Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has caused significant morbidity and mortality in high-risk populations. Several therapeutics have been developed to reduce the risk of complications related to COVID-19, hospitalizations, and death. In several studies, nirmatrelvir-ritonavir (NR) was reported to reduce the risk of hospitalizations and death. We aimed to evaluate the efficacy of NR in preventing hospitalizations and death during the Omicron predominant period.
Methods
We retrospectively evaluated patients from June 1, 2022, through September 24, 2022. There were a total of 25,939 documented COVID-19 cases. Using propensity matching, we matched 5754 patients treated with NR with untreated patients.
Results
Postmatching, the median age of the NR-treated group was 58 years (interquartile range, 43-70 years) and 42% were vaccinated. Postmatching composite outcome of the 30-day hospitalization and mortality in the NR-treated group were 0.9% (95% confidence interval [CI]: 0.7%−1.2%) versus 2.1% (95% CI: 1.8%−2.5%) in the matched control group, with a difference of −1.2 (−1.7, −0.8), P value <.01. The difference rates (NR vs. control) in 30-day all-cause hospitalizations and mortality were −1.2% (95% CI: −1.6% to −0.7%, P value <.01) and −0.1% (95% CI: −0.2% to 0.0%, P value = 0.29), respectively. We found similar finding across different age groups (≥65 vs. <65) and the vaccinated group.
Conclusion
We report a significant benefit with the use of NR in reducing hospitalizations among various high-risk COVID-19 groups during the Omicron BA.5 predominant period.
Keywords: COVID-19, Omicron variant, Nirmatrelvir-ritonavir
Clinical Significance.
-
•
Coronavirus disease 2019 (COVID-19) infections continue to inflict high-risk populations with significant mortality and morbidity with the emergence of new variants resulting from random mutations that evade immunity.
-
•
Several studies have reported a reduction in hospitalizations and death among patients treated with nirmatrelvir-ritonavir (Paxlovid).
-
•
Despite the reported benefits of nirmatrelvir-ritonavir, greater health care provider awareness is required because many at-risk individuals are not being offered this important treatment option.
Alt-text: Unlabelled box
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused a significant number of deaths in the United States (US) and globally. With ongoing worldwide infections, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to inflict high-risk populations with significant mortality and morbidity through the emergence of new variants from resulting random mutations that evade immunity.1 In November 2021, the Omicron variant became the dominant SARS-CoV-2 globally and was identified as a variant of concern (VOC). Subsequently, the subvariants BA.1, BA.1.1, and BA.2 were reported in 2022. Most recently, the subvariants BA.4, BA.5, and later subvariants BA.2.75, BA.4.6, BF.7, BQ.1, and BQ.1.1 were reported in the US and deemed nonsusceptible to anti-SARS-CoV-2 neutralizing anti-pike monoclonal antibodies (mAbs).2, 3, 4, 5 As a result, the Food and Drug Administration (FDA) revoked the Emergency Use Authorization (EUA) of bebtelovimab on November 30, 2022.6 Nirmatrelvir, an oral protease inhibitor active against the viral protease MPRO, which is important in viral replication through the cleavage of the 2 viral polyproteins, has potent antiviral activity against all human coronaviruses. When administered with ritonavir, nirmatrelvir therapeutic concentrations are increased.7, 8, 9 A randomized controlled trial of nirmatrelvir-ritonavir (NR), brand name Paxlovid, in nonhospitalized, high-risk, SARS-CoV-2 nonimmune adults provided the data for the FDA EUA approval of NR during the B.1.617.2 (delta) variant predominant period.10 , 11 In December 2021, the FDA issued an EUA for the use of NR in the treatment of mild-to-moderate COVID-19 in high-risk patients.7, 8, 9 In a recent study during the Omicron variant surge, rates of hospitalization and deaths related to COVID-19 were significantly lower in adults 65 years of age or older treated with NR than among younger patients who had received such treatment, regardless of previous SARS-CoV-2 immunity.12 Moreover, several studies had reported on the effective prevention of hospitalizations and deaths using MAbs in high-risk patients.13, 14, 15 Meanwhile, spike protein mutations in SARS-CoV-2 Omicron subvariants resulted in the reduced susceptibility of previously authorized MAbs such as bamlanivimab-etesevimab, casirivimab-imdevimab, and sotrovimab. Therefore, on February 11, 2022, the FDA granted EUA for bebtelovimab (BEB) as an alternative therapy for high-risk patients with mild to moderate COVID-19.16, 17, 18, 19, 20
In a recent real-world study of BEB, researchers evaluated high-risk outpatients during the predominantly SARS-CoV-2 Omicron BA.2 subvariants period and compared outcomes to those treated with NR. According to the authors, the rate of progression to severe disease after treatment with BEB did not result in a significant difference when compared to the NR-treated group. This led to the use of BEB as a valuable option for high-risk patients for whom NR use may have been challenging at the time.21 Based on the results of this study and the National Institutes of Health (NIH) recommendations at the time, many centers, including ours, used BEB in patients at risk for NR-associated drug interactions and adverse reactions such as in solid organ transplant recipients.22 However, after the FDA revoked the BEB EUA on November 20, 2022, oral antivirals and remdesivir became the only viable treatment options for patients at-risk for severe COVID-19 infections.5 , 6 Therefore, we aimed to evaluate the 30-day all-cause hospitalization and/or mortality in patients with mild to moderate COVID-19 treated with NR in our hospitals and clinics. Furthermore, we evaluated these outcomes among patients who were vaccinated, immunocompromised, and elderly (≥65 years of age).
Methods
This study was approved by the institutional review board of the University of Arizona with a waiver of patient consent, given the retrospective nature of the study. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Overview
This study is an observational retrospective cohort analysis of patients with COVID-19 from June 1, 2022, through September 24, 2022. The patient follow-up date was censored on October 24, 2022. Patients prescribed NR and those untreated were captured from electronic health records (EHRs) in the Banner Health Care System (a nonprofit, large health care organization), which houses 30 hospitals and several clinics across the western US. High-risk patients with mild to moderate COVID-19 infection within 5 days of symptoms onset may be prescribed NR under the FDA EUA (https://www.bannerhealth.com/staying-well/health-and-wellness/wellness/covid/treatment). Oral antivirals and remdesivir are currently available treatment options because the FDA revoked BEB EUA on November 30, 2022 (https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region).
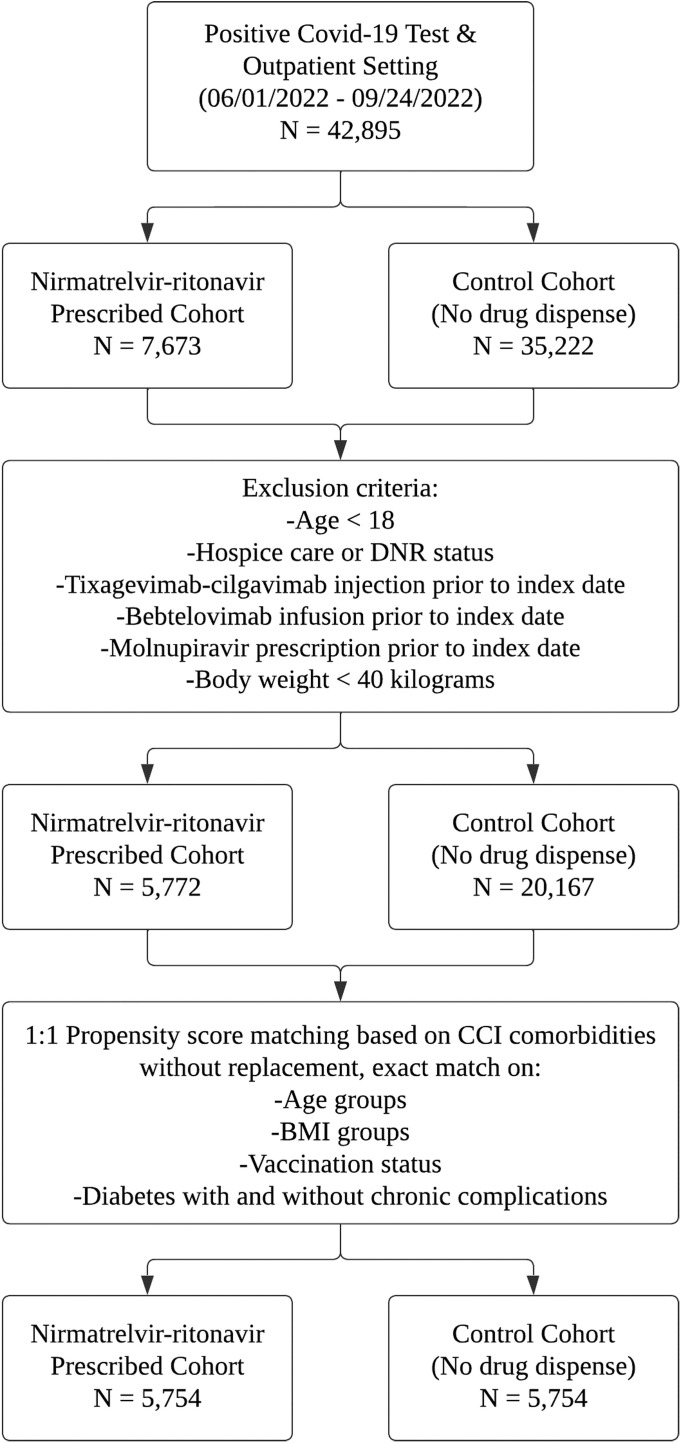
A total of 42,895 outpatients positive for COVID-19 (positive PCR or direct antigen tests) were selected from the Banner Cerner-EHRs. Exclusion criteria included age younger than 18 years; those in hospice, emergency department, or inpatient settings; receipt of tixagevimab-cilgavimab injection or BEB infusion, molnupiravir use prior to the index date, and/or weight less than 40 kg (Figure ). The resulting study cohort included 5772 patients prescribed NR and 20,167 patients untreated in the Banner system. The study index date for cohorts was determined as the date of the completed NR prescription date or the date of the first positive COVID-19 test. The index dates were used as enrollment dates for the study. Demographic and clinical covariates of both cohorts were extracted from the EHRs for propensity matching and analysis (Table 1 ). The clinical covariates were derived from the Charlson Comorbidity Index. The postmatch cohort consisted of 5754 pairs (N = 11,508). Primary outcomes included the occurrence of 30-day all-cause hospitalization and/or mortality after the index date. For subgroup analysis, patients were grouped and rematched under vaccination status, immunocompromised status, and age (<65 or ≥65) categories. The rate of emergency department visits within 30 days of enrollment was considered a secondary outcome of the study.
Figure.
Flow chart.
Table 1.
Patient Characteristics and Covariate Balance Before and After Propensity Matching.
| After Propensity Matching |
Before Propensity Matching |
|||||
|---|---|---|---|---|---|---|
| Nirmatrelvir-Ritonavir Cohort | Untreated Control Cohort | SMD | Nirmatrelvir-Ritonavir Cohort | Untreated Control Cohort | SMD | |
| N = 5754 | N = 5754 | N = 5772 | N = 20,167 | |||
| Age | 58.0 [43.0,70.0] | 58.0 [42.0,70.0] | 58.0 [43.0,70.0] | 46.0 [31.0,63.0] | ||
| Age Groups | ||||||
| 18-35 | 785 (13.6) | 785 (13.6) | 0.00 | 788 (13.7) | 6572 (32.6) | −0.55 |
| 35-50 | 1348 (23.4) | 1348 (23.4) | 0.00 | 1355 (23.5) | 4890 (24.2) | −0.02 |
| 50-60 | 1045 (18.2) | 1045 (18.2) | 0.00 | 1047 (18.1) | 3013 (14.9) | 0.08 |
| 60-70 | 1253 (21.8) | 1253 (21.8) | 0.00 | 1255 (21.7) | 2662 (13.2) | 0.21 |
| >70 | 1323 (23.0) | 1323 (23.0) | 0.00 | 1327 (23.0) | 3030 (15.0) | 0.19 |
| Sex | ||||||
| Male | 2295 (39.9) | 2396 (41.6) | −0.04 | 2300 (39.8) | 7844 (38.9) | 0.02 |
| Fully Vaccinated | ||||||
| Yes | 2418 (42.0) | 2418 (42.0) | 0.00 | 2422 (42.0) | 7756 (38.5) | 0.07 |
| No | 2080 (36.1) | 2080 (36.1) | 0.00 | 2088 (36.2) | 6560 (32.5) | 0.08 |
| Unknown | 1256 (21.8) | 1256 (21.8) | 0.00 | 1262 (21.9) | 5851 (29.0) | −0.17 |
| Race/Ethnicity | ||||||
| White | 4090 (71.1) | 4007 (69.6) | 0.03 | 4103 (71.1) | 12652 (62.7) | 0.18 |
| Black | 240 (4.2) | 281 (4.9) | −0.04 | 242 (4.2) | 1183 (5.9) | −0.08 |
| Hispanic | 827 (14.4) | 758 (13.2) | 0.03 | 829 (14.4) | 4009 (19.9) | −0.16 |
| Asian/Pacific Islander | 101 (1.8) | 108 (1.9) | −0.01 | 101 (1.7) | 358 (1.8) | −0.00 |
| Native American/Alaskan | 66 (1.1) | 54 (0.9) | 0.02 | 67 (1.2) | 250 (1.2) | −0.01 |
| Unknown | 430 (7.5) | 546 (9.5) | −0.08 | 430 (7.4) | 1715 (8.5) | −0.04 |
| BMI Group | ||||||
| ≤20 | 143 (2.5) | 143 (2.5) | 0.00 | 146 (2.5) | 897 (4.4) | −0.12 |
| 20-25 | 1000 (17.4) | 1000 (17.4) | 0.00 | 1001 (17.3) | 4340 (21.5) | −0.11 |
| 25-30 | 1637 (28.4) | 1637 (28.4) | 0.00 | 1638 (28.4) | 5354 (26.5) | 0.04 |
| 30-35 | 1299 (22.6) | 1299 (22.6) | 0.00 | 1300 (22.5) | 3809 (18.9) | 0.09 |
| 35-40 | 743 (12.9) | 743 (12.9) | 0.00 | 745 (12.9) | 2007 (10.0) | 0.09 |
| >40 | 657 (11.4) | 657 (11.4) | 0.00 | 663 (11.5) | 1661 (8.2) | 0.10 |
| Unknown | 275 (4.8) | 275 (4.8) | 0.00 | 279 (4.8) | 2099 (10.4) | −0.26 |
| Time period | ||||||
| 6/01-30/2022 | 1864 (32.4) | 1887 (32.8) | −0.01 | 1869 (32.4) | 6856 (34.0) | −0.03 |
| 7/01-31/2022 | 2115 (36.8) | 2091 (36.3) | 0.01 | 2123 (36.8) | 7161 (35.5) | 0.03 |
| 8/01-31/2022 | 1267 (22.0) | 1246 (21.7) | 0.01 | 1270 (22.0) | 4311 (21.4) | 0.02 |
| 9/01-24/2022 | 508 (8.8) | 530 (9.2) | −0.01 | 510 (8.8) | 1839 (9.1) | 0.01 |
| Myocardial Infarction | 165 (2.9) | 147 (2.6) | 0.02 | 166 (2.9) | 598 (3.0) | −0.01 |
| Heart Failure | 237 (4.1) | 231 (4.0) | 0.01 | 240 (4.2) | 870 (4.3) | −0.01 |
| Cerebrovascular Disease | 241 (4.2) | 220 (3.8) | 0.02 | 242 (4.2) | 822 (4.1) | 0.01 |
| Hemiplegia or Paraplegia | 49 (0.9) | 51 (0.9) | −0.00 | 50 (0.9) | 186 (0.9) | −0.01 |
| Peripheral Vascular Disease | 314 (5.5) | 289 (5.0) | 0.02 | 315 (5.5) | 754 (3.7) | 0.07 |
| Chronic Pulmonary Disease | 1567 (27.2) | 1456 (25.3) | 0.04 | 1575 (27.3) | 4275 (21.2) | 0.14 |
| Dementia | 33 (0.6) | 43 (0.7) | −0.02 | 33 (0.6) | 170 (0.8) | −0.04 |
| Hypertension | 2337 (40.6) | 2285 (39.7) | 0.02 | 2351 (40.7) | 5900 (29.3) | 0.23 |
| Diabetes Without Chronic Complications | 1055 (18.3) | 1055 (18.3) | 0.00 | 1067 (18.5) | 2565 (12.7) | 0.15 |
| Diabetes With Chronic Complications | 293 (5.1) | 293 (5.1) | 0.00 | 305 (5.3) | 945 (4.7) | 0.03 |
| Renal Mild-Moderate-Advanced Disease (CKD stages 1-4) | 255 (4.4) | 252 (4.4) | 0.00 | 258 (4.5) | 917 (4.5) | −0.00 |
| Renal Severe Disease (CKD stage 5 and ESRD) | 20 (0.3) | 21 (0.4) | 0.00 | 20 (0.3) | 226 (1.1) | −0.13 |
| Mild Liver Disease | 454 (7.9) | 427 (7.4) | 0.02 | 457 (7.9) | 1154 (5.7) | 0.08 |
| Moderate to Severe Liver Disease | 43 (0.7) | 44 (0.8) | 0.00 | 43 (0.7) | 229 (1.1) | −0.05 |
| Peptic Ulcer Disease | 80 (1.4) | 72 (1.3) | 0.01 | 80 (1.4) | 264 (1.3) | 0.01 |
| Rheumatic Disease | 178 (3.1) | 160 (2.8) | 0.02 | 179 (3.1) | 504 (2.5) | 0.03 |
| Malignancy | 200 (3.5) | 210 (3.6) | −0.01 | 201 (3.5) | 461 (2.3) | 0.07 |
| Lymphoproliferative Disease | 110 (1.9) | 84 (1.5) | 0.03 | 111 (1.9) | 212 (1.1) | 0.06 |
| Metastatic Solid Tumor | 79 (1.4) | 70 (1.2) | 0.01 | 79 (1.4) | 163 (0.8) | 0.05 |
| HIV | 17 (0.3) | 19 (0.3) | −0.01 | 17 (0.3) | 60 (0.3) | −0.00 |
| Opportunistic Infection | 362 (6.3) | 350 (6.1) | 0.01 | 365 (6.3) | 1236 (6.1) | 0.01 |
| Solid Organ Transplant | 16 (0.3) | 14 (0.2) | 0.01 | 16 (0.3) | 114 (0.6) | −0.05 |
BMI = body mass index; CKD = chronic kidney disease; ESRD = end-stage renal disease; HIV = human immunodeficiency virus; SD = standard deviation; SMD = standardized mean difference.
Data are presented as mean (SD) for continuous measures, and n (%) for categorical measures.
Multivariable Propensity Matching
The cohorts were 1:1 propensity matched without replacement across 26 covariates. Because a randomization was not an option for this retrospective observational study, a propensity matching was performed to adjust for the difference between the treated and untreated cohorts to reduce covariate imbalances and to minimize selection bias. An optimal matching algorithm that minimizes the sum of the absolute pairwise distance across the matched sample was used for matching. This algorithm was determined best over the nearest neighbors and complete matching algorithms per the covariate balance and the count of unmatched individuals. A generalized linear model with a logistic regression link was used to calculate pairwise distances. Pairs were matched exactly based on vaccination status, body mass index, age, and diabetes. (ie, a fully vaccinated patient from the NR-prescribed cohort could only be matched to a fully vaccinated patient from the untreated cohort). The clinical (Charlson Comorbidity Index-based) variables, demographic covariates, and monthly time variable were included in the model to calculate the propensity score (PS). Postmatch time variable balance showed that the index dates of treated and untreated cohorts were within a month of each other to account for monthly COVID-19 variant changes. Covariate balance was assessed by comparing pre- and postmatch standardized mean differences (SMDs), reported in Table 1. MatchIt package from the statistical computing software R was used to build the propensity models.
Statistical Analysis
For each outcome, event count, percentage of the event, and 95% confidence intervals were reported. Exact McNemar test was used to compute the percentage difference between the prescribed and untreated groups. P values of the test were reported. P values less than 0.05 were considered statistically significant. For subgroup analysis, vaccination status (fully vaccinated: yes or no), immunocompromised status (malignancy, lymphoproliferative disorders, hematopoietic stem cell transplant, solid organ transplantation, HIV: yes or no) and age (<65 vs ≥65 years old) were assessed to compare the differences in primary outcomes. A patient was considered fully vaccinated (greater than 2 doses of mRNA vaccines) if at least 14 days passed before they were enrolled in the study. Using only the observations that were matched originally, cohorts were rematched within subgroups using the same propensity model.23
Results
Patient Characteristics
From June 1, 2022, through September 24, 2022, a total of 25,939 patients were diagnosed with COVID-19; of these, 5772 patients were treated with NR. Table 1 shows the baseline characteristics of the NR-treated and untreated control cohorts before and after PS matching. All post-PS-matching covariate SMDs were below a 0.05 threshold, indicating an optimal matching and statistically insignificant differences among the variables between the 2 groups at baseline. In the post-PS-matched cohort, the median age of patients in the NR treatment arm was 58 years (interquartile range 43-70 years); 60.1% were female; 71.1% were of the white race; and 42% were fully vaccinated. Some of the high-risk characteristics included age ≥60 years (44.8%); body mass index ≥35 kg/m2 (24.3%); diabetes mellitus (23.4%); chronic lung disease (27.2%); kidney disease–any stage (4.7%); solid organ transplantation (0.3%); cancer (6.8%); and HIV disease (0.3%).
Primary and Secondary Outcomes
The incidence of the composite outcome in the pre-PS matched NR and untreated control cohorts were 0.9% and 1.8%, respectively (data not shown). Table 2 shows the results of the composite outcome within 30 days in the post-propensity-matched cohorts. Compared to the untreated control group, the incidence of patients with the composite outcome in the NR-treated group within 30 days is 0.9% (95% CI: 0.7%-1.2%) versus 2.1% (95% CI: 1.8%-2.5%) (difference −1.2 [−1.7, −0.8], P value <.01). The difference rate (NR minus untreated control) in all-cause hospitalizations and mortality within 30 days were −1.2% (95% CI: −1.6% to −0.7%, P value <.01) versus −0.1% (95% CI: −0.2% to 0.0%, P value = 0.29). Visits to the emergency department after the index date was lower in NR cohort compared with the untreated control cohort (difference −1.0% (95% CI: −1.6% to −0.3%, P value <.01). The number needed to treat (1/0.021−0.009) with NR was 83 to avoid 1 hospitalization and/or death over 30 days.
Table 2.
The Primary and Secondary Outcomes in the Postpropensity Score-Matched Cohorts
| Primary Outcomes | ||||||
|---|---|---|---|---|---|---|
| Nirmatrelvir-Ritonavir Cohort |
Untreated Control Cohort |
|||||
| N (%) | 95% CI* | N (%) | 95% Cl* | Difference in % with 95% CI* | P value | |
| Composite outcome within 30 days of enrollment | 52 (0.9) | 0.7, 1.2 | 122 (2.1) | 1.8, 2.5 | −1.2 (−1.7, −0.8) | <.01 |
| All-cause hospitalization within 30 days of enrollment | 50 (0.9) | 0.6, 1.1 | 118 (2.1) | 1.7, 2.5 | −1.2 (−1.6, −0.7) | <.01 |
| Mortality within 30 days of enrollment | 2 (0.0) | 0.0, 0.0 | 6 (0.1) | 0.0, 0.2 | −0.1 (−0.2, 0.0) | .29 |
| Secondary Outcomes | ||||||
|---|---|---|---|---|---|---|
| Nirmatrelvir-Ritonavir Cohort |
Untreated Control Cohort |
|||||
| N (%) | 95% CI* | N (%) | 95% Cl* | Difference in % with 95% CI* | P value | |
| Emergency visit within 30 days of index date | 158 (2.7) | 2.3, 3.2 | 213 (3.7) | 3.2, 4.2 | −1.0 (−1.6, −0.3) | <.01 |
CI = confidence interval.
The Clopper-Pearson method was used to calculate 95% CIs for the outcome percentages using the R package (Exactci). CI for difference in paired proportions between the treatment and control cohorts.
Subgroup Analysis
Table 3 shows the primary composite outcomes for the propensity-matched NR-treated and untreated cohorts, stratified by vaccination status, immunocompromised status, and age. A significantly lower rate of primary outcome events occurred in both the vaccinated (% difference −1.0, [95% CI: −1.7, −0.4], P <.01) and unvaccinated (% diff −1.2, [95% CI: −3.1, −1.0], P <.01) groups treated with NR compared to their untreated counterparts. Patients 65 age and older (% diff −1.9, [95% CI: −2.9, −0.9], P <.01) and patients younger than 65 (% diff −0.8, [95% CI: −1.3, −0.3], P <.01) experienced a significantly lower rate of primary composite events with NR. Immunocompetent patients treated with NR experienced fewer primary composite outcome events (% diff −1.2, [95% CI: −1.6, −0.7], P <.01). However, NR treatment did not reach statistical significance in the rate of primary composite outcomes among immunocompromised patients (% diff −2.1, [95% CI: −6.0, 1.7], P = .33), but the relative risk reduction of 44% is clinically meaningful.
Table 3.
The Primary Composite Outcome Stratified by Patient Vaccination Status,* Age Groups (<65, ≥65 Years Old), and Immunocompromised Status† Among the Propensity-Matched Study Cohort
| Nirmatrelvir-Ritonavir Cohort |
Untreated Control Cohort |
|||||
|---|---|---|---|---|---|---|
| N (%) | 95% CIǂ | N (%) | 95% Clǂ | Difference in % with 95% CIǂ | P value | |
| Fully vaccinated N = 4836 |
11 (0.5) | 0.2, 0.8 | 36 (1.5) | 1.0, 2.1 | −1.0 (−1.7, −0.4) | <.01 |
| Not fully vaccinated N = 4160 |
41 (2.0) | 1.4, 2.7 | 84 (4.0) | 3.2, 5.0 | −2.1 (−3.1, −1.0) | <.01 |
| Immunocompromised N = 484 |
6 (2.5) | 0.9, 5.3 | 11 (4.5) | 2.3, 8.0 | −2.1 (−6.0, 1.7) | .33 |
| Not Immunocompromised N = 10,582 |
39 (0.7) | 0.5, 1.0 | 100 (1.9) | 1.5, 2.3 | −1.2 (−1.6, −0.7) | <.01 |
| Age ≥65 N = 3972 |
26 (1.3) | 0.9, 1.9 | 63 (3.2) | 2.4, 4.0 | −1.9 (−2.9, −0.9) | <.01 |
| Age <65 N = 7258 |
25 (0.7) | 0.4, 1.0 | 55 (1.5) | 1.1, 2.0 | −0.8 (−1.3, −0.3) | <.01 |
CI = confidence interval.
This analysis included the patients with known vaccination status only.
Immunocompromised status includes malignancy, lymphoproliferative disorders, and hematopoietic stem cell transplants, solid organ transplants, and patients with HIV.
The Clopper-Pearson method was used to calculate 95% CIs for the outcome percentages using the R package (Exactci). CI for difference in paired proportions between the treatment and control cohorts.
Discussion
In the current study, we report on the efficacy of NR in reducing the risk of hospitalization among a large cohort of diverse-matched patients during the COVID-19 Omicron variant predominant period. In addition, we found that this benefit persisted among different age and vaccination groups. Our secondary outcomes revealed a reduction in visits to the emergency department but not a reduction in the mortality rate. NR has been reported to reduce the rates of 28-day hospitalizations or death in high-risk patients with COVID-19 and, therefore, received the FDA's EUA approval.9 Since its approval, multiple studies have reported on the efficacy of NR in reducing hospitalizations among COVID-19 patients.12 , 24 However, in contrast to prior studies, our cohort included a large and diverse group of patients that were matched based on underlying characteristics and comorbidities.
Our cohort included patients during the period during which the Omicron variant was predominant (BA.2, BA.2.12.1, and BA.5). This is important to consider because the FDA withdrew the last available mAb, BEB, due to its reduced efficacy against Omicron variants on November 30, 2022.6 Therefore, NR provides a preferable antiviral treatment option in outpatient settings, especially with the presumed lower efficacy of molnupiravir and the logistic challenges accompanying the administration of intravenous remdesivir in the outpatient settings.25 , 26 Despite the reported lower rates of hospitalizations and mortality during the Omicron period,27 we have shown that there was a significant (∼50%) reduction in the rates of hospitalizations with the use of NR. Therefore, the benefit of NR persisted in preventing hospitalizations in high-risk patients during this epidemiological shift.
In our study period, the 30 days mortality was very low at 0.1% among untreated groups compared to previous reports in the literature.27 Although previous studies reported that NR reduced mortality mainly among elderly patients, we found that the benefits persisted across all age groups, which could be related to low mortality rates in our cohort. Moreover, while Arbel et al12 concluded that NR was beneficial only among patients 65 years or older, the efficacy of NR in reducing rates of hospitalizations was statistically significant across different age groups in our study.
Immunocompromised patients are at the highest risk for COVID-19 complications due to their compromised immune response to infection, reduced immunogenicity, and increased risk of post-COVID-19 superimposed bacterial and fungal infections. Therefore, providing COVID-19 therapies in a timely manner can help reduce such complications. Indeed, in our cohort, the untreated immunocompromised patients had worse outcomes and higher percentages of complications compared to the immunocompetent group. This was corroborated by another study of solid organ transplant recipients during the Omicron variants period.28 However, although NR reduced such complications, these results were not statistically significant. This is likely due to our small sample size of matched immunocompromised patients and potentially to the lower rates of NR prescriptions in this population.29
COVID-19 vaccines have emerged as an effective method in reducing COVID-19 complications; however, with the emergence of SARS-CoV-2 variants, their effectiveness was shown to be reduced.30 In our study, we showed that NR led to a reduction in hospitalizations regardless of vaccination status. Most recent NR-related studies also report on the reduction in mortality among hospitalized and nonhospitalized vaccinated patients.31 , 32
Propensity matching cannot replace gold standard randomization process in clinical trials or account for unmeasured confounders, but it can reduce bias in estimating treatment effects in nonrandomized observational data, such as ours. The propensity score is the probability of receiving a treatment of interest based on patient characteristics and clinical setting and is used to adjust for differences between groups (pseudo-randomization). Our post-match SMDs were below 0.10 (mostly <0.05) indicating acceptable matching. We believe that our propensity matching is optimal and high quality because it used a large sample size, matching based 26 covariates (and 4 exact matching), and the postmatch SMDs being mostly <0.05, indicating perfect matching and adjustment for covariate imbalances.
The strengths of our study include the real-world experience in a large population, matched based on multiple risk factors, which reduced confounding. Moreover, our study included an analysis of the effects of NR among vaccinated individuals. We investigated the outcome of visits to the emergency department separately from hospitalizations and death, which can help provide insight into the intervention's cost-to-benefit ratio. However, our study has several limitations, including the retrospective design, which could have biased data collection. This may be seen in the number of deaths and hospitalizations being lower than what has been reported by Arizona Department of Health Services. Although patients were prescribed NR, it is unknown from our data set if they received the medication or adhered to therapy. Also, while we included vaccination status among different groups, we did not account for acquired immunity from previous infections or reduced immune responses secondary to the patients’ underlying comorbidities. Lastly, while our data is obtained from a large hospital system, data outcomes of patients that were possibly hospitalized outside the Banner health system and the state of Arizona are lacking.
In conclusion, we found that NR helps prevent COVID-19 hospitalization and visits to the emergency department in different high-risk groups. Moreover, beyond the reduction of COVID-19 complications, NR can help prevent health care systems from becoming overwhelmed with visits related to COVID-19 and, therefore, permit cases other than COVID-19 to be treated adequately.
Footnotes
Funding: None.
Conflicts of Interest: ABG, SM, BT, IM, RCW, and BT report none. MMA-O reports an honorarium from Shionogi Inc. and La Jolla Pharmaceuticals for serving on their advisory board. TTZ reports a research grant with AiCuris.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Dong E, H D, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602(7898):664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration (FDA). Evusheld Healthcare Providers 2023. Available at: https://www.fda.gov/media/154701/. Accessed January 12, 2022.
- 5.Food and Drug Administration (FDA). Bebtelovimab Health Care Provider Fact Sheet 2023. Available at: https://www.fda.gov/media/156152/download. Accessed January 12, 2022.
- 6.Food and Drug Administration (FDA). FDA Announces Bebtelovimab Is Not Currently Authorized in Any US Region. Available at:https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region. Accessed January 12, 2022.
- 7.Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem. 2016;59(14):6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration (FDA). Paxlovid 2021. Available at: https://www.fda.gov/media/155050/download. Accessed January 12, 2022.
- 10.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, non-hospitalized adults with COVID-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin EJ, Baden LR. The potential of intentional drug development. N Engl J Med. 2022;386(15):1463–1464. doi: 10.1056/NEJMe2202160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 14.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant. N Engl J Med. 2022;386(10):995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruel T, Hadjadj J, Maes P, et al. Serum neutralization of SARS-CoV-2 omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28(6):1297–1302. doi: 10.1038/s41591-022-01792-5. [DOI] [PubMed] [Google Scholar]
- 19.Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv. Available at: https://www.medrxiv.org/content/10.1101/2022.03.10.22272100v1. Accessed March 12, 2022.
- 20.Food and Drug Administration (FDA). Coronavirus (COVID-19) update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant. Available at:https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-monoclonal-antibody-treatment-covid-19-retains. Accessed January 12, 2022.
- 21.Razonable RR, O'Horo JC, Hanson SN, et al. Comparable outcomes for bebtelovimab and ritonavir-boosted nirmatrelvir treatment in high-risk patients with coronavirus disease-2019 during severe acute respiratory syndrome coronavirus 2 ba.2 omicron epoch. J Infect Dis. 2022;226(10):1683–1687. doi: 10.1093/infdis/jiac346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institutes of Health (NIH). Anti-SARS-CoV-2 monoclonal antibodies | COVID-19 treatment guidelines. Available at: https://www.ncbi.nlm.nih.gov/pubmed/. Accessed January 12, 2022.
- 23.Wang SV, Jin Y, Fireman B, et al. Relative performance of propensity score matching strategies for subgroup analyses. Am J Epidemiol. 2018;187(8):1799–1807. doi: 10.1093/aje/kwy049. [DOI] [PubMed] [Google Scholar]
- 24.Amani B, Amani B. Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: a rapid review and meta-analysis. J Med Virol. 2023;95(2):e28441. doi: 10.1002/jmv.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in non-hospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adjei S, Hong K, Molinari NM, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and delta variant pandemic periods - United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1182–1189. doi: 10.15585/mmwr.mm7137a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cochran W, Shah P, Barker L, et al. COVID-19 clinical outcomes in solid organ transplant recipients during the omicron surge. Transplantation. 2022;106(7):e346–e3477. doi: 10.1097/TP.0000000000004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salerno DM, Jennings DL, Lange NW, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;22(8):2083–2088. doi: 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–1222. doi: 10.1016/S0140-6736(22)01586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with COVID-19. Clin Infect Dis. 2023;76(4):563–572. doi: 10.1093/cid/ciac673. [DOI] [PMC free article] [PubMed] [Google Scholar]