cytokine deprivation-induced apoptosis but does mediate c-Myc-induced apoptosis. (A) Bax+/+ (squares) and Bax−/− (triangles) pre-B cells were cultured in medium with (open symbols) or without (solid symbols) IL-7, and their viability was determined at intervals by trypan blue dye exclusion. The data are representative of two independent experiments. (B) 4-HT was added to the indicated primary pre-B-cell cultures to activate Myc-ER, and their viability was determined at intervals thereafter by trypan blue dye exclusion. Apoptosis was confirmed by analysis of subdiploid DNA content after staining with propidium iodide. Steady-state levels of apoptosis in the wild-type primary pre-B cells are indicated at the zero hour time point. (Inset) The protein levels of Myc-ER in Bax −/− (−/−) and Bax +/+ (+/+) pre-B cells infected with a retrovirus encoding Myc-ER-GFP and pre-B cells infected with the GFP vector control (V) retrovirus were determined by immunoblotting with an antibody for Myc. The data are the mean of three independent experiments, and error bars represent one standard deviation.
To address whether Bax influences Myc-induced apoptosis, we infected primary wild-type and Bax-deficient pre-B cells with a Myc-ER-GFP (previously referred to as Myc-ERTM-GFP [5, 6]) expressing retrovirus or with a control GFP-only expressing retrovirus and then sorted for virus-infected cells by FACS. There was a modest decrease in the viability in wild-type pre-B cells infected with the Myc-ER encoding retrovirus compared to Bax-deficient pre-B cells infected with the same virus (Fig. 1B). This is most likely due to the somewhat leaky nature of Myc-ER expression system (5, 63). Activation of Myc-ER with 4-HT led to rapid apoptosis in the wild-type pre-B cells cultured in IL-7-containing medium, whereas the Bax-null cells were very resistant to Myc-induced apoptosis (Fig. 1B). Few wild-type pre-B cells (<10%) were alive after 24 h of Myc activation, whereas Bax-null pre-B cells were greater than 60% viable at this interval. Primary pre-B cells died by apoptosis upon Myc-ER activation, as determined by DNA fragmentation analysis (data not shown) and cleavage of caspase targets such as PARP (Fig. 2A). Thus, Bax is an important mediator of Myc-induced apoptosis in primary pre-B cells.
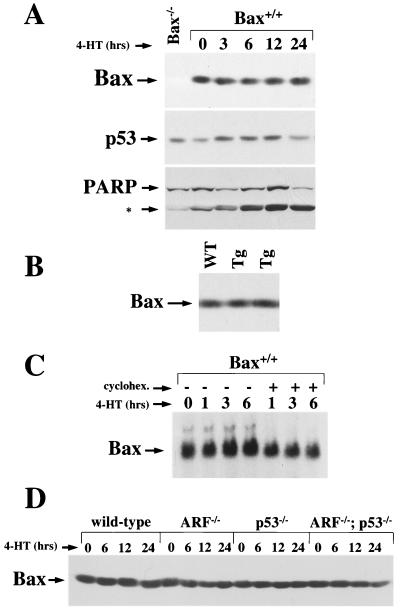
FIG. 2.
Myc does not upregulate Bax protein expression in B cells. (A) 4-HT was added to wild-type pre-B-cell cultures infected with the Myc-ER encoding retrovirus to activate Myc-ER. At the indicated intervals cells were collected and protein lysates were made. Equal quantities of protein were assessed by immunoblotting with antibodies specific for Bax, p53, or PARP. The 85- kDa caspase cleavage fragment of PARP is denoted by an asterisk. (B) Equal quantities of protein from FACS sorted IgM+/CD19+ splenic B cells from one wild-type (WT) and two precancerous Eμ-myc transgenics (Tg) were assessed by immunoblotting with an antibody specific for Bax. (C) Total RNA was isolated from Myc-ER-infected wild-type pre-B cells activated with 4-HT for the indicated intervals. Pre-B cells pretreated with cycloheximide are indicated by a plus sign. The expression of bax transcripts was assessed by Northern blot analyses utilizing bax cDNA. (D) 4-HT was added to wild-type, ARF−/−, p53−/−, and ARF−/−p53−/− pre-B-cell cultures infected with the Myc-ER-encoding retrovirus to activate Myc-ER. At the indicated intervals, cells were collected and protein lysates were prepared. Equal quantities of protein were evident by immunoblotting with antibodies specific for Bax, and the levels of Myc-ER protein expressed in the four genotypes were equivalent (5).
bax has been suggested to be a direct transcriptional target of c-Myc in immortal human tumor cell lines (32). However, Myc activation in primary pre-B cells was not associated with changes in Bax protein (Fig. 2A) whereas, as expected (5), p53 expression was increased upon Myc activation. Moreover, Bax levels were unaltered in IgM+ splenic B cells from precancerous Eμ-myc transgenic mice compared to wild-type littermate controls (Fig. 2B). Therefore, Myc activation or overexpression does not alter Bax protein levels in B cells ex vivo or in vivo. Only a very slight increase in bax RNA levels was observed following Myc activation in pre-B cells, and this was prevented by pretreatment of the cells with cycloheximide (Fig. 2C). Since cycloheximide blocks new protein synthesis, the modest changes in bax RNA induced by Myc activation are indirect. Furthermore, the modest upregulation of bax RNA by Myc did not result in any increase in Bax protein levels (Fig. 2A).
Immortal cell lines, such as those used by Mitchell et al. (32), generally inactivate p53 or ARF to become established in vitro (11, 19), and bax has been reported to be a transcriptional target of p53 (34, 35). Therefore, it was possible that the loss of p53 and/or ARF influences Bax expression independent of Myc and/or could alter the response of Bax to Myc activation. To address this issue, primary pre-B cells lacking p53 and/or ARF were infected with the Myc-ER encoding retrovirus. Loss of ARF and/or p53 did not significantly alter the steady-state levels of Bax protein (Fig. 2D). Moreover, activation of Myc-ER with 4-HT did not result in alteration of Bax protein levels in ARF-, p53-, or ARF/p53-double null primary pre-B cells (Fig. 2D). Therefore, loss of p53 and/or ARF does not influence Bax expression, even when Myc is activated.
Bax loss accelerates Myc-induced lymphomagenesis.
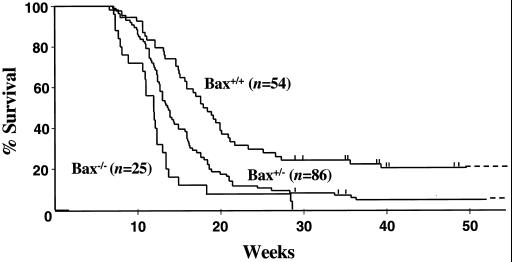
Loss of ARF or p53 impairs Myc-mediated apoptosis and consequently accelerates Myc-induced lymphomagenesis (5, 50, 63). To test the genetic contribution of Bax to Myc-induced lymphomagenesis, we crossed Eμ-myc transgenic mice onto the Bax-null background. Congenic C57BL/6 Eμ-myc transgenic mice were mated to C57BL/6 × 129/svj Bax+/− mice, since Bax−/− males and females have fertility problems (22, 43). F1 littermates were intercrossed to obtain Bax+/+, Bax+/−, and Bax−/− Eμ-myc transgenic mice, and these transgenics were carefully monitored for disease development. Notably, Bax loss accelerated the course of lymphoma development, decreasing the average age of survival from 21.7 weeks in Bax+/+ Eμ-myc transgenics to 12.6 weeks in Bax-null Eμ-myc transgenics (Fig. 3). None of the Bax−/− Eμ-myc transgenic mice survived past 30 weeks, whereas 22% (12 of 54) of the Bax+/+ Eμ-myc transgenics lived longer than 30 weeks. Bax haploinsufficency also accelerated tumor development, since Bax+/− Eμ-myc transgenic mice had a mean life span of 16.0 weeks (Fig. 3). Therefore, Bax impairs Myc-induced lymphomagenesis. FACS analysis with lymphoid-specific antibodies showed that the Bax-null and Bax+/− Eμ-myc transgenics develop pre-B- and B-cell lymphoma typical of wild-type Eμ-myc transgenics (data not shown) and not the primitive lymphoid tumor described in the Eμ-myc/Eμ-bcl-2 double-transgenic mice (54). Predictably, Southern blot analysis revealed that all but one of the lymphomas that arose in Bax-null Eμ-myc transgenics were clonal (data not shown), which is characteristic of the lymphomas that develop in Eμ-myc transgenics (1).
FIG. 3.
Myc-induced lymphomagenesis is accelerated by Bax loss. The genotypes of the mice are indicated next to the Kaplan-Meier survival curves, and the numbers of mice in each group are denoted by the n values. Vertical lines indicate ages of surviving mice: 0 Bax−/−, 3 Bax+/−, and 10 Bax+/+ mice. The average life spans of Bax+/+, Bax+/−, and Bax−/− Eμ-myc transgenics were 21.7, 16.0, and 12.6 weeks, respectively. Pre-B- and/or B-cell lymphoma was documented in all of the animals.
Inactivation of p53 or ARF occurs in a mutually exclusive fashion in over half of all Eμ-myc lymphomas (5). Bax appears to function as a tumor suppressor in some scenarios (45), although Bax-null mice do not spontaneously develop cancer (20, 22). If Bax functioned as a bona fide tumor suppressor in Eμ-myc transgenic mice, one would predict that Bax would be inactivated in a subset of lymphomas and that lymphomas arising in Bax+/− Eμ-myc transgenics would suffer inactivating mutations of the remaining wild-type Bax allele. However, we failed to detect loss of heterozygosity of Bax in the tumors analyzed from Bax+/− Eμ-myc transgenics (n = 15), nor did we observe deletion of Bax in any lymphomas from Bax+/+ Eμ-myc transgenics (n > 50) (data not shown). Furthermore, all tumors derived from wild-type and Bax+/− Eμ-myc transgenics expressed Bax protein (Fig. 4A and data not shown). In previous studies Bax has been shown to be inactivated in tumor cells by frameshift and missense point mutations (4, 30, 31, 45). However, sequencing full-length Bax cDNA derived from 16 tumors from Bax+/+ and Bax+/− Eμ-myc transgenics failed to reveal any tumor-specific changes in Bax sequence. All tissue derived from these mice displayed a single nucleotide change (213T to C) in the BH3 domain of Bax, yet this change is silent and maintains aspartate at codon 71. Thus, inactivating mutations of Bax either do not occur or are rare in pre-B- and B-cell lymphomas arising in Eμ-myc transgenics, even though Eμ-myc transgenics that lack Bax develop lymphomas at an accelerated rate. Therefore, Bax does not function as a classic tumor suppressor but rather appears to act as a modifier of lymphoma development in Eμ-myc transgenic mice.
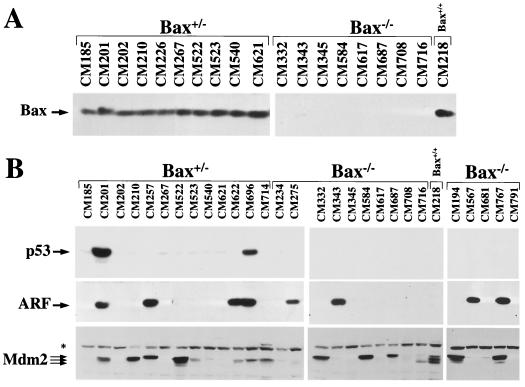
FIG. 4.
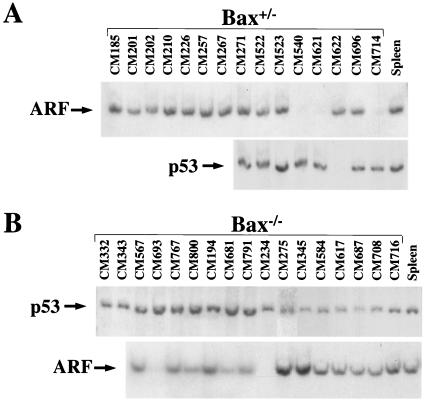
Western blot analysis of lymphomas arising in Bax+/− and Bax−/− Eμ-myc transgenic mice. Levels of Bax (A), p53 (B, top), p19ARF (B, middle), and Mdm2 (B, bottom) protein in whole-cell extracts of tumors from Bax+/− and Bax−/− Eμ-myc transgenic mice were assessed by immunoblotting with antibodies specific for each protein. Protein extracts from a tumor arising in a Bax+/+ Eμ-myc transgenic mouse that contains the p92, p90, and p85 Mdm2 isoforms were run to show the location of these isoforms in panel B and as a blotting control for Bax expression in panel A. The asterisk in panel B marks the position of a nonspecific background band detected with the Mdm2 antibody.
Bax loss selectively bypasses p53 mutations that arise during Myc-induced lymphomagenesis.
ARF-null animals are tumor prone and spontaneously develop sarcomas and lymphomas within 8 months of age (19). ARF is upregulated after Myc activation, and loss of ARF impairs Myc-induced apoptosis and accelerates lymphomagenesis in Eμ-myc transgenic mice (5, 50). To determine whether the loss of Bax influences tumorigenesis initiated in ARF-deficient mice, we crossed ARF- and Bax-deficient mice, and F1 mice were then mated to generate ARF/Bax-double null mice. The ARF−/−Bax−/− mice were monitored for tumor development and compared with the tumor latency in ARF−/−Bax+/+ littermates. We found that Bax deficiency does not alter the survival of ARF-null mice, since the average age of survival for Bax+/+ ARF−/− (48.9 weeks, n = 37) and Bax−/− ARF−/− (47.6 weeks, n = 39) mice was essentially equivalent. Therefore, loss of Bax does not influence the overall survival of ARF-null mice. At face value this could suggest that Bax is in the same pathway as ARF. On the other hand, the results could indicate that ARF and Bax exist in separate pathways that function independently of each other. In support of the latter concept, biallelic deletion of ARF occurred in lymphomas from both Bax−/− Eμ-myc transgenics (12%) and Bax+/− Eμ-myc transgenic mice (20%) (Fig. 5; Table 1), indicating that Bax loss does not prevent inactivation of ARF. The percentage of tumors with ARF deletions was slightly lower than previously reported (5), but this could be due to experimental variation or (more likely) to the different background of this cross from the one previously reported. Indeed, genetic background effects are evident when the differences in average survival of wild-type Eμ-myc transgenics, 22 weeks in the Bax background (Fig. 3) and 33 weeks in the ARF background, are compared (5).
FIG. 5.
Southern blot analysis of Bax+/− and Bax−/− Eμ-myc lymphomas. AflII and BamHI restriction fragments containing ARF exon 1β and p53 exons 2 to 10, respectively, from genomic DNA isolated from lymphomas arising in Bax+/− (A) and Bax−/− (B) Eμ-myc transgenic mice. Genomic DNA from the spleen of a wild-type littermate was used as a control in both panels A and B. Lack of a band denotes biallelic deletion of that gene.
TABLE 1.
p53, ARF, and Mdm2 protein expression in lymphomas from Bax+/− and Bax−/− Eμ-myc transgenics
| Eμ-myc transgenic (no. of tumors analyzed) | Finding (% tumors analyzed) | Mouse | Expression of:
|
||
|---|---|---|---|---|---|
| p53 | p19ARF | Mdm2 | |||
| Bax+/− (15) | ARF inactivation (20) | ||||
| CM540 | Basal | Undetected (deleted) | Low | ||
| CM621 | Basal | Undetected (deleted) | Undetected | ||
| CM714 | Low | Undetected (deleted) | Moderately overexpressed | ||
| p53 inactivation (26) | |||||
| CM201 | Mutant | Overexpressed | Overexpressed | ||
| CM271 | Mutant | Overexpressed | Overexpressed | ||
| CM622 | Deleted | Overexpressed | Moderately overexpressed | ||
| CM696 | Mutant | Overexpressed | Moderately overexpressed | ||
| ARF overexpressed, p53 wild type (7) |
|||||
| CM257 | Low | Overexpressed | Overexpressed | ||
| Mdm2 overexpression only (27) | |||||
| CM210 | Basal | Undetected | Overexpressed | ||
| CM226 | Low | Undetected | Moderately overexpressed | ||
| CM522 | Basal | Undetected | Overexpressed | ||
| CM523 | Basal | Undetected | Moderately overexpressed | ||
| No detectable alteration (20) | |||||
| CM185 | Basal | Undetected | Undetected | ||
| CM202 | Low | Undetected | Undetected | ||
| CM267 | Basal | Undetected | Undetected | ||
| Bax−/− (17) | ARF inactivation (12) | ||||
| CM234 | Basal | Undetected (deleted) | Low | ||
| CM693 | Basal | Undetected (deleted) | Overexpressed | ||
| p53 inactivation (0) | |||||
| ARF overexpressed, p53 wild type (24) |
|||||
| CM275 | Low | Overexpressed | Low | ||
| CM343 | Low | Overexpressed | Undetected | ||
| CM567 | Low | Overexpressed | Low | ||
| CM767 | Low | Overexpressed | Overexpressed | ||
| Mdm2 overexpression only (35) | |||||
| CM194 | Basal | Undetected | Overexpressed | ||
| CM332 | Basal | Undetected | Overexpressed | ||
| CM584 | Basal | Undetected | Overexpressed | ||
| CM687 | Basal | Undetected | Overexpressed | ||
| CM716 | Basal | Undetected | Moderately overexpressed | ||
| CM800 | Basal | Undetected | Moderately overexpressed | ||
| No detectable alteration (29) | |||||
| CM345 | Basal | Undetected | Undetected | ||
| CM617 | Basal | Undetected | Undetected | ||
| CM681 | Basal | Undetected | Low | ||
| CM708 | Basal | Undetected | Undetected | ||
| CM791 | Basal | Undetected | Undetected | ||
The frequency of p53 alterations in Bax+/− Eμ-myc transgenics was similar to that reported for wild-type Eμ-myc transgenic mice (5), since approximately one-quarter of the lymphomas arising in Bax+/− Eμ-myc transgenics sustained mutations or deletions in p53 (Fig. 4B and 5A; Table 1). As a consequence of p53 mutations, these tumors (CM201, CM271, and CM696) displayed high levels of p53 protein and concomitant increases in ARF protein (Fig. 4B and data not shown), presumably due to the loss of feedback control of ARF expression by p53 (47). One tumor (CM622) from a Bax+/− Eμ-myc transgenic had deleted both alleles of p53, as determined by Southern blot analysis (Fig. 5A). Strikingly, not a single lymphoma arising in Bax-null Eμ-myc transgenics contained mutant p53, nor was p53 deleted in any of these tumors (Fig. 4B and 5B; Table 1). Therefore, Bax loss selectively circumvents the requirement for p53 mutations and deletions during Myc-induced tumorigenesis but does not alter the frequency of alterations in ARF.
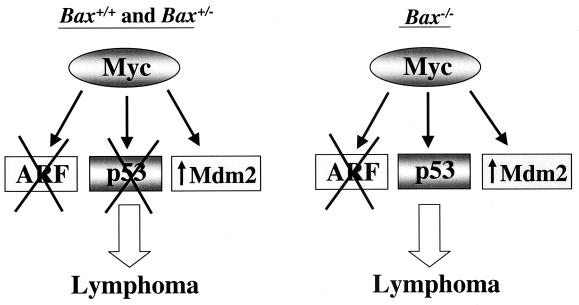
Normally, Mdm2 protein is expressed at very low levels. However, half of all lymphomas arising in Eμ-myc transgenics overexpress Mdm2 protein, and this also occurs in tumors bearing deletions of ARF or mutations or deletions of p53 (5). Mdm2 was also overexpressed in approximately half (56%) of all lymphomas arising in Bax-null and Bax+/− Eμ-myc transgenics, regardless of their ARF or p53 status (Fig. 4B and Table 1). The frequency of Mdm2 overexpression in tumors lacking alterations in ARF or p53 was somewhat higher in Bax−/− (35%) and Bax+/− (27%) Eμ-myc transgenics (Table 1) compared to lymphomas arising in wild-type (16%) Eμ-myc transgenic mice (5). This difference may be due to the lack of p53 alterations in Bax-deficient Eμ-myc transgenic mice. Overall, lymphomas from Bax−/− Eμ-myc transgenics showed a frequency of alterations in the ARF-Mdm2-p53 pathway (71%) similar to that of Bax+/− (80%) (Table 1) and wild-type (80%) (5) Eμ-myc transgenics. Therefore, Bax loss selectively eliminates the requirement for p53 mutations or deletions during lymphomagensis, without significantly influencing the frequency of alterations in ARF and Mdm2 (Fig. 6).
FIG. 6.
Schematic describing the outcome of molecular events that occur in lymphomas in Bax+/+, Bax+/−, and Bax−/− Eμ-myc transgenic mice. On the left, the majority of lymphomas that arise in Bax+/+ and Bax+/− Eμ-myc transgenic mice have alterations in ARF (deletion, X) or p53 (mutation or deletion, X) and/or Mdm2 (overexpression, ↑) (Fig. 4 and 5 and Table 1; see also reference 5). On right, a similar frequency of alterations in ARF and Mdm2 still occurs in lymphomas from Bax-null Eμ-myc transgenic mice; however, no p53 mutations or deletions are observed in these lymphomas (Fig. 4 and 5, Table 1). Therefore, Myc targets ARF and Mdm2 but not p53 in the absence of Bax during Myc-induced lymphomagenesis.
DISCUSSION
B cells lacking mediators of Myc-induced apoptosis have an accelerated course of lymphoma development in Eμ-myc transgenic mice (5, 17, 50). For example, deleting ARF or p53, both mediators of Myc-induced apoptosis, accelerates lymphomagenesis in Eμ-myc transgenic mice (5, 50). Here, by using in vivo models, we extend these observations to the proapoptotic Bcl-2 family member Bax and show that intersecting apoptotic pathways play a crucial role in Myc-induced lymphomagenesis. Loss of Bax in pre-B cells confers resistance to Myc-induced apoptosis and accelerates pre-B- and B-cell lymphoma development in Eμ-myc transgenic mice. This is consistent with the finding that Bax-null mouse embryo fibroblasts (MEFs) are more resistant to Myc-induced apoptosis (32). Therefore, Bax is a mediator of Myc-induced apoptosis and inhibits Myc-initiated tumorigenesis.
The balance of proapoptotic and antiapoptotic Bcl-2 family members regulates the susceptibility of cells to apoptosis (reviewed in reference 23). An excess of Bax induces cell death, whereas overexpression of Bcl-2 or Bcl-XL suppresses apoptosis induced by a variety of apoptotic stimuli. In immortal human cells bax has been reported to be a transcriptional target of both p53 (34, 35) and Myc (32), yet we failed to detect any direct or significant increase in Bax expression upon Myc activation in primary murine pre-B cells. Nevertheless, activation of p53 can induce bax and suppress bcl-2 expression in certain cell types (33–35). Myc, on the other hand, upregulates p53 and ARF (5, 63) and suppresses Bcl-XL expression, and the latter is independent of either p53 or ARF in primary murine hematopoietic cells (6). Thus, Myc activation alone is sufficient to alter the ratio of pro- and antiapoptotic Bcl-2 family members with the net result being an excess of Bax, which leads to apoptosis. Bax loss short-circuits this response and confers survival to cells overexpressing Myc.
p53 is a mediator of Myc-induced apoptosis (5, 63), and Bax plays an important role in p53-dependent apoptosis in some cell types in vitro (28, 35) and in vivo (52, 62). This study confirms and extends these results by linking Myc with p53 and Bax in vivo. Lymphomas arising in Bax-deficient Eμ-myc transgenic mice lack p53 mutations and deletions, whereas 28% of tumors from wild-type Eμ-myc transgenics (5) and 27% of lymphomas from Bax+/− Eμ-myc transgenic mice sustain mutations or deletions in p53. Therefore, under selective pressure from Myc, Bax-deficient B cells differ from their wild-type counterparts by sustaining wild-type p53 expression. These results imply that during transformation mutation of p53 is unnecessary when Bax is absent, supporting the observations that Bax is downstream from p53 (10). This is consistent with the observation that there is no cooperative effect on the rate of tumorigenesis in mice lacking both p53 and Bax compared to mice deficient in p53 alone (20). Moreover, when the statuses of ARF and p53 were analyzed in lymphomas that arise in Eμ-myc transgenic mice, biallelic deletion of ARF or p53 deletion or mutation occurred in a mutually exclusive fashion (5). Thus, although ARF and p53 can function in the same tumor suppressor pathway, our results demonstrate that this pathway must bifurcate, since only Bax and p53 are in the same Myc-induced pathway (Fig. 6). Indeed, this study provides formal genetic proof that ARF, Mdm2, and p53 have different targets.
One of the most intriguing outcomes of these studies is the finding that that Bax loss influences p53 status independent of ARF (Fig. 6). Bax deficiency did not affect the frequency of ARF deletions in Eμ-myc lymphomas nor tumor latency in ARF-null mice, suggesting that Bax and ARF are not in the same pathway, or that Bax resides in a position in the pathway that does not influence ARF. However, further analysis has demonstrated that Bax loss does affect the tumor spectrum in ARF-null mice (C. M. Eischen and J. L. Cleveland, unpublished data). Thus, there is cooperativity at least at some level, and ARF and Bax must function in separate pathways.
Even though ARF and p53 function in the same tumor suppressor pathway, p53 has been reported to function independently of ARF in certain situations and vice versa. For example, gamma irradiation-induced apoptosis is p53 dependent and still occurs in ARF-null cells (19), and ARF can still induce cell cycle arrest in fibroblasts lacking p53 and Mdm2 (58). Our data from Bax-null Eμ-myc transgenic mice reveals a role for Bax that is dependent on p53 but independent of ARF. Myc thus affects the p53 pathway in two ways: by upstream activation through ARF and by downstream activation of Bax. However, the loss of Bax does seem to disrupt the ARF-Mdm2-p53 pathway by other means. The increased percentage of lymphomas in Bax-null Eμ-myc transgenics that overexpressed ARF (Table 1) suggests that Bax expression could influence the ARF-Mdm2-p53 pathway by somehow targeting proteins that regulate ARF expression, such as Bmi-1 (16), Dmp-1 (13), JunD (60), Tbx2 (15), and Twist (27). Alternatively, Bax status could disrupt the delicate feedback control mechanisms that regulate the expression of ARF, Mdm2, and p53 (47, 53).
Mdm2 is a negative regulator of p53, and we therefore predicted that, since the loss of Bax bypassed requirements for inactivating p53, then the frequency of Mdm2 overexpression would be decreased as well. Surprisingly, Mdm2 was overexpressed in 50% (16 of 32) of all of the lymphomas analyzed, as shown in wild-type Eμ-myc transgenics (5), regardless of Bax status. Mdm2 has many targets other than p53 (e.g., E2F-1, DP-1, p300, and pRb) (reviewed in reference 36) and appears to function independently of the p53 pathway in certain scenarios. For example, Mdm2 is overexpressed in a third of all lymphomas that had mutated or deleted p53 (5), and haploinsufficiency of Mdm2 alters the tumor spectrum in p53-null mice (29). Moreover, Mdm2 transgene overexpression resulted in altered mammary gland development (26) and tumorigenesis (18) in p53−/− mice. Therefore, selection for Mdm2 overexpression in Eμ-myc lymphomas is not influenced by Bax status and is also not necessarily linked to alterations in p53 or ARF (Fig. 6).
Bax does not function as a classic tumor suppressor in Eμ-myc-induced lymphomas, as do ARF and p53 (reviewed in reference 51). However, Bax tumor suppressor function has been reported in some scenarios. First, a Bax frameshift mutation occurs in a subset of colon adenocarcinomas (45), and cells with this mutation display a survival advantage when transplanted into nude mice (14). Second, Bax deficiency accelerates brain and breast tumor development in SV40 large T antigen-transgenic mice (52, 62). Finally, there is increased foci formation in transformation assays by using Bax-deficient MEFs (28). In contrast, Bax is not mutated in any of the Bax+/+ or Bax+/− Eμ-myc tumors analyzed. Additionally, there was no loss of heterozygosity of Bax in mammary carcinomas from Bax+/− C3 (1)/SV40 large T antigen-transgenic mice (52) or in lymphomas from Bax+/− Eμ-myc transgenics (Fig. 4A). These latter findings are consistent with the observation that Bax is infrequently mutated in most types of human B-cell lymphoma (42). Moreover, Bax-null mice do not spontaneously develop cancer (22), and loss of Bax did not influence the survival of ARF-null mice. Therefore, Bax may function as a classic tumor suppressor under very specific circumstances but acts as a modifier in most situations, such as Myc-induced lymphomas. Our observations also indicate that targets in addition to Bax must contribute to p53-dependent tumor suppression.
It has been known for over a decade that Bcl-2 and Myc can cooperate in transformation. The malignancy in Eμ-myc/Eμ-bcl-2 double-transgenic mice is composed of primitive lymphoid cells (54), rather than the pre-B and/or mature B cells typical of Eμ-myc lymphomas (1). Although loss of Bax also accelerates lymphomagenesis in Eμ-myc transgenics, flow cytometric analysis clearly indicates that these lymphomas are of pre-B- and/or mature B-cell origin. Thus, overexpression of Bcl-2 blocks Myc-induced apoptosis and alters the differentiation of the lymphoid cell, while Bax loss alters the sensitivity to Myc-induced apoptosis without overtly affecting B-cell differentiation. Thus, Bcl-2 and Bax appear to provide different developmental roles when B-cell precursors are forced to proliferate by Myc expression. Although Bcl-2 can inhibit the apoptotic effects of Bax (reviewed in reference 23), Bcl-2 and Bax can regulate apoptosis independently of each other (21). Moreover, Bcl-2 is overexpressed at the same frequency in Bax−/− and Bax+/+ Eμ-myc lymphomas (Eischen and Cleveland, unpublished). Therefore, it will be interesting to evaluate the status of p53, ARF, and Mdm2 in the tumors from Eμ-myc/Eμ-bcl-2 double transgenics in order to determine whether Bcl-2 and Bax function in common or distinct pathway(s) in relationship to p53.
ACKNOWLEDGMENTS
We thank Gerard Zambetti for many helpful discussions and for critical review of the manuscript, Robert Hawley and Derek Persons for retroviral vectors, Alan Harris and Charles Sidman for providing breeders for Eμ-myc transgenic mice, Richard Cross for superb assistance with FACS, and Cynthia Wetmore for assistance with p53 sequencing. We also appreciate the outstanding technical support of Chunying Yang, Elsie White, and Rose Mathew.
This work was supported in part by National Institutes of Health grants CA76379 and DK44158 (J.L.C.), CA71907 and CA56819 (M.F.R.), Cancer Center Core grant CA21765, NIH Postdoctoral Grant CA81695 (C.M.E.), and by the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital.
REFERENCES
- 1.Adams J M, Harris A W, Pinkert C A, Corcoran L M, Alexander W S, Cory S, Palmiter R D, Brinster R L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 2.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 3.Barak Y, Juven T, Haffner R, Oren M. Mdm2 expression is induced by wild-type p53 activity. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brimmell M, Mendiola R, Mangion J, Packham G. BAX frameshift mutations in cell lines derived from human haemopoietic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene. 1998;16:1803–1812. doi: 10.1038/sj.onc.1201704. [DOI] [PubMed] [Google Scholar]
- 5.Eischen C M, Weber J D, Roussel M F, Sherr C J, Cleveland J L. Disruption of the ARF-Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eischen C M, Woo D, Roussel M F, Cleveland J L. Apoptosis triggered by Myc-induced suppression of Bcl-XL or Bcl2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 8.Fanidi A, Harrington E A, Evan G I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 9.Gross A, McDonnell J M, Korsmeyer S J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 11.Harvey D M, Levine A J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 12.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue K, Roussel M F, Sherr C J. Induction of ARF tumor suppressor gene expression and cell cycle arrest by transcription factor DMP1. Proc Natl Acad Sci USA. 1999;96:3993–3998. doi: 10.1073/pnas.96.7.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ionov Y, Yamamoto H, Krajewski S, Reed J C, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci USA. 2000;97:10872–10877. doi: 10.1073/pnas.190210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs J J, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof P M, van Welsem T, van de Vijver M J, Koh E Y, Daley G Q, van Lohuizen M. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nat Genet. 2000;26:291–299. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs J J, Kieboom K, Marino S, DePinho R A, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs J J, Scheijen B, Voncken J W, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13:2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones S N, Sands A T, Hancock A R, Vogel H, Donehower L A, Linke S P, Wahl G M, Bradley A. The tumorigenic potential and cell growth characteristics of p53- deficient cells are equivalent in the presence or absence of Mdm2. Proc Natl Acad Sci USA. 1996;93:14106–14111. doi: 10.1073/pnas.93.24.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 20.Knudson C M, Johnson G M, Lin Y, Korsmeyer S J. Bax accelerates tumorigenesis in p53-deficient mice. Cancer Res. 2001;61:659–665. [PubMed] [Google Scholar]
- 21.Knudson C M, Korsmeyer S J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 22.Knudson C M, Tung K S, Tourtellotte W G, Brown G A, Korsmeyer S J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 23.Korsmeyer S J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- 24.Lindsten T, Ross A J, King A, Zong W X, Rathmell J C, Shiels H A, Ulrich E, Waymire K G, Mahar P, Frauwirth K, Chen Y, Wei M, Eng V M, Adelman D M, Simon M C, Ma A, Golden J A, Evan G, Korsmeyer S J, MacGregor G R, Thompson C B. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundgren K, Montes de Oca Luna R, McNeill Y B, Emerick E P, Spencer B, Barfield C R, Lozano G, Rosenberg M P, Finlay C A. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997;11:714–725. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- 27.Maestro R, Dei Tos A P, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach D H, Hannon G J. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207–2217. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCurrach M E, Connor T M, Knudson C M, Korsmeyer S J, Lowe S W. Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonnell T J, Montes de Oca Luna R, Cho S, Amelse L L, Chavez-Reyes A, Lozano G. Loss of one but not two mdm2 null alleles alters the tumour spectrum in p53 null mice. J Pathol. 1999;188:322–328. doi: 10.1002/(SICI)1096-9896(199907)188:3<322::AID-PATH372>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Meijerink J P, Mensink E J, Wang K, Sedlak T W, Sloetjes A W, de Witte T, Waksman G, Korsmeyer S J. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- 31.Meijerink J P, Smetsers T F, Sloetjes A W, Linders E H, Mensink E J. Bax mutations in cell lines derived from hematological malignancies. Leukemia. 1995;9:1828–1832. [PubMed] [Google Scholar]
- 32.Mitchell K O, Ricci M S, Miyashita T, Dicker D T, Jin Z, Reed J C, El-Deiry W S. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000;60:6318–6325. [PubMed] [Google Scholar]
- 33.Miyashita T, Harigai M, Hanada M, Reed J C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 34.Miyashita T, Krajewski S, Krajewska M, Wang H G, Lin H K, Liebermann D A, Hoffman B, Reed J C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 35.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 36.Momand J, Wu H H, Dasgupta G. MDM2–master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 37.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 38.Motoyama N, Wang F, Roth K A, Sawa H, Nakayama K I, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh D Y. Massive cell death of immature hematopoietic cells and neurons in Bcl-x- deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K, Negishi I, Kuida K, Sawa H, Loh D Y. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packham G, Cleveland J L. c-Myc and apoptosis. Biochim Biophys Acta. 1995;1242:11–28. doi: 10.1016/0304-419x(94)00015-t. [DOI] [PubMed] [Google Scholar]
- 41.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nunez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng H, Aiello A, Packham G, Isaacson P G, Pan L. Infrequent bax gene mutations in B-cell lymphomas. J Pathol. 1998;186:378–382. doi: 10.1002/(SICI)1096-9896(199812)186:4<378::AID-PATH203>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Perez G I, Robles R, Knudson C M, Flaws J A, Korsmeyer S J, Tilly J L. Prolongation of ovarian life span into advanced chronological age by Bax- deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 44.Quelle D E, Zindy F, Ashmun R A, Sherr C J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 45.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed J C, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 46.Reed J C, Miyashita T, Takayama S, Wang H G, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 47.Robertson K D, Jones P A. The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and downregulated by wild-type p53. Mol Cell Biol. 1998;18:6457–6473. doi: 10.1128/mcb.18.11.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roussel M F, Theodoras A M, Pagano M, Sherr C J. Rescue of defective mitogenic signaling by D-type cyclins. Proc Natl Acad Sci USA. 1995;92:6837–6841. doi: 10.1073/pnas.92.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitt C A, McCurrach M E, de Stanchina E, Wallace-Brodeur R R, Lowe S W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherr C J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 52.Shibata M A, Liu M L, Knudson M C, Shibata E, Yoshidome K, Bandey T, Korsmeyer S J, Green J E. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J. 1999;18:2692–2701. doi: 10.1093/emboj/18.10.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stott F J, Bates S, James M C, McConnell B B, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden K H, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strasser A, Harris A W, Bath M L, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 55.Tao W, Levine A J. p19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaux D L, Cory S, Adams J M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c- myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 57.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 58.Weber J D, Jeffers J R, Rehg J E, Randle D H, Lozano G, Roussel M F, Sherr C J, Zambetti G P. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 2000;14:2358–2365. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber J D, Taylor L J, Roussel M F, Sherr C J, Bar-Sagi D. Nucleolar ARF sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 60.Weitzman J B, Fiette L, Matsuo K, Yaniv M. JunD protects cells from p53-dependent senescence and apoptosis. Mol Cell. 2000;6:1109–1119. doi: 10.1016/s1097-2765(00)00109-x. [DOI] [PubMed] [Google Scholar]
- 61.Wu X, Bayle J H, Olson D, Levine A J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 62.Yin C, Knudson C M, Korsmeyer S J, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 63.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]