Abstract
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with high pathogenicity and infectiousness has become a sudden and lethal pandemic worldwide. Currently, there is no accepted specific drug for COVID-19 treatment. Therefore, it is extremely urgent to clarify the pathogenic mechanism and develop effective therapies for patients with COVID-19. According to several reliable reports from China, traditional Chinese medicine (TCM), especially for three Chinese patent medicines and three Chinese medicine formulas, has been demonstrated to effectively alleviate the symptoms of COVID-19 either used alone or in combination with Western medicines. In this review, we systematically summarized and analyzed the pathogenesis of COVID-19, the detailed clinical practice, active ingredients investigation, network pharmacology prediction and underlying mechanism verification of three Chinese patent medicines and three Chinese medicine formulas in the COVID-19 combat. Additionally, we summarized some promising and high-frequency drugs of these prescriptions and discussed their regulatory mechanism, which provides guidance for the development of new drugs against COVID-19. Collectively, by addressing critical challenges, for example, unclear targets and complicated active ingredients of these medicines and formulas, we believe that TCM will represent promising and efficient strategies for curing COVID-19 and related pandemics.
Keywords: COVID-19, Huashi Baidu Formula, Jinhua Qinggan Granules, Lianhua Qingwen Capsule, Qingfei Paidu Decoction, SARS-CoV-2, traditional Chinese medicine, Xuanfei Baidu Formula, Xuebijing Injection
1. Introduction
The coronavirus disease 2019 (COVID-19) is a highly contagious respiratory illness caused by a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that swiftly spreads to several cities and nations, leading to a global pathogenic pandemic (Xu et al., 2020, Ye et al., 2020). Until now, there have been approximately 300 million confirmed illnesses with over 5 million deaths according to the latest WHO coronavirus (COVID-19) dashboard (https://covid19.who.int/). COVID-19 patients have several similar symptoms of ordinary flu, such as cough, fever, tiredness, anorexia and muscular discomfort (Kevadiya et al., 2021). Furthermore, anosmia and dysgeusia are also usually diagnosed and are COVID-19-specific manifestations (Salian et al., 2021). Due to the clinical similarities between COVID-19 and regular influenza, COVID-19 patients are often unaware of their illness or misdiagnosed in the clinic, thereby progressing to the severe stage without immediate treatment. It is worth noting that SARS-CoV-2 is able to spread by direct touch, respiratory droplets, aerosolized urine or feces in confined spaces and maintain infectivity for the entire incubation period, making COVID-19 more dangerous than common flu (Otto et al., 2021). SARS-CoV-2 penetrates and infects cells that expressed angiotensin-converting enzyme 2 (ACE2) receptors, thus contributing to virus internalization (Zhou et al., 2020). Structurally, SARS-CoV-2 has a positive-sense single-stranded RNA (+ssRNA) encased in a spiked capsid with a large genome, which decides complex machinery to infect host cells (Zhang & Holmes, 2020). Despite these tough challenges, COVID-19 has no effective therapeutic strategy except for some regular supportive care (Remali & Aizat, 2020). Therefore, it remains a pressing need to investigate interventions to mitigate the risk of COVID-19 and its complications.
Traditional Chinese medicine (TCM), mainly derived from natural plants, is extensively used in the prevention or treatment of several chronic diseases, such as pneumonia, psoriasis, asthma, diabetes, and chronic gastropathy, under the guidance of the theory of TCM. Notably, TCM therapies have shown advantages in treating longer respiratory diseases by effectively alleviating symptoms, decreasing related complications, and improving the living quality of patients. Infectious illnesses, also historically named as ‘Wen Yi’ or ‘Yi Bing’ (Huang et al., 2021), are not new for the Chinese medical field. From ancient times till now, several epidemics motivate Chinese people to build a medical defense system with TCM and fight against the contagion spread by droplets. Prior pandemics, such as severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS) as well as seasonal epidemics caused by multiple influenza viruses, have been efficiently controlled with TCM prescriptions (Remali & Aizat, 2020). It is generally recognized that TCM prevents the development and spread of influenza mainly via interfering with the natural resistance of host cells against viral infections and enhancing the immunity of the human body (You et al., 2020, Lyu et al., 2021, Qian et al., 2021).
With deeper cognitions about mechanisms of COVID-19 at the cellular and molecular level, TCM has played a significant role in the treatment of virus infection and garnered renewed attention during the COVID-19 pandemic. Early intervention of TCM improves cure rates, shortens the course of the disease, delays disease development and lowers death rates in COVID-19 patients (Ding et al., 2017, Huang et al., 2021, Ji et al., 2020). Therefore, the National Administration of Traditional Chinese Medicine also takes the engagement of TCM as a highlight in COVID-19 treatment (https://www.satcm.gov.cn/). Furthermore, the National Health Commission of China (NHC-China) has officially added TCM in the 3rd–8th editions of the diagnosis and treatment guideline of COVID-19 and advised Jinhua Qinggan Granules, Lianhua Qingwen Capsule and Xuebijing Injection (three Chinese patent medicines) and Qingfei Paidu Decoction, Huashi Baidu Formula, and Xuanfei Baidu Formula (three TCM formulas) in clinic prophylaxis and treatment (Luo et al., 2020, Zhang et al., 2020). Except for the above-mentioned formulas, some Chinese herbal injections, including Reduning Injection, Shenfu Injection, Shengmai Injection and Shenmai Injection are used as supplemental treatments for critically ill patients with COVID-19 (Lyu et al., 2021). Based on many COVID-19 clinical cases, three Chinese patent medicines and three Chinese medicine formulas have definite effects on the treatment of clinical symptoms since they can effectively relieve cough, fever, and tiredness symptoms in patients, reduce the proportion of severe cases as well as decrease the duration of fever (Huang et al., 2021). Additionally, an active ingredient isolated from these medicines and formulas effectively alleviated injury symptoms in hACE2 transgenic mice infected with SARS-CoV-2 (Song et al., 2021). Collectively, TCM, especially for three Chinese patent medicines and three Chinese medicine formulas, may become a promising therapeutic strategy in COVID-19 combat.
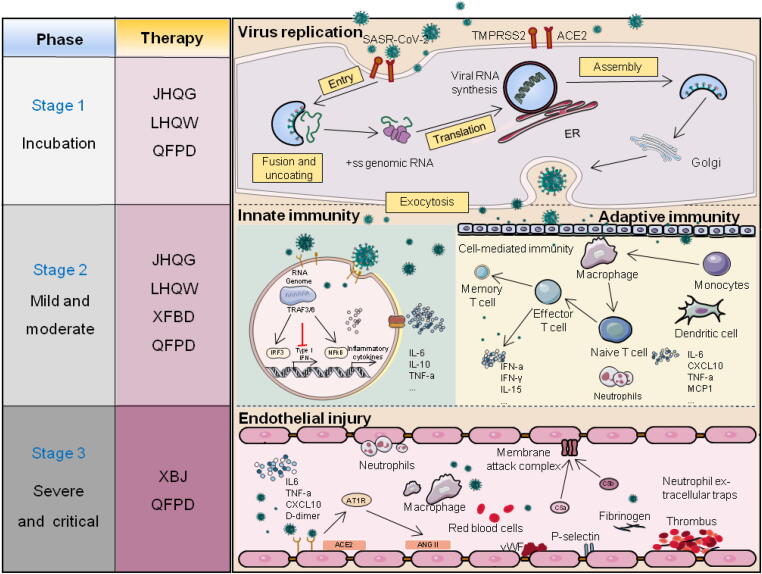
In this review, we mainly focus on the protective effects and potential mechanism of three Chinese patent medicines and three Chinese medicine formulas on the treatment of COVID-19. Meanwhile, this review comprehensively summarizes recent clinical and experimental studies, highlights findings and antiviral mechanisms neglected by prior studies, and ultimately provides a novel perspective for TCM-based prevention and treatment of COVID-19 (Fig. 1).
Fig. 1.
Protective role of three Chinese patent medicines and three Chinese medicine formulas in COVID-19. JHQG: Jinhua Qinggan Granule; LHQW: Lianhua Qingwen Granule; QFPD: Qingfei Paidu Granule; XFBD: Xuanfei Baidu Granule; XBJ: Xuebijing Injection.
2. Pathogenic mechanisms of COVID-19
2.1. Characteristic structural proteins and crucial proteinases of SARS-CoV-2
Genome sequencing of cultured isolated samples from the upper respiratory tract and bronchoalveolar lavage fluid of COVID-19 patients clearly showed that SARS-CoV-2 was comprised of four structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N), 16 nonstructural proteins (NSP 1–16) and nine accessory proteins with unknown functions (Lu et al., 2020, Zhang and Holmes, 2020). For structural proteins, S protein was a type of essential surface protein that mediated the combination of the virus with host cells (V'Kovski et al., 2021, Weisblum et al., 2020). It has been confirmed that SARS-CoV-2 and previous SARS-CoV both exploit the same cell entry receptor, ACE2. ACE2 also shows high affinity with receptor-binding domain (RBD) of S protein, in which transmembrane protease serine 2 (TMPRSS2) promotes the fusion between virus and host cells by cleaving the spike S1/2 cleavage sites (Harrison et al., 2020, Perico et al., 2021). Furthermore, cleaving into micro functional units from polyprotein replies on the proteolytic enzymes and is indispensable for the replication and spread of coronaviruses (Báez-Santos et al., 2014, Báez-Santos et al., 2015). NSP 1–16 were reported to be cleaved from two polyproteins (polyprotein1a (pp1a) and polyprotein1b (pp1b)) encoded by open reading frame 1a (ORF1a) and reading frame 1a (ORF1b), respectively, and the former encoded important proteases like papain-like protease (PLpro) and 3C-like protease (3CLpro) (Perico et al., 2021). Among these necessary proteases for viral replication and transmission, PLpro is implicated for cleavage of I interferon regulatory factor 3 (IRF3) whereas the 3CLpro is responsible for the cleavage of NLR family pyrin domain containing 12 (NLRP12) and TGF-β activated kinase 1binding protein 1 (TAB1) (Klemm et al., 2020), which further trigger and upgrade the generation of cytokines and host innate immune response against SARS-CoV-2 (Moustaqil et al., 2021, Shin et al., 2020). Interestingly, these two proteinases also offer benefits for the immune evasion process. PLpro is closely associated with proteinaceous post-translational modifications cleavage, IFN-β transcription inhibition and the impairment of innate T cell antiviral responses (Taefehshokr, Taefehshokr, Hemmat, & Heit, 2020). In addition, 3CLpro inhibits the IFN-β level and participates in immune evasion due to its self-hydrolytic cleavage activity (Chen et al., 2019, Liu et al., 2020). These studies may establish a framework for explaining the specific immune response observed in COVID-19 patients.
2.2. Immune response elicited by SARS-CoV-2 infection
2.2.1. First step of virus infection
A great number of studies suggests that the interaction between SARS-CoV-2 and ACE2 is the primary entry point for understanding the pathogenic mechanism and exploring the therapeutic targets of COVID-19. The primary viral tropism of SARS-CoV-2 to the lung depends on its strong binding activity with ACE2 receptor, which further causes the structural change of this receptor and allows the fusion of each other membranes with assistance from TMPRSS2-, furin- and cathepsin B/L-mediated elevation of polybasic cleavage sites S1/S2(Melenotte et al., 2020). The proteolytic process activates S protein, allowing viral-host membrane fusion and viral RNA released into the host cytoplasm. In the cytoplasm, viral RNA replicates its genetic materials and assembles new viral particles (Harrison et al., 2020). The widespread expression of ACE2 in multiple organs contributes to SARS-CoV-2-induced infection of several tissues, including but not limited to lung, heart, kidney, liver, intestine, testis and vascular endothelium (Cheng et al., 2007, Zhang et al., 2021, Zhang et al., 2021, Zhang et al., 2021). Notably, the high co-expression of ACE2 and TMPRSS2 was found in type II alveolar epithelial cells in the lung and closely followed by nasal epithelial cells, indicating that the latter may be the primary targets for initial viral infection and are crucial for subsequent viral spread and clearance. Besides, given that severe COVID-19 patients showed platelet hyperactivation and aggregation, the expression of ACE2 and TMPRSS2 on platelets is also regarded as an intriguing result (Zhang et al., 2020), which further promotes the release of coagulation factors, the production of inflammatory cytokines, and the development of leukocyte-platelet aggregates and subsequent thromboembolic consequences. Notably, numerous studies have reported that new mediators may boost the susceptibility and infectivity of SARS-CoV-2 in consideration of the moderate expression of ACE2. As a result, a variety of cellular mediators/receptors as angiotensin II receptor type 2 (AGTR2), CD147, furin, glucose-regulated protein 78 (GRP78), the receptor for advanced glycation end products (RAGE), heparan sulfate, neuropilin-1 (NRP1), and sialic acids have been explored and gradually proven to be engaged in facilitating SARS-CoV-2 to infect host cells (Kyrou, Randeva, Spandidos, & Karteris, 2021). Collectively, ACE2, as the binding receptor of SARS-CoV-2, is regarded as a potential therapeutic target for the clinical treatment of SARS-CoV-2.
2.2.2. Innate immunity associated with COVID-19
Once the virus recognizes the ACE2 receptor, innate immune system will be the front-line defense against viral infection. After colonizing target cells, SARS-CoV-2 rapidly activates innate immune cells by activating different intracellular pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors and melanoma differentiation-associated gene 5 (MDA5) that sense aberrant RNA structures (Schultze & Aschenbrenner, 2021). Following PRR activation, the virus generally triggers molecular signaling cascades and activates downstream transcription factors such as nuclear factor-κB (NF-κB) and IRFs, which establish the original defense of cellular antiviral action by stimulating interferon-stimulated genes (ISGs), as well as promoting the release of several cytokines and chemokines (Onabajo et al., 2020). Regrettably, early COVID-19 clinical cases showed that SARS-CoV-2 generated relatively low levels of IFN I and II in host cells, resulting in only modest upregulation levels of ISGs and proinflammatory cytokines including interleukin 1 β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α), as well as various chemokines, resulting in an inadequate type I IFN response (Park & Iwasaki, 2020). Based on these observations, SARS-CoV-2 seems to have an extraordinary way to antagonize the IFN system and achieve immune evasion. In another word, SARS-CoV-2 appears to target the type I IFN system for destroying the coordinated balance between the innate and adaptive immune responses.
Furthermore, cytokines, chemokines, hormones and metabolites regulate immune cells both locally and systemically in response to innate cytokine storm and immunosuppression (Ye, Wang, & Mao, 2020). The immune response to SARS-CoV-2 infection has initially involved in the activation of macrophages and a subsequent formation of cytokine storm, which seems to be irreplaceable for the initiation and propagation of this hyper-inflammatory reaction (Ye et al., 2020). A pro-inflammatory cascade induced by macrophage activation, including increased levels of IL-2, IL-6, granulocyte colony-stimulating factor (G-CSF), C-X-C motif chemokine ligand 10 (CXCL10), CCL2 and CCL7, is linked to a higher incidence of thrombosis, multiple organ injury and even mortality (Hanff, Mohareb, Giri, Cohen, & Chirinos, 2020). Moreover, these macrophages expressed ACE2 on their surface could interact with spike, promote the activation and formation of neutrophil extracellular traps (NETs) and thus accelerate the release of more cytokines (Middleton et al., 2020, Ramasamy and Subbian, 2021, Veras et al., 2020). These findings suggest that unbalanced innate defense responses may exacerbate lung damage and hyper-inflammatory reactions in severe cases of COVID-19.
2.2.3. Complement activation and COVID-19
The complement system is an important component of innate immune response and comprises over 30 different proteins, including nature proteins, regulatory proteins and complement receptors. It has been well accepted that the complement system can be activated by three main pathways: the classical pathway, the lectin pathway and the alternative pathway. These three pathways all promote the formation of C3 convertases that cleave C3 to generate the pro-inflammatory peptide C3a and a large amount of C3b, and thus opsonize pathogens (Perico et al., 2021). Meanwhile, complement activation produces the proinflammatory C3a, C4a, and C5a molecules that recruit inflammatory cytokines and stimulate neutrophils. However, unrestrained activation of the complement system contributes to acute and chronic inflammation, thrombosis, endothelial cell injury, and ultimately leads to multi-systemic organ failure or even death in patients with COVID-19 (Noris, Benigni, & Remuzzi, 2020). Most recently, unrestrained activation of complement system induced by SARS-CoV-2 was reported to induce the production of C3a and C5a, promote the secretion and production of IL-6 from alveolar macrophages and thus exacerbate ARDS (acute respiratory distress syndrome) (Nilsson et al., 2022). These findings imply that the inhibition of unrestrained activation of the complement system might be an ideal therapeutic method for the clinical treatment of SARS-CoV-2.
2.2.4. Adaptive immunity associated with COVID-19
The majority of viral infections can be influenced by the adaptive immune system, which is mainly involved in B cells, CD4+ T cells and CD8+ T cells. Unlike innate immunity, the adaptive immune system plays a pivotal but different role in the clearance and prevention of SARS-CoV-2 by activating cytotoxic T-cells that destroy infected cells and B cells that produce neutralizing antibodies against virus-specific antigens. The significance of T cell responses to respiratory coronaviruses has been extensively reviewed. Indeed, most of the SARS-CoV-2-induced infections showed detectable T cell responses with obvious CD4+ T cells outnumbering CD8+ T cells (Noh, Jeong, Kim, & Shin, 2021). However, it’s also worthy to note that lymphopenia is a common feature characterized by markedly reduced numbers of CD4+ T cells, CD8+T cells and B cells in some severe COVID-19 patients (Sette & Crotty, 2021). Considering its essential role in the antigen presentation and subsequent induction of adaptive immunity, lymphopenia may be partially explained by an abnormal innate immune response with a feature of low IFN-I (van Eijk et al., 2021). Besides, an increasing number of clinical findings revealed that decreased blood lymphocytes were usually related to an increased number of neutrophils both in blood and bronchoalveolar lavage fluid of COVID-19 patients. Furthermore, the concentration of cytokines might have an impact on the proliferation and survival of T cells in severe COVID-19 patients (de Candia, Prattichizzo, Garavelli, & Matarese, 2021). These findings suggest that improved function of damaged lymphocytes will alleviate adaptive immunological resistance to SARS-CoV-2 in COVID-19 patients, which is also a pivotal target for pharmaceutical intervention.
2.3. Endothelium injury to COVID-19
Generally, SARS-CoV-2 infects the respiratory epithelium of patients, followed by alveolar damage resulting from the increase of cytokines and chemokines, the migration of immune cells and the endothelial injury closely related to the complement system. According to a previous analysis of post-mortem tissue from COVID-19 patients, SARS-CoV-2 has also shown susceptibility and tendency to infect pulmonary endothelial cells (Varga et al., 2020). Notably, COVID-19 syndrome often originates in the lungs and extends to many other organs, such as the heart and kidney (Guan et al., 2020, Noris et al., 2020).About 14% of COVID-19 patients appeared dyspnea, lung opacities, and developed respiratory failure, multiorgan dysfunction or even failure in severe cases (Solomon, Heyman, Ko, Condos, & Lynch, 2021). Indeed, SARS-CoV-2 may alter vascular homeostasis by directly infecting endothelial cells via ACE2 receptor. Mounting evidence suggested that the loss of vessel barrier integrity and the development of a pro-coagulative endothelium contributed to the initiation and progression of ARDS in COVID-19, which might be attributed to induced endothelin, activated inflammatory cell infiltration, and hyperactivity of angiotensin II (Ang II) after ACE2 binding with SARS-CoV-2 (Perico et al., 2021). Given the role of ACE2 played in the conversion of Ang II, endothelial cells with lower ACE2 expression in response to SARS-CoV-2 infection may result in the less synthesis of Ang 1–7 and subsequent a local pro-thrombotic endothelial cell phenotype (Erfinanda et al., 2021). On the other hand, reduced ACE2 expression in COVID-19 patients also activated the kallikrein-kinin system (KKS) to increase vascular permeability, which might break the tight balance between the KKS and renin-angiotensin systems and further lose the control of endothelial cell thromboresistance (Gando & Wada, 2021).
3. Application of three Chinese patent medicines and three Chinese medicine formulas in COVID-19
Numerous clinical studies have shown that TCM occupies an extremely important place in the clinical prevention and management of COVID-19 (Ren, Zhang, & Wang, 2020). TCM has been incorporated in the COVID-19 diagnostic and treated program (Trial version 6) publicized by NHC-China (http://www.nhc.gov.cn/xcs/new_index.shtml). Meanwhile, several impactful TCM prescriptions have been mainly promoted and the most noteworthy of them are three Chinese patent medicines and three Chinese medicine formulas (Table 1).
Table 1.
Studies of Chinese herbal medicines on treatment for COVID-19.
| Chinese herbal prescriptions | Prescription compositions | Clinical manifestation | Mechanism of action | References |
|---|---|---|---|---|
| Qingfei Paidu Decoction | Ephedrae Herba; Glycyrrhizae Radix et Rhizoma; Armeniacae Semen Amarum; Gypsum Fibrosum; Cinnamomi Ramulus; Alismatis Rhizoma; Polyporus; Atractylodis Macrocephalae Rhizoma; Poria; Bupleuri Radix; Hedysarum multijugum Maxim.; Arum ternatum Thunb.; Zingiberis Rhizoma Recens; Asteris Radix et Rhizoma; Farfarae Flos; Belamcandae Rhizoma; Asari Radix et Rhizoma; Dioscoreae Rhizoma; Aurantii Fructus Immaturus; Citri Exocarpium Rubrum; Pogostemonis Herba | Time of nucleic acid conversion, duration of hospital stays and symptoms of fever, cough ↓ ↓ Risk of mortality, acute liver injury and acute kidney injury↓ Anti-viral and anti-inflammatory responses, and energy metabolism↑ |
NF-κB signaling pathway↓, thrombin (F12, F13, F9), and TLR signaling pathway↑ CRP, LC, IL10↑. LDH, CK, CK-MB, BUN,IL6, CCL2, TNF-α, PTGS1/2, CYP1A1, and CYP3A4↓ miRNA (MIR301↓ MIR183/130B↑ CDK7 ↑; TF (LXR) ↓; ACE2 and CD147↓; Interacting with Cdc20, Ido1, Ifng, Ptger4, Spi1, and Tnf↑ |
(Chen et al., 2020, Mou et al., 2021, Shi et al., 2020, Wang et al., 2021, Wang et al., 2020, Xin et al., 2020, Yang et al., 2020, Zhang et al., 2021, Zhao et al., 2021) |
| Xuanfei Baidu Recipe | Ephedrae Herba; Glycyrrhizae Radix et Rhizoma; Armeniacae Semen Amarum; Gypsum Fibrosum; Coicis Semen; Atractylodis Rhizoma; Pogostemonis Herba; Artemisia annua L.; Polygoni Cuspidati Rhizoma et Radix; Verbenae Herb; Phragmitis Rhizoma; Lepidii Semen Descurainiae Semen; Citri Grandis Exocarpium | Fever, cough, fatigue, appetite↓ Profibrotic macrophage responses↑ infiltration of neutrophils↓ activation and migration of macrophages ↓ immunity balance, inflammation, hepatic and biliary metabolism and energy metabolism balance↑ |
IL-6/STAT3 signaling pathway↓ IgG, IgM, TNF-α, IFN-γ, IL-2, IL-4, IL-6, α-SMA, IL-4, IL-6, and iNOS↓ Splenic Lymphocytes, CD4+ and CD8+ T Cells↑ WBC, LC↑; CRP, ESR↓ |
(Wang et al., 2020, Wang et al., 2022; Wang, Wang et al., 2022, Wendisch et al., 2021, Yan et al., 2021) |
| Huashi Baidu Granule | Ephedrae Herba, Armeniacae Semen Amarum, Gypsum Fibrosum, Paeoniae Radix Rubra, Lepidii Semen Descurainiae Semen, Glycyrrhizae Radix et Rhizoma, Pinelliae Rhizoma, Poria, Tsaoko Fructus, Pogostemonis Herba, Atractylodis Rhizoma, Hedysarum multijugum Maxim., Magnoliae Officinalis Cortex, Rhei Radix et Rhizoma | Cough, fever, fatigue, chest discomfort, ground glass area of chest CT, clinical remission, and viral negative conversion time↓ Rate of viral negative conversion and clinical remission↑ |
Lactate dehydrogenase, hemoglobin↑ IL-6, leucocyte, neutrophils, and total bilirubin ↓ MAPK3, MAPK8, TNF, TP53 |
(Liu et al., 2021, Shi et al., 2021) |
| Lianhua Qingwen Granules | Forsythiae Fructus, Lonicerae Japonicae Flos, Ephedrae Herba, Armeniacae Semen Amarum, Gypsum Fibrosum, Isatidis Radix, Dryopteridis Crassirhizomatis Rhizoma, Houttuyniae Herba, Rhei Radix et Rhizoma, Rhodiolae Crenulatae Radix et Rhizoma, L-menthol, Glycyrrhizae Radix et Rhizoma | Time of nucleic acid conversion and lung inflammation extinction ↓ Fever, fatigue, coughing, anorexia, nausea, vomiting, limb pain, chest tightness and shortness↓ Regulating coagulation function, airway microenvironment and immune response |
Modifying morphology of virions; the number of viruses↓ IL-10, LC, ALB and HGB↑; TNF-α, IL-6, CCL-2, CXCL-10, ESR, CRP, D-dimer, and amyloid A↓ Regulating AKT1, JUN, MAPK8, MAPK8, VEGFA, CASP3, PTGS3, MAPK1, MAPK3, CXCL8 Regulating INF-γ signaling pathway, IL-23 signaling pathway, FcγR-mediated phagocytosis, and leukocyte mediated immunity Blocking COVID-19 binding to ACE2 |
(Hu et al., 2021, Liu et al., 2021, Niu et al., 2020, Runfeng et al., 2020, Shen et al., 2021, Xia et al., 2020, Xiao et al., 2020; S. Zheng et al., 2020) |
| Xuebijing Injection | Carthami Flos, Paeoniae Radix Rubra, Chuanxiong Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Sinensis Radix | Systemic inflammatory response syndrome or/and multiple-organ failure↓ Time of ICU care, mechanical ventilation,pneumonia severity index, and mortality↓ |
GSK-3β/ CREB, GSK-3β/ NF-κB and ER stress signaling pathway↑ Oxygenation index, LC, WBC↑; CRP, PCT, D-dimer, AST, ALT↓ Binding of 3CLpro, S protein, and ACE2↑ IL-10↑, IL-6, IL-17, TNF-α↓ Balance of Tregs and Th17 cells↑ Regulating GAPDH, ALB, EGFR, MAPK1, CASP3, STAT3, MAPK8, PTGS2, JUN, IL-2, ESR1, and MAPK14 |
(Cao et al., 2021, Chen et al., 2018, Chen et al., 2020, Ji et al., 2020, José et al., 2020, Shang et al., 2019, Shin et al., 2020, Song et al., 2019, Song et al., 2020, Xing et al., 2020b, Zheng et al., 2020, Zhong et al., 2020) |
| Jinhua Qinggan Granule | Lonicerae Japonicae Flos, Gypsum Fibrosum, Ephedrae Herba, Armeniacae Semen Amarum, Scutellariae Radix, Forsythiae Fructus, Fritillariae Thunbergii Bulbus, Anemarrhenae Rhizoma, Arctii Fructus, Artemisiae Annuae Herba, Menthae Haplocalycis Herba | Fever, cough, poor appetite, diarrhea, fatigue, and mortality↓ | IL-1, IL-6, TNF-α↓. WBC↑ |
(An et al., 2021, Chen et al., 2020, Liu et al., 2020) |
| Time and rate of viral clearance and absorption of pneumonia inflammatory exudate↑ | ||||
| anti-inflammatory effect of western medicine↑; usage rate of antibiotics↓ |
3.1. Lianhua Qingwen Granules in treatment of COVID-19
Lianhua Qingwen Granules (LHQW), a representative TCM recipe developed from suitable modifications of Maxing Shigan decoction (MXSG) and Yinqiao San (YQS), including 13 natural herbs (Forsythiae Fructus, Lonicerae Japonicae Flos, Ephedrae Herba, Armeniacae Semen Amarum, Gypsum Fibrosum, Isatidis Radix, Dryopteridis Crassirhizomatis Rhizoma, Houttuyniae Herba, Rhei Radix et Rhizoma, Rhodiolae Crenulatae Radix et Rhizoma, L-menthol, Glycyrrhizae Radix et Rhizoma), has been recorded as a kind of broad-spectrum antiviral drugs in the treatment of some viral epidemics, such as SARS and H1N1 disease (Zhang, Morris-Natschke, Cheng, Lee, & Li, 2020).Considerable research efforts have been devoted to investigating the effect and mechanism of LHQW, which are not only used as therapeutic medicine but also become a significant source for pharmacological drug discovery. Moreover, the National Administration of Traditional Chinese Medicine also has authorized LHQW to ameliorate moderate respiratory symptoms like coughing, exhaustion, and fever induced by SARS-CoV-2 (NATCM, http://www.satcm.gov.cn/).
Recently, a prospective multicenter randomized controlled trial from Zhong Nanshan’s team revealed that 284 patients who received LHQW capsule medication for 14 d showed several improved COVID-19 symptoms, such as fever, fatigue, coughing, and chest computed tomographic manifestations, as well as the shortener time of LHQW treatment compared with other groups without LHQW (Hu et al., 2021). Another similar clinical retrospective study within 248 moderate COVID-19 cases revealed that COVID-19 patients receiving LHQW showed significantly reduced erythrocyte sedimentation rates and higher cure rates by improving endothelial dysfunction, secondary fibrinolysis, airway microenvironment and regulating the immune response (Shen et al., 2021). In addition to being taken alone, the combination of LHQW and Huoxiang Zhengqi Pills exerted significant clinical advantages, such as mitigating nausea, vomiting, and limbing muscle fatigue, and lowering antibiotic utilization, decreasing the percentage of patients who transitioned to severe condition, based on a randomized controlled trial of 283 COVID-19 patients (Xiao et al., 2020). Moreover, 108 patients with COVID-19 were either taken both arbidol and LHQW or arbidol alone. Patients in the arbidol and LHQW group showed a higher level of lymphocytes, shorter duration of nucleic acid detection and better extinction of lung inflammation than patients in the arbidol group (Liu et al., 2021, Liu et al., 2021).
3.2. Xuebijing Injection in treatment of COVID-19
Xuebijing Injection (XBJ), a widely used TCM injection, is composed of Carthami Flos, Paeoniae Radix Rubra, Chuanxiong Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Sinensis Radix. It has been approved to treat sepsis, ARDS as well as previous infectious diseases caused by H1N1, H7N9, MERS, Ebola virus and dengue virus without causing obvious side effects (Song et al., 2020). Considering its clinical curative effects, NHC-China approved XBJ for the treatment of COVID-19 critical patients, particularly accompanied with inflammatory response syndrome (SIRS) and multi-organ dysfunction [“Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7),” 2020].
A prospective randomized controlled study of 710 cases with severe community-acquired pneumonia showed that the use of XBJ significantly improved the pneumonia severity index, decreased mechanical ventilation time, intensive care unit (ICU) stay time and even mortality (Song et al., 2019). A Meta-analysis involved total 2072 cases also showed that, besides the shortening of ICU stay and mechanical ventilated operation, XBJ treatment remarkably reduced the serum levels of C-reactive protein (CRP), white blood cell (WBC), procalcitonin (PCT), D-dimer, TNF-a and IL-6 in COVID-19 patients without changing the incidence of adverse reactions (Wang, Zhu, Guo, & Wang, 2020).
3.3. Jinhua Qinggan Granule in treatment of COVID-19
Furthermore, Jinhua Qinggan Granule (JHQG) is composed of 13 herbs, including Lonicerae Japonicae Flos, Gypsum Fibrosum, Ephedrae Herba, Armeniacae Semen Amarum, Scutellariae Radix, Forsythiae Fructus, Fritillariae Thunbergii Bulbus, Anemarrhenae Rhizoma, Arctii Fructus, Artemisia annua L., Menthae Haplocalycis Herba, Glycyrrhizae Radix et Rhizoma and has also been recommended as one of the major therapeutic prescriptions for COVID-19 in China.
Accumulating evidence showed that JHQG prominently improved clinical typical symptoms of patients infected by SARS-CoV-2 (Chen, Song, Gao, Zhao, & Ma, 2020). Furthermore, JHQG was also reported to markedly promote the absorption of pneumonia inflammatory exudate and shorten the duration of positive nucleic acid detection based on a clinical case of 44 patients (Liu et al., 2020, Liu et al., 2020). It is worth noting that in a randomized controlled trial of 123 COVID-19 cases, JHQG supplemented with Western prescription oseltamivir minimized antibiotic use and markedly alleviated COVID-19 clinical symptoms (An et al., 2021).
3.4. Qingfei Paidu Decoction in treatment of COVID-19
Qingfei Paidu Decoction (QFPD) is a well-designed combination of four ancient prescriptions, including MSXG, Xiao Chaihu Decoction (XCH), Wu Ling San (WLS), Shegan Mahuang Decoction (SGMH) for treating exogenous fever caused by cold in Zhang Zhongjing's Treatise on Febrile and Miscellaneous Diseases. Because QFPD is effective for patients with SARS-CoV-2 infection at all stages, NHC-China promoted QFPD as a general prescription in the diagnostic and treatment plan of COVID-19 in the 7th Version of COVID-19 Diagnostic and Treatment.
It is worthy to note that QFPD is composed of multiple concordant prescriptions, including MXSG, XCH, SGMH, and WLS, which all contribute to the clinical efficacy of QFPD (Wang et al., 2021, Wang et al., 2022). According to retrospective multicenter cohort research, QFPD effectively resulted in better clinical outcomes, including fewer days of viral shedding, faster recovery times as well as shorter hospital stays (Shi et al., 2020). It has also been reported that QFPD could restore the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), D-dimer, CRP, erythrocyte sedimentation rate (ESR) and the percentage of lymphocytes, which might contribute to its anti-virus, anti-inflammation and pulmonary protection effects in clinic (Ye et al., 2020). In addition, national retrospective registry researchers focused on the correlation between QFPD and mortality of COVID-19 patients and found that QFPD was associated with a significantly lower risk of in-hospital mortality, without additional risk of hepatic injury or acute kidney injury usually observed in COVID-19 patients (Zhang et al., 2021). More importantly, QFPD was reported to not only alleviate the symptoms and inflammatory response in the lung but also has the potential to repair multi-organ impairment in the clinic (Xin et al., 2020). According to a systematic review and Meta-analysis including 16 clinical studies with 11 237 patients, the efficacy and safety of QFPD treatment in COVID-19 patients have been methodically evaluated. The use of QFPD was reported to significantly reduce the time for nucleic acid conversion and the duration of symptoms recovery (Wang et al., 2021).
3.5. Other TCM formulas in treatment of COVID-19
Except for prescriptions mentioned above, some other Chinese medicines formulas show curative effects on COVID-19 as well. Huashi Baidu Decoction (HSBD), is a compound granule composed of 14 Chinese herbs, including Ephedrae Herba, Armeniacae Semen Amarum, Gypsum Fibrosum, Paeoniae Radix Rubra, Lepidii Semen Descurainiae Semen, Glycyrrhizae Radix et Rhizoma, Pinelliae Rhizoma, Poria, Tsaoko Fructus, Pogostemonis Herba, Atractylodis Rhizoma, Hedysarum multijugum Maxim., Magnoliae Officinalis Cortex, Rhei Radix et Rhizoma. Since 2020, NHC-China and NATCM-China have recommended HSBD as one of the principal Chinese herbal formulas for the treatment of COVID-19. From a single-center, open-label, randomized controlled trial of 204 COVID-19 cases, compared with routine treatment alone, combination with HSBD more efficiently alleviated the common COVID-19 symptoms without generating substantial negative transformation in the SARS-CoV-2 test or severe side effects. (Liu et al., 2021) Another non-randomized controlled trial of 60 patients with COVID-19 demonstrated that HSHD could enhance the efficacy of lopinavir-ritonavir pharmaceutical compositions in the COVID-19 treatment in terms of the quarantine period. In addition, a combination of HSBD with other TCM injections, such as Xiyanping Injection (XYP) and XBJ, also displayed better antiviral effects than it used alone (Shi et al., 2021). Xuanfei Baidu Recipe (XFBD), another recipe of the Chinese herbal preparations for COVID-19 therapy, is composed of three empirical prescriptions, including MXSG, Mayi Xinggan Decoction (MYXG) and Tingli Dazao Xiefei Decoction (TLDZ). Although XFBD is not applied in clinical as much as LHQW, it effectively suppressed inflammatory reactions, improved immune system function and alleviated the clinical symptoms of COVID-19 patients, as evidenced by the increased number of WBC and lymphocytes and reduced CRP and ESR in a pilot randomized clinical trial (Xiong, Wang, Du, & Ai, 2020).
4. Mechanism of three Chinese patent medicines and three Chinese medicine formulas in COVID-19 treatment
4.1. Mechanism of LHQW in treatment of COVID-19
4.1.1. Targeting ACE2 receptor
Evidence so far focuses on the role of LHQW in the blockade of receptor recognition and viral replication. A recent study conducted by Nanshan Zhong et al. revealed that LHQW administration suppressed the plaque formation of SARS-COV-2, and decreased virions or modified the virions surface of infected cells (Runfeng et al., 2020). Meanwhile, Niuand his colleagues integrated clinical data and molecular docking analysis and reported that several critical drugs like Forsythiae Fructus and Lonicerae Japonicae Flos of LHQW might effectively antagonize the binding of SARS-CoV-2 with ACE2 in the surface of host cells (Niu et al., 2020). While a network pharmacology article speculated that LHQW might directly regulate AKT1, a member of the MAPK cascade downstream of ACE2, and thus inhibit platelet activation and SARS-CoV-2 infection. Furthermore, six active compounds of LHQW, namely beta-carotene, kaempferol, luteolin, naringenin, quercetin and wogonin, were able to enter the active pocket of Akt1, thereby exerting potential therapeutic effects against COVID-19 (Xia et al., 2020).
4.1.2. Targeting cytokine responses
Except for targeting ACE2 receptor and affecting SARS-CoV-2 invasion and replication, LHQW also processes advantages in regulating immune response and suppressing cytokine storm. Nanshan Zhong’s team first demonstrated that LHQW significantly decreased the mRNA levels of TNF-α, IL-6, CCL-2, and CXCL-10 in Vero E6 cells infected by SARS-COV-2, which are diagnostic markers of COVID-19 patients (Runfeng et al., 2020). Zheng et al. applied extensive detailed network pharmacology analysis and found that LHQW regulated multiple inflammatory processes, such as interferon-gamma (IFN-γ)-, IL-23- and Fc γ receptor-mediated signaling pathway, improved cytokine storm and ACE2-expression-disorder-caused symptoms (Zheng et al., 2020, Zheng et al., 2020). In addition, a study with network pharmacology and molecular docking analyses reported that several important ingredients of LHQW, like balangan and ethanol extracts could eliminate oxygen free radicals by lowering the production and release of inflammatory mediators such as TNF-α, IL-6 and IL-10 that are considered as critical markers for prognosis estimation of COVID‐19 (Xia et al., 2020).
4.2. Mechanism of QFPD in treatment of COVID-19
4.2.1. Targeting ACE2 receptor
Currently, several studies have shown that multiple organ dysfunction caused by COVID-19, including throat, liver, kidney, and pancreas, may be attributed to the wide distribution of ACE2 across these tissues. Recently, emerging evidence indicated that QFPD targets were highly enriched in a variety of COVID-19-related disorders. Additionally, QFPD shared several common targets with SARS-CoV-2 proteins and ACE2-related genes including Mpro, 3CLpro and Nsp14-16 (Chen et al., 2020), indicating that QFPD may simultaneously influence multiple stages of virus infection. Furthermore, Zhao et al. demonstrated that several critical compounds isolated from QFPD, such as baicalin and glycyrrhizic acid, might bind to six host proteins that interact with SARS-CoV-2 proteins and thus contribute to the anti-virus effects of QFPD (Zhao et al., 2021, Zhao et al., 2021).
4.2.2. Targeting virus infection and replication of SARS-CoV-2
It is becoming gradually clear that host cellular miRNAs and cyclin-dependent kinase 7 (CDK7) have crucial roles in the regulation of viral infection and replication. Based on an intriguing new network pharmacology investigation, QFPD was classified into five functional units using the compatibility principle of TCM. More specifically, miRNAs such as miRNA183 and miRNA130A/B/301 were linked to four QFPD functional units with potential anti-viral activities. Furthermore, CDK7 was enriched in 80% of QFPD formulations, indicating that QFPD might impact COVID-19 virus replication viaCDK7-mediated cellular cycle and RNA polymerase II transcription. In addition, innovative functional units of network pharmacology study also explored that the common GO terms of QFPD targets were significantly enriched in small molecule metabolic process, oxidoreductase activity, lipid binding, lipid metabolic process and homeostatic process, suggesting that QFPD may exert anti-viral activity through the regulation of metabolic function (Chen et al., 2020). Additionally, it has been generally acknowledged that lipid metabolic reprogramming occupies an important place in virus replication and immune responses, which could be a novel meaningful and applicable target for anti-SARS-CoV-2 therapy (Ganeshan and Chawla, 2014, Yuan et al., 2019). The innovative study indicated that QFPD might exert anti-viral activity and anti-inflammatory responses through metabolic function. Network pharmacology analysis made by Chen et al. indicated that endocrine system pathways, including the peroxisome proliferator-activated receptors (PPARs), small molecule metabolic process, lipid binding, lipid metabolism and adipocytokine signaling pathways were significantly enhanced in more than four formulations in QFPD including MSXG, XCH, SGMH, and WLS. Moreover, the ability of QFPD to protect COVID-19 and its targeted drug-attacks, like cell division cycle 20 (CDC20), indoleamine 2,3-dioxygenase 1 (IDO1) and IFN-γ were associated with bacterial and viral responses, cytokine, and immune system (Chen et al., 2020).
4.2.3. Targeting immune responses of virus infection
Given the importance of inflammatory cytokine storm and immune response in COVID-19 progression, the latest studies have examined whether QFPD suppresses some pathways associated with immune cell activation. Based on the combination of network pharmacology and experiment validation of QFPD and XFBD, researchers found that both QFPD and XFBD had a significant inhibitory impact on the production of pro-inflammatory markers such as IL-6, TNF-α, MCP-1, and CXCL10 in THP-1-derived M1 macrophages, while did not influence the type 1 IFN signaling pathway in A549 cells. Furthermore, the pinocytosis capability of macrophages and the phosphorylation of IκBα and NF-κB p65 during macrophage polarization were dramatically reduced after treatment with QFPD and XFBD (Shi et al., 2021). This study suggested that QFPD and XFBD might improve the damaged innate immunity induced by SARS-CoV-2 through the inhibition of pro-inflammatory cytokine production, NF-kB signaling pathway and pinocytosis activity in activated macrophages. Subsequently, in an experimental study combined with network pharmacology of QFPD and its major component MXSG against SARS-CoV-2, a total of 129 compounds in QFPD were extracted and identified, mainly belonging to flavonoids, glycosides, carboxylic acids, and saponins. According to the transcriptome results, thrombin and TLR signaling pathways were indicated to be critical pathways for QFPD- and MXSG-mediated anti-inflammatory effects in a rat model of LPS-induced pneumonia. Furthermore, glycyrrhizin acid, a major component of MXSG, was reported to block TLR agonist-induced IL-6 production in macrophages and alleviate subsequent cytokines storm (Yang et al., 2020). Furthermore, Zhao et al. demonstrated that QFPD significantly repressed platelet aggregation and the activities of IL6, CCL-2, TNF-α, NF-κB, CYP1A1 and CYP3A4, and upregulated IL10 expression and they also pointed out four key compounds in QFPD, including baicalin, glycyrrhizin acid, hesperidin and hyperoxide (Zhao et al., 2021).
4.2.4. Targeting coagulation system of virus infection
It is well-acknowledged that the coagulation system could be largely affected by SARS-CoV-2. The transcriptome results showed that QFPD was closely related to complement system and coagulation cascades including such as coagulation factor XII (F12), F13b and F9, which were involved in the conversion of zymogen to serine protease and eventually formed thrombin. Furthermore, according to the data from an established liquid chromatography-mass spectrometry (LC-MS) method, ephedrine, as a main active ingredient of MXSG (a prescription belongs to QFPD), exerted anti-platelet action and attenuated the damage of coagulation system (Yang et al., 2020). The result suggests that MXSG might play an important role in vascular protection and coagulation function improvement thus increase the overall effect of QFPD prescription.
4.3. Mechanism of XBJ in treatment of COVID-19
4.3.1. Targeting virus infection and replication of SARS-CoV-2
Recently, XBJ was shown to protect against SARS-CoV-2-induced Vero E6 cell death, and thus reduce the average size and number of the plaque in a dose-dependent manner (Patel et al., 2020). Meanwhile, according to network pharmacology and molecular docking analyses completed by Xing et al., anhydrosafflor yellow B, salvianolic acid B and rutin in XBJ could directly target COVID-19 crucial proteins like 3CLpro, S protein and ACE2 and thus exert anti-inflammatory and antiviral response against COVID-19 (Xing, Hua, Shang, Ge, & Liao, 2020a).
4.3.2. Targeting immune responses of virus infection
An increasing number of research have recently implicated that XBJ might become an effective pharmacotherapy for immunological imbalance in COVID-19 by regulating immune cell function and inflammatory cytokine release. It was reported that XBJ improves survival rate in a murine model of polymicrobial sepsis after cecal ligation and puncture (CLP) surgeries, partially through preventing cytokine storm, inhibiting inflammation and regulating the balance of Tregs and Th17 cells (Chen et al., 2018). Based on network pharmacology, Zheng et al. further obtained 144 potential COVID-19 targets of XBJ and they suggested that XBJ exerted its therapeutic effects by regulating NF-κB, MAPK, PI3K-Akt, vascular endothelial growth factor, TLR, TNF and renin-angiotensin system signaling pathways (Zheng et al., 2020).
4.3.3. Protecting multiple organ damage caused by virus infection
Until now, XBJ is one of the most effective TCM drugs approved for treating sepsis and multiple organ dysfunction in the clinic. Lots of complications have happened in the severe and critical phase of COVID-19, including septicemia and multiple organ damage like cardiac, hepatic and renal injury (Chen et al., 2020, José et al., 2020). A recent study also showed that the pretreatment of XBJ markedly reduced serum levels of AST and ALT and inhibited the release of pro-inflammatory factors into serum, more importantly, improved liver function by upregulating glycogen synthase kinase 3 beta (GSK-3β) and strengthening the binding of phospho-cAMP-response element-binding protein (p-CREB) to calcium-binding protein (CBP) (Cao et al., 2021). In an interesting experimental study combined with network pharmacology analysis, XBJ enhanced the viability of renal cells, abolished the colonization of Candida albicans in kidneys and rescued mice from lethal candida-induced sepsis (Shang et al., 2019).
4.4. Mechanism of XFBD in treatment of COVID-19
XFBD, which has shown a curative effect in epidemics induced by coronaviruses since SARS 2013, is approved to treat COVID-19 and achieve clinical efficiency nowadays. Thus, it is necessary to explore the regulatory mechanism in depth. Wang et al. applied network pharmacology and found that there were 109 of XFBD targets associated with both the disease targets of COVID-19 and the disease pathways of viral infection, energy metabolism, parasites and bacterial infection and lung injury (Wang et al., 2020, Wang et al., 2022, Wang et al., 2020). Meanwhile, Zhao et al. combined a comprehensive research system to explore the pharmacological mechanism and bioactive ingredients of XFBD. They profiled 154 compounds in XFBD and identified the inflammatory pathways as primary targets. Then they verified that XFBD decreased pulmonary inflammation and serum proinflammatory cytokines levels in an acute inflammation mice model induced by LPS, and inhibited macrophage activation and migration in a zebrafish wounding model and RAW 264.7 cell lines. Moreover, either single herbs or effective compounds isolated from XFBD that inhibiting macrophage-related immunoreaction were also identified (Zhao et al., 2021). It has been demonstrated that XFBD inhibited the expression and secretion of IL-6 and TNF-α and the activity of iNOS in both LPS-treated RAW264.7 macrophages and LPS-induced acute lung injury mice through the PD-1/IL17A pathway (Wang, Wang, et al., 2022), suggesting the possibility of XFBD in regulating the infiltration of neutrophils and macrophages in COVID-19 patients. Furthermore, XFBD protected against cyclophosphamide (CY)-induced acute inflammation, which significantly suppressed mouse serum levels of TNF-α, IFN-γ, IgG, and IgM and the expression of IL-2, IL-4, and IL-6 in the spleen, enhanced splenic lymphocyte proliferation response and reduced the counts of CD4+ and CD8+ T lymphocytes (Yan et al., 2021). Therefore, XFBD might play a crucial role in preventing immunosuppression and become a potential candidate for immune modification and therapy. What is more serious is that SARS-CoV-2 has been testified to induce evident pulmonary fibrosis and develop into acute respiratory distress syndrome (ARDS) (Wendisch et al., 2021). A recent study showed that XFBD effectively inhibited the expression of α-SMA, IL-4, IL-6, iNOS and the migration of fibroblasts, prevented macrophage infiltration and eventually bleomycin-induced pulmonary fibrosis by inhibiting IL-6/STAT3 signaling (Wang, Sang, et al., 2022).
4.5. Mechanism of other Chinese patent medicines and Chinese medicine formulas in treating COVID-19
In addition to the above TCM, there are a few but significant studies that explore the potential mechanism of other Chinese patent medicines and Chinese medicine formulas in curing COVID-19. Gong et al. applied network pharmacology and molecular docking methods and speculated that JHQG might affect TNF, PI3K/Akt, and HIF-1 signaling pathways via directly binding ACE2 and thus regulating its targets including prostaglandin-endoperoxide synthase 1 (PTGS1), PTGS2, heat shock protein 90 alpha family class B member 1 (HSP90AB1), heat shock protein 90 alpha family class A member 1 (HSP90AA1), and nuclear receptor coactivator 2 (NCOA2) (Gong, 2020). Similarly, Tao et al. also used above methods to investigate the molecular targets and mechanisms of HSBD in the treatment of COVID-19 and they found that HSBD largely regulated the signaling pathways of TNF, PI3K-Akt, NOD-like receptor, MAPK and HIF-1, of which top two compounds, baicalein and quercetin, had high affinity with ACE2 (Tao et al., 2020).
5. Discussion
COVID-19 pneumonia, a sudden and devasting health problem, has rapidly spread and inflicted immense losses on human lives and properties on a global scale over two years. SARS-CoV-2, the pathogen of COVID-19 with ever-changing and increasingly dangerous variants, such as variant Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and latest Omicron (B.1.1.529), is considered as a kind of highly transmissible pathogenic coronavirus, transmits through complicated ways like respiratory droplets, aerosol, and surface contamination(Mistry et al., 2021). From a clinical diagnostic perspective, apart from severe flu-like symptoms, patients infected with COVID-19 are more likely to undertake life-threatening severe symptoms including fulminant diseases like sepsis, ARDS and multi organ failure (Harrison et al., 2020, Wiersinga et al., 2020). For analyzing the pathogenesis of COVID-19, the characteristic structural proteins and proteases, and how SARS-CoV2 attaches to and infects host cells have been summarized. Up to date, most innovative drugs are mainly focused on the abnormal immunologic process affected by SARS-CoV-2. However, the unstable variants, expensive costs and limited therapeutic time may be the challenge for drug discovery. Even worse, except for some regular antivirus agents like remdesivir, broad-spectrum antibiotics, reliever medications, and vaccines injection, there is no effective specific drug for COVID-19, aggravating tremendous economic burden and increasing global morbidity. Therefore, it is urgent to provide complementary and alternative therapies for the treatment of COVID-19 patients.
TCM is a well-accepted therapy with long-lasting application history in China, which combines ancient medical theories with clinical evidence. Under the guidelines of ‘Yi Bing’ theory (Huang et al., 2021), several herbal formulas are effective in treating COVID-19 and have been recommended in clinical practice by NHC-China for precaution, treatment, and prognosis of this epidemic since its outbreak from 2019 (Luo et al., 2020). Mounting clinical and laboratory studies demonstrated that medication combined with TCM showed better effects than taken Western medicine alone in treating the COVID-19.Notably, three Chinese patent medicines and three Chinese medicine formulas are beneficial for relieving the typical clinical symptoms, improving the lung features and directing regulating inflammatory and immune response, and have been approved to symptomatically treat COVID-19 in different stages with significant therapeutic efficacy. Among them, LHQW, recommended by the Chinese Academy of Engineering (CAE), is quite effective in reducing symptoms of mild and moderate COVID-19 patients, as well as effectively limiting the severe pneumonia conversion rate. Besides, pharmacodynamic studies indicate that LHQW may significantly inhibit the SARS-CoV-2 activity and reduce the abnormal activation of cytokines (Wu et al., 2020, Xiao et al., 2020). In addition, long-term clinical reports show that QFPD, the first and common prescription proposed by NHC-China, plays a crucial role in preventing the conversion of common COVID-19 symptoms to severe and critical stages. In a recent review, Boli Zhang’s team also points out that QFPD can ameliorate extensive adverse symptoms and is also suitable for the treatment of COVID-19 in mild, moderate, severe and critical stages (Lyu et al., 2021). As previously demonstrated, QFPD seems to significantly reduce viral activity, pathological immune response and coagulation activation induced by SARS-CoV-2 (Shi et al., 2021, Yang et al., 2020). It is also worth mentioning that XBJ, which is refined from ‘XuefuZhuyu Decoction (XFZY)’ and applied in the clinic since SARS 2003, is extensively utilized in the severe and critical stage of COVID-19 patients with sepsis and multiple organ damage. Except for the decline of severe illness conversion and improvement of recovery rate, XBJ also has the advantage of improving COVID-19-induced systemic inflammatory response and multiple organ failure (Cao et al., 2021, Wang et al., 2019) (https://www.satcm.gov.cn/). Therefore, for COVID-19 patients, different TCM formulas may be used symptomatically to treat their different stages of disease. In particular, we elaborate and emphasize the characteristic immunological process of SARS-CoV-2 infection in great detail, however, most of the clinical or experimental studies of three Chinese patent medicines and three Chinese medicine formulas mainly focused on the regulatory effects of TCM on the typical inflammatory signaling pathways or inflammatory factors without investigating the changes of interaction between SARS-CoV-2 and ACE2, complement activation and T cell subsets. Thus, the anti-virus activities and potential mechanism of the above-reported TCMs still need extensive research. If possible, we expect that this review may offer a promising research direction for Chinese medicines against COVID-19 in the future.
Based on the comprehensive analysis of the frequency of current drugs used in COVID-19 treatment, Scutellariae Radix, Glycyrrhizae Radix et Rhizoma, Hedysarum multijugum Maxim., Saposhnikoviae Radix, Atractylodis Macrocephalae Rhizoma, Lonicerae Japonicae Flos, and Forsythiae Fructus are the most high-frequency and promising therapeutic medicines (Xin et al., 2020). Notably, Scutellariae Radix and licorice the most common herbs. Scutellariae Radix, an essential TCM, has been widely used over for 2000 years in China that exerts obvious therapeutic effects on the treatment of multiple respiratory diseases. Baicalein, the leading chemical constituent of Scutellariae Radix, exerts anti-inflammation and broad-spectrum antiviral effects. Recent studies showed that Baicalein could inhibit the activity and replication of SARS-CoV-2 and relieve the inflammatory infiltration in lung tissue infected with SARS-CoV-2 (Huang et al., 2020, Huang et al., 2020, Song et al., 2021). In addition, some previous studies showed the antiviral activity of glycyrrhizin (belongs to licorice is the main ingredient of MXSG in QFPD) in inhibiting SARS-COV2. Moreover, glycyrrhizin also inhibits the main protease Mpro to block the replication of SARS-CoV-2 (van de Sand et al., 2021). Based on the results of in vitro and in vivo experiments and structure–activity relationship analysis, another two ingredients isolated from licorice, glycyrrhizin acid and licorice-saponin A3 have been testified to potently inhibit SARS-CoV-2 infection and replication by targeting the nsp7 protein and the spike protein receptor binding domain, respectively (van de Sand et al., 2021). Furthermore, other herbs also show potential as promising therapeutic candidates for the treatment of COVID-19 patients. Thus, we speculate that these active herbs or related ingredients contribute to the protective anti-virus effects of three Chinese patent medicines and three Chinese medicine formulas in COVID-19 therapy.
6. Conclusion
Currently, the spread of COVID-19 is still uncontrollable with the fact that the numbers of confirmed and death cases are continuously rising, and no specific medicine has been discovered for COVID-19. After verified by sufficient evidence from the clinic and laboratory, TCM, especially for three Chinese patent medicines and three Chinese medicine formulas, can alleviate the symptoms of COVID-19 from mild, moderate to severe and critical phases by affecting multiple effective targets, thus making it an irreplaceable option for COVID-19 treatment. In summary, considering the critical role of TCM in clinical practice, clarifying regulatory mechanisms, investigating complex ingredients and improving the practice guidelines of TCM in treating COVID-19 will not only benefit the international public health care at present but also contribute to the establishment of epidemic prevention system in the future.
Authors’ contributions
XL conceived the original idea. XG supervised the study. XL, KJ, YL and TL prepared the manuscript. All authors have approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202006 to XG and XL); Beijing Municipal Science & Technology Commission (No. 7212174 to XL); National Natural Science Foundation of China (No. 82004045 to XL); Beijing Nova Program of Science & Technology (No. Z191100001119088 to XL); and the Young Talents Promotion Project of China Association of Traditional Chinese Medicine (No. 2020-QNRC2-01 to XL).
Contributor Information
Xiaohong Gu, Email: guxh1003@126.com.
Xiaojiaoyang Li, Email: xiaojiaoyang.li@bucm.edu.cn.
References
- An X., Xu X., Xiao M., Min X., Lyu Y., Tian J.…Tong X. Efficacy of Jinhua Qinggan Granules combined with western medicine in the treatment of confirmed and suspected COVID-19: A randomized controlled trial. Frontiers in Medicine (Lausanne) 2021;8 doi: 10.3389/fmed.2021.728055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Santos Y.M., Barraza S.J., Wilson M.W., Agius M.P., Mielech A.M., Davis N.M.…Mesecar A.D. X-ray structural and biological evaluation of a series of potent and highly selective inhibitors of human coronavirus papain-like proteases. Journal of Medical Chemistry. 2014;57(6):2393–2412. doi: 10.1021/jm401712t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Research. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Li Z., Ren Y., Wang M., Yang Z., Zhang W.…Nie S. Xuebijing protects against septic acute liver injury based on regulation of GSK-3β pathway. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.627716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Song Y.P., Gao K., Zhao L.T., Ma L. Efficacy and safety of Jinhua Qinggan Granules for coronavirus disease 2019 (COVID-19): A protocol of a systematic review and Meta-analysis. Medicine (Baltimore) 2020;99(24):e20612. doi: 10.1097/MD.0000000000020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang D., Sun Z., Gao L., Zhu X., Guo J.…Xiao S. Arterivirus nsp4 antagonizes interferon beta production by proteolytically cleaving NEMO at multiple sites. Journal of Virology. 2019;93(12) doi: 10.1128/JVI.00385-19. e00385-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R.…Cao Y.B. Protection against COVID-19 injury by Qingfei Paidu Decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomedicine Pharmacotherapy. 2020;129 doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Feng Y., Shen X., Pan G., Fan G., Gao X.…Zhu Y. Anti-sepsis protection of Xuebijing Injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. Journal of Ethnopharmacology. 2018;211:358–365. doi: 10.1016/j.jep.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Chen Y.M., Zheng Y., Yu Y., Wang Y., Huang Q., Qian F.…Zhang Y.Z. Blood molecular markers associated with COVID-19 immunopathology and multi-organ damage. Embo Journal. 2020;39(24):e105896. doi: 10.15252/embj.2020105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clinical Microbiology Reviews. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Candia P., Prattichizzo F., Garavelli S., Matarese G. T Cells: Warriors of SARS-CoV-2 Infection. Trends in Immunology. 2021;42(1):18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). (2020). Chinese Medecine Journal (Engl), 133(9), 1087-1095. [DOI] [PMC free article] [PubMed]
- Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q.…Zhang F. The Chinese prescription Lianhuaqingwen Capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complementary Alternative Medicine. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfinanda L., Ravindran K., Kohse F., Gallo K., Preissner R., Walther T., Kuebler W.M. Oestrogen-mediated upregulation of the Mas receptor contributes to sex differences in acute lung injury and lung vascular barrier regulation. European Respiratory Journal. 2021;57(1) doi: 10.1183/13993003.00921-2020. [DOI] [PubMed] [Google Scholar]
- Gando S., Wada T. Thromboplasminflammation in COVID-19 Coagulopathy: Three viewpoints for diagnostic and therapeutic strategies. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.649122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annul Reviews Immunology. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P.-Y. Exploring active compounds of Jinhua Qinggan Granules for prevention of COVID-19 based on network pharmacology and molecular docking. Chinese Traditional and Herbal Drugs. 2020;51(7):1685–1693. [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X.…Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. New England Journal Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. American Journal of Hematology. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends in Immunology. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B.…Zhong N.S. Efficacy and safety of Lianhuaqingwen Capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Zhang P., Zhang Z., Youn J.Y., Wang C., Zhang H., Cai H. Traditional Chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacology Therapy. 2021;225 doi: 10.1016/j.pharmthera.2021.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Liu Y., Zhang Y., Zhang R., Zhu C., Fan L.…Shi Y. Baicalein inhibits SARS-CoV-2/VSV replication with interfering mitochondrial oxidative phosphorylation in a mPTP dependent manner. Signal Transduction and Targeted Therapy. 2020;5(1):266. doi: 10.1038/s41392-020-00353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacology Research. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., Bai Q., Wu X., Zhang D.W., Wang S., Shen J.L., Fei G.H. Unique synergistic antiviral effects of Shufeng Jiedu Capsule and oseltamivir in influenza A viral-induced acute exacerbation of chronic obstructive pulmonary disease. Biomedicine Pharmacotherapy. 2020;121 doi: 10.1016/j.biopha.2019.109652. [DOI] [PubMed] [Google Scholar]
- José R.J., Williams A., Manuel A., Brown J.S., Chambers R.C. Targeting coagulation activation in severe COVID-19 pneumonia: Lessons from bacterial pneumonia and sepsis. European Respiratory Reviews. 2020;29(157) doi: 10.1183/16000617.0240-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevadiya B.D., Machhi J., Herskovitz J., Oleynikov M.D., Blomberg W.R., Bajwa N.…Gendelman H.E. Diagnostics for SARS-CoV-2 infections. Nature Materials. 2021;20(5):593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm T., Ebert G., Calleja D.J., Allison C.C., Richardson L.W., Bernardini J.P.…Komander D. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. Embo Journal. 2020;39(18):e106275. doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I., Randeva H.S., Spandidos D.A., Karteris E. Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduction Target Therapy. 2021;6(1):21. doi: 10.1038/s41392-020-00460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yang W., Liu Y., Lu C., Ruan L., Zhao C.…Huang L. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Shi F., Tu P., Chen C., Zhang M., Li X., Li C. Arbidol combined with the Chinese medicine Lianhuaqingwen capsule versus arbidol alone in the treatment of COVID-19. Medicine (Baltimore) 2021;100(4):e24475. doi: 10.1097/MD.0000000000024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liang C., Xin L., Ren X., Tian L., Ju X.…Jian Y. The development of Coronavirus 3C-Like protease (3CL(pro)) inhibitors from 2010 to 2020. European Journal Medicine Chemistry. 2020;206 doi: 10.1016/j.ejmech.2020.112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li X., Gou C., Li L., Luo X., Zhang C.…Wang X. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. Journal Traditional Chinese Medicine. 2020;40(3):467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H.…Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Gao Y., Zou J., Zhang S., Chen H., Liu Q.…Wang S. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chinese Medine. 2020;15:94. doi: 10.1186/s13020-020-00375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu M., Fan G., Xiao G., Wang T., Xu D., Gao J.…Zhang B. Traditional Chinese medicine in COVID-19. Acta Pharmaceutica Sinica B. 2021;11(11):3337–3363. doi: 10.1016/j.apsb.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melenotte C., Silvin A., Goubet A.G., Lahmar I., Dubuisson A., Zumla A.…Zitvogel L. Immune responses during COVID-19 infection. Oncoimmunology. 2020;9(1):1807836. doi: 10.1080/2162402X.2020.1807836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P.…Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M.…Pepper M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou J.F., Lin X.Z., Su H.L., Lu H.L., Liu Q.B., Liang B.…Zhou X.L. Anti-hepatitis B virus activity and hepatoprotective effect of des(rhamnosyl) verbascoside from Lindernia ruellioides in vitro. Phytotherapy Research. 2021;35(8):4555–4566. doi: 10.1002/ptr.7159. [DOI] [PubMed] [Google Scholar]
- Moustaqil M., Ollivier E., Chiu H.P., Van Tol S., Rudolffi-Soto P., Stevens C.…Gambin Y. SARS-CoV-2 proteases PLpro and 3CLpro cleave IRF3 and critical modulators of inflammatory pathways (NLRP12 and TAB1): Implications for disease presentation across species. Emerging Microbes Infections. 2021;10(1):178–195. doi: 10.1080/22221751.2020.1870414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Persson B., Eriksson O., Fromell K., Hultström M., Frithiof R.…Ekdahl K.N. How the innate immune system of the blood contributes to systemic pathology in COVID-19-induced ARDS and provides potential targets for treatment. Frontiers in Immunology. 2022;13 doi: 10.3389/fimmu.2022.840137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Wang R.L., Wang Z.X., Zhang P., Bai Z.F., Jing J.…Xiao X.H. Rapid establishment of traditional Chinese medicine prevention and treatment of 2019-nCoV based on clinical experience and molecular docking. China Journal of Chinese Materia Medica. 2020;45(6):1213–1218. doi: 10.19540/j.cnki.cjcmm.20200206.501. [DOI] [PubMed] [Google Scholar]
- Noh J.Y., Jeong H.W., Kim J.H., Shin E.C. T cell-oriented strategies for controlling the COVID-19 pandemic. Nature Reviews Immunology. 2021;21(11):687–688. doi: 10.1038/s41577-021-00625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Internatinal. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onabajo O.O., Banday A.R., Stanifer M.L., Yan W., Obajemu A., Santer D.M.…Prokunina-Olsson L. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nature Genetics. 2020;52(12):1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S.P., Day T., Arino J., Colijn C., Dushoff J., Li M.…Ogden N.H. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Current Biology. 2021;31(14):R918–R929. doi: 10.1016/j.cub.2021.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A., Iwasaki A. Type I and Type III interferons - induction, signaling, evasion, and application to Combat COVID-19. Cell Host Microbe. 2020;27(6):870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K., Harrison S., Elkhashab M., Trotter J., Herring R., Rojter S.…Noureddin M. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: A phase 2 randomized controlled trial. Hepatology (Baltimore, Md.) 2020;72(1):58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nature Reviews Nephrology. 2021;17(1):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Xu H., Lv D., Liu W., Chen E., Zhou Y.…Fan X. Babaodan controls excessive immune responses and may represent a cytokine-targeted agent suitable for COVID-19 treatment. Biomedicine Pharmacotherapy. 2021;139 doi: 10.1016/j.biopha.2021.111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S., Subbian S. Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clinical Microbiology Reviews. 2021;34(3) doi: 10.1128/CMR.00299-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remali J., Aizat W.M. A review on plant bioactive compounds and their modes of action against coronavirus infection. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.589044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacology Research. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S.…Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacology Research. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian V.S., Wright J.A., Vedell P.T., Nair S., Li C., Kandimalla M.…Kandimalla K.K. COVID-19 transmission, current treatment, and future therapeutic strategies. Molecular Pharmaceutics. 2021;18(3):754–771. doi: 10.1021/acs.molpharmaceut.0c00608. [DOI] [PubMed] [Google Scholar]
- Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang T., Yu Q., Ren T., Wang X.T., Zhu H., Gao J.M.…Li M.C. Xuebijing Injection maintains GRP78 expression to prevent Candida albicans-induced epithelial death in the kidney. Frontiers in Pharmacology. 2019;10:1416. doi: 10.3389/fphar.2019.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Li J., Tu S., Wu Y., Peng Y., Chen G., Chen C. Positive effects of Lianhuaqingwen granules in COVID-19 patients: A retrospective study of 248 cases. Journal of Ethnopharmacology. 2021;278 doi: 10.1016/j.jep.2021.114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Guo L., Liu B., Bian Y., Chen R., Chen S.…Huang L. Efficacy and safety of Chinese herbal medicine versus lopinavir-ritonavir in adult patients with coronavirus disease 2019: A non-randomized controlled trial. Phytomedicine. 2021;81 doi: 10.1016/j.phymed.2020.153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H.…Wang Y. Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study. Pharmacology Research. 2020;161 doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A.…Dikic I. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587(7835):657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J.J., Heyman B., Ko J.P., Condos R., Lynch D.A. CT of post-acute lung complications of COVID-19. Radiology. 2021;301(2):E383–E395. doi: 10.1148/radiol.2021211396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhang L., Xu Y., Yang D., Zhang L., Yang S.…Du G. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochemical Pharmacology. 2021;183 doi: 10.1016/j.bcp.2020.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K.…Bai C. XueBiJing Injection versus placebo for critically Ill patients with severe community-acquired pneumonia: A randomized controlled trial. Critical Care Medicine. 2019;47(9):e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhang M., Yin L., Wang K., Zhou Y., Zhou M., Lu Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2) Internatinal Journal of Antimicrobial Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taefehshokr N., Taefehshokr S., Hemmat N., Heit B. Covid-19: Perspectives on innate immune evasion. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q., Du J., Li X., Zeng J., Tan B., Xu J.…Chen X.L. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Development and Industry Pharmacy. 2020;46(8):1345–1353. doi: 10.1080/03639045.2020.1788070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V'Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nature Reviews Microbiology. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sand L., Bormann M., Alt M., Schipper L., Heilingloh C.S., Steinmann E.…Krawczyk A. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13(4):609. doi: 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk L.E., Binkhorst M., Bourgonje A.R., Offringa A.K., Mulder D.J., Bos E.M.…van Goor H. COVID-19: Immunopathology, pathophysiological mechanisms, and treatment options. Journal of Pathology. 2021;254(4):307–331. doi: 10.1002/path.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S.…Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C.…Cunha F.Q. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. Journal of Experimental Medicine. 2020;217(12) doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Shi Q.P., Ding F., Jiang X.D., Tang W., Yu M.L., Cheng J.Q. Reevaluation of the post-marketing safety of Xuebijing injection based on real-world and evidence-based evaluations. Biomedicine Pharmacotherapy. 2019;109:1523–1531. doi: 10.1016/j.biopha.2018.10.190. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhu J., Guo J., Wang Q. Could Xuebijing Injection reduce the mortality of severe pneumonia patients? A systematic review and Meta-analysis. Evidencend Based Complementary Alternative Medicine. 2020;2020:9605793. doi: 10.1155/2020/9605793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhu H., Li M., Liu Y., Lai H., Yang Q.…Ge L. Efficacy and safety of Qingfei Paidu Decoction for treating COVID-19: A wystematic review and Meta-analysis. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.688857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.Q., Yang S.J., Xie C.G., Shen Q.L., Li M.Q., Lei X.…Huang M. Clinical observation of Qingfeipaidu Decoction in the treatment of COVID-19. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(1):13–18. [Google Scholar]
- Wang Y., Li X., Zhang J.H., Xue R., Qian J.Y., Zhang X.H.…Zhang B.L. mechanism of Xuanfei Baidu Tang in treatment of COVID-19 based on network pharmacology. China Journal of Chinese Materia Medica. 2020;45(10):2249–2256. doi: 10.19540/j.cnki.cjcmm.20200325.401. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sang X., Shao R., Qin H., Chen X., Xue Z.…Yang J. Xuanfei Baidu Decoction protects against macrophages induced inflammation and pulmonary fibrosis via inhibiting IL-6/STAT3 signaling pathway. Journal of Ethnopharmacology. 2022;283 doi: 10.1016/j.jep.2021.114701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang X., Li Y., Xue Z., Shao R., Li L.…Yang J. Xuanfei Baidu Decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacology Research. 2022;176 doi: 10.1016/j.phrs.2022.106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.…Bieniasz P.D. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendisch D., Dietrich O., Mari T., von Stillfried S., Ibarra I.L., Mittermaier M.…Sander L.E. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184(26):6243–6261. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. Jama. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu L., Chen Y., Ma Y., Yang Z., Yang N., Deng W.…Lin L. Clinical practice guideline on treating influenza in adult patients with Chinese patent medicines. Pharmacology Research. 2020;160 doi: 10.1016/j.phrs.2020.105101. [DOI] [PubMed] [Google Scholar]
- Xia Q.D., Xun Y., Lu J.L., Lu Y.C., Yang Y.Y., Zhou P.…Wang S.G. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Proliferation. 2020;53(12):e12949. doi: 10.1111/cpr.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y.…Tong X. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacology Research. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L.…Yu R. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with western medicine for COVID-19 treatment. Biomedicine Pharmacotherapy. 2020;129 doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Hua Y.R., Shang J., Ge W.H., Liao J. Traditional Chinese medicine network pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chinese Journal of Nature Medicine. 2020;18(12):941–951. doi: 10.1016/S1875-5364(20)60038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Hua Y.R., Shang J., Ge W.H., Liao J. Traditional Chinese medicine networpk pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chinese Journal of Nature Medicine. 2020;18(12):941–951. doi: 10.1016/S1875-5364(20)60038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial. Integrative Medicine Research. 2020;9(3) doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang Y., Li X., Li X.X. Analysis on prevention plan of coronavirus disease 2019 (COVID-19) by traditional Chinese medicine in various regions. Chinese Traditional and Herbal Drugs. 2020;51(4):866–872. [Google Scholar]
- Yan H., Lu J., Wang J., Chen L., Wang Y., Li L.…Zhang H. Prevention of cyclophosphamide-induced immunosuppression in mice with traditional Chinese medicine Xuanfei Baidu Decoction. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.730567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R.…Wang Y. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against coronavirus disease 2019 (COVID-19): In silico and experimental study. Pharmacology Research. 2020;157 doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. Journal of Infection. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. European Radiology. 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y.N., Yan H., Wang S.C., Luo X.H., Zhao X. Therapeutic strategy of traditional Chinese medicine for COVID-19. Drug Evaluation Research. 2020;43(4):613–619. [Google Scholar]
- Yuan S., Chu H., Chan J.F., Ye Z.W., Wen L., Yan B.…Yuen K.Y. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nature Communications. 2019;10(1):120. doi: 10.1038/s41467-018-08015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zheng X., Bai X., Wang Q., Chen B., Wang H.…Li J. Association between use of Qingfei Paidu Tang and mortality in hospitalized patients with COVID-19: A national retrospective registry study. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2021.153531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xiang R., Huo S., Zhou Y., Jiang S., Wang Q., Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduction Target Therapy. 2021;6(1):233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y.…Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of Hematology Oncology. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wan N., Huang X.Y., Luo J., Li Y., Zhang Y.T., Qiu T., Wu Z.F., Yang M. Application of aromatic Chinese materia medica in corona virus disease 2019 (COVID-19) Chinese Traditional and Herbal Drugs. 2021;52(11):3408–3417. [Google Scholar]
- Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.J., Morris-Natschke S.L., Cheng Y.Y., Lee K.H., Li R.T. Development of anti-influenza agents from natural products. Medicine Research Reviews. 2020;40(6):2290–2338. doi: 10.1002/med.21707. [DOI] [PubMed] [Google Scholar]
- Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F.…Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., Liu, H., Wang, Y., Wang, S., Xun, D., Wang, Y., … Zhang, B. (2021). Multimodal identification by transcriptomics and multiscale bioassays of active components in Xuanfeibaidu Formula to suppress macrophage-mediated immune response. Engineering (Beijing), 20, 63–76. [DOI] [PMC free article] [PubMed]
- Zheng S., Baak J.P., Li S., Xiao W., Ren H., Yang H.…Wen C. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in Corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.J., Yan Q., Ni Y.S., Zhan S.F., Yang L.L., Zhuang H.F.…Jiang Y. Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology. BioData Mining. 2020;13:17. doi: 10.1186/s13040-020-00227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Rao Z., Rao J., Han G., Wang P., Jiang T.…Wang X. Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell. 2020;19(8):e13186. doi: 10.1111/acel.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W.…Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]